Abstract
The embryonic urogenital sinus mesenchyme (UGM) induces prostate epithelial morphogenesis in development. The molecular signals that drive UGM-mediated prostatic induction have not been defined. We hypothesized that the TGF-β signaling directed the prostatic induction. UGM from TGF-β type II receptor stromal conditional knockout mice (Tgfbr2fspKO) or control mice (Tgfbr2floxE2/floxE2)was recombined with wild-type adult mice bladder urothelial cells. The resulting urothelium associated with Tgfbr2floxE2/floxE2 UGM was instructively differentiated into prostatic epithelium, as expected. In contrast, the urothelium associated with Tgfbr2fspKO UGM permissively maintained the phenotype of bladder epithelial cells. Microarray analysis of UGM tissues suggested the down-regulation of multiple Wnt ligands and the up-regulation of the Wnt antagonist, Wif 1, by the Tgfbr2fspKO UGM compared with Tgfbr2floxE2/floxE2 UGM. The overexpression of Wif-1 by wild-type UGM resulted in the inhibition of prostatic induction. These data suggest that the stromal TGF-β activity mediated by paracrine Wnt is necessary for the induction of prostatic differentiation. As Wnt ligands mediate differentiation and maintain the stem cell phenotype, the contribution of mouse stem cells and somatic cells to prostatic epithelium in the tissue recombination models was tested. The directed differentiation of mouse embryonic stem cells by UGM is suggested by a threshold number of mouse stem cells required in prostatic differentiation. To determine the contribution of somatic cells, the adult bladder epithelial compartment was labeled with green-fluorescent vital dye (CMFDA) and the stem-like cells marked by bromodeoxyuridine (BrdU) label-retention. The resulting prostatic epithelia of the tissue recombinants maintained the CMFDA dye, suggesting minimal cell division. Thus, the UGM can induce endoderm-derived epithelia and stem cells to form prostate through a transdifferentiation mechanism that requires stromal TGF-β signaling to mediate epithelial Wnt activity.
Keywords: Urogenital, Mesenchyme, Prostate, TGF-β, Wnt, Stem cell
1. Introduction
The importance of mesenchymal cells in organogenesis is established in many organs including the prostate. The prostate develops from the embryonic urogenital sinus, having both mesodermally derived urogenital sinus mesenchyme (UGM) and endodermally derived epithelial cells. In the presence of androgen, the epithelium undergoes proliferation and differentiation into luminal and basal subtypes. Concurrently, the UGM proliferates and differentiates into prostatic smooth muscle (Cunha et al., 1987; Hayward and Cunha, 2000). Tissue recombination models have been extensively used to examine mesenchymal-epithelial interactions during the process of prostatic organ formation. They have provided important insights into the role of paracrine signaling. Yet, the mechanism for this mesenchymal programming is still not understood. The instructive nature of the UGM toward prostatic epithelial development suggests that paracrine factors are involved in this process (Shima et al., 1995). Many androgen-regulated cytokines and growth factors play important roles in controlling proliferation and differentiation of both epithelial and stromal cells (Cunha et al., 1992, 1995; Thomson and Cunha, 1999; Hayward et al., 1998). The accumulation of TGF-β1 protein is reported to localize in the mesenchyme of fetal and neonatal prostate (Timme et al., 1994). TGF-β signals by the binding of type I and II TGF-β receptors at the cell surface to activate cytoplasmic signaling molecules including the Smad proteins (Massague and Gomis, 2006). However, the role of TGF-β signaling on UGM inductivity is not known. We have used the tissue recombination model system to study the signaling mechanisms between the stromal and epithelial compartments in vivo.
The mechanisms gleaned through tissue recombination modeling often recapitulate native tissue development (Thomson et al., 2002; Donjacour and Cunha, 1995). Tissue recombination studies illustrated the ability of UGM to instructively induce prostatic differentiation from a number of endodermally derived epithelia including those of the prostate, bladder, and urethra (Boutin et al., 1991a, b; Cunha et al., 1983). When applied to human embryonic stem cells, UGM induces prostatic epithelium in the context of teratoma-like growth (Taylor et al., 2006). Using a similar model, we have reported bladder epithelial induction from mouse embryonic stem cells by embryonic bladder mesenchyme (Oottamasathien et al., 2007). Although it is established that the UGM can differentiate pluripotent embryonic stem cells, it is not known whether the differentiation of the adult bladder epithelia into prostate is a result of the differentiation of resident tissue-specific stem cells or the adult urothelium itself. If it were the latter, the studies would reveal the plasticity of non-transformed adult epithelia and, further, somatic cell differentiation in prostate development.
In the present study, we identify the role of TGF-β in UGM induction of prostatic differentiation through the use of tissue recombination techniques. We employed the TGF-β type II receptor conditional knockout mouse that has a loss of TGF-β responsivity in the mesenchymal compartment (Tgfbr2fspKO) (Bhowmick et al., 2004). The differences between normal UGM from the Tgfbr2floxE2/floxE2 mouse and Tgfbr2fspKO UGM on instructing adult mouse bladder epithelial differentiation were compared. The results suggested that regulation of Wnt paracrine signaling mediates the role of TGF-β in the inductive effects of the UGM on the adjacent epithelium. Further, the prostatic inductivity of the UGM was extended to transdifferentiation of mouse stem and somatic cells.
2. Materials and methods
2.1. Animals and urogenital sinus
Tgfbr2floxE2/floxE2 and Tgfbr2fspKO mice bred on the C57Bl/6 background were generated as described previously (Bhowmick et al., 2004). Tgfbr2floxE2/floxE2 mice having loxP sites at introns 1 and 2 of Tgfbr2 were our control mice. Tgfbr2fspKO mice are homozygous conditional knockout of the Tgfbr2 in fibroblasts. Adult male athymic nude mice and C57Bl/6 mice were purchased from Harlan (Indianapolis, IN). All animal procedures were approved by the Vanderbilt Institutional Animal Care and Use Committee.
The urogenital sinuses from mouse or rat embryos (E16 and E18 day, respectively) were separated under a dissecting microscope into epithelial and mesenchymal (UGM) components as described previously (Staack et al., 2003). Rat UGMs were pooled together and further digested with collagenase into single cells (Hayward et al., 1999).
2.2. Tissue recombination and grafting
UGM from Tgfbr2floxE2/floxE2 or Tgfbr2fspKO mice was recombined with 1/6 of the adult bladder epithelial cells (approximately 30,000 cells) from one mouse in a 50-μl rat-tail collagen and incubated overnight at 37 °C. For the recombination of rat UGM with mouse ES cells, each 50-μl collagen contained 300,000 rat UGM cells with 500-5000 ES cells. The ES cells were maintained and cultured with a feeder layer of mitotically inactivated primary mouse embryonic fibroblasts as described before (Oottamasathien et al., 2007). The mouse-derived tissue recombinants were grafted under the renal capsule of syngenic C57Bl/6, whereas the tissue recombinants containing rat UGM were xenografted under the renal capsule of athymic nude mice. The grafts were analyzed after 4 weeks following paraformaldehyde fixation and paraffin embedding.
2.3. Wif 1 lenti-virus production and transduction of UGM
Mouse Wif 1 cDNA clone in pCMV-SPORT6 vector (clone ID, 3984128) was purchased from Open Biosystems (Huntsville, AL). It was subcloned into pLenti6/V5-DEST Gateway vector (Invitrogen Life Technologies, Carlsbad, CA) through Gateway BP and LR recombination reactions using Gateway Technology with Clonase II (Invitrogen) according to the user manual. The Wif 1 lenti-virus was generated by transfection of Wif 1 in pLenti6/V5-DEST in 293FT cells using ViraPower Packaging Mix from Invitrogen. Then the supernatants with virus were collected and stored at -80 °C for further use.
Wif 1 overexpression UGMs were generated by incubation of UGMs from Tgfbr2floxE2/floxE2 mice embryos with Wif 1 lenti-virus-containing medium overnight. Since UGMs lose the inductivity 2 or 3 days in vitro, the transduction rate was determined by two parallel control experiments. In one, the UGMs were transduced with EGFP lenti-virus generated at the same time with Wif 1 lenti-virus, and the green fluorescence of the UGM was observed after 3-4 days. In the second experiment, primary cultured Tgfbr2floxE2/floxE2 mice prostate fibroblast cells were transduced with Wif 1 lenti-virus at the same condition as UGMs, then the cells were selected by blasticidin, and it was noticed that nearly 100% cells survived.
2.4. BrdU injection and uroepithelial cells preparation
Tgfbr2floxE2/floxE2 mice (7-9 weeks old) received one daily intraperitoneal injection of 0.1-ml 10 mg/ml 5-bromo-2-deoxyuridine (BrdU, Sigma) in sterile saline for 12 days. The urinary bladders were excised from these mice 1 month after the last injection of BrdU (Kiel et al., 2007; Lawson et al., 2005; Taupin, 2007; Yan et al., 2007; Kempermann et al., 2003). The urothelial cells of the bladder from either BrdU-injected or control mice were separated by EDTA incubation and micromanipulation (Oottamasathien et al., 2006). The bladder epithelia were labeled with CellTracker CMFDA (5-chloromethylfluorescein diacetate, Molecular Probes, Eugene, OR) at 37 °C for 1 h and washed prior to tissue recombination (Cheng et al., 2005).
For counting of BrdU- and CMFDA-labeled urothelial cells, the cells were collected on a slide by Cytospin (Thermo Shandon, Pittsburgh, PA), 5 min at 1000 rpm. After fixation with ice-cold acetone, the cells were treated with 2 N HCl at room temperature for half an hour before incubation with BrdU antibody for immunofluorescent staining. Slides were evaluated using epifluorescence microscopy.
We determined the threshold of CMFDA CellTracker dye detection following cell division under fluorescence microscopy. Simply, a specified cell number was incubated with CMFDA, washed, and allowed to divide in culture. The cells were counted and visualized at regular intervals until the cells were not visible by epi-fluorescence microscopy.
2.5. Antibodies and immunohistochemistry
Tissue sections were deparaffinized with xylene and hydrated in graded ethanol. The tissues were treated with hydrogen peroxide (1%) and antigen retrieval was performed by boiling in unmasking reagent (1:100, Vector Labs) prior to blocking. The primary antibodies used in this study were as follows: biotin-conjugated anti-BrdU (Molecular Probes, 1:100), rabbit polyclonal androgen receptor (N-20), p63 (H-137), Wif-1 (H-180), goat polyclonal FoxA1 (C-20) (Santa Cruz Biotechnology, 1:1000), phospho-Smad2 (Ser465/467) (Cell Signaling, 1:1000), mouse monoclonal anti-prostatic acid phosphatase (Sigma-Aldrich, 1:200), and uroplakin (a kind gift from Dr. T.T. Sun, New York University School of Medicine, Department of Dermatology, New York). All antibodies were diluted in PBS containing 10% fetal bovine serum (FBS) and incubated overnight at 4 °C. The secondary antibodies and detection reagents, from Dako (Dako, Carpinteria, CA), were applied the next day according to the manufacturer's instruction. The immunohistochemical stainings were visualized and captured using a Nikon Coolscope (Nikon Instrument Inc., Melville, NY).
2.6. Microarray and real-time PCR confirmation
Tgfbr2floxE2/floxE2 UGMs from three embryos were pooled together as a common reference control. Tgfbr2fspKO UGMs from three embryos were collected individually. They were placed directly in SuperAmplification lysis buffer provided by the Miltenyi Biotec Company (Auburn, CA), stored on dry ice, and immediately transported to the company for superamplification of cDNA and microarray analysis using Agilent whole mouse genome oligomicroarray (44k). The three Tgfbr2fspKO UGM cDNA samples were directly compared with the pooled Tgfbr2floxE2/floxE2 in three separate arrays. The response measures, average fold changes, from direct comparison within each array were extracted for each gene of three Tgfbr2fspKO UGM cDNA samples, and data were analyzed using the EASE software (http://david.abcc.ncifcrf.gov/ease/ease.jsp). To confirm the differential expression of genes of interest from the microarray analysis, reverse transcription and quantitative real-time PCR were performed using iScript and iQ SYBR Green supermix, respectively (Bio-Rad, Hercules, CA). Relative quantitation was calculated by the ΔΔCt method normalized to GAPDH (Dorak, 2006).
3. Results
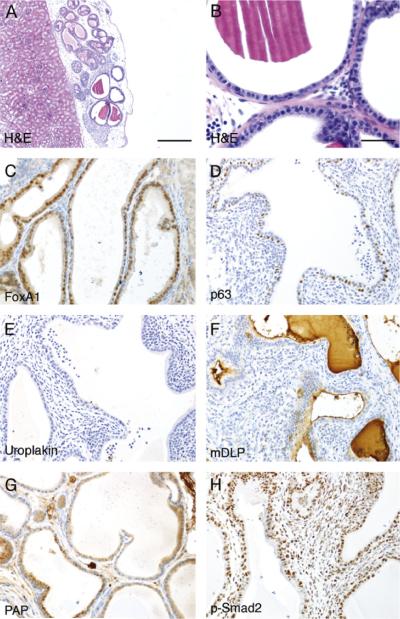
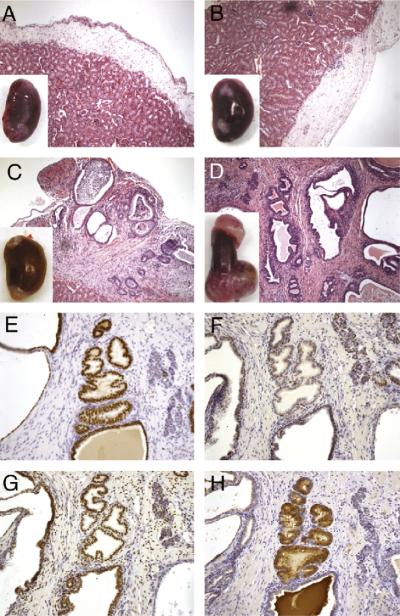
Tissue recombination techniques have been used to study stromal-epithelial interactions and recapitulate development of various tissues under the renal capsule. We have used tissue recombination to study the role of stromal TGF-β signaling in epithelial differentiation. Recombinations of UGM from Tgfbr2floxE2/floxE2 15-18-day mouse embryos with adult mouse wild-type bladder epithelia were grown under the renal capsules of syngeneic mice for 1 month. The grafts developed into glandular structures with surrounding stromal differentiation, as shown in Fig. 1. (A summary of all the tissue recombination grafts is shown in Table 1.) The immunohistochemical staining showed no expression of the urothelial differentiation marker, uroplakin. However, as expected, the expressions of prostate secretory protein differentiation markers, dorsal lateral prostate (mDLP), and prostate acid phosphatase (PAP) were detected in the epithelium (Fig. 1). p63, a basal cell marker, showed the integrity of the glandular structure of the grafted tissue. The staining for FoxA1 in the epithelium, a transcription factor in the cells developed from endoderm, confirmed the origin of the epithelium from urothelial cells (Lee et al., 2005). Additionally, phosphorylated Smad2, an indication of activated TGF-β signaling, was localized to the nuclei of both stromal and epithelial cells. Together, the data were consistent with previous reports that Tgfbr2floxE2/floxE2 UGM, similar to wild-type UGM, was inductive of bladder epithelial differentiation to prostatic epithelium.
Fig. 1.
Tissue recombination experiment of Tgfbr2floxE2/floxE2 UGM with adult bladder epithelia. Bladder epithelia from wild-type C57Bl/6 mice were combined with UGM from Tgfbr2floxE2/floxE2 mouse embryo into collagen gel plug and grafted under the renal capsule of synergistic mice for 1 month. The histology of the resulting grafts was visualized by H&E staining at (A) 200× and (B) 400× magnification. Immunohistochemistry for the (C) endoderm marker FoxA1, (D) basal cell marker p63, (E) bladder urothelial marker uroplakin, (F) differentiated prostate epithelial markers mDLP secreted protein, and (G) PAP is shown in representative pictures as indicated. (H) Immunohistochemical staining for phosphorylated Smad2 indicated TGF-β signaling in both stromal and epithelial cells. The immunohistochemically stained sections were counterstained with hematoxylin (blue). Scale bar represents 200 μm (A) and 25 μm (B-H).
Table 1.
Number, type, and results of tissue recombinants
| Wt | UGM | KO | UGM | Wif 1 | UGM | Rat | UGM | |
|---|---|---|---|---|---|---|---|---|
| BLE | 40 | Prostate | 30 | Bladder | 8 | Bladder | ||
| BLE-labeled | 24 | Prostate | ||||||
| mES | 22 | Teratoma |
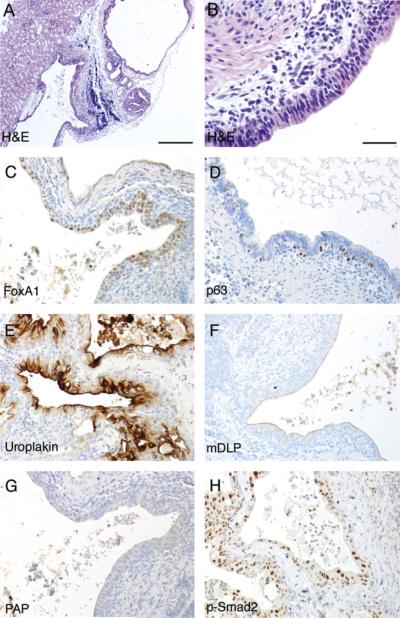
Next we determined the role of TGF-β responsiveness of the UGM in the induction of prostate differentiation. UGM knocked out for the expression of Tgfbr2 was either obtained from Tgfbr2fspKO mouse embryos or generated by applying Creadenovirus on Tgfbr2floxE2/floxE2 UGM. The important difference between the two sources of UGM was the extent of Tgfbr2 recombination. The Tgfbr2fspKO UGM had approximately 50% detectible recombination of the Tgfbr2 gene, and the ex vivo adenoviral-Cre transduction resulted in approximately 80% recombination as determined by phosphorylated-Smad2 expression (data not shown). However, both Tgfbr2fspKO and Cre-adenoviral UGM behaved in a similar manner in that they did not yield glandular prostatic structures when recombined with bladder epithelia. The grafted tissues were histologically similar to transitional epithelia surrounding the luminal space (Fig. 2). Immunohistochemical staining indicated uroplakin expression in the epithelium, suggesting the maintenance of urothelial differentiation. The lack of mDLP secretory protein and PAP expression in the grafts further suggested the loss of prostatic induction by the Tgfbr2 knockout UGM. Phosphorylated-Smad2 immuno-localization confirmed epithelial TGF-β signaling, with substantial loss of stromal detection. The representative panels shown in Fig. 2 were from Cre-adenovirus-transduced Tgfbr2floxE2/floxE2 mouse UGM. Thus, stromal TGF-β signaling played an important role in the UGM induction of prostatic differentiation from bladder urothelium.
Fig. 2.
Tissue recombination experiment of Tgfbr2fspKO UGM with adult bladder epithelia. Bladder epithelia from wild-type C57Bl/6 mice were combined with UGM from Tgfbr2fspKO mouse embryo into collagen gel plug and grafted under the renal capsule of synergistic mice for 1 month. The histology of the resulting grafts was visualized by H&E staining at (A) 200× and (B) 400× magnification. Immunohistochemistry for the (C) endoderm marker FoxA1, (D) basal cell marker p63, (E) bladder urothelial marker uroplakin, (F) differentiated prostate epithelial markers mDLP secreted protein, and (G) PAP is shown in representative pictures as indicated. (H) Immunohistochemical staining for phosphorylated Smad2 indicated TGF-β signaling in the epithelial compartment, diminished in the stromal cells. The immunohistochemically stained sections were counterstained with hematoxylin (blue). Scale bar represents 200 μm (A) and 25 μm (B-H).
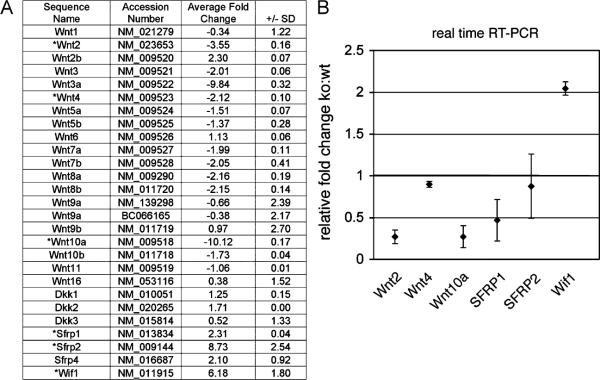
To further identify the mechanism how TGF-β imparts UGM prostate identity on the adjacent epithelium, gene expression profiles were compared between the Tgfbr2floxE2/floxE2 and Tgfbr2fspKO UGM. The Wnt signaling emerged from our analysis as a prime candidate to be the primary regulated signaling pathway. Correspondingly, many Wnt ligands were down-regulated and the secreted Wnt antagonists (Wif 1, SFRP1, and SFRP2) were consistently up-regulated by the Tgfbr2fspKO UGM (Fig. 3A). Among those genes, Wnt2, Wnt4, Wnt10a, SFRP1, and SFRP2 and Wif 1 had greater than two-fold change and a significant p-value (p<0.0001) by microarray analysis. These changes were further validated by real-time PCR with the exception of SFRP1 and SFRP2 (Fig. 3B). As many Wnt ligands are stromally secreted with cognate receptors expressed by the epithelia, Wnts and their antagonists were good candidates for mediating stromal-epithelial interactions.
Fig. 3.
Microarray analysis indicated that the loss of prostatic inductivity by Tgfbr2fspKO UGM was associated with the down-regulation of many Wnt ligands and the up-regulation of Wnt antagonists. (A) The list illustrates the accession number, gene name, and fold change (± standard deviation) comparing three Tgfbr2fspKO UGM to three pooled Tgfbr2floxE2/floxE2 UGMs. The genes suggested to have greater than two-fold change and significant p-value by microarray analysis were validated by real-time RT-PCR (asterisk). (B) The graph indicates mean±standard deviation of real-time RT-PCR data (p<0.01, n = 3).
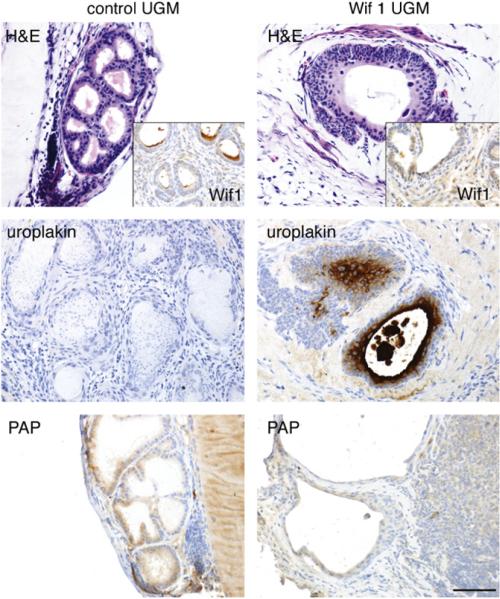
To determine the role of paracrine Wnt signaling in mediating prostate induction by the UGM, Wif 1 was overexpressed by wild-type UGM. Wif 1 is a pan-antagonist for both canonical and non-canonical Wnt signaling by direct binding to Wnt ligands. Wif 1 lenti-virus-transduced UGM and control UGM were recombined with bladder epithelia and grafted in mice for 1 month. Histology of recombinants generated using Wif 1-expressing UGM demonstrated the maintenance of bladder transitional epithelial differentiation expressing uroplakin and no PAP (Fig. 4). In contrast, the control UGM induced prostate luminal epithelial differentiation with associated epithelia-expressed PAP and without uroplakin expression. Taken together, these data demonstrated that TGF-β signaling regulated the inductivity of UGM through inhibition of Wnt signaling in the adjacent epithelium.
Fig. 4.
Tissue recombinations of mouse adult bladder epithelia associated with wild-type control UGM (left) were compared with Wif 1-overexpressed wild-type UGM (right). The resulting prostatic histology of the control was illustrated by H&E staining and PAP immunohistochemical expression with the absence of Wif 1 (inset), and uroplakin expression. In contrast, the expression of Wif 1 by the UGM resulted in a bladder-like histology, confirmed by positive immunohistochemistry staining for Wif 1 and uroplakin with the absence of PAP localization. Scale bar represents 50 μm.
The importance of paracrine Wnt signaling in prostatic induction increased the possibility that prostatic induction by the UGM is through directed differentiation of the residing stem cells in the bladder epithelial compartment (Blank et al., 2008; Blanpain et al., 2007). To initially determine the number of stem cells required in prostatic tissue induction by UGM, mouse embryonic stem (mES) cells were tested in tissue recombinant experiments in male SCID host mice. We found that when 500 or 1000 mES cells were recombined with 300,000 rat UGM cells and xenografted for 6 weeks no tissue grew (Fig. 5A, B). However, under the same conditions, 1500 and 5000 mES cells were induced to develop teratoma containing prostatic structures (Fig. 5C, D). The prostatic structures comprised 10-20% of each graft. The prostatic phenotype was confirmed by immunohistochemistry staining of FoxA1, p63, AR, and mDLP secretory protein (Fig. 5E-H). The representative pictures shown in Fig. 5E-H were from the recombinants using 1500 mES cells with UGM. Greater mES cell numbers (5000) grew into larger tissue recombinants with similar structures and compositions (Fig. 5D). These recombination experiments indicate that the threshold number of mouse stem cells can be induced to form prostate by the UGM.
Fig. 5.
Tissue recombination of mES cells with rat UGM. Rat UGM cells (300,000) were recombined with (A) 500, (B) 1000, (C) 1500, and (D) 5000 mES cells and xenografted under the renal capsule of nude mice. The resulting grafts after 6 weeks varied in gross size (inset) and histology (A-D). The recombinant grafts with UGM and the minimum 1500 mES developed teratomas which contained 10-20% prostatic structures. Positive immunohistology staining for (E) FoxA1, (F) p63, (G) AR, and (H) mDLP secreted protein supported our interpretation of prostatic differentiation. The sections were nuclear counterstained with hematoxylin (blue).
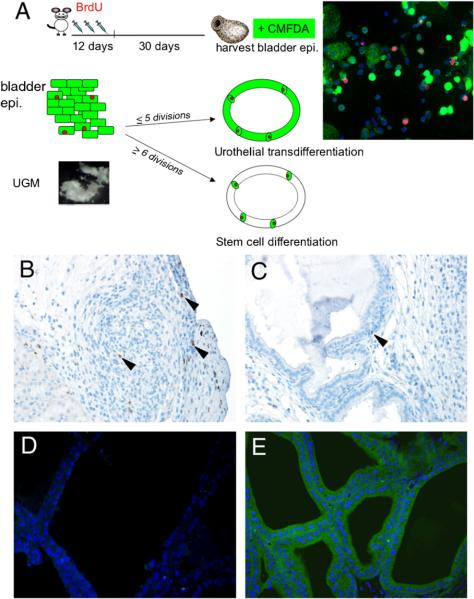
To differentiate the contribution of adult stem cell differentiation from the alternative hypothesis of the transdifferentiation of urothelial cells, a set of experiments illustrated in Fig. 6A were designed. BrdU label-retention methods were used to mark potential stem cells of the donor mouse bladders 4 weeks prior to harvesting (Kempermann et al., 2003; Kiel et al., 2007). The bladder epithelia were further labeled with a green-fluorescent vital dye, CMFDA, following harvesting. The CMFDA fluorescent probe can be retained in living cells through several generations. It can pass freely through cell membranes, but once inside the cell is transformed into a membrane-impermeable reaction product. The CMFDA dye can be inherited by daughter cells after cell division and is not transferred to adjacent cells in a population. In vitro, we have observed that the fluorescence can be detected up to five cell divisions, but was undetectable after six divisions. Following isolation from the bladder and cytospin, urothelial cells were counted. By this method an average of 200,000 epithelial cells were isolated from one adult bladder. qThe number of BrdU label-retaining cells was about 3600 (~1.8% of total). We recombined approximately 30,000 bladder epithelial cells, containing about 600 BrdU label-retaining cells, with a single-embryo UGM (approximately 90,000 cells) to form each graft. If most of the urothelial cells transdifferentiated into prostate, after five or less divisions in 1 month, the green CMFDA vital dye would be visible in the harvested grafts. However, if only the stem cells of the bladder, originally only ~600 in number, differentiated to prostate, six or more cell divisions would be needed to develop into the prostatic tissues of the size observed. As the CMFDA concentration is diluted in each cell division, most of the glandular cells would likely not have green CMFDA fluorescence. There may be only a few undifferentiated stem cells co-staining of BrdU and CMFDA in either scenario.
Fig. 6.
Tissue recombination of Tgfbr2floxE2/floxE2 UGM with labeled adult bladder epithelia. (A) The diagram illustrates the strategy for identifying BrdU label-retaining cells by the injection of BrdU in wild-type donor mice for 12 consecutive days, then harvesting the bladder 30 days later. Following the isolation of the bladder epithelia, the cells were further labeled with CMFDA. Dual labeling was confirmed by cytospin and fluorescence detection. The panel on the right illustrates urothelial cells immunostained for BrdU in red and green fluorescence of CMFDA. Two outcomes of the tissue recombinants with the labeled bladder epithelia could be observed: (1) If majority of the resulting prostatic epithelium in the grafts maintained detectable green CMFDA-labeled dye, it would suggest urothelial transdifferentiation as only a few cell divisions would be required (p5 cell divisions). (2) Alternatively, if only a few green cells were present maintaining BrdU (red), then it is likely that many more cell divisions (≥6) would be required, suggesting bladder stem cell induction as the primary means of prostate differentiation. Immunohistochemistry was used to detect BrdU in tissue recombinants after 1 month of grafting for (B) the control graft with bladder epithelial cells only (no mesenchyme) and (C) in the tissue recombinants with both bladder epithelia and UGM. Arrowheads indicate cells positive for BrdU staining. (D) Fluorescence microscopy of the UGM+urothelium tissue recombinant grafts showed little autofluorescence when the control epithelia were not labeled with CMFDA. (E) However, tissue recombinants with bladder epithelia pre-labeled with CMFDA showed nearly all epithelial cells were green 1 month after grafting. The tissue recombinants with UGM and bladder epithelial grafts in panels (C-E) had prostatic histology.
The recombinant tissues utilizing the CMFDA- and BrdU-labeled bladder epithelia were harvested and analyzed by microscopy. Fig. 6A illustrates the red immunofluorescence for BrdU and green for CMFDA from the bladder epithelia used in the tissue recombination experiments. Immunohistochemistry illustrated the BrdU staining in the grafts of bladder epithelium alone (control, i.e., no mesenchyme) and the recombinant grafts with UGM (UGM+urothelium), respectively (Fig. 6B, C). The adult bladder epithelium-only grafts had a few scattered BrdU-positive cells in the small pool of surviving epithelia, with no ductal structure. The UGM enabled prostatic structure formation with few cells maintaining BrdU staining in the epithelial compartment. There was a reduction in apparent BrdU-labeled cells in the grafts compared to the approximate 600 BrdU-labeled stem cells in the starting material (see Fig. 6A). To detect CMFDA fluorescence in the epithelia, the grafts were observed under fluorescent microscopy (Fig. 6D, E). The negative control of the bladder epithelium not labeled with CMFDA had no significant green autofluorescence. However, the green fluorescence of the originally CMFDA-labeled bladder epithelia was still detectable in all of the resulting glandular prostate epithelium 4 weeks after grafting. The apparent reduction of BrdU label-retaining cells and the striking retention of CMFDA indicated that most of the adult bladder epithelia were transdifferentiated to prostatic epithelia with a likely contribution of stem cells in prostatic induction by the UGM.
4. Discussion
The role of TGF-β signaling in UGM prostate inductivity was revealed through tissue recombination with adult bladder epithelium. The embryonic UGM is capable of inducing prostate-like morphogenesis in endodermally derived epithelial cells from fetal or adult bladder, urethra, or vagina (Cunha et al., 1983, 1987; Boutin et al., 1991a). Growth factors such as TGF-β, fibroblast growth factor-7 (FGF-7, also known as keratinocyte growth factor or KGF), insulin-like growth factors, Wnt ligands, epidermal growth factor, heparin-binding growth factors, and platelet-derived growth factor have been correlated with epithelial differentiation of the prostate (Shima et al., 1995; Timme et al., 1994; Cunha et al., 1992, 1995; Sugimura et al., 1996; Thomson et al., 1997). The ability of UGM to induce prostatic differentiation is likely to be regulated by more than one factor; these factors cross-talk at multiple levels. The TGF-β family has been extensively studied and is believed to be important in mediating the interaction between stromal cells and epithelium during development and pathogenesis. The UGM has been suggested to be the major site of TGF-β isoform expression, and accumulation of TGF-β1 protein was localized to the mesenchyme surrounding the developing prostate buds throughout the perinatal period (Timme et al., 1994). The TGF-β target genes, such as uPA and c-myc, were also expressed more in the UGM compared to the urogenital epithelia (Timme et al., 1994; Haughney et al., 1998). Our present work illustrates the necessity of TGF-β signaling in the UGM in prostate epithelial induction, and further implicates Wnt epithelial activity in this induction.
The Wnt ligands are usually stromally secreted with the cognate receptors on adjacent epithelial cells. Once the Wnt ligands activate the frizzled receptors at the cell surface, Wnt signaling is initiated through canonical or non-canonical pathways (Clevers, 2006; Kohn and Moon, 2005). TGF-β and Wnt signaling cross-talk in development and cancer can be both cooperative and antagonistic (Letamendia et al., 2001; Labbe et al., 2000, 2007; Attisano and Labbe, 2004). However, our understanding of the interactions of these pathways at a paracrine level is incomplete. In the presence of both Wnt and TGF-β, Smad3 and Smad4 bind to LEF1/TCFs to enhance β-catenin transcriptional activation of target genes (Labbe et al., 2000; Letamendia et al., 2001). TGF-β is also reported to promote the physical interaction of smad7 with β-catenin and LEF1/TCF, and accumulates β-catenin in a p38 MAP kinase-dependent manner (Edlund et al., 2005). The studies described here suggest that TGF-β action on the stromal cells activates Wnt signaling, which then acts, presumably in a paracrine manner on adjacent epithelial cells, to elicit critical aspects of prostatic differentiation.
The comparison of the gene expression profiles between UGM from Tgfbr2floxE2/floxE2 and Tgfbr2fspKO mice indicated the down-regulation of most Wnt ligands and the up-regulation of Wnt antagonists (Fig. 3). However, most Wnt signaling target genes, such as VEGF, MMPs, and CD44, were not changed (Supplemental Table). These data further implied that the decreased Wnt signaling in the Tgfbr2fspKO UGM was not acting in an autocrine but probably in a paracrine manner. The role for Wnt signaling was confirmed by antagonizing the pathway in wild-type UGM by the transduction of Wif 1. Bladder epithelium was not converted to prostate when Wnt signaling was blocked in UGM tissues (Fig. 4). However, Tgfbr2fspKO mice develop prostates, albeitwith a transformed phenotype (Bhowmick et al., 2004). It would suggest that the signal intensity involved in prostate induction of embryonic urogenital sinus epithelia may differ from that required to re-program adult urothelia to the prostate. The role of Wnt signaling in cancerous transformation of the prostate is well documented (Voeller et al., 1998; Bruxvoort et al., 2007; Chesire and Isaacs, 2003; Bierie et al., 2003). Interestingly, our studies indicate that the same pathway is necessary for the developmental differentiation of the prostate.
During prostatic development, the mesenchyme induces epithelial ductal morphogenesis and differentiation. In tissue recombination models involving heterotypic epithelia, the origin of the resultant epithelial component is unclear. Two possible explanations have been discussed: transdifferentiation from apparently “terminally” differentiated urothelium and, alternatively, induction of a stem cell population residing in the epithelium (Kinbara et al., 1996). Work showing the formation of human prostate tissue from embryonic stem cells supported the possibility of urothelial-resident stem cells differentiating to prostatic epithelia (Taylor et al., 2006). Our data with mES cells further supported this line of reasoning (Fig. 5). However, the data also suggest that the number of stem-like label-retaining cells residing in the bladder epithelial compartment is not sufficient to develop into prostate alone (Figs. 5 and 6). Our experiment with mES cell-UGM recombinations was similar to previous publications with human ES cells, where a minimum number of stem cells were needed for prostatic glandular development (Taylor et al., 2006). Indeed, the UGM-mediated induction of mES cells and the adult label-retaining cells are not the same. The BrdU label-retention studies in fact normally overestimate the number of stem-like cells in the tissue (Kiel et al., 2007; Kurzrock et al., 2008). However, the prostatic specificity induced in tissue recombinants from a variety of epithelial sources by UGM suggests common inductive mechanisms for this model and likely in normal prostate development. The results with CMFDA vital dye-labeled cells are the most persuasive in supporting the alternative hypothesis of urothelial transdifferentiation, where the newly developed prostatic epithelia maintained the dye. If the prostate was a result of stem cell differentiation, much of the epithelium would have been replaced by cells with no visible dye. This indicated there was transdifferentiation of the urothelia itself. The contribution of the terminally differentiated apical umbrella cells of bladder epithelium in prostatic transdifferentiation is unlikely. The contribution of resident stem cells cannot be ruled out, but is apparently not the primary means of prostate formation in this model. Yamanaka and colleagues reported the successful induction of pluripotent stem cells from both mouse embryonic and adult fibroblast cultures by defined factors (Aoi et al., 2008; Nakagawa et al., 2008; Takahashi et al., 2007a, b; Takahashi and Yamanaka, 2006). This implied that any differentiated cell can be reprogrammed to stem cells under certain factors, and the reprogrammed stem cells can be further induced to any tissues with the respective mesenchyme. Our studies further support the role of Wnt signaling activity in the reprogramming of adult urothelia to enable prostatic transdifferentiation. Although in natural prostatic development urothelial cells are not a contributor, our studies illustrate the capacity and mechanism of the UGM to transdifferentiate somatic cells.
Supplementary Material
Appendix A. Supplementary material
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.diff.2008.09.012.
References
- Aoi T, Yae K, Nakagawa M, Ichisaka T, Okita K, Takahashi K, Chiba T, Yamanaka S. Generation of pluripotent stem cells from adult mouse liver and stomach cells. Science. 2008 doi: 10.1126/science.1154884. [DOI] [PubMed] [Google Scholar]
- Attisano L, Labbe E. TGFbeta and Wnt pathway cross-talk. Cancer Metastasis Rev. 2004;23:53–61. doi: 10.1023/a:1025811012690. [DOI] [PubMed] [Google Scholar]
- Bhowmick NA, Chytil A, Plieth D, Gorska AE, Dumont N, Shappell S, Washington MK, Neilson EG, Moses HL. TGF-beta signaling in fibroblasts modulates the oncogenic potential of adjacent epithelia. Science. 2004;303:848–851. doi: 10.1126/science.1090922. (New York, NY) [DOI] [PubMed] [Google Scholar]
- Bierie B, Nozawa M, Renou JP, Shillingford JM, Morgan F, Oka T, Taketo MM, Cardiff RD, Miyoshi K, Wagner KU, Robinson GW, Hennighausen L. Activation of beta-catenin in prostate epithelium induces hyperplasias and squamous transdifferentiation. Oncogene. 2003;22:3875–3887. doi: 10.1038/sj.onc.1206426. [DOI] [PubMed] [Google Scholar]
- Blank U, Karlsson G, Karlsson S. Signaling pathways governing stem-cell fate. Blood. 2008;111:492–503. doi: 10.1182/blood-2007-07-075168. [DOI] [PubMed] [Google Scholar]
- Blanpain C, Horsley V, Fuchs E. Epithelial stem cells: turning over new leaves. Cell. 2007;128:445–458. doi: 10.1016/j.cell.2007.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boutin EL, Battle E, Cunha GR. The response of female urogenital tract epithelia to mesenchymal inductors is restricted by the germ layer origin of the epithelium: prostatic inductions. Differentiation. 1991a;48:99–105. doi: 10.1111/j.1432-0436.1991.tb00248.x. [DOI] [PubMed] [Google Scholar]
- Boutin EL, Sanderson RD, Bernfield M, Cunha GR. Epithelial-mesenchymal interactions in uterus and vagina alter the expression of the cell surface proteoglycan, syndecan. Dev. Biol. 1991b;148:63–74. doi: 10.1016/0012-1606(91)90317-v. [DOI] [PubMed] [Google Scholar]
- Bruxvoort KJ, Charbonneau HM, Giambernardi TA, Goolsby JC, Qian CN, Zylstra CR, Robinson DR, Roy-Burman P, Shaw AK, Buckner-Berghuis BD, Sigler RE, Resau JH, Sullivan R, Bushman W, Williams BO. Inactivation of Apc in the mouse prostate causes prostate carcinoma. Cancer Res. 2007;67:2490–2496. doi: 10.1158/0008-5472.CAN-06-3028. [DOI] [PubMed] [Google Scholar]
- Cheng N, Bhowmick NA, Chytil A, Gorksa AE, Brown KA, Muraoka R, Arteaga CL, Neilson EG, Hayward SW, Moses HL. Loss of TGF-beta type II receptor in fibroblasts promotes mammary carcinoma growth and invasion through upregulation of TGF-alpha-, MSP- and HGF-mediated signaling networks. Oncogene. 2005;24:5053–5068. doi: 10.1038/sj.onc.1208685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesire DR, Isaacs WB. Beta-catenin signaling in prostate cancer: an early perspective. Endocr.-Relat. Cancer. 2003;10:537–560. doi: 10.1677/erc.0.0100537. [DOI] [PubMed] [Google Scholar]
- Clevers H. Wnt/beta-catenin signaling in development and disease. Cell. 2006;127:469–480. doi: 10.1016/j.cell.2006.10.018. [DOI] [PubMed] [Google Scholar]
- Cunha GR, Fujii H, Neubauer BL, Shannon JM, Sawyer L, Reese BA. Epithelial-mesenchymal interactions in prostatic development. I. Morphological observations of prostatic induction by urogenital sinus mesenchyme in epithelium of the adult rodent urinary bladder. J. Cell Biol. 1983;96:1662–1670. doi: 10.1083/jcb.96.6.1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunha GR, Donjacour AA, Cooke PS, Mee S, Bigsby RM, Higgins SJ, Sugimura Y. The endocrinology and developmental biology of the prostate. Endocr. Rev. 1987;8:338–362. doi: 10.1210/edrv-8-3-338. [DOI] [PubMed] [Google Scholar]
- Cunha GR, Alarid ET, Turner T, Donjacour AA, Boutin EL, Foster BA. Normal and abnormal development of the male urogenital tract. Role of androgens, mesenchymal-epithelial interactions, and growth factors. J. Androl. 1992;13:465–475. [PubMed] [Google Scholar]
- Cunha GR, Foster B, Thomson A, Sugimura Y, Tanji N, Tsuji M, Terada N, Finch PW, Donjacour AA. Growth factors as mediators of androgen action during the development of the male urogenital tract. World J. Urol. 1995;13:264–276. doi: 10.1007/BF00185969. [DOI] [PubMed] [Google Scholar]
- Donjacour AA, Cunha GR. Induction of prostatic morphology and secretion in urothelium by seminal vesicle mesenchyme. Development. 1995;121:2199–2207. doi: 10.1242/dev.121.7.2199. (Cambridge, England) [DOI] [PubMed] [Google Scholar]
- Dorak M. Real-Time PCR. Oxford; New York: 2006. [Google Scholar]
- Edlund S, Lee SY, Grimsby S, Zhang S, Aspenstrom P, Heldin CH, Landstrom M. Interaction between Smad7 and beta-catenin: importance for transforming growth factor beta-induced apoptosis. Mol. Cell. Biol. 2005;25:1475–1488. doi: 10.1128/MCB.25.4.1475-1488.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haughney PC, Hayward SW, Dahiya R, Cunha GR. Species-specific detection of growth factor gene expression in developing murine prostatic tissue. Biol. Reprod. 1998;59:93–99. doi: 10.1095/biolreprod59.1.93. [DOI] [PubMed] [Google Scholar]
- Hayward SW, Cunha GR. The prostate: development and physiology. Radiol. Clin. N. Am. 2000;38:1–14. doi: 10.1016/s0033-8389(05)70146-9. [DOI] [PubMed] [Google Scholar]
- Hayward SW, Haughney PC, Rosen MA, Greulich KM, Weier HU, Dahiya R, Cunha GR. Interactions between adult human prostatic epithelium and rat urogenital sinus mesenchyme in a tissue recombination model. Differentiation. 1998;63:131–140. doi: 10.1046/j.1432-0436.1998.6330131.x. [DOI] [PubMed] [Google Scholar]
- Hayward SW, Haughney PC, Lopes ES, Danielpour D, Cunha GR. The rat prostatic epithelial cell line NRP-152 can differentiate in vivo in response to its stromal environment. Prostate. 1999;39:205–212. doi: 10.1002/(sici)1097-0045(19990515)39:3<205::aid-pros9>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Kempermann G, Gast D, Kronenberg G, Yamaguchi M, Gage FH. Early determination and long-term persistence of adult-generated new neurons in the hippocampus of mice. Development. 2003;130:391–399. doi: 10.1242/dev.00203. [DOI] [PubMed] [Google Scholar]
- Kiel MJ, He S, Ashkenazi R, Gentry SN, Teta M, Kushner JA, Jackson TL, Morrison SJ. Haematopoietic stem cells do not asymmetrically segregate chromosomes or retain BrdU. Nature. 2007;449:238–242. doi: 10.1038/nature06115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinbara H, Cunha GR, Boutin E, Hayashi N, Kawamura J. Evidence of stem cells in the adult prostatic epithelium based upon responsiveness to mesenchymal inductors. Prostate. 1996;29:107–116. doi: 10.1002/(SICI)1097-0045(199608)29:2<107::AID-PROS6>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Kohn AD, Moon RT. Wnt and calcium signaling: beta-catenin-independent pathways. Cell Calcium. 2005;38:439–446. doi: 10.1016/j.ceca.2005.06.022. [DOI] [PubMed] [Google Scholar]
- Kurzrock EA, Lieu DK, Degraffenried LA, Chan CW, Isseroff RR. Label-retaining cells of the bladder: candidate urothelial stem cells. Am. J. Physiol. Renal Physiol. 2008 doi: 10.1152/ajprenal.00533.2007. [DOI] [PubMed] [Google Scholar]
- Labbe E, Letamendia A, Attisano L. Association of Smads with lymphoid enhancer binding factor 1/T cell-specific factor mediates cooperative signaling by the transforming growth factor-beta and wnt pathways. Proc. Natl. Acad. Sci. USA. 2000;97:8358–8363. doi: 10.1073/pnas.150152697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labbe E, Lock L, Letamendia A, Gorska AE, Gryfe R, Gallinger S, Moses HL, Attisano L. Transcriptional cooperation between the transforming growth factor-beta and Wnt pathways in mammary and intestinal tumorigenesis. Cancer Res. 2007;67:75–84. doi: 10.1158/0008-5472.CAN-06-2559. [DOI] [PubMed] [Google Scholar]
- Lawson DA, Xin L, Lukacs R, Xu Q, Cheng D, Witte ON. Prostate stem cells and prostate cancer. Cold Spring Harbor Symp. Quant. Biol. 2005;70:187–196. doi: 10.1101/sqb.2005.70.003. [DOI] [PubMed] [Google Scholar]
- Lee CS, Friedman JR, Fulmer JT, Kaestner KH. The initiation of liver development is dependent on Foxa transcription factors. Nature. 2005;435:944–947. doi: 10.1038/nature03649. [DOI] [PubMed] [Google Scholar]
- Letamendia A, Labbe E, Attisano L. Transcriptional regulation by Smads: crosstalk between the TGF-beta and Wnt pathways. J. Bone Jt. Surg. Am. 2001;83-A(Suppl 1):S31–S39. [PubMed] [Google Scholar]
- Massague J, Gomis RR. The logic of TGFbeta signaling. FEBS Lett. 2006;580:2811–2820. doi: 10.1016/j.febslet.2006.04.033. [DOI] [PubMed] [Google Scholar]
- Nakagawa M, Koyanagi M, Tanabe K, Takahashi K, Ichisaka T, Aoi T, Okita K, Mochiduki Y, Takizawa N, Yamanaka S. Generation of induced pluripotent stem cells without Myc from mouse and human fibroblasts. Nat. Biotechnol. 2008;26:101–106. doi: 10.1038/nbt1374. [DOI] [PubMed] [Google Scholar]
- Oottamasathien S, Williams K, Franco OE, Thomas JC, Saba K, Bhowmick NA, Staack A, Demarco RT, Brock JW, III, Hayward SW, Pope J.C.t. Bladder tissue formation from cultured bladder urothelium. Dev. Dyn. 2006;235:2795–2801. doi: 10.1002/dvdy.20886. [DOI] [PubMed] [Google Scholar]
- Oottamasathien S, Wang Y, Williams K, Franco OE, Wills ML, Thomas JC, Saba K, Sharif-Afshar AR, Makari JH, Bhowmick NA, DeMarco RT, Hipkens S, Magnuson M, Brock JW, III, Hayward SW, Pope J.C.t., Matusik RJ. Directed differentiation of embryonic stem cells into bladder tissue. Dev. Biol. 2007;304:556–566. doi: 10.1016/j.ydbio.2007.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shima H, Tsuji M, Elfman F, Cunha GR. Development of male urogenital epithelia elicited by soluble mesenchymal factors. J. Androl. 1995;16:233–241. [PubMed] [Google Scholar]
- Staack A, Donjacour AA, Brody J, Cunha GR, Carroll P. Mouse urogenital development: a practical approach. Differentiation. 2003;71:402–413. doi: 10.1046/j.1432-0436.2003.7107004.x. [DOI] [PubMed] [Google Scholar]
- Sugimura Y, Foster BA, Hom YK, Lipschutz JH, Rubin JS, Finch PW, Aaronson SA, Hayashi N, Kawamura J, Cunha GR. Keratinocyte growth factor (KGF) can replace testosterone in the ductal branching morphogenesis of the rat ventral prostate. Int. J. Dev. Biol. 1996;40:941–951. [PubMed] [Google Scholar]
- Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Okita K, Nakagawa M, Yamanaka S. Induction of pluripotent stem cells from fibroblast cultures. Nat. Protoc. 2007a;2:3081–3089. doi: 10.1038/nprot.2007.418. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007b;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- Taupin P. Protocols for studying adult neurogenesis: insights and recent developments. Regenerative Med. 2007;2:51–62. doi: 10.2217/17460751.2.1.51. [DOI] [PubMed] [Google Scholar]
- Taylor RA, Cowin PA, Cunha GR, Pera M, Trounson AO, Pedersen J, Risbridger GP. Formation of human prostate tissue from embryonic stem cells. Nat. Meth. 2006;3:179–181. doi: 10.1038/nmeth855. [DOI] [PubMed] [Google Scholar]
- Thomson AA, Cunha GR. Prostatic growth and development are regulated by FGF10. Development. 1999;126:3693–3701. doi: 10.1242/dev.126.16.3693. [DOI] [PubMed] [Google Scholar]
- Thomson AA, Foster BA, Cunha GR. Analysis of growth factor and receptor mRNA levels during development of the rat seminal vesicle and prostate. Development. 1997;124:2431–2439. doi: 10.1242/dev.124.12.2431. [DOI] [PubMed] [Google Scholar]
- Thomson AA, Timms BG, Barton L, Cunha GR, Grace OC. The role of smooth muscle in regulating prostatic induction. Development. 2002;129:1905–1912. doi: 10.1242/dev.129.8.1905. (Cambridge, England) [DOI] [PubMed] [Google Scholar]
- Timme TL, Truong LD, Merz VW, Krebs T, Kadmon D, Flanders KC, Park SH, Thompson TC. Mesenchymal-epithelial interactions and transforming growth factor-beta expression during mouse prostate morphogenesis. Endocrinology. 1994;134:1039–1045. doi: 10.1210/endo.134.3.8119140. [DOI] [PubMed] [Google Scholar]
- Voeller HJ, Truica CI, Gelmann EP. Beta-catenin mutations in human prostate cancer. Cancer Res. 1998;58:2520–2523. [PubMed] [Google Scholar]
- Yan L, Han Y, He Y, Xie H, Liu J, Zhao L, Wang J, Gao L, Fan D. Cell tracing techniques in stem cell transplantation. Stem Cell Rev. 2007;3:265–269. doi: 10.1007/s12015-007-9004-y. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.