Abstract
The present study compared the immunolocalization of Kim-1, renal papillary antigen (RPA)-1, and RPA-2 with that of inducible nitric oxide synthase (iNOS) and nitrotyrosine in kidneys of gentamicin sulfate (Gen)- and cisplatin (Cis)-treated rats. The specificity of acute kidney injury (AKI) biomarkers, iNOS, and nitrotyrosine was evaluated by dosing rats with valproic acid (VPA). Sprague-Dawley (SD) rats were injected subcutaneously (sc) with 100 mg/kg/day of Gen for six or fourteen days; a single intraperitoneal (ip) dose of 1, 3, or 6 mg/kg of Cis; or 650 mg/kg/day of VPA (ip) for four days. In Gen-treated rats, Kim-1 was expressed in the epithelial cells, mainly in the S1/S2 segments but less so in the S3 segment, and RPA-1 was increased in the epithelial cells of collecting ducts (CD) in the cortex. Spatial expression of iNOS or nitrotyrosine with Kim-1 or RPA-1 was detected. In Cis-treated rats, Kim-1 was expressed only in the S3 segment cells, and RPA-1 and RPA-2 were increased in the epithelial cells of medullary CD or medullary loop of Henle (LH), respectively. Spatial expression of iNOS or nitrotyrosine with RPA-1 or RPA-2 was also identified. These findings suggest that peroxynitrite formation may be involved in the pathogenesis of Gen and Cis nephrotoxicity and that Kim-1, RPA-1, and RPA-2 have the potential to serve as site-specific biomarkers for Gen or Cis AKI.
Keywords: cisplatin, gentamicin, Kim-1, nitrotyrosine, RPA-1, RPA-2
Introduction
Gentamicin (Gen), an aminoglycoside antibiotic, and cisplatin (Cis; Cis-platin, cis-diamminedichloroplatinum II), an antineoplastic agent, have been widely used as model nephrotoxicants for evaluating biomarkers for acute kidney injury (AKI) in rats. Kidney injury molecule-1 (Kim-1) has been shown to be a sensitive and specific biomarker for studying Gen- and Cis-induced renal injury (Amin et al. 2004; Cristofori et al. 2007; Espandiari, Zhang, Rosenzweig, Vaidya et al. 2007; Espandiari, Zhang, Rosenzweig, Zhou et al. 2007; Vaidya and Bonventre 2006; Vaidya et al. 2006; Zhou et al. 2008). Recently renal papillary antigen (RPA)-1 has been suggested to be a sensitive biomarker for Gen-induced kidney injury (Shaw et al. 2007) and was shown to be a sensitive and specific biomarker of renal papillary collecting duct (CD) injury induced by N-phenylanthranillic acid (NPAA) (Harpur et al. 2008).
Cis and Gen are currently two of the most widely used nephrotoxicants for elucidating the role of oxidative and nitrosative stress in experimental rodent models (Table 1). It is, therefore, of interest to know whether any relationship exists between site-specific generation of inducible nitric oxide synthase (iNOS) and nitrotyrosine and site-selective tubular injury, as well as the relationship, if any, between peroxynitrite formation and the expression of the renal biomarkers Kim-1, RPA-1, and RPA-2. Previous studies have suggested that iNOS activation with nitrotyrosine production in injured nephron segments is involved in the induction of Kim-1 and up-regulation of RPA-1 and RPA-2 following exposure to the nephrotoxicants Gen, mercury, and chromium (Zhang, Brown et al. 2008). Peroxynitrite formation may represent a common final pathway in drug-dependent toxicity (Denicola and Radi 2005), and thus selective inhibition of peroxynitrite formation and iNOS has become an important strategy to ameliorate Gen-, Cis-, potassium dichromate-, and ischemia/perfusion-induced renal injury (Table 1).
Table 1.
Immimohistochemical changes in iNOS and nitrotyrosine via inhibition of oxidative and nitrosative stress in animals treated with nephrotoxicants or exposed to nephrotoxic insult.
| Rodent | Nephrotoxicant or nephrotoxic insult |
Protective agents | Actions | IHC for markers of oxidative and nitrosative stress |
References |
|---|---|---|---|---|---|
| Rat | Gen | S-allylmercaptocysteine | Hydroxyl radical scavenger | IHC for 4-hydroxy-2-nonenal ↓ | Pedraza-Chaverri et al., 2004 |
| IHC for nitrotyrosine ↓ | |||||
| IHC for dinitrophenol ↓ | |||||
| Rats | Cis | 5, 10, 15, 20-tetrakis (4-sulfonato-phenyl) porphyrinato iron (III) | Peroxynitrite decomposition catalyst | IHC for nitrotyrosine ↓ | Chirino et al., 2004 |
| Rats | Cis | N-[3-(aminomethyl)benzyl] acetamidine | Selective iNOS inhibitor | IHC for nitrotyrosine ↓ | Chirino et al., 2008 |
| IHC for 4-hydroxy-2-nonenal ↓ | |||||
| Rats | Ischemia/reperfusion injury | L-N (6)-(l-iminoethyl) lysine | Selective iNOS inhibitor | IHC for 3-nitrotyrosine-protein adducts↓ | Walker et al., 2000 |
| Rats | Ischemia/reperfusion injury | L-N (6)-(l-iminoethyl) lysine & aminoethyl-isothiourea | Selective iNOS inhibitors | IHC for nitrotyrosine ↓ | Chatterjee, Patel et al., 2002 |
| Rats & mice | Ischemia/reperfusion injury | GW274150 | Selective iNOS inhibitor | IHC for nitrotyrosine & PARP↓ | Chatterjee et al., 2003 |
| IHC for poly[adenosine diphosphate (ADP)-ribose] ↓ | |||||
| Rats | Ischemia/reperfusion injury | N-[3-(aminomethyl)benzyl] acetamidine | Selective iNOS inhibitor | IHC for nitrotyrosine ↓ | Mark et al., 2005 |
| IHC for iNOS ↓ | |||||
| Rats | Ischemia | L-N (6)-(l-iminoethyl) lysine | Selective iNOS inhibitor | IHC for iNOS ↓ | Lee et al., 2005 |
| IHC for peroxynitrite ↓ | |||||
| Rats | Ischemia/reperfusion injury | Tempol | Radical scavenger | IHC for nitrotyrosine ↓ | Chatterjee et al., 2000 |
| IHC for poly [adenosine diphosphate (ADP)-ribose] ↓ | |||||
| Rats | Ischemia/reperfusion injury | Lipoteichoic acid from Staphylococcus aureus | Antioxidant | IHC for nitrotyrosine ↓ | Chatterjee, Zacharowski et al., 2002 |
| IHC for iNOS ↓ | |||||
| Rats | Potassium dichromate | Garlic powder | Antioxidant | IHC for nitrotyrosine ↓ | Pedraza-Chaverri et al., 2008 |
| IHC for 4-hydroxy-2-nonenal ↓ |
The objective of the present study was to determine if an association exists between the induction of iNOS and nitrotyrosine and the expression of the site-specific biomarkers Kim-1, RPA-1, and RPA-2 in Sprague-Dawley (SD) rats administered Gen or Cis. Histopathological evaluation of kidney injury, the terminal deoxynucleotidyl transferase-mediated [TdT] dUTP nick-end labeling (TUNEL) assay for in situ apoptosis detection and immunohistochemical staining for Kim-1, RPA-1, and RPA-2 were used to study the relationships between iNOS and nitrotyrosine expression and induction or up-regulation of site-specific biomarkers. The hepatotoxicant valproic acid (VPA) (Espandiari, Zhang, Rosenzweig et al. 2008) produces negligible renal injury and was therefore chosen as a control agent to evaluate the specificity of the three biomarkers for renal injury.
Materials and Methods
Animals
Male ten-, twenty-five-, forty-, and eighty-day-old SD rats (Harlan, Indianapolis, IN) were used in this study. Rats were maintained in plastic cages in a controlled environment at 21° C–23° C with a twelve-hour light-dark cycle, fed Purina rodent laboratory chow (Purina Mills, St. Louis, MO), and provided water ad libitum. The protocol was approved by the Institutional Animal Care and Use committee of the FDA White Oak Animal Program (Silver Spring, MD). Animal care procedures for the study conformed to the 1996 ILAR (Institute of Laboratory Animal Resources) Guide for the Care and Use of Laboratory Animals (NRC 1996).
Experimental Procedures
Study 1: Gentamicin
Male ten-day-old rats received a single daily subcutaneous (sc) injection of 100 mg/kg/day of Gen (Sigma-Aldrich Chemical Co., St. Louis, MO, USA) for six consecutive days, and eighty-day old rats received this dose for six or fourteen days. Age-matched controls were given saline by a single daily sc injection. Rats were anesthetized with isoflurane and euthanized by exsanguination twenty-four hours after the final injection.
Study 2: Cisplatin
Male ten-, forty-, and eighty-day-old rats were injected intraperitoneally (ip) with a single dose of 1, 3, or 6 mg/kg Cis (Sigma-Aldrich Chem. Co.). Age-matched controls were given a single ip injection of saline. Rats were euthanized seventy-two hours after the final injection.
Study 3: Valproic Acid
Male twenty-five-day-old rats were injected ip with 650 mg/kg/day of VPA (Sigma-Aldrich) for four days. Age-matched controls were given a single ip injection of saline. All rats were euthanized twenty-four hours after the last dose.
Histopathological Studies
At necropsy, both kidneys were collected. One kidney was prepared for histopathology evaluation, and the other was frozen for mRNA expression analyses in another study. The kidney to be studied for histopathology was sagittally cut and divided into dorsal and ventral “halves.” The two tissue sections were fixed in phosphate-buffered 10% formalin, embedded in paraffin, sectioned at a thickness of 5 mm, and stained with hemotoxylin-eosin (H&E). Periodic acid-Schiff (PAS), Masson trichrome, and a silver preparation for basement membrane were used in selected cases. Unstained, formalin-fixed, paraffin-embedded tissue sections were used for the immunohistochemistry (IHC) studies.
Grading System for Renal Lesions
The frequency and severity of renal lesions were assessed by microscopic examination of at least four tissue sections (two H&E- and two TUNEL-stained sections) and varying numbers of specially stained tissue sections.
To obtain a comprehensive assessment of the severity of renal toxicity, alterations to the glomeruli, tubules, interstitium, and renal blood vessels (arteries and arterioles) were assessed as described previously (Duarte et al. 1997). However, changes in AKI biomarkers are believed to be most closely related to changes in tubular epithelial cells, and thus these alterations were used as the basis for assessing lesion severity on a semi-quantitative scoring scale of 0 to 5. In addition, alterations of glomeruli are believed to be secondary factors relevant to tubular AKI. Alterations to the interstitium and vessels were not as significant as those to the tubules and glomeruli.
The criteria of a semiquantitative scoring scale of 0-5 were as follows:
0 = Normal tubules, glomerulus, interstitium, and vessels.
1 = Scant number of tubular epithelial cells showing minimal degeneration, mild tubular dilatation, small number of proteinaceous casts, no regeneration, no definitely significant necrosis or apoptosis. No changes in the glomerulus, interstitium, and vessels.
2 = < 25% of tubular epithelial cells showing mild degeneration (large cytoplasmic vacuoles, a few hya-line droplets in the cytoplasm), mild degree of tubular dilation and proteinaceous casts, slight change in tubular brush border loss, acute tubular necrosis in individual cell or small group of cells, a few apoptotic cells, and no regeneration. No changes in the glomerulus, interstitium, and vessels.
3 = 25%-50% of tubular epithelial cells showing moderate degeneration (multiple large-sized vacuoles, multiple foci of hyaline droplets), mild regeneration, moderate tubular brush border loss, moderate acute tubular necrosis in small group of tubules, and increased number of apoptotic cells. Little involvement of mild glomerular vacuolization. No changes in the interstitium and vessels.
4 = 51%-75% of tubular epithelial cells showing extensive moderate degeneration; moderate regeneration; severe tubular brush border loss; severe acute tubular necrosis; and a large number of apoptotic cells, with apoptotic bodies in clusters of tubules. Little involvement of mild glomerular vacuolization and interstitial lymphocytic infiltration.
5 = >75% of tubular epithelial cells showing severe degeneration, regeneration, severe tubular brush border loss, acute tubular necrosis, and a large number of apoptotic cells with numerous apoptotic bodies. Mild involvement of glomerular injury (vacuolization, mesangial cell proliferation, and increase in mesangial matrix) and interstitial lymphocytic infiltration. No significant changes in the vessels.
Immunohistochemical Studies
TUNEL Assay
Renal tubular epithelial cell apoptosis was detected using the TACS in situ apoptotic detection kit (Trevigen, Inc., Gaithersburg, MD). The TUNEL procedures were as follows. The formalin-fixed, paraffin-embedded tissue sections were mounted on glass slides coated with amino-propyltriethoxysilane. They were then treated with proteinase K for fifteen minutes and 2% H2O2 for five minutes. These steps were followed by incubation with a reaction mixture (TdT, MnCl2, and dNTP) at 37° C for one hour, then with streptavidin-horseradish peroxidase for ten minutes. In the final step, uncolored soluble chromogen (Trevigen blue label) was enzymatically converted into an insoluble blue-colored complex, precipitating at the site of the reaction following incubation for three minutes. These sections were then counterstained with Red Counterstain C. The DNA fragmentation of apoptotic nuclei was indicated by a blue color (Zhang et al. 1999).
Indirect Immunoperoxidase Procedures
Immunohistochemical assays for iNOS (BD Biosciences, San Diego, CA); nitrotyrosine (Cell Science, Norwood, MA); Kim-1 (mouse anti-rat-Kim-1 ectodomain monoclonal antibody, MARKE) (Harvard Medical School, Boston, MA); RPA-1 (Cat. #BIO87CD, Argutus Medical, Dublin, Ireland); and RPA-2 (Cat. #BIO88LH, Argutus Medical) were performed as described previously (Zhang, Brown et al. 2008). The formalin-fixed, paraffin-embedded, poly-L-lysine–coated slides were treated with antigen retrieval solution for twenty minutes, 0.3% H2O2 for thirty minutes, and 5% normal horse serum for thirty minutes. These sections were incubated with primary monoclonal antibodies (1:50 dilution for Kim-1; 1:100 dilution for iNOS, nitrotyrosine, RPA-1, and RPA-2) overnight at 4°C, followed by incubation with a biotinylated secondary antibody for one hour, and avidin-biotinylated horseradish peroxide complex for thirty minutes. The peroxidase reaction was carried out with 0.05% 3′3′ -diaminobenzidine for ten minutes. For all mAbs, a positive reaction was indicated by a brown color (Zhang, Brown et al. 2008).
For negative control staining, primary mAb iNOS was replaced by the isotype mouse IgG2a (BD Biosciences, Cat. #550339), primary mAb nitrotyrosine by mouse IgG2b (Cell Sciences, Cat. #CNCH003-100), primary mAb IgG RPA-1 by mouse serum IgG1 (DAKO, Cat. #X0931), and primary mAb IgM RPA-2 by mouse IgM (DAKO, Cat. #X0942). The isotype mouse nonimmune IgG matched for the primary mAb Kim-1 is not available, so the Kim-1 mAb was omitted from the incubation step for negative control staining.
Statistics
The nonparametric Kruskal-Wallis rank test was used to assess difference in renal lesion scores. A p value < .05 was considered statistically significant.
Results
Histopathology
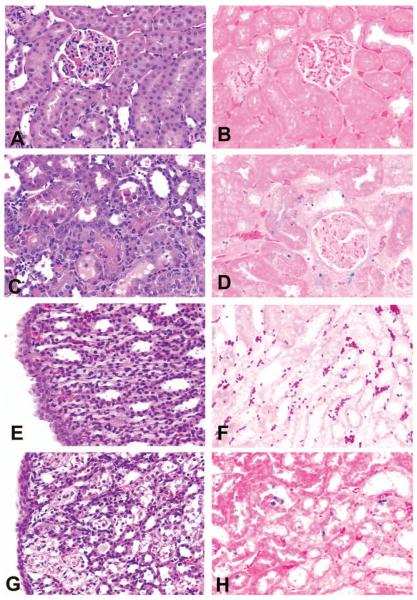
In control rats in the Gen study, no renal injury was found in any proximal tubular segment (Figures 1A and 1B). In Gen-treated rats, epithelial cell necrosis (Figure 1C) and apoptosis (Figure 1D) were located predominantly in the S1/S2 segments of proximal tubules and to a lesser extent in the S3 segment. In control rats in the Cis study, neither tubular epithelial cell necrosis nor apoptosis was found in medullary CD or LH (Figures 1E and 1F). In contrast, in forty-day-old rats treated with 3 mg/kg of Cis, epithelial cell necrosis and apoptosis primarily occurred in the S3 segments of proximal straight tubules (figure not shown), CD (figure not shown), and LH in the medulla (Figures 1G and 1H). Although the epithelial cell injury (degeneration, regeneration, necrosis, and apoptosis) induced by Gen and Cis was similar, the injury was located in the S1/S2 segments (proximal convoluted tubules) and the S3 segments of proximal straight tubules for Gen nephrotoxicity and in the medullary CD or medullary LH for Cis nephrotoxicity (Figures 1C, 1D, 1G, and 1H).
Figure 1.
Representative photomicrographs demonstrating renal alterations in control, gentamicin (Gen)-, or cisplatin (Cis)-treated rats. (A) Neither tubular epithelial cell necrosis nor (B) apoptosis were found in the S1/S2 segments in eighty-day-old, saline-treated control rats in the Gen study. (C) Tubular epithelial cell necrosis and (D) apoptosis were found in the S1/S2 segments in eighty-day-old rats treated with 100 mg/kg/day of Gen for fourteen days. (E) Neither tubular epithelial cell necrosis nor (F) apoptosis were found in the tubular epithelial cells of the collecting ducts or the loop of Henle of saline-treated, forty-day-old control rats in the Cis study. (G) Tubular epithelial cell necrosis and (H) apoptosis were found in the tubular epithelial cells of the loop of Henle in forty-day-old rats treated with 3 mg/kg Cis. All figures ×400.
Renal injury was more severe in Gen- and Cis-treated adult rats (forty or eighty days old) than in immature rats (ten days old), and renal injury was greater in rats treated with Gen for fourteen days compared to those treated for six days (Table 2). In the Cis study, renal injury in aged rats treated with 6 mg/kg of Cis (average lesion score = 3.1) was more severe than renal injury in aged rats treated with 3 mg/kg of Cis (average lesion score = 2.8) or 1 mg/kg of Cis (average lesion score = 0.7).
Table 2.
Immunolocalization of Kim-1, RPA-1, and RPA-2 in SD rats treated with Gen or Cis.
| Age and sex (days old) |
Renal lesion scorea (0-5) |
IHC staining intensity for specific immunoreactivityb |
Nonspecific binding in necrotic proximal tubular cells |
||||
|---|---|---|---|---|---|---|---|
| Treatment | Kim-1 | RPA-1 | RPA-2 | RPA-1 | RPA-2 | ||
| 10, 80, ♂ | Saline for Gen | 0 | −, S1/S2,S3 | CL | CL | − | − |
| 10, ♂ | Gen 100 mg/kg/day × 6 days | 2* | ++, S1/S2,S3 | +, CD (cortex) | +, LH | + | + |
| 80, ♂ | Gen 100 mg/kg/day × 6 days | 3.3* | +++, S1/S2,S3 | ++, CD (cortex) | +, LH | + | + |
| 80, ♂ | Gen 100 mg/kg/day × 14 days | 5* | +++, S1/S2,S3 | +++, CD (cortex) | +, LH | + | + |
| 10, 40, 80, ♂ | Saline for Cis | 0 | −, S1/S2,S3 | CL | CL | − | − |
| 10, ♂ | Cis, 1, 3, 6 mg/kg | 0.3 (0-2.0) | +, S3; −, S1/S2 | +, CD (medulla) | +, LH | − | − |
| 40, ♂ | Cis, 1, 3, 6 mg/kg | 2.6* (0.5-4.0) | ++, S; −, S1/S2 | ++, CD (medulla) | ++, LH | − | − |
| 80, ♂ | Cis, 1, 3, 6 mg/kg | 3.25* (1.5-5) | ++, S3; −, S1/S2 | +++, CD (medulla) | +++, LH | − | − |
Abbreviations: CL, constitutive level of RPA-1 or RPA-2 immunostaining intensity; S1/S2, the S1 & S2 segments of proximal convoluted tubules; S3, the S3 segments of proximal straight tubule.
For Gen-nephrotoxicity, renal lesion score indicates average score from each treatment group. For Cis-nephrotoxicity, renal lesion score indicates average score from 3 treatment groups followed by reference range of 3 groups (in parentheses).
IHC keys: Scores of −, +, ++, and +++ represent no positive reaction, weak, moderate, and strong positive reaction, respectively.
Statistically significant different as compared with saline-treated with saline (p < .05).
Immunolocalization of iNOS and Nitrotyrosine
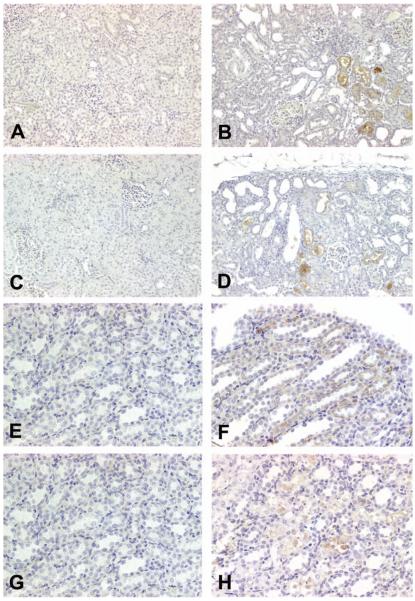
In saline-treated rats, expression of iNOS (Figure 2A) and nitrotyrosine (Figure 2C) was not found in either the renal cortex or the medulla. In contrast, in Gen-treated rats, iNOS (Figure 2B) and nitrotyrosine (Figure 2D) were expressed in the cytoplasm of epithelial cells in the proximal tubules. Moreover, iNOS and nitrotyrosine were also expressed in the flat epithelial cells in the dilated tubules (Figures 2B and 2D), the morphology of which was identical to that detected in RPA-1–stained CD in Gen-treated rats (Figures 4B–4D). In saline-treated control rats in the Cis study, iNOS (Figure 2E) and nitrotyrosine (Figure 2G) were not apparent in the epithelial cells of CD or LH in the medulla. In contrast, in Cis-treated rats, iNOS was expressed predominantly in the epithelial cells of LH of the medulla, although slight expression of iNOS was also discernible in the epithelial cells of CD in the medulla (Figure 2F). Nitrotyrosine expression was found only in the epithelial cells of LH of the medulla (Figure 2H).
Figure 2.
Representative photomicrographs demonstrating expression of inducible nitric oxide synthase (iNOS) and nitrotyrosine in control, gentamicin (Gen)-, or cisplatin (Cis)-treated rats. (A) Expression of iNOS was not found in the epithelial cells of proximal tubules in eighty-day-old, saline-treated control rats in the Gen study; ×200. (B) Expression of iNOS was observed in the epithelial cells of proximal tubules in eighty-day-old rats treated with 100 mg/kg/day of Gen for fourteen days; ×200. (C) Nitrotyrosine expression was not found in the epithelial cells of proximal tubules in eighty-day-old, saline-treated control rats in the Gen study; ×200. (D) Nitrotyrosine expression was found predominantly in the epithelial cells of proximal tubules in eighty-day-old rats treated with 100 mg/kg/day of Gen for fourteen days; ×200. (E) No iNOS expression was found in the medulla of forty-day-old, saline-treated control rats in the Cis study; ×400. (F) Expression of iNOS was found predominantly in the tubular epithelial cells of the loop of Henle, but slight expression of iNOS was also discernible in some of the collecting duct epithelial cells in forty-day-old rats treated with 3 mg/kg Cis; ×400. (G) No nitrotyrosine expression was found in the medulla in forty-day-old, saline-treated control rats in the Cis study; ×400. (H) Nitrotyrosine expression was found in the tubular epithelial cells of the loop of Henle, but not in the collecting duct tubular cells in forty-day-old rats treated with 3 mg/kg Cis. ×400.
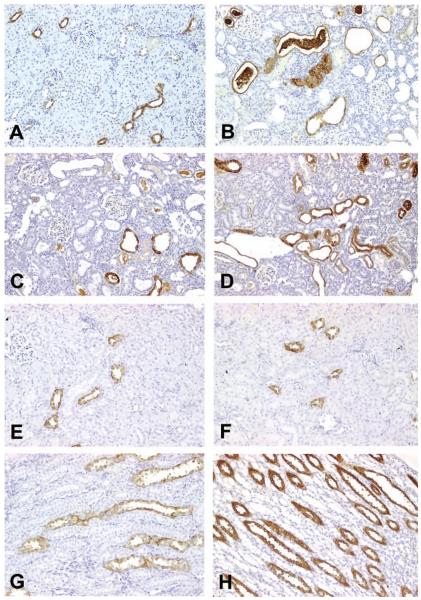
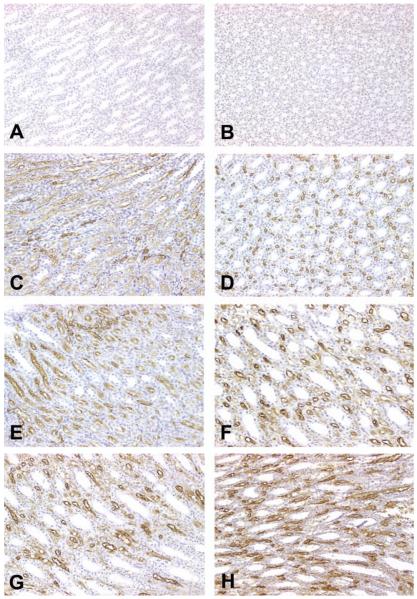
Figure 4.
Representative photomicrographs demonstrating renal papillary antigen (RPA)-1 expression in control, gentamicin (Gen)-, or cisplatin (Cis)-treated rats. (A) In eighty-day-old, saline-treated control rats in the Gen study, constitutive expression of RPA-1 was identified in the collecting duct (CD) epithelial cells in the cortex. Note: normal kidney displayed a few small-sized CDs in the cortex. (B-D) In eighty-day-old rats treated with 100 mg/kg/day of Gen for fourteen days, CDs were of remarkable dilatation. Renal papillary antigen-1 was expressed in the epithelial cells of dilated CD tubules, some of which contained amorphous materials stained by RPA-1. Note: nonspecific binding of RPA-1 was also identified in the epithelial cells of proximal tubules (at the center of 4B). (E) In eighty-day-old, saline-treated control rats in the Cis study, RPA-1 was expressed in a few CD of the cortex. (F) In eighty-day-old rats treated with 6 mg/kg Cis, RPA-1 was expressed in the cortical CD, which was comparable to that observed in control rats. (G) In eighty-day-old, saline-treated control rats in the Cis study, RPA-1 was expressed in epithelial cells of some CD in the medulla. (H) In eighty-day-old rats treated with 6 mg/kg Cis, RPA-1 was expressed in epithelial cells of almost all CD in the medulla, and immunostaining intensity was markedly increased. All figures ×200.
Immunolocalization of Kim-1 in Gen and Cis Nephrotoxicity
Saline-treated, ten-day-old rats did not exhibit positive immunoperoxidase staining for Kim-1 in any segment of the nephron in any renal tissue sections examined (Figure 3A). In contrast, in Gen-treated, ten-day-old rats, Kim-1 expression was located predominantly in the cortex and slightly in the outer stripe (Figure 3B). In saline-treated, ten-day-old rats, Kim-1 expression was not detected in the S1/S2 or S3 segments (Figure 3C). However, in Gen-treated, ten-day-old rats, Kim-1 expression was detected primarily in the S1/S2 segments (Figure 3D). In eighty-day-old rats treated with 100 mg/kg Gen for fourteen days, Kim-1 was expressed in the proximal convoluted tubular cells of the S1/S2 segments (Figure 3E) and in the proximal straight tubular cells of the S3 segments (Figure 3F). In Cis nephrotoxicity, Kim-1 induction was predominantly localized to epithelial cells of the S3 segments, proximal straight tubules (Figures 3G and 3H) in rats treated with either 3 mg/kg (Figure 3G) or 6 mg/kg Cis (Figure 3H). In both Gen and Cis nephrotoxicity, the extent of Kim-1 expression in the S1/S2 or S3 segments increased with time for Gen-treated rats and dose for Cis-treated rats. In addition, the immunostaining intensity correlated with the severity of tubular epithelial cell injury (Table 2).
Figure 3.
Representative photomicrographs demonstrating Kim-1 expression in control, gentamicin (Gen)-, or cisplatin (Cis)-treated rats. (A) No Kim-1 expression was found in the cortex and the outer stripe in ten-day-old, saline-treated control rats in the Gen study; ×50. (B) Kim-1 expression was located in the cortex and the outer stripe in ten-day-old rats treated with 100 mg/kg/day of Gen for six days; ×50. (C) No Kim-1 expression was found in the epithelial cells of proximal tubules in ten-day-old, saline-treated control rats in the Gen study; ×200. (D) Kim-1 expression was located in the epithelial cells of proximal tubules in ten-day-old rats treated with 100 mg/kg/day of Gen for six days; ×200. (E and F) Kim-1 expression was located in the proximal tubular epithelial cells in rats treated with 100 mg/kg/day of Gen for fourteen days; ×200. (G) Kim-1 expression was found in the proximal tubular epithelial cells in eighty-day-old rats treated with 3 mg/kg Cis; ×200. (H) Kim-1 expression was found in the proximal tubular epithelial cells in eighty-day-old rats treated with 6 mg/kg Cis; ×400.
Immunolocalization of RPA-1 in Gen and Cis Nephrotoxicity
Positive staining with mAb RPA-1 (isotype IgG) and negative control staining with the nonimmune mouse IgG1 were performed concurrently. Using the negative control antiserum (mouse IgG1), RPA-1–like expression was not found in CD in the cortex and medulla in rats treated with saline (figure not shown). However, weak nonspecific binding of the RPA-1 antibody was identified in necrotic proximal tubular cells in Gen-treated rats but not in saline control rats (figure not shown). The results were similar to those in a previous study of Gen nephrotoxicity in rats (Zhang, Shaw et al. 2008).
Using the mAb RPA-1 in ten-day-old, saline-treated control rats in the Gen study, constitutive (basal) levels of RPA-1 expression were found mainly in the inner medullary CD epithelial cells and sporadically in the outer medullary and the cortical CD epithelial cells (Figure 4A). The lumens of normal CDs were very small and narrow (Figure 4A). In contrast, in ten-day-old rats treated with 100 mg/kg Gen, RPA-1 was expressed in the epithelial cells of CD in the cortex (Figures 4B-4D). The lumens of CD stained with RPA-1 appeared irregular in shape and larger. Strong, positive-staining amorphous materials were found in some of the lumens (Figure 4B). It was evident that nonspecific binding of RPA-1 occurred in the epithelial cells of proximal tubules (Figure 4B). No significant alterations of RPA-1 expression were found in the medullae of ten-day-old rats treated with 100 mg/kg Gen. In contrast to the intensive staining of cortical CD in the Gen study, RPA-1 expression was comparable between saline-treated rats (Figure 4E) and Cis-treated rats (Figure 4F). In eighty-day-old, saline-treated control rats in the Cis study, constitutive levels of RPA-1 expression were detected in a few CD epithelial cells in the medulla (Figure 4G). In contrast, in eighty-day-old rats treated with 3 mg/kg Cis, RPA-1 expression was increased in the medullary CD, particularly in the proliferative tubular epithelial cells of the dilated CD (Figure 4H). In Cis-treated rats, RPA-1 expression in the epithelial cells of the medullary CD increased with rat age (Table 2).
Immunolocalization of RPA-2 in Gen and Cis Nephrotoxicity
The negative control antiserum (mouse IgM) for RPA-2 showed no staining in the cortical and medullary LH (Figures 5A and 5B) in saline-treated control rats, but nonspecific binding of the anti RPA-2 was detected in the necrotic proximal epithelial cells in Gen-treated rats (figure not shown), which is similar to the findings of our previous Gen study (Zhang, Shaw et al. 2008). In Gen-treated rats, RPA-2 was expressed in the epithelial cells in the thick ascending segment of LH, but the staining intensity was weak and blurred (figures not shown). In addition, nonspecific binding of RPA-2 was also located in the cytoplasm of necrotic proximal cells or their debris in rats treated with 100 mg/kg Gen for six days.
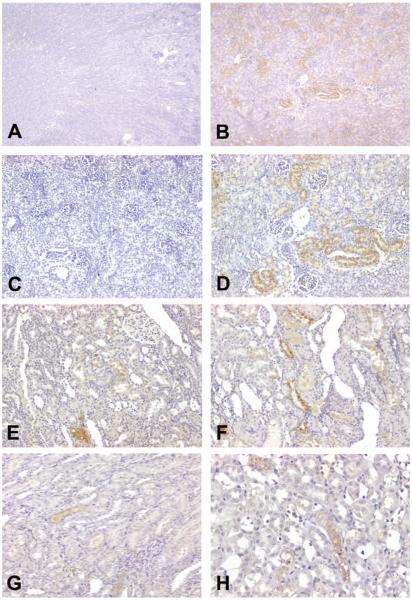
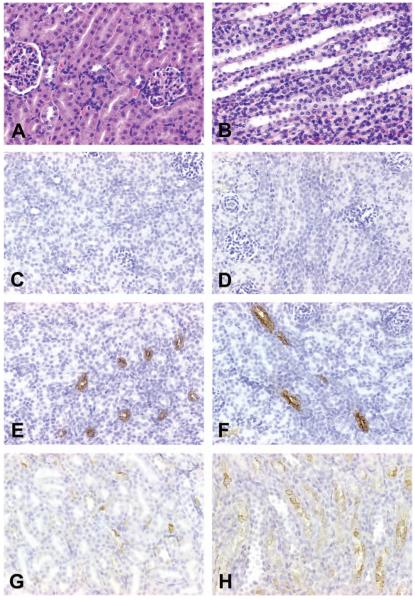
Figure 5.
Representative photomicrographs demonstrating RPA-2 expression in control and cisplatin (Cis)-treated rats. (A and B) RPA-2 expression was not found in the medulla the loop of Henle (LH) in saline-treated, eighty-day-old rats when running negative control staining using nonimmune mouse IgM. (C) Constitutive levels of RPA-2 expression, with faint and indistinct staining in most LH and distinct staining in a few LH, in the medullary LH of saline-treated, eighty-day-old rats. (D-H) Increased RPA-2 expression in the medullary LH in eighty-day-old rats treated with 1 mg/kg of Cis (D), 3 mg/kg of Cis (E and F), and 6 mg/kg of Cis (G and H). All figures ×400.
In control saline-treated, eighty-day-old rats in the Cis study, constitutive levels of RPA-2, with weak and blurry staining in most of the LH and distinct staining in some of the LH, were found in the medulla (Figure 5C). In contrast, RPA-2 expression was increased in the tubular epithelial cells of LH in the medulla in eighty-day-old rats treated with 1 mg/kg Cis (Figure 5D), 3 mg/kg Cis (Figures 5E and 5F), and 6 mg/kg Cis (Figures 5G and 5H). The staining intensity increased with the increase in dose (1, 3, or 6 mg/kg). Expression of RPA-2 in Cis-injured tubular epithelial cells of the medullary LH also increased with rat age (Table 2).
Histopathology and Immunolocalization of Kim-1, RPA-1, and RPA-2 in VPA Study
The hepatotoxicant VPA induced hepatocyte degeneration (cytoplasmic vacuolization) and necrosis and hemorrhage (Espandiari et al. 2008), but it did not induce renal lesions in any segment of the cortex (Figure 6A) or the medulla (Figure 6B). In twenty-five-day-old rats treated with 650 mg/kg VPA, Kim-1 was not expressed in the epithelial cells of proximal tubules (Figure 6C) and in the outer stripe (Figure 6D). Constitutive levels of RPA-1 were expressed in the tubular epithelial cells of CD in the corticomedullary junction (Figure 6E) and in the cortex (Figure 6F). Very low constitutive levels of RPA-2 were expressed in the tubular epithelial cells of LH in the cortex (Figure 6G) and in the corticomedullary junction (Figure 6H). Expression of iNOS and nitrotyrosine was not found in saline-and VPA-treated rats (figure not shown).
Figure 6.
Representative photomicrographs demonstrating expression of Kim-1, RPA-1, and RPA-2 in rats treated with 650 mg/kg/day of Valproic acid (VPA). (A) Normal epithelial cells of proximal tubules; H&E stain. (B) Normal tubular epithelial cells of the LH in the medulla; H&E stain. (C) No Kim-1 was expressed in the epithelial cells of proximal tubules in the cortex. (D) No Kim-1 expression in the tubular epithelial cells of proximal tubules in the outer stripe. (E) Constitutive level of RPA-1 was expressed in the epithelial cells of CD in the medullary ray. (F) Constitutive levels of RPA-1 were expressed in the tubular epithelial cells of CD in the corticomedullary junction. (G) Constitutive levels of RPA-2 were expressed in the tubular epithelial cells of LH in the cortex. (H) Constitutive levels of RPA-2 expression in the tubular epithelial cell of LH in the corticomedullary junction. All figures ×400.
Discussion
In the present study, the most interesting of the observations made included two distinctive IHC expression patterns for AKI biomarkers and markers of oxidative/nitrosative stress: (1) in Gen-treated rats, Kim-1 expression in the S1/S2 segments and the S3 segment, RPA-1 expression in the cortical CD, and an association of Kim-1 and RPA-1 with spatial expression of iNOS or nitrotyrosine; and (2) in Cis-treated rats, Kim-1 expression in the S3 segment, RPA-1 expression in the medullary CD and RPA-2 expression in the medullary LH, and an association of Kim-1, RPA-1, and RPA-2 with spatial expression of iNOS or nitrotyrosine. These two IHC patterns appeared to be correlated with the degree of severity of the epithelial cell necrosis and apoptosis in the Gen and Cis study, respectively. These patterns will be discussed individually below. Although these findings remain to be more precisely defined in future studies and the quantitative IHC data for these biomarkers remain to be determined, they may provide morphological and IHC clues when searching for novel AKI biomarkers and may help elucidate the possible mechanism of Gen and Cis nephrotoxicity.
Immunohistochemical Patterns of iNOS, Nitrotyrosine, Kim-, RPA-1, and RPA-2 Expression in the S1/S2 and S3 Segments Associated with Gen Nephrotoxicity
Microscopic examination demonstrated that Gen-induced AKI was predominantly localized to the S1/S2 segments, with minor involvement of the S3 segment. The IHC data showed that induction of iNOS and nitrotyrosine, in association with expression of Kim-1, was greater in the S1/S2 segments of the cortex than in the S3 segments in the corticomedullary rays. Nonspecific binding of anti-RPA-1 and anti-RPA-2 was identified in the necrotic proximal cells of the same segments. These spatial distributions of AKI biomarkers and oxidative and nitrosative stress markers lend support to our hypothesis that NO production was greater in the S1/S2 segments. However, lack of evidence obtained from specially designed studies (such as quantification of iNOS and nitrotyrosine activity in micro-dissected segments or use of NOS knockout animals) leaves this hypothesis inconclusive at present. In addition, the specialized histological features and functional properties of various nephron segments may also contribute to the topographic distribution of renal injury. The morphologically distinct segments (S1, S2, and S3) of rat kidney proximal tubule cells have been investigated in detail by Maunsback (1966a, 1966b, 1966c). In the rat kidney, the S1 segment has a tall brush border, a prominent endocytic system, numerous mitochondria, and extensive invaginations of the basolateral plasma membrane; the S2 segment has a less prominent brush border than the S1 segment and large secondary lysosomes; and the S3 segment has a tall brush border, less prominent endocytic system than in the S1 and S2 segments, sparse basolateral invaginations, and scattered smaller mitochondria (Madsen and Tisher 2004; Maunsback 1966a, 1966b, 1966c). The prominent endocytic activity and abundant mitochondria in S1-segment epithelia and abundant secondary lysosomes in the S2 segment epithelia with lower endocytic activity and fewer mitochondria may explain why there is general predilection for injury in the S1/S2 segments than in the S3 segment in Gen nephrotoxicity, since it is generally accepted that Gen is taken into the cell mainly by endocytosis (Greaves 1990). It is postulated that damage to other segments may occur in Gen nephrotoxicity, in terms of physiological function of downstream nephron effects resulting from hyperfiltration and functional nephron loss. Other biochemical and molecular mechanisms also play a role in Gen nephrotoxicity; however, a detailed discussion is beyond the scope of this article.
Immunohistochemical Patterns of iNOS, Kim-1, RPA-1, and RPA-2 Expression in the S3 Segment, CD, and LH associated with Cis Nephrotoxicity
Cis-induced AKI was limited almost exclusively to the medulla, specifically, the S3 segments in the outer medulla, and the LH and CD in the outer and the inner medulla. At the sites of Cis-induced AKI, the induction of iNOS and nitrotyrosine was associated with increased expression of Kim-1, RPA-1, and RPA-2. The mechanisms by which Cis induced iNOS and nitrotyrosine; increased the expression of Kim-1, RPA-, and RPA-2; and caused renal injury are not yet fully elucidated.
Immediately after Cis administration, the proximal and distal tubular reabsorption rates of sodium and water are significantly decreased (Daugaard et al. 1987; Daugaard 1990). The reduction of reabsorption capacities in the S1/S2 segments and the S3 segments occur concomitantly with reduced renal blood flow rates, increased renal vascular resistance, and increased urinary levels of sodium and potassium. Therefore, it is possible that the CD and LH, which are permeable to water and salts, will be overburdened by increased urinary sodium, potassium, and water (Daugaard et al. 1987). As a result, the competing burdens of high oxygen demand and insufficient oxygen supply in the LH and CD segments may make these segments more susceptible to hypoxic injury.
A Common Final Pathway Leading to Nephrotoxicity via Nitrotyrosine Generation
Activation of iNOS has been implicated in the mechanisms of various forms of renal injury induced by both nephrotoxicants (e.g., Gen, Cis, chromate) and other forms of renal insult (e.g., ischemia/reperfusion) (Table 1). There is growing evidence to suggest that most of the cytotoxicity attributed to NO is rather a result of peroxynitrite, which is formed by the reaction between superoxide anion and nitric oxide (NO•) (Pacher et al. 2007). Therefore, attenuation of the toxic levels of nitrotyrosine and selective inhibition of iNOS have become pharmacological strategies against nitrosative stress-mediated toxicity in vivo (Denicola and Radi 2005; Pacher et al. 2007). Recent IHC studies have demonstrated that the hydroxyl radical scavenger S-allylmercaptocysteine (a garlic-derived compound) decreased expression of nitrotyrosine, dinitrophenol, and 4-hydroxy-2-nonenal in kidney tissue sections (Pedraza-Chaverri et al. 2004). Concurrent attenuation of increases in serum creatinine, blood urea nitrogen (BUN), and urinary N-acetyl-β-D-glucosaminidase (NAG), as well as reduced kidney damage (proximal tubular cell necrosis) in rats treated with Gen was also observed. These findings suggest that oxidative and nitrosative stress are implicated in Gen nephrotoxicity (Pedraza-Chaverri et al. 2004). In addition, the peroxynitrite decomposition catalyst 5,10,15,20-tetrakis (4-sulfonatophenyl) porphyrinato iron (III) (a soluble complex to catalyze the destruction of peroxynitrite) decreased expression of nitrotyrosine in the kidney tissue sections and attenuated levels of serum creatinine, BUN, NAG, and total proteins in Cis-treated rats. These findings demonstrated that Cis nephrotoxicity was peroxynitrite mediated (Chirino et al. 2004). Recently, a selective and irreversible iNOS inhibitor, N-[3-(aminomethyl)benzyl] accetamine (1400W), was shown to suppress oxidative stress (decreased renal cortex malondialdehyde content and decreased expression of 4-hydroxy-2-nonenal in the kidney tissue sections) and nitrosative stress (decreased expression of nitrotyrosine in the tissue sections). These alterations were accompanied by a reduction in kidney damage (increased proteinuria and decreased creatinine clearance) and improvement of renal function). These results suggest that NO•-derived iNOS contributes to Cis nephrotoxicity (Chirino et al. 2008).
In contrast to previous studies, the present study demonstrated increased expression of iNOS and nitrotyrosine in the epithelial cells of proximal tubules, CD, or LH in Gen- and Cis-treated rats (Figures 2). Therefore, these findings support the hypothesis of a common final pathway leading to Gen or Cis nephrotoxicity via nitrotyrosine generation (the production of NO and superoxide); however, further studies of decreased formation of toxic levels of peroxynitrite are required to reach specific conclusions.
Kim-1, RPA-1, and RPA-2 as Site-specific Biomarkers for Gen and Cis Nephrotoxicity
The present study demonstrated that the expression of Kim-1, RPA-1, and RPA-2 was significantly increased in Gen- and Cis-induced renal tubular epithelial cell injury as compared with their expression in rats treated with saline or VPA, a non-nephrotoxicant. The present study also demonstrated that the immunolocalizations of Kim-1, RPA-1, and RPA-2 were distinctly different.
Kim-1 was mainly expressed in the S1/S2 segments and less in the S3 segments of the cortex in Gen-induced renal injury, whereas it was expressed mainly in the S3 segment in Cis nephrotoxicity. The immunolocalization of Kim-1 is clearly consistent with histopathology for Gen versus Cis nephrotoxicity. These findings demonstrate that Kim-1 is a specific and sensitive biomarker for proximal tubular injury.
The anti-RPA-1 monoclonal antibody recognizes an antigen in the epithelial cells of CD (Falkenberg et al. 1996; Hildebrand et al. 1999). A recent study (Harpur et al. 2008) has shown urinary RPA-1 to be a sensitive and specific biomarker of renal papillary CD injury after treatment with N-phenylanthranillic acid (NPAA), a drug that targets papillary CD. However, the present study revealed that RPA-1 expression was increased in the epithelial cells of CD in the cortex in Gen nephrotoxicity (Figures 4B-4D), in association with morphological evidence of cellular injury, whereas RPA-1 expression was increased in the epithelial cells of CD in the medulla in Cis nephrotoxicity (Figure 4H). These findings may explain our earlier study of Gen nephrotoxicity in rats (Shaw et al. 2007), in which the urinary levels of RPA-1 were significantly elevated four- to twelve-fold higher (dose-dependent) than controls at seventy-two hours after treatment. Therefore, RPA-1 has the potential to serve as a novel biomarker for cortical CD injury induced by Gen, as well as for papillary CD injury induced by NPAA.
The monoclonal anti-RPA-2 recognizes an antigen in the epithelial cells of LH (Falkenberg et al. 1996; Hildebrand et al. 1999). Scant information has been published on RPA-2 distribution and induction in drug-induced nephrotoxicity. The present study demonstrated that RPA-2 expression was increased in the epithelial cells of LH in the medulla (Figures 5C-5H) in association with morphological evidence of cellular injury in Cis nephrotoxicity. No urinary RPA-2 data are available at this time. Although the information regarding RPA-2 in this study is incomplete, the up-regulation of RPA-2 was clearly visible, and RPA-2 has the potential to serve as a novel biomarker for Cis nephrotoxicity.
Acknowledgments
The authors wish to thank Alan Knapton (CDER, FDA) for assistance in figure layout.
Abbreviations
- AKI
acute kidney injury
- CD
collecting ducts
- Cis
cisplatin
- DCT
distal convoluted tubule
- Gen
gentamicin sulfate
- IHC
immunohistochemistry
- iNOS
inducible nitric oxide synthase
- Kim-1
kidney injury molecule-1
- LH
loop of Henle
- NO
nitric oxide
- RPA-1
renal papillary antigen-1
- RPA-2
renal papillary antigen-2
- S1/S2 segments
proximal convoluted tubules
- S3 segment
proximal straight tubules
- VPA
valproic acid
Footnotes
Conflict of Interest: The authors have not declared any conflict of interests.
This report is not an official U.S. Food and Drug Administration guidance or policy statement. No official support or endorsement by the U. S. Food and Drug Administration is intended or should be inferred.
For reprints and permissions queries, please visit SAGE's Web site at http://www.sagepub.com/journalsPermissions.nav
References
- Amin RP, Vickers AE, Sistare F, Thompson KL, Roman RJ, Lawton M, Kramer J, Hamadeh HK, Collins J, Grissom S, Bennett L, Tucker CJ, Wild S, Kind C, Oreffo V, Davis JW, II, Curtiss S, Naciff JM, Cunningham M, Tennant R, Stevens J, Car B, Bertram TA, Afshari CA. Identification of putative gene-based markers of renal toxicity. Environ Health Perspect. 2004;112:465–79. doi: 10.1289/ehp.6683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee PK, Cuzzocrea S, Brown PA, Zacharowski K, Stewart KN, Mota-Filipe H, Thiemermann C. Tempol, a membrane-permeable radical scavenger, reduces oxidant stress-mediated renal dysfunction and injury in the rat. Kidney Int. 2000;58:658–73. doi: 10.1046/j.1523-1755.2000.00212.x. [DOI] [PubMed] [Google Scholar]
- Chatterjee PK, Patel NS, Kvale EO, Cuzzocrea S, Brown PA, Stewart KN, Mota-Filipe H, Thiemermann C. Inhibition of inducible nitric oxide synthase reduces renal ischemia/reperfusion injury. Kidney Int. 2002;61:862–71. doi: 10.1046/j.1523-1755.2002.00234.x. [DOI] [PubMed] [Google Scholar]
- Chatterjee PK, Zacharowski K, Cuzzocrea S, Brown PA, Stewart KN, Mota-Fiilipe H, Thiemermann C. Lipoteichoic acid from Staphylococcusaureus reduces renal ischemia/reperfusion injury. Kidney Int. 2002;62:1249–63. doi: 10.1111/j.1523-1755.2002.kid580.x. [DOI] [PubMed] [Google Scholar]
- Chatterjee PK, Patel NS, Sivarajah A, Kvale EO, Dugo L, Cuzzocrea S, Brown PA, Stewart KN, Mota-Filipe H, Britti D, Yaqoob MM, Thiemermann C. GM274150, a potent and highly selective inhibitor of iNOS, reduced experimental renal ischemia/reperfusion injury. Kidney Int. 2003;63:853–65. doi: 10.1046/j.1523-1755.2003.00802.x. [DOI] [PubMed] [Google Scholar]
- Chirino YI, Hernandez-Pando R, Pedraza-Chaverri J. Peroxynitrite decomposition catalyst ameliorates renal damage and protein nitration in cisplatin-induced nephrotoxicity in rats. BMC Pharmacol. 2004;30:20. doi: 10.1186/1471-2210-4-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirino YI, Trujillo J, Sanchez-Gonzalez DJ, Martinez-Martinez CM, Cruz C, Bobadilla NA, Pedraza-Chaverri J. Selective iNOS inhibition reduces renal damage induced by cisplatin. Toxicol Lett. 2008;176:48–57. doi: 10.1016/j.toxlet.2007.10.006. [DOI] [PubMed] [Google Scholar]
- Cristofori P, Zanetti E, Fregona D, Piaia A, Trevisan A. Renal proximal tubule segment-specific nephrotoxicity: An overview on biomarkers and histopathology. Toxicol Pathol. 2007;35:270–75. doi: 10.1080/01926230601187430. [DOI] [PubMed] [Google Scholar]
- Daugaard G. Cisplatin nephrotoxicity: experimental and clinical studies. Dan Med Bull. 1990;37:1–12. [PubMed] [Google Scholar]
- Daugaard G, Abildggard U, Larsen S, Holstein-Rathlou NH, Amtorp O, Olesen HP, Leyssac PP. Functional and histopathological changes in dog kidney after administration of cisplatin. Renal Physiol. 1987;10:54–64. doi: 10.1159/000173114. [DOI] [PubMed] [Google Scholar]
- Denicola A, Radi R. Peroxynitrite and drug-dependent toxicity. Toxicology. 2005;208:273–88. doi: 10.1016/j.tox.2004.11.023. [DOI] [PubMed] [Google Scholar]
- Duarte CD, Zhang J, Ellis S. The SHR as a small animal model for radiocontrast renal failure. Relation of nephrotoxicity to animal's age, gender, strain, and dose of radiocontrast. Renal Failure. 1997;19:723–43. doi: 10.3109/08860229709037213. [DOI] [PubMed] [Google Scholar]
- Espandiari P, Zhang J, Rosenzweig BA, Vaidya VS, Sun J, Schnackenberg L, Herman EH, Knapton A, Bonventre JV, Beger RD, Thompson KL, Hanig J. The utility of a rodent model in detecting pediatric drug-induced nephrotoxicity. Toxicol Sci. 2008;99:637–48. doi: 10.1093/toxsci/kfm184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espandiari P, Zhang J, Rosenzweig B, Zhou Y, Vaidya VS, Herman E,H, Miller T,J, Knapton A, Honchel R, Weaver J, Noory L, Adeyemo O, Benedick M, Goering P, Brown R, Bonventre JV, Thompson K, Sadrieh N, Hanig J. Age-related differences in susceptibility to cisplatin-induced renal toxicity; International Congress of Toxicology; Montreal, Canada. July 15–17, 2007.2007. [Google Scholar]
- Espandiari P, Zhang J, Schnackenberg LK, Miller TJ, Knapton A, Herman EH, Beger RD, Hanig J. Age-related differences in susceptibility to toxic effects of valproic acid in rats. J Applied Toxicol. 2008;27:628–37. doi: 10.1002/jat.1314. [DOI] [PubMed] [Google Scholar]
- Falkenberg FW, Hilderbrand H, Lutte L, Schwengberg S, Henke B, Greshake D, Schmidt B, Friederich A, Rinke M, Schluter G, Bombard E. Urinary antigens as markers of papillary toxicity. I: Identification and characterization of rat kidney papillary antigens with monoclonal antibodies. Arch Toxicol. 1996;71:80–92. doi: 10.1007/s002040050361. [DOI] [PubMed] [Google Scholar]
- Greaves P. Urinary Tract. In: Greaves P, editor. Histopathology of Preclinical Toxicity Studies. Elseviser Science Publishers B. V.; Amsterdam, the Netherlands: 1990. pp. 497–583. chap. IX. [Google Scholar]
- Harpur E, Schuster K, Betton G, Bounous D, Ennulat D, Reifker B, Mylecraine L, Hoffman D, Gautier J. Comparative performance of novel markers of nephrotoxicity in the rat. Toxicologist. 2008;102:361. Abstract 1758. [Google Scholar]
- Hildebrand H, Rinke M, Schluter G, Bomhard E, Falkenberg FW. Urinary antigens as markers of papillary toxicity. II: Application of monoclonal antibodies for the determination of papillary antigens in rat urine. Arch Toxicol. 1999;73:233–45. doi: 10.1007/s002040050612. [DOI] [PubMed] [Google Scholar]
- Lee CC, Lee YY, Chang CK, Lin MT. Selective inhibition of inducible nitric oxide synthase attenuates renal ischemia and damage in experimental heatstroke. J Pharmacol Sci. 2005;99:68–76. doi: 10.1254/jphs.fp0050300. [DOI] [PubMed] [Google Scholar]
- Madsen KM, Tisher CC. Anatomy of the Kidney. In: Brenner BM, editor. Brenner and Rector's The Kidney. 7th ed. Vol. 1. Saunders; Philadelphia: 2004. pp. 18–72. [Google Scholar]
- Mark LA, Robinson AV, Schulak JA. Inhibition of nitric oxide synthase reduces renal ischemia/reperfusion injury. J Surg Res. 2005;129:236–41. doi: 10.1016/j.jss.2005.06.019. [DOI] [PubMed] [Google Scholar]
- Maunsbach AB. The influence of different fixatives and fixation methods on the ultrastructure of rat kidney proximal tubule cells. I. Comparison of different perfusion fixation methods and of glutaraldehyde, formaldehyde and osmium tetroxide fixative. J Ultrastruct Res. 1966a;15:242–82. doi: 10.1016/s0022-5320(66)80109-0. [DOI] [PubMed] [Google Scholar]
- Maunsbach AB. The influence of different fixatives and fixation methods on the ultrastructure of rat kidney proximal tubule cells. II. Effects of varying osmolality, ionic strength, buffer system, and fixative concentration of glutaraldehyde. J Ultrastruct Res. 1966b;15:283–309. doi: 10.1016/s0022-5320(66)80110-7. [DOI] [PubMed] [Google Scholar]
- Maunsbach AB. Observation on the segmentation of the proximal tubule in the rat kidney: comparison of results from phase contrast, fluorescence and electron microscopy. J Ultrastruct Res. 1966c;16:239–58. doi: 10.1016/s0022-5320(66)80060-6. [DOI] [PubMed] [Google Scholar]
- National Research Council (NRC) Guide for the Care and Use of Laboratory Animals. National Academy Press; Washington, DC: 1996. pp. 227–33. [Google Scholar]
- Pacher P, Beckman JS, Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiol Rev. 2007;87:315–424. doi: 10.1152/physrev.00029.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedraza-Chaverri J, Barrera D, Maldonado PD, Chirino YI, Macias-Ruvalcaba NA, Medina-Campos ON, Castro L, Salcedo MI, Hernandez-Pando R. S-allymercaptocystein scavenges hydroxyl radical and singlet oxygen in vitro and attenuates gentamicin-induced oxidative and nitrosative stress and renal damage in vivo. BMC Clin Pharmacol. 2004;4:5. doi: 10.1186/1472-6904-4-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedraza-Chaverri J, Yam-Canul P, Chirino YI, Sanchez-Gonzalez DJ, Martinez-Martinez C. M. Cruz C., Medina-Campos ON. Protective effects of garlic powder against potassium dichromate-induced oxidative stress and nephrotoxicity. Food Chem Toxicol. 2008;46:619–27. doi: 10.1016/j.fct.2007.09.088. [DOI] [PubMed] [Google Scholar]
- Shaw M, Vaidya VS, Zhou Y, Ferguson M, Zhang J, Brown RP, Keenan JV, Bonventre JV, Jr., Goering PL. Alpha-GST, GST-Yb1, RPA-1, and Kim-1 are sensitive biomarkers of subclinical renal injury in gentamicin-treated rats. Toxicologist. 2007;91:97. Abstract 464. [Google Scholar]
- Vaidya VS, Bonventre JV. Mechanistic biomarkers for cytotoxic acute kidney injury. Expert Opin Drug Metab Toxicol. 2006;2:697–713. doi: 10.1517/17425255.2.5.697. [DOI] [PubMed] [Google Scholar]
- Vaidya VS, Ramirez V, Ichimura T, Bobadilla NA, Bonventre JV. Urinary kidney injury molecule-1: a sensitive quantitative biomarker for early detection of kidney tubular injury. Am J Physiol Renal Physiol. 2006;290:F517–29. doi: 10.1152/ajprenal.00291.2005. [DOI] [PubMed] [Google Scholar]
- Vijayan FP, Rani VK, Vineesh VR, Sudha KS, Michael M, Padikkala J. Protective effect of Cyclea peltata Lam on cisplatin-induced nephrotoxicity and oxidative damage. J Basic Clin Physiol Pharmacol. 2007;18:101–14. doi: 10.1515/jbcpp.2007.18.2.101. [DOI] [PubMed] [Google Scholar]
- Walker LM, Walker PD, Imam SZ, Ali SF, Mayeux PR. Evidence for peroxynitrite formation in renal ischemia-reperfusion injury: studies with the inducible nitric oxide synthase inhibitor L-N (6)-(11minothyl) lysine. J Pharmacol Exp Ther. 2000;295:417–22. [PubMed] [Google Scholar]
- Zhang J, Duarte CG, Ellis S. Contrast medium- and mannitol-induced apoptosis in heart and kidney of SHR rats. Toxicol Pathol. 1999;27:427–35. doi: 10.1177/019262339902700406. [DOI] [PubMed] [Google Scholar]
- Zhang J, Brown RP, Shaw M, Vaidya VS, Zhou YZ, Espandiari P, Sadrieh N, Stratmeyer M, Keenan J, Kilty CG, Bonventre JV, Goering PL. Immunolocalization of Kim-1, RPA-1, RPA-2 in kidney of gentamicin-, mercury-, or chromium-treated rats: relationship to renal distributions of iNOS and nitrotyrosine. Toxicol Pathol. 2008;36:397–409. doi: 10.1177/0192623308315832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Shaw M, Goering PL. Renal papillary antigen-1 (RPA-1) cross reactivity in necrotic renal proximal tubules: Significance of immunohistochemistry and histopathology. Toxicol Pathol. 2008;36:891–93. [Google Scholar]
- Zhou Y, Vaidya VS, Brown RP, Zhang J, Rosenzweig BA, Thompson KL, Miller TJ, Bonventre JV, Goering PL. Comparison of kidney injury molecule-1 and other nephrotoxicity biomarkers in urine and kidney following acute exposure to gentamicin, mercury, and chromium. Toxicol Sci. 2008;101:159–70. doi: 10.1093/toxsci/kfm260. [DOI] [PMC free article] [PubMed] [Google Scholar]