Abstract
Thrombosis underlies numerous life-threatening cardiovascular syndromes. Development of thrombosis-specific molecular imaging agents to detect and monitor thrombogenesis and fibrinolysis in vivo could improve the diagnosis, risk stratification, and treatment of thrombosis syndromes. To this end, we have synthesized efficient multimodal nanoagents targeted to two different constituents of thrombi, namely, fibrin and activated factor XIII. These agents are targeted via the conjugation of peptide-targeting ligands to the surface of fluorescently labeled magnetic nanoparticles. As demonstrated by in vitro and in vivo studies, both nanoagents possess high affinities for thrombi, and enable mutimodal fluorescence and magnetic resonance imaging.
Introduction
Thrombosis is characterized by the accumulation of platelets and fibrin induced by the activation of coagulation factors. Thrombosis underlies several life-threatening cardiovascular syndromes including myocardial infarction, stroke, and pulmonary embolism, leading causes of mortality in the developed world (1). It is thus important to localize and characterize thrombi in vivo, in order to determine treatment options and their resultant efficacy.
Several conventional and molecular imaging strategies have been developed in order to visualize thrombus formation (2). Platelets, which adhere to the damaged vessel wall, have been fluorescently or radioactively labeled in order to visualize their accumulation (3–5). Alternately, agents targeted to specific molecules within the thrombus have been investigated (3, 4, 6–9). We have previously reported on the synthesis of a peptide-based fluorescent imaging agent targeted to activated factor XIII (FXIIIa) (9). FXIIIa is responsible for the cross-linking of fibrin α- and γ-chains, increasing fibrinolytic resistance, and is a hallmark of biologically acute thrombi, as its activity diminishes over time. This peptide agent is covalently cross-linked into clots actively undergoing thrombogenesis by FXIIIa, thereby exhibiting a time-dependent increase in the fluorescence signal and as such may be utilized to distinguish acute from subacute thrombi in vivo.
Another thrombosis-targeting strategy utilizes the noncovalent binding of a fibrin-avid peptide to thrombi. The tripeptide Gly-Pro-Arg comprises the N-terminal sequence of the α-chain of fibrin, and can inhibit fibrin and thrombin clotting (10). Subsequent iterations have identified the peptide Gly-Pro-Arg-Pro-Pro (GPRPP) as a highly avid fibrin-binding agent possessing increased resistance to proteolysis (8, 11). This pentapeptide has previously been used to scintigraphically image pulmonary emboli in swine by the addition of a C-terminal radionuclide binding sequence, Gly-(D)-Ala-Gly-Gly (GAGG), and labeling with 99mTc. However, few fibrin-targeted molecular imaging agents are available for high-resolution in vivo optical/near-infrared fluorescence imaging, a modality that offers utility in noninvasive imaging (12), intravital microscopy (2), as well as high-resolution intravascular molecular imaging catheters compatible with human coronary arteries (13).
Herein, we discuss the synthesis and characterization of two multimodal thrombus-targeted nanoagents exhibiting either covalent or noncovalent binding to thrombi. Functionalization of fluorescently labeled cross-linked iron oxide (CLIO) nano-particles with targeting peptides for fibrin or FXIIIa yields thrombus-targeted agents detectable by both magnetic resonance (MR) and optical imaging modalities (Table 1). These agents utilize two spectrally distinct fluorescence channels, which allows for the simultaneous monitoring of thrombus formation, the determination of biological thrombus age, and the efficacy of thrombolytic therapies.
Table 1. Thrombus-Targeted Imaging Agents.
| agent | targeting sequence | target | fluorophore |
|---|---|---|---|
| CLIO-Cy7 | n/a | n/a | Cy7 |
| CLIO-GPRPP | GPRPPGGSKGC-NH2 | fibrin | Cy7 |
| CLIO-GPSPP | GPSPPGGSKGC-NH2 | fibrin control | Cy7 |
| CLIO-VT680 | n/a | n/a | VT680 |
| CLIO-FXIII | GNQEQVSPLTLLKC-NH2 | FXIIIa | VT680 |
| CLIO-CXIII | GNAEQVSPLTLLKC-NH2 | FXIIIa control | VT680 |
Experimental Procedures
General
All solvents and reagents used were of reagent grade or better and were used as received. Cross-linked iron oxide nanoparticles (CLIO) were synthesized as described previously and obtained from the Center for Molecular Imaging Research Chemistry Core (14, 15). All peptides were synthesized by the Peptide Synthesis/Protein Sequencing Core at the Massachusetts General Hospital using conventional Fmoc synthesis strategies on Rink amide resin. The peptides (0.1 mmol scale) were received on Rink amide resin with the N-terminus t-butoxucarbonyl (Boc)-protected. High performance liquid chromatography (HPLC) was performed on a Varian ProStar HPLC. Gradients were run with buffer A (H2O/0.1% trifluoroacetic acid (TFA)) and buffer B (acetonitrile/10% buffer A). For analytical HPLC, a Varian C-18 reversed-phase column was used with dimensions of 250 mm × 4.6 mm. For semipreparative HPLC, a Varian C-18 reversed-phase column was used with dimensions of 250 mm × 21.2 mm. UV–vis spectra were recorded on a Cary 50 spectrophotometer and fluorescence emission spectra on a Cary Eclipse spectrofluorometer. Electrospray ionization (ESI) mass spectra were recorded on a Waters Micromass ZQ mass spectrometer in the solvents indicated. Near-infrared fluorophores in the far-red (VT680, 660 nm excitation and 735 nm emission) or near-infrared channel (Cy7, 762 nm excitation and 800 nm emission) were employed for agent synthesis to enable translatable near-infrared fluorescence imaging. Fluorescence reflectance imaging was accomplished on either a Siemens BonSAI or Olympus OV100 system. To augment spin dephasing-induced MRI signal changes from iron oxide nanoparticles, T2*-weighted MR imaging was conducted on a 4.7 T Bruker Pharmascan.
Peptide Cleavage and Purification
The peptide resin was swelled in N,N-dimethylformamide (DMF) for 15 min and filtered. The peptide was then cleaved from the resin and deprotected by reaction with TFA/triisopropylsilane/water (95: 2.5:2.5, 5 mL) for 3 h. The resin was filtered, and the peptide contained in the supernatant was precipitated by the addition of excess methyl tert-butyl ether (MTBE). The precipitate was recovered following centrifugation and washed twice more with MTBE. The resulting crude peptide was purified by HPLC.
GNQEQVSPLTLLKC Peptide for Factor XIII (FXIII) Targeting
The peptide was purified by HPLC (gradient, 80% buffer A to 20% buffer A over 30 min; retention time (rt) = 12.8 min). ESI+ MS (30 V, H2O/0.1% TFA) m/z = 1715.0 (MH+).
GNAEQVSPLTLLKC Peptide Control (CXIII)
The above FXIII peptide underwent a single amino acid substitution (Q → A) to generate a control peptide with weak binding by FXIII (9). The CXIII peptide was purified by HPLC (gradient, 80% buffer A to 20% buffer A over 30 min; rt = 13.3 min). ESI+ MS (30 V, H2O/0.1% TFA) m/z = 1658.1 (MH+).
GPRPPGGSKGC Peptide for Fibrin Targeting (GPRPP)
The peptide was purified by HPLC (gradient, 100% buffer A to 60% buffer A over 30 min; rt = 10.1 min). ESI+ MS (30 V, H2O/0.1% TFA) m/z = 941.8 (MH+).
GPSPPGGSKGC Peptide Control Peptide (GPSPP)
The control peptide with minimal binding to fibrin was purified by HPLC (gradient, 100% buffer A to 60% buffer A over 30 min; rt = 11.1 min). ESI+ MS (30 V, H2O/0.1% TFA) m/z = 1011.8 (MH+).
CLIO-VT680
To CLIO (20 mg) in PBS (2.37 mL) was added the succinimidyl ester of VivoTag 680 (1 mg, VisEn Medical) dissolved in 400 μL of dimethylsulfoxide (DMSO). The reaction was allowed to proceed for 16 h, at which time it was purified by filtration through Sephadex G-25 to yield 3 dyes per CLIO, as determined spectrophotometrically.
CLIO-Cy7
To CLIO (20 mg) in PBS (2.37 mL) was added the succinimidyl ester of Cy7 (1 mg, GE Healthcare) dissolved in 400 μL of dimethylsulfoxide (DMSO). The reaction was allowed to proceed for 16 h, at which time it was purified by filtration through Sephadex G-25 to yield 2 dyes per CLIO, as determined spectrophotometrically.
FXIII-CLIO-VT680 (CLIO-FXIII)
To CLIO-VT680 (5 mg) in PBS (602 μL) was added succinimidyl iodoacetate (SIA, 5 mg) dissolved in DMSO (100 μL) The reaction proceeded for 4 h, at which time it was purified by filtration through Sephadex G-25. To the resulting particle was added the FXIII peptide (5 mg) dissolved in DMSO (200 μL). The reaction was allowed to proceed for 16 h, at which time it was purified by filtration through Sephadex G-25. This procedure typically conjugates 10–12 peptides per particle, as described previously (16).
CXIII-CLIO-VT680 (CLIO-CXIII)
CLIO-CXIII was synthesized as described above for CLIO-FXIII using the CXIII peptide.
GPRPPGGSKGC-CLIO-Cy7 (CLIO-GPRPP)
To CLIO-Cy7 (5 mg) in PBS (531 μL) was added succinimidyl iodoacetate (SIA, 5 mg) dissolved in DMSO (100 μL) The reaction proceeded for 3 h, at which time it was purified by filtration through Sephadex G-25. To the resulting particle was added the GPRPP peptide (5 mg) dissolved in DMSO (200 μL). The reaction was allowed to proceed for 16 h, at which time it was purified by filtration through Sephadex G-25. This procedure typically conjugates 10–12 peptides per particle, as described previously (16).
GPSPPGGSKGC-CLIO-Cy7 (CLIO-GPSPP)
CLIO-GPSPP was synthesized as described above for CLIO-GPRPP using the GPSPP peptide.
In Vitro Clot Binding
Clots were created from fresh frozen plasma (FFP) in 96 well plates. Each well received 180 μL of FFP, 10 μL of 0.4 M CaCl2, and 10 μL of thrombin (0.1 U/μL PBS). The plate was incubated at 37 °C for 90 min to form plasma clots. Thereafter, 6 μg of various nanoparticle preparations (∼2 mg Fe/mL) was added to respective wells and then incubated at 37 °C for an additional 30 min. The clots were then washed twice with PBS and centrifuged at 500 rpm for 10 min. They were then washed twice more with PBS and imaged by fluorescence reflectance imaging on the BonSAI system in the Cy7 or VT680 channels (exposure times from 1–10 s).
For magnetic resonance (MR) imaging, representative clots from the above FRI experiment were embedded in 2% agar and imaged using a T2*-weighted pulse sequence with the following imaging parameters: multislice 2D fast low angle shot (FLASH) sequence, repetition time (TR) 500 ms, echo time (TE) 8 ms, flip angle 30°, matrix 128 × 128, in-plane resolution 0.3 × 0.3 mm, slice thickness 0.8 mm, and 8 averages. Following MR imaging, the gel was imaged on the OV100 system for correlation of fluorescence and magnetic resonance images.
In Vivo Thrombosis Binding
Animal studies were approved by the Hospital Subcommittee of Research Animal Care. In vivo thrombi were induced by ferric chloride (FeCl3) injury to the jugular vein (6, 9). Mice were anesthetized using an intraperiotenal (IP) ketamine (50 mg/mL, 330 μL), xylazine (100 mg/mL, 50 μL), and sterile saline (380 μL) mixture. Each mouse received a 60 μL IP induction dose of the resulting mixture, followed by 30–50 μL IP hourly for continued anesthesia. A paramedian neck incision was then made exposing approximately 1 cm of the jugular vein. FeCl3 was locally applied to the jugular vein using a 2 mm × 1 mm strip of Whatman No. 1 filter paper soaked in 10% FeCl3 in distilled water. The filter paper was applied to the anterior surface of the vein for 5 min. Thereafter, the filter paper was removed, and the injured vessel was irrigated with sterile saline. At 30 min after injury, a respective thrombus-targeted agent (CLIO-GPRPP, CLIO-FXIII) or control agent (CLIO-GPSPP, CLIO-CXIII) was injected i.v. via a tail vein catheter. All agents were injected at 7.5 mg Fe/kg mouse (200 μL total volume). Thrombus binding by imaging agents was determined by ex vivo fluorescence reflectance imaging (FRI) of the resected thrombosed and contralateral control jugular veins. At 30 min after injection of the agent, the mice were sacrificed by cervical dislocation and perfused with 20 mL saline. The jugular vein containing the thrombus and contralateral control jugular vein were then excised and imaged in the Cy7 (fibrin) or VT680 (FXIII) channel on an OV100 FRI system.
Image Analysis
Image analyses were performed with ImageJ 1.37v software (NIH) using manual region-of-interest tracing. Signal-to-background ratios (SBRs) were determined using the formula SBR = mean thrombus signal/mean background signal. For in vitro FRI studies, background was defined as the fluorescence signal from the clots incubated with PBS. For ex vivo FRI studies, background was defined as the nonthrombosed contralateral jugular vein. The statistical significance of the results was calculated using either a one way ANOVA or unpaired t-test, depending upon the number of groups in the comparison.
Results and Discussion
Thrombus-Targeted Probe Synthesis
The fibrin-avid pentapeptide Gly-Pro-Arg-Pro-Pro was synthesized with the addition of the C-terminal sequence Gly-Gly-Ser-Lys-Gly-Cys (GGSKGC), in order to impart functionality on the peptide. GGSKGC has been used with peptides derived from phage screening libraries and contains an amine for modification via amide bond formation as well as a free thiol for conjugation via disulfide or thioester bonds. The peptide, Gly-Pro-Arg-Pro-Pro-Gly-Gly-Ser-Lys-Gly-Cys (GPRPP peptide) was synthesized by typical Fmoc methods, with Boc-protection at the N-terminus. This allows for the glycine to remain unmodified after cleavage from the resin, preventing a possible loss of binding affinity due to steric interference. Similar to the fibrin-targeting peptide, an analogous control peptide with scant FXIII avidity was synthesized with one amino acid replaced (9). The sequence Gly-Pro-Ser-Pro was previously shown to have minimal fibrin binding (10) and thus served as a suitable control peptide. This peptide sequence was initially identified in defective human fibrinogen (fibrinogen Detroit) (17). The peptide, Gly-Pro-Ser-Pro-Pro-Gly-Gly-Ser-Lys-Gly-Cys (GPSPP peptide), was synthesized as described above for the GPRPP peptide.
The fibrin-targeted nanoparticulate agents (GPRPP-CLIO and GPSPP-CLIO) were synthesized by modification of cross-linked iron oxide (CLIO) nanoparticles with the respective peptides. CLIO consists of a 5 nm iron oxide core surrounded by a dextran coating that has been cross-linked with epichlorohydrin and aminated (14). Initially, the particles were appended with Cy7 by reaction of the amines with the succinimidyl ester of the dye to yield 2 fluorophores per particle, as determined spectrophotometrically. The resulting magnetofluorescent particle was then reacted with succinimidyl iodoacetate (SIA) in order to impart reactivity toward sulfhydryl groups and subsequently reacted with the C-terminal cysteine of the peptide. Often, peptides are modified with a chromophore, such as fluorescein (FITC), prior to conjugation to the particle, in order to calculate the number of peptides per particle. In this case, this is not possible as the ultimate utility of these particles is the monitoring of clot formation in vivo, and the FITC channel is often necessary for fluorescence angiography during multichannel intravital microscopy. As the procedure for the conjugation of the peptide to the particle is well established, it is assumed that there are approximately 10 peptides per nanoparticle (16).
The nanoagent targeted to FXIIIa and its corresponding control (CXIII) were synthesized using the peptide sequences previously reported (9, 18). The N-terminus of α2-antiplasmin, N13QEQVSPLTLLK24, is specifically cross-linked into thrombi by the transglutaminase FXIIIa via a covalent bond between the glutamine 14 residue and the ε-amino group of a lysine residing within fibrin. In order to facilitate use as an imaging agent, this sequence was modified at its N-terminus with a glycine and at its C-terminus with a tryptophan spacer and a cysteine, to allow for conjugation to a particle. The control peptide sequence was identical to the FXIII peptide with the exception of an alanine replacement of glutamine 14. This modification results in negligible binding of the peptide to FXIII (9, 18).
Analogous to the fibrin-targeted nanoparticle synthesis, the FXIII and CXIII peptides were conjugated to CLIO via the C-terminal cysteine after modification of the particle surface with SIA. The main difference between the nanoplatforms utilized for FXIIIa and fibrin is the wavelength of the fluorescent dye. For CLIO-FXIII, we chose to utilize VT680-labeled CLIO, as this will allow for simultaneous in vivo imaging of the accumulation of the two agents as the excitation and emission wavelengths of Cy7 and VT680 are spectrally distinct, which enables simultaneous fluorescence imaging of FXIII and fibrin via multichannel fluorescence imaging approaches. Specifically, these agents can be utilized to localize thrombi via the fibrin-targeted nanoparticle and determine thrombus age and lysis potential via the FXIII-targeted agent.
In Vitro Clot Binding
The targeting ability of the synthesized agents was tested with fresh frozen plasma (FFP) clots. Clots were formed in 96-well plates by the addition of CaCl2 and thrombin to FFP, followed by incubation for 90 min. The agents were then incubated with the clots for an additional 30 min and were washed and centrifuged to remove the unbound agent.
Fibrin
The plate was initially imaged by FRI in the Cy7 channel. Clots incubated with the fibrin-targeted imaging agent (CLIO-GPRPP) demonstrated considerably greater NIRF signal than clots incubated with control agents CLIO-GPSPP and CLIO-Cy7 (Figure 1B). The target-to-background ratio (SBR) of CLIO-GPRPP clots (16.6 ± 1.7) was more than 3-fold the SBR of control CLIO-GPSPP (5.0 ± 1.2) and CLIO-Cy7 (5.3 ± 0.8).
Figure 1.
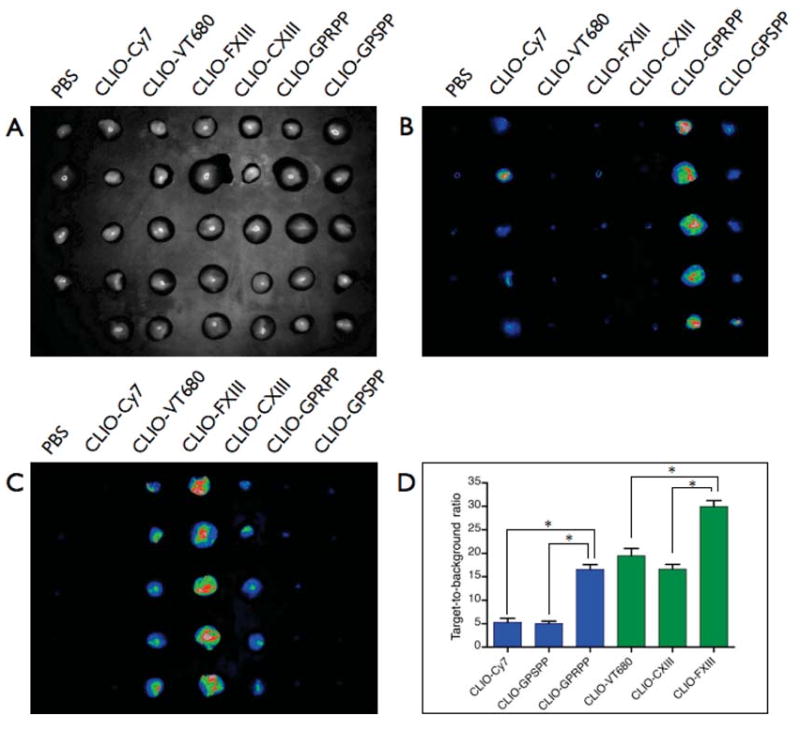
Fluorescence reflectance imaging of in vitro binding of agents to FFP clots (A, bright field image) as imaged in the (B) Cy7 and (C) VT680 channels. (D) The target-to-background ratios of the targeted agents were significantly greater than those of the control agents. All differences were statistically significant (*P < 0.0001).
FXIIIa
When imaged in the VT680 channel, clots incubated with the FXIIIa-targeted imaging agent (CLIO-FXIII) demonstrated considerably greater NIRF signal than clots incubated with control agents CLIO-CXIII and CLIO-VT680 (Figure 1C). The SBR of CLIO-FXIII clots (30 ± 2.8) was more than 1.5-fold higher than the SBR of control CLIO-CXIII (16.6 ± 2.3) and CLIO-VT680 (19.5 ± 3.4). This high level of control agent background suggests an intrinsic avidity of VT680-bearing CLIO for thrombi, given the much lower SBRs obtained for CLIO-Cy7 incubated clots.
MRI
The multimodal capabilities of the nanoagents was next tested by MRI. Representative FFP clots from the above experiments were embedded within 2% agar (Figure 2A) and then underwent T2*-weighted MR imaging at 4.7 T. Consistent with the well-known MRI signal effects of iron oxide nanoparticles, clots binding targeted CLIO agents (CLIO-GPRPPP and CLIO-FXIII) demonstrated strong MRI signal loss on T2*-weighted images resulting in high thrombus-to-agar contrast-to-noise ratios (CNR) of 50.4 and 30.6, respectively (Figure 2B). In contrast, clots incubated with the control agents (CLIO-CXIII and CLIO-GPSPP) demonstrated negligible signal changes resulting in low CNR values (8.6 and −1.2, respectively) (Figure 2B). FRI of the agar clot phantom after MRI confirmed increased deposition of the targeted FXIII nanoparticle in the VT680 channel (Figure 2C) and targeted fibrin nanoparticles in the Cy7 channel (Figure 2D), further illustrating the multimodal nature of the agents.
Figure 2.
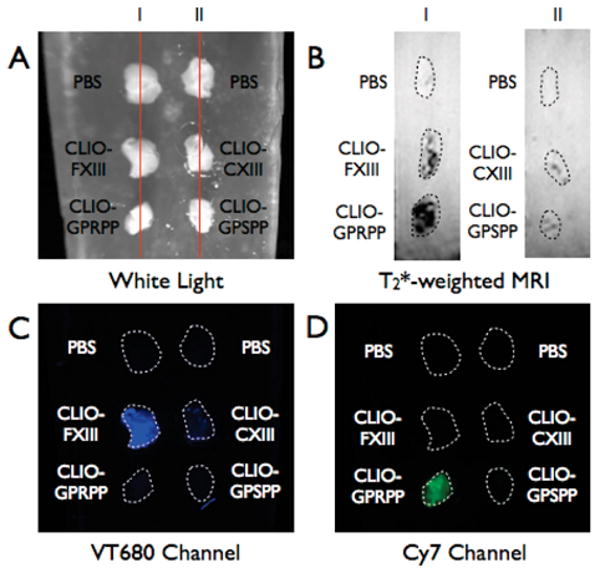
FRI and MRI imaging of FFP clots embedded in 2% agar. (A) White light image of FFP clots. Lines depict where sagittal view of clots was acquired in MRI. (B) T2*-weighted MR image of FFP clots depicting strong T2 decreases in clots incubated with CLIO-FXIII and CLIO-GPRPP. (I) and (II) correlate with lines (I) and (II) of the white light image. (C) FRI image of the gel in the VT680 channel demonstrating the binding of CLIO-FXIII to the FFP clot. (D) FRI image of the gel in the Cy7 channel demonstrating the binding of CLIO-GPRPP to the FFP clot.
In Vivo Thrombosis Binding
The targeting ability of the nanoagents was tested in vivo in an FeCl3-induced thrombosis model in wild-type C57Bl6 mice. Application of 10% FeCl3 laden filter paper directly to the jugular vein for 5 min induced thrombus formation. After 30 min, mice were injected intravenously with targeted or control agents, followed by a 30 min circulation time. The thombosed jugular vein and contralateral normal jugular vein were resected and imaged by FRI. As is evident in Figure 3, the fibrin and FXIII-targeted agents preferentially accumulated within thrombi and generated higher SBR ratios as compared to those of their respective control agents. CLIO-GPRPP displayed a 1.6-fold greater SBR than the control particle, CLIO-GPSPP (SBR = 2.19 ± 0.33 vs 1.37 ± 0.21, respectively), while the SBR for CLIO-FXIII was 2.7-fold greater than that of CLIO-CXIII (SBR = 26.8 ± 3.2 vs 9.7 ± 2.6, respectively). The differences in the magnitude of the SBRs can be attributed to the sensitivity of the instrument in the multiple fluorescent channels. The longest wavelength channel, Cy7, is much less sensitive than the VT680 channel due to fewer photons being transmitted by the OV100 broadband light source. Longer acquisition times are thus required for image formation, which concomitantly produces greater background signals.
Figure 3.
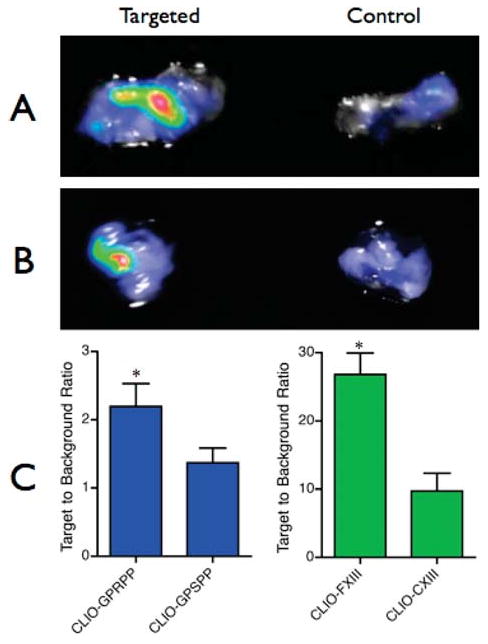
In vivo targeting of acute thrombi. Representative ex vivo FRI composite images of fibrin-targeted (A) and FXIII-targeted (B) nanoagents, and their respective control agents. The fluorescence images of the fibrin-targeted agent were acquired in the Cy7 channel and the FXIII-targeted agent in the VT680 channel. (C) Ex vivo FRI target-to-background ratios for all agents studied (*P < 0.05).
Conclusions
In this work, we demonstrate the synthesis of two novel multimodal nanoagents targeted to key molecules in thrombi, namely, fibrin and FXIIIa. In vitro clot studies demonstrated strong binding of the agents, which is detectable by near-infrared fluorescence and magnetic resonance imaging. In vivo thrombosis studies in mice further demonstrated that these nanoagents readily accumulate within vascular thrombi preferentially over analogously synthesized control agents. Further in vivo studies will utilize these agents to characterize the biological profile of fibrin and factor XIII in acute and subacute thrombi, to examine the role of fibrin and FXIIIa in predicting fibrinolysis and stent thrombosis, and to image fibrin-rich and FXIII-rich thrombi in a variety of thrombosis syndromes.
Supplementary Material
Acknowledgments
This work was supported by NIH grants U24-CA092782 (to R.W.) and U01-HL080731 (to J.R.M., F.A.J., and R.W.), American Heart Association Scientist Development Grant (to F.A.J.), Howard Hughes Medical Institute Career Development Award (to F.A.J.), and NIH grants U54-CA119349 (to R.W.) and U54-CA126515 (to R.W.). We thank Dr. Ashok Khatri for synthesizing the peptide, Dr. Matthias Nahrendorf and Gregory Wojtkiewicz, MS for assistance with MRI, and Zach Mueller for assistance with FRI.
Footnotes
Supporting Information Available: HPLC traces of all peptides synthesized and ex vivo FRI images acquired during this study. This material is available free of charge via the Internet at http://pubs.acs.org.
Literature Cited
- 1.Murray CJ, Lopez AD. Alternative projections of mortality and disability by cause 1990–2020: Global Burden of Disease Study. Lancet. 1997;9064:1498–1504. doi: 10.1016/S0140-6736(96)07492-2. [DOI] [PubMed] [Google Scholar]
- 2.Jaffer FA, Libby P, Weissleder R. Molecular imaging of cardiovascular disease. Circulation. 2007;9:1052–1061. doi: 10.1161/CIRCULATIONAHA.106.647164. [DOI] [PubMed] [Google Scholar]
- 3.Flaumenhaft R, Tanaka E, Graham GJ, De Grand AM, Laurence RG, Hoshino K, Hajjar RJ, Frangioni JV. Localization and quantification of platelet-rich thrombi in large blood vessels with near-infrared fluorescence imaging. Circulation. 2007;1:84–93. doi: 10.1161/CIRCULATIONAHA.106.643908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Massberg S, Enders G, Matos FC, Tomic LI, Leiderer R, Eisenmenger S, Messmer K, Krombach F. Fibrinogen deposition at the postischemic vessel wall promotes platelet adhesion during ischemia-reperfusion in vivo. Blood. 1999;11:3829–3838. [PubMed] [Google Scholar]
- 5.Sim DS, Merrill-Skoloff G, Furie BC, Furie B, Flaumenhaft R. Initial accumulation of platelets during arterial thrombus formation in vivo is inhibited by elevation of basal cAMP levels. Blood. 2004;6:2127–2134. doi: 10.1182/blood-2003-04-1133. [DOI] [PubMed] [Google Scholar]
- 6.Jaffer FA, Tung CH, Gerszten RE, Weissleder R. In vivo imaging of thrombin activity in experimental thrombi with thrombin-sensitive near-infrared molecular probe. Arterioscler, Thromb, Vasc Biol. 2002;11:1929–1935. doi: 10.1161/01.atv.0000033089.56970.2d. [DOI] [PubMed] [Google Scholar]
- 7.Falati S, Gross P, Merrill-Skoloff G, Furie BC, Furie B. Real-time in vivo imaging of platelets, tissue factor and fibrin during arterial thrombus formation in the mouse. Nat Med. 2002;10:1175–1181. doi: 10.1038/nm782. [DOI] [PubMed] [Google Scholar]
- 8.Thakur ML, Pallela VR, Consigny PM, Rao PS, Vessileva-Belnikolovska D, Shi R. Imaging vascular thrombosis with 99mTc-labeled fibrin alpha-chain peptide. J Nucl Med. 2000;1:161–168. [PubMed] [Google Scholar]
- 9.Jaffer FA, Tung CH, Wykrzykowska JJ, Ho NH, Houng AK, Reed GL, Weissleder R. Molecular imaging of factor XIIIa activity in thrombosis using a novel, near-infrared fluorescent contrast agent that covalently links to thrombi. Circulation. 2004;2:170–176. doi: 10.1161/01.CIR.0000134484.11052.44. [DOI] [PubMed] [Google Scholar]
- 10.Laudano AP, Doolittle RF. Synthetic peptide derivatives that bind to fibrinogen and prevent the polymerization of fibrin monomers. Proc Natl Acad Sci USA. 1978;7:3085–3089. doi: 10.1073/pnas.75.7.3085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aruva MR, Daviau J, Sharma SS, Thakur ML. Imaging thromboembolism with fibrin-avid 99mTc-peptide: evaluation in swine. J Nucl Med. 2006;1:155–162. [PMC free article] [PubMed] [Google Scholar]
- 12.Ntziachristos V, Tung CH, Bremer C, Weissleder R. Fluorescence molecular tomography resolves protease activity in vivo. Nat Med. 2002;8:757–761. doi: 10.1038/nm729. [DOI] [PubMed] [Google Scholar]
- 13.Jaffer FA, Vinegoni C, John MC, Aikawa E, Gold HK, Finn AV, Ntziachristos V, Libby P, Weissleder R. Real-time catheter molecular sensing of inflammation in proteolytically active atherosclerosis. Circulation. 2008;18:1802–1809. doi: 10.1161/CIRCULATIONAHA.108.785881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Josephson L, Tung CH, Moore A, Weissleder R. High-efficiency intracellular magnetic labeling with novel superparamagnetic-Tat peptide conjugates. Bioconjugate Chem. 1999;2:186–191. doi: 10.1021/bc980125h. [DOI] [PubMed] [Google Scholar]
- 15.Perez JM, O'Loughin T, Simeone FJ, Weissleder R, Josephson L. DNA-based magnetic nanoparticle assembly acts as a magnetic relaxation nanoswitch allowing screening of DNA-cleaving agents. J Am Chem Soc. 2002;12:2856–2857. doi: 10.1021/ja017773n. [DOI] [PubMed] [Google Scholar]
- 16.Wunderbaldinger P, Josephson L, Weissleder R. Tat peptide directs enhanced clearance and hepatic permeability of magnetic nanoparticles. Bioconjugate Chem. 2002;2:264–268. doi: 10.1021/bc015563u. [DOI] [PubMed] [Google Scholar]
- 17.Blomback M, Blomback B, Mammen EF, Prasad AS. Fibrinogen Detroit--a molecular defect in the N-terminal disulphide knot of human fibrinogen? Nature. 1968;5137:134–137. doi: 10.1038/218134a0. [DOI] [PubMed] [Google Scholar]
- 18.Tung CH, Ho NH, Zeng Q, Tang Y, Jaffer FA, Reed GL, Weissleder R. Novel Factor XIII Probes for Blood Coagulation Imaging. ChemBioChem. 2003;9:897–899. doi: 10.1002/cbic.200300602. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


