Abstract
Background
One of the major obstacles hindering the clinical development of a cell-free, hemoglobin-based oxygen carrier (HBOC) is systemic vasoconstriction.
Methods and results
Experiments were performed in healthy mice and lambs by infusion of either murine tetrameric hemoglobin (0.48 g/kg) or glutaraldehyde-polymerized bovine hemoglobin (HBOC-201, 1.44 g/kg). We observed that intravenous (IV) infusion of either murine tetrameric hemoglobin or HBOC-201 induced prolonged systemic vasoconstriction in wild-type mice, but not in mice congenitally deficient in endothelial nitric oxide (NO) synthase (NOS3). Treatment of wild-type mice by breathing NO at 80 parts per million (ppm) in air for 15 or 60 min, or with 200 ppm NO for 7 min, prevented the systemic hypertension induced by subsequent IV administration of murine tetrameric hemoglobin or HBOC-201 and did not result in conversion of plasma hemoglobin to methemoglobin. IV administration of sodium nitrite (48 nmol) 5 min before infusion of murine tetrameric hemoglobin also prevented the development of systemic hypertension. In awake lambs, breathing NO at 80 ppm for 1 h prevented the systemic hypertension caused by subsequent infusion of HBOC-201.
Conclusions
These findings demonstrate that HBOCs can cause systemic vasoconstriction by scavenging NO produced by NOS3. Moreover, in two species, inhaled NO, administered before the IV infusion of HBOCs, can prevent systemic vasoconstriction without causing methemoglobinemia.
Keywords: nitric oxide, hemoglobin, hypertension, vasoconstriction
Hemoglobin (Hb)-based oxygen carriers (HBOC) have been investigated for clinical use as blood substitutes.1-3 These agents offer the potential to treat patients with anemia or hemorrhage in situations where standard blood transfusions are not readily available (e.g., traumatic injuries), the safety of the blood supply is not assured (e.g. in countries with a high prevalence of HIV infections and/or insufficient safeguards), or when religious beliefs preclude standard transfusions.4
Historically, the major problems associated with infusion of HBOC include a relatively brief circulating half-life, renal toxicity, and, most importantly, the diffuse vasoconstriction potentially leading to coronary and cerebral vasospasm.5 The first two problems have been addressed by producing highly-purified and chemically-crosslinked Hb molecules.1 However, the problem of HBOC-induced vasoconstriction remains unsolved. In human clinical trials, it has been suggested that the gastrointestinal side effects (nausea, vomiting, loss of appetite) and the chest and abdominal pain associated with HBOC administration are the direct results of vasoconstriction.6
The mechanisms responsible for HBOC-induced vasoconstriction are incompletely understood. Winslow has proposed an “autoregulation theory” suggesting that enhanced plasma O2 delivery by cell-free Hb may trigger arteriolar vasoconstriction.6 Alternatively, it has been proposed that the scavenging of endothelium-derived nitric oxide (NO) by cell-free Hb is responsible for the HBOC-induced vasoconstriction. Reiter and co-workers have suggested that, in patients with sickle-cell disease, consumption of NO by high plasma concentrations of cell-free Hb can predispose these patients to vaso-occlusive crises.7,8 A free-Hb-induced “NO deficiency” has also been implicated in the pathogenesis of other human disorders such as hemolysis-associated smooth muscle dystonia, vasculopathy, and endothelial dysfunction.9 Administration of NO donor compounds, such as nitroglycerin or sodium nitroprusside, can attenuate HBOC-induced vasoconstriction but may also cause systemic hypotension.
Inhaled NO is a selective pulmonary vasodilator that has been used to treat pulmonary hypertension and to increase systemic oxygenation in babies and adults, as well as to prevent chronic lung disease associated with prematurity.10 Recent evidence suggests that inhaled NO may affect the systemic vasculature leading to vasodilation when endogenous NO synthesis is inhibited11 (although this is not evident in mice12). Moreover, inhaled NO can ameliorate ischemia-reperfusion injury of peripheral organs.13,14 Inhaled NO may exert systemic effects via interaction with circulating cells as they transit the lungs. Alternatively, some NO, once inhaled, may escape scavenging by Hb and be converted to relatively stable products that can regenerate NO in the periphery.15 Recently, Minneci et al reported that, in dogs, the systemic vasoconstriction induced by intravenous (IV) infusion of cell-free Hb was prevented by concurrent breathing of NO (80 parts per million (ppm)).16 However, breathing NO caused cell-free Hb to be converted to methemoglobin (metHb), disabling the oxygen-carrying capacity of the infused Hb. Our studies had two objectives. First, we sought to determine whether or not scavenging of endogenously-synthesized NO was responsible for the systemic hypertension caused by HBOC infusion. Second, we sought to develop a strategy whereby NO inhalation could be employed to prevent the systemic vasoconstriction induced by HBOC administration without causing its oxidation to metHb. Preparations of HBOCs including murine tetrameric Hb (containing 100% tetramer) and HBOC-201 (a crosslinked bovine Hb containing 3% tetramer) were studied in awake and anesthetized mice, and HBOC-201 was tested in awake lambs. We report that the ability of HBOCs to induce hypertension in mice requires the enzyme responsible for endothelial NO synthesis, NO synthase 3 (NOS3). Moreover, pretreatment with inhaled NO in both species prevents the systemic hypertension caused by subsequent infusion of HBOCs without causing methemoglobinemia.
METHODS
All animal experiments were approved by the Subcommittee on Research Animal Care at Massachusetts General Hospital, Boston, MA. A detailed description of the methods used to prepare murine tetrameric Hb solution, HBOC-201, and metHb, to obtain invasive hemodynamic measurements, and to measure Hb, metHb and nitrite levels are provided in the Online Data Supplement.
Measurements of systolic blood pressure in awake mice
Systolic blood pressure (SBP) was measured with a non-invasive blood pressure system (XBP 1000, Kent Scientific, Torrington, CT) in awake wild type (WT) and NOS3−/− mice. Briefly, the mouse was initially placed in a restrainer (Kent Scientific, Torrington, CT) for a short period (about 1 min), then maintained in the restrainer for longer times to acclimate to the device, as judged by the absence of agitation. After a few days of practice sessions, mice remained comfortable for prolonged periods. Whole blood (1.44 g Hb/kg), murine tetrameric Hb (0.48 g/kg), or HBOC-201 (1.44 g/kg) was administered over 1 min via a tail vein.
Six groups of WT mice were studied. A control group received an infusion of murine whole blood. A second group received an infusion of murine tetrameric Hb. A third group received an infusion of HBOC-201. A fourth group of mice breathing 80 ppm NO beginning 1 h before and continuing after infusion of murine tetrameric Hb. A fifth group was pretreated with various concentrations of inhaled NO (80 ppm for 1 h, 80 ppm for 15 min, and 200 ppm for 7 min) followed by discontinuation of NO breathing and the infusion of murine tetrameric Hb. A sixth group of mice was pretreated with inhaled NO at 80 ppm for 15 min followed by discontinuation of NO breathing and infusion of HBOC-201.
Three groups of NOS3−/− mice were studied. The first received an infusion of murine whole blood served as a control group. The second received an infusion of murine tetrameric Hb, and a third received an infusion of HBOC-201.
Effect of sodium nitrite on the hypertensive response to HBOC
Sodium nitrite (Sigma-Aldrich, St. Louis, MO) was dissolved in PBS, and the pH was adjusted to 7.4. A final volume of 50 μl PBS solution containing 48 nmol sodium nitrite was administered via a tail vein and followed 5 min later by infusion of murine tetrameric Hb solution (0.48 g/kg).
Effect of inhaled NO on systemic blood pressure after challenge with HBOC-201 in awake lambs
Awake, spontaneously breathing lambs were studied. Lactated Ringer's solution was administered at 10 ml/kg/hr. All measurements and samples were obtained at baseline, and before and at the end of each treatment. In all eleven lambs, venous blood was withdrawn into a heparinized syringe and stored at 4°C for two days before reinfusion. Three groups of lambs were studied. One group (n=3) received an infusion of autologous whole blood (warmed at 37°C, 1.44 g Hb/kg over 20 min) while breathing at FiO2=0.3. A second group (n=3) received an infusion of HBOC-201 (1.44 g/kg over 20 min) while breathing at FiO2=0.3. A third group (n=5) breathed 80 ppm NO at FiO2=0.3 for 1 h, followed by discontinuation of NO gas breathing and infusion of HBOC-201 (1.44 g/kg over 20 min) while breathing at FiO2=0.3.
Statistical analysis
All values are expressed as mean±SEM. Data was analyzed using repeated measures ANOVA with interaction. A paired t-test with a Holm-Sidak adjustment was used to compare the changes in clearance of murine tetrameric Hb or HBOC-201. A multilinear regression model analysis was tested in the invasive hemodynamic measurements in anesthetized mice. Detailed explanations of the statistical methods are provided in the Online Data Supplement. P values less than 0.05 were considered significant.
The authors had full access to and take responsibility for the integrity of the data. All authors have read and agree to the manuscript as written.
RESULTS
Clearance of murine tetrameric Hb or HBOC-201
A murine tetrameric Hb solution was prepared from lysed murine red blood cells and 0.48 g/kg was administered to mice via a tail vein over one minute. Blood samples were taken from the lateral saphenous vein every 15 min to measure plasma Hb levels. Administration of murine tetrameric Hb increased plasma Hb levels to 143±8 μM (mean±SEM, n=6) at 15 min. Thereafter, plasma Hb levels declined to 106±9 μM at 1 h and 25±2 μM by 3 h (p<0.008 differs versus 15 min for both). After administration of HBOC-201 (1.44 g/kg over one minute, n=5), plasma Hb levels increased to 391±8 μM at 15 min and remained elevated at 3 h (334±12 μM).
Effect of infusion of tetrameric Hb or HBOC-201 on systolic blood pressure in awake mice
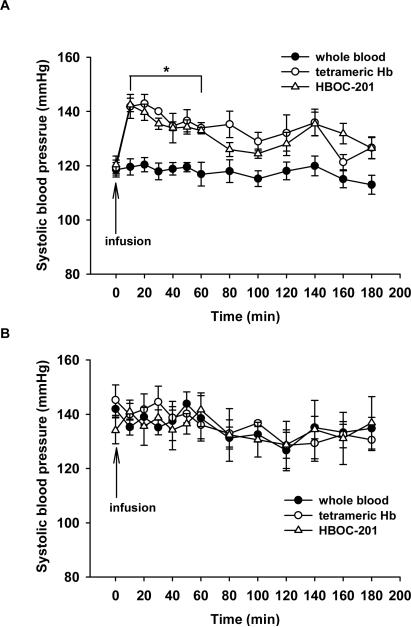
Infusion of murine whole blood (1.44 g Hb/kg, n=7) did not change SBP in awake mice (control group), as measured using a non-invasive tail cuff method. In contrast, infusion of murine tetrameric Hb into mice (n=5) caused immediate and prolonged systemic hypertension (Figure 1A). SBP increased from 119±3 at baseline to 142±4 mmHg at 10 min (p<0.001).
Figure 1.
(A) In WT mice, tail-cuff systolic blood pressure (SBP, mmHg) was measured before and after IV infusion of whole blood (n=7), murine tetrameric Hb (n=5), or HBOC-201 (n=5). *p<0.001 differs versus whole blood infusion group. (B) In NOS3−/− mice, SBP was measured before and after IV infusion of whole blood (n=5), murine tetrameric Hb (n=5), or HBOC-201 (n=5). There was no increase of SBP after infusion of murine tetrameric Hb or HBOC-201.
IV infusion of HBOC-201 increased SBP at 10 min (from 120±3 to 142±2 mmHg, p<0.05, n=5; Figure 1A), and hypertension persisted for more than 1 h (p<0.001).
To examine the role of endothelium-derived NO in the vasoconstriction induced by tetrameric Hb, we investigated whether the administration of murine tetrameric Hb or HBOC-201 would cause vasoconstriction in mice lacking NOS3. Adult NOS3−/−mice (8- to 10-week-old) were hypertensive, as described previously.17 Administration of either murine tetrameric Hb (n=5) or HBOC-201 (n=5) did not increase SBP in NOS3−/− mice (Figure 1B). Infusion of phenylephrine (1 mg/kg) increased systemic blood pressure in anesthetized NOS3-deficient mice from 140±7 mmHg to 189±9 mmHg (n=3, P=0.0016) confirming the ability of NOS3−/− mice to respond to vasoconstrictors. We found a statistically significant interaction between infusion treatments and times in WT mice (P=0.009), but not in NOS3−/− mice. We also compared the vasoconstrictor effects of murine tetrameric Hb or HBOC-201 between WT and NOS3−/− mice, which revealed a statistically significant difference between these two genotypes (P=0.022). These results support the hypothesis that the scavenging of endothelium-derived NO by cell-free Hb is responsible for the vasopressor response observed after the administration of HBOC.
Invasive hemodynamic measurements in anesthetized mice
To further explore the mechanisms by which infusion of tetrameric Hb causes systemic hypertension in awake WT mice, we performed invasive hemodynamic measurements in anesthetized WT (n=15) and NOS3−/− mice (n=13) before and 3 min after infusion of murine tetrameric Hb or whole murine blood, as a control. At baseline, left ventricular (LV) end-diastolic pressure (LVEDP), maximum rate of developed LV pressure (dP/dtmax), minimum rate of developed LV pressure (dP/dtmin), time constant of isovolumic relaxation (τ), and central venous pressure (CVP) were similar between genotypes (Table 1). LV end-systolic pressure (LVESP), arterial elastance (Ea), and systemic vascular resistance (SVR) were greater at baseline in NOS3−/− than in WT mice (p<0.001 for all three). Infusion of murine whole blood did not change the heart rate (HR), LVESP, LVEDP, cardiac output (CO), SVR, Ea, τ, or CVP in either genotype. However, infusion of murine tetrameric Hb in WT mice increased LVESP, LVEDP, SVR, Ea, and τ and decreased CO without affecting dP/dtmax, dP/dtmin, or CVP. In contrast, the infusion of murine tetrameric Hb into NOS3−/− mice did not alter HR, LVESP, LVEDP, CO, dP/dtmax, dP/dtmin, SVR, Ea, τ, or CVP. These results suggest that infusion of tetrameric Hb causes systemic vasoconstriction and impairs cardiac diastolic function via a mechanism which depends on NOS3.
Table 1.
Comparison of cardiac function and systemic hemodynamic measurements in WT and NOS3−/− mice before and after infusion of murine tetrameric Hb solution.
| WT |
NOS3−/− |
WT + iNO |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Baseline (n=7) |
Whole Blood |
Baseline (n=8) |
Tetrameric Hb |
Baseline (n=6) |
Whole Blood |
Baseline (n=7) |
Tetrameric Hb |
Baseline (n=12) |
Tetrameric Hb |
|
| HR (bpm) | 623±14 | 623±15 | 608±13 | 602±12 | 561±14 | 564±12 | 545±14 | 553±18 | 607±19 | 603±20 |
| LVESP (mmHg) | 101±3 | 100±4 | 101±3 | 158±5* | 120±5† | 116±4 | 118±4 | 117±5 | 100±2 | 101±4‡ |
| LVEDP (mmHg) | 6±1 | 6±1 | 6±1 | 12±1* | 6±1 | 7±2 | 6±1 | 8±2 | 5±1 | 6±1‡ |
| dP/dtmax (mmHg/s) | 13800±1200 | 13480±1340 | 12060±1170 | 12260±750 | 13400±1100 | 12040±860 | 12340±840 | 13736±606 | 12420±970 | 11230±750 |
| dP/dtmin (mmHg/s) | −12100±630 | −12800±980 | −11730±540 | −10110±640 | −14150±800 | −14070±670 | −12900±850 | −12290±620 | −11610±700 | −10650±430 |
| CO (ml/min) | 12±1 | 12±1 | 12±1 | 10±1* | 9±1 | 10±1 | 9±1 | 9±1 | 12±1 | 12±1 |
| SVR (dynes*sec/cm2) | 7±0 | 7±0 | 9±1 | 16±1* | 15±5† | 16±4 | 15±2 | 15±2 | 8±1 | 8±1‡ |
| Ea (mmHg/μl) | 5±0 | 5±0 | 5±0 | 9±1* | 8±1† | 8±1 | 8±1 | 8±1 | 5±1 | 5±0‡ |
| τ (msec) | 5±0 | 5±0 | 5±0 | 8±1* | 6±1 | 6±1 | 6±0 | 6±1 | 5±1 | 5±0‡ |
| CVP (mmHg) | 3±0 | 3±0 | 3±0 | 3±0 | 3±0 | 4±0 | 3±0 | 4±0 | 3±0 | 4±0 |
WT: infusion of whole blood or murine tetrameric Hb with breathing air in WT mice; NOS3−/−: infusion of whole blood or murine tetrameric Hb with breathing air in NOS3−/− mice; WT + iNO: breathing 80 ppm NO in air for 15 min, followed by discontinuation of NO gas breathing and infusion of murine tetrameric Hb solution in WT mice; HR: heart rate; LVESP: left ventricular end-systolic pressure; LVEDP: left ventricular end-diastolic pressure; dP/dtmax: maximum rate of developed left ventricular pressure; dP/dtmin: minimum rate of developed left ventricular pressure; CO: cardiac output; SVR: systemic vascular resistance. Ea: arterial elastance; τ: time constant of isovolumic relaxation; CVP: central venous pressure. Values are mean±SEM.
p<0.001 differs versus baseline.
p< 0.05 differs versus WT baseline.
p<0.001 differs versus infusion of tetrameric Hb in WT mice without iNO.
Effect of inhaled NO on systemic blood pressure after challenge with murine tetrameric Hb or HBOC-201 in awake mice
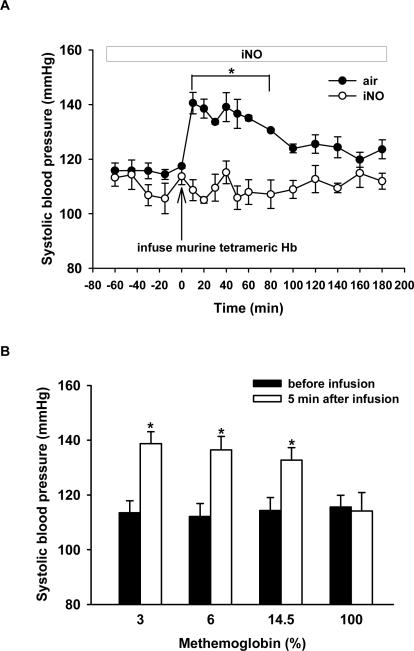
To examine whether or not inhaled NO can prevent the systemic vasoconstriction induced by tetrameric Hb infusion in mice, we investigated the impact of breathing 80 ppm NO on the vasoconstrictor response to murine tetrameric Hb. Inhalation of 80 ppm NO in air, beginning 1 h before and continuing during and after administration of murine tetrameric Hb, completely prevented the increase of SBP (117±1 versus 141±4 mmHg at 10 min, p=0.004, n=5; see Figure 2A).
Figure 2.
(A) Tail-cuff SBP (mmHg) was measured in awake WT mice after infusion of murine tetrameric Hb solution while either breathing air or breathing 80 ppm NO in air (iNO) (n=5 in each group). *p=0.004 differs versus group breathing air without NO. (B) Tail-cuff SBP (mmHg) was measured before and after IV infusion of murine tetrameric Hb containing various concentrations of metHb (3%, 6%, 14.5%, and 100%, respectively n=5 for each group). *p<0.001 differs versus before infusion.
Blood samples were taken 10 min after infusion of murine tetrameric Hb into mice breathing air supplemented with or without NO, and plasma metHb levels were measured. In mice continuously breathing 80 ppm NO for 1 h after administration of murine tetrameric Hb, plasma metHb levels were much greater than those detected in mice breathing air without NO (74±10% versus 4±1%, p<0.001). These observations suggest that inhaled NO prevents the vasoconstrictor effects of murine tetrameric Hb by oxidizing it to metHb, which appears not to scavenge endothelium-derived NO.16
To investigate whether or not metHb can produce systemic hypertension, SBP was measured after infusing various concentrations of metHb (from 3% to 100%) in mice (n=5). Infusion of murine tetrameric Hb with 100% metHb did not alter SBP, suggesting that metHb does not scavenge NO. However, injecting murine tetrameric Hb containing lower concentrations of metHb (3, 6, and 14.5%) increased SBP (Figure 2B).
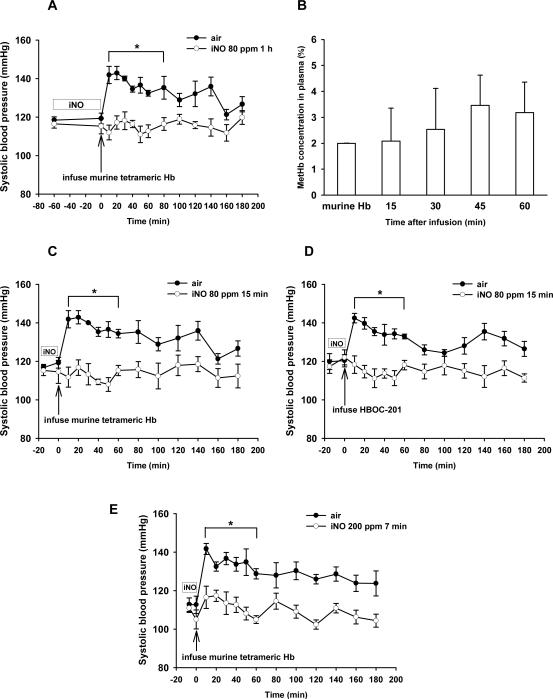
Since breathing NO during administration of murine tetrameric Hb oxidized the infused Hb to metHb (impairing its ability to carry oxygen), we investigated whether or not pretreatment with inhaled NO could attenuate or prevent the systemic hypertension associated with administration of murine tetrameric Hb or HBOC-201 without causing plasma methemoglobinemia. Mice breathed NO in air (80 ppm for 1 h, 80 ppm for 15 min, or 200 ppm for 7 min), immediately followed by IV administration of murine tetrameric Hb, and SBP was measured serially. Blood samples were withdrawn every 15 min after murine tetrameric Hb infusion to monitor plasma Hb and metHb levels. Breathing NO at 80 ppm for 1 h blocked the systemic vasoconstriction induced by subsequent infusion of murine tetrameric Hb (n=5, Figure 3A). The tetrameric Hb that we prepared from murine blood contained 2±0% metHb. Pretreatment with inhaled NO did not increase plasma metHb levels (2±1% at 15 min and 3±1% at 60 min; Figure 3B). When the duration of NO inhalation (80 ppm) was decreased from 1 h to 15 min, NO pretreatment was still able to prevent the systemic hypertension induced by the subsequent infusion of murine tetrameric Hb or HBOC-201 (Figure 3C and 3D, n=5). Similarly, pretreatment with 200 ppm NO breathing for 7 min prevented the systemic hypertension following infusion of murine tetrameric Hb (Figure 3E, n=5). However, pretreatment by breathing 80 ppm NO for 5 min was unable to prevent the systemic hypertension induced by the subsequent infusion of murine tetrameric Hb (data not shown). Invasive hemodynamic measurements revealed that pretreatment of WT mice with inhaled NO at 80 ppm for 15 min abolished the increase of LVESP, LVEDP, SVR, Ea, and τ induced by murine tetrameric Hb infusion (n=12, Table 1). These observations demonstrate that pretreatment with inhaled NO for short periods can prevent the systemic vasoconstriction and diastolic dysfunction produced by HBOC infusion without causing its oxidation to metHb.
Figure 3.
(A) Tail-cuff SBP (mmHg) was measured after pretreatment by breathing 80 ppm NO for 1 h and subsequent infusion of murine tetrameric Hb solution (n=5). Additional mice received the murine tetrameric Hb solution without NO pretreatment (n=7). (B) Plasma metHb concentration (%) at various times after infusion of murine tetrameric Hb following pretreatment by breathing 80 ppm NO in air for 1 h (n=5). (C) SBP was measured after infusion of murine tetrameric Hb solution in mice pretreated without or with breathing 80 ppm NO for 15 min (n=5 in each group). (D) SBP was measured after infusion of HBOC-201 in mice pretreated without or with breathing 80 ppm NO for 15 min (n=5 in each group). (E) SBP was measured after infusion of murine tetrameric Hb solution in mice pretreated without or with breathing 200 ppm NO for 7 min (n=5 in each group). *p<0.05 differs versus group breathing air without NO.
Effect of infusion of sodium nitrite on the hypertensive response to HBOC
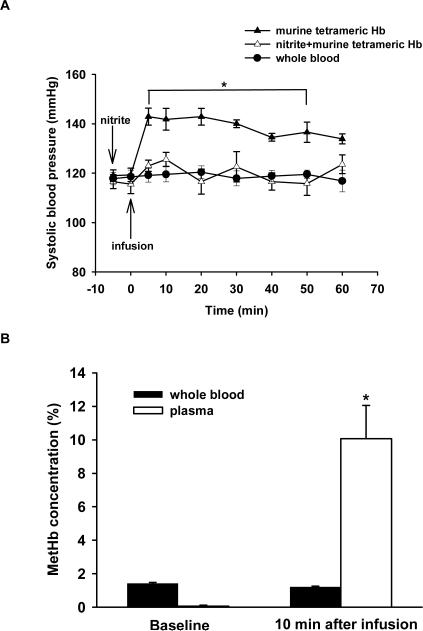
Breathing NO leads to the accumulation of NO metabolites including nitrite,11,14 and nitrite may be converted back to NO via nitrite reductases including deoxyHb.18 To examine whether or not nitrite administration could prevent the systemic hypertension caused by challenge with murine tetrameric Hb, sodium nitrite (48 nmol, 0.13 mg/kg) was administered intravenously 5 min before murine tetrameric Hb was infused. This dose of sodium nitrite chosen is based on the findings of Duranski et al19, who reported that intraventricular injection of nitrite (48 nmol) reduced myocardial infarct size in mice subjected to cardiac ischemia and reperfusion.19 Five minutes after nitrite (48 nmol) was infused into mice, plasma nitrite levels were 1.9-fold greater than baseline values (0.58±0.09 μM versus 0.31±0.06 μM, p<0.02, n=7). Nitrite administration did not alter blood pressure before murine tetrameric Hb was infused. Administration of nitrite blocked the systemic hypertension caused by the subsequent infusion of murine tetrameric Hb (n=5, p<0.05 differs versus both the whole group and the nitrite plus murine tetrameric Hb group, Figure 4A). However, 10 min after murine tetrameric Hb infusion, the plasma metHb level increased to 10±2% (p=0.001 differs versus baseline, p=0.002 differs versus pretreatment with inhaled NO, Figure 4B). This concentration of metHb (10±2%) is insufficient to account for the ability of nitrite to block HBOC-induced hypertension because infusion of murine tetrameric Hb solution containing 14.5% metHb caused systemic hypertension (Figure 2B).
Figure 4.
(A) Tail-cuff SBP (mmHg) was measured in awake mice before and after infusion of whole blood (n=7), murine tetrameric Hb (n=5), or nitrite (48 nmol) followed by murine tetrameric Hb (n=5). *p<0.05 differs versus both the whole blood group and the nitrite plus murine tetrameric Hb group. (B) MetHb concentration (%) in whole blood and plasma (n=5) at baseline and 10 min after infusion of nitrite. *p<0.05 differs versus baseline plasma level.
Effect of inhaled NO on systemic blood pressure after challenge with HBOC-201 in awake lambs
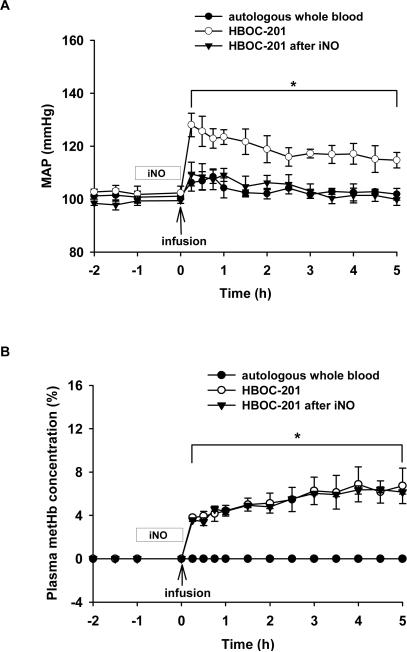
Since, in mice, pretreatment with inhaled NO at 80 ppm for 1 h prevented the systemic hypertension induced by subsequent administration of murine tetrameric Hb or HBOC-201, we investigated whether this effect could be reproduced in a larger species, the awake normovolemic, non-anemic lamb. Mean arterial pressure (MAP) was continuously monitored in each group (Figure 5A). Infusion of autologous whole blood (1.44 g Hb/kg) did not significantly alter MAP. MAP increased immediately after infusion of HBOC-201 (1.44 g/kg) in the group breathing at FiO2=0.3 without NO (p<0.05 differs versus autologous whole blood group). In contrast, pretreatment with inhaled NO blocked the systemic hypertensive effects of HBOC-201 challenge (p<0.05 differs from HBOC-201 without inhaled NO). After administration of HBOC-201, plasma metHb levels in the group pretreated with inhaled NO were not different from those in the group that was not pretreated with inhaled NO (Figure 5B).
Figure 5.
(A) Mean arterial pressure (MAP, mmHg) of awake lambs after infusion of autologous whole blood while breathing at FiO2=0.3 (n=3), HBOC-201 (n=3), or HBOC-201 after pretreatment with breathing 80 ppm NO for 1 h (n=5). * p<0.05 differs from autologous blood and from HBOC-201 after inhaled NO. (B) Plasma metHb concentration (%) before and after infusion of either autologous whole blood or HBOC-201 with or without pretreatment by inhaled NO. *p<0.05 autologous whole blood differs from HBOC-201 with or without iNO pretreatment. Pretreatment with inhaled NO did not increase plasma metHb.
DISCUSSION
In the current study, we report that the vasoconstriction caused by administration of murine tetrameric Hb or HBOC-201 depends on NOS3 in mice. Pretreatment with inhaled NO prevented the systemic hypertension induced by administration of murine tetrameric Hb or HBOC-201 without causing methemoglobinemia. Invasive hemodynamic measurements confirmed that pretreatment by breathing 80 ppm NO for 15 min blocked the systemic hypertension and diastolic dysfunction induced by subsequent infusion of murine tetrameric Hb. We also report that in awake lambs, pretreatment with inhaled NO (80 ppm for 1 h) prevented the systemic hypertension caused by subsequent infusion of HBOC-201.
The “autoregulation theory” suggests that enhanced plasma O2 delivery by cell-free Hb triggers vasoconstriction.6,20 Our study casts doubt on the “autoregulation theory”, since tetrameric Hb delivered oxygen similarly to the arterioles of NOS3−/− and WT mice, yet did not produce systemic vasoconstriction in the former. In NOS3-deficient mice, infusion of phenylephrine increased the systemic blood pressure confirming the ability of NOS3−/− mice to vasoconstrict. Thus, our results provide evidence that the scavenging of endothelium-derived NO (synthesized by NOS3) by cell-free tetrameric Hb is the primary mechanism responsible for the vasoconstriction observed after administration of HBOC-containing tetramer. Our results support and extend prior studies which demonstrated that chemical inhibition of all three NOS isoforms with Nω-nitro-L-arginine methyl ester (L-NAME) prevented the vasoconstriction induced by tetrameric Hb transfusion in cats.21
A major limitation to the clinical application of artificial blood transfusion has been the concern that some HBOCs have caused coronary vasoconstriction that would reduce coronary perfusion and result in myocardial ischemia.22-25 Several Hb modifications have been attempted to limit vasoconstriction after HBOC administration. One strategy has been to genetically engineer the heme pocket of Hb to reduce its NO affinity.26,27 Another strategy has been to attenuate extravasation of HBOCs through endothelial junctions by producing larger Hb molecules, such as polyhemoglobin or conjugated Hb.1
We evaluated an alternate strategy to prevent HBOC-induced vasoconstriction by pretreatment with inhaled NO. Similar to the observations of Minneci et al16, we observed that although breathing 80 ppm NO during and after the administration of tetrameric Hb prevented systemic hypertension, the plasma ferrous Hb was rapidly oxidized resulting in high plasma metHb levels. MetHb is unable to bind and transport oxygen to tissues. In contrast, NO breathing did not increase metHb levels inside the red cell, apparently due to the ample metHb reductase activity in this compartment.1 Intravenous administration of murine tetrameric Hb containing 100% metHb did not produce a systemic vasopressor response providing in vivo evidence that metHb in plasma does not significantly scavenge NO produced by the endothelium. However, concurrent inhalation of high levels of NO with HBOC administration will not enable HBOC-based therapies because the oxygen-carrying capacity of the plasma Hb is largely abrogated.
A most important finding of our current study is that breathing high levels of NO before but not during administration of HBOC prevented the development of systemic hypertension in awake mice. The prevention of systemic hypertension by NO breathing did not come at the cost of oxidizing the cell-free Hb to metHb. Moreover, we report that in awake instrumented lambs, breathing 80 ppm NO for 1 h prevented the systemic hypertension induced by subsequent challenge with HBOC-201.
The pulmonary vasodilator effects of inhaled NO are rapidly dissipated after NO breathing is discontinued, and whether or not breathing NO can modulate systemic vascular tone remains controversial.10 One hypothesis is that during NO breathing, NO-exposed blood cells are responsible for the systemic effects of inhaled NO. Stamler and colleagues proposed that NO can react with Cys93 of the Hb β chain and can be converted back to NO in the periphery.28-30 Alternatively, accumulating evidence shows that breathing NO increases plasma levels of NO metabolites including nitrite.11,14,31 Nitrite has been implicated as a potential mediator of the systemic vasodilation induced by tissue hypoxia, 18,19,32,33 and nitrite formed during NO inhalation could be responsible for the ability of inhaled NO pretreatment to prevent HBOC-induced systemic hypertension. We observe that sodium nitrite infusion increased plasma nitrite levels and prevented the systemic hypertension caused by the subsequent infusion of HBOC. However, nitrite infusion raised plasma metHb levels, partially inactivating oxygen transport. Nonetheless, since infusion of tetrameric Hb containing equivalent levels of metHb (14.5%) still caused systemic hypertension, these findings suggest that nitrite can prevent HBOC-induced hypertension via a mechanism that does not require oxidation of Hb.
The findings that pretreatment with inhaled NO prevents the systemic vasoconstriction induced by HBOCs in two species suggest this strategy may be applicable to humans. If the studies of pretreatment with inhaled NO in the mouse and sheep can be extrapolated to human beings, breathing NO may enable transfusion with artificial blood for the following reasons. First, pretreatment with inhaled NO prevented the subsequent systemic vasoconstriction noted after IV administration of Hb solutions containing tetrameric Hb. Second, inhaled NO pretreatment did not cause plasma methemoglobinemia whereas continuing NO inhalation at 80 ppm during cell-free Hb transfusion dramatically increased plasma metHb levels. Third, because inhaling NO does not cause hypotension, it will likely be feasible to deliver the gas non-invasively while IV access is obtained, for example, in hypotensive trauma patients, prior to HBOC infusion and resuscitation.
In conclusion, we report that vasoconstriction induced by administration of HBOC is abolished in NOS3−/− mice. Pretreatment with inhaled NO prevents the systemic hypertension induced by subsequent IV administration of HBOC without causing plasma methemoglobinemia. Our data support the definitive link between cell-free Hb and endothelial NO consumption. Pretreatment with NO inhalation may provide a novel strategy, which can enable the transfusion of artificial blood without causing systemic hypertension.
Supplementary Material
Acknowledgements
The authors thank and acknowledge the assistance provided in mice and sheep experiments by Dr. Rong Liu, in sheep experiments by Dr. Oleg V. Evgenov, and in statistical analysis by Dr. Hui Zheng of the Massachusetts General Hospital Biostatistics Center.
Source of funding
This work was supported by US Public Health Service Grant HL-42397 to W.M. Zapol.
Footnotes
Conflict of Interest Disclosures
Dr. Zapol receives royalties on patents licensed by Massachusetts General Hospital to Linde Corp. and INO Therapeutics on inhaled nitric oxide. Drs. Zapol and Bloch serve on the Scientific Advisory Board of INO Therapeutics LLC. Other authors (Drs. Yu, Volpato, Ichinose, and Raher) have no relationships that are relevant to the topic of the manuscript.
References
- 1.Chang TMS. Blood Substitutes: Principles, Methods, Products and Clinical Trials. Vol. 1. Karger Landes Systems; Basel, New York: 1997. [Google Scholar]
- 2.Chang TMS. Therapeutic applications of polymeric artificial cells. Nat Rev Drug Discovery. 2005;4:221–235. doi: 10.1038/nrd1659. [DOI] [PubMed] [Google Scholar]
- 3.Hare GMT, Mazer CD. Hemoglobin-based oxygen carriers. in Perioperative Transfusion Medicine. In: Spiess BD, Spence RK, Shander A, editors. 2nd edition Lippincott Williams and Wilkins; 2005. pp. 253–271. [Google Scholar]
- 4.Gannon CJ, Napolitano LM. Severe anemia after gastrointestinal hemorrhage in a Jehovah's witness: new treatment strategies. Crit Care Med. 2002;30:1893–1895. doi: 10.1097/00003246-200208000-00036. [DOI] [PubMed] [Google Scholar]
- 5.Jahr JS, Nesargi SB, Lewis K, Johnson C. Blood substitutes and oxygen therapeutics: an overview and current status. Am J Ther. 2002;9:437–443. doi: 10.1097/00045391-200209000-00012. [DOI] [PubMed] [Google Scholar]
- 6.Winslow RM. Current status of blood substitute research: towards a new paradigm. J Int Med. 2003;253:508–517. doi: 10.1046/j.1365-2796.2003.01150.x. [DOI] [PubMed] [Google Scholar]
- 7.Reiter CD, Wang X, Tanus-Santos JE, Hogg N, Cannon RO, 3rd, Schechter AN, Gladwin MT. Cell-free hemoglobin limits nitric oxide bioavailability in sickle-cell disease. Nat Med. 2002;8:1383–1389. doi: 10.1038/nm1202-799. [DOI] [PubMed] [Google Scholar]
- 8.Gladwin MT, Sachdev V, Jison MJ, Shizukuda Y, Plehn JF, Minter K, Brown B, Coles WA, Nichols JS, Ernst I, Hunter LA, Blackwelder WC, Schechter AN, Rodgers GP, Castro O, Ognibene FP. Pulmonary hypertension as a risk factor for death in patients with sickle cell disease. N Engl J Med. 2004;350:886–895. doi: 10.1056/NEJMoa035477. [DOI] [PubMed] [Google Scholar]
- 9.Rother RP, Bell L, Hillmen P, Gladwin MT. The clinical sequelae of intravascular hemolysis and extracellular plasma hemoglobin a novel mechanism of human disease. J Am Med Assoc. 2005;293:1653–1662. doi: 10.1001/jama.293.13.1653. [DOI] [PubMed] [Google Scholar]
- 10.Bloch KD, Ichinose F, Roberts JD, Jr., Zapol WM. Inhaled NO as a therapeutic agent. Cardiovasc Res. 2007;75:339–348. doi: 10.1016/j.cardiores.2007.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cannon RO, 3rd, Schechter AN, Panza JA, Ognibene FP, Pease-Fye ME, Waclawiw MA, Shelhamer JH, Gladwin MT. Effects of inhaled nitric oxide on regional blood flow are consistent with intravascular nitric oxide delivery. J Clin Invest. 2001;108:279–287. doi: 10.1172/JCI12761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hataishi R, Zapol WM, Bloch KD, Ichinose F. Inhaled nitric oxide does not reduce systemic vascular resistance in mice. Am J Physiol Heart Circ Physiol. 2006;290:H1826–H1829. doi: 10.1152/ajpheart.00938.2005. [DOI] [PubMed] [Google Scholar]
- 13.Fox-Robichaud A, Payne D, Hasan SU, Ostrovsky L, Fairhead T, Reinhardt P, Kubes P. Inhaled NO as a viable antiadhesive therapy for ischemia/reperfusion injury of distal microvascular beds. J Clin Invest. 1998;101:2497–2505. doi: 10.1172/JCI2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hataishi R, Rodrigues AC, Neilan TG, Morgan JG, Buys E, Shiva S, Tambouret R, Jassal DS, Raher MJ, Furutani E, Ichinose F, Gladwin MT, Rosenzweig A, Zapol WM, Picard MH, Bloch KD, Scherrer-Crosbie M. Inhaled nitric oxide decreases infarction size and improves left ventricular function in a murine model of myocardial ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol. 2006;291:H379–H384. doi: 10.1152/ajpheart.01172.2005. [DOI] [PubMed] [Google Scholar]
- 15.Gaston B. Summary: systemic effects of inhaled nitric oxide. Proc Am Thorac Soc. 2006;3:170–172. doi: 10.1513/pats.200506-049BG. [DOI] [PubMed] [Google Scholar]
- 16.Minneci PC, Deans KJ, Zhi H, Yuen PS, Star RA, Banks SM, Schechter AN, Natanson C, Gladwin MT, Solomon SB. Hemolysis-associated endothelial dysfunction mediated by accelerated NO inactivation by decompartmentalized oxyhemoglobin. J Clin Invest. 2005;115:3409–3417. doi: 10.1172/JCI25040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang PL, Huang Z, Mashimo H, Bloch KD, Moskowitz MA, Bevan JA, Fisherman MA. Hypertension in mice lacking the gene for endothelial nitric oxide synthase. Nature. 1995;377:239–242. doi: 10.1038/377239a0. [DOI] [PubMed] [Google Scholar]
- 18.Cosby K, Partovi KS, Crawford JH, Patel RP, Reiter CD, Martyr S, Yang BK, Waclawiw MA, Zalos G, Xu X, Huang KT, Shields H, Kim-Shapiro DB, Schechter AN, Cannon RO, 3rd, Gladwin MT. Nitrite reduction to nitric oxide by deoxyhemoglobin vasodilates the human circulation. Nat Med. 2003;9:1498–1505. doi: 10.1038/nm954. [DOI] [PubMed] [Google Scholar]
- 19.Duranski MR, Greer JJ, Dejam A, Jaganmohan S, Hogg N, Langston W, Patel RP, Yet SF, Wang X, Kevil CG, Gladwin MT, Lefer DJ. Cytoprotective effects of nitrite during in vivo ischemia-reperfusion of the heart and liver. J Clin Invest. 2005;115:1232–1240. doi: 10.1172/JCI22493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vandegriff KD, Malavalli A, Wooldridge J, Lohman J, Winslow RM. MP4, a new nonvasoactive PEG-Hb conjugate. Transfusion. 2003;43:509–516. doi: 10.1046/j.1537-2995.2003.00341.x. [DOI] [PubMed] [Google Scholar]
- 21.Sampei K, Ulatowski JA, Asano Y, Kwansa H, Bucci E, Koehler RC. Role of nitric oxide scavenging in vascular response to cell-free hemoglobin transfusion. Am J Physiol Heart Circ Physiol. 2005;289:H1191–1201. doi: 10.1152/ajpheart.00251.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vogel WM, Dennis RC, Cassidy G, Apstein CS, Valeri CR. Coronary constrictor effect of stroma-free hemoglobin solutions. Am J Physiol Heart Circ Physiol. 1986;251:H413–420. doi: 10.1152/ajpheart.1986.251.2.H413. [DOI] [PubMed] [Google Scholar]
- 23.Hare GMT, Hum KM, Kim SY, Barr A, Baker AJ, Mazer CD. Increased cerebral tissue oxygen tension after extensive hemodilution with a hemoglobin-based oxygen carrier. Anesth Analg. 2004;99:528–535. doi: 10.1213/01.ANE.0000136769.65960.D1. [DOI] [PubMed] [Google Scholar]
- 24.Poli de Figueiredo LF, Mathru M, Solanki D, Macdonald VM, Hess JR, Kramer GC. Pulmonary hypertension and systemic vasoconstriction may offset the benefits of acellular hemoglobin blood substitutes. J Trauma. 1997;42:847–856. doi: 10.1097/00005373-199705000-00015. [DOI] [PubMed] [Google Scholar]
- 25.Motterlini R, Macdonald VW. Cell-free hemoglobin potentiates acetylcholine-induced coronary vasoconstriction in rabbit hearts. J Appl Physiol. 1993;75:2224–2233. doi: 10.1152/jappl.1993.75.5.2224. [DOI] [PubMed] [Google Scholar]
- 26.Doherty DH, Doyle MP, Curry SR, Vali RJ, Fattor TJ, Olson JS, Lemon DD. Rate of reaction with nitric oxide determines the hypertensive effect of cell-free hemoglobin. Nature Biotechnol. 1998;16:672–676. doi: 10.1038/nbt0798-672. [DOI] [PubMed] [Google Scholar]
- 27.Dobschuetz VE, Hutter J, Hoffmann T, Messmer K. Recombinant human hemoglobin with reduced nitric oxide-scavenging capacity restores effectively pancreatic microcirculatory disorders in hemorrhagic shock. Anesthesiology. 2004;100:1484–1490. doi: 10.1097/00000542-200406000-00021. [DOI] [PubMed] [Google Scholar]
- 28.Gaston B, Singel D, Doctor A, Stamler JS. S-nitrosothiol signaling in respiratory biology. Am J Respir Crit Care Med. 2006;173:1186–93. doi: 10.1164/rccm.200510-1584PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hess DT, Matsumoto A, Kim SO, Marshall HE, Stamler JS. Protein S-nitrosylation: purview and parameters. Nat Rev Mol Cell Biol. 2005;6:150–66. doi: 10.1038/nrm1569. [DOI] [PubMed] [Google Scholar]
- 30.Singel DJ, Stamler JS. Chemical physiology of blood flow regulation by red blood cells: the role of nitric oxide and S-nitrosohemoglobin. Annu Rev Physiol. 2005;67:99–145. doi: 10.1146/annurev.physiol.67.060603.090918. [DOI] [PubMed] [Google Scholar]
- 31.Lecour S, Clermont G, du Toit E, Gilson L, Maupoil V, Lowe S, Dupuis P, Girard C, Rochette L. Evidence for the extrapulmonary localization of inhaled nitric oxide. Heart Dis. 2003;5:372–377. doi: 10.1097/01.hdx.0000098613.53486.08. [DOI] [PubMed] [Google Scholar]
- 32.Gladwin MT, Raat NJ, Shiva S, Dezfulian C, Hogg N, Kim-shapiro DB, Patel RP. Nitrite as a vascular endocrine nitric oxide reservoir that contributes to hypoxic signaling, cytoprotection and vasodilation. Am J Physiol Heart Circ Physiol. 2006;291:2026–2035. doi: 10.1152/ajpheart.00407.2006. [DOI] [PubMed] [Google Scholar]
- 33.Hunter CJ, Dejam A, Blood AB, Shields H, Kim-Shapiro DB, Machado RF, Tarekegn S, Mulla N, Hopper AO, Schechter AN, Power GG, Gladwin MT. Inhaled nebulized nitrite is a hypoxia-sensitive NO-dependent selective pulmonary vasodilator. Nat Med. 2004;10:1122–1127. doi: 10.1038/nm1109. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.