Abstract
Intrauterine growth rate is associated with body distribution in adulthood suggesting differential response of fetal fat depots to nutritional modifications. We hypothesize that there is regional differences in fetal adipogenesis, in part, due to depot-specific regulation of the availability of insulin growth factors. In near-term baboon fetuses (n = 3–5), the subcutaneous abdominal vs. omental preadipocytes had (1) more extensive lipid accumulation as assessed by BODIPY (lipid staining) to DAPI (nuclei) absorbance ratios (mean ± SEM; 0.51 ± 0.21, 0.35 ± 0.09, p < 0.05), (2) lower (p < 0.05) secretion of IGF-binding protein 4 (9.6 ± 1.2 vs. 17.4 ± 2.8 ng/ml) and its protease pregnancy associated plasma protein A (24.6 ± 1.9 vs. 39.1 ± 6.3 µIU/ml), (3) lower protein expression of IGF2 “clearance” receptor in cell lysate (0.28 ± 0.03 vs. 0.53 ± 0.02 OD U/mm2, p < 0.05); all variables were intermediate in femoral preadipocytes. The regional variation of the adipogenesis and the IGF regulatory pathway set the stage for differential responsiveness of fat depots to external signals.
Keywords: Fat, Adipose, Preadipocyte, Differentiation, Adipogenesis, Insulin-like growth factor, Pregnancy associated plasma protein, Non-human primates, Fetus
Fat accumulation in the upper body is associated with metabolic abnormalities in obese adult individuals [1] whereas preferential accumulation of fat in the lower body seems to be less detrimental or even protective against adverse metabolic effects [2]. Therefore, studying mechanisms regulating differential regional adipose expansion is important. Previous studies in human adults have reported that in vitro cellular kinetic properties of preadipocytes vary among fat depots in favor of higher adipogenic capacity of preadipocytes from abdominal subcutaneous (SQ ABD) vs. femoral (FEM) [3] and visceral fat depots [4,5]. IGF1, a potent stimulator of adipogenesis, acts in an autocrine/paracrine fashion when in free form [6,7]. Preadipocyte differentiation in response to this growth factor varies among fat depots [8]. Furthermore, evidence of depot-related differences in the transcription of IGF1 binding protein 2 (IGFBP-2) [9] and pregnancy associated plasma protein (PAPP-A), a protease that cleaves IGFBP2/IGFBP4 and decreases their affinity to bind IGF1 [10], suggests that regional variation in regulation of IGF1 bioavailability may play a role in body fat distribution.
Epidemiological studies and animal research have demonstrated an association between restriction of fetal growth and development of upper-body fat distribution later in life suggesting that fetal development of adipose tissue may be a critical period in regards to subsequent fat distribution [11,12]. Both IGF1 and IGF2 are important for adipose tissue growth during fetal development [13]. However, the cellular kinetics of preadipocytes and the regulation of IGF1/IGF2 bioavailability in various fetal adipose depots are unknown.
In this study, we used near-term baboon fetuses, a non-human primate model whose developmental anatomy and physiology are closer to humans than that of other animal models [14], to measure the cellular kinetics of preadipocytes isolated from SQ ABD, omental (OM), and FEM depots. We also measured the secretion of IGFBP-4 and PAPP-A, and the protein levels of IGF1 and -2 receptors (IGF1R and IGF2R, respectively) in cultured fetal stromovascular cell cultures.
Methods
SV cultures
SV cultures
Adipose tissue samples from OM, SQ ABD, and FEM were collected from six near-term (165 days gestation; term gestation is 184 days) baboon fetuses (1 female and 5 males) from ad libitum fed pregnant baboons at the Southwest Foundation for Biomedical Research (SFBR, San Antonio TX). Details of housing and environmental enrichment have been published elsewhere [15]. Cesarean sections were performed under general anesthesia using standard techniques as previously described [16]. All procedures were approved by the SFBR and University of Texas Health Science Center, San Antonio Institutional Animal Care and Use Committees. Adipose tissue was removed from the fetus under aseptic conditions, placed in HBSS buffer and shipped at room temperature to the Pennington Biomedical Research Center.
Within ~24 h from collection, the adipose tissue was digested enzymatically and the isolated stromovascular (SV) cells were cultured as previously described [17]. Third passages were frozen until samples from all fetuses were available for batch processing in a single assay.
Preadipocyte kinetics
Adipogenesis
Cells in 10% FBS/DMEM-F12 (1:1) medium were seeded in duplicate onto fibronectin (2.5 µg/cm2)-coated 96 well plates (1.5 × 104 cells/cm2). After 24 h, preadipocytes were differentiated using serum-free, chemically-defined medium as described previously [18] with modifications: DMEM-F12 (1:1) medium plus 10 mg/mL transferin, 33 µM biotin, 17 µM calcium pantothenate, 0.5 µM insulin, 0.1 µM dexamethasone, 0.2 nM triiodothyronine, 0.5 µM Roziglitazone, and 540 µM IBMX (the last two components for the first 2 days only) for 9 days. Cultures were stained for lipids (BODIPY, 10 µg/ml) and DNA (DAPI, 300 nM); both reagents from Invitrogen, Carlsbad, CA. The absorbance from BODIPY and DAPI was measured on a FlexStation 3 fluorescent microplate reader and the BODIPY-to-DAPI (B:D) absorbance ratio was calculated.
Proliferation
Cells in 10% FBS/DMEM-F12 were seeded in triplicates separately in two 96-well plates (0.5 × 104 cells/cm2). DNA content of each well was quantified using CyQuant® NF Cell Proliferation Assay Kit (Invitrogen, Carlsbad, CA) after 1 (plate one) and 4 (plate two) days. The percent change in fluorescence intensity of the nuclei dye [(day 4–day 1) × 100/day 1] was used as an index of proliferation.
Apoptosis
Cells in 10% FBS/DMEM-F12 were seeded in 2 sets of triplicates in a 96 well plate (1.0 × 104 cells/cm2) and grown to confluence. Apoptosis was induced in one set of the triplicates by switching to serum-free DMEM-F12 (1:1) medium enriched with 10 nM TNFα and 10 µg/ml cycloheximide for 4 h as previously described [7]. The levels of cytoplasmic nucleosomes were measured using Roche’s Cell Death Detection ELISAPLUS kit (Roche Applied Science, Indianapolis, IN). The ratio of the absorbance in the induced to the respective non-induced cultures provided an index of apoptosis.
Secretion of IGFBP-4 and PAPP-A
Media conditioned by confluent cultures from one female and three male fetuses for 72 h were collected and frozen at −80 °C until assayed. The levels of the IGFBP-4 and PAPP-A were determined using RayBio®Human IGFBP-4 ELISA Kit (RayBiotech Inc., Norcross, GA) and ultra sensitive PAPP-A ELISA (provided to Dr. Conover by Diagnostic Systems Laboratories, Inc., Webster, TX) and normalized for DNA content (DAPI absorbance). To verify that secretion of PAPP-A corresponds to its activity, conditioned media from 2 fetuses was incubated with 125IGFBP-4 in the absence and presence of IGF2 at 37 °C for 24 h. IGF2 binds to 125IGFBP-4 increasing its susceptibility to cleavage by PAPP-A [19]. The cleaved 18-kDa radio-labeled fragments and the residual intact PAPP-A were separated by SDS–PAGE and visualized by auto-radiography.
Immunoblotting for IGF receptors
Cells cultured in 10% FBS/DMEM-F12, were lysed on ice in 50 mM Hepes, 15 mM NaCl, 1 mM MgCl2, 1 mM CaCl2, 2 mM EDTA, 10% glycerol, 1% Triton X-100, 5 µL/mL protease inhibitors (phosphotase 1, phosphotase 2 cocktails), and 3 mg/mL benzamidine. The lysates were stored at −80 °C until assayed. Protein concentrations were determined by bicinchoninic assay (Pierce, Rockford, IL). Samples (30 µg for IGF1R and 25 µg for IGF2R) were loaded onto a precasted minigels (7.5% for IGF1R and 4–15% gradient for IGF2R; Criterion/BioRad, Hercules, CA) and electrotransferred onto the polyvinylidene difluoride membranes (Roche, Indianapolis, IN). The membranes were incubated in 0.1% TBS-T buffer containing mouse monoclonal antibodies against IGF1R (1:100, Calbiochem, San Diego, CA) or IGF2R (2 µg/mL; Abcam, Cambridge, MA). Rabbit polyclonal β-actin (1:1000, Cell Signaling Technology, Danvers, MA) or mouse monoclonal glyceraldehyde 3-phosphate dehydrogenase (0.12 µg/mL; Biogenesis, Poole, UK) antibodies (suitable for the pore sizes of the gels used) were applied as loading controls. Antigen–antibody complexes were detected using respective secondary antibodies coupled with horseradish peroxidase (GE Healthcare Bio-Sciences Corp., Piscataway, NJ) and the Pierce enhanced chemiluminescence detection system (Thermo Fisher Sci., Waltham, MA). The density of the bands on the X-ray film were measured using Quantity One® software and a VersaDoc Imaging System (Model 3000; BioRad, Hercules, CA) and the ratios of IGF1R-to-β-actin and IGF2R-to-GAPDH densities were analyzed.
Statistical analysis
The data were analyzed using SAS statistical software version 9.1.3 (Cary, NC). All values are expressed as means ± SEM. Differences among depots were tested using analysis of variance with identification data and replicates within depot as random effects and depot as fixed effect. P values less than 0.05 were considered statistically significant.
Results
Regional preadipocyte kinetics
The adipogenesis in SQ ABD SV cultures was greater (p < 0.05) than that in FEM and OM depots as assessed by B:D ratios (Table 1). However, we found no difference in the indices for proliferation and sensitivity to apoptotic stimuli among depots.
Table 1.
Cell kinetics of baboon fetal SV cultures by fat depot.
| Omental | Subcutaneous abdominal |
Femoral | |
|---|---|---|---|
| Differentiation (n = 4) | |||
| Lipid content per well, AU of BODIPY-to-DAPI Fluorescence | 0.35 ± 0.09a | 0.51 ± 0.21b | 0.37 ± 0.07a |
| Proliferation (n = 5) | |||
| Percent increase in DNA amount, AU of “CyQuant” fluorescence | 111 ± 11 | 185 ± 38 | 143 ± 25 |
| Apoptosis (n = 5) | |||
| Induced fold increase in cytoplasmic nucleosomes | 1.10 ± 0.07 | 1.43 ± 0.21 | 1.22 ± 0.07 |
Data are means ± SEM.
p < 0.05 between depots.
Secreted proteins
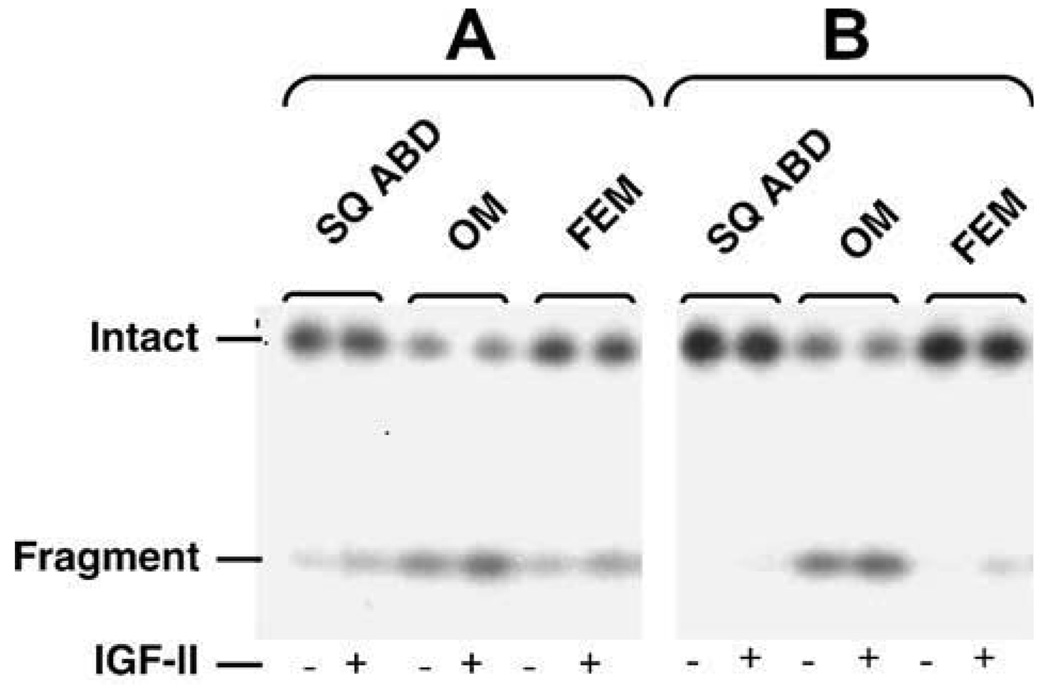
The differences in the secretion of IGFBP-4 among depots had a similar profile as those of the PAPP-A (Table 2). Omental SV cultures secreted significantly (p < 0.05) larger amounts of IGFBP-4 and PAPP-A than SQ ABD. The levels of IGFBP-4 and PAPP-A secreted by FEM SV cultures were intermediate. The cleavage of 121I-IGFBP-4 by secreted PAPP-A in a cell-free assay resulted in loss of intact IGFBP-4 and generation of 18-kDa radio-labeled fragments to the highest degree in media conditioned by OM SV cultures (Fig. 1). This proteolysis occurred in the absence of exogenous IGF2 and did not change after addition of IGF2.
Table 2.
Concentrations of IGFBP-4 and PAPP-A in media conditioned by baboon fetal SV confluent cultures by fat depot (n = 4).
| Omental | Subcutaneous Abdominal | Femoral | |
|---|---|---|---|
| IGFBP-4, ng/ml | 17.4 ± 2.8a | 9.6 ± 1.2b | 12.9 ± 1.3ab |
| PAPP-A, µIU/ml | 39.1 ± 6.3a | 24.6 ± 1.9b | 27.8 ± 1.2ab |
Data are means ± SEM. Different letters in the superscripts indicate a difference among depots, p < 0.05.
Fig. 1.
125I-IGFBP-4 protease activity of PAPP-A. Conditioned media from OM, SQ ABD, and FEM SV cultures from 2 baboon fetuses were incubated with 125I-IGFBP-4 and 5 nM IGF-II at 37 °C for 24 h. The radio-labeled fragment (18 kDa) resulting from the proteolysis of 125I-IGFBP-4 by PAPP-A and the remaining intact 125I-IGFBP-4 were separated on SDS gel and visualized by auto-radiography. Conditioned media from OM SV cultures exhibited IGF-II independent greatest loss of intact 125IGFBP-4s coupled with greatest generation of 18-kDa fragments indicating highest PAPP-A proteolytic activities.
Protein expression of IGF receptors
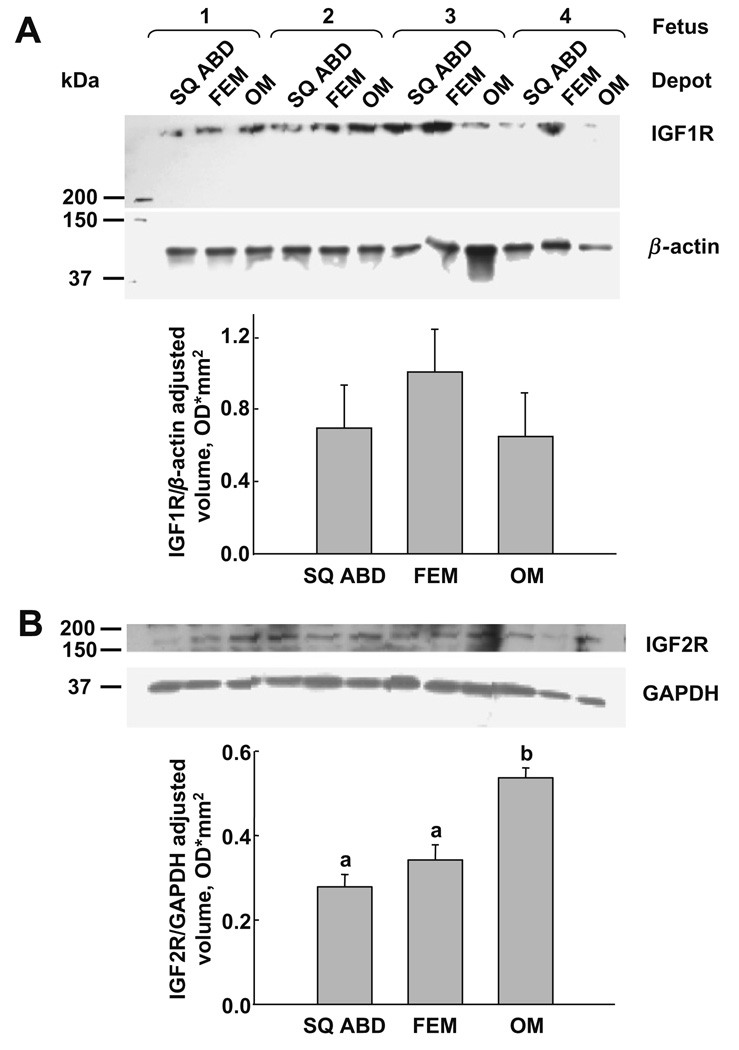
The Western blot analyses (Fig. 2) show the protein bands of IGF1R [~260 kDa (A)] and IGF2R [~180 kDa (B)]. The quantification of the density of the protein bands (Fig. 2, bar graphs) after normalization revealed no differences in the IGF1R expression among fat depots (A) but higher (p < 0.05) expression of IGF2R in cultures from OM than SQ ABD and FEM depots (B).
Fig. 2.
Immunoblots for IGF receptors in adipose-derived SV cultures by fat depots (n = 4). (A) IGF1R protein expression was similar among depots. Mean ± SEM; SQ ABD, 0.69 ± 0.24; FEM, 1.00 ± 0.24; OM, 0.65 ± 0.24 U/mm2 (B) IGF2R protein expression was higher in OM compared to both SQ ABD and FEM fat depots. Mean ± SEM: SQ ABD, 0.28 ± 0.03a; FEM, 0.34 ± 0.04ab; OM, 0.53 ± 0.02b U/mm2. Different letters in the superscripts indicate a difference among depots, p < 0.05.
Discussion
We report that, in near-term baboon fetuses, the main difference in the preadipocyte kinetics among depots was in the adipogenesis. The observed higher rate of lipid accumulation in subcutaneous vs. omental preadipocytes is consistent with previous studies in fetal lambs [20], adult pigs [21], and in some [22,23] but not all [24,25] investigations in humans. Evaluating the composition of the differentiation media suggest that in preadipocytes with high propensity for differentiation, i.e. from pigs and sheep, differences can be detected under “suboptimal” conditions of media lacking PPARγ agonists. Adding PPARγ agonists to the differentiation cocktail [20,21] likely drives the differentiation to its maximal degree that overrides potential differences. In contrast, human SV cultures appear to have a weaker capacity to differentiate as differences among depots cannot be detected when “suboptimal” medium is employed [24,25] but is apparent with the addition of PPARγ agonists [22,23]. Baboon fetal SV cells resemble those of humans, as they required PPARγ agonists for differentiation. Recent gene expression studies report depot-differences in the transcription of genes regulating adipogenesis which are involved fundamentally in embryonic development and pattern specification [10,26–29]. This suggests that the differences in the preadipocyte dynamic among anatomical sites may be, to some extent, innate regional variations in adipogenesis. Our data in fetal preadipocytes further supports this notion. We only observed a trend for higher proliferation rates in SQ ABD compared to OM and FEM depots. Others have been able to detect differences between OM and SQ ABD in children after at least 7 days of mitogenic stimulation of SV cultures with serum containing medium [8].
IGF1 and IGF2 are important regulators of intrauterine growth and adipose tissue development. IGF1 mediates the effect of extrinsic factors including those of adipogenic hormones and nutrient manipulations [6,11]. Modifications of IGF2 expression due to genetic variations affect fetal growth and adipose tissue development leading to higher adiposity later in life [30–32]. IGF1 and IGF2 exert their biological effects by binding to IGF1R [33] only when they are in free forms. The IGF1/IGF2 bioavailability is controlled by binding to IGFBPs, which in turn is regulated by proteases such as PAPP-A. PAPP-A increases IGF-I bioavailability indirectly, via degradation of the inhibitory IGFBP-2, -4, -5. Among the six IGFBPs, IGFBP-4 has been shown to play a role in regulating differentiation of fetal preadipocytes [34] and is the main substrate for PAPP-A. To our knowledge, this is the first report of higher secretion of IGFBP-4 in OM than SQ ABD preadipocytes. Since IGFBP-4 binds IGF1, a decrease in free IGF1 available to bind IGF1R is expected. This mechanism would explain the lower level of preadipocyte differentiation in OM vs. SQ ABD depot given similar levels of PAPP-A. However, the secretion of PAPP-A had a similar profile of inter-depot differences as IGFBP-4 with higher concentrations in media from OM preadipocytes than SQ ABD preadipocytes. These data is consistent with the previously reported higher protein and gene expression of PAPP-A in OM vs. SQ ABD preadipocytes in human adults [10,35]. Our data also suggest that the higher secretion of PAPP-A by OM preadipocytes translates into higher in vitro 125IGFBP-4 protease activity. However, the proteolytic activity of PAPP-A may be further regulated by other factors including a membrane bound PAPP-A [36] and by the physiologic inhibitor the precursor form of Eosinophil Major Basic Protein [37]. The functional relevance of the interaction between IGFBP-4 and PAPP-A in the regulation of IGF1 bioavailability needs to be addressed in future functional studies.
IGFBPs bind also to IGF2. In addition, IGF2 bioavailability is also controlled by its “clearance” when bound to IGF2R. It is known that IGF2R plays a role in internalization, lysosomal trafficking, and degradation of IGF2 [38]. The higher levels of the protein expression of IGF2R in the OM vs. SQ ABD preadipocytes may lead to lower bioavailability and activity of IGF2 resulting in the observed restrained differentiation in this depot. Furthermore, it is known that IGF2R may degrade other proteins, in addition to IGF2, including leukemia inhibitory factor [38] and may activate proteolytically transforming growth factor β [39], both of which also inhibit adipogenesis or lipid accumulation [40,41] providing supplementary mechanisms of regulating adipogenesis.
In conclusion, the distinct adipogenesis and protein expression of components of the IGF regulatory system in preadipocytes from different fat depots is apparent during late-term fetal development. This suggests that fat distribution phenotype may have in part developmental genetic origin and that it may be influenced by potential regional variation in the preadipocyte responsiveness to adaptive changes in IGFs and other growth factors as a result of alterations in maternal or postnatal nutrition or disease.
Acknowledgments
We thank Shantele Thomas, Kenneth Benson (Pennington Biomedical Research Center), Laurie K. Bale and Sean C. Harrington (Mayo Clinic), and Dr. Mark Nijland (University of Texas Health Science Center at San Antonio) for their help.
This work was supported by Grants P30 DK072476, P01 HD21350, and R01 DK060412-06.
References
- 1.Reaven GM. Banting lecture 1988. Role of insulin resistance in human disease. Diabetes. 1988;37:1595–1607. doi: 10.2337/diab.37.12.1595. [DOI] [PubMed] [Google Scholar]
- 2.Snijder MB, Dekker JM, Visser M, Bouter LM, Stehouwer CD, Yudkin JS, Heine RJ, Nijpels G, Seidell JC. Trunk fat and leg fat have independent and opposite associations with fasting and postload glucose levels: the Hoorn study. Diabetes Care. 2004;27:372–377. doi: 10.2337/diacare.27.2.372. [DOI] [PubMed] [Google Scholar]
- 3.Hauner H, Wabitsch M, Pfeiffer EF. Differentiation of adipocyte precursor cells from obese and nonobese adult women and from different adipose tissue sites. Horm. Metab. Res. Suppl. 1988;19:35–39. [PubMed] [Google Scholar]
- 4.Tchkonia T, Giorgadze N, Pirtskhalava T, Tchoukalova Y, Karagiannides I, Forse RA, DePonte M, Stevenson M, Guo W, Han J, Waloga G, Lash TL, Jensen MD, Kirkland JL. Fat depot origin affects adipogenesis in primary cultured and cloned human preadipocytes. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2002;282:R1286–R1296. doi: 10.1152/ajpregu.00653.2001. [DOI] [PubMed] [Google Scholar]
- 5.Niesler CU, Siddle K, Prins JB. Human preadipocytes display a depot-specific susceptibility to apoptosis. Diabetes. 1998;47:1365–1368. doi: 10.2337/diab.47.8.1365. [DOI] [PubMed] [Google Scholar]
- 6.Hausman GJ. The influence of insulin, triiodothyronine (T3) and insulin-like growth factor-I (IGF-1) on the differentiation of preadipocytes in serum-free cultures of pig stromal-vascular cells. J. Anim. Sci. 1989;67:3136–3143. doi: 10.2527/jas1989.67113136x. [DOI] [PubMed] [Google Scholar]
- 7.Smith PJ, Wise LS, Berkowitz R, Wan C, Rubin CS. Insulin-like growth factor-I is an essential regulator of the differentiation of 3T3-L1 adipocytes. J. Biol. Chem. 1988;263:9402–9408. [PubMed] [Google Scholar]
- 8.Grohmann M, Sabin M, Holly J, Shield J, Crowne E, Stewart C. Characterization of differentiated subcutaneous and visceral adipose tissue from children: the influences of TNF-alpha and IGF-I. J. Lipid Res. 2005;46:93–103. doi: 10.1194/jlr.M400295-JLR200. [DOI] [PubMed] [Google Scholar]
- 9.Vohl MC, Sladek R, Robitaille J, Gurd S, Marceau P, Richard D, Hudson TJ, Tchernof A. A survey of genes differentially expressed in subcutaneous and visceral adipose tissue in men. Obes. Res. 2004;12:1217–1222. doi: 10.1038/oby.2004.153. [DOI] [PubMed] [Google Scholar]
- 10.Tchkonia T, Lenburg M, Thomou T, Giorgadze N, Frampton G, Pirtskhalava T, Cartwright A, Cartwright M, Flanagan J, Karagiannides I, Gerry N, Forse RA, Tchoukalova Y, Jensen MD, Pothoulakis C, Kirkland JL. Identification of depot-specific human fat cell progenitors through distinct expression profiles and developmental gene patterns. Am. J. Physiol. Endocrinol. Metab. 2007;292:E298–E307. doi: 10.1152/ajpendo.00202.2006. [DOI] [PubMed] [Google Scholar]
- 11.Law CM, Barker DJ, Osmond C, Fall CH, Simmonds SJ. Early growth and abdominal fatness in adult life. J. Epidemiol. Commun. Health. 1992;46:184–186. doi: 10.1136/jech.46.3.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vickers MH, Breier BH, Cutfield WS, Hofman PL, Gluckman PD. Fetal origins of hyperphagia, obesity, and hypertension and postnatal amplification by hypercaloric nutrition. Am. J. Physiol. Endocrinol. Metab. 2000;279:E83–E87. doi: 10.1152/ajpendo.2000.279.1.E83. [DOI] [PubMed] [Google Scholar]
- 13.Louveau I, Gondret F. Regulation of development and metabolism of adipose tissue by growth hormone and the insulin-like growth factor system. Domest. Anim. Endocrinol. 2004;27:241–255. doi: 10.1016/j.domaniend.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 14.Comuzzie AG, Cole SA, Martin L, Carey KD, Mahaney MC, Blangero J, VandeBerg JL. The baboon as a nonhuman primate model for the study of the genetics of obesity. Obes. Res. 2003;11:75–80. doi: 10.1038/oby.2003.12. [DOI] [PubMed] [Google Scholar]
- 15.Schlabritz-Loutsevitch NE, Howell K, Rice K, Glover EJ, Nevill CH, Jenkins SL, Bill Cummins L, Frost PA, McDonald TJ, Nathanielsz PW. Development of a system for individual feeding of baboons maintained in an outdoor group social environment. J. Med. Primatol. 2004;33:117–126. doi: 10.1111/j.1600-0684.2004.00067.x. [DOI] [PubMed] [Google Scholar]
- 16.Schlabritz-Loutsevitch NE, Hubbard GB, Dammann MJ, Jenkins SL, Frost PA, McDonald TJ, Nathanielsz PW. Normal concentrations of essential and toxic elements in pregnant baboons and fetuses (Papio species) J. Med. Primatol. 2004;33:152–162. doi: 10.1111/j.1600-0684.2004.00066.x. [DOI] [PubMed] [Google Scholar]
- 17.Tchkonia T, Tchoukalova YD, Giorgadze N, Pirtskhalava T, Karagiannides I, Forse RA, Koo A, Stevenson M, Chinnappan D, Cartwright A, Jensen MD, Kirkland JL. Abundance of two human preadipocyte subtypes with distinct capacities for replication, adipogenesis, and apoptosis varies among fat depots. Am. J. Physiol. Endocrinol. Metab. 2005;288:E267–E277. doi: 10.1152/ajpendo.00265.2004. [DOI] [PubMed] [Google Scholar]
- 18.Hauner H, Petruschke T, Russ M, Rohrig K, Eckel J. Effects of tumour necrosis factor alpha (TNF alpha) on glucose transport and lipid metabolism of newly-differentiated human fat cells in cell culture. Diabetologia. 1995;38:764–771. doi: 10.1007/s001250050350. [DOI] [PubMed] [Google Scholar]
- 19.Qin X, Byun D, Lau KH, Baylink DJ, Mohan S. Evidence that the interaction between insulin-like growth factor (IGF)-II and IGF binding protein (IGFBP)-4 is essential for the action of the IGF-II-dependent IGFBP-4 protease. Arch. Biochem. Biophys. 2000;379:209–216. doi: 10.1006/abbi.2000.1872. [DOI] [PubMed] [Google Scholar]
- 20.Soret B, Lee HJ, Finley E, Lee SC, Vernon RG. Regulation of differentiation of sheep subcutaneous and abdominal preadipocytes in culture. J. Endocrinol. 1999;161:517–524. doi: 10.1677/joe.0.1610517. [DOI] [PubMed] [Google Scholar]
- 21.Samulin J, Lien S, Grindflek E, Berget I, Ruyter B, Sundvold H. Depot specific differences during adipogenesis of porcine stromal-vascular cells. Cell Biol. Int. 2008;32:525–531. doi: 10.1016/j.cellbi.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 22.Adams M, Montague CT, Prins JB, Holder JC, Smith SA, Sanders L, Digby JE, Sewter CP, Lazar MA, Chatterjee VK, O’Rahilly S. Activators of peroxisome proliferator-activated receptor gamma have depot-specific effects on human preadipocyte differentiation. J. Clin. Invest. 1997;100:3149–3153. doi: 10.1172/JCI119870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hutley LJ, Newell FM, Joyner JM, Suchting SJ, Herington AC, Cameron DP, Prins JB. Effects of rosiglitazone and linoleic acid on human preadipocyte differentiation. Eur. J. Clin. Invest. 2003;33:574–581. doi: 10.1046/j.1365-2362.2003.01178.x. [DOI] [PubMed] [Google Scholar]
- 24.Shahparaki A, Grunder L, Sorisky A. Comparison of human abdominal subcutaneous versus omental preadipocyte differentiation in primary culture. Metabolism. 2002;51:1211–1215. doi: 10.1053/meta.2002.34037. [DOI] [PubMed] [Google Scholar]
- 25.Van Harmelen V, Rohrig K, Hauner H. Comparison of proliferation and differentiation capacity of human adipocyte precursor cells from the omental and subcutaneous adipose tissue depot of obese subjects. Metabolism. 2004;53:632–637. doi: 10.1016/j.metabol.2003.11.012. [DOI] [PubMed] [Google Scholar]
- 26.Gesta S, Bluher M, Yamamoto Y, Norris AW, Berndt J, Kralisch S, Boucher J, Lewis C, Kahn CR. Evidence for a role of developmental genes in the origin of obesity and body fat distribution. Proc. Natl. Acad. Sci. USA. 2006;103:6676–6681. doi: 10.1073/pnas.0601752103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu Y, Kim JY, Zhou S, Smas CM. Differential screening identifies transcripts with depot-dependent expression in white adipose tissues. BMC Genomics. 2008;9:397. doi: 10.1186/1471-2164-9-397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cowherd RM, Lyle RE, Miller CP, McGehee RE., Jr Developmental profile of homeobox gene expression during 3T3-L1 adipogenesis. Biochem. Biophys. Res. Commun. 1997;237:470–475. doi: 10.1006/bbrc.1997.7160. [DOI] [PubMed] [Google Scholar]
- 29.Cantile M, Procino A, D’Armiento M, Cindolo L, Cillo C. HOX gene network is involved in the transcriptional regulation of in vivo human adipogenesis. J. Cell. Physiol. 2003;194:225–236. doi: 10.1002/jcp.10210. [DOI] [PubMed] [Google Scholar]
- 30.Murphy R, Baptista J, Holly J, Umpleby AM, Ellard S, Harries LW, Crolla J, Cundy T, Hattersley AT. Severe intrauterine growth retardation and atypical diabetes associated with a translocation breakpoint disrupting regulation of the insulin-like growth factor 2 gene. J. Clin. Endocrinol. Metab. 2008;93:4373–4380. doi: 10.1210/jc.2008-0819. [DOI] [PubMed] [Google Scholar]
- 31.Vykoukalova Z, Knoll A, Dvorak J, Cepica S. New SNPs in the IGF2 gene and association between this gene and backfat thickness and lean meat content in Large White pigs. J. Anim. Breed. Genet. 2006;123:204–207. doi: 10.1111/j.1439-0388.2006.00580.x. [DOI] [PubMed] [Google Scholar]
- 32.Le Stunff C, Fallin D, Bougneres P. Paternal transmission of the very common class I INS VNTR alleles predisposes to childhood obesity. Nat. Genet. 2001;29:96–99. doi: 10.1038/ng707. [DOI] [PubMed] [Google Scholar]
- 33.Frasca F, Pandini G, Scalia P, Sciacca L, Mineo R, Costantino A, Goldfine ID, Belfiore A, Vigneri R. Insulin receptor isoform A, a newly recognized, high-affinity insulin-like growth factor II receptor in fetal and cancer cells. Mol. Cell Biol. 1999;19:3278–3288. doi: 10.1128/mcb.19.5.3278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hausman GJ, Richardson RL, Simmen FA. Secretion of insulin-like growth factor (IGF)-I and -II and IGF binding proteins (IGFBPs) in fetal stromal-vascular (S-V) cell cultures obtained before and after the onset of adipogenesis in vivo. Growth Dev. Aging. 2002;66:11–26. [PubMed] [Google Scholar]
- 35.Kirkland JL, Tchkonia T, Cleveland-Donovan K, Giorgadze N, Gagua M, Karagiannides I, Pothoulakis C, Conover C, Boney C. Regional variation in Bioavailability and Responsiveness to IGF-1 in Human Preadipocytes. Obesity. 2007;15 Suppl:A14. [Google Scholar]
- 36.Sun IY, Overgaard MT, Oxvig C, Giudice LC. Pregnancy-associated plasma protein A proteolytic activity is associated with the human placental trophoblast cell membrane. J. Clin. Endocrinol. Metab. 2002;87:5235–5240. doi: 10.1210/jc.2002-020561. [DOI] [PubMed] [Google Scholar]
- 37.Kalli KR, Chen BK, Bale LK, Gernand E, Overgaard MT, Oxvig C, Cliby WA, Conover CA. Pregnancy-associated plasma protein-A (PAPP-A) expression and insulin-like growth factor binding protein-4 protease activity in normal and malignant ovarian surface epithelial cells. Int. J. Cancer. 2004;110:633–640. doi: 10.1002/ijc.20185. [DOI] [PubMed] [Google Scholar]
- 38.Kornfeld S. Structure and function of the mannose 6-phosphate/insulinlike growth factor II receptors. Annu. Rev. Biochem. 1992;61:307–330. doi: 10.1146/annurev.bi.61.070192.001515. [DOI] [PubMed] [Google Scholar]
- 39.Dennis PA, Rifkin DB. Cellular activation of latent transforming growth factor beta requires binding to the cation-independent mannose 6-phosphate/insulin-like growth factor type II receptor. Proc. Natl. Acad. Sci. USA. 1991;88:580–584. doi: 10.1073/pnas.88.2.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ailhaud G. Autocrine/paracrine effectors of adipogenesis. Ann. Endocrinol. (Paris) 2002;63:83–85. [PubMed] [Google Scholar]
- 41.Shin SM, Kim K, Kim JK, Yoon SR, Choi I, Yang Y. Dexamethasone reverses TGF-beta-mediated inhibition of primary rat preadipocyte differentiation. FEBS Lett. 2003;543:25–30. doi: 10.1016/s0014-5793(03)00371-5. [DOI] [PubMed] [Google Scholar]