Abstract
Kv4.2 is the major voltage-gated K+ (Kv) channel α subunit responsible for the somatodendritic transient or A-type current ISA that activates at subthreshold membrane potentials. Stable association of Kv4.2 with diverse auxiliary subunits and reversible Kv4.2 phosphorylation regulate ISA function. Two classes of auxiliary subunits play distinct roles in modulating the biophysical properties of Kv4.2: dipeptidyl-peptidase-like Type II transmembrane proteins typified by DPPX-S, and cytoplasmic Ca2+ binding proteins known as K+ channel interacting proteins (KChIPs). Here, we characterize the convergent roles that DPPX-S and KChIPs play as component subunits of Kv4.2 channel complexes. We co-expressed DPPX-S with Kv4.2 in heterologous cells and found a dramatic redistribution of Kv4.2, releasing it from intracellular retention and allowing plasma membrane expression, as well as altered Kv4.2 phosphorylation, detergent solubility, and stability. These changes are remarkably similar to those obtained upon co-expression of Kv4.2 with the structurally distinct KChIPs1–3 auxiliary subunits. KChIP4a, which negatively affects the impact of other KChIPs on Kv4.2, also inhibits the effects of DPPXS, consistent with the formation of a ternary complex of Kv4.2, DPPX-S and KChIPs early in channel biosynthesis. Tandem MS analyses reveal that co-expression with DPPX-S or KChIP2 leads to a pattern of Kv4.2 phosphorylation in heterologous cells similar to that observed in brain, but lacking in cells expressing Kv4.2 alone. In conclusion, transmembrane DPPX-S and cytoplasmic KChIPs exert similar effects on Kv4.2 trafficking, despite having distinct structures and binding sites on Kv4.2, but distinct effects on Kv4.2 gating.
The somatodendritic potassium current ISA 1 is a rapidly inactivating potassium current that activates at subthreshold membrane potentials to regulate neuronal excitability by attenuating back-propagation of action potentials into dendrites, and by limiting propagation of dendritic synaptic signals to the soma (1, 2). ISA function is reversibly modulated by phosphorylation allowing for a dynamic role in regulating dendritic excitability and synaptic function (2, 3). The voltage-gated potassium, or Kv, channels that mediate this current are homo- or hetero-tetramers of transmembrane pore-forming and voltage-sensing primary or α subunits of the Kv4 family, predominantly Kv4.2 and Kv4.3 (4–9). Kv4 channel complexes also contain two major families of auxiliary subunits; a family of Ca2+-binding cytoplasmic proteins, known as K+ channel interacting proteins, or KChIPs (10), and a family of dipeptidyl peptidase-like (DPP) Type II transmembrane proteins with short, alternatively-spliced cytoplasmic domains, typified by DPPX-S (11, 12). KChIPs regulate diverse aspects of the biophysical and biochemical properties of Kv4.2 channels (10, 13); [reviewed in (14)], and also impact their intracellular trafficking (10, 13, 15, 16). DPPX-S and related DPP proteins exert effects on the biophysical properties of coexpressed Kv4.2 distinct from those of KChIPs [reviewed in (14)], and also exhibit extensive colocalization with mammalian brain Kv4 α subunits (17). Electrophysiological analyses suggest the formation of ternary channel complexes of Kv4 α subunits, KChIPs and DPPX in heterologous cells (18, 19) that recapitulate native ISA currents observed in certain brain neurons (19–22). Recent studies have identified mutations in DPPX in humans that are associated with neurological disorders, including autism (23) and amyotrophic lateral sclerosis (24).
Previous studies have shown that KChIP1–3 (but not KChIP4a) co-expression rescues Kv4.2 from intracellular retention by inducing forward trafficking of the channel complex (10, 13, 16). Correlated with this forward trafficking is a shift in the electrophoretic mobility of Kv4.2 on SDS-PAGE due to increased constitutive phosphorylation (13). In vitro phosphorylation studies using purified kinases and Kv4.2-GST fusion proteins identified several kinases that phosphorylate multiple sites on the cytoplasmic C-terminus of Kv4.2, along with a single phosphorylation site on the N-terminus (25, 26). In vivo studies have shown that activation of these kinases has diverse effects on Kv4.2 from modulating channel gating to increasing surface expression (27–30). To better understand the molecular mechanisms underlying DPPX-S regulation of Kv4 channels, here we investigate the convergent effects of DPPX-S and KChIP co-expression on the intracellular trafficking and molecular characteristics of Kv4.2 channels.
EXPERIMENTAL PROCEDURES
Plasmids, Site-Directed Mutagenesis, and Transfection of COS-1 cells
COS-1 cells were grown in Dulbecco’s modified Eagle’s medium with the addition of 10% bovine calf serum, 50 units/ml penicillin, and 50 μg/ml streptomycin (Invitrogen, Carlsbad, CA). Cells were cultured on plastic tissue culture dishes, or on poly-L-lysine coated glass cover slips in Petri dishes, and maintained in a humidified incubator at 37º C at 5% CO2. Human DPPX-S (a generous gift of Dr. Steve Goldstein, University of Chicago), rat Kv4.2 (31), and rat KChIP (10) cDNAs, in mammalian expression vectors, were transfected into cells with Polyfect (Qiagen, Valencia, CA) reagents for biochemical experiments or Lipofectamine (Invitrogen) for immunofluorescence experiments, following the manufacturer’s protocols. In all experiments, total cDNA levels used in transfections were balanced by adding varying amounts of empty pRBG4 vector. Mutagenesis of the recombinant rat Kv4.2 cDNA in the pRBG4 vector was performed using the Quick-Change site-directed mutagenesis kit (Stratagene, La Jolla, CA) following the manufacturer’s instructions.
Immunofluorescence
Cells expressing DPPX-S, Kv4.2, and/or KChIPs were stained 48 hrs posttransfection using surface or permeabilized cell immunofluorescence protocols (32). In brief, cells were fixed with 4% paraformaldehyde in PBS (10 mM sodium phosphate, 150 mM NaCl, pH 7.4), and stained with the ectodomain-directed anti-Kv4.2 IgG3 mouse monoclonal antibody (K57/40) to detect the cell surface pool of Kv4.2. Cells were then permeabilized with 0.1% TX-100, and stained with a cytoplasmic-directed anti-Kv4.2 IgG1 monoclonal antibody (L28/4), generated against the C-terminus of Kv4.2, to detect total cellular Kv4.2. A rabbit anti-HA epitope tag antibody (Invitrogen) was used to detect HA-tagged DPPX-S, and previously described (33) mouse monoclonal antibodies were used for staining KChIPs. Bound primary antibodies were detected using Alexa Fluor 350-, Alexa Fluor 488-, and Alexa Fluor 594- conjugated mouse isotype- or species-specific secondary antibodies (Invitrogen) and viewed under indirect immunofluorescence.
Immunoblotting, immunoprecipitation, and SDS PAGE-COS-1 cell lysates were prepared essentially as previously described (34, 35). In brief, cells were washed on ice with Dulbecco's Phosphate-Buffered Saline (DPBS), then harvested and centrifuged in DPBS for 5 min at 1000 x g. The cell pellet was then lysed by incubating for 20 minutes at 4°C in a buffer containing TBS (10 mM Tris, 150 mM NaCl, pH 8.0), 1 mM EDTA, 1.0% Triton-X 100, and a protease inhibitor mixture containing 2 μg/ml aprotinin, 1 μg/ml leupeptin, 2 μg/ml antipain, and 10 μg/ml benzamidine and 0.2 mM phenylmethylsulfonyl fluoride), followed by centrifugation for 20 minutes at 16,000 x g to pellet the nuclei and cell debris. For immunoprecipitation reactions, affinity-purified polyclonal antibodies (5 μg/ml) or antisera (1 μl) was added to the lysate, and the mixture was incubated on a rotator at 4 °C for 16 h, followed by the addition of protein A agarose, and incubation for an additional one hour. Reducing SDS sample buffer was then added to the protein A agarose. For immunoblots, detergent extracts were directly added to 2X reducing SDS sample buffer and run on 9% SDS polyacrylamide gels made with lauryl sulfate (Sigma, St. Louis, MO) as the SDS source to accentuate gel shifts caused by differences in phosphorylation state (34, 36). Immunoblots were probed with mouse monoclonal antibody K57/41 for Kv4.2, rabbit anti-HA for DPPX-S, and S552P for Kv4.2 phosphorylated at pS552, and immunoreactivity detected by HRP-conjugated secondary antibodies and ECL. Quantitation of immunoreactivity was performed by densitometry and analysis using Image J software. Three independent immunoblots of independent samples were analyzed, and values subjected to pairwise statistical Student’s T-tests.
Generation of a Phosphospecific Antibody Against Kv4.2 pS552
A rabbit polyclonal antiserum “S552P” against the pS552 phosphorylation site was raised against two synthetic peptides containing the sequence VSGSHRGpSVQELST and with artificial Cys added to either the N- or C-terminus for conjugation to keyhole limpet hemocyanin (Twenty First Century Biochemicals, Marlboro, MA).
Cycloheximide and Alkaline Phosphatase Treatments
Cycloheximide treatment was accomplished as previously described (13). For alkaline phosphatase treatment, a crude COS-1 membrane fraction was prepared by hypotonic lysis of COS-1 cells in 10 mM Tris pH 8.0 and homogenization in a pre-chilled Dounce homogenizer. Samples were then centrifuged at 1,000 x g for 5 minutes and the resultant supernatant centrifuged at 100,000 x g for 1 hr. Pelleted membranes were resuspended in 10 mM Tris pH 8.0. AP treatment was performed as previously described (34).
Kv4.2 Phosphorylation Site Analysis by Tandem Mass Spectrometry
Rat brain membranes (RBM) were prepared as previously described (37). For immunoprecipitation reactions, 20 mg of RBM protein was diluted into 10 ml of lysis buffer. Affinity-purified polyclonal Kv4.2N antibody (5 μg/ml) was added, and the mixture was incubated on a rotator at 4 °C for 16 h, followed by addition of protein A agarose, and incubation for an additional one hour. Following washes in lysis buffer, immunoprecipitation products were fractionated by SDS PAGE, and stained with coomassie blue stain. The Kv4.2 band was excised and subjected to in gel trypsin digestion (38).
Digested peptides were analyzed by LC-MS/MS on an LTQ with a Michrom Paradigm LC and CTC Pal autosampler. Peptides were separated with a 90 min gradient using a Michrom 200 μm x 150 mm Magic C18AQ reversed phase column at 2 μl/min. Peptides were directly loaded onto an Agilent ZORBAX 300SB C18, reversed phase trap cartridge, which, after loading, was switched in-line with a Michrom Magic C18 AQ 200 um x 150 mm C18 column connected to a Thermo-Finnigan LTQ ion trap mass spectrometer through a Michrom Advance Plug and Play nano-spray source. Peptides were separated using a gradient of 2–40% B (A = 0.1% Formic Acid, B= 100% Acetonitrile) in 75 minutes (90 minute total run time), 40–80% in 4 minutes, hold 1 minute, then 80–2% in 10 minutes. MS and MS/MS spectra were acquired using a top 10 method, where the top 10 ions in the MS scan were subjected to automated low energy collisioninduced dissociation. MS/MS spectra were interpreted through Mascot searches (Matrix Science) with an MS/MS tolerance of 0.6 Da and with phosphorylation on Ser, Thr, and Tyr residues allowed. Each filtered MS/MS spectra exhibiting possible phosphorylation was manually checked and validated. Existence of a 98 Da mass loss (-H3PO4: phosphopeptide specific CID neutral loss) and any ambiguity of phosphorylation sites were carefully examined (38).
RESULTS
DPPX-S Co-Expression Leads to Changes in the Intracellular Trafficking of Kv4.2 in COS-1 Cells
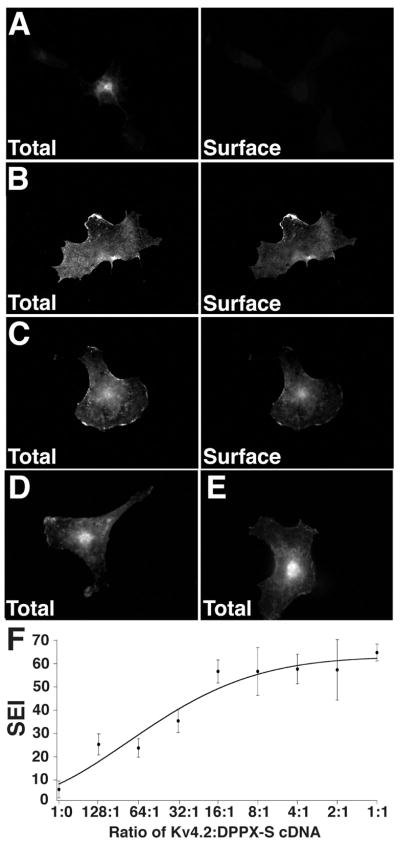
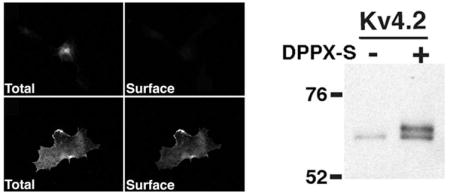
To address the effects of DPPX-S co-expression on Kv4.2 channels, we first analyzed the immunofluorescence-staining pattern of Kv4.2 in COS-1 cells either transfected alone or with DPPX-S. Consistent with previous studies (10, 13, 15, 16), virtually all intact cells that were expressing Kv4.2 alone had no detectable surface staining with an ectodomain directed anti- Kv4.2 antibody, and strong perinuclear Kv4.2 staining after detergent permeabilization (Figure 1A).
FIGURE 1.
DPPX-S co-expression leads to changes in the subcellular localization of Kv4.2 expressed in COS-1 cells. (A–C) COS-1 cells were transfected with (A) Kv4.2 alone; or (B) Kv4.2 plus DPPX-S; and (C) Kv4.2 plus KChIP2 at 1:1 cDNA ratios, and stained for total and cell surface Kv4.2 to determine the extent of Kv4.2 cell surface expression in transfected cells. Total cellular Kv4.2 pool (left panels). Cell surface Kv4.2 pool (right panels). (D–E) COS-1 cells transfected with either (D) DPPX-S alone or (E) DPPX-S and Kv4.2. Cells were permeabilized and stained for DPPX-S. Scale bar = 20 μm. (F) Dose-response curve showing changes in surface expression index (SEI: the percentage of Kv4.2-expressing cells with Kv4.2 cells surface expression) in response to increasing amounts of co-transfected DPPX-S cDNA. Three independent dishes were assayed for each Kv4.2/DPPX-S ratio. Approximately 100 Kv4.2 expressing cells were counted from each sample. Data are presented as the mean +/− S.E.
Co-expression of Kv4.2 with DPPX-S in COS-1 cells at a 1:1 cDNA ratio led to a dramatic change in the subcellular localization of Kv4.2, leading to a loss of the bulk of intracellular staining and strong cell surface staining (Figure 1B). The change in Kv4.2 localization in response to DPPXs coexpression was similar to that seen upon coexpression of KChIP2 (Figure 1C). DPPX-S exhibits both intracellular and plasma membrane-associated expression in the absence or presence of Kv4.2 co-expression (Figure 1D–E).
To determine if this DPPX-S-dependent trafficking of Kv4.2 to the cell surface occurs in a dose-dependent manner, cells transfected with a constant amount of Kv4.2 cDNA were co-transfected with increasing amounts of DPPX-S cDNA, stained for cell surface and total Kv4.2, and the percentage of Kv4.2 expressing cells with detectable surface staining determined. DPPXS increased cell surface expression of Kv4.2 in a dose dependent manner, and reached maximal effect at a 16:1 ratio of Kv4.2 to DPPX-S cDNA (Figure 1F). The percentage of Kv4.2- expressing cells with Kv4.2 surface staining reached a plateau at ≈ 60%, similar to the maximum value obtained upon co-expression with KChIP2 (13).
DPPX-S Induces Changes in the Molecular Characteristics of Kv4.2
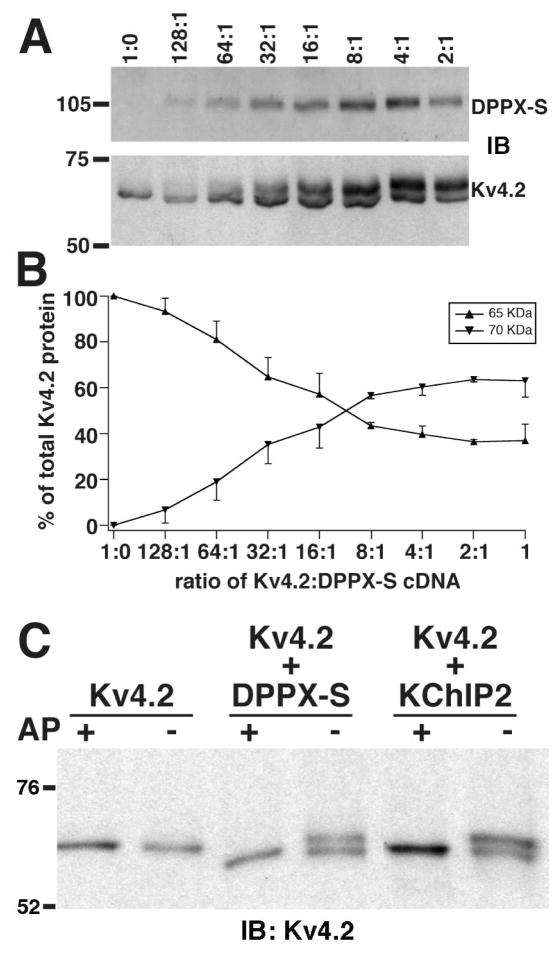
As previously reported (13), when analyzed by SDS-PAGE Kv4.2 expressed alone in COS-1 cells exists as a single pool of expressed protein with an Mr ≈ 65 kDa (Figure 2A), distinct from the Mr ≈ 70 kDa present in brain (13). However, upon co-expression with increasing amounts of DPPX-S cDNA, there is a dose-dependent induction of the appearance of an additional Mr ≈ 70 kDa band (Figure 2A, B). Note that the dose-dependent effects of DPPX-S co-expression on the shift in Mr and on cell surface trafficking expression (Figure 1F) were quite similar, both reaching a plateau at ≈ 60%, suggesting that the appearance of the Mr ≈ 70 kDa form of Kv4.2 may be associated with forward trafficking of the channel complex to the cell surface. In addition, it is quite remarkable that DPPX-S and KChIP2 have such a maximal effect on both the Mr shift and cell surface trafficking of Kv4.2 despite being such distinctly different proteins.
FIGURE 2.
DPPX-S induces a dose-dependent shift in the Mr of Kv4.2 on SDS gels. COS-1 cells were co-transfected with a fixed amount of Kv4.2 cDNA and increasing amounts of DPPXS. (A) Detergent extracts were immunoblotted for Kv4.2 and DPPX-S. (B) Quantitative analysis of the DPPX-S dose dependent effects on the electrophoretic mobility of Kv4.2. The intensities of the immunoreactivity of the Mr ≈ 65 kDa form (upward facing arrowheads and error bars) and Mr ≈ 70 kDa form (downward facing arrowhead and error bars) at each cDNA ratio were measured as the percentage of each form relative to the total Kv4.2 pool. (C) Crude membrane fractions from COS-1 cells expressing Kv4.2 alone, Kv4.2 + DPPX-S, or Kv4.2 + KChIP2 were incubated in the presence (+) or absence (−) of alkaline phosphatase (AP) and immunoblotted for Kv4.2.
DPPX-S Co-Expression Causes Increased Phosphorylation of Kv4.2
Our next objective was to determine if the DPPX-S-dependent mobility shift of Kv4.2 was due to an increase in its phosphorylation, as previously observed for the effects of KChIP2 co-expression (13). A crude membrane fraction prepared from COS-1 cells co-transfected with Kv4.2 and DPPX-S was subjected to treatment with the broad-spectrum alkaline phosphatase (AP) from calf intestine, followed by analysis by SDS-PAGE and immunoblotting. As found with cell extracts coexpressing KChIP2, as well as adult rat brain membranes (13), AP treatment of cell lysates coexpressing DPPX-S yielded a shift in the electrophoretic mobility of Kv4.2 on SDS gels (Figure 2C). When quantified by densitometry, the percentage of the total Kv4.2 pool that was present in the Mr = 65 kDa form in lysates from cells expressing Kv4.2 alone 90.0 ± 3.0% (n = 3), was significantly different than that in cells co-expressing DPPX-S (47.0 ± 1.1%, n = 3, p = 0.002) or KChIP2 (53.7 ± 0.8%, n = 3, p =0.003). AP treatment yielded a shift in the electrophoretic mobility of the Mr = 70 kDa pool such that it now co-migrated with the Mr = 65 kDa Kv4.2 pool, which now totaled 93.7±3.9%, 92.5±5.9% (p = 0.84 versus Kv4.2 alone, n = 3) or 88.5±6.1% (p = 0.44 versus Kv4.2 alone, n = 3) of the total Kv4.2 pool in cells expressing Kv4.2 alone, or with co-expression of KChIP2 or DPPX-S, respectively. These results confirm that co-expression of DPPX-S, like KChIP2, induces a significant increase in Kv4.2 phosphorylation that is eliminated by treatment with AP (Figure 2C).
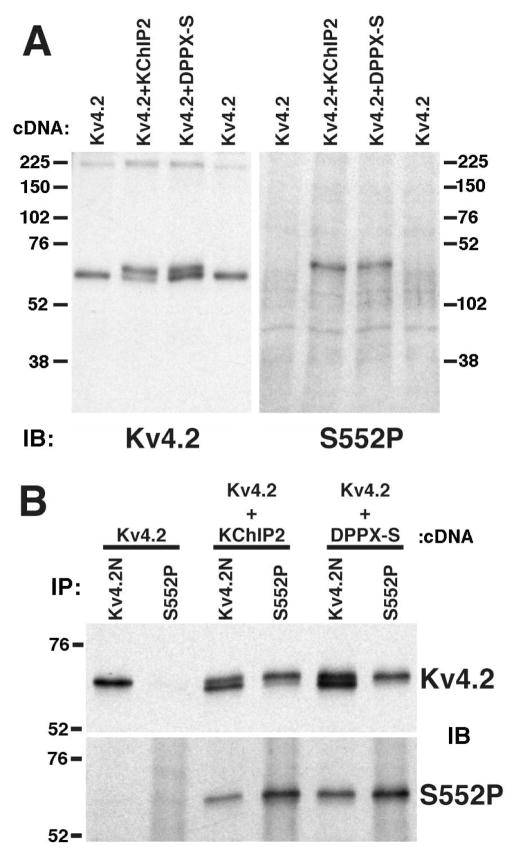
Kv4.2 is phosphorylated in vitro at Serine 552 (pS552) by purified PKA (25), and phosphorylation at this site is induced in heterologous cells upon co-expression with KChIP2 (13). As DPPX-S induces increased overall phosphorylation of Kv4.2 (Figure 2C), we examined if DPPX-S induced increased phosphorylation at pS552. We first examined phosphorylation at this site on the Mr ≈ 70 kDa population of Kv4.2 using a phosphospecific antibody (“S552P”) specific for Kv4.2 phosphorylated at pS552. On immunoblots of COS-1 cells expressing Kv4.2, Kv4.2 plus DPPX-S, and Kv4.2 plus KChIP2, the S552P phosphospecific antibody recognized only the Mr ≈ 70 kDa population of Kv4.2 present in the samples obtained from cells coexpressing DPPX-S and KChIP2, and not the Mr ≈ 65 kDa population in these cells or in cells expressing Kv4.2 alone (Figure 3A, right panel) that was apparent when this same immunoblot was reprobed with a general anti-Kv4.2 antibody (Figure 3A, left panel). To further confirm that DPPX-S was inducing increased phosphorylation at pS552, immunoprecipitation reactions were performed with either the phosphospecific S552P antibody, or the phospho-independent anti- Kv4.2 antibody Kv4.2N, from detergent extracts of COS-1 cells expressing Kv4.2 alone, or Kv4.2 co-expressed with DPPX-S or KChIP2, followed by immunoblot analyses with either a general anti-Kv4.2 antibody or S552P. As judged by immunoblotting for Kv4.2, immunoprecipitation reactions with the S552P antibody did not yield any Kv4.2 from extracts from cells expressing Kv4.2 alone, while those performed with the Kv4.2N antibody yielded the expected Mr = 65 kDa population of Kv4.2 present in these cells (Figure 3B). Immunoprecipitation reactions with S552P antibody did, however, yield Kv4.2 from cells coexpressing either KChIP2 or DPPX-S. Immunoblotting of these samples with the phosphoindependent anti-Kv4.2 antibody (Figure 3B, upper panel) clearly illustrate the specificity of the S552P antibody. S552P selectively immunoprecipitated only the Mr = 70 kDa population of Kv4.2, while the Kv4.2N immunoprecipitation reactions contained both the Mr = 65 and 70 kDa forms of Kv4.2. Moreover, immunoprecipitation reactions immunoblotted with the S552P phospho-specific antibody again revealed staining of only the Mr = 70 kDa form of Kv4.2, including the Kv4.2N immunoprecipitation reactions which yielded both the Mr = 65 and 70 kDa forms of Kv4.2 (Figure 3B, lower panel).
FIGURE 3.
DPPX-S-dependent phosphorylation of Kv4.2 at Serine 552. (A) Detergent extracts of COS-1 cells expressing Kv4.2 alone, Kv4.2 and KChIP2, and Kv4.2 and DPPX-S were immunoblotted with phospho-independent (K57/41, left panel) or phospho-specific (S552P) antibody (right panel). Note that the S552P antibody recognizes only the Mr ≈ 70 kDa form of Kv4.2. (B) Kv4.2 from COS-1 cell lysates expressing Kv4.2, Kv4.2 and KChIP2, and Kv4.2 and DPPX-S was subjected to immunoprecipitation using an N-terminal directed phosphoindependent antibody (Kv4.2N) or the phospho-specific S552P antibody. Samples were immunoblotted with anti-Kv4.2 (K57/41; upper panel) or S552P (lower panel) antibodies.
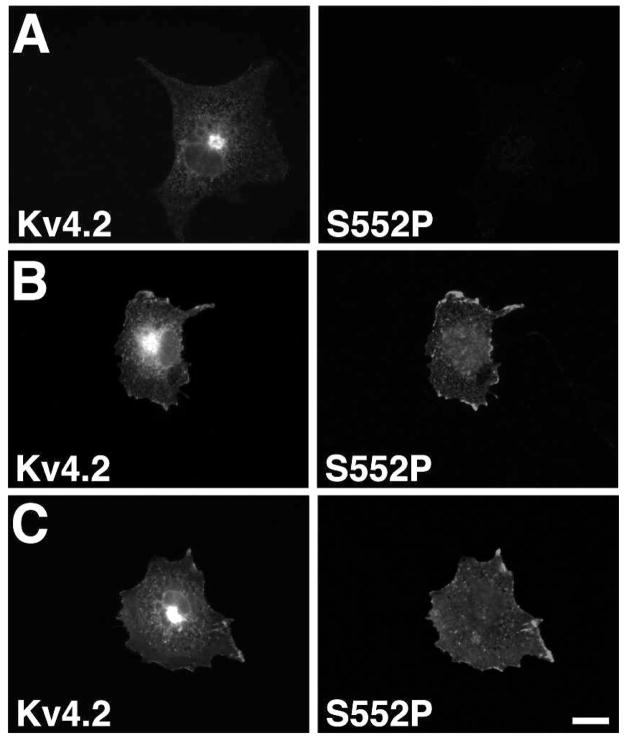
Immunofluorescence experiments revealed staining patterns consistent with the immunoblots and immunoprecipitation reactions, in that detergent-permeabilized COS-1 cells expressing Kv4.2 alone yielded no S552P antibody staining despite a robust signal with a phosphorylation independent anti-Kv4.2 antibody (Figure 4A). However, co-expression of Kv4.2 with DPPX-S (Figure 4B) or KChIP2 (Figure 4C) led to robust S552P staining, which in both cases appeared to be specific for the plasma membrane-associated Kv4.2 pool with little staining of the perinuclear intracellular Kv4.2 pool. Taken together, the results show that DPPX-S coexpression leads to enhanced Kv4.2 phosphorylation at pS552, and that this phosphorylation is correlated with the forward trafficking of the channel complex.
FIGURE 4.
Phosphorylation at Serine 552 is specific to the cell surface Kv4.2 pool. (A–C) COS- 1 cells were transfected with Kv4.2 alone (A), Kv4.2 and DPPX-S (B), or with Kv4.2 and KChIP2 (C) at 1:1 cDNA ratios and stained for Kv4.2 using phospho-independent (K57/41, left panels) or S552P phosphospecific (right panels) antibodies. Scale bar = 20 μm.
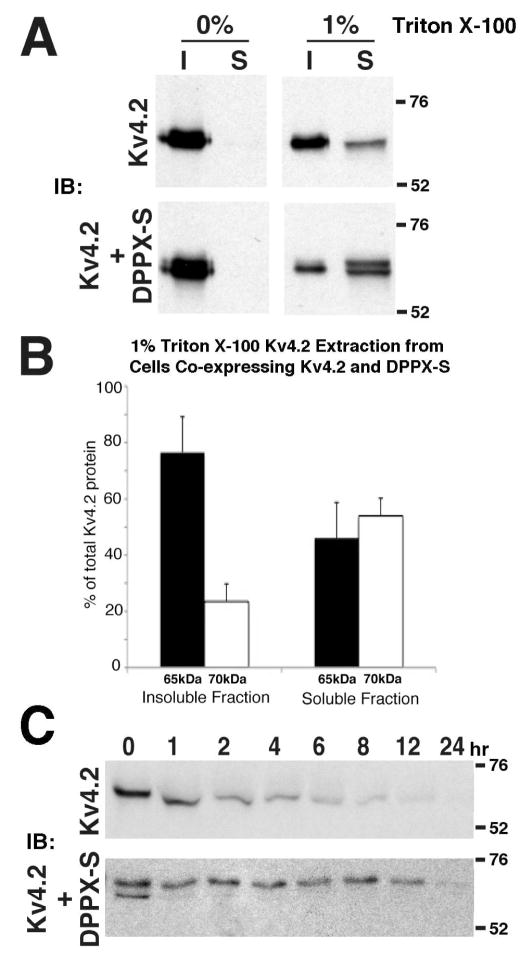
The Detergent Solubility and Stability of Kv4.2 is Altered by DPPX-S Co-Expression
We next analyzed the effects of DPPX-S co-expression and the subsequent increase in Kv4.2 phosphorylation on the biochemical properties of Kv4.2. We first examined the effects of the nonionic detergent Triton X-100 on the solubility of Kv4.2 alone and co-expressed with DPPXS. Solubility under these conditions can roughly indicate the folding state of a membrane protein (39), providing a useful assay to identify if DPPX-S-induced phosphorylation of Kv4.2 correlates with folding state. In the absence of detergent, Kv4.2 expressed alone or co-expressed with DPPX-S was found to be completely insoluble (Figure 5A). Inclusion of 1% Triton X-100 in the lysis buffer led to only a moderate level of extraction of the Mr = 65 kDa form of Kv4.2 from cells expressing Kv4.2 alone or co-expressing DPPX-S. However, the Mr = 70 kDa form of Kv4.2 present in cells co-expressing DPPX-S was almost completely extracted in this detergent solution (Figure 5A), suggesting that it has biochemical properties distinct from the Mr = 65 kDa form of Kv4.2. When quantified by densitometry (Figure 5C), the Mr = 70 kDa form represented only 23.5±12.7% (n=3) of the Kv4.2 in the detergent-insoluble pool, but represented a significantly higher (p=0.033) fraction (53.9±6.3%, n=3) of the Kv4.2 in the detergent-soluble pool. In our previously published results for the effects of KChIP2 co-expression on the detergent solubility of Kv4.2, both the Mr = 70 kDa and Mr = 65 kDa forms of Kv4.2 were completely soluble in 1% Triton X-100. This may represent a difference in the effects of these two auxiliary subunits on Kv4.2, or differences in the specific details of the assays (here, extracts 13 were subjected to centrifugation at 16,000 x g for 20 min versus 13,000 x g for 2 min in Shibata et al., 2003). The basis for the increased detergent solubility is unknown, and could represent enhancement of Kv4.2 folding (39) or association with different lipid domains (40).
FIGURE 5.
Molecular characteristics of Kv4.2 are altered upon co-expression with DPPX-S. (A) COS-1 cells expressing Kv4.2 alone or Kv4.2 and DPPX-S at a 1:1 ratio were harvested and solubilized in lysis buffer with and without the non-ionic detergent Triton X-100 as indicated. Detergent insoluble (I) and soluble (S) cell fractions were separated by centrifugation and immunoblotted for Kv4.2 (K57/41). (B) Quantitative analysis of the detergent solubility of the DPPX-S dependent 70 kDa population of Kv4.2 with 1% Triton X-100. The intensities of the immunoreactivity of the Mr ≈ 65 kDa form (black columns and error bars) and Mr ≈ 70 kDa form (white columns and error bars) for the insoluble and soluble fractions of Kv4.2 were measured as a percentage of each form relative to the total pool. (C) Immunoblot analysis of the half-life of Kv4.2 in the absence and presence of DPPX-S. COS-1 cells expressing Kv4.2 alone or Kv4.2 and DPPX-S were incubated in the presence of cycloheximide (100 μg/ml) for the indicated time and immunoblotted for Kv4.2.
To determine if DPPX-S co-expression also affected the stability of Kv4.2, COS-1 cells expressing Kv4.2 alone, or co-expressing Kv4.2 and DPPX-S were incubated for various times with the protein synthesis inhibitor cycloheximide. Inhibiting further Kv4.2 synthesis allowed for immunoblot analyses of Kv4.2 stability by comparing samples harvested at different time points after cycloheximide treatment. The Mr = 65 kDa form of Kv4.2 from cells expressing Kv4.2 alone is relatively unstable compared to the Mr = 70 kDa form of Kv4.2 present in cells coexpressing Kv4.2 and DPPX-S (Figure 5C). These results suggest that proper Kv4.2 folding may be dependent on not only auxiliary subunit binding, but also on DPPX-S- or KChIP2-dependent Kv4.2 phosphorylation.
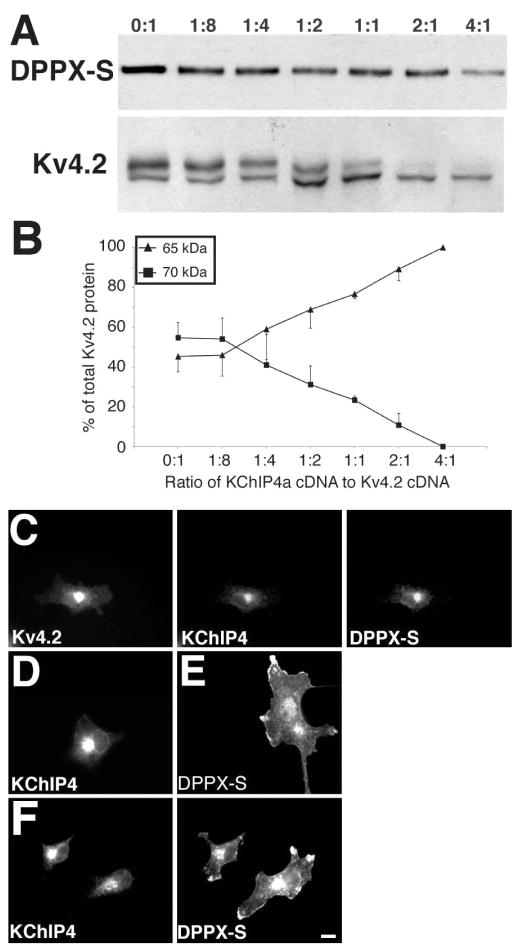
KChIP4a Inhibition of DPPX-S-Dependent Modulation of Kv4.2
Co-expression with the KChIP4a auxiliary subunit was previously found to not only in itself fail to promote efficient cell surface expression and phosphorylation of Kv4.2 as do other KChIPs, but to induce a dosedependent inhibition of the KChIP2-dependent effects (13). To determine whether incorporation of KChIP4a into ternary complexes with Kv4.2 and DPPX-S could suppress the DPPX-Sinduced effects on Kv4.2 intracellular trafficking and phosphorylation, cells transfected with a constant ratio of Kv4.2 and DPPX-S cDNA were transfected with increasing amounts of KChIP4a cDNA. We found that KChIP4a did indeed exhibit a dose-dependent inhibition of DPPX-S-induced Kv4.2 phosphorylation (Figure 6A, B). Dose-dependent inhibition of DPPX-S dependent cell surface expression of Kv4.2 by KChIP4a was also observed in immunofluorescence experiments (Figure 6C). That the DPPX-S pool in cells co-expressing Kv4.2, KChIP4a and DPPX-S was now associated with the KChIP4a:Kv4.2 intracellular staining as opposed to its normal plasma membrane-associated localization (Figure 6E) supports a model whereby Kv4.2 and the two auxiliary subunits comprise ternary complexes. This model is further supported in that Kv4.2 is required to mediate the association between KChIP4a and DPPX-S that results in intracellular retention of DPPX-S. Cells expressing KChIP4a and DPPX-S in the absence of Kv4.2 exhibit the distinct staining patterns of the respective auxiliary subunits (Figure 6F; intracellular/perinuclear staining for KChIP4a, and plasma membrane-associated for DPPXS).
FIGURE 6.
KChIP4a co-expression inhibits the DPPX-S induced effects on Kv4.2. (A) COS-1 cells were triple-transfected with Kv4.2 and DPPX-S at a 2:1 ratio (1 and 0.5 μg) and increasing amounts (0–2 μg) of KChIP4a cDNA was added as indicated. Detergent extracts of transfected cells were immunoblotted with anti-Kv4.2 and anti-DPPX antibodies. (B) Quantitative analysis of the KChIP4a inhibition of DPPX-S dependent effects on the electrophoretic mobility of Kv4.2. The intensities of the immunoreactivity of the Mr ≈ 65 kDa form (up facing arrowheads and error bars) and Mr ≈ 70 kDa form (squares and down facing error bars) at each cDNA ratio were measured as the percentage of each form relative to the total Kv4.2 pool. (C) Triple label immunofluorescence staining of COS-1 cells triple-transfected with Kv4.2, DPPX-S, and KChIP4a at 2:1:1 cDNA ratios. (D–E) COS-1 cells transiently transfected with KChIP4a (D) or DPPX-S (E) cDNA were stained for KChIP4a (K55/82) or DPPX (anti-HA). (F) COS-1 cells co-transfected with KChIP4a and DPPX-S cDNA at a 1:1 ratio were double-stained for KChIP4a and DPPX-S. Scale bar = 20 μm.
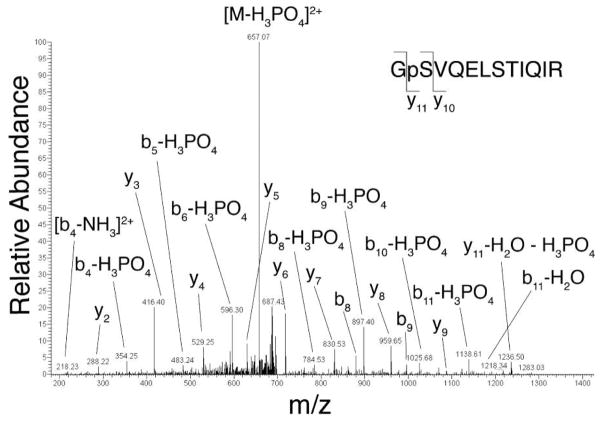
Tandem MS Identification of In Vivo Kv4.2 Phosphorylation Sites
Previous studies using in vitro phosphorylation of recombinant fragments of Kv4.2 led to the identification of a number of phosphorylation sites on Kv4.2 (25, 26). Here, we used tandem mass spectrometry as an unbiased approach to identify in vivo phosphorylation sites on the Kv4.2 α subunit in brain and heterologous cells. Kv4.2 was purified by immunoprecipitation from both crude rat brain membranes and COS-1 cell lysates from cells expressing Kv4.2 alone, or co-expressing Kv4.2 and KChIP2, or Kv4.2 and DPPX-S. The proteins were digested with trypsin for analysis by liquid chromatography tandem mass spectrometry (LC-MS/MS) (38, 41, 42). An example of a typical LC-MS/MS result, in this case for a doubly charged, singly phosphorylated Kv4.2 peptide with a mass to charge (m/z) ratio of 1409.69 is shown in Figure 7. The tandem mass spectrum had an almost inclusive array of y-ion and b-ion series describing the sequence GpSVQELSTIQIR (amino acids 551–562) that allowed for the unambiguous assignment of the pS552 phosphorylation site. Similar analyses of additional rat brain Kv4.2 peptides led to the identification of a total of four serine phosphorylation sites (pS548, pS552, pS572, and pS575). This set of sites is also phosphorylated on recombinant Kv4.2 co-expressed with DPPX-S or KChIP2 in heterologous cells, but not in cells expressing Kv4.2 alone (Table 1).
FIGURE 7.
Identification of Kv4.2 phosphorylation sites by tandem mass spectrometry. MS/MS spectrum of Kv4.2 phosphopeptide GpSVQELSTIQIR from COS-1 cells co-expressing Kv4.2 and DPPX-S.
Table 1.
In VivoKv4.2 Phosphorylation Sites
| COS-1 Cells |
||||
|---|---|---|---|---|
| Phosphorylation Site | Kv4.2 | Kv4.2 + DPPX-S | Kv4.2 + KChIP2 | Rat Brain |
| pS548 | − | − | + | + |
| pS552 | + | + | + | + |
| pS572 | − | + | + | + |
| pS575 | − | + | + | + |
+: phosphorylation site detected
−: phosphorylation site not detected
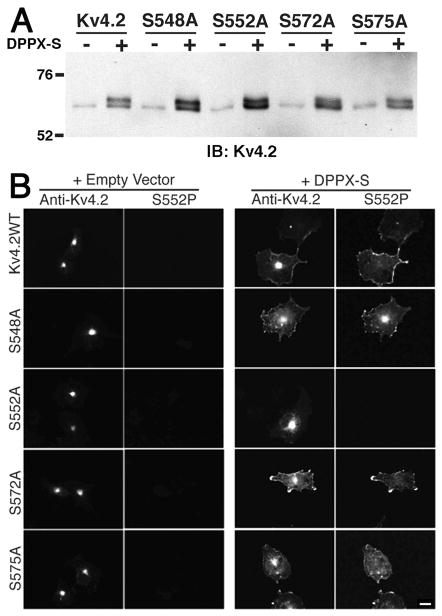
We next examined whether eliminating these phosphorylation sites with site-directed S548A, S552A, S572A, or S575A mutations would affect the DPPX-S dependent effects on Kv4.2 trafficking and increased Mr. We found that these point mutations had no effect on the relative mobility of Kv4.2 when either expressed alone, or in the presence of DPPX-S, when compared to wild-type Kv4.2 (Figure 8A). Immunoblot analyses with the S552P phosphospecific antibody suggested that the S548A, S572A, and S575A point mutations did not grossly affect the DPPX-S dependent phosphorylation of Kv4.2 at pS552, although S552P immunoreactivity was eliminated in the S552A mutant (data not shown). Immunofluorescence analysis of these point mutants expressed in COS-1 cells with and without DPPX-S yielded a pattern of total and cell surface staining indistinguishable from that of wild-type Kv4.2 (Figure 8B).
FIGURE 8.
Effects of phosphorylation site point mutations. (A) Effects of phosphorylation site mutations on the Mr of Kv4.2 on SDS PAGE. Solubilized Kv4.2 from detergent extracts of COS- 1 cells expressing wild-type Kv4.2 or phosphorylation site mutants S548A, S552A, S572A, and S575A alone or with DPPX-S were size fractionated on SDS PAGE and immunoblotted for Kv4.2. (B) Effects of phosphorylation site mutations on the localization of Kv4.2 in transfected cells. Kv4.2 expressed with empty vector RBG4 (left panels) or with DPPX-S (right panels) were double-stained for anti-Kv4.2 and S552P as indicated. Scale bar = 20 μm.
DISCUSSION
DPPX-S Dramatically Impacts the Molecular Properties of Kv4.2
We have shown here that co-expression of DPPX-S dramatically changes the molecular properties of Kv4.2 α subunits expressed in COS-1 cells, and that these effects are remarkably similar to those previously observed upon co-expression of KChIPs1–3 (13). Our data here confirm our previous results that in the absence of auxiliary subunits, the Kv4.2 α subunit tends to misfold and aggregate intracellularly as indicated by a perinuclear localization and lack of cell surface expression, and insolubility in non-ionic detergents. Kv4.2 expressed alone also exhibits reduced stability, and is hypophosphorylated relative to Kv4.2 found in native brain. DPPX-S, like KChIPs1–3, is able to rescue Kv4.2 from these aberrant molecular traits, and yield a population of Kv4.2 whose molecular properties more closely resemble the native Kv4.2 found in mammalian brain. As such, DPPX-S and KChIPs1–3 play a similar and fundamental role in determining the intracellular trafficking and molecular properties of Kv4.2. This is remarkable given the differences in structure, subcellular localization, and Kv4 α subunit binding site of the transmembrane DPPX-S and cytoplasmic KChIPs [reviewed in (43)] and is in sharp contrast to the distinct effects of these two classes of auxiliary subunits on Kv4.2 gating (14).
The Mechanistic Basis for the Similar Effects of Structurally Distinct Auxiliary DPPX-S and KChIPs on Kv4.2
Numerous studies have suggested that KChIPs1–3 act on Kv4.2 by binding and masking hydrophobic segments in the Kv4.2 N-terminus (13, 44–49). The prevailing model suggests that in the absence of KChIPs1–3, the hydrophobic segments on multiple Kv4.2 α subunits oligomerize, leading to aggregation and misfolding, and ultimately intracellular retention, hypophosphorylation and enhanced degradation. The masking of these hydrophobic segments upon their binding to KChIPs allows for proper assembly of Kv4.2 tetramers, and expression of a population of Kv4.2 with enhanced detergent solubility, phosphorylation and stability, and increased plasma membrane localization (13). Interestingly, although the transmembrane DPPX-S has a structure distinct from that of the KChIPs (50), and exerts distinct effects on the gating of Kv4.2 channels [reviewed in (43)], we found that DPPX-S has remarkably similar effects to those of KChIPs on the plasma membrane expression, detergent solubility, phosphorylation and stability of Kv4.2. The type II transmembrane DPPX-S has a short cytoplasmic N-terminus and a very prominent extracellular domain, and based on studies of the highly related DPPY (51), associates with the S1–S2 transmembrane domain region of Kv4.2 as opposed to the N-terminus. As such it appears unlikely that DPPX-S is mediating its effects on Kv4.2 through a mechanism similar to that defined for KChIPs.
Previously we found that unlike KChIPs1–3, KChIP4a could not rescue the aberrant phenotype of Kv4.2 in heterologous cells (13). A recent NMR study suggests a possible structural basis for these findings, in that the unique N-terminus of KChIP4a binds to the Kv4.2 binding site in the KChIP core domain and occludes Kv4.2 binding (48). As such, inclusion of KChIP4a subunits in octameric (Kv4)4(KChIP)4 complexes (52), even those containing KChIPs1–3 subunits, would lead to unmasked Kv4.2 N-termini free to oligomerize and induce intracellular retention, aggregation and hypophosphorylation of Kv4.2. We show here that KChIP4a also interferes with the facilitating effects of DPPX-S co-expression on Kv4.2. As it is unlikely that the facilitating effects of DPPX are through the mechanism of masking of the Kv4.2 N-terminus proposed for KChIPs1–3, it is just as unlikely that KChIP4a interferes with the DPPX effects by occluding such a masking mechanism.
A recent report provides a striking alternative view of the role of the KChIP4a Nterminus in regulating Kv4 channels. The authors provide compelling evidence that the Nterminus of KChIP4a is not cytoplasmic but instead forms a transmembrane segment (53), bringing into question the results of structural studies suggesting an intramolecular binding of a cytoplasmic KChIP4a N-terminus to other cytoplasmic KChIP4a domains. A more general model including these new results posits that if Kv4.2 is provided with partner proteins (e.g., KChIPs1–3, DPPX) that promote cell surface trafficking, either by directly masking a retention signal, or by providing an alternative and stronger cell surface expression signal, then Kv4.2 is phosphorylated and moves to the cell surface. Otherwise, in the absence of these partner proteins, or in the presence of these together with KChIP4a, Kv4.2 remains in its hypophosphorylated state where it is retained intracellularly and targeted for degradation.
Auxiliary Subunits and Kv4.2 Phosphorylation
Here we used tandem LC-MS/MS to identify four in vivo phosphorylation sites on the Kv4.2 C-terminus that are constitutively phosphorylated in both rat brain and in COS-1 cells in which Kv4.2 is co-expressed with DPPXS or KChIP2. One of these sites (pS552) was defined in previous studies using recombinant fragments of Kv4.2 as substrates for phosphorylation by purified PKA (25). The other three sites (pS548, pS572, and pS575) are novel. Consistent with our immunoblot analyses published previously (13), and presented here, our LC-MS/MS analyses show that Kv4.2 expressed alone in heterologous cells is hypophosphorylated. Only one of the four sites identified on brain Kv4.2, and on Kv4.2 from cells co-expressing auxiliary subunits, was detected on Kv4.2 purified from cells expressing Kv4.2 alone. It is intriguing that the one site detected was pS552, given the lack of detectable reaction of samples from cells expressing Kv4.2 alone with the S552P phosphospecific antibody in immunoblot, immunoprecipitation and immunofluorescence experiments presented here and previously (13). This presumably reflects different levels of sensitivity between the different detection methods. It is also formally possible that in cells expressing Kv4.2 alone it exists in a state that is phosphorylated at S552 but refractory to binding of our S552P antibody in immunoblots, immunoprecipitation, and immunofluorescence experiments, and that DPPX-S and KChIP2 co-expression reveals this immunoreactivity. Note that we have not applied more quantitative LC-MS/MS approaches [e.g., SILAC methods; (42)] here to determine the levels of Kv4.2 with phosphorylation at distinct sites in the presence and absence of auxiliary subunits. That similar patterns of phosphorylation were obtained for Kv4.2 purified from brain and from heterologous cells co-expressing DPPX-S and KChIP2 does suggest that the predominant pattern of Kv4.2 phosphorylation in brain neurons can be constitutively recapitulated in heterologous cells, but requires co-expression of auxiliary subunits. It is intriguing that a previous study found that the PKA-dependent modulation of Kv4.2 that has been observed in neurons could only be obtained for Kv4.2 expressed in Xenopus oocytes when it was co-expressed with KChIPs (27). Our studies showing convergent effects of DPPX-S and KChIPs on Kv4.2 phosphorylation suggest that DPPX-S may also be able to confer the sensitivity to PKA modulation (in this case via pS552) observed in native Kv4.2 channels on Kv4.2 in heterologous cells.
Similar to our previous findings on the effects of KChIP co-expression (13), phosphorylation at S552P strongly correlates with plasma membrane expression of Kv4.2. This is especially intriguing given that a recent study in cultured hippocampal neurons suggests that phosphorylation at S552P is associated with activity-dependent internalization of Kv4.2 from dendritic spines (54). We did not observe any obvious effects of mutating the pS552 phosphorylation site, or any of the other phosphorylation sites defined here, on plasma membrane expression of Kv4.2 in heterologous cells. As such, phosphorylation may be indicative of plasma membrane expression and not a determinant thereof. However, enhanced phosphorylation at these sites is associated with fundamental changes in the molecular properties of Kv4.2, including the enhanced detergent solubility and stability that are restricted to the more highly phosphorylated Mr = 70 kDa form (as opposed to the Mr = 65 kDa form) of Kv4.2. The link between the increased Kv4.2 phosphorylation and these distinct biochemical properties is not yet clear. Future studies will allow for insights into the mechanism whereby phosphorylation is linked to changes in the molecular properties of Kv4.2, and the role of phosphorylation at the novel sites we have identified in our LC-MS/MS analyses in regulating expression, localization and function of Kv4.2 in heterologous cells and neurons. Note that in addition to the studies described above, previous work has revealed diverse effects of C-terminal phosphorylation on Kv4.2 expression. Ca2+/calmodulin-dependent protein kinase II-dependent phosphorylation of Kv4.2 at S438 and S459 increases plasma membrane expression of Kv4.2 (30). A recent study using a phosphospecific antibody specific for ERK phosphorylation sites at T602, T607, and S616 suggests that Kv4.2 phosphorylation is also increased at one or more of these sites in response to kainate-induced status epilepticus, and that this correlates with decreased plasma membrane expression of Kv4.2 (55). These results suggest that although phosphorylation at pS552 may be enough to mediate the internalization of Kv4.2, there are other phosphorylation sites playing additional roles in Kv4.2 trafficking and localization. While we did not observe any effects of the S552A, or other mutations described here, on the plasma membrane trafficking or overall phosphorylation levels of Kv4.2 in our heterologous cell system, presumably phosphorylation at one or more of these sites is associated with fundamental changes in the molecular properties of Kv4.2, and may also be important in the modulation of channel gating. A comprehensive LC-MS/MS based analysis of changes in Kv4.2 phosphorylation in response to increased synaptic activity and status epilepticus will allow for a better understanding of the effects of these stimuli on Kv4.2 phosphorylation, and their role in regulating activity-dependent changes in Kv4.2 expression
Insights into the role of Kv4.2-associated auxiliary subunits in mammalian brain
DPPXS and KChIPs have distinct effects on the gating of Kv4.2 channels [reviewed in (14)]. The distinct patterns of expression of DPPX-S and KChIPs in mammalian brain (10, 18, 33, 56–58) have been proposed to underlie cell-specific differences in the functional properties of Kv4.2- based ISA (43). However, we suggest that in addition to these subtype-specific effects of DPPX-S and KChIPs on Kv4.2 channels, these structurally distinct auxiliary subunits exert convergent effects on fundamental aspects of Kv4.2 biology
Acknowledgments
We thank Dr. Kang-Sik Park for help with LC-MS/MS, and Dr. JoAnne Engebrecht for a careful reading of the manuscript.
Footnotes
This work was supported by NIH grant RO1 NS42225 (to JST), and by predoctoral training support for ES from NIH training grant T32 GM007377.
Abbreviations: AP, alkaline phosphatase; DPP, dipeptidyl peptidase-like; ISA, somatodendritic subthreshold A-type current; KChIPs, K+ channel interacting proteins; Kv, voltage-gated potassium; LC-MS/MS; liquid chromatography tandem mass spectrometry; MS, mass spectrometry; RBM, rat brain membranes.
References
- 1.Losonczy A, Makara JK, Magee JC. Compartmentalized dendritic plasticity and input feature storage in neurons. Nature. 2008;452:436–441. doi: 10.1038/nature06725. [DOI] [PubMed] [Google Scholar]
- 2.Kim J, Hoffman DA. Potassium channels: newly found players in synaptic plasticity. Neuroscientist. 2008;14:276–286. doi: 10.1177/1073858408315041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Johnston D, Hoffman DA, Magee JC, Poolos NP, Watanabe S, Colbert CM, Migliore M. Dendritic potassium channels in hippocampal pyramidal neurons. J Physiol. 2000;525:75–81. doi: 10.1111/j.1469-7793.2000.00075.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Serodio P, Rudy B. Differential expression of Kv4 K+ channel subunits mediating subthreshold transient K+ (A-type) currents in rat brain. J Neurophysiol. 1998;79:1081–1091. doi: 10.1152/jn.1998.79.2.1081. [DOI] [PubMed] [Google Scholar]
- 5.Song WJ, Tkatch T, Baranauskas G, Ichinohe N, Kitai ST, Surmeier DJ. Somatodendritic depolarization-activated potassium currents in rat neostriatal cholinergic interneurons are predominantly of the A type and attributable to coexpression of Kv4.2 and Kv4.1 subunits. J Neurosci. 1998;18:3124–3137. doi: 10.1523/JNEUROSCI.18-09-03124.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shibata R, Wakazono Y, Nakahira K, Trimmer JS, Ikenaka K. Expression of Kv3.1 and Kv4.2 genes in developing cerebellar granule cells. Dev Neurosci. 1999;21:87–93. doi: 10.1159/000017370. [DOI] [PubMed] [Google Scholar]
- 7.Tkatch T, Baranauskas G, Surmeier DJ. Kv4.2 mRNA abundance and Atype K(+) current amplitude are linearly related in basal ganglia and basal forebrain neurons. J Neurosci. 2000;20:579–588. doi: 10.1523/JNEUROSCI.20-02-00579.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen X, Yuan LL, Zhao C, Birnbaum SG, Frick A, Jung WE, Schwarz TL, Sweatt JD, Johnston D. Deletion of Kv4.2 gene eliminates dendritic Atype K+ current and enhances induction of long-term potentiation in hippocampal CA1 pyramidal neurons. J Neurosci. 2006;26:12143–12151. doi: 10.1523/JNEUROSCI.2667-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nerbonne JM, Gerber BR, Norris A, Burkhalter A. Electrical remodelling maintains firing properties in cortical pyramidal neurons lacking KCND2- encoded A-type K+ currents. J Physiol. 2008;586:1565–1579. doi: 10.1113/jphysiol.2007.146597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.An WF, Bowlby MR, Betty M, Cao J, Ling HP, Mendoza G, Hinson JW, Mattsson KI, Strassle BW, Trimmer JS, Rhodes KJ. Modulation of A-type potassium channels by a family of calcium sensors. Nature. 2000;403:553–556. doi: 10.1038/35000592. [DOI] [PubMed] [Google Scholar]
- 11.Nadal MS, Ozaita A, Amarillo Y, Vega-Saenz de Miera E, Ma Y, Mo W, Goldberg EM, Misumi Y, Ikehara Y, Neubert TA, Rudy B. The CD26-related dipeptidyl aminopeptidase-like protein DPPX is a critical component of neuronal A-type K+ channels. Neuron. 2003;37:449–461. doi: 10.1016/s0896-6273(02)01185-6. [DOI] [PubMed] [Google Scholar]
- 12.Nadal MS, Amarillo Y, Vega-Saenz de Miera E, Rudy B. Evidence for the presence of a novel Kv4-mediated A-type K(+) channel-modifying factor. J Physiol. 2001;537:801–809. doi: 10.1111/j.1469-7793.2001.00801.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shibata R, Misonou H, Campomanes CR, Anderson AE, Schrader LA, Doliveira LC, Carroll KI, Sweatt JD, Rhodes KJ, Trimmer JS. A fundamental role for KChIPs in determining the molecular properties and trafficking of Kv4.2 potassium channels. J Biol Chem. 2003;278:36445–36454. doi: 10.1074/jbc.M306142200. [DOI] [PubMed] [Google Scholar]
- 14.Covarrubias M, Bhattacharji A, De Santiago-Castillo JA, Dougherty K, Kaulin YA, Na-Phuket TR, Wang G. The neuronal Kv4 channel complex. Neurochem Res. 2008;33:1558–1567. doi: 10.1007/s11064-008-9650-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.O'Callaghan DW, Hasdemir B, Leighton M, Burgoyne RD. Residues within the myristoylation motif determine intracellular targeting of the neuronal Ca2+ sensor protein KChIP1 to post-ER transport vesicles and traffic of Kv4 K+ channels. J Cell Sci. 2003;116:4833–4845. doi: 10.1242/jcs.00803. [DOI] [PubMed] [Google Scholar]
- 16.Hasdemir B, Fitzgerald DJ, Prior IA, Tepikin AV, Burgoyne RD. Traffic of Kv4 K+ channels mediated by KChIP1 is via a novel post-ER vesicular pathway. J Cell Biol. 2005;171:459–469. doi: 10.1083/jcb.200506005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Clark BD, Kwon E, Maffie J, Jeong HY, Nadal M, Strop P, Rudy B. DPP6 Localization in Brain Supports Function as a Kv4 Channel Associated Protein. Front Mol Neurosci. 2008;1:8. doi: 10.3389/neuro.02.008.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jerng HH, Kunjilwar K, Pfaffinger PJ. Multiprotein assembly of Kv4.2, KChIP3 and DPP10 produces ternary channel complexes with ISA-like properties. J Physiol. 2005;568:767–788. doi: 10.1113/jphysiol.2005.087858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maffie J, Rudy B. Weighing the evidence for a ternary protein complex mediating A-type K+ currents in neurons. J Physiol. 2008;586:5609–5623. doi: 10.1113/jphysiol.2008.161620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Amarillo Y, De Santiago-Castillo JA, Dougherty K, Maffie J, Kwon E, Covarrubias M, Rudy B. Ternary Kv4.2 channels recapitulate voltage-dependent inactivation kinetics of A-type K+ channels in cerebellar granule neurons. J Physiol. 2008;586:2093–2106. doi: 10.1113/jphysiol.2007.150540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim J, Nadal MS, Clemens AM, Baron M, Jung SC, Misumi Y, Rudy B, Hoffman DA. Kv4 accessory protein DPPX (DPP6) is a critical regulator of membrane excitability in hippocampal CA1 pyramidal neurons. J Neurophysiol. 2008;100:1835–1847. doi: 10.1152/jn.90261.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maffie J, Blenkinsop T, Rudy B. A novel DPP6 isoform (DPP6-E) can account for differences between neuronal and reconstituted A-type K(+) channels. Neurosci Lett. 2009;449:189–194. doi: 10.1016/j.neulet.2008.10.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marshall CR, Noor A, Vincent JB, Lionel AC, Feuk L, Skaug J, Shago M, Moessner R, Pinto D, Ren Y, Thiruvahindrapduram B, Fiebig A, Schreiber S, Friedman J, Ketelaars CE, Vos YJ, Ficicioglu C, Kirkpatrick S, Nicolson R, Sloman L, Summers A, Gibbons CA, Teebi A, Chitayat D, Weksberg R, Thompson A, Vardy C, Crosbie V, Luscombe S, Baatjes R, Zwaigenbaum L, Roberts W, Fernandez B, Szatmari P, Scherer SW. Structural variation of chromosomes in autism spectrum disorder. Am J Hum Genet. 2008;82:477–488. doi: 10.1016/j.ajhg.2007.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van Es MA, van Vught PW, Blauw HM, Franke L, Saris CG, Van den Bosch L, de Jong SW, de Jong V, Baas F, van't Slot R, Lemmens R, Schelhaas HJ, Birve A, Sleegers K, Van Broeckhoven C, Schymick JC, Traynor BJ, Wokke JH, Wijmenga C, Robberecht W, Andersen PM, Veldink JH, Ophoff RA, van den Berg LH. Genetic variation in DPP6 is associated with susceptibility to amyotrophic lateral sclerosis. Nat Genet. 2008;40:29–31. doi: 10.1038/ng.2007.52. [DOI] [PubMed] [Google Scholar]
- 25.Anderson AE, Adams JP, Qian Y, Cook RG, Pfaffinger PJ, Sweatt JD. Kv4.2 phosphorylation by cyclic AMP-dependent protein kinase. J Biol Chem. 2000;275:5337–5346. doi: 10.1074/jbc.275.8.5337. [DOI] [PubMed] [Google Scholar]
- 26.Adams JP, Anderson AE, Varga AW, Dineley KT, Cook RG, Pfaffinger PJ, Sweatt JD. The A-type potassium channel Kv4.2 is a substrate for the mitogen-activated protein kinase ERK. J Neurochem. 2000;75:2277–2287. doi: 10.1046/j.1471-4159.2000.0752277.x. [DOI] [PubMed] [Google Scholar]
- 27.Schrader LA, Anderson AE, Mayne A, Pfaffinger PJ, Sweatt JD. PKA modulation of Kv4.2-encoded A-type potassium channels requires formation of a supramolecular complex. J Neurosci. 2002;22:10123–10133. doi: 10.1523/JNEUROSCI.22-23-10123.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yuan LL, Adams JP, Swank M, Sweatt JD, Johnston D. Protein kinase modulation of dendritic K+ channels in hippocampus involves a mitogen-activated protein kinase pathway. J Neurosci. 2002;22:4860–4868. doi: 10.1523/JNEUROSCI.22-12-04860.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hoffman DA, Johnston D. Downregulation of transient K+ channels in dendrites of hippocampal CA1 pyramidal neurons by activation of PKA and PKC. J Neurosci. 1998;18:3521–3528. doi: 10.1523/JNEUROSCI.18-10-03521.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Varga AW, Yuan LL, Anderson AE, Schrader LA, Wu GY, Gatchel JR, Johnston D, Sweatt JD. Calcium-calmodulin-dependent kinase II modulates Kv4.2 channel expression and upregulates neuronal A-type potassium currents. J Neurosci. 2004;24:3643–3654. doi: 10.1523/JNEUROSCI.0154-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Roberds SL, Tamkun MM. Cloning and tissue-specific expression of five voltage-gated potassium channel cDNAs expressed in rat heart. Proc Natl Acad Sci U S A. 1991;88:1798–1802. doi: 10.1073/pnas.88.5.1798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tiffany AM, Manganas LN, Kim E, Hsueh YP, Sheng M, Trimmer JS. PSD-95 and SAP97 exhibit distinct mechanisms for regulating K(+) channel surface expression and clustering. J Cell Biol. 2000;148:147–158. doi: 10.1083/jcb.148.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rhodes KJ, Carroll KI, Sung MA, Doliveira LC, Monaghan MM, Burke SL, Strassle BW, Buchwalder L, Menegola M, Cao J, An WF, Trimmer JS. KChIPs and Kv4 alpha subunits as integral components of A-type potassium channels in mammalian brain. J Neurosci. 2004;24:7903–7915. doi: 10.1523/JNEUROSCI.0776-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shi G, Kleinklaus AK, Marrion NV, Trimmer JS. Properties of Kv2.1 K+ channels expressed in transfected mammalian cells. J Biol Chem. 1994;269:23204–23211. [PubMed] [Google Scholar]
- 35.Manganas LN, Trimmer JS. Subunit composition determines Kv1 potassium channel surface expression. J Biol Chem. 2000;275:29685–29693. doi: 10.1074/jbc.M005010200. [DOI] [PubMed] [Google Scholar]
- 36.Ward GE, Garbers DL, Vacquier VD. Effects of extracellular egg factors on sperm guanylate cyclase. Science. 1985;227:768–770. doi: 10.1126/science.2857502. [DOI] [PubMed] [Google Scholar]
- 37.Trimmer JS. Immunological identification and characterization of a delayed rectifier K+ channel polypeptide in rat brain. Proc Natl Acad Sci U S A. 1991;88:10764–10768. doi: 10.1073/pnas.88.23.10764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Park KS, Mohapatra DP, Misonou H, Trimmer JS. Graded regulation of the Kv2.1 potassium channel by variable phosphorylation. Science. 2006;313:976–979. doi: 10.1126/science.1124254. [DOI] [PubMed] [Google Scholar]
- 39.Marquardt T, Helenius A. Misfolding and aggregation of newly synthesized proteins in the endoplasmic reticulum. J Cell Biol. 1992;117:505–513. doi: 10.1083/jcb.117.3.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Martens JR, Navarro-Polanco R, Coppock EA, Nishiyama A, Parshley L, Grobaski TD, Tamkun MM. Differential targeting of Shaker-like potassium channels to lipid rafts. J Biol Chem. 2000;275:7443–7446. doi: 10.1074/jbc.275.11.7443. [DOI] [PubMed] [Google Scholar]
- 41.Mann M, Ong SE, Gronborg M, Steen H, Jensen ON, Pandey A. Analysis of protein phosphorylation using mass spectrometry: deciphering the phosphoproteome. Trends Biotechnol. 2002;20:261–268. doi: 10.1016/s0167-7799(02)01944-3. [DOI] [PubMed] [Google Scholar]
- 42.Park KS, Yang JW, Seikel E, Trimmer JS. Potassium channel phosphorylation in excitable cells: providing dynamic functional variability to a diverse family of ion channels. Physiology (Bethesda) 2008;23:49–57. doi: 10.1152/physiol.00031.2007. [DOI] [PubMed] [Google Scholar]
- 43.Jerng HH, Pfaffinger PJ, Covarrubias M. Molecular physiology and modulation of somatodendritic A-type potassium channels. Mol Cell Neurosci. 2004;27:343–369. doi: 10.1016/j.mcn.2004.06.011. [DOI] [PubMed] [Google Scholar]
- 44.Bahring R, Dannenberg J, Peters HC, Leicher T, Pongs O, Isbrandt D. Conserved Kv4 N-terminal domain critical for effects of Kv channel-interacting protein 2.2 on channel expression and gating. J Biol Chem. 2001;276:23888–23894. doi: 10.1074/jbc.M101320200. [DOI] [PubMed] [Google Scholar]
- 45.Scannevin RH, Wang K, Jow F, Megules J, Kopsco DC, Edris W, Carroll KC, Lu Q, Xu W, Xu Z, Katz AH, Olland S, Lin L, Taylor M, Stahl M, Malakian K, Somers W, Mosyak L, Bowlby MR, Chanda P, Rhodes KJ. Two N-terminal domains of Kv4 K(+) channels regulate binding to and modulation by KChIP1. Neuron. 2004;41:587–598. doi: 10.1016/s0896-6273(04)00049-2. [DOI] [PubMed] [Google Scholar]
- 46.Pioletti M, Findeisen F, Hura GL, Minor DL., Jr Three-dimensional structure of the KChIP1-Kv4.3 T1 complex reveals a cross-shaped octamer. Nat Struct Mol Biol. 2006;13:987–995. doi: 10.1038/nsmb1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang H, Yan Y, Liu Q, Huang Y, Shen Y, Chen L, Chen Y, Yang Q, Hao Q, Wang K, Chai J. Structural basis for modulation of Kv4 K+ channels by auxiliary KChIP subunits. Nat Neurosci. 2007;10:32–39. doi: 10.1038/nn1822. [DOI] [PubMed] [Google Scholar]
- 48.Schwenk J, Zolles G, Kandias NG, Neubauer I, Kalbacher H, Covarrubias M, Fakler B, Bentrop D. NMR analysis of KChIP4a reveals structural basis for control of surface expression of Kv4 channel complexes. J Biol Chem. 2008;283:18937–18946. doi: 10.1074/jbc.M800976200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cui YY, Liang P, Wang KW. Enhanced trafficking of tetrameric Kv4.3 channels by KChIP1 clamping. Neurochem Res. 2008;33:2078–2084. doi: 10.1007/s11064-008-9688-7. [DOI] [PubMed] [Google Scholar]
- 50.Strop P, Bankovich AJ, Hansen KC, Garcia KC, Brunger AT. Structure of a human A-type potassium channel interacting protein DPPX, a member of the dipeptidyl aminopeptidase family. J Mol Biol. 2004;343:1055–1065. doi: 10.1016/j.jmb.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 51.Ren X, Hayashi Y, Yoshimura N, Takimoto K. Transmembrane interaction mediates complex formation between peptidase homologues and Kv4 channels. Mol Cell Neurosci. 2005;29:320–332. doi: 10.1016/j.mcn.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 52.Kim LA, Furst J, Butler MH, Xu S, Grigorieff N, Goldstein SA. Ito Channels Are Octomeric Complexes with Four Subunits of Each Kv4.2 and K+ Channelinteracting Protein 2. J Biol Chem. 2004;279:5549–5554. doi: 10.1074/jbc.M311332200. [DOI] [PubMed] [Google Scholar]
- 53.Jerng HH, Pfaffinger PJ. Multiple Kv channel-interacting proteins contain an N-terminal transmembrane domain that regulates Kv4 channel trafficking and gating. J Biol Chem. 2008;283:36046–36059. doi: 10.1074/jbc.M806852200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hammond RS, Lin L, Sidorov MS, Wikenheiser AM, Hoffman DA. Protein kinase a mediates activity-dependent Kv4.2 channel trafficking. J Neurosci. 2008;28:7513–7519. doi: 10.1523/JNEUROSCI.1951-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lugo JN, Barnwell LF, Ren Y, Lee WL, Johnston LD, Kim R, Hrachovy RA, Sweatt JD, Anderson AE. Altered phosphorylation and localization of the A-type channel, Kv4.2 in status epilepticus. J Neurochem. 2008;106:1929–1940. doi: 10.1111/j.1471-4159.2008.05508.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.de Lecea L, Soriano E, Criado JR, Steffensen SC, Henriksen SJ, Sutcliffe JG. Transcripts encoding a neural membrane CD26 peptidase-like protein are stimulated by synaptic activity. Brain Res Mol Brain Res. 1994;25:286–296. doi: 10.1016/0169-328x(94)90164-3. [DOI] [PubMed] [Google Scholar]
- 57.Xiong H, Kovacs I, Zhang Z. Differential distribution of KChIPs mRNAs in adult mouse brain. Brain Res Mol Brain Res. 2004;128:103–111. doi: 10.1016/j.molbrainres.2004.06.024. [DOI] [PubMed] [Google Scholar]
- 58.Holmqvist MH, Cao J, Hernandez-Pineda R, Jacobson MD, Carroll KI, Sung MA, Betty M, Ge P, Gilbride KJ, Brown ME, Jurman ME, Lawson D, Silos- Santiago I, Xie Y, Covarrubias M, Rhodes KJ, Distefano PS, An WF. Elimination of fast inactivation in Kv4 A-type potassium channels by an auxiliary subunit domain. Proc Natl Acad Sci U S A. 2002;99:1035–1040. doi: 10.1073/pnas.022509299. [DOI] [PMC free article] [PubMed] [Google Scholar]