Abstract
Objective
To develop a speech recognition index that summarizes data collected through an array of age-appropriate hierarchical speech recognition tests in a longitudinal study.
Study Design
Prospective cohort.
Setting
Six tertiary referral centers in the Childhood Development after Cochlear Implantation (CDaCI) Study.
Patients
One hundred eighty-eight children implanted at age 5 years or younger and 97 age-comparable normal-hearing controls.
Intervention
Cochlear implantation.
Main Outcome Measures
Outcome measures were the following: Infant-Toddler Meaningful Auditory Integration Scale, Meaningful Auditory Integration Scale, Early Speech Perception Test, Pediatric Speech Intelligibility Test, Multisyllabic Lexical Neighborhood Test, Lexical Neighborhood Test, and Hearing in Noise Test, obtained before implantation and at 6, 12, 18, and 24 months postimplant.
Results
A speech recognition cumulative index, speech recognition index in quiet (SRI-Q), was created to combine information from tests administered in quiet. This index allows simultaneous display of data from all tests in the speech recognition hierarchy and is sensitive to improvements in performance over time as a function of age. SRI-Q also provides a composite of performance on multiple tests, allowing both the tracking of “growth curve” in speech recognition across a wide age range over an extended follow-up period and the comparison of normal-hearing and implanted children on multiple measures. The data range for individual tests is also preserved for ease of interpretation.
Conclusion
SRI-Q allows tracking of global development of speech recognition over time as children progress through a hierarchy of speech perception measures and complements the more detailed assessments obtained from individual tests within the hierarchy.
Keywords: Hierarchical testing, Pediatric cochlear implantation, Speech recognition, Summary index
Tracking development of auditory perception in early childhood is highly challenging because children are in a phase of rapid acquisition of auditory milestones and demonstrate a wide range of perceptual capabilities. Strategies used by previous investigators to overcome these challenges tend to fall into 2 categories: those that used instruments valid over a broad developmental range but provided only gross assessment (e.g., Beadle et al. [1]) and those that used age-specific instruments for more detailed assessment but required subgroup analyses within each instrument (e.g., Eisenberg et al. [2]). Instruments valid over a broad developmental range typically use a scale that classifies auditory abilities into a limited number of categories that may not be very sensitive to auditory outcomes. In contrast, instruments that target narrower developmental ranges are better suited to study the acquisition of speech perception skills with greater detail but are subject to floor and/or ceiling observations when study participants are outside the developmental range of a given instrument.
A longitudinal study that tracks development of speech recognition over time in early childhood should use an array of developmentally appropriate measures. The study protocol should entail cross-sectional assessment at each follow-up interval using instruments specifically designed for the range of ages and abilities represented within the study population and address the need to switch to other age- or ability-appropriate instruments as children develop throughout the course of follow-up. Furthermore, longitudinal tracking of postimplant development among children who are deaf and hard of hearing is frequently complicated by potential delays in speech perception and language abilities, rendering the suitability of test instruments based on age less appropriate for children with a cochlear implant (CI).
To meet these challenges, a hierarchical approach to speech recognition assessment has been formulated in the “Childhood Development after Cochlear Implantation” (CDaCI) study. This approach combines a battery of developmentally appropriate measures of auditory perceptual capabilities to assess children from toddler through kindergarten ages. In this article, we propose a strategy for tracking children's speech perception development longitudinally over a wide range of ages at enrollment and postimplant follow-up. This strategy uses a summary index that combines the outcomes acquired with an array of speech recognition instruments targeting different stages of auditory skill acquisition and developmental abilities.
Methods
Participants
The CDaCI cohort includes 188 children with sensorineural hearing loss who received CIs at a mean age of 2.2 years (standard deviation [SD], 1.2 yr) and 97 age-comparable normal-hearing (NH) children (mean age at enrollment, 2.3 yr; SD, 1.1 yr). Children were enrolled through 6 tertiary referral centers: House Ear Institute, Johns Hopkins University, University of Miami, University of Michigan, University of North Carolina, and University of Texas at Dallas, between November 2002 and December 31, 2004. Fifty-five percent of the CDaCI cohort participants are female subjects, and 26% are minorities. The sex and race composition are similar between the CI and NH groups.
Children were assessed at baseline (before implant surgery for the CI group) and at 6-month follow-up intervals for 3 years. A detailed description of the research design, eligibility criteria, study protocol, and baseline characteristics of the cohort has been reported (3). The institutional review boards of each participating center approved the study protocol. Written informed consent was obtained from each participating family. This report used data collected from baseline through the 24-month follow-up.
Speech Recognition Hierarchy
The CDaCI speech recognition hierarchy was developed and structured according to the child's age and functional hearing ability. The details of this hierarchy have been described previously (2). Briefly, the Preschool Battery that targets the emerging abilities of toddlers through approximately 4 years consists of the Infant-Toddler Meaningful Auditory Integration Scale (IT-MAIS) (4), Meaningful Auditory Integration Scale (MAIS) (5), Early Speech Perception Test (ESP) (6), Pediatric Speech Intelligibility Test (PSI) (7), Multisyllabic Lexical Neighborhood Test (MLNT) (8), and the Lexical Neighborhood Test (LNT) (9). This battery incorporates measures that assess pattern perception and word and sentence recognition presented in closed and open sets. The IT-MAIS and MAIS are parent questionnaires and criteria-referenced rating scales assessing the child's meaningful use of sound with sensory devices in their home environments and everyday listening situations. The ESP and PSI are closed-set tasks, in which a limited number of potential choices are available to the listener. The MLNT and LNT are open-set tasks that use no predefined response alternatives so that the number of choices is unlimited. The School-Age Battery extends the upper age range over which children can be assessed, incorporating the Phonetically Balanced Word Lists–Kindergarten (PBK) (10) and the Hearing in Noise Test for Children (HINT-C) (11). Both are open-set tasks suitable for assessing word and sentence recognition in children aged 5 years and older.
For each measure in the hierarchy, a criterion level of performance is required before proceeding to the next level of difficulty. Testing is discontinued on a specific measure once ceiling is reached on 2 consecutive test intervals. A consequence of this progression is that fewer numbers of children are assessed on the more difficult tests.
Analysis
Speech recognition data obtained for all measures from baseline to the 24-month postimplant follow-up were explored graphically to display the range and growth pattern of scores over the ranges of ages assessed using each test instrument and to identify the age at which mean outcome approached the ceiling for that instrument. Data were analyzed separately for the CI and NH groups. Outcome data for the PSI, MLNT, LNT, PBK, and HINT-C were in the form of percent correct scores (0–100%). For this analysis, only PSI and HINT-C scores obtained in quiet were used. Total scores (ratings) for the IT-MAIS and MAIS questionnaires were converted to percentages for ease of comparison with the other speech recognition measures. Because the IT-MAIS and MAIS largely tap the same abilities in younger (IT-MAIS) and older (MAIS) children, these measures were combined to form a single MAIS score. It was also observed that the fitted linear regression between MLNT and LNT scores across all children (intercept = 4.18, slope = 0.91) was very close to the diagonal line. Thus, when both were available, the scores for each child from these 2 instruments were averaged and labeled as M/LNT. Finally, results for the ESP, which classifies performance into 1 of 4 speech perception categories, were reassigned values of 25 (Category 1), 50 (Category 2), 75 (Category 3), and 100 (Category 4), for visual integration with other speech perception data. Scatter plots and nonparametric smoothing curves tracking growth of individual speech observations over chronological age were produced to guide the creation of a speech recognition index under quiet testing conditions (SRI-Q).
The trajectories of SRI-Q by age from baseline and each of the 4 follow-up visits were examined for stability over time among NH children. The developmental trajectories of speech recognition among CI children for each of the 4 post-CI study visits were also estimated and compared with the estimated growth in speech recognition in the NH children to explore postimplant growth in speech recognition among CI children.
Results
Speech Recognition Test Results at Baseline
At baseline, the IT-MAIS/MAIS score for NH children approached ceiling, with an overall mean score of 97.1% (Table 1). Approximately two thirds of NH children were evaluated with ESP, and almost all had consistent word identification (Table 2). Approximately one third of NH children were evaluated with PSI and, on average, had 94% correct sentence identification under quiet testing conditions. In contrast, CI children at baseline had an average IT-MAIS/MAIS score of 23%. Approximately one quarter of CI children were evaluated with ESP at baseline, and among them, 58% were in the detection stage of speech recognition.
Table 1. Speech recognition test results at baseline for 188 children who later received cochlear implantation and 97 normal-hearing children in the Childhood Development After Cochlear Implantation Study.
| CI children | NH children | |||
|---|---|---|---|---|
| n | Mean (SD) | n | Mean (SD) | |
| IT-MAIS/MAIS | 186 | 23.0 (25.3) | 93 | 97.1 (7.7) |
| ESP score | 50 | 47.5 (30.8) | 63 | 99.0 (3.9) |
| PSI (quiet) | 12 | 65.4 (33.7) | 31 | 94.2 (12.3) |
| MLNT/LNT | 9 | 11.1 (12.7) | 21 | 82.1 (14.9) |
| PBK | 0 | — (—) | 2 | 86.5 (9.2) |
| HINT-C (quiet) | 0 | — (—) | 1 | 96 (—) |
CI indicates cochlear implant;NH, normal hearing;SD, standard deviation; IT-MAIS, Infant-Toddler Meaningful Auditory Integration Scale; MAIS, Meaningful Auditory Integration Scale; ESP, Early Speech Perception Test; PSI, Pediatric Speech Intelligibility Test; MLNT, Multisyllabic Lexical Neighborhood Test; LNT, Lexical Neighborhood Test; PBK, Phonetically Balanced Word Lists–Kindergarten; HINT-C; Hearing in Noise Test for Children.
Table 2. Results for Early Speech Perception Test.
| CI children | NH children | |||
|---|---|---|---|---|
| Early Speech Perception Test category | n | % | n | % |
| 1 | 29 | 58 | 0 | 0 |
| 2 | 8 | 16 | 0 | 0 |
| 3 | 2 | 4 | 1 | 1.6 |
| 4 | 11 | 22 | 62 | 98.4 |
Speech Recognition Development in NH Children
Figure 1A shows the scatter plot of all available speech outcome observations as a function of age at testing among 97 NH children between baseline and the 24-month follow-up visit. To reflect their position in the hierarchy, range of the speech measures were rescaled to 100 to 200 for ESP, 200 to 300 for PSI in quiet, 300 to 400 for M/LNT, 400 to 500 for PBK, and 500 to 600 for HINT-C in quiet, resulting in a range of scores from 0 to 600.
Fig. 1.
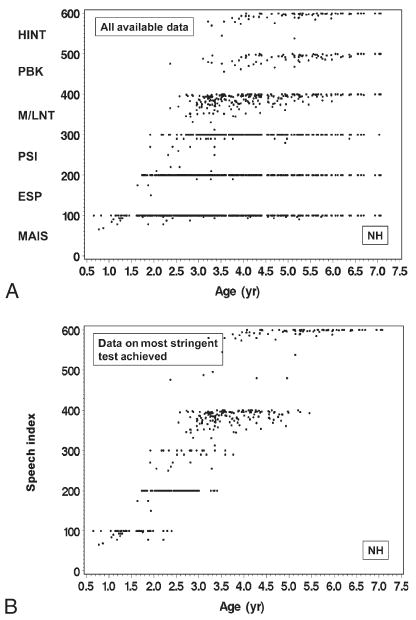
All available data (A) from the CDaCI speech recognition hierarchy from baseline to 24-month follow-up in 97 NH children. SRI-Q index (B), the result from the most stringent test of the CdaCI speech recognition hierarchy with which a child was evaluated at a given visit, from baseline to 24-month follow-up among 97 NH children.
For NH children, the average IT-MAIS/MAIS and ESP scores approached ceiling around age of 2 years, indicating that speech recognition growth as reflected by these instruments was mostly truncated after that age. The average PSI scores in quiet approached ceiling at approximately 3.5 years. As described earlier, the growth in MLNT and LNT results was quite similar in NH children, with both measures showing average scores of 60 to 70% correct at the age of 2.5 years and approaching ceiling (100% correct) around age 6.5 years. Therefore, the combined M/LNT scores are presented.
The nonzero score from the most stringent test in the hierarchy at each visit was converted into a summary score, the speech recognition index in quiet (SRI-Q), for that visit. The SRI-Q has a possible value of between 0 and 600 points (Fig. 1B). This index allows us to visually extract information regarding which was the most stringent test with which each child could be assessed at the visit and how the child fared in that test. Comparing Figure 1, A and B, we can see that, in general, NH children who reached the ceiling on the IT-MAIS/MAIS, ESP, or PSI test were able to take on more stringent tests during the same visit. Similarly, many of those NH children who scored well on MLNT, LNT, or PBK were able to move on to the HINT-C in quiet.
Speech Recognition Development in CI Children
Figure 2A shows the scatter plot of all available speech outcome observations as a function of age at testing among 188 CI children between baseline and the 24-month follow-up visit. At baseline, most CI children were only able to be evaluated using the IT-MAIS/MAIS. During the postimplant follow-up visits, however, many CI children achieved ceiling on the IT-MAIS/MAIS, ESP, and PSI, although some children who were older than 5 years still did not reach ceiling on the IT-MAIS/MAIS. Compared with the NH children, speech recognition outcomes for CI children were more variable. This contrast can be seen more clearly when the SRI-Q score for CI children (Fig. 2B) was compared with the SRI-Q score for NH children (Fig. 1B). For example, among those aged 5 years or older at the time of testing, some CI children were still able to be evaluated only with IT-MAIS/MAIS or ESP, whereas many others could be evaluated with HINT-C in quiet, similar to most of their NH peers. In general, most CI children were able to be evaluated with MLNT/LNT or more difficult tests by their 24-month follow-up visit.
Fig. 2.
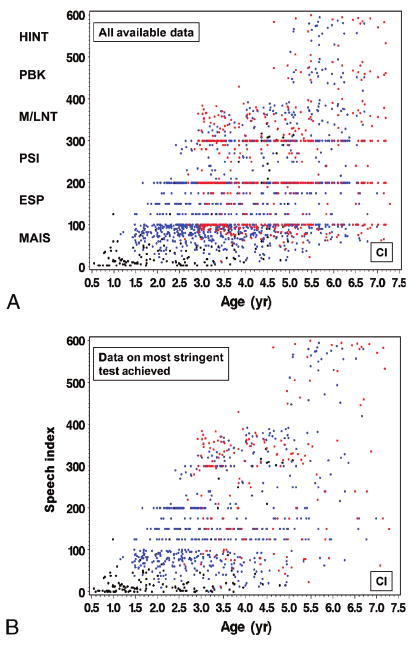
All available data (A) from the CdaCI speech recognition hierarchy from baseline to 24-month follow-up in 188 CI children. SRI-Q index (B), the result from the most stringent test of the CdaCI speech recognition hierarchy with which a child was evaluated at a given visit, from baseline to 24-month follow-up among 188 CI children. Black indicates measures assessed at baseline; blue, measures assessed at 6-, 12-, or 18-month follow-up; red, measures assessed at 24-month follow-up.
Growth Trajectories in Speech Recognition Over 24 Months of Follow-Up
By connecting up to 5 SRI-Q scores, a speech recognition developmental trajectory can be tracked for each CI child from baseline up to the 24-month post-CI evaluation (Fig. 3). The growth in speech recognition development over the first 24 months post-CI was spread widely among CI children. A substantial number of CI children implanted at younger ages demonstrated growth patterns very similar in range or well into the trajectories of the NH children. A few children implanted at older ages showed slower trajectories of development after implantation. In contrast, the trajectories of speech recognition development among NH children showed much less variability, forming a much tighter band of normal development. This range provides a reference for evaluating postimplant speech recognition in CI children.
Fig. 3.
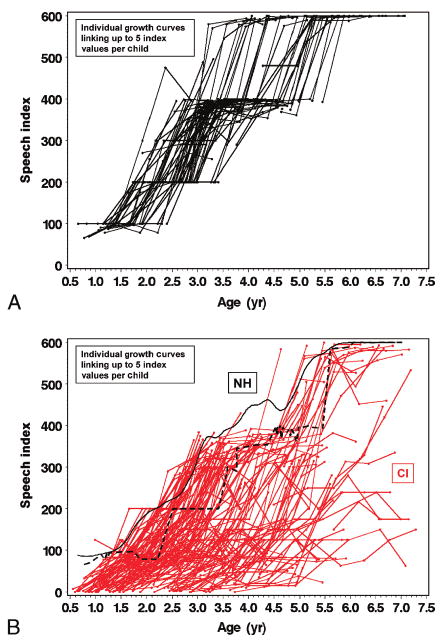
Growth trajectories between baseline and 24-month follow-up visit using SRI-Q index for (A) 97 NH children in black solid lines and (B) 188 CI children in red solid lines. The black solid curve (B) indicates the nonparametric mean trajectory of SRI-Q index by age for all 97 NH children. The black dashed line indicates the estimated lower boundary of SRI-Q score, by age, achieved by the NH children.
Discussion
We explored the potential use of a simple index, the SRI-Q, in tracking the longitudinal growth in development of speech recognition skills for young CI and NH children. This index, which summarizes the results from an array of age- or ability-appropriate speech recognition tests, allows simultaneous display of data from multiple instruments over time. It has face validity and provides a global indicator that complements test results from individual instruments.
The SRI-Q index score identifies both the most stringent measure in the speech recognition hierarchy with which a child was able to be evaluated in a given follow-up visit and the actual test score obtained on that test. This approach preserves the ranking of most difficult test achieved while allowing the interpretation of results within the original range of 0 to 100% for the corresponding test.
Using the SRI-Q, we identified a region of normal growth trajectory in speech recognition development over a 2-year period in 97 CDaCI NH children aged 5 years or younger at baseline. This provides a reference for comparing longitudinal postimplant speech recognition development in CI children.
One limitation of our method is the need to incorporate test results obtained in the presence of noise (HINT-C) or competing speech (PSI), or to create a separate index for such test results. This will be done when more data obtained under noise/competition testing conditions become available. Another limitation is that the SRI-Q index did not completely distinguish children who achieved a ceiling score on 1 measure but who were not able to be tested with the next more stringent measure in the hierarchy from those children who achieved a lower score on the first test but who were able to move up to a more difficult test (e.g., a child with M/LNT score of 90% who was not tested with PBK versus a second child with M/LNT of 30% and PBK of 5%). In the former case, a given child's SRI-Q would be based on incomplete data that could underestimate or not necessarily reflect his/her speech perception ability. Conversely, SRI-Qs for children who were able to move through the hierarchy as designed reflect only their performance on the most stringent measure tested but not on individual measures at lower levels that could contain useful information. A more comprehensive approach to address incomplete progression through the hierarchy and use as much of the data as possible will require sophisticated statistical modeling. Nevertheless, in our data set, those children with scores close to ceiling on a given test usually had better SRI-Q scores than those with lower scores on the same test when both were evaluated with a more stringent measure. Furthermore, the current approach provides a simple way for clinicians to summarize useful information without the need to transform test scores through a series of regression coefficients and equations.
The ability to position a child's speech recognition at given points in time relative to the normal developmental trajectory may prove helpful in screening children for abnormal development and in evaluating the progress of rehabilitation for children with developmental delays or disabilities. The SRI-Q index provides a simple way to track and compare growth trajectories in speech recognition development in both CI and NH children based on a hierarchy of age-appropriate instruments, thus overcoming some of the limitations associated with using a single broad-ranged test instrument. This SRI-Q index also provides a single summary value that can be used to study the relationships between speech recognition development and developmental indices in language or other important childhood developmental domains.
Acknowledgments
This research project was supported by Grant R0l DC004797 from the National Institute on Deafness and Other Communication Disorders.
The funding agencies played no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; or preparation, review, or approval of the manuscript. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute on Deafness and Other Communication Disorders or the National Institutes of Health.
Appendix
CDaCI Investigative Team: House Ear Institute, Los Angeles, CA: Laurie S. Eisenberg, PhD, CCC-A (PI); Karen Johnson, Ph.D., CCC-A (coordinator); Jean DesJardin, Ph.D. (data collection); Melinda Gillinger (data collection); William Luxford, M.D. (surgeon); Amy Martinez, M.A., CCC-A (data collection); Leslie Visser-Dumont, MA, CCC-A (data collection); Carren Stika, Ph.D. (data collection); Sophie Ambrose, M.A. (data collection); Dianne Hammes-Ganguly, M.A. (data collection).
Johns Hopkins University, Listening Center, Baltimore, MD: John K. Niparko, M.D. (PI); Jennifer Mertes, Au.D., CCC-A (coordinator); Steve Bowditch, M.S., CCC-A (data collection); Jill Chinnici, M.A., CCC-A (data collection); Howard Francis, M.D. (surgeon); Rick Ostrander, ED.D. (data collection); Jennifer Yeagle, M.Ed., CCC-A (data collection).
Johns Hopkins University, The River School, Washington, DC: Nancy Mellon (administration); Mary O'Leary Kane, M.A., CCCSLP (coordinator); Sarah Wainscott (data collection); Jennifer Wallace, M.S., CCC-SLP (data collection).
University of Miami, Miami, FL: Annelle Hodges, Ph.D. (PI); Thomas Balkany, M.D. (surgeon); Alina Lopez, M.A., CCC-SLP/A (coordinator); Leslie Goodwin, M.S.N., CCRC (data collection); Stacy Payne, M.A., CCC-A (data collection).
University of Michigan, Ann Arbor, MI: Teresa Zwolan, Ph.D. (PI); Amy Donaldson, M.A., CCC-A (coordinator); H. Alexander Arts, M.D. (surgeon); Brandi Butler, M.A., CCC-A (data collection); Hussam El-Kashlam, M.D. (surgeon); Krista Heavner, M.S., CCC-SLP (data collection); Mary Beth O'Sullivan, M.S., CCC-A (data collection); Steve Telian, M.D. (surgeon); Ellen Thomas, M.A., CCC-SLP (data collection); Anita Vereb, M.S., CCC-A (former coordinator).
University of North Carolina, Carolina Children's Communicative Disorders Program, Chapel Hill, NC: Carolyn J. Brown, M.S. (PI); Holly F.B. Teagle, Au.D., (coordinator); Craig A. Buchman, M.D. (surgeon); Carlton Zdanski, M.D. (surgeon); Hannah Eskridge, M.S.P. (data collection); Harold C. Pillsbury, M.D. (surgeon).
University of Texas at Dallas, Callier Advanced Hearing Research Center, Dallas, TX: Emily A. Tobey, Ph.D., CCC-SLP (PI); Betty Loy, Au.D., CCC-A (coordinator); Paul Bauer, M.D. (surgeon); Angela Boyd, B.A. (data collection); Laura Cantu, B.S. (data collection); Carol Cokely, Ph.D., CCC-A (data collection); Sarah Florence, M.S., CCC-A (data collection); Janee Gisclair, M.S., CCC-A (data collection); Laura Levitan, B.A. (data collection); Joy Penrad (data collection); Shannon Raby, M.A., CCC-SLP (data collection); Jamie Rasmus, B.S. (data collection); Peter Roland, M.D. (surgeon); Heather MacFadyen, M.S., CCC-SLP (data collection); Donise Pearson, M.S., CCC-SLP (data collection); Deborah M. Rekart, Ph.D. (former coordinator); Lauren Sacar, B.A. (data collection); Melissa Sweeney, M.S., CCC-SLP (data collection); Linsey Wagner, B.A. (data collection); Nicole Weissner, B.A. (data collection); Berkley Williams, M.A., CCC-SLP (data collection).
Resource Centers: Data Coordinating Center, Johns Hopkins University, Welch Center for Prevention, Epidemiology and Clinical Research, Baltimore, MD: Nancy E. Fink, M.P.H. (PI); Nae-Yuh Wang, Ph.D. (Co-PI of Data Coordinating Center and Study Statistician); Patricia Bayton (data assembly); Thelma Vilche (data assembly); Daniel Habtemariam (data assembly).
Psychometrics Center, University of Miami, Department of Psychology, Coral Gables, FL: Alexandra Quittner, Ph.D. (PI); David Barker (data analysis); Pam Leibach (data analysis); Ivette Cruz (data analysis).
Study Oversight Committees: Executive Committee: John K. Niparko, M.D. (chair); Laurie S. Eisenberg, Ph.D.; Nancy E. Fink, M.P.H.; Alexandra L. Quittner, Ph.D.; Emily A. Tobey, Ph.D.; Nae-Yuh Wang, Ph.D.
External Advisors: Noel Cohen, M.D.; Julia Evans, Ph.D.; Ann Geers, Ph.D.; Karen Iler Kirk, Ph.D.
References
- 1.Beadle EAR, McKinley DJ, Nikolopoulos TP, Brough J, O'Donoghue GM, Archbold SM. Long-term functional outcomes and academic-occupational status in implanted children after 10 to 14 years of cochlear implant use. Otol Neurotol. 2005;26:1152–60. doi: 10.1097/01.mao.0000180483.16619.8f. [DOI] [PubMed] [Google Scholar]
- 2.Eisenberg LS, Johnson KC, Martinez AS, et al. CDaCI Investigative Team Speech recognition at 1-year follow-up in the childhood development after cochlear implantation (CDaCI) study: methods and preliminary findings. Audiol Neurotol. 2006;11:259–68. doi: 10.1159/000093302. [DOI] [PubMed] [Google Scholar]
- 3.Fink NF, Wang NY, Visaya J, et al. CDaCI Investigative Team Childhood Development after Cochlear Implantation Study (CDaCI): design and baseline characteristics. Cochlear Implants Int. 2007;8:92–116. doi: 10.1179/cim.2007.8.2.92. [DOI] [PubMed] [Google Scholar]
- 4.Zimmerman-Phillips S, Robbins AM, Osberger MJ. Assessing cochlear implant benefit in very young children. Ann Otol Rhinol Laryngol. 2000;109:42–3. doi: 10.1177/0003489400109s1217. [DOI] [PubMed] [Google Scholar]
- 5.Robbins AM, Renshaw JJ, Berry SW. Evaluating meaningful auditory integration in profoundly hearing-impaired children. Am J Otol. 1991;12:144–50. [PubMed] [Google Scholar]
- 6.Moog JS, Geers AE. Early Speech Perception Test for Profoundly Hearing-Impaired Children. St Louis, MO: Central Institute for the Deaf; 1990. [Google Scholar]
- 7.Jerger S, Jerger J. Pediatric Speech Intelligibility Test. St. Louis, MO: Auditec of St. Louis; 1984. [Google Scholar]
- 8.Kirk KI, Pisoni DB, Osberger MJ. Lexical effects of spoken word recognition by pediatric cochlear implant users. Ear Hear. 1995;16:470–81. doi: 10.1097/00003446-199510000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kirk KI, Diefendorf AO, Pisoni DB, Robbins AM. Assessing speech perception in children. In: Mendel LL, Danhauer JL, editors. Audiologic Evaluation and Management and Speech Perception Assessment. San Diego, CA: Singular Publishing Group; 1997. pp. 101–32. [Google Scholar]
- 10.Haskins H. A phonetically balanced test of speech discrimination for children [master's thesis] Evanston, IL: Northwestern University; 1949. [Google Scholar]
- 11.Gelnett D, Sumida A, Nilsson M, Soli SD. Annu Meet Am Acad Audiol, Dallas. 1995. Development of the Hearing in Noise Test for Children (HINT-C) [Google Scholar]


