Abstract
We investigated the effects of age and gender on emotional perception and physiology using electrodermal skin conductance response (SCR) and examined whether SCR related to subjective perceptions of emotional pictures. Older adults found pictures to be more positive and arousing than younger participants. Older women rated pictures more extremely at both ends of the valence continuum: they rated positive pictures more positively and negative pictures more negatively. Elders were less likely to show measurable SCRs. However, magnitude of SCRs when a response occurred did not differ between young and old. Subjective ratings of emotion correlated with physiological responses in younger participants, but they were unrelated in older participants. Thus, in older adults the perception of emotional events was disconnected from the physiological state induced by emotion.
1. Introduction
Emotional experiences change in aging (Lawton et al., 1992; Levenson et al., 1994; Mroczek and Kolarz, 1998). Well-being studies suggest that old age brings greater emotional control, less negative affect, and results in a positive outlook (Lawton et al., 1992; Mroczek and Kolarz, 1998). The mechanisms behind these age-related changes remain to be explained fully, however. Here we tie together the social cognitive and neurobiological perspectives. Our overarching hypothesis is that decreased physiological responsiveness to negative emotions in aging is due in part to loss of amygdala function, which results in relative preservation of response to positive events over negative events.
Life span theories of emotion characterize old age as a time of positive emotional well-being and enhanced emotional control. Older adults experience less negative affect than younger adults and comparable or higher levels of positive affect (Carstensen et al., 2000; Mroczek and Kolarz, 1998). Older adults show a more complex understanding of their emotions, as shown by more vivid descriptions of their emotions in which they integrate their subjective experience within a larger context (Labouvie-Vief et al., 1989). Factor analysis of older adults' emotional reports also show a greater number of dimensions, suggesting that they have a more differentiated emotional experience (Carstensen et al., 2000). Furthermore, older adults report greater “control” over their emotions (Lawton et al., 1992), as defined as deliberate attempts to regulate emotional experiences. This does not seem to be merely a self-report bias, as older adults' daily emotional experiences show that they maintain positive states longer and are more likely to transition out of negative states (Carstensen et al., 2000).
Older adults differ from the young in emotional processing, as well. Older adults attend less to negative faces (Isaacowitz et al., 2006; Mather and Carstensen, 2003) and show greater amygdala activation for positive as compared to negative pictures (Mather et al., 2004). This differential amygdala activity suggests that older adults are less aroused by negative pictures. In fact, younger adults in the study by Mather and colleagues (2004) rated negative pictures as more arousing than did older adults. Thus both behavioral and neuroimaging outcomes suggest a relatively greater arousal to positive emotions among older adults. Emotional memory shows a similar pattern: Older adults remember relatively more positive than negative information, be it words (Thomas and Hasher, 2006), faces (Leigland et al., 2004), or pictures (Charles et al., 2003; Mather and Knight, 2005), as compared to younger people. These age differences are striking because a large literature shows that, among young people, negative information is more salient or stronger than positive information (for reviews see Baumeister et al., 2001; Rozin and Royzman, 2001). Multiple lines of evidence thus support the idea that the negativity bias shown by young adults decreases with age. In older adulthood, people instead show relatively greater focus on positive emotions.
Carstensen's socioemotional selectivity theory (SST) posits that psychological mechanisms account for these age differences in emotional experiences. According to this theory, awareness of endings promotes emotional goals, which in turn accounts for older adults' greater focus on positive affect (Carstensen, 1995; Carstensen et al., 1999). In other words, older adults' decreased levels of negative affect, decreased arousal to negative affect, and decreased attention to negative affect may all reflect a motivational difference between young and old, with the old focusing more strongly on maintaining positive affective states. Age differences in emotional experiences may also reflect age-related changes in physiological systems, however. Physiological studies suggest there is age-related loss of physiological arousal (Smith et al., 2005; Tsai et al., 2000), which could mediate the increase in emotional control.
A number of physiological systems respond to arousing stimuli regardless of the stimulus' valence (Tsai et al., 2000; Witvliet and Vrana, 1995) and older adults have lower responses in these systems (Lau et al., 2001). Older people have a diminished cardiovascular response when discussing emotional conflicts or viewing emotional films (Kunzmann et al., 2005; Levenson et al., 1994; Tsai et al., 2000). Older adults show diminished startle response (N1 and P3 amplitudes in electrophysiological recordings), heart rate deceleration, and corrugators' EMG response (e.g., eyebrow wrinkling) to emotional pictures (Smith et al., 2005), and diminished skin conductance and respiration rates to negative (disgust) films (Kunzmann et al., 2005). Nevertheless, effects are not consistent across all measures. Other studies report no age differences in SCR (Tsai et al., 2000) or higher startle-blink responses among older participants as compared to younger participants (Smith et al., 2005).
Age differences are also found in the amygdala and frontal regions, both of which are implicated in the processing of emotion and memory for emotional material (Gunning-Dixon et al., 2003; Gur et al., 1994). Specifically, both regions respond to emotionally arousing stimuli (Kensinger and Schacter, 2006) and more amygdala activity is associated with better memory for emotionally arousing material (Hamann et al., 1999). Thus, one possibility is that atrophy and other physiological changes of the amygdala and frontal cortex account for age differences in emotional processing and emotional experiences.
Despite evidence of age differences in physiological and brain systems related to emotions, relatively few studies have linked age-related physiological changes to age-related changes in emotional experiences. The aim of our study was to investigate age and gender differences in perceptions and physiological responses to emotional pictures. Our goal was to link subjective and objective measures of emotional experience in order to understand how aging affects emotional experiences and emotional memory. We used measures that have been used in the past to assess the amygdala's role in modulating hippocampal dependent memory in functional imaging studies (Hamann et al., 1999) and studies of patients with amygdala damage (Siebert et al., 2003) in order to relate findings to a potential neural basis. We hypothesized that older adults would have lower physiological responses (SCR) than younger adults. In addition, we examined the relations between skin conductance, conscious arousal ratings, and memory performance. We hypothesized that older participants would show disproportionately higher arousal for positively valenced information as we and others have reported previously, and that this would be associated with a differential SCR to positive stimuli. We did not expect gender differences in emotional perceptions or responses.
2. Method
2.1. Participants
We recruited participants in two age groups: older adults aged 65 - 85 and younger adults aged 24 - 40. Participants were recruited through advertisements and a database of previous study participants. Inclusion criteria required that individuals understand English and have adequate hearing and vision to view computer and paper-pencil tasks (with correction if necessary). Medical histories were obtained via phone interview or in person. Exclusion criteria included smoking or excessive drinking (> 3 alcoholic beverages /day), history of neurological disorders (e.g. stroke, epilepsy, or head trauma), significant medical problems (e.g. uncontrolled hypertension), psychiatric conditions (e.g. history of major depression or schizophrenia), or current use of medications likely to affect cognition such as anti-anxiety agents (e.g. Prozac). Participants were not on any hormone therapies or supplementation (including birth control). The Mini-Mental Status Examination (MMSE; Folstein et al., 1975) and Geriatric Depression Scale (GDS; Yesavage et al., 1983) were used to exclude older participants with possible dementia (MMSE < 26) or depression (GDS > 10). Three volunteers were excluded due to low MMSE scores and two were excluded due to high GDS scores. Two additional participants (one old, one young) were excluded because their performance and behavior during testing suggested they were unable or unwilling to follow task directions.
Older participants (n = 52) had fewer years of education than younger participants (n = 52), but were matched for Wechsler Adult Intelligence Scale-Revised (WAIS-R) Vocabulary subtest scores (Wechsler, 1981). The vocabulary subtest is highly correlated with both verbal and full-scale IQ scores. This subtest provides a standardized approximation of functional intelligence because formal schooling experience often differs between younger and older adults, even if functional intelligence is similar. All participants provided written informed consent and were paid for their involvement in the study.
Participants completed other cognitive tasks not related to the current study during the test sessions.
2.2. Procedures
2.2.1. SCR-picture task
In order to assess age-related changes in physiologic arousal we recorded SCR using the Biopac MP100A system in tandem with the GSR100C transducer module (sensitivity = .7 nanoSiemens). We used a constant voltage method that included a DC excitation voltage of .5V gain of 5microSiemens/Volt, a low pass filter of 1.0 Hz and a high pass filter of 0.05 Hz. Six-mm Ag/AgCl electrodes were placed on the distal fingers of the non-dominant hand with a 5% saline electrode paste.
Previous research shows that 1 out of 4 people do not show measurable SCRs to an orienting stimulus (Venables and Mitchell, 1996). In order to exclude nonresponders from the SCR dataset, participants performed two tasks to measure their physiological responsiveness. Subjects took 2 deep breaths, and then viewed 5 pictures for 2 seconds each. Both of these activities typically yield a measurable SCR (Greenwald et al., 1989; Hay et al., 1997; Lim et al., 2003). A response was determined to be any rise in skin conductance equal to or greater than 0.1 microsiemens (μS). If no response was evident to either the breaths or the pictures, the subject was classified a nonresponder. Sixty-eight participants showed a response, but due to equipment failure we were unable to collect full SCR data on two participants. Only participants who showed a response completed the remainder of the task and only their data is reported (66 participants; nold = 25; nyoung = 41). Older participants were more likely to be classified as non-responders (χ2(1, N = 102) = 9.49, p < .01). There were no gender differences in the frequency of non-responders. Older responders had higher WAIS-R and MMSE scores (p`s < .05) than non-responders.
We recorded SCRs during the last two-minutes of a 10 minute rest period (eyes closed) to establish a baseline skin conductance level. Participants then viewed a set of 70 pictures selected from the IAPS. The pictures viewed during the SCR task were matched for arousal across three valence categories (negative, neutral, and positive), and to the picture set used for the Picture-memory measure (see Table 1) as per the published norms. Participants viewed each picture for 2 seconds, at which time the picture disappeared and the participants rated each picture for valence, arousal, and complexity. Participants had 18 - 19 seconds to rate the picture before the next picture was presented (making the inter-stimulus interval 20 - 21 seconds). This interval was chosen to permit the SCR adequate time to show at least half-recovery to baseline (Dawson et al., 2000).
Table 1.
Normative Valence and Arousal Ratings of Picture Sets
| SCR Valence Norms M (SD) | SCR Arousal Norms M (SD) | Memory Valence Norms M (SD) | Memory Arousal Norms M (SD) | |
|---|---|---|---|---|
| Negative Pictures | 3.48 (.36)* | 5.00 (.96) | 2.73 (.54) | 4.99 (.47) |
| Neutral Pictures | 5.97 (.97)* | 4.53 (.37) | 5.00 (.67) | 4.79 (.93) |
| Positive Pictures | 7.31 (.23)* | 4.97 (1.23) | 7.57 (.39) | 5.07 (.58) |
Note. Negative and neutral pictures in the SCR task had higher normative valence ratings than those used in the Memory task, ts > 6.17, p < .001. Positive pictures in the SCR task had lower normative valence ratings, t (df=47.91) = 2.98, p < .01. Arousal ratings were matched across pictures sets in all valence categories.
All SCR data was square-root transformed prior to data analysis to correct for non-normality of responses. A skin conductance response to a picture was identified as the highest peak that started within the 6-second window following stimulus onset. Peaks were defined as changes in skin conductance amplitude of .01 μS or greater. This criterion permitted us to detect slight changes in skin conductance that may occur to pictures of low arousal. We measured the maximum peak height in response to a picture. If a participant's SCR graph did not show a peak in the 6-second window following picture presentation, we coded it as a non-response (zero). SCR for each valence category is the mean of all SCRs, including the non-responses, to negative, neutral, or positive pictures.
2.2.2. Picture-memory task
Both parts of this task (picture rating and picture memory) took place before the SCR task to insure recognition performance was not confounded by exposure to pictures in the SCR task. Participants viewed 90 pictures selected from the International Affective Picture System (IAPS; Lang et al., 1999). The pictures were divided equally among three a priori valence categories: negative, neutral, and positive (30 pictures in each). These three categories had similar average arousal levels as reported in the IAPS normative data (range 3.7 - 7.0). They also had similar picture content (i.e. there were similar numbers of pictures of people, animals, scenes or food in each category). None of the rated memory pictures overlapped with pictures seen subsequently in the SCR task. There were slight differences in the valence levels of the memory pictures as compared to those used in the SCR task (See Table 1). That is, the memory pictures were more negative by valence norms for both negative and neutral pictures, but more positive for the positive pictures as compared to the SCR task pictures. Participants viewed each picture for 2 seconds, the same as in the SCR task, and rated the picture (valence, arousal, and complexity) to encourage encoding. The intertrial interval varied according to the subjects' response times in making the ratings.
Participants were not told that they would be asked to remember the pictures later. After a one week (old participants) or two week (young participants) retention interval, participants completed a yes/no recognition test comprised of 180 pictures, 90 previously-viewed pictures (targets) interspersed with 90 novel pictures. We used a shorter retention interval for older participants in order to match the overall memory performance between younger and older participants so that age differences in memory for negative versus neutral and positive information was not contaminated simply by poorer memory in the elderly. This permits the interpretation of differential retention for one valence over another in old versus young participants. The 90 novel pictures and targets were matched for valence, arousal, and content of the target pictures as per the IAPS norms. Recognition was assessed as the percentage of pictures identified correctly (both targets and novel pictures) within each a priori valence category.
2.3. Analyses
We analyzed age and gender differences in arousal ratings, valence ratings, and SCR using a multivariate analysis of variance (MANOVA). MANOVA can be used to analyze the dependent variables without the assumptions of sphericity or compound symmetry (Howell, 2002). Average subjective ratings and SCRs to positive, negative, and neutral pictures were within-subject outcome variables and gender and age were between-subject variables. Bonferroni adjustments on post-hoc comparisons served to protect an alpha level of .05.
In order to assess within-person relations between subjective arousal and physiological response, we used a mixed effects models (Laird & Ware, 1982). This approach allows us to examine the association of interest: does an individual's subjective perception of a particular picture relate to the individual's physiological response to that picture? Between-person associations can differ from within-person associations in terms of both magnitude and direction (Tennen et al., 2000). Thus, we chose an analysis that takes into account the multiple within-subject measurements.
The use of mixed effects models presented a challenge not faced with the between-person analyses. Individuals may not show a measurable physiological response to all stimuli. In fact, within many individuals, the distribution of SCR was essentially bimodal, with responses at zero (no response), and then a range of responses of varying magnitudes. We thus analyzed SCRs from those pictures where a person showed a response, so that we model the relation between SCR and subjective arousal rating for pictures to which individuals responded (PROC MIXED procedure in SAS). SCR values, picture ratings, and valence category are within-subjects variables; gender and age are between-subject variables. When there was a significant interaction, models were then fitted stratified by gender or age as appropriate. Tests for interactions and main effects were an F-test in the mixed effects model. When investigating pairwise comparisons, a Bonferonni adjustment was used to control for multiple comparisons.
3. Results
3.1. Sample Descriptives
Within the final sample of responders, men had fewer years of education than women (F(1,62) = 7.66, p < .01) and older people had fewer years of education than younger people (F(1,62) = 4.29, p < .05; See Table 2), but groups were matched for WAIS-R scores. There were no significant age X gender interactions for education or vocabulary scores. Older men were younger (t(23) = 2.13, p < .05) and tended to have lower MMSE scores (t(23) = 1.83, p < .09) than the older women.
Table 2.
Participant Characteristics
| Older Women (n = 13) M (SD) | Older Men (n = 12) M (SD) | Younger Women (n = 20) M (SD) | Younger Men (n = 21) M (SD) | |
|---|---|---|---|---|
| Age | 74.0 (5.7) | 69.8 (4.0)† | 29.0 (3.4) | 31.9 (5.7) |
| Education** | 16.2 (2.3) | 14.3 (2.8) | 16.9 (2.0) | 15.9 (1.6) |
| WAIS-R | 57.3 (5.5) | 57.9 (5.5) | 55.8 (7.5) | 58.2 (5.4) |
| MMSE | 29.1 (.6) | 28.3 (1.3)† | ||
| GDS | 3.2 (2.5) | 3.2 (3.6) |
Note: This table includes the characteristics of those subjects who were “responders” (had SCRs) and participated in the entire study.
Education = years of education; WAIS-R = Wechsler Adult Intelligence Scale-Revised; MMSE = Mini-Mental Status Exam; GDS = Geriatric Depression Scale. Within each row, means with differing superscripts are significantly different from one another.
Older adults had fewer years of education than younger adults (p < .05); women had more education than men (p < .01).
Older men were younger (p < .05) and tended to have lower MMSE scores (p < .09) than the older women.
3.2. SCR-Picture task
3.2.1. Subjective arousal ratings
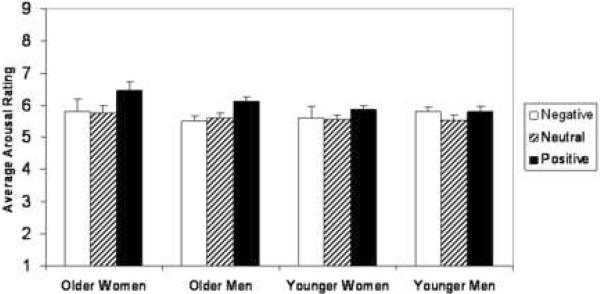
Arousal ratings did not differ between age groups nor were there sex differences (p's > .27). Arousal ratings did differ by valence category (F(2,61) = 26.25, p < .001). This association depended on age (three-way interaction: F(2,61) = 3.15, p < .05). Post-hoc analyses revealed that older participants rated positive pictures as more arousing than negative (t(24) = 2.57; p < .05) or neutral pictures (t(24) = 5.83, p < .05; See Figure 1). Younger participants rated positive pictures as more arousing than neutral pictures only (t(40) = 3.76, p < .05). In addition, older participants rated positive pictures as more arousing than did the young (t(64) = 2.50, p < .05). Old and young did not differ in the ratings of neutral or negative pictures (p's > .40).
Figure 1. Arousal Ratings by Age and Gender.
Older adults rated positive pictures more arousing than both negative and neutral pictures. Younger adults rated positive pictures as more arousing than neutral pictures only. In addition, older adults rated positive pictures as more arousing than did younger adults. Error bars indicate SEM.
3.2.2. Subjective valence ratings
Valence ratings differed by valence category (F(2,61) = 351.76, p < .001). Valence ratings in each of the 3 categories were significantly different from each other, in accordance with the a priori categorization: negative pictures were rated lower than neutral pictures, which in turn were rated lower than positive pictures (p's < .001). Older participants rated pictures more positively than younger participants (F(1,62) = 4.09, p < .05). There was a significant three-way age X gender X valence interaction (F(2,61) = 4.46, p < .05). To examine this 3-way interaction, we analyzed age and gender effects on valence ratings using a 2 × 2 analysis of variance (ANOVA) within each valence category. Age X gender interactions arose for both negative (F(1,62) = 6.76, p < .05) and positive pictures (F(1,62) = 4.51, p < .05), primarily because older women were more extreme in their ratings than other groups: more positive for positive pictures and more negative for negative pictures. For neutral pictures, there was a significant main effect of age only, with older participants rating neutral pictures as more positive than younger participants (F(1,26) = 4.53, p < .05; See Figure 2).
Figure 2. Valence Ratings by Age and Gender.
Older women rated negative pictures more negatively, and positive pictures more positively, than other groups. Error bars indicate SEM.
3.2.4. Skin conductance responses
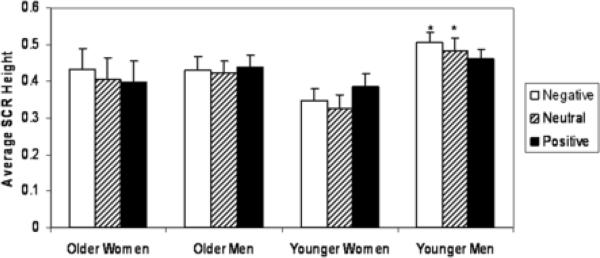
Women tended to have lower SCRs than men (F(1,62) = 3.93, p < .06), although young and old did not differ in their average SCR (F(1,62) = .01, ns). SCR did not differ across valence categories (F(2,61) = 2.50, p < .10), in line with the study design. These main effects were qualified by a significant 3-way interaction between age, gender, and valence category (F(2,61) = 4.47, p < .05). We stratified analyses by age group and found a valence X gender effect in young people only (F(2,78) = 5.82, p < .01). Young men had higher SCRs to negative and neutral pictures compared to young women. Old men and women had similar SCRs within all valence categories.
Participants may vary in their average SCR due to differences in the height of their SCRs or because some people have frequent non-responses, e.g. no change in skin conductance when viewing a picture. We sought to characterize the gender difference in young people more fully in an exploratory analysis that analyzed both maximum SCR height and number of non-responses. Young women's maximum SCR values tended to be lower than young men's for negative pictures only (interaction F(2,37) = 3.02, p < .07; post-hoc p < .05) and they had more frequent non-responses than young men for negative pictures (gender X valence interaction: F(2,38) = 3.35, p < .05; post-hoc p < .05). In other words, young women had lower average SCR to negative pictures both because they tended to have a lower response and because they were less likely than young men to show an arousal response to the pictures.
3.1.5. Relation between subjective and objective arousal responses
We tested whether subjective ratings of arousal related to physiological arousal as indexed by SCR. The mixed model analysis examined the relation between arousal ratings and SCRs to the pictures within each person. The initial model included age, gender, arousal rating, and baseline skin conductance level as predictors of peak SCR. Neither gender (F(1,61) = .53, ns) nor age (F(1,61) = 3.23, p = .08) were significantly related to SCR. The main effect of arousal was significant, such that an increase of 1 point on the rating scale for arousal was associated with a .01 (± .002) increase in SCR height (F(1,2812) = 14.15, p < .001). In addition, there was a significant age X arousal interaction (F(1,2812) = 3.90, p < .05). Subjective arousal ratings were related to SCRs for younger people only (F(1,1735) = 16.45, p < .001). This pattern remained when we added valence category to the model.
3.2. Picture Memory Task1
Despite the attempt to match young and older people's retention by using a longer retention interval for the young, younger people recognized pictures better than older people (F(1,62) = 20.22, p < .001). In addition, memory differed according to the valence of the pictures (F(2,61) = 22.68, p < .001). Participants remembered negative pictures better than neutral (posthoc t (65) = 3.77, p < .01) or positive pictures (posthoc t (65) = 6.77, p < .01). Participants remembered neutral pictures better than positive pictures as well (posthoc t (65) = 3.51, p < .01). Higher SCRs in the SCR task were not correlated to better memory in any of the valence categories (p's> .08).
3.3. Effect of beta-blockers
Seven of the 25 older participants were taking beta-blockers. Beta blockers have been associated with loss of response and memory for negative affective stimuli as well as loss of amygdala activation to negative stimuli (van Stegeren et al., 2005). However, our results are not due to beta blocker effects. Visual inspection of means and analyses revealed that older participants on beta-blockers had similar SCRs, picture ratings, and memory as those not on beta-blockers (p's > .35). In addition, deleting the participants taking beta-blockers did not change the overall pattern of results in most cases, although the loss of sample size caused some statistically significant effects to become borderline or disappear.
4. Discussion
We found that older adults perceived pictures differently than younger adults. Older adults rated positive pictures as more arousing than negative or neutral pictures, and more arousing than younger adults. These findings are consistent with the idea that older adults focus more on positive affect (Carstensen, 1995). In addition, older people rated the valence of pictures more positively than young people. However, older women rated pictures more extreme at both end of the valence continuum: they rated positive pictures more positively and negative pictures more negatively. A few other studies show similar results, with older adults rating positive pictures as more positive and more arousing than younger adults (Mather et al., 2004; Smith et al., 2005). These findings suggest that the IAPS arousal norms based on responses of college students may not provide an appropriate index of “normative” arousal for older people. Thus, converging evidence points to systematic age differences in perception of affective pictures.
Elders also differed from young with regard to the SCR responses. Older adults were less likely to show measurable SCRs (e.g., non-responders) as compared to the young. Few studies that measure SCR among older people report rates of non-responding so it is difficult to compare these results to other samples. Studies of much younger subjects report non-response rates to orienting stimuli to be around 25% (Venables and Mitchell, 1996). A similar rate was reported in a sample of older adults (Denburg et al., 2003). The higher rate of non-responders among older adults in our study may have occurred by chance or may be due to a more stringent criterion for an SCR in the screening phase of the current study (.10 versus .05 in Venables & Mitchell, 1996). If the higher rate of nonresponders is due to our criteria for a response, it would suggest that older adults show smaller responses than do younger adults. Age-related physiological changes unrelated to emotion, such as changes in skin or hypothyroidism, may decrease skin conductance (Dolu et al., 1999). Inclusion of non-responders may obscure age differences in physiologic arousal. Thus, it is critical to include information on how non-responders are identified in order make comparisons across studies.
Non-responders differed from responders on a few characteristics. In the elderly, responders had higher vocabulary and MMSE scores, suggesting that at least for some elderly, cognitive functional loss is associated with lack of arousal-induced physiologic responses. Thus, the reported SCR findings in the elderly may not be generalizable to all elderly.
Although our primary focus was on age differences in emotional experiences, we did find some gender differences. In particular, our older women evidenced more extreme subjective valence ratings than the other groups, rating negative pictures as more unpleasant and positive pictures as more pleasant. Other researchers also report this pattern for women's ratings (Greenwald et al., 1989), although it was true only for older women in the SCR portion of the current study. Few studies of age differences in emotional experiences report testing for gender effects, so it is unclear whether gender differences are generally not found or rather that gender was not included in analytic models.
We found also that younger women had lower SCR as compared to younger men for both negative and neutral pictures. Findings about gender differences in emotional responses are inconsistent across studies and response systems. Some studies report greater SCR in men as compared to women when viewing positive pictures (Bradley et al., 2001) or fearful films (Kring and Gordon, 1998). Other studies report that women are more physiologically responsive to negative stimuli (Bradley et al., 2001), although others find no gender differences in SCR to negative stimuli (Kring and Gordon, 1998). In light of these inconsistent patterns, we hesitate to over-interpret the gender difference found here.
Our analytic method looked at the relation between people's arousal rating and their SCR for each picture. One of the most interesting findings is that older adults' reports of arousal did not match their physiological response, whereas arousal ratings were related to physiological responses in the young. Previous research showing a positive correlation between subjective and physiological arousal responses relied largely on college-aged samples (Greenwald et al., 1989; Lang et al., 1993). Other studies have noted minimal coherence between conscious and physiological emotional systems (Bonanno and Keltner, 2004; Mauss et al., 2005), but have not investigated the effect of age. The current study adds a unique piece to this body of knowledge by showing that coherence between conscious awareness of arousal and physiological arousal is lower in older people.
Interestingly, amygdala damage also impairs the relationship between SCR and arousal ratings: Arousal ratings correlate positively with SCR in healthy participants, but not in patients with amygdala damage (Glascher and Adolphs, 2003; Lang et al., 1993). Thus, one intriguing possibility is that age-related changes in amygdala function causes the disconnection between conscious awareness and objective physiological arousal in older adults. The amygdala shows atrophy or degeneration even in healthy older adults (Mu et al., 1999), with a predicted 8-12% loss of amygdala volume between the ages of 30 and 80 years (Allen et al., 2005; but see Grieve et al., 2005 for opposite findings). Such changes may account for the disconnection between subjective and physiological responses observed in the elderly.
Other age-related changes may also affect emotional experiences. For example, hormone levels decrease with age; dramatically for women at menopause, but a smaller, gradual decline occurs also for men. In a recent study, emotional responses and memory were compared between older women on and off hormone therapy and younger women. Estrogen enhanced arousal to negative material. Older women on estrogen therapy rated negative pictures as more arousing than positive pictures and gave negative pictures higher arousal ratings than older women not on hormone therapy or younger women (Pruis et al. in press). Higher arousal did not lead to better memory, however. Estrogen receptors are located in the amygdala (Osterlund et al., 2000) and this study suggests that some age-related changes in emotional responses may be modified by the presence of estrogen. Future studies may examine whether the presence of estrogen maintains the correlation between subjective perceptions of arousal and physiological indices of arousal. We note that none of the participants in the current study were on hormonal treatments.
In conclusion, emotional perceptions differed in the old and the young. SCR was related to objective arousal ratings in the young but not the old, suggesting a disconnection between older participants' conscious awareness and physiologic arousal. SST is often evoked to explain age differences in emotional experiences (e.g., Charles et al., 2003): Older adults are better able to regulate their emotional reactions, focus more on positive emotions, and therefore are less affected by negative stimuli. What might be construed as a bias for negative information in the young essentially goes away with age. However, this explanation does not account for the disconnection between older adults' cognitive and physiological responses to pictures. Similarly, age-related declines in neuroticism may decrease reactivity to emotionally arousing stimuli, but it is unclear why personality changes should lead to a disconnection between subjective and physiological responses to pictures. The disconnection occurred for both positive and negative emotions and thus cannot be ascribed to lack of attention to or reduced sensitivity to negative emotions in particular. It is, however, consistent with impairment in amygdala function or possibly other structures (e.g., prefrontal cortex) in the emotion circuit (Nagai et al., 2004; Williams et al., 2006; Williams et al., 2001).
Older adults may rely on different processes and thus different areas of the brain to process emotion, such as the prefrontal cortex. It may be that analysis of emotional stimuli by the prefrontal cortex is less reliant on autonomic arousal mechanisms (Gunning-Dixon et al., 2003). One recent study suggests that in older adults the regulation (inhibition) of negative affect does not induce frontal activity to the degree found in younger subjects (Urry et al., 2006). The current study points to an intriguing age difference in emotional experience. Future studies using multiple measures of physiological response such as functional neuroimaging in the elderly will shed light on this issue.
Figure 3. SCR by Age and Gender.
Young men had higher SCRs to negative and neutral pictures as compared to young women. Error bars indicate SEM of group averaged responses.
Acknowledgements
This work was supported by: NIH Grant R01- AG12611 (JS Janowsky) and M01 RR000334. The authors would like to thank Karla Schilling for her assistance with data collection.
Footnotes
Disclosure Statement: The authors have no conflicts of interest to report.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
To avoid repetition, we do not report the full valence and arousal ratings analyses here as they replicate the findings reported under the SCR pictures. We do note, however, one slight difference: all women (not just older women) rated valence more extremely than men, providing lower ratings for negative pictures and higher ratings for positive pictures. Nonetheless, older women rated negative pictures lower than all other groups.
Reference List
- Allen JS, Bruss J, Brown CK, Damasio H. Normal neuroanatomical variation due to age: the major lobes and a parcellation of the temporal region. Neurobiol. Aging. 2005;26(9):1245–1260. doi: 10.1016/j.neurobiolaging.2005.05.023. [DOI] [PubMed] [Google Scholar]
- Baumeister RF, Bratslavsky E, Finkenauer C, Vohs KD. Bad is stronger than good. Review of General Psychology. 2001;5:323–370. [Google Scholar]
- Bonanno GA, Keltner D. The coherence of emotion systems: Comparing “online” measures of appraisal and facial expressions, and self-report. Cogn. Emot. 2004;18:431–444. [Google Scholar]
- Bradley MM, Codispoti M, Sabatinelli D, Lang PJ. Emotion and motivation II: sex differences in picture processing. Emotion. 2001;1(3):300–319. [PubMed] [Google Scholar]
- Carstensen LL. Evidence for a life-span theory of socioemotional selectivity. Curr. Dir. Psychol. Sci. 1995;4:151–155. doi: 10.1177/09637214211011468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carstensen LL, Isaacowitz DM, Charles ST. Taking time seriously. A theory of socioemotional selectivity. Am. Psychol. 1999;54(3):165–181. doi: 10.1037//0003-066x.54.3.165. [DOI] [PubMed] [Google Scholar]
- Carstensen LL, Pasupathi M, Mayr U, Nesselroade JR. Emotional experience in everyday life across the adult life span. J. Pers. Soc. Psychol. 2000;79(4):644–655. [PubMed] [Google Scholar]
- Charles ST, Mather M, Carstensen LL. Aging and emotional memory: the forgettable nature of negative images for older adults. J. Exp. Psychol. Gen. 2003;132(2):310–324. doi: 10.1037/0096-3445.132.2.310. [DOI] [PubMed] [Google Scholar]
- Dawson ME, Schell AM, Filion DL. The electrodermal system. In: Cacioppo JT, Tassinary LG, Berntson GG, editors. Handbook of psychophysiology. 2 ed. Cambridge University Press; New York: 2000. pp. 200–223. [Google Scholar]
- Denburg NL, Buchanan TW, Tranel D, Adolphs R. Evidence for preserved emotional memory in normal older persons. Emotion. 2003;3(3):239–253. doi: 10.1037/1528-3542.3.3.239. [DOI] [PubMed] [Google Scholar]
- Dolu N, Suer C, Ozesmi C, Kelestimur F, Ozcan Y. Electrodermal activity in hypothyroid patients and healthy subjects. Thyroid. 1999;9(8):787–790. doi: 10.1089/thy.1999.9.787. [DOI] [PubMed] [Google Scholar]
- Folstein M, Folstein S, McHugh P. Mini-mental state: A practical method of grading the cognitive status of patients for the clinician. J. Psychiatr. Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Glascher J, Adolphs R. Processing of the arousal of subliminal and supraliminal emotional stimuli by the human amygdala. J. Neurosci. 2003;23(32):10274–10282. doi: 10.1523/JNEUROSCI.23-32-10274.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwald MK, Cook EWI, Lang PJ. Affective judgment and psychophysiological response: Dimensional covariation in the evaluation of pictorial stimuli. J. Psychophysiol. 1989;3:51–64. [Google Scholar]
- Grieve SM, Clark CR, Williams LM, Peduto AJ, Gordon E. Preservation of limbic and paralimbic structures in aging. Hum. Brain Mapp. 2005;25(4):391–401. doi: 10.1002/hbm.20115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunning-Dixon FM, Gur RC, Perkins AC, Schroeder L, Turner T, Turetsky BI, Chan RM, Loughead JW, Alsop DC, Maldjian J, Gur RE. Age-related differences in brain activation during emotional face processing. Neurobiol. Aging. 2003;24(2):285–295. doi: 10.1016/s0197-4580(02)00099-4. [DOI] [PubMed] [Google Scholar]
- Gur RC, Skolnick BE, Gur RE. Effects of emotional discrimination tasks on cerebral blood flow: regional activation and its relation to performance. Brain Cogn. 1994;25(2):271–286. doi: 10.1006/brcg.1994.1036. [DOI] [PubMed] [Google Scholar]
- Hamann SB, Ely TD, Grafton ST, Kilts CD. Amygdala activity related to enhanced memory for pleasant and aversive stimuli. Nat. Neurosci. 1999;2(3):289–293. doi: 10.1038/6404. [DOI] [PubMed] [Google Scholar]
- Hay JE, Taylor PK, Nukada H. Auditory and inspiratory gasp-evoked sympathetic skin response: age effects. J. Neurol. Sci. 1997;148(1):19–23. doi: 10.1016/s0022-510x(96)05221-5. [DOI] [PubMed] [Google Scholar]
- Howell D. Statistical methods for psychology. 5th ed. Duxbury; Pacific Grove, CA: 2002. [Google Scholar]
- Isaacowitz DM, Wadlinger HA, Goren D, Wilson HR. Is there an age-related positivity effect in visual attention? A comparison of two methodologies. Emotion. 2006;6(3):511–516. doi: 10.1037/1528-3542.6.3.511. [DOI] [PubMed] [Google Scholar]
- Kensinger EA, Schacter DL. Processing emotional pictures and words: effects of valence and arousal. Cogn Affect. Behav. Neurosci. 2006;6(2):110–126. doi: 10.3758/cabn.6.2.110. [DOI] [PubMed] [Google Scholar]
- Kring AM, Gordon AH. Sex differences in emotion: expression, experience, and physiology. J. Pers. Soc. Psychol. 1998;74(3):686–703. doi: 10.1037//0022-3514.74.3.686. [DOI] [PubMed] [Google Scholar]
- Kunzmann U, Kupperbusch CS, Levenson RW. Behavioral inhibition and amplification during emotional arousal: a comparison of two age groups. Psychol. Aging. 2005;20(1):144–158. doi: 10.1037/0882-7974.20.1.144. [DOI] [PubMed] [Google Scholar]
- Labouvie-Vief G, Hakim-Larson J, Bulka D. Speaking about feelings: Conceptions of emotions across the life span. Psychol. Aging. 1989;4:425–437. doi: 10.1037//0882-7974.4.4.425. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Greenwald MK, Bradley MM, Hamm AO. Looking at pictures: affective, facial, visceral, and behavioral reactions. Psychophysiology. 1993;30(3):261–273. doi: 10.1111/j.1469-8986.1993.tb03352.x. [DOI] [PubMed] [Google Scholar]
- Lang P, Bradley M, Cuthbert B. The International Affective Picture System (IAPS): Instruction manual and affective ratings. 1999. University of Florida, The Center for Research in Psychophysiology; 1999. [Google Scholar]
- Lau AW, Edelstein BA, Larkin KT. Psychophysiological arousal in older adults: a critical review. Clin. Psychol. Rev. 2001;21(4):609–630. doi: 10.1016/s0272-7358(00)00052-0. [DOI] [PubMed] [Google Scholar]
- Lawton MP, Kleban MH, Rajagopal D, Dean J. Dimensions of affective experience in three age groups. Psychol. Aging. 1992;7(2):171–184. doi: 10.1037//0882-7974.7.2.171. [DOI] [PubMed] [Google Scholar]
- Leigland LA, Schulz LE, Janowsky JS. Age related changes in emotional memory. Neurobiol. Aging. 2004;25(8):1117–1124. doi: 10.1016/j.neurobiolaging.2003.10.015. [DOI] [PubMed] [Google Scholar]
- Levenson RW, Carstensen LL, Gottman JM. The influence of age and gender on affect, physiology, and their interrelations: a study of long-term marriages. J. Pers. Soc. Psychol. 1994;67(1):56–68. doi: 10.1037//0022-3514.67.1.56. [DOI] [PubMed] [Google Scholar]
- Lim CL, Seto-Poon M, Clouston PD, Morris JG. Sudomotor nerve conduction velocity and central processing time of the skin conductance response. Clin. Neurophysiol. 2003;114(11):2172–2180. doi: 10.1016/s1388-2457(03)00204-9. [DOI] [PubMed] [Google Scholar]
- Mather M, Canli T, English T, Whitfield S, Wais P, Ochsner K, Gabrieli JD, Carstensen LL. Amygdala responses to emotionally valenced stimuli in older and younger adults. Psychol. Sci. 2004;15(4):259–263. doi: 10.1111/j.0956-7976.2004.00662.x. [DOI] [PubMed] [Google Scholar]
- Mather M, Carstensen LL. Aging and attentional biases for emotional faces. Psychol. Sci. 2003;14(5):409–415. doi: 10.1111/1467-9280.01455. [DOI] [PubMed] [Google Scholar]
- Mather M, Knight M. Goal-directed memory: the role of cognitive control in older adults' emotional memory. Psychol. Aging. 2005;20(4):554–570. doi: 10.1037/0882-7974.20.4.554. [DOI] [PubMed] [Google Scholar]
- Mauss IB, Levenson RW, McCarter L, Wilhelm FH, Gross JJ. The tie that binds? Coherence among emotion experience, behavior, and physiology. Emotion. 2005;5(2):175–190. doi: 10.1037/1528-3542.5.2.175. [DOI] [PubMed] [Google Scholar]
- Mroczek DK, Kolarz CM. The effect of age on positive and negative affect: a developmental perspective on happiness. J. Pers. Soc. Psychol. 1998;75(5):1333–1349. doi: 10.1037//0022-3514.75.5.1333. [DOI] [PubMed] [Google Scholar]
- Mu Q, Xie J, Wen Z, Weng Y, Shuyun Z. A quantitative MR study of the hippocampal formation, the amygdala, and the temporal horn of the lateral ventricle in healthy subjects 40 to 90 years of age. AJNR Am. J. Neuroradiol. 1999;20(2):207–211. [PMC free article] [PubMed] [Google Scholar]
- Nagai Y, Critchley HD, Featherstone E, Trimble MR, Dolan RJ. Activity in ventromedial prefrontal cortex covaries with sympathetic skin conductance level: a physiological account of a “default mode” of brain function. Neuroimage. 2004;22(1):243–251. doi: 10.1016/j.neuroimage.2004.01.019. [DOI] [PubMed] [Google Scholar]
- Osterlund MK, Keller E, Hurd YL. The human forebrain has discrete estrogen receptor alpha messenger RNA expression: high levels in the amygdaloid complex. Neuroscience. 2000;95(2):333–342. doi: 10.1016/s0306-4522(99)00443-1. [DOI] [PubMed] [Google Scholar]
- Pruis TA, Neiss MB, Leigland LA, Janowsky JS. Estrogen modifies arousal and memory for emotional events in older women. Neurobiol. Aging. doi: 10.1016/j.neurobiolaging.2007.11.009. (in press pending minor revisions) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozin P, Royzman EB. Negativity bias, negativity dominance, and contagion. Pers. Soc. Psychol. Rev. 2001;5:296–320. [Google Scholar]
- Siebert M, Markowitsch HJ, Bartel P. Amygdala, affect and cognition: evidence from 10 patients with Urbach-Wiethe disease. Brain. 2003;126(Pt 12):2627–2637. doi: 10.1093/brain/awg271. [DOI] [PubMed] [Google Scholar]
- Smith DP, Hillman CH, Duley AR. Influences of age on emotional reactivity during picture processing. J. Gerontol. B Psychol. Sci. Soc. Sci. 2005;60(1):P49–56. doi: 10.1093/geronb/60.1.p49. [DOI] [PubMed] [Google Scholar]
- Tennen H, Affleck G, Armeli S, Carney MA. A daily process approach to coping. Linking theory, research, and practice. Am. Psychol. 2000;55(6):626–636. doi: 10.1037//0003-066x.55.6.626. [DOI] [PubMed] [Google Scholar]
- Thomas RC, Hasher L. The influence of emotional valence on age differences in early processing and memory. Psychol. Aging. 2006;21(4):821–825. doi: 10.1037/0882-7974.21.4.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai JL, Levenson RW, Carstensen LL. Autonomic, subjective, and expressive responses to emotional films in older and younger Chinese Americans and European Americans. Psychol. Aging. 2000;15(4):684–693. doi: 10.1037//0882-7974.15.4.684. [DOI] [PubMed] [Google Scholar]
- Urry HL, van Reekum CM, Johnstone T, Kalin NH, Thurow ME, Schaefer HS, Jackson CA, Frye CJ, Greischar LL, Alexander AL, Davidson RJ. Amygdala and ventromedial prefrontal cortex are inversely coupled during regulation of negative affect and predict the diurnal pattern of cortisol secretion among older adults. J. Neurosci. 2006;26(16):4415–4425. doi: 10.1523/JNEUROSCI.3215-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venables PH, Mitchell DA. The effects of age, sex and time of testing on skin conductance activity. Biol. Psychol. 1996;43(2):87–101. doi: 10.1016/0301-0511(96)05183-6. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale - Revised. Psychological Corporation; New York: 1981. [Google Scholar]
- Williams LM, Brown KJ, Palmer D, Liddell BJ, Kemp AH, Olivieri G, Peduto A, Gordon E. The mellow years?: neural basis of improving emotional stability over age. J. Neurosci. 2006;26(24):6422–6430. doi: 10.1523/JNEUROSCI.0022-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams LM, Phillips ML, Brammer MJ, Skerrett D, Lagopoulos J, Rennie C, Bahramali H, Olivieri G, David AS, Peduto A, Gordon E. Arousal dissociates amygdala and hippocampal fear responses: evidence from simultaneous fMRI and skin conductance recording. Neuroimage. 2001;14(5):1070–1079. doi: 10.1006/nimg.2001.0904. [DOI] [PubMed] [Google Scholar]
- Witvliet CV, Vrana SR. Psychophysiological responses as indices of affective dimensions. Psychophysiology. 1995;32(5):436–443. doi: 10.1111/j.1469-8986.1995.tb02094.x. [DOI] [PubMed] [Google Scholar]
- Yesavage J, Brink T, Rose T. Development and validation of a geriatric depression screening scale: A preliminary report. J. Psychiatr. Res. 1983;17:37–49. doi: 10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]