Abstract
Following HIV diagnosis, linkage to outpatient treatment, antiretroviral initiation, and longitudinal retention in care represent the foundation for successful treatment. While prior studies have evaluated these processes in isolation, a systematic evaluation of successive steps in the same cohort of patients has not yet been performed. To ensure optimal long-term outcomes, a better understanding of the interplay of these processes is needed. Therefore, a retrospective cohort study of patients initiating outpatient care at the University of Alabama at Birmingham 1917 HIV/AIDS Clinic between January 2000 and December 2005 was undertaken. Multivariable models determined factors associated with: late diagnosis/linkage to care (initial CD4 < 350 cells/mm3), timely antiretroviral initiation, and retention across the first two years of care. Delayed linkage was observed in two-thirds of the overall sample (n = 567) and was associated with older age (odds ratio [OR] = 1.31 per 10 years; 95% confidence interval [CI] = 1.06–1.62) and African American race (OR = 2.45; 95% CI = 1.60–3.74). Attending all clinic visits (hazard ratio [HR] = 6.45; 95% CI = 4.47–9.31) and lower initial CD4 counts led to earlier antiretroviral initiation. Worse retention in the first 2 years was associated with younger age (OR = 0.68 per 10 years; 95% CI = 0.56–0.83), higher baseline CD4 count, and substance abuse (OR = 1.78; 95% CI = 1.16–2.73). Interventions to improve timely HIV diagnosis and linkage to care should focus on older patients and African Americans while efforts to improve retention should address younger patients, those with higher baseline CD4 counts, and substance abuse. Missed clinic visits represent an important obstacle to the timely initiation of antiretroviral therapy. These data inform development of interventions to improve linkage and retention in HIV care, an emerging area of growing importance.
Introduction
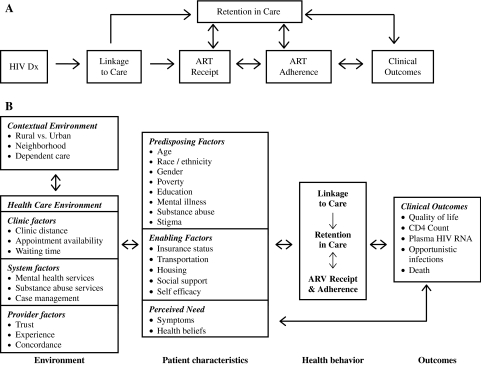
Combination antiretroviral therapy (ART) has led to dramatic improvements in HIV-related morbidity and mortality in the age of modern HIV care. Successful establishment of HIV treatment necessitates that HIV diagnosis is followed by timely linkage to outpatient care, prompt initiation of ART and prophylactic medications when indicated, and subsequent adherence to prescribed medications.1–5 For the benefits of treatment to be fully realized, patients must progress through this sequence of steps while remaining engaged in uninterrupted HIV clinical care (Fig. 1A).
FIG. 1.
A: Blueprint for HIV treatment success: requisite processes from initial diagnosis to long-term clinical outcomes. (adapted from Giordano et al.1 and Samet et al.3). B: Adaptation of the behavioral model of health services utilization to provide a conceptual framework to evaluate the relationships between patient characteristics and their contextual and health care environmental factors in contributing to health behaviors outlined in the “blueprint for HIV treatment success” that ultimately influence clinical outcomes.
Previous studies have evaluated these processes individually, in isolation from neighboring steps.4,6 For example, delays in establishing HIV care after diagnosis and factors related to presentation for care with advanced infection have been described.4,7–9 In addition, Giordano and colleagues6 found that nearly half of patients attending a clinic intake visit were not subsequently seen in follow-up, therefore failing to fully establish outpatient treatment after initial linkage. While prior studies have contributed important insights, the evaluation of multiple successive steps among the same cohort of patients establishing initial outpatient treatment has not yet been performed.
To ensure optimal outcomes for HIV-infected individuals, a better understanding of the multiple successive steps in the blueprint for HIV treatment success is needed. Evaluation of patient and contextual factors posing difficulties with each of these steps may allow for targeted interventions focusing on individuals who are particularly susceptible. We suggest this blueprint may serve as a framework to study how environmental and patient factors influence the processes of linkage and retention in care, and ART receipt and adherence to ultimately influence clinical outcomes (Fig. 1B).10 Here, we present findings from a study conducted in an effort to provide insight into each process in the proposed blueprint with the intention of identifying patient populations for informed interventions aimed at optimizing the longitudinal care process and ultimately, clinical outcomes.
Methods
Sample and procedure
The University of Alabama at Birmingham (UAB) 1917 HIV/AIDS Clinic has provided comprehensive HIV services for over 6000 HIV-infected individuals since 1988. Currently, the clinic provides primary HIV care for over 1500 active patients, who participate in the UAB 1917 HIV/AIDS Clinic Cohort Observational Database Project (UAB 1917 Clinic Cohort). A retrospective review of medical records of patients establishing primary HIV care at the UAB 1917 HIV/AIDS Clinic between January 1, 2000 and December 31, 2005 was undertaken. The current study includes patients whose initial visit to the 1917 Clinic represented their initial outpatient HIV appointment. Patients who had previously received outpatient HIV treatment elsewhere were excluded from this study. These criteria were applied to focus on a cohort of patients establishing initial linkage to outpatient HIV treatment. Study information was retrieved via a combination of queries of the UAB 1917 Clinic Cohort Database and abstraction of patient medical records by trained abstractors. The UAB 1917 Clinic Cohort Database is a 100% quality controlled observational cohort study that has been described in detail elsewhere, and was recently recognized for excellence in information integrity.11–13 Briefly, detailed sociodemographic, psychosocial, clinical, and pharmacy information across a wide range of domains is captured from patients receiving care at the clinic, and recorded in the database. The UAB Institutional Review Board reviewed and approved this study protocol.
Statistical analysis
Descriptive statistics were computed for all study variables to ensure assumptions of statistical tests to be employed were met. All statistical analyses were performed using SAS Software, version 9.1.3 (SAS Institute, Cary, NC), and are described in detail below for each dependent variable analyzed in this study.
Independent variables
Variables were selected a priori based upon review of the literature and a behavioral model of health services utilization and included patient sociodemographic information (age, gender, race, HIV risk factor and health insurance), medical history (year of first arrived visit, history of affective mental health disorder, substance abuse, alcohol abuse and antiretroviral medication initiation), laboratory [baseline CD4 and plasma HIV viral load (VL)] and clinic utilization measures (attendance to scheduled primary HIV provider appointments).5–7,10,12,14–17
Dependent variables
Late presentation for HIV care
Defined as an initial CD4 count below 350 cells/mm3, this threshold was selected as it represents the CD4 count below which initiation of antiretroviral therapy is recommended.18,19 Since much debate has focused on the optimal time to start ART, particularly as it relates to patients with CD4 counts above 350 cells/mm3, we sought to evaluate the proportion of new patients for whom this discussion was germane. Univariate and multivariable logistic regression models were used to identify factors associated with late presentation for outpatient HIV care (CD4 count < 350 cells/mm3).
Time to start ART
Evaluation of the time to antiretroviral therapy initiation was undertaken for all study patients. Individuals who initiated therapy prior to their first clinic visit (e.g., during an inpatient hospitalization) were excluded. Survival methods were used to evaluate factors related to initiation of ART in the first year of care in the overall population and then stratified by initial CD4 count (≥350 cells/mm3, < 350 cells/mm3). The stratified analysis was performed to separately evaluate patients with and without an indication for ART on initial presentation for care according to antiretroviral treatment recommendations. Univariate and multivariable Cox proportional hazards (PH) models were applied to evaluate factors associated with faster initiation of ART following an initial attended visit while adjusting for covariates. Of particular interest was the role of missed visits as it relates to delayed ART initiation. For this analysis, only missed visits occurring before ART initiation were incorporated in the appointment attendance measure. Patients were censored either at the time of loss to follow-up (due to death or failure to return to subsequent clinic visits) or at 1 year after their initial attended visit.
Early retention in outpatient HIV care
Retention in care was measured as the number of 6-month blocks during which at least one clinic visit was attended over the 2-year period following an initial attended visit (range, 1–4). This measure, that we refer to as persistence in care, has been widely used to evaluate retention in HIV treatment.5,6,14,15,17,20 Patients who died within 2 years of their first attended visit were excluded from this measure because they did not have complete exposure history to generate data for all four 6-month blocks. Univariate and multivariable ordinal logistic regression analysis (proportional odds assumption test) were used to identify factors related to early retention in care. For analytic purposes, worse retention in care was modeled as the outcome measure.
Results
Between January 1, 2000 and December 31, 2005, 567 patients established initial outpatient HIV care at the UAB 1917 HIV/AIDS Clinic and are included in this study. Among the study sample, 197 patients entered care in 2000–2001 (35%), 174 in 2002–2003 (31%), and 196 in 2004–05 (35%; Table 1). The mean age (±standard deviation [SD]) was 38 ± 9 years, and the majority of patients were African American (55%), men who have sex with men (MSM; 51%), and male (75%). Nearly half of the sample (49%) had private health insurance while over one third were uninsured (36%). Alcohol abuse and illicit drug abuse was recorded in 19% and 26% of patients, respectively. The baseline CD4 count was < 350 cells/mm3 in 63% of patients (n = 354), and the median CD4 count was 239 ± 284 cells/mm3. The baseline plasma HIV viral load (VL) was < 100,000 copies/mL in 70% of patients (n = 390; Table 1).
Table 1.
Baseline Characteristics of Five Hundred Sixty-Seven Patients Establishing Initial Outpatient HIV Care at the UAB 1917 HIV/AIDS Clinic; January 1, 2000–December 31, 2005
| Characteristic | Mean ± standard deviation or n (%) |
|---|---|
| Year of entry into care | |
| 2000–2001 | 197 (34.7) |
| 2002–2003 | 174 (30.7) |
| 2004–2005 | 196 (34.6) |
| Age (range, 19–70 years) | 37.8 ± 9.4 |
| Gender | |
| Male | 427 (75.3) |
| Female | 140 (24.7) |
| Race | |
| White | 257 (45.3) |
| African American | 310 (54.7) |
| HIV risk factor | |
| MSM | 281 (50.5) |
| Heterosexual | 228 (40.9) |
| IDU or MSM/IDU | 48 ( 8.6) |
| Health insurance | |
| Private | 278 (49.0) |
| Public | 86 (15.2) |
| Uninsured | 203 (35.8) |
| Affective mental health disorder | |
| No | 301 (53.1) |
| Yes | 266 (46.9) |
| Substance abuse | |
| No | 422 (74.4) |
| Yes | 145 (25.6) |
| Alcohol abuse | |
| No | 457 (80.6) |
| Yes | 110 (19.4) |
| Baseline CD4 count | |
| <200 cells/mm3 | 256 (45.6) |
| 200–350 cells/mm3 | 98 (17.5) |
| ≥350 cells/mm3 | 207 (36.9) |
| Baseline viral load (log10) | 4.4 ± 1.1 |
| Antiretroviral therapy started in first year | |
| No | 185 (32.6) |
| Yes | 382 (67.4) |
| Baseline viral load | |
| <100,000 | 390 (69.6) |
| ≥100,000 | 170 (30.4) |
| Retention (persistence) in the first 2 yearsa | |
| 1 | 84 (15.9) |
| 2 | 64 (12.1) |
| 3 | 70 (13.2) |
| 4 | 312 (58.9) |
Retention in care was measured as the number of 6-month intervals during which at least one clinic visit was attended over the two year period following an initial outpatient HIV primary care visit (range 1–4).
MSM, Men who have sex with men; IDU, injection drug use.
Late presentation for HIV care (baseline CD4 < 350 cells/mm3)
Among the study sample, 561 patients (99%) had baseline CD4 values available for analysis. Sixty-three percent of these patients (n = 354) had an immunologic indication for ART at their first visit based upon an initial CD4 count below 350 cells/mm3. In multivariable analysis, late presentation for care (baseline CD4 < 350 cells/mm3) was associated with older age (OR = 1.31 per 10 years; 95% CI = 1.06–1.62), African American race (OR = 2.45; 95% CI = 1.60–3.74), and baseline VL > 100,000 (OR = 5.81; 95% CI = 3.54–9.56; Table 2).
Table 2.
Characteristics Associated with Late Presentation for Outpatient HIV Care Among Five Hundred Sixty-One patients Establishing Initial Treatment at the UAB 1917 HIV/AIDS Clinic; January 1, 2000–December 31, 2005
| Characteristic (n = 561) | CD4 < 350 (n = 354) | CD4 ≥ 350 (n = 207) | Crude OR (95% CI) CD4 < 350 | Adjusted OR (95% CI) CD4 < 350a |
|---|---|---|---|---|
| Year of entry into care | ||||
| 2000–2001 | 121 (34.2) | 73 (35.3) | 1.0 | |
| 2002–2003 | 112 (31.6) | 59 (28.5) | 1.15 (0.75–1.76) | 1.21 (0.75–1.94) |
| 2004–2005 | 121 (34.2) | 75 (36.2) | 0.97 (0.65–1.47) | 0.86 (0.54–1.35) |
| Age (years)b | 38.6 ± 9.5 | 36.3 ± 9.2 | 1.29 (1.07–1.56)c | 1.31 (1.06–1.62)d |
| Gender | ||||
| Male | 268 (75.7) | 154 (74.4) | 1.0 | 1.0 |
| Female | 86 (24.3) | 53 (25.6) | 0.93 (0.63–1.39) | 0.63 (0.36–1.09) |
| Race | ||||
| White | 140 (39.6) | 113 (54.6) | 1.0 | 1.0 |
| African American | 214 (60.4) | 94 (45.4) | 1.84 (1.30–2.60)c | 2.45 (1.60–3.74)c |
| HIV risk factor | ||||
| MSM | 174 (49.9) | 106 (52.0) | 1.0 | 1.0 |
| Heterosexual | 145 (41.6) | 80 (39.2) | 1.10 (0.77–1.59) | 1.03 (0.61–1.73) |
| IVDU or MSM/IVDU | 30 ( 8.6) | 18 (8.8) | 1.02 (0.54–1.91) | 1.00 (0.48–2.07) |
| Health insurance | ||||
| Private | 170 (48.0) | 104 (50.2) | 1.0 | 1.0 |
| Public | 61 (17.2) | 24 (11.6) | 1.56 (0.91–2.65) | 1.05 (0.57–1.93) |
| Uninsured | 123 (34.8) | 79 (38.2) | 0.95 (0.66–1.38) | 0.82 (0.54–1.24) |
| Baseline Viral Load | ||||
| < 100,000 | 207 (59.1) | 181 (87.9) | 1.0 | 1.0 |
| ≥ 100,000 | 143 (40.9) | 25 (12.1) | 5.00 (3.13–8.00)c | 5.81 (3.54–9.56)d |
Multivariable logistic regression model characteristics; Hosmer-Lemeshow goodness of fit statistic P = 0.724, C-statistic = 0.738.
Odds ratio in 10-year increment.
p < 0.01; dp < 0.05, statistically significant variables presented in boldface.
Time to start ART
Among the study sample, 536 patients (95%) who had not started antiretroviral therapy prior to their first outpatient clinic visit were included in this analysis. Among this sample, 349 (66%) started antiretroviral therapy in the first year of care. In Cox PH analysis of the overall sample, lower baseline CD4 values (CD4 200–350 HR = 5.43; 95% CI = 3.75–7.87; and CD4 < 200 HR = 7.82; 95% CI = 5.51–11.1) as well as attendance to all scheduled primary HIV care clinic visits (HR = 6.45; 95%CI = 4.47–9.31) were associated with faster initiation of ART (Table 3). In Cox PH models stratified by ART indication based on baseline CD4 counts (<350 versus ≥ 350 cells/mm3),18,19 only plasma HIV VL > 100,000 copies per milliliter (HR = 1.33; 95% CI = 1.04–1.69 if CD4 < 350 cells/mm3 and HR = 2.29; 95% CI = 1.12–4.66 if CD4 ≥ 350 cells/mm3) and perfect clinic attendance (HR = 6.66; 95% CI = 4.27–10.41 if CD4 < 350 cells/mm3 and HR = 5.48; 95% CI = 2.76–10.88 if CD4 ≥ 350 cells/mm3) were associated with faster initiation of antiretroviral therapy in both groups (Table 3).
Table 3.
Factors Associated with Faster ART Initiation During the First Year of Care Among Five Hundred Thirty-Six Patientsa at the UAB 1917 HIV/AIDS Clinic Overall and Stratified by Baseline CD4 Value
| |
Overall (n = 536) |
Stratified by CD4 value |
||
|---|---|---|---|---|
| Characteristic | Crude HR (95% CI) | Adjusted HR (95% CI) | CD4 < 350 Adjusted HR (95% CI) | CD4 ≥ 350 Adjusted HR (95% CI) |
| Age (per 10 years) | 1.17 (1.04–1.31)b | 1.00 (0.88–1.13) | 0.99 (0.86–1.14) | 0.99 (0.73–1.33) |
| Gender | ||||
| Male | 1.0 | 1.0 | 1.0 | 1.0 |
| Female | 0.96 (0.75–1.23) | 0.90 (0.65–1.23) | 0.86 (0.6–1.22) | 1.76 (0.74–4.16) |
| Race | ||||
| White | 1.0 | 1.0 | 1.0 | 1.0 |
| African American | 0.99 (0.80–1.22) | 0.95 (0.74–1.24) | 1.00 (0.75–1.34) | 0.60 (0.3–1.2) |
| HIV risk factor | ||||
| MSM | 1.0 | 1.0 | 1.0 | 1.0 |
| Heterosexual | 1.08 (0.87–1.35) | 1.26 (0.94–1.69) | 1.22 (0.89–1.68) | 1.57 (0.66–3.75) |
| IDU or MSM/IDU | 0.89 (0.60–1.32) | 1.15 (0.72–1.85) | 1.18 (0.69–2.01) | 1.44 (0.48–4.35) |
| Health insurance | ||||
| Private | 1.0 | 1.0 | 1.0 | 1.0 |
| Public | 0.97 (0.71–1.33) | 1.14 (0.82–1.61) | 1.13 (0.78–1.63) | 1.81 (0.76–4.3) |
| Uninsured | 0.82 (0.65–1.04) | 0.88 (0.69–1.14) | 0.89 (0.68–1.17) | 0.84 (0.44–1.59) |
| History of affective mental health disorder | ||||
| No | 1.0 | 1.0 | 1.0 | 1.0 |
| Yes | 1.00 (0.81–1.24) | 1.09 (0.87–1.37) | 1.13 (0.88–1.45) | 0.87 (0.49–1.55) |
| History of substance abuse | ||||
| No | 1.0 | 1.0 | 1.0 | 1.0 |
| Yes | 0.78 (0.61–1.00)c | 0.95 (0.69–1.31) | 0.84 (0.58–1.2) | 1.03 (0.48–2.22) |
| History of alcohol abuse | ||||
| No | 1.0 | 1.0 | 1.0 | 1.0 |
| Yes | 0.69 (0.52–0.92)c | 0.93 (0.68–1.26) | 1.03 (0.73–1.44) | 0.64 (0.31–1.34) |
| Baseline CD4 count (cells/mm3) | ||||
| <200 | 9.29 (6.81–12.66)b | 7.82 (5.51–11.1)b | — | — |
| 200–350 | 5.99 (4.20–8.55)b | 5.43 (3.75–7.87)b | ||
| ≥350 | 1.0 | 1.0 | ||
| Baseline viral load | ||||
| <100,000 | 1.0 | 1.0 | 1.0 | 1.0 |
| ≥100,000 | 2.37 (1.90–2.95)b | 1.24 (0.97–1.58) | 1.33 (1.04–1.69)c | 2.29 (1.12–4.66)c |
| Attended all scheduled visits | ||||
| No | 1.0 | 1.0 | 1.0 | 1.0 |
| Yes | 7.32 (5.22–10.26)b | 6.45 (4.47–9.31)b | 6.66 (4.27–10.41)b | 5.48 (2.76–10.88)b |
Patients who initiated ART prior to their first outpatient visit at the clinic were excluded from analyses (n = 31).
p < 0.01; statistically significant variables presented in boldface.
p < 0.05.
ART, antiretroviral therapy; HR, hazard ratio; CI, confidence interval; MSM, men who have sex with men; IDU, injection drug use.
Early retention in outpatient HIV care
Thirty-seven of the initial 567 patients (7%) in the study sample died within 2 years of their initial attended clinic visit and were therefore excluded from this analysis. Among the remaining 530 patients, 84 (16%) attended a clinic visit in only the first 6-month interval during the 2 years following the index visit, 64 (12%) of patients in 2 of 4 intervals, 70 (13%) in 3 of 4, and 312 (59%) in all four 6-month intervals (Table 4). In multivariable ordinal logistic regression analysis, early retention in care was worse in those with higher baseline CD4 counts (OR 2.65 if CD4 200–349 cells/mm3; 95% CI = 1.6–4.38 and OR = 2.48 if CD4 ≥ 350 cells/mm3; 95% CI = 1.6–3.86), and those with a history of substance abuse (OR = 1.67; 95% CI = 1.02–2.71). Older patients had better retention (OR = 0.70 per 10 years; 95% CI = 0.57–0.86), as did patients with a history of an affective mental health disorder (OR, 0.45; 95% CI = 0.31–0.67). No other factors were associated with early retention in care during the first 2 years after establishment of outpatient HIV treatment (Table 4).
Table 4.
Characteristics Associated with Worse Retention in HIV Care During the Two Years After an Initial Outpatient Visit Among Five Hundred Thirty Patients Establishing Treatment at the UAB 1917 HIV/AIDS Clinic; January 1, 2000–December 31, 2005
| |
Retention (persistence) in HIV carea |
|
|
|||
|---|---|---|---|---|---|---|
| Characteristic | 1 (n = 84) | 2 (n = 64) | 3 (n = 70) | 4 (n = 312) | Crude OR (95% CI) | Adjusted OR (95% CI)b |
| Year of entry into care | ||||||
| 2000–2001 | 23 (27.4) | 24 (37.5) | 20 (28.6) | 115 (36.9) | 1.0 | 1.0 |
| 2002–2003 | 29 (34.5) | 20 (31.2) | 24 (34.3) | 93 (29.8) | 1.33 (0.88–2.01) | 1.26 (0.81–1.98) |
| 2004–2005 | 32 (38.1) | 20 (31.2) | 26 (37.1) | 104 (33.3) | 1.28 (0.86–1.92) | 1.30 (0.84–2.02) |
| Age (years)c | 36.4 ± 9.2 | 34.6 ± 6.7 | 35.6 ± 8.5 | 38.9 ± 9.8 | 0.70 (0.58–0.84)d | 0.7 (0.57–0.86)d |
| Gender | ||||||
| Male | 61 (72.6) | 48 (75.0) | 46 (65.7) | 246 (78.9) | 1.0 | 1.0 |
| Female | 23 (27.4) | 16 (25.0) | 24 (34.3) | 66 (21.1) | 1.38 (0.94–2.02) | 1.45 (0.86–2.46) |
| Race | ||||||
| White | 31 (36.9) | 26 (40.6) | 28 (40.0) | 159 (51.0) | 1.0 | 1.0 |
| African American | 53 (63.1) | 38 (59.4) | 42 (60.0) | 153 (49.0) | 1.60 (1.14–2.24)d | 1.23 (0.81–1.86) |
| HIV risk factor | ||||||
| MSM | 41 (49.4) | 32 (50.8) | 27 (39.1) | 173 (55.6) | 1.0 | 1.0 |
| Heterosexual | 32 (38.6) | 27 (42.9) | 38 (55.1) | 113 (36.3) | 1.32 (0.93–1.88) | 1.01 (0.61–1.66) |
| IDU or MSM/IDU | 10 (12.0) | 4 (6.4) | 4 (5.8) | 25 (8.1) | 1.35 (0.73–2.51) | 0.99 (0.47–2.08) |
| Health insurance | ||||||
| Private | 39 (46.4) | 28 (43.7) | 29 (41.4) | 173 (55.5) | 1.0 | 1.0 |
| Public | 14 (16.7) | 11 (17.2) | 10 (14.3) | 40 (12.8) | 1.54 (0.94–2.52) | 1.59 (0.91–2.80) |
| Uninsured | 31 (36.9) | 25 (39.1) | 31 (44.3) | 99 (31.7) | 1.46 (1.01–2.10)e | 1.28 (0.85–1.91) |
| Affective mental health disorder | ||||||
| No | 60 (71.4) | 36 (56.3) | 37 (52.9) | 139 (44.6) | 1.0 | 1.0 |
| Yes | 24 (28.6) | 28 (43.7) | 33 (47.1) | 173 (55.4) | 0.48 (0.34–0.68)d | 0.45 (0.31–0.67)d |
| Substance abuse | ||||||
| No | 56 (66.7) | 44 (68.8) | 48 (68.6) | 244 (78.2) | 1.0 | 1.0 |
| Yes | 28 (33.3) | 20 (31.2) | 22 (31.4) | 68 (21.8) | 1.63 (1.13–2.37)d | 1.67 (1.02–2.71)e |
| Alcohol abuse | ||||||
| No | 69 (82.1) | 54 (84.4) | 54 (77.1) | 250 (80.1) | 1.0 | 1.0 |
| Yes | 15 (17.9) | 10 (15.6) | 16 (22.9) | 62 (19.9) | 0.90 (0.59–1.38) | 0.8 (0.49–1.29) |
| Baseline CD4 count | ||||||
| < 200 cells/mm3 | 18 (22.2) | 21 (33.3) | 25 (35.7) | 164 (52.6) | 1.0 | 1.0 |
| 200–349 cells/mm3 | 21 (25.9) | 13 (20.6) | 13 (18.6) | 47 (15.0) | 2.70 (1.68–4.33)d | 2.65 (1.6–4.38)d |
| ≥ 350 cells/mm3 | 42 (51.9) | 29 (46.0) | 32 (45.7) | 101 (32.4) | 2.63 (1.79–3.87)d | 2.48 (1.6–3.86)d |
| Baseline viral load (log10) | 4.1 ± 1.1 | 4.3 ± 1.0 | 4.3 ± 1.1 | 4.5 ± 1.1 | 0.83 (0.72–0.97)e | 0.91 (0.77–1.08) |
Retention in care was measured as “persistence,” or the number of 6-month blocks during which at least one clinic visit was attended over the 2-year period following an initial outpatient HIV primary care visit (range, 1–4).
Multivariable ordinal logistic regression model characteristics: C-statistic = 0.695.
Odds ratio in 10-year increment.
p < 0.01; statistically significant variables presented in boldface.
p < 0.05.
OR, odds ratio; CI, confidence interval; MSM, men who have sex with men; IDU, injection drug use.
Discussion
This study is the first to examine and characterize multiple successive steps in the establishment of treatment among a cohort of patients initiating outpatient HIV care. We found that older individuals and African Americans were more likely to have delayed linkage to care. After attending a first clinic appointment, only subsequent missed visits and higher baseline CD4 count, not sociodemographic factors, delayed the initiation of ART thereby placing patients at risk for poor clinical outcomes. While older patients were more likely to present for initial outpatient care with advanced infection (CD4 count < 350 cells/mm3), retention in care during the first two years was better in this group. In addition to younger patients, those with higher baseline CD4 counts and substance abuse disorders had worse early retention in outpatient treatment after initial linkage to care. In aggregate, these findings provide important insights into the early processes of establishing HIV care and highlight risk factors that threaten to break the continuum. Accordingly, this study identifies priority populations for targeted interventions designed to optimize patient assimilation into the care process and ultimately improve treatment outcomes. Such insight will likely prove important to the efforts of our health system to effectively engage the expected influx of newly diagnosed patients with HIV anticipated in response to the revised CDC HIV testing recommendations.21,22 Case management and outreach interventions hold promise,23–25 and our findings suggest such initiatives may require specific attention to identified subgroups at increased risk of poor linkage and retention in care.
In the blueprint for HIV treatment success (Fig. 1A), diagnosis represents only the first step and must be followed by timely linkage to care. Studies have shown that delayed establishment of care following HIV diagnosis is common and is associated with worse long-term outcomes.4,26 In a previous study, our group further identified risk factors for failure to establish care among new patients to our clinic; the “no-show phenomenon” was more common in females, African Americans, and those without private health insurance.12 In the present study, late diagnosis and linkage to care was defined as initiation of outpatient HIV treatment with a baseline CD4 count below 350 cells/mm3. Older patients and those with high baseline plasma HIV viral load (>100,000 copies per milliliter) values experienced late diagnosis and linkage to care. African Americans were also more likely to establish care with advanced HIV infection, which is possibly related to disparities in access to healthcare or distrust and stigma observed in this population in the United States.27–29 Our results point to a need for particular emphasis on older patients and African Americans when designing interventions to promote timely HIV diagnosis and linkage to outpatient care. This is highly relevant as health care systems implement revised CDC HIV testing recommendations that advocate routine, opt-out HIV testing in all health care settings. Older patients and African Americans may particularly benefit if HIV testing programs focus on these susceptible priority populations who currently experience greater difficulties with timely HIV diagnosis and linkage to care.
Following linkage to care, initiation of ART is the next milestone in the blueprint for HIV treatment success. It is pertinent to point out that while current guidelines advocate initiating antiretroviral therapy when CD4 counts fall below 350 cells/mm3, discussion on the optimal time to start ART continues, with many advocating higher CD4 thresholds (<500 cells/mm3) than recommended by current guidelines.19,30–35 Pragmatically, this distinction matters little in our clinic where nearly two thirds of patients enter care with CD4 counts less than 350 cells/mm3 and 79% (n = 441) enter care below the proposed 500 cells/mm3 threshold.36 While research and debate on the optimal timing of ART initiation are important, interventions designed to diagnose and link patients to care earlier are needed before the benefits of such changes can be broadly realized.
Patients starting ART at lower CD4 counts have suboptimal improvements of CD4 counts and higher rates of clinical progression and mortality relative to those starting therapy at higher CD4 levels.30,34,37 As expected, the strongest predictor for more expeditious ART initiation was baseline CD4 count. Compared to patients with baseline CD4 counts higher than 350 cells/mm3, those with lower baseline values had significantly higher likelihood of antiretroviral initiation. In concordance to observations in the overall sample, Cox PH analyses stratifying patients by baseline CD4 count (≥350 cells/mm3 and < 350 cells/mm3) revealed perfect clinic visit attendance was conducive to timely ART initiation. Patients who missed visits after establishing care had significantly longer delays in ART initiation, even when treatment was indicated based on baseline CD4 counts. Hence, engaging patients in the care process and avoiding missed visits following initial linkage is paramount to avoiding therapeutic delays and allowing for optimal short- and long-term clinical outcomes.
Following linkage to outpatient treatment, poor retention in care presents a major obstacle to optimal HIV treatment and favorable outcomes. A recent study found nearly half of patients attending an initial intake clinic visit failed to return for a provider visit and successfully establish outpatient care.6 In the current study, retention in care was analyzed as the number of 6-month blocks during which at least one clinic visit was attended over the 2-year period following an initial visit. Worse early retention in outpatient care was associated with younger age, a history of substance abuse and a higher baseline CD4 count. Because patients with higher baseline CD4 counts are typically asymptomatic and often not receiving antiretroviral therapy, they may have less motivation to remain in care compared to those with symptomatic disease and those receiving antiretroviral treatment. We also posit the improved dosing schedules and tolerability of contemporary antiretroviral medications may facilitate better retention in care among those initiating treatment in more recent years.38,39 Collectively, these findings suggest particular efforts to educate patients on the importance of retention in care will need to focus on those with less advanced immunologic disease when establishing care, perhaps with added emphasis for those not receiving antiretroviral therapy. Also, while efforts to improve timely diagnosis and initial linkage to care may need to focus on older patients, interventions to maintain retention in care should pay particular attention to younger patients.
While patients with substance abuse disorders had worse early retention, those with a diagnosis of an affective mental health disorder were found to have better retention in care. Of note, other studies utilizing provider diagnosis of mental health disorders have similarly found improved outcomes among HIV-infected patients with diagnosed mental health disorders.40,41 One potential explanation may relate to high rates of undiagnosed and untreated mental health disorders among those without an identified condition contributing to inferior outcomes in this group, which has been cited as a putative rationale in these earlier studies and may apply to the current study as well.
Our findings should be interpreted with respect to the limitations of our study. As a retrospective study from a single HIV cohort, our findings may not be generalizable to other national or international settings, though our analysis may provide insights applicable to such settings. As with all observational studies we are able to identify associations but cannot attribute causality. Patients with poor retention in care may have transferred care elsewhere without notifying the clinic and have been improperly categorized to a worse retention category. Furthermore, one visit per 6-month interval may represent too conservative an estimate of retention in care, although this measure has been widely used in prior studies.14,15,17,20 Last, this study focused on patient level factors and did not evaluate how environmental factors influence the processes outlined in the blueprint (Fig. 1B). We believe this study is an important step and intend to carry out future studies evaluating the role environmental factors, and suggest others continue to engage in such research to advance the field.
In summary, our findings suggest that efforts to improve timely HIV diagnosis and linkage to care should focus on older patients and African Americans, while emphasizing appointment adherence is vital for the timely initiation of ART. Younger patients, those with substance abuse disorders and patients with higher baseline CD4 counts may require specific attention to ensure retention in outpatient treatment following linkage to care. Interventions designed to address each element of the blueprint of treatment success should be informed by empiric data to ensure that appropriate emphasis and targeted interventions are deployed among patient groups experiencing the greatest challenges. The expected influx of newly diagnosed patients who need to navigate the successive steps in the establishment of HIV care highlights the importance of our findings and urgent need to develop evidence-based interventions for widespread dissemination to ensure optimal clinical outcomes.
Acknowledgments
Data presented in part at 3rd International Conference on HIV Treatment Adherence, Jersey City, New Jersey, from March 17–18, 2008.
The UAB 1917 HIV/AIDS Clinic Cohort Observational Database project receives financial support from the following: UAB Center for AIDS Research (grant P30-AI27767), CFAR-Network of Integrated Clinical Systems, CNICS (grant 1 R24 AI067039-1), and the Mary Fisher CARE Fund. This study was supported by Grant Number K23MH082641 from the National Institute of Mental Health (MJM), the Ruth L. Kirschstein National Research Service Award (JHW). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Mental Health, the National Institutes of Health, or any other agency providing support for this study.
Author Disclosure Statement
J.H.W. has received research funding from the Bristol-Myers Squibb Virology Fellows Research Program for the 2006–2008 Academic Years. M.J.M. has received recent research funding from Tibotec and Bristol-Myers Squibb. M.S.S. has received recent research funding or consulted for: Adrea Pharmaceuticals, Avexa, Boehringer Ingelheim, Bristol-Myers Squibb, Gilead, GlaxoSmithKline, Merck, Monogram Biosciences, Panacos, Pfizer, Progenics, Roche, Serono, Tanox, Tibotec, Trimeris, and Vertex.
References
- 1.Giordano TP. Suarez-Almazor ME. Grimes RM. The population effectiveness of highly active antiretroviral therapy: Are good drugs good enough? Curr HIV/AIDS Rep. 2005;2:177–183. doi: 10.1007/s11904-005-0013-7. [DOI] [PubMed] [Google Scholar]
- 2.Cheever LW. Engaging HIV-infected patients in care: Their lives depend on it. Clin Infect Dis. 2007;44:1500–1502. doi: 10.1086/517534. [DOI] [PubMed] [Google Scholar]
- 3.Janssen RS. Holtgrave DR. Valdiserri RO. Shepherd M. Gayle HD. De Cock KM. The serostatus approach to fighting the HIV epidemic: Prevention strategies for infected individuals. Am J Public Health. 2001;91:1019–1024. doi: 10.2105/ajph.91.7.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Samet JH. Freedberg KA. Savetsky JB. Sullivan LM. Stein MD. Understanding delay to medical care for HIV infection: The long-term non-presenter. AIDS. 2001;15:77–85. doi: 10.1097/00002030-200101050-00012. [DOI] [PubMed] [Google Scholar]
- 5.Giordano TP. Gifford AL. White AC, Jr., et al. Retention in care: A challenge to survival with HIV infection. Clin Infect Dis. 2007;44:1493–1499. doi: 10.1086/516778. [DOI] [PubMed] [Google Scholar]
- 6.Giordano TP. Visnegarwala F. White AC, Jr., et al. Patients referred to an urban HIV clinic frequently fail to establish care: Factors predicting failure. AIDS Care. 2005;17:773–783. doi: 10.1080/09540120412331336652. [DOI] [PubMed] [Google Scholar]
- 7.Mugavero MJ. Castellano C. Edelman D. Hicks C. Late diagnosis of HIV infection: the role of age and sex. Am J Med. 2007;120:370–373. doi: 10.1016/j.amjmed.2006.05.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Keruly JC. Moore RD. Immune status at presentation to care did not improve among antiretroviral-naive persons from 1990 to 2006. Clin Infect Dis. 2007;45:1369–1374. doi: 10.1086/522759. [DOI] [PubMed] [Google Scholar]
- 9.Dybul M. Bolan R. Condoluci D, et al. Evaluation of initial CD4+T cell counts in individuals with newly diagnosed human immunodeficiency virus infection, by sex and race, in urban settings. J Infect Dis. 2002;185:1818–1821. doi: 10.1086/340650. [DOI] [PubMed] [Google Scholar]
- 10.Andersen RM. Revisiting the behavioral model and access to medical care: Does it matter? J Health Soc Behav. 1995;36:1–10. [PubMed] [Google Scholar]
- 11.Willig JH. Westfall AO. Allison J, et al. Nucleoside reverse-transcriptase inhibitor dosing errors in an outpatient HIV clinic in the electronic medical record era. Clin Infect Dis. 2007;45:658–661. doi: 10.1086/520653. [DOI] [PubMed] [Google Scholar]
- 12.Mugavero MJ. Lin HY. Allison JJ, et al. Failure to establish HIV care: characterizing the "no show" phenomenon. Clin Infect Dis. 2007;45:127–130. doi: 10.1086/518587. [DOI] [PubMed] [Google Scholar]
- 13.Chen RY. Accortt NA. Westfall AO, et al. Distribution of health care expenditures for HIV-infected patients. Clin Infect Dis. 2006;42:1003–1010. doi: 10.1086/500453. [DOI] [PubMed] [Google Scholar]
- 14.Ashman JJ. Conviser R. Pounds MB. Associations between HIV-positive individuals' receipt of ancillary services and medical care receipt and retention. AIDS Care. 2002;14(Suppl 1):S109–118. doi: 10.1080/09540120220149993a. [DOI] [PubMed] [Google Scholar]
- 15.Conviser R. Pounds MB. Background for the studies on ancillary services and primary care use. AIDS Care. 2002;14(Suppl 1):S7–14. doi: 10.1080/09540120220149993. [DOI] [PubMed] [Google Scholar]
- 16.Giordano TP. White AC., Jr. Sajja P, et al. Factors associated with the use of highly active antiretroviral therapy in patients newly entering care in an urban clinic. J Acquir Immune Defic Syndr. 2003;32:399–405. doi: 10.1097/00126334-200304010-00009. [DOI] [PubMed] [Google Scholar]
- 17.Lo W. MacGovern T. Bradford J. Association of ancillary services with primary care utilization and retention for patients with HIV/AIDS. AIDS Care. 2002;14(Suppl 1):S45–57. doi: 10.1080/0954012022014992049984. [DOI] [PubMed] [Google Scholar]
- 18.Panel on Antiretroviral Guidelines for Adult and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1–infected adults and adolescents. Department of Health and Human Services. Jan 29, 2008. www.aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf. [Feb 8;2008 ]. pp. 1–128.www.aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf
- 19.Hammer SM. Saag MS. Schechter M, et al. Treatment for adult HIV infection: 2006 recommendations of the International AIDS Society-USA panel. JAMA. 2006;296:827–843. doi: 10.1001/jama.296.7.827. [DOI] [PubMed] [Google Scholar]
- 20.Sherer R. Stieglitz K. Narra J, et al. HIV multidisciplinary teams work: support services improve access to and retention in HIV primary care. AIDS Care. 2002;14(Suppl 1):S31–44. doi: 10.1080/09540120220149975. [DOI] [PubMed] [Google Scholar]
- 21.Branson BM. Handsfield HH. Lampe MA, et al. Revised recommendations for HIV testing of adults, adolescents, and pregnant women in health-care settings. MMWR Recomm Rep. 2006;55:1–17. ; quiz CE11–14. [PubMed] [Google Scholar]
- 22.Saag MS. Which policy to ADAP-T: Waiting lists or waiting lines? Clin Infect Dis. 2006;43:1365–1367. doi: 10.1086/508664. [DOI] [PubMed] [Google Scholar]
- 23.Cabral HJ. Tobias C. Rajabiun S, et al. Outreach program contacts: do they increase the likelihood of engagement and retention in HIV primary care for hard-to-reach patients? AIDS Patient Care STDs. 2007;21(Suppl 1):S59–67. doi: 10.1089/apc.2007.9986. [DOI] [PubMed] [Google Scholar]
- 24.Gardner LI. Metsch LR. Anderson-Mahoney P, et al. Efficacy of a brief case management intervention to link recently diagnosed HIV-infected persons to care. AIDS. 2005;19:423–431. doi: 10.1097/01.aids.0000161772.51900.eb. [DOI] [PubMed] [Google Scholar]
- 25.Rajabiun S. Mallinson RK. McCoy K, et al. “Getting me back on track”: the role of outreach interventions in engaging and retaining people living with HIV/AIDS in medical care. AIDS Patient Care STDs. 2007;21(Suppl 1):S20–29. doi: 10.1089/apc.2007.9990. [DOI] [PubMed] [Google Scholar]
- 26.Samet JH. Freedberg KA. Stein MD, et al. Trillion virion delay: Time from testing positive for HIV to presentation for primary care. Arch Intern Med. 1998;158:734–740. doi: 10.1001/archinte.158.7.734. [DOI] [PubMed] [Google Scholar]
- 27.Whetten K. Leserman J. Whetten R, et al. Exploring lack of trust in care providers and the government as a barrier to health service use. Am J Public Health. 2006;96:716–721. doi: 10.2105/AJPH.2005.063255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reif S. Geonnotti KL. Whetten K. HIV Infection and AIDS in the Deep South. Am J Public Health. 2006;96:970–973. doi: 10.2105/AJPH.2005.063149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pence BW. Reif S. Whetten K, et al. Minorities, the poor, and survivors of abuse: HIV-infected patients in the US deep South. South Med J. 2007;100:1114–1122. doi: 10.1097/01.smj.0000286756.54607.9f. [DOI] [PubMed] [Google Scholar]
- 30.Egger M. May M. Chene G, et al. Prognosis of HIV-1-infected patients starting highly active antiretroviral therapy: A collaborative analysis of prospective studies. Lancet. 2002 Jul 13;360:119–129. doi: 10.1016/s0140-6736(02)09411-4. [DOI] [PubMed] [Google Scholar]
- 31.Holmberg SD. Palella FJ., Jr. Lichtenstein KA. Havlir DV. The case for earlier treatment of HIV infection. Clin Infect Dis. 2004;39:1699–1704. doi: 10.1086/425743. [DOI] [PubMed] [Google Scholar]
- 32.Phillips AN. Gazzard BG. Clumeck N. Losso MH. Lundgren JD. When should antiretroviral therapy for HIV be started? BMJ. 2007;334:76–78. doi: 10.1136/bmj.39064.406389.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Phillips AN. Lepri AC. Lampe F. Johnson M. Sabin CA. When should antiretroviral therapy be started for HIV infection? Interpreting the evidence from observational studies. AIDS. 2003;17:1863–1869. doi: 10.1097/00002030-200309050-00004. [DOI] [PubMed] [Google Scholar]
- 34.Phillips AN. Gazzard B. Gilson R, et al. Rate of AIDS diseases or death in HIV-infected antiretroviral therapy-naive individuals with high CD4 cell count. AIDS. 2007;21:1717–1721. doi: 10.1097/QAD.0b013e32827038bf. [DOI] [PubMed] [Google Scholar]
- 35.Sax PE. Updated DHHS treatment guidelines. AIDS Clin Care. 2006;18:105. [PubMed] [Google Scholar]
- 36.Braithwaite RS. Roberts MS. Chang CC, et al. Influence of alternative thresholds for initiating HIV treatment on quality-adjusted life expectancy: A decision model. Ann Intern Med. 2008;148:178–185. doi: 10.7326/0003-4819-148-3-200802050-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moore RD. Keruly JC. CD4+ cell count 6 years after commencement of highly active antiretroviral therapy in persons with sustained virologic suppression. Clin Infect Dis. 2007;44:441–446. doi: 10.1086/510746. [DOI] [PubMed] [Google Scholar]
- 38.Moyle G. Gatell J. Perno CF. Ratanasuwan W. Schechter M. Tsoukas C. Potential for New antiretrovirals to address unmet needs in the management of HIV-1 infection. AIDS Patient Care STDs. 2008;22:459–471. doi: 10.1089/apc.2007.0136. [DOI] [PubMed] [Google Scholar]
- 39.Willig JH. Abroms S. Westfall AO, et al. Increased regimen durability in the era of once daily fixed-dose combination antiretroviral therapy. AIDS. 2008;22:1951–1960. doi: 10.1097/QAD.0b013e32830efd79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Himelhoch S. Moore RD. Treisman G. Gebo KA. Does the presence of a current psychiatric disorder in AIDS patients affect the initiation of antiretroviral treatment and duration of therapy? J Acquir Immune Defic Syndr. 2004;37:1457–1463. doi: 10.1097/01.qai.0000136739.01219.6d. [DOI] [PubMed] [Google Scholar]
- 41.Pence BW. Ostermann J. Kumar V. Whetten K. Thielman N. Mugavero MJ. The influence of psychosocial characteristics and race/ethnicity on the use, duration, and success of antiretroviral therapy. J Acquir Immune Defic Syndr. 2008;47:194–201. doi: 10.1097/QAI.0b013e31815ace7e. [DOI] [PubMed] [Google Scholar]