Abstract
Similar to infants born with persistent pulmonary hypertension of the newborn (PPHN), there is an increase in circulating endothelin-1 (ET-1) and decreased cGMP-mediated vasodilation in an ovine model of PPHN. These abnormalities lead to vasoconstriction and vascular remodeling. Our previous studies have demonstrated that reactive oxygen species (ROS) levels are increased in pulmonary arterial smooth muscle cells (PASMC) exposed to ET-1. Thus the initial objective of this study was to determine whether the development of pulmonary hypertension in utero is associated with elevated production of the ROS hydrogen peroxide (H2O2) and if this is associated with alterations in antioxidant capacity. Second we wished to determine whether chronic exposure of PASMC isolated from fetal lambs to H2O2 would mimic the decrease in soluble guanylate cyclase expression observed in the ovine model of PPHN. Our results indicate that H2O2 levels are significantly elevated in pulmonary arteries isolated from 136-day-old fetal PPHN lambs (P 0.05). In addition, we determined that catalase and glutathione peroxidase expression and activities remain unchanged. Also, we found that the overnight exposure of fetal PASMC to a H2O2-generating system resulted in significant decreases in soluble guanylate cyclase expression and nitric oxide (NO)-dependent cGMP generation (P 0.05). Finally, we demonstrated that the addition of the ROS scavenger catalase to isolated pulmonary arteries normalized the vasodilator responses to exogenous NO. As these scavengers had no effect on the vasodilator responses in pulmonary arteries isolated from age-matched control lambs this enhancement appears to be unique to PPHN. Overall our data suggest a role for H2O2 in the abnormal vasodilation associated with the pulmonary arteries of PPHN lambs.
Keywords: reactive oxygen species; vasodilation; guanosine 3′,5′-cyclic monophosphate
Normal pulmonary vascular tone is regulated by a complex interaction of vasoactive substances produced by the vascular endothelium (6–8, 31). These include nitric oxide (NO) and endothelin-1 (ET-1). NO is an endothelium-derived relaxing factor synthesized by the oxidation of L-arginine after activation of endothelial NO synthase (eNOS) (19). ET-1, a 21-amino acid polypeptide produced by vascular endothelial cells (EC), has potent vasoactive properties and is mitogenic for pulmonary vascular smooth muscle cells (SMC) (29). The complex pulmonary vasoactive effects of ET-1 are mediated by at least two receptors: ETA receptors, located on vascular SMC, mediate vasoconstriction, whereas ETB receptors, located on vascular EC, mediate vasodilation (2, 21, 22). Data indicate that NO and ET-1 regulate each other through an autocrine feedback loop (16).
Abnormal regulation of endothelial function is implicated in the pathophysiology of a number of pulmonary hypertensive disorders. In persistent pulmonary hypertension of the newborn (PPHN), pulmonary vascular resistance does not decrease normally at birth, resulting in pulmonary hypertension, right-to-left shunting and hypoxemia (23). Newborns who die of PPHN exhibit an increase both in the thickness of the smooth muscle layer within small pulmonary arteries and an extension of this muscle to nonmuscular arteries (9). Often, microvascular thrombi occlude these arteries, and there is proliferation of adventitial tissues (17).
Surgical ligation of the ductus arteriosus in the fetal lamb generates a model of PPHN that resembles the human condition (1, 3, 18, 32). In this model, similar to infants born with PPHN, there is an increase in the expression of the genes that induce pulmonary vasoconstriction and a reduction in those that induce vasodilation (3). In addition, we have previously shown that ET-1 stimulates H2O2 levels in isolated pulmonary arterial smooth muscle cells (PASMC). However, the effects of increased generation of H2O2 on vasodilator signaling in PPHN are not well understood. Thus the objective of this study was to determine whether there is relationship between increased reactive oxygen species (ROS) production in PPHN lambs and to determine whether this is associated with decreases in cGMP signaling. Finally, we wished to determine whether ROS scavenging could help to alleviate the abnormal vasodilator responses observed in the pulmonary arteries of the PPHN lambs.
MATERIALS AND METHODS
Prenatal ligation of the ductus arteriosus
The technique for creating pulmonary hypertension by prenatal ligation of the ductus arteriosus has been described previously (3). In brief, time-dated pregnant ewes (mixed breed) were operated on at 127 days of gestation (term = 146 days). After 9 days, the fetus was delivered by cesarean section, and the umbilical cord was clamped (n = 6). Twin fetuses served as controls (n = 6). Before the first breath, lambs were killed by rapid exsanguination through a direct cardiac puncture. The Laboratory Animal Care Committee at the State University of New York at Buffalo and Northwestern University approved all protocols.
Cell culture
Primary cultures of fetal (F) PASMC from sheep were isolated by the explant technique as described previously (24, 26, 29). In brief, a segment of the main pulmonary artery from 136-day-old fetal lambs was excised and placed in a sterile 10-cm dish containing DMEM supplemented with 1 g/l of glucose. The segment was stripped of adventitia with a sterile forceps. The main pulmonary artery segment was then cut longitudinally to open the vessel, and the endothelial layer was removed by gentle rubbing with a cell scraper. The vessel was then cut into 2-mm segments, inverted, and placed on a collagen-coated 35-mm tissue culture dish. A drop of DMEM containing 10% fetal bovine serum (HyClone), antibiotics (Media-Tech), and antimycotics (MediaTech) was then added, and the cells were grown overnight at 37°C in a humidified atmosphere with 5% CO2-95% air. The next day, an additional 2 ml of complete medium were added. The growth medium was subsequently changed every 2 days. When SMC islands could be observed under the microscope, the tissue segment was removed, and the individual cell islands were subcloned. Identity was confirmed as FPASMC by immunostaining (>99% positive) with antibodies against α-smooth muscle actin, calponin, and caldesmon. This was taken as evidence that cultures were not contaminated with fibroblasts or with endothelial cells. All cultures for subsequent experiments were maintained in DMEM supplemented with 10% fetal calf serum (Hyclone), antibiotics (MediaTech), and antimycotics (MediaTech) at 37°C in a humidified atmosphere with 5% CO2-95% air. Cells were utilized at passage 3. The Laboratory Animal Care Committee at Northwestern University approved all protocols.
In situ detection of H2O2
Fifth-generation pulmonary arteries (with first generation being the main branch of the pulmonary artery, 2–3 mm in diameter) were obtained from control and ductal ligation lambs. These were embedded in tissue freezing medium [Tris-buffered saline (TBS), Fisher], frozen, then cut into 30-μm-thick sections, and placed on glass slides. Slides were stored at − 80°C until used. The sections were exposed to 5 μM dichlorodihydrofluorescein diacetate (H2DCF-DA, Molecular Probes) with or without 250 U/ml polyethylene glycol (PEG)-catalase (Sigma) or the NOS inhibitor nitro-L-arginine methyl ester (L-NAME, 1 mM) in PBS. PEG-catalase was used to confirm that the oxidation of H2DCF-DA is H2O2 dependent, and L-NAME was used to determine whether an uncoupled NOS was involved in the generation of H2O2 in the lung sections. Slides were incubated in a light-protected humidified chamber at 37°C for 30 min. Slides were washed three times in PBS and observed under a Nikon Eclipse TE-300 fluorescent microscope with excitation at 485 nm and emission at 530 nm. Fluorescent images were captured with a CoolSnap digital camera, and the average fluorescent intensities (to correct for differences in cell number due to variability in slice thickness) were quantified with Metamorph imaging software (Fryer). Data are presented as PEG-catalase-inhibitable H2DCF-DA fluorescence.
Protein preparation and Western blotting
For Western blot analyses in the PPHN model, peripheral lung parenchyma and fifth-generation pulmonary arteries and veins were rapidly isolated and frozen in liquid nitrogen. Protein extracts were prepared as previously described (3). Proteins were separated on polyacrylamide gels and transferred to Hybond-polyvinylidene difluoride (PVDF) membranes. After blocking, the membranes were incubated for 1 h at room temperature with the appropriate dilution of antibody: catalase (RBI, 1:2,000) and glutathione peroxidase-1 (GPX-1, 1:500). The rabbit polyclonal GPX-1-specific antiserum was prepared by Biosynthesis (Lewisville, TX) using the sequence NH2-CDIETLLSQQSGNS-OH as previously described (15).
For Western blot analyses in FPASMC, cells were seeded on a six-well plate at a density of 500,000 cells/well and allowed to attach overnight. Subsequently, cells were treated or not with 0.015 U/ml glucose oxidase (from Aspergillus niger, Recombinant; Calbiochem, La Jolla, CA) for 12 h. Cells were then harvested in lysis buffer containing 5% (vol/vol) protease inhibitor cocktail and allowed to lyse for 15 min on ice. Samples were then centrifuged at 12,000 g for 10 min, and supernatant was collected. Samples were loaded on a 4–20% gradient gel and transferred to a PVDF membrane. Blots were blocked overnight at 4°C with 5% nonfat milk in TBS-Tween. A polyclonal antibody raised against soluble guanylate cyclase-α (sGC-α, 1:500 dilution) was used to probe the membrane for 3 h at room temperature.
After being washed to remove excess primary antibody, the membranes were incubated with an appropriate secondary antibody conjugated with horseradish peroxidase. The protein bands were visualized by chemiluminescent procedures (3). Band intensities were determined with Kodak ImageStation software.
Determination of GPX and catalase activity
Catalase and total GPX activities were measured as previously described (15). In brief, for the catalase assay peripheral lung or fifth-generation arterial or vein tissues were homogenized in assay buffer containing 1:1.55 KH2/Na2 (pH 7.0). Each sample containing 40 μg of protein was added to 1 ml of buffer and 1 ml of H2O2 solution. Absorbance was measured at 260 nm at room temperature for 30 s. For total GPX activity, peripheral lung or fifth-generation arterial or vein tissues were homogenized in Tris·HCl, pH 7.2, (vol 3:1). Homogenate was centrifuged at 3,000 g for 10 min at 4°C. Supernatant was removed and further centrifuged at 12,000 g for 15 min at 4°C. Samples containing 40 μg of protein were added to the assay buffer consisting of 100 mM Tris·HCl, pH 7.2, 3 mM EDTA, 1 mM sodium azide (Sigma Chemical, St. Louis, MO), 1.2 mM cumene hydroperoxide, 0.5 mM NADPH, and 1 unit GSH reductase (Sigma Chemical) in a final volume of 1 ml. The rate of NADPH oxidation was measured spectrophotometrically at 340 nm.
Determination of cGMP levels
FPASMC were seeded on a six-well plate at a density of 500,000 cells/well and allowed to attach overnight. Subsequently, cells were treated or not with 0.015 U/ml glucose oxidase for 12 h. Just before being harvested, cells were treated with or without spermine NONOate (50 μM) in the presence of 4-{[3′,4′-(methylenedioxy)benzyl]amino}-6-methoxyquinazoline [1 μM, a phosphodiesterase (PDE) 5 inhibitor] for 30 min to determine total cGMP accumulation. Cells were harvested in enzyme immunoassay buffer provided by the enzyme immunoassay kit and allowed to freeze-thaw through three cycles. Samples were then centrifuged at 12,000 g for 10 min, and supernatant was collected. The samples were tested for cGMP levels according to the manufacturer’s directions (Cayman Chemical).
Isolated vessel protocol
We have previously described the techniques for the study of isolated vessels in detail (4). Here we used fifth-generation pulmonary arteries with inside diameters of ≤ 500 μm. Vessel rings were mounted on stainless steel hooks and placed in water-jacketed chambers containing Krebs-Ringer solution. The buffer was maintained at 37°C and aerated with a gas mixture of 94% O2 and 6% CO2 to maintain a pH of 7.40, a PCO2 of 38 Torr and a PO2 of >500 Torr. Vessels were pretreated for 20 min with 10−5 M indomethacin and 10−6 M propranolol to prevent the formation of vasoactive prostaglandins and β-adrenergic receptor activation, respectively. Vessels were preconstricted with a submaximal concentration of norepinephrine. Cumulative concentration-response curves for S-nitrosyl-acetyl-penicillamine (SNAP, 10−9 to 5 × 10−6 M) were developed. Some vessels were pretreated for 20 min with PEG-bound catalase (1,200 U/ml) before relaxation with SNAP.
Statistical analysis
The relative fluorescent intensity was calculated for H2DCF-DA and expressed as fold change (vs. untreated cells) ± SD. Chemiluminescence intensities were determined for secondary antibodies and expressed ± SD. Catalase and GPX activities were determined and expressed ± SD. Comparisons between treatment groups were made by the unpaired t-test using the GB-STAT software program. A P < 0.05 was considered statistically significant.
RESULTS
Initially we used the fluorescent dye H2DCF-DA to determine whether H2O2 is elevated in pulmonary arteries isolated from ligated lambs. The results obtained indicated that H2DCF-DA fluorescence was fourfold higher in the pulmonary arteries isolated from ligated lambs relative to controls (Fig. 1). H2DCF-DA is also sensitive to other ROS, but cotreatment with cell-permeable PEG-catalase significantly reduced fluorescence in the ductal ligation samples, suggesting that H2O2 is the predominantly detected species (Fig. 1). In addition, we cotreated the pulmonary arteries with the NOS inhibitor L-NAME (1 mM). However, this did not significantly reduce the level of H2DCF-DA fluorescence in pulmonary arteries isolated from either control or ligated lambs (data not shown).
Fig. 1.
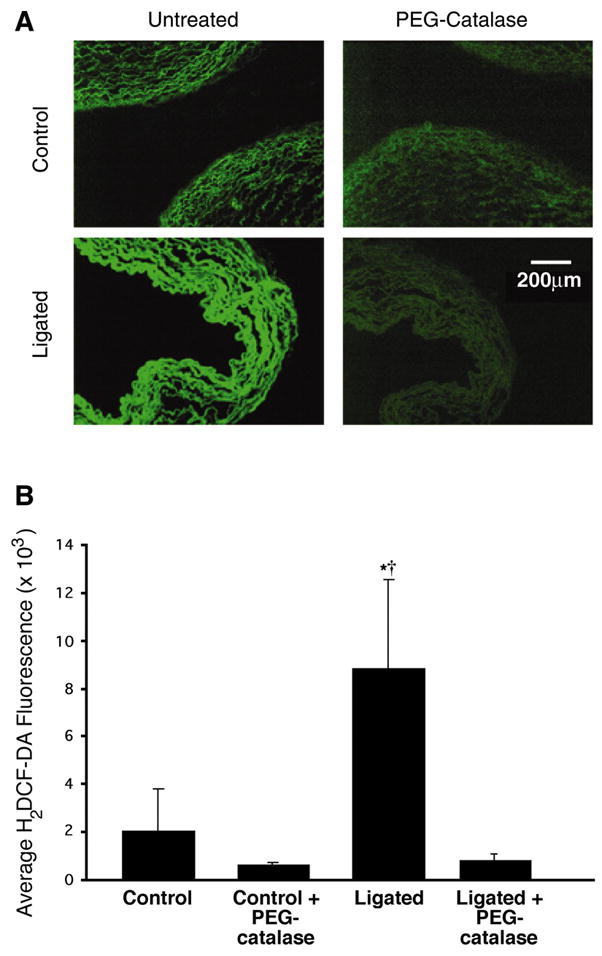
In situ detection of H2O2 in late-gestation fetal lamb lungs. A: unfixed frozen sections (30 μm) of pulmonary arteries prepared from near-term fetal lambs. Control sections and sections after ligation of the ductus arteriosus in utero (ligated) were incubated with dichlorodihydrofluorescein diacetate (H2DCF-DA, 5 μmol/l) and visualized by fluorescent microscopy. Under identical imaging conditions, H2DCF-DA fluorescence is markedly increased in the pulmonary vessels of the ductal ligation lambs. Preincubation with polyethylene glycol (PEG)-catalase (50 units) reduces the H2DCF-DA fluorescent signal and indicates its specificity for H2O2. B: average H2DCF-DA fluorescence intensities from images in A were determined. Values are means ± SD; n = 6. *P < 0.05 vs. control; †P < 0.05 vs. ligated + PEG-catalase.
Using whole lung and isolated vessel homogenates, we then determined whether the increase in H2O2 was associated with changes in lung antioxidant enzymes. Western blot analysis showed that both catalase (Fig. 2) and GPX-1 (Fig. 3) levels were unchanged in lung and isolated vessels from ligated lambs and controls. Similarly, total catalase and GPX activities were unchanged (Fig. 4).
Fig. 2.
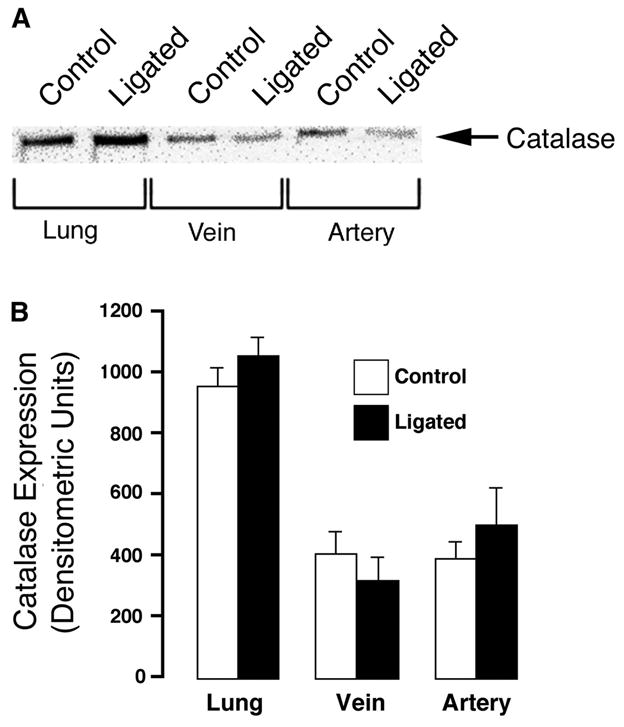
Catalase expression in peripheral lung tissue and 5th-generation pulmonary arteries and veins isolated from near-term fetal lambs. Control and after ligation of the ductus arteriosus in utero (ligated). A: protein extracts (40 μg), prepared from peripheral lung tissue, isolated 5th-generation pulmonary arteries, or pulmonary veins were separated on a 12% denaturing polyacrylamide gel, electrophoretically transferred to Hybond membranes, and analyzed using a specific antiserum raised against catalase protein. A representative blot is shown. B: there are no significant differences in densitometric values for catalase protein in peripheral lung tissue and 5th-generation isolated pulmonary arteries and veins from 5 control and 5 ligated near-term fetal lambs. Values are means ± SD.
Fig. 3.
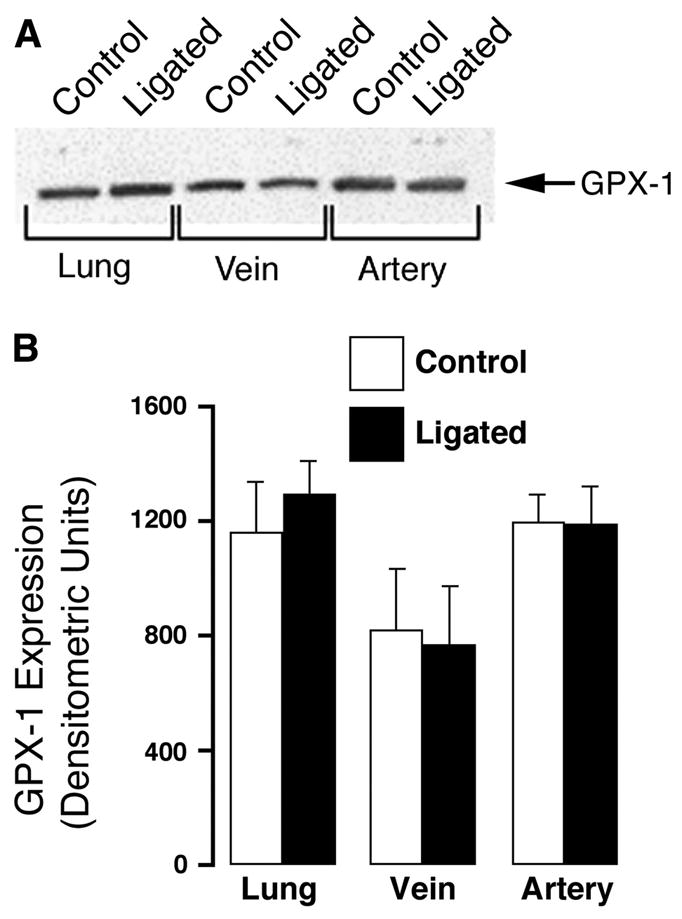
Glutathione peroxidase-1 (GPX-1) expression in peripheral lung tissue and 5th-generation pulmonary arteries and veins isolated from near-term fetal lambs. Control and after ligation of the ductus arteriosus in utero (ligated). A: protein extracts (40 μg), prepared from peripheral lung tissue, isolated 5th-generation pulmonary arteries, or pulmonary veins were separated on a 12% denaturing polyacrylamide gel, electrophoretically transferred to Hybond membranes, and analyzed using a specific antiserum raised against GPX-1 protein. A representative blot is shown. B: there are no significant differences in densitometric values for GPX-1 protein in peripheral lung tissue and 5th-generation isolated pulmonary arteries and veins from 5 control and 5 ligated near-term fetal lambs. Values are means ± SD.
Fig. 4.
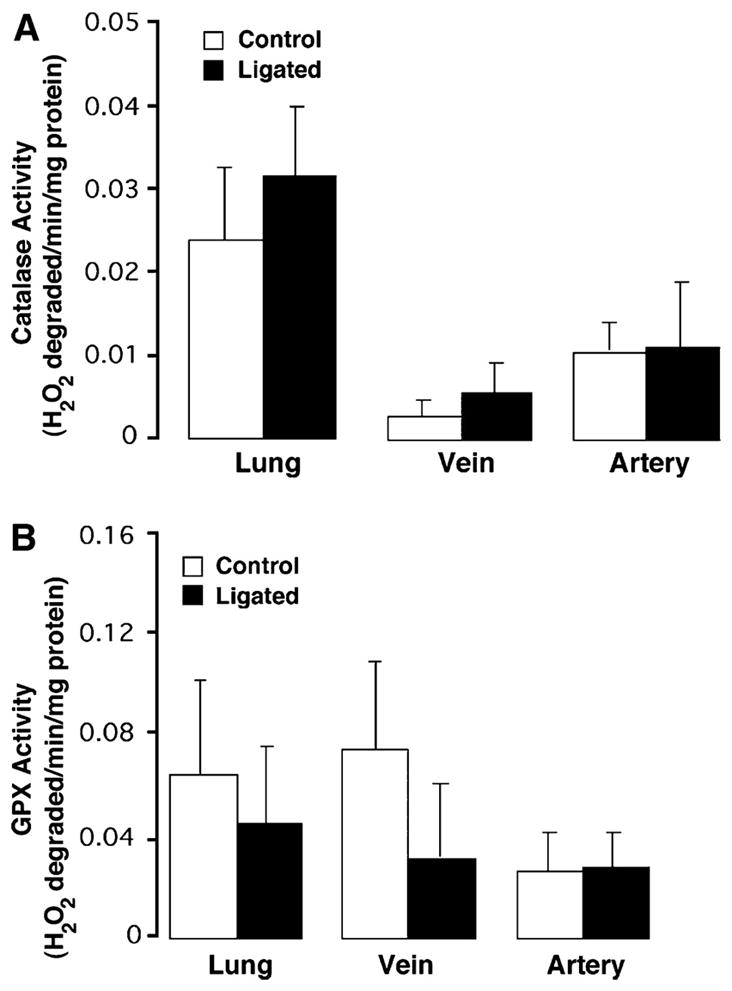
Catalase and GPX activity in peripheral lung tissue and 5th-generation pulmonary arteries and veins isolated from near-term fetal lambs. Control and after ligation of the ductus arteriosus in utero (ligated). Catalase (A) and GPX (B) activities were determined in protein extracts (40 μg), prepared from peripheral lung tissue, isolated 5th-generation pulmonary arteries, or pulmonary veins from both control (n = 5) and ligated (n = 5) lambs. Values are expressed as units/μg protein. Values are means ± SD. There are no significant differences in either catalase or GPX activities in peripheral lung tissue and 5th-generation isolated pulmonary arteries and veins in control and ligated near-term fetal lambs.
We have previously demonstrated that sGC expression is significantly reduced in the PPHN lamb model (3). However, the mechanism for this reduction has not been determined. Thus we next investigated whether the increased H2O2 we observed in the ligated lambs could be involved in reducing the sGC expression and cGMP signaling observed in this model. PASMC isolated from late-gestation fetal lambs were exposed to glucose oxidase as an H2O2-generating system for 12 h. The data obtained indicate that H2O2 significantly decreases both sGC-α expression and the generation of cGMP in response to challenge with an NO donor (Fig. 5).
Fig. 5.
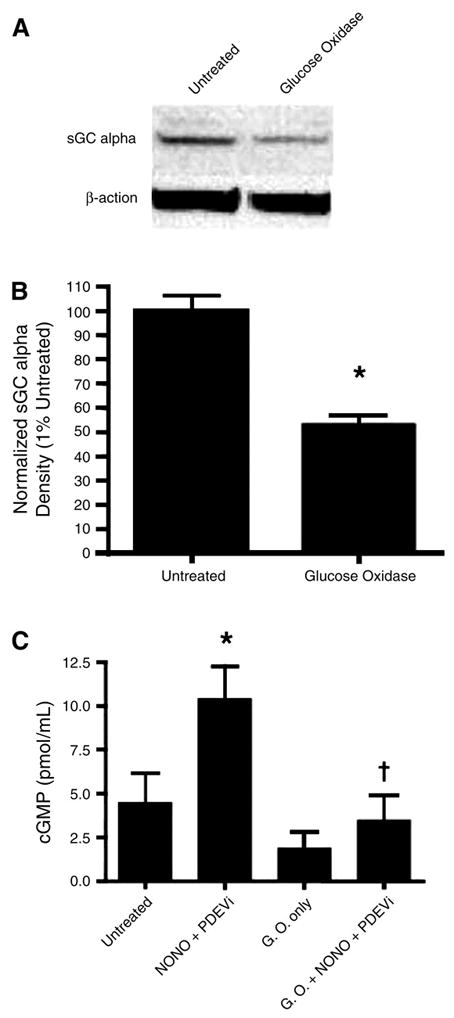
Exposure of fetal pulmonary arterial smooth muscle cells to H2O2 decreases soluble guanylate cyclase (sGC) expression and cGMP signaling. Fetal pulmonary arterial smooth muscle cells (PASMC) were exposed or not to the H2O2-generating system glucose oxidase (0.015 U/ml, 12 h). Cells were then either harvested to examine sGC expression or treated with the NO donor spermine NONOate (30 μM, 30 min) in the presence of the phosphodiesterase (PDE) 5 inhibitor 4-{[3′,4′-(methylenedioxy)benzyl]amino}-6-methoxyquinazoline (1 μM), and total accumulation of cGMP was determined. A: protein extracts (25 μg) prepared from fetal PASMC exposed or not to glucose oxidase (GO, 0.015 U/ml, 12 h) were separated on a 4–20% denaturing polyacrylamide gel, electrophoretically transferred to Hybond membranes, and analyzed using a specific antiserum raised against sGC-α. Blots were stripped and reprobed for β-actin as a loading control. Shown is a representative blot from 3 independent experiments. B: H2O2 decreases sGC-α expression in fetal PASMC. Band density for sGC-α was normalized to β-actin and expressed as the percentage in untreated cells (100%). Values are means ± SD; n = 3. *P < 0.05 vs. untreated cells. C: H2O2 decreases NO-dependent cGMP generation in fetal PASMC. Cells were activated with 50 μM spermine NONOate (NONO) for 30 min in the presence of a phosphodiesterase 5 inhibitor (PDEVi) that was added to the sample to prevent degradation of cGMP. *P < 0.05 vs. untreated cells; †P < 0.05 vs. NO-treated and PDEV-exposed cells.
Finally we performed in vivo functional assays on fifth-generation pulmonary arteries from control and PPHN lambs. Pulmonary arteries from PPHN lambs showed significantly blunted relaxations to the NO donor SNAP (Fig. 6). Pretreatment with the cell-permeable ROS scavenger PEG-catalase enhanced the relaxations to SNAP in pulmonary arteries from PPHN lambs (Fig. 6). Pretreatment with PEG-catalase had no significant effect on SNAP relaxations in pulmonary arteries isolated from control lambs (Fig. 6).
Fig. 6.
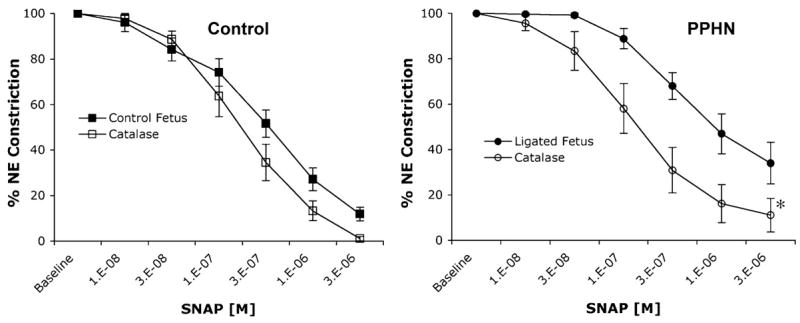
Cumulative concentration-response curves for the NO donor S-nitrosyl-acetyl-penicillamine (SNAP) in isolated 5th-generation pulmonary arteries isolated from near-term fetal lambs. Control arteries and arteries with PPHN after in utero ligation of the ductus arteriosus (Ligated Fetus). Arterial rings from PPHN lambs have attenuated responses to SNAP compared with controls. Pretreatment with PEG-catalase (1,200 U/ml) had no effect on arterial rings from control lambs but significantly enhanced SNAP relaxations in rings isolated from PPHN lambs. All rings were pretreated with indomethacin and propranolol. Relaxations are expressed as percentages of a plateau constriction in response to norepinephrine (100%). Data are means ± SE; n = 3–9 lambs for each data point. *P < 0.05 vs. SNAP alone.
DISCUSSION
The ductal ligation lamb model of PPHN displays abnormal regulation of the ET-1 and NO signaling cascades such that ET-1 levels are elevated and eNOS expression is decreased (3). Overall we have shown previously that there is an increase in the expression of the genes that induce pulmonary vasoconstriction and a reduction in those that induce vasodilation (3). Thus, in PPHN, ET-1-mediated vasoconstriction coupled with reduced NO-mediated vasodilation is likely to make a significant contribution to pulmonary hypertension. However, the mechanism responsible for this apparent coordinated regulation of vasodilator and vasoconstrictor gene expression is not well understood.
We have previously shown that ET-1 stimulates the proliferation of FPASMC via a pathway involving ETA-mediated superoxide synthesis (29), and superoxide production is increased in the smooth muscle layer of pulmonary arteries of ductal ligation PPHN lambs (4). Because ROS appear to play a central role in PPHN, we also examined the effects of scavenging ROS on the vasodilator response of pulmonary arteries to exogenous NO. We chose to look at the effects of H2O2 since this ROS is more stable than superoxide and may be a more likely intercellular signaling molecule between FPASMC and fetal pulmonary arterial endothelial cells (FPAEC). Indeed our recently published study indicates that ET-1-mediated increases in H2O2 within the SMC can produce decreases in eNOS gene expression in cocultured EC (25). Thus, in this study, we determined whether the development of PPHN in vivo also resulted in an increase in H2O2 levels. In addition, we wished to determine whether increases in H2O2 levels could also account for the decreased cGMP signaling associated with PPHN both in the lamb model of ductal ligation (3) and in children born with the disease (5).
Our finding that H2O2 levels are elevated in isolated ductal ligation vessels is in agreement with our previous studies looking at levels of superoxide (4). However, in that study, we found that SOD activity was decreased in ductal ligation arteries (4), which would be predicted to yield less H2O2. Because catalase and GPX activities are not altered in the PPHN vessels, elevated superoxide generation likely accounts for the increased levels of H2O2, despite the lower SOD activity. However, Cys55 in bovine CuZn-SOD is involved in the formation of an internal disulfide bond that is essential for activity. It has been suggested that this cysteine residue is sensitive to oxidative modification, which can result in enzyme inhibition (20). Thus the reduction in SOD activity may be simply a function of an overtaxed enzymatic system (4). However, aberrations in alternative pathways of H2O2 catalysis in PPHN cannot be ruled out.
In the ductal ligation lamb, ETA receptor blockade attenuates pulmonary hypertension and SMC growth (12). Conversely, prolonged infusion of fetal lambs with an ETB receptor antagonist generates increases in pulmonary artery pressure, pulmonary vascular resistance, and muscularization of small pulmonary arteries (11). Our data complement these studies by demonstrating that scavenging ROS can attenuate the altered vasodilator response in the pulmonary arteries of hypertensive lambs. These data suggest a central role for ROS in producing the altered vasodilator responses seen in PPHN. In our proposed pathway, the increased ET-1 released during the development of PPHN activates ETA receptors on FPASMC, resulting in increased ROS generation through NADPH oxidase activation. The subsequent increase in ROS induces FPASMC proliferation (29) and vascular remodeling. In addition, the H2O2 released may result in the decreased expression of the vasodilator proteins eNOS and sGC. Indeed we have recently shown that the ET-1-mediated elevation of H2O2 levels in FPASMC leads to a concomitant increase in cocultured FPAEC, which produces a decrease in eNOS transcription and subsequent decrease in eNOS protein expression and activity (25). In addition, our new data presented in this study indicate that increased generation of H2O2 in PASMC results in a decrease in sGC-α expression and a reduction in NO-dependent cGMP accumulation. Both of these events would result in a reduction in NO signaling, and we speculate that the increased H2O2 generation in the lungs of ductal ligation lambs may explain the apparent coordinated increases in vasoconstrictor gene expression with decreased vasodilator gene expression. Indeed previous studies have shown that oxidative stress will increase ET-1 gene expression (13, 14). However, further studies will be required to determine whether H2O2 can also increase the expression of the ETA receptor and PDE5 that are vasoconstrictor proteins and decrease the expression of the vasodilator protein ETB receptor.
In addition, less NO signaling to the FPASMC to stimulate vasodilation and inhibit SMC growth would result in an increase in vasoconstriction and potentially a stimulation of SMC proliferation. Indeed, we have recently shown that exogenous NO attenuates FPASMC proliferation by stimulating G0/G1 growth (28). Furthermore, activation of NO production in rat aortic EC inhibits the proliferation of rat aortic SMC in coculture (10), suggesting that endothelium-derived NO can influence the growth of adjacent SMC. Overall, the predicted effects of these events are reduced NO-mediated vasodilation, elevated ET-1-mediated vasoconstriction, and increased ROS-mediated vascular remodeling. Indeed our data demonstrate that fifth-generation pulmonary arteries isolated from PPHN lambs had significantly decreased relaxations to the NO donor SNAP compared with controls. The addition of PEG-catalase to pulmonary arteries isolated from PPHN lambs significantly enhanced their relaxations to SNAP. As PEG-catalase had no effect in control pulmonary arteries, this enhancement appears to be unique to PPHN. These data demonstrate that excess ROS inhibit relaxations in PPHN, and indicate that our in vitro data are recapitulated in vivo.
In conclusion our data establish a link between increased H2O2 generation and the abnormal vasodilator responses of the pulmonary arteries in lambs with PPHN. In addition, we speculate that increased generation of H2O2 in the lungs of ductal ligation lambs may explain the apparent coordinated increases in vasoconstrictor gene expression with decreased vasodilator gene expression. Our data suggest that antioxidants could be a potential therapy to reduce or prevent the pulmonary vascular remodeling associated with PPHN. Indeed, we have previously found that antioxidants and inhibitors of NADPH oxidase attenuate ET-1-mediated FPASMC proliferation (29), and recently we showed that EUK-134, a synthetic SOD/catalase mimetic, inhibits serum-induced FPASMC growth (26). However, the effect of this type of treatment in vivo is unknown and will require new studies utilizing the ductal ligation model to directly assess this issue.
Acknowledgments
The authors also gratefully acknowledge Sylvia Gugino for expert technical assistance in isolating the pulmonary vessels and HL-61284 to Dr. Jeff Fineman at University of California San Francisco.
GRANTS
This research was supported in part by National Institutes of Health Grants HL-60190 (to S. M. Black), HD-39110 (to S. M. Black), HL-67841 (to S. M. Black), HL-72123 (to S. M. Black), HL-70061 (to S. M. Black), and HL-54705 (to R. H. Steinhorn) and American Heart Association National Office Grant 0330292N (to S. Wedgwood).
References
- 1.Abman SH, Shanley PF, Accurso FJ. Failure of postnatal adaptation of the pulmonary circulation after chronic intrauterine pulmonary hypertension in fetal lambs. J Clin Invest. 1989;83:1849–1858. doi: 10.1172/JCI114091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arai H, Hori S, Aramori I, Ohkubo H, Nakanishi S. Cloning and expression of a cDNA encoding an endothelin receptor. Nature. 1990;348:730–732. doi: 10.1038/348730a0. [DOI] [PubMed] [Google Scholar]
- 3.Black SM, Johengen MJ, Soifer SJ. Coordinated regulation of genes of the nitric oxide and endothelin pathways during the development of pulmonary hypertension in fetal lambs. Pediatr Res. 1998;44:821–830. doi: 10.1203/00006450-199812000-00001. [DOI] [PubMed] [Google Scholar]
- 4.Brennan LA, Steinhorn RH, Wedgwood S, Mata-Greenwood E, Roark EA, Russell JA, Black SM. Increased superoxide generation is associated with pulmonary hypertension in fetal lambs: a role for NADPH oxidase. Circ Res. 2003;92:683–691. doi: 10.1161/01.RES.0000063424.28903.BB. [DOI] [PubMed] [Google Scholar]
- 5.Christou H, Adatia I, Van Marter L, Kane J, Thompson J, Stark A, Wessel D, Kourembanas S. Effect of inhaled nitric oxide on endothelin-1 and cyclic guanosine 5′-monophosphate plasma concentrations in newborn infants with persistent pulmonary hypertension. J Pediatr. 1997;130:603–611. doi: 10.1016/s0022-3476(97)70245-2. [DOI] [PubMed] [Google Scholar]
- 6.Fineman JR, Soifer SJ, Heymann MA. Regulation of pulmonary vascular tone in the perinatal period. Annu Rev Physiol. 1995;57:115–134. doi: 10.1146/annurev.ph.57.030195.000555. [DOI] [PubMed] [Google Scholar]
- 7.Furchgott RF, Vanhoutte PM. Endothelium-derived relaxing and contracting factors. FASEB J. 1989;3:2007–2018. [PubMed] [Google Scholar]
- 8.Hassoun P, Thappa V, Landman M, Fanburg B. Endothelin 1: mitogenic activity on pulmonary artery smooth muscle cells and release from hypoxic endothelial cells. Proc Exp Bio Med. 1992;199:165–170. doi: 10.3181/00379727-199-43342. [DOI] [PubMed] [Google Scholar]
- 9.Haworth S, Reid L. Persistent fetal circulation: newly recognized structural features. J Pediatr. 1976;88:614–620. doi: 10.1016/s0022-3476(76)80021-2. [DOI] [PubMed] [Google Scholar]
- 10.Ignarro LJ, Buga GM, Wei LH, Bauer PM, Wu G, del Soldato P. Role of the arginine-nitric oxide pathway in the regulation of vascular smooth muscle cell proliferation. Proc Natl Acad Sci USA. 2001;98:4202–4208. doi: 10.1073/pnas.071054698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ivy DD, Parker TA, Abman SH. Prolonged endothelin B receptor blockade causes pulmonary hypertension in the ovine fetus. Am J Physiol Lung Cell Mol Physiol. 2000;279:L758–L765. doi: 10.1152/ajplung.2000.279.4.L758. [DOI] [PubMed] [Google Scholar]
- 12.Ivy DD, Parker TA, Ziegler JW, Galan HL, Kinsella JP, Tuder RM, Abman SH. Prolonged endothelin A receptor blockade attenuates chronic pulmonary hypertension in the ovine fetus. J Clin Invest. 1997;99:1179–1186. doi: 10.1172/JCI119274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kahler J, Ewert A, Weckmuller J, Stobbe S, Mittmann C, Koster R, Paul M, Meinertz T, Munzel T. Oxidative stress increases endothelin-1 synthesis in human coronary artery smooth muscle cells. J Cardiovasc Pharmacol. 2001;38:49–57. doi: 10.1097/00005344-200107000-00006. [DOI] [PubMed] [Google Scholar]
- 14.Kahler J, Mendel S, Weckmuller J, Orzechowski HD, Mittmann C, Koster R, Paul M, Meinertz T, Munzel T. Oxidative stress increases synthesis of big endothelin-1 by activation of the endothelin-1 promoter. J Mol Cell Cardiol. 2000;32:1429–1437. doi: 10.1006/jmcc.2000.1178. [DOI] [PubMed] [Google Scholar]
- 15.Khan JY, Black SM. Developmental changes in murine brain antioxidant enzymes. Pediatr Res. 2003;54:77–82. doi: 10.1203/01.PDR.0000065736.69214.20. [DOI] [PubMed] [Google Scholar]
- 16.Luscher TF, Yang Z, Tschudi M, von Segesser L, Stulz P, Boulanger C, Siebenmann R, Turina M, Buhler FR. Interaction between endothelin-1 and endothelium-derived relaxing factor in human arteries and veins. Circ Res. 1990;66:1088–1094. doi: 10.1161/01.res.66.4.1088. [DOI] [PubMed] [Google Scholar]
- 17.Mecham R, Whitehouse L, Wren D, Parks W, Griffin G, Senior R, Crouch E, VŒlkel N, Stenmark K. Smooth muscle-mediated connective tissue remodeling in pulmonary hypertension. Science. 1987;237:423–426. doi: 10.1126/science.3603030. [DOI] [PubMed] [Google Scholar]
- 18.Morin FC., III Ligating the ductus arteriosus before birth causes persistent pulmonary hypertension in the newborn lamb. Pediatr Res. 1989;25:245–250. doi: 10.1203/00006450-198903000-00005. [DOI] [PubMed] [Google Scholar]
- 19.Palmer RMJ, Ashton DS, Moncada S. Vascular endothelial cells synthesize nitric oxide from L-arginine. Nature. 1988;333:664–666. doi: 10.1038/333664a0. [DOI] [PubMed] [Google Scholar]
- 20.Rypniewski WR, Mangani S, Bruni B, Orioli PL, Casati M, Wilson KS. Crystal structure of reduced bovine erythrocyte superoxide dismutase at 1.9 A resolution. J Mol Biol. 1995;251:282–296. doi: 10.1006/jmbi.1995.0434. [DOI] [PubMed] [Google Scholar]
- 21.Sakurai T, Yanagisawa M, Takuwa Y, Miyazaki H, Kimura S, Goto K, Masaki T. Cloning of a cDNA encoding a non-isopeptide-selective subtype of the endothelin receptor. Nature. 1990;348:732–735. doi: 10.1038/348732a0. [DOI] [PubMed] [Google Scholar]
- 22.Shetty SS, Okada T, Webb RL, DelGrande D, Lappe RW. Functionally distinct endothelin B receptors in vascular endothelium and smooth muscle. Biochem Biophys Res Commun. 1993;191:459–464. doi: 10.1006/bbrc.1993.1240. [DOI] [PubMed] [Google Scholar]
- 23.Steinhorn R, Millard S, Morin F., III Persistent pulmonary hypertension of the newborn: role of nitric oxide and endothelin in pathophysiology and treatment. Clin Perinatol. 1995;22:405–428. [PubMed] [Google Scholar]
- 24.Wedgwood S, Black SM. Combined superoxide dismutase/catalase mimetics alter fetal pulmonary arterial smooth muscle cell growth. Antioxid Redox Signal. 2004;6:191–197. doi: 10.1089/152308604771978507. [DOI] [PubMed] [Google Scholar]
- 25.Wedgwood S, Black SM. Endothelin-1 decreases endothelial NOS expression and activity through ETA receptor-mediated generation of hydrogen peroxide. Am J Physiol Lung Cell Mol Physiol. 2005;288:L480–L487. doi: 10.1152/ajplung.00283.2004. [DOI] [PubMed] [Google Scholar]
- 26.Wedgwood S, Black SM. Induction of apoptosis in fetal pulmonary arterial smooth muscle cells by a combined superoxide dismutase/catalase mimetic. Am J Physiol Lung Cell Mol Physiol. 2003;285:L305–L312. doi: 10.1152/ajplung.00382.2002. [DOI] [PubMed] [Google Scholar]
- 28.Wedgwood S, Black SM. Molecular mechanisms of nitric oxide-induced growth arrest and apoptosis in fetal pulmonary arterial smooth muscle cells. Nitric Oxide. 2003;9:201–210. doi: 10.1016/j.niox.2003.11.005. [DOI] [PubMed] [Google Scholar]
- 29.Wedgwood S, Dettman R, Black S. ET-1 stimulates pulmonary arterial smooth muscle cell proliferation via induction of reactive oxygen species. Am J Physiol Lung Cell Mol Physiol. 2001;281:L1058–L1067. doi: 10.1152/ajplung.2001.281.5.L1058. [DOI] [PubMed] [Google Scholar]
- 31.Wiklund N, Persson M, Gustafsson L, Moncada S, Hedqvist P. Modulatory role of endogenous nitric oxide in pulmonary circulation in vivo. Eur J Pharmacol. 1990;185:123–124. doi: 10.1016/0014-2999(90)90221-q. [DOI] [PubMed] [Google Scholar]
- 32.Wild LM, Nickerson PA, Morin FC., III Ligating the ductus arteriosus before birth remodels the pulmonary vasculature of the lamb. Pediatr Res. 1989;25:251–257. doi: 10.1203/00006450-198903000-00006. [DOI] [PubMed] [Google Scholar]


