Abstract
Background
Exposure to environmental tobacco smoke (ETS) is associated with the development of serious health consequences in children with cancer due to preexisting disease and treatment-related vulnerabilities. The purpose of the current investigation was to identify predictors of non-participation in a randomized intervention trial to reduce ETS exposure among pediatric cancer patients.
Methods
One hundred and fifty-three families of pediatric cancer patients met study eligibility criteria. Parents of 117 (76%) patients agreed to study participation, whereas thirty-six (24%) parents declined (non-participants). Data were collected with respect to participant sociodemographic, medical, and treatment-related characteristics.
Results
Univariate analyses indicated that families whose primary caregivers were females or smokers were more likely to be non-participants in the ETS reduction trial (P=0.045 and P=0.009, respectively). Medical features that significantly associated with study non-participation included CNS tumor diagnosis (P=0.030), no history of chemotherapy (P=0.012), history of surgery prior to study recruitment (P=0.036), and having future radiation therapy planned post study recruitment (P=0.009). Multivariable logistic regression modeling revealed that study non-participation was associated with the primary caregiver being a smoker (OR=6.48, P=0.002) or female (OR=8.56, P=0.023), and patient CNS tumor diagnosis (OR=4.63, P=0.021).
Conclusions
Although a large percentage of eligible participants enrolled in the ETS reduction trial, findings suggest that future recruitment strategies of families should be tailored to parental smoking status and gender, as well as child diagnosis and treatment.
Keywords: environmental tobacco smoke exposure (ETS), second hand smoke, clinical trial enrollment, intervention, participation rates, pediatric oncology, pediatric cancer
Introduction
Exposure to environmental tobacco smoke (ETS) represents a serious public health threat, and remains a preventable cause of morbidity and mortality among children. The National Health and Nutrition Examination Survey III (NHANES) indicates that 40% of US children live in a home with at least one smoker [1]. Similarly, the National Youth Tobacco Survey reveals that 44% of adolescents live in smoking households, with 6.2 million reporting direct exposure to ETS [2]. These prevalence rates of ETS exposure are concerning and suggest that a significant proportion of US youth are exposed to this environmental contaminant.
Adverse health effects have been reported in children exposed to ETS including increased risk of pneumonia, bronchitis, respiratory illness, wheezing, middle ear effusions and otitis media [3, 4, 5, 6]. Children whose parents smoke experience these conditions at disproportional rates, and risk of complication increases with higher levels of exposure [7]. Among middle- and high school-aged youth, ETS exposure causes a variety of problems such as acute lower and upper respiratory tract illness, asthma or exacerbation of existing asthmatic symptoms, and reduced lung function and growth [8]. Lower serum levels of Vitamin C in exposed children provide preliminary evidence of the direct adverse metabolic consequences of ETS [9]. Second-hand smoke exposure in healthy youths is associated with adverse health outcomes; hence, reductions in exposure should yield health benefits.
Some preliminary work has been conducted to establish the prevalence of ETS exposure within the childhood cancer population. Tyc and colleagues (2004) investigated the prevalence of parental smoking in households of 303 children newly diagnosed with cancer [10]. Consistent with the population-based findings, approximately 45% lived in households with one or more smoking parent. Additionally, patients of smoking households experienced more respiratory problems than those from non-smoking households. These findings suggest that many cancer patients have regular and repeated ETS exposure from parents and other caregivers, and consequently are at increased risk for the development of deleterious health conditions.
These rates of exposure are particularly troubling as ETS represents a serious health threat for children on treatment for cancer due to their disease and treatment-related vulnerabilities [10]. Adverse health effects for pediatric patients secondary to treatment-related toxicities include compromised pulmonary, respiratory, and cardiovascular functioning. Extended exposure to ETS may, therefore, place these patients at high risk for cardiovascular or pulmonary disease [11]. Selective subgroups of patients treated with cardiopulmonary toxic agents [12] and/or thoracic radiation therapy [13] may also develop restrictive lung disease exacerbated by ETS exposure. Likewise, an association between anthracycline therapy and risk of congestive heart failure in childhood cancer survivors [14] suggests that patients receiving this therapeutic agent may be especially susceptible to tobacco-related health problems. Pediatric cancer patients are already at risk for developing second cancers, and exposure to ETS may exacerbate these vulnerabilities [15, 16, 17]. Clearly, second-hand smoke exposure in this population places patients at high risk for the development of future tobacco-related complications. As a result, the provision of familial-based ETS exposure interventions may be beneficial not only in terms of reducing second-hand smoke exposure, but in reducing the initiation of smoking by children due to reduced opportunities to observe smoking modeled by parents.
Exposure to ETS has harmful health consequences for pediatric patients, and the available data suggest that many childhood cancer patients are regularly exposed. Using other successful ETS reduction interventions tailored for pediatric patients, our clinical trial is the first to explore the feasibility and efficacy of a parent-based second-hand smoke reduction intervention within the childhood cancer population [18, 19]. Despite the reported success of these interventions in other pediatric populations, previous research has found that there are potential barriers associated with non-participation in such clinical trials [20, 21]. Among families with asthmatic children, for example, participation rates on ETS intervention trials has ranged from only 31%-71%, and factors such as race, distance to hospital, and parent smoking status have been identified as being barriers to study participation [22, 23, 24]. The purpose of the current investigation was to identify and explore sociodemographic, medical, and treatment-related predictors of participation/non-participation in a randomized intervention trial to reduce ETS exposure among pediatric cancer patients.
Methods
Participant eligibility was determined through successful completion of 2 steps: 1) Initial chart review; and 2) In-person participant eligibility screening. Patient eligibility criteria included being a nonsmoker, younger than 18 years of age, in active treatment for cancer, and being at least 30 days post diagnosis (or date of recurrence/relapse). Of those who met these initial criteria, a more in-depth participant review occurred in which patient health status was determined. To remain eligible for study participation, patients could not have a high risk prognosis, be on the bone marrow transplant (BMT) service, nor be in medical/social crisis. Patients and their families who met these initial criteria were eligible to participate in the in-person screening (i.e. step 2 of eligibility determination).
Parents of pediatric patients were approached and screened within the clinic setting and were asked about their household smoking status and child's ETS exposure. If the caregiver reported no smokers in the home, then the family was study ineligible. If household smokers were reported to be in the home, then parents would be further questioned as to whether the patient experienced ETS exposure in the home or car setting. If the parent responded “no,” they were deemed study ineligible. If the parent responded “yes” to patient exposure (and the patient denied active tobacco use), then the family was deemed study eligible.
Eligible families were informed of the clinical trial investigating an intervention to reduce ETS exposure among pediatric cancer patients. In this randomized two-group design, caregivers were informed that they would receive either brief advice about secondhand smoke exposure or a three-month, multi-component behavioral intervention. The experimental group (i.e., behavioral program) was six sessions in length which occurred either at the hospital or via telephone. Caregivers were told prior to study enrollment that they would not be required to quit smoking as part of study participation; rather the goal of the study was to reduce patient ETS exposure. Furthermore, the person receiving the formal intervention (target parent) did not have to be a smoker (e.g., the non-smoking spouse of a smoker), but had to be willing to implement the ETS reduction strategies within their home. Potential participants were also told that intervention measures (study-related questionnaires, caregiver report of child exposure, and child's urine cotinine) would be collected 5 times over the 12 month study, and that enrollment would consist of signing an informed consent and obtaining child assent (patients aged ≥ 7 years). Families were told to think about their interest in participating in the trial over the next several days, and that a member of the research team would approach them at a later time for study enrollment if they were interested. It was this final group that was considered eligible to participate in the intervention (N=153), and whose data are considered in this study. Figure 1 outlines participant eligibility.
Figure 1.
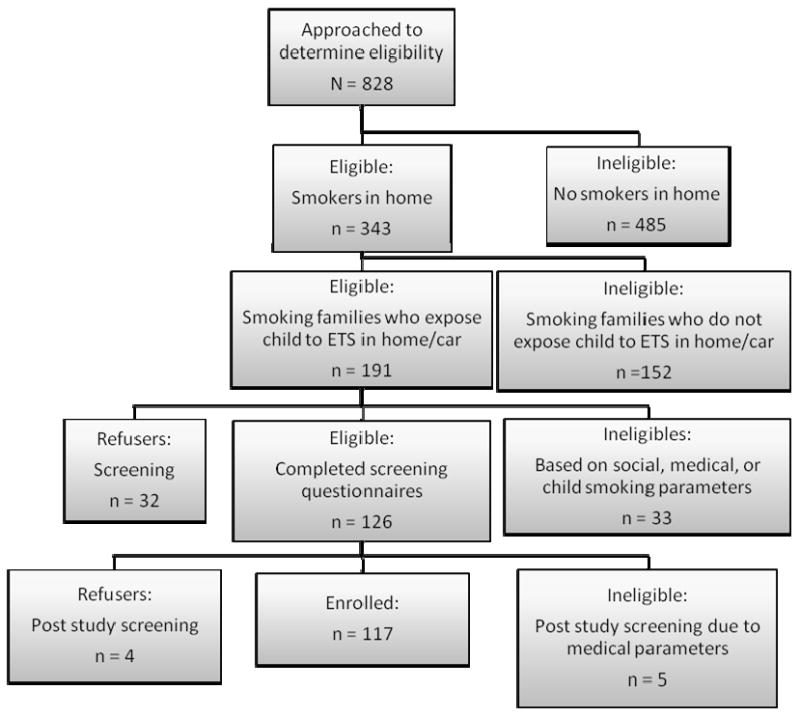
Outline of Parental Eligibility and Participation on an Environmental Tobacco Smoke (ETS) Reduction Trial for Pediatric Oncology Patients. Totals: 117 enrolled, 36 refused, 675 ineligible
The association of sociodemographic, medical, and treatment-related variables with study non-participation was investigated using Pearson chi-square or Fisher's exact tests for nominal categorical variables, Mantel-Haenzel or exact Mantel-Haenzel tests for ordered categorical variables, and t-tests for continuous variables [25]. Demographic factors considered included child and parent age (in years), gender, and race, along with education level (parents only). Social or familial factors included socioeconomic status [26] (high, medium, low), caregiver marital status (married, divorced/separated/never married/unknown), caregiver smoking status (yes/no), household density (0 siblings, 1 sibling, ≥ 2 siblings, unknown), and distance from home to hospital (<35 miles, ≥ 35 miles - <300 miles, ≥ 300 miles). Medical and treatment variables included child diagnosis (Central Nervous System (CNS) tumor, leukemia/lymphoma, solid tumor), time since diagnosis (0-30 days, 31-90 days, 91-180 days, >180 days), number of hospitalized days in last month (0 days, 1-7 days, 8-14 days, 15-30 days), number of therapeutic protocols (0, 1, 2, 3-6), and history of relapse, surgery, chemotherapy, or radiation treatment or planned future surgery, chemotherapy or radiation (all categorized yes/no). Variables significant in univariate analyses at the 0.10 alpha level were further investigated using multivariable exact logistic regression to explore which variables predicted study non-enrollment [25] after adjusting for other variables in the model using SAS Release 9.1.3 (SAS Institute, Cary, NC). All reported p-values are two-sided and not adjusted for multiple comparisons.
Results
Of those families who met study eligibility (n=191), 33 were deemed ineligible due to social crisis (e.g. parental psychopathology or unstable living situation), medical crisis (e.g. relapse, BMT), or patient tobacco use. Five additional families became ineligible prior to final study enrollment, due to medical reasons: 2 patients relapsed, and 3 transferred to BMT clinic. Of the 153 families who met full study eligibility criteria, approximately 76% (n=117) enrolled on the ETS reduction trial, whereas 24% (n=36) refused participation.
Regarding differences between those who enrolled or refused study participation, chi-square analyses revealed that families whose primary caregivers were female or smokers were more likely to be non-participants in the ETS reduction trial (P=0.045 and P=0.009, respectively). Additionally, patient medical features which significantly associated with non-participation included diagnosis of CNS tumor (P=0.030), no history of chemotherapy prior to study recruitment (P=0.012), history of surgery prior to study recruitment (P=0.036), and having future radiation therapy planned post study recruitment (P=0.009). Sociodemographic and medical/treatment-related characteristics by familial participation are detailed in Tables I and II, respectively.
Table I. Sociodemographic characteristics by participation status on trial to reduce environmental tobacco smoke (ETS) exposure among pediatric cancer patients.
| Total (N = 153) |
Participants (n = 117) |
Non-Participants (n = 36) |
P-Value* | |
|---|---|---|---|---|
| Child characteristics | M (SD) | M (SD) | M (SD) | |
| Age | 8.4 (5.0) | 8.3 (5.0) | 8.8 (5.1) | 0.720 |
| Gender | n (%) | n (%) | n (%) | |
| Male | 82 (54) | 61 (52) | 21 (58) | |
| Female | 71 (46) | 56 (48) | 15 (42) | 0.514 |
| Race | ||||
| White | 119 (78) | 90 (77) | 29 (81) | |
| Non-White | 34 (22) | 27 (23) | 7 (19) | 0.647 |
| Target parent characteristics | M (SD) | M (SD) | M (SD) | |
| Age | 33.8 (8.7) | 33.7 (8.7) | 34.0 (8.8) | 0.921 |
| Gender | n (%) | n (%) | n (%) | |
| Male | 20 (13) | 19 (16) | 1 (3) | |
| Female | 133 (87) | 98 (84) | 35 (97) | 0.045 |
| Race | ||||
| White | 125 (82) | 94 (80) | 31 (86) | |
| Non-White | 28 (18) | 23 (20) | 5 (14) | 0.434 |
| Education level | ||||
| Did not complete high school | 35 (23) | 25 (21) | 10 (28) | |
| Completed high school | 59 (39) | 46 (39) | 13 (36) | |
| Some college/technical degree | 46 (30) | 37 (32) | 9 (25) | |
| College degree or higher | 11 (7) | 8 (7) | 3 (8) | 0.564 |
| Unknown | 2 (1) | 1 (1) | 1 (3) | |
| Family characteristics | n (%) | n (%) | n (%) | |
| Socio-economic status1 | ||||
| High | 36 (24) | 29 (25) | 7 (19) | |
| Medium | 34 (22) | 29 (25) | 5 (14) | |
| Low | 83 (54) | 59 (50) | 24 (67) | 0.172 |
| Primary caregiver martial status | ||||
| Married | 86 (56) | 63 (54) | 23 (64) | |
| Divorced/separated/never married/unknown | 67 (44) | 54 (46) | 13 (36) | 0.288 |
| Primary caregiver smoking status | ||||
| Smoker | 115 (75) | 82 (70) | 33 (92) | |
| Non-smoker | 38 (25) | 35 (30) | 3 (8) | 0.009 |
| Household density2 | ||||
| No siblings | 42 (27) | 30 (25) | 12 (33) | |
| 1 sibling | 57 (37) | 43 (37) | 14 (39) | |
| 2 or more siblings | 53 (35) | 44 (38) | 9 (25) | 0.177 |
| Unknown | 1 (1) | 0 (0) | 1 (3) | |
| Distance home to hospital (miles)3 | ||||
| <35 miles | 21 (14) | 15 (13) | 6 (17) | |
| ≥35 miles and <300 miles | 57 (37) | 45 (38) | 12 (33) | |
| ≥300 miles | 75 (49) | 57 (49) | 18 (50) | 0.850 |
Hollingshead's Four Factor Index of Social Status [26]: High=Major business & professional, or medium business, minor professional, Medium=Skilled craftsman, clerical, sales workers, Low=Machine operators, semi-skilled workers, or unskilled laborers, menial service workers;
Number of siblings/cousins residing in household ≤18 years;
Groupings for distance determined by hospital transportation assistance policy, where 35 miles or more and less than 300 miles are eligible for bus, rail or mileage assistance, and 300 miles or more are eligible for air transportation assistance in addition to bus/rail/mileage assistance.
T-test was used for continuous variables, Pearson chi-square test or Fisher's exact test was used for nominal categorical variables, and Mantel-Haenszel chi-square test was used for ordered category variables.
Table II. Medical and treatment-related characteristics by participation status on ETS reduction trial among pediatric cancer patients.
| Total (N = 153) |
Participants (n = 117) |
Non-participants (n = 36) |
P-Value* | |
|---|---|---|---|---|
| n (%) | n (%) | n (%) | ||
| Child cancer diagnosis | ||||
| CNS Tumor | 16 (10) | 8 (7) | 8 (22) | |
| Leukemia / Lymphoma | 96 (63) | 77 (66) | 19 (53) | |
| Solid Tumor | 41 (27) | 32 (27) | 9 (25) | 0.030 |
| Time since diagnosis (days) | ||||
| 0-30 days | 2 (1) | 2 (2) | 0 (0) | |
| 31-90 days | 73 (48) | 56 (48) | 17 (47) | |
| 91-180 days | 29 (19) | 22 (19) | 7 (20) | |
| >180 days | 49 (32) | 37 (31) | 12 (33) | 0.754 |
| History of relapse (prior to recruitment) | ||||
| No | 137 (90) | 104 (89) | 33 (92) | |
| Yes | 16 (10) | 13 (11) | 3 (8) | 0.764 |
| Number of days hospitalized (30 days prior to recruitment) | ||||
| 0 days | 91 (60) | 67 (57) | 24 (67) | |
| 1-7 days | 43 (28) | 34 (29) | 9 (25) | |
| 8-14 days | 16 (10) | 14 (12) | 2 (5) | |
| > 14 days | 3 (2) | 2 (2) | 1 (3) | 0.384 |
| History of surgery (prior to recruitment) | ||||
| No | 91 (59) | 75 (64) | 16 (44) | |
| Yes | 62 (41) | 42 (36) | 20 (56) | 0.036 |
| History of chemotherapy (prior to recruitment) | ||||
| No | 10 (7) | 4 (3) | 6 (17) | |
| Yes | 143 (93) | 113 (97) | 30 (83) | 0.012 |
| History of radiation (prior to recruitment) | ||||
| No | 121 (79) | 96 (82) | 25 (69) | |
| Yes | 32 (21) | 21 (18) | 11 (31) | 0.104 |
| Planned surgery (post recruitment) | ||||
| No | 112 (73) | 83 (71) | 29 (81) | |
| Yes | 41 (27) | 34 (29) | 7 (19) | 0.265 |
| Planned chemotherapy (post recruitment) | ||||
| No | 21 (14) | 16 (14) | 5 (14) | |
| Yes | 132 (86) | 101 (86) | 31 (86) | 0.999 |
| Planned radiation (post recruitment) | ||||
| No | 111 (73) | 91 (78) | 20 (56) | |
| Yes | 42 (27) | 26 (22) | 16 (44) | 0.009 |
| Total number of therapeutic and non-therapeutic protocols enrolled on (prior to recruitment) | ||||
| No protocols | 26 (17) | 21 (18) | 5 (14) | |
| 1 protocol | 46 (30) | 32 (28) | 14 (39) | |
| 2 protocols | 34 (22) | 25 (21) | 9 (25) | |
| 3-6 protocols | 47 (31) | 39 (33) | 8 (22) | 0.484 |
T-test was used for continuous variables, Pearson chi-square test or Fisher's exact test was used for nominal categorical variables, and Mantel-Haenszel chi-square test was used for ordered category variables.
Since history of chemotherapy and surgery, and planned radiation therapy were highly associated with diagnosis (all Ps<0.001, data not shown), treatment variables were not further investigated in the multivariable analyses. Additionally, an initial model indicated that the odds of non-participation did not significantly differ for leukemia and solid tumor diagnoses (P=0.847, data not shown), so these categories were combined. Exact multivariable logistic regression modeling revealed that families with caregivers who were active smokers were over 6 times as likely to be non-participants than those with non-smoking parents (OR=6.48, P=0.002), and families with female caregivers were over 8 times as likely be non-participants than those with male caregivers (OR=8.56, P=0.023). Families who had a child with CNS tumor were also almost 5 times as likely to be non-participants than families of children with leukemia/lymphoma or solid tumors (OR=4.63, P=0.021). Results from the multivariate logistic regression modeling can be found in Table III.
Table III. Exact conditional logistic regression analyses investigating predictors of nonparticipation on ETS reduction trial among pediatric cancer patients.
| Parameter1 | Odds Ratio | 95% CIs | P-Value |
|---|---|---|---|
| Model | |||
| Smoking Target Parent | 6.48 | 1.74 – 37.05 | 0.002 |
| Female Target Parent | 8.56 | 1.22 - 376.10 | 0.023 |
| CNS Tumor child diagnosis | 4.63 | 1.23 – 18.61 | 0.021 |
Reference groups are non-smoking target parent, male target parent, and leukemia and other sold tumors combined for CNS tumor.
Discussion
ETS exposure has deleterious health implications for all children. Childhood cancer patients are at a relatively higher risk for the development of health complications as a result of ETS exposure due to variables associated with the course of disease, treatment, and risk of further adverse health consequences after treatment. As children with cancer are exposed to significant levels of ETS, interventions are needed to reduce second-hand smoke exposure as a means of reducing medical risk in this fragile population.
As with all clinical research, recruitment procedures and participation rates can influence the composition of a study sample, and therefore affect the generalizability of research results. In order to reduce participant characteristic bias across study conditions, we employed random assignment in our study design. However, the representitiveness of any sample is dependent on who consents to study participation. As a result, determining which participant characteristics predict study enrollment is an important step, not only to identify appropriate covariates when examining the efficacy of clinical trials, but to inform study recruitment and retention strategies for other scientists engaging in pediatric behavioral health research [20].
In this regard, our study aimed to identify demographic, social, medical, and treatment-related variables related to study non-participation and found that smokers were less likely to enroll than non-smokers. This finding is somewhat troubling given that smoking caregivers presumably place their children at the greatest risk for exposure, and ideally, would be the primary target for ETS intervention enrollment. It is possible that non-smokers are more likely to participate in these trials due to the perceived reduced threat that smokers may associate with tobacco-based intervention. As non-smokers, they are not guilty of producing ETS, and are perhaps less burdened by influencing changes in smoking behavior that reduce health risk in their child with cancer. As tobacco use frequently increases for smokers under stressful circumstances [27], an intervention aimed at changing their smoking behavior may be less appealing given the demands of their child's medical treatment.
In addition to smokers, female caretakers were also less likely to enroll on study as compared to males. Table I shows that 87% of eligible study participants were female, suggesting that mothers were the primary caretakers of patients in our sample. As our institutional typically pays for one parent to accompany their child during medical visits, an initial interpretation of this finding relates to increased parenting stress burden. Many of these mother-child dyads are traveling (and sometimes relocating) outside of their home community for cancer treatment, and the additional burden associated with adjusting to the demands of cancer treatment (in the absence of immediate family and established support systems) could reduce maternal time/energy to participate in interventions of this nature. Conversely, the majority of eligible males (who enrolled on study at a 95% participation rate) had a female spouse or familial support locally during the period of their child's cancer treatment, potentially reducing paternal parenting stress burden. Although this explanation of gender enrollment differences seems plausible, it is important to note that significant differences in SES, marital status, household density, or distance from home to hospital (all factors which associate with parenting stress burden) were not identified across study groups.
Diagnostic type was also a predictor of study non-participation. Families of children with CNS tumors were almost 5 times more likely to be non-participants than those with leukemia or solid tumors. Because the prognosis for children with CNS tumors is generally worse than those with other types of cancer, it is hypothesized that these families are experiencing higher levels of distress. It may be that the motivation to change smoking behaviors within these households is relatively lower. Also, the increased demands associated with treating a brain tumor may place participation in preventative behavioral health interventions at a lower priority when allocating familial resources.
The findings of our study must be interpreted within the context of its limitations. Although we investigated the influence of sociodemographic, medical and treatment-related variables on study participation, we were not able consider the role of psychological variables (e.g., perceived benefit of study participation, mother/father conflict about smoking, maternal assertiveness, etc.) due to lack of data among those who refused study participation. These types of psychological variables could have influenced study participation. Future studies should devise systems of collecting more in-depth information on non-participants, while at the same time, respecting their decision to decline study participation. Due to the number of factors considered and our sample size, we were also unable to test the effect of interacting variables on study enrollment.
Although 76% of eligible participants enrolled in the ETS reduction trial, findings suggest that future familial recruitment strategies should be tailored to parental smoking status and gender, as well as child diagnosis and treatment. Additionally, interventions should employ strategies that gently persuade smokers to participate in tobacco trials that reduce potential threat and defensiveness. Developing and implementing interventions designed to increase study enrollment among groups underrepresented in ETS-based clinical trials should reduce the likelihood of sampling bias while increasing the generalizability of our research findings. This approach should allow for the testing, development, and implementation of empirically-sound ETS interventions which in turn may facilitate the ultimate goal of reducing health risk among pediatric patients on-treatment for cancer.
References
- 1.Gergen PJ, Fowler JA, Maurer KR, et al. The burden of environmental smoke exposure on the respiratory health of children 2 months of age through 5 years of age in the United States: Third National Health and Nutrition Examination Survey, 1988-1994. Pediatrics. 1998;101(2):e8. doi: 10.1542/peds.101.2.e8. [DOI] [PubMed] [Google Scholar]
- 2.Farrelly MC, Chen J, Thomas KY, et al. Youth exposure to environmental tobacco smoke. American Legacy Foundation First Look Report. 2001;6:6–24. [Google Scholar]
- 3.Etzel RA. Environmental tobacco smoke. Immunol Allergy Clin N Am. 1994;14:621–633. [Google Scholar]
- 4.Environmental Protection Agency, Office of Research and Development, Office of Air and Radiation. Respiratory health effects of passive smoking: lung cancer and other disorders. Washington, DC: Environmental Protection Agency; 1992. Publication EPA/600/6-90/006F. [Google Scholar]
- 5.Centers for Disease Control, Center for Health Promotion and Education, Office on Smoking and Health. The health consequences of involuntary smoking: a report of the Surgeon General. Rockville, MD: US Department of Health and Human Services; 1986. Publication CDC 87-9398. [Google Scholar]
- 6.Environmental tobacco smoke: measuring exposures and assessing health effects. Vol. 28. National Research Council; Washington, DC: National Academy Press; 1986. [PubMed] [Google Scholar]
- 7.DiFranza JR, Lew RA. Morbidity and mortality in children associated with the use of tobacco products by other people. Pediatrics. 1996;97:560–568. [PubMed] [Google Scholar]
- 8.Cook DG, Strachan DP. Health effects of passive smoking −10: Summary of effects of parental smoking on the respiratory health of children and implications for research. Thorax. 1999;54:357–366. doi: 10.1136/thx.54.4.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Strauss RS. Environmental tobacco smoke and serum Vitamin C levels in children. Pediatrics. 2001;107:540–542. doi: 10.1542/peds.107.3.540. [DOI] [PubMed] [Google Scholar]
- 10.Tyc VL, Throckmorton-Belzer L, Klosky JL, et al. Smoking among parents of pediatric cancer patients and children's exposure to environmental tobacco smoke. Journal of Child Health Care. 2004;8(4):286–298. doi: 10.1177/1367493504047319. [DOI] [PubMed] [Google Scholar]
- 11.US Department of Health and Human Services (DHHS) The Health Consequences of Smoking. Chronic Obstructive Lung Disease: A Report of the Surgeon General, DHHS publication no. (PHS) 84-50205. Rockville, MD: US Department of Health and Human Services, Public Health Service Office on Smoking and Health; 1995. [Google Scholar]
- 12.O'Driscoll BR, Hasleton PS, Taylor PM, et al. Active lung fibrosis up to 17 years after chemotherapy with carmustine (BCNU) in childhood. New England Journal of Medicine. 1990;323:378–382. doi: 10.1056/NEJM199008093230604. [DOI] [PubMed] [Google Scholar]
- 13.Benoist MR, Lemerle J, Jean R. Effects on pulmonary function of whole lung irradiation for Wilms' tumor in children. Thorax. 1982;37:175–180. doi: 10.1136/thx.37.3.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lipshultz SE, Colan SD, Gelbar RD, et al. Late cardiac effects of doxorubicin therapy for acute lymphoblastic leukemia in childhood. N Engl J Med. 1991;324:808–815. doi: 10.1056/NEJM199103213241205. [DOI] [PubMed] [Google Scholar]
- 15.Meisler L. ‘Late Effects of Childhood Cancer Therapy’, Current Problems in Pediatrics. 1993;23:102–31. doi: 10.1016/0045-9380(93)90019-9. [DOI] [PubMed] [Google Scholar]
- 16.Neglia JP, Meadows AT, Robison LL, et al. Second malignant neoplasms after acute lymphoblastic leukemia in childhood. New England Journal of Medicine. 1991;325(19):1330–6. doi: 10.1056/NEJM199111073251902. [DOI] [PubMed] [Google Scholar]
- 17.Robison GC, Mertens A. Second Tumors After Treatment of Childhood Malignancies. Hematology and Oncology Clinics of North America. 1993;7(2):401–15. [PubMed] [Google Scholar]
- 18.Hovell MF, Meltzer SB, Wahlgren DR, et al. Asthma management and environmental tobacco smoke exposure reduction in Latino children: a controlled trial. Pediatrics. 2002;111(5):946–956. doi: 10.1542/peds.110.5.946. [DOI] [PubMed] [Google Scholar]
- 19.Winickoff JP, Hillis VJ, Palfrey JS, et al. A smoking cessation intervention for parents of children who are hospitalized for respiratory illness: The STOP tobacco outreach program. Pediatrics. 2003;112:1127–1133. doi: 10.1542/peds.111.1.140. [DOI] [PubMed] [Google Scholar]
- 20.Betan EJ, Roberts MC, McCluskey-Fawcett K. Rates of participation for clinical child and pediatric psychology research: issues in methodology. Journal of Clinical Child Psychology. 1995;24:227–235. [Google Scholar]
- 21.Zebracki K, Drotar D, Kirchner HL, et al. Predicting attrition in a pediatric asthma intervention study. Journal of Pediatric Psychology. 2003;28(8):519–528. doi: 10.1093/jpepsy/jsg042. [DOI] [PubMed] [Google Scholar]
- 22.Wilson SR, Yamada EG, Sudhaker R, et al. A controlled trial of an environmental tobacco smoke reduction intervention in low-income children with asthma. Chest. 2001;120:1709–1722. doi: 10.1378/chest.120.5.1709. [DOI] [PubMed] [Google Scholar]
- 23.Krieger JW, Takaro TK, Song L, Weaver M. The Seattle-King County Healthy Homes Project: a randomized, controlled trial of a community health worker intervention to decrease exposure to indoor asthma triggers. American Journal of Public Health. 2005;95(4):652–9. doi: 10.2105/AJPH.2004.042994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Irvine L, Crombie IK, Clark RA, Slane PW, Feyerabend C, Goodman KE, Cater JI. Advising parents of asthmatic children on passive smoking: randomised controlled trial. BMJ (Clinical Research Ed) 1999;318(7196):1456–9. doi: 10.1136/bmj.318.7196.1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Agresti A. Categorical Data Analysis. 2nd. New York: John Wiley & Sons; 2002. [Google Scholar]
- 26.Hollingshead AB. Four factor index of social status. Department of Sociology, Yale University; New Haven, CT: 1975. [Google Scholar]
- 27.Shadel WJ, Mermelstein RJ. Cigarette smoking under stress: the role of coping expectancies among smokers in a clinic-based smoking cessation program. Health Psychology. 1993;12(6):443–450. doi: 10.1037//0278-6133.12.6.443. [DOI] [PubMed] [Google Scholar]


