Abstract
Outdoor air pollution at levels occurring in many urban areas around the world has substantial adverse effects on health. Children in general, and children with asthma in particular, are sensitive to the adverse effects of outdoor air pollutants, including ozone, nitrogen oxides, and respirable particulate matter. A growing number of studies also show that children living in environments near traffic have increased risks of new-onset asthma, asthma symptoms, exacerbations, school absences, and asthma-related hospitalizations. The large population of children exposed to high levels of outdoor air pollutants and the substantial risks for adverse health effects present unexploited opportunities to reduce the burden of asthma. Because the evidence indicates significant adverse effects of air pollution at current levels, there is clearly a need to reduce levels of regulated pollutants such as ozone, as well as unregulated pollutants in tailpipe emissions from motor vehicles. Achieving this long-term goal requires the active involvement of physicians and medical providers to ensure that the health of children is at the top of the list of competing priorities for regulatory policy decision-making. Clinical approaches include treatment to control asthma and patient education to reduce adverse effects of the disease. Reduction in exposures also can be approached at a policy level through changes in schools and school bus operations. Beyond clinical and public health approaches to reduce exposure, another strategy to be used before clean air goals are met is to decrease the susceptibility of children to air pollution. Emerging research indicates that dietary supplementation for individuals with low antioxidant levels is one promising approach to reducing susceptibility to air pollution. A second approach involves induction of enzymatic antioxidant defenses, especially for individuals with at-risk genetic variants of key antioxidant enzymes.
Keywords: air pollution, asthma, children, genetic susceptibility
We are all too familiar with the brown haze produced by photochemical smog in many urban regions of the world. The adverse health effects of several pollutants in smog have been studied extensively, and such pollutants now are recognized to be substantial threats to health at levels once thought to be safe. Ambient air pollutants with clear evidence for adverse health effects (criteria pollutants) have been identified and regulated by a variety of agencies.1–8 Criteria pollutants include ozone, nitrogen oxides such as nitrogen dioxide, and respirable particulate matter (PM). Other pollutants, such as volatile organic compounds or ultrafine particles, also may have adverse health effects.9
Elevated levels of regional pollutants in smog affect large geographic areas. A rapidly emerging issue involves the health effects of air pollutants produced locally, largely through traffic-related combustion of fossil fuels. These local exposures differ from regional exposures because the emissions include larger amounts of reactive gases and ultrafine particles. Very high local concentrations of these and other combustion products are observed on or near major highways and roadways.10 These unregulated local emissions seem to be highly toxic and pose a major new clinical and public health challenge.11,12 Although exposure to fresh tailpipe emissions is common, levels of the most toxic of these pollutants currently are not included in the air pollution regulatory framework. It should also be noted that most outdoor pollutants penetrate into buildings and make substantial contributions to indoor pollution levels.13
Children are vulnerable to the effects of air pollution because their lungs and immune systems are developing, they are more active in environments with high levels of pollutants (eg, while participating in sports in the afternoon), and they receive higher doses, relative to adults, because of differences in breathing rates and patterns. The inflamed and hyperreactive airways of children with asthma create a new level of vulnerability. Consistent with the increased vulnerability of children, a large and growing number of studies show that children living near traffic or high levels of ozone, nitrogen dioxide, or PM have increased risks of adverse respiratory effects.5,6,14–16
Many of the adverse effects of air pollution seem to be mediated through increased levels of oxidative and nitrosative stress.17,18 Research indicates that children’s vulnerability may be further increased by inadequate antioxidant defenses18–21 attributable to low levels of small-molecule antioxidants, such as vitamins C and E, or variations in the expression or function of enzymatic antioxidants, such as glutathione-S-transferases (GSTs).18 These antioxidant defense elements offer promising chemoprevention targets that have the potential to reduce the burden of asthma.
METHODS
The spectrum of air pollutants and related health effects in children with and without asthma and the air pollution-related opportunities to reduce the burden of asthma are illustrated primarily with data from studies conducted in southern California, as part of the Children’s Health Study (CHS) and the California Health Interview Survey. The CHS is one of the largest and most comprehensive investigations of the long-term effects of air pollution on the respiratory health of children.22,23 To maximize the differences in air pollution concentrations and mixtures, >6000 schoolchildren were selected from classrooms in 12 communities across the region. Beginning in 1996 and continuing until high school graduation, yearly questionnaires assessed the development of respiratory symptoms and current activity patterns, and lung function was measured annually through spirometry. School absences were monitored to evaluate the effects of pollution on acute respiratory illnesses.24 Outdoor concentrations of ozone, PM of <2.5-μm diameter, PM of <10-μm diameter, and nitrogen dioxide were measured at central monitoring stations.
In the CHS study of air pollution and new-onset asthma, a cohort of >4000 children was monitored from fourth grade until high school graduation. New-onset cases of asthma were ascertained annually. Regional air pollutants were monitored continuously during the 8-year follow-up period. CHS findings were also used to examine the relationship between living near a major road and asthma onset before study entry. Lifetime history of doctor-diagnosed asthma and prevalent asthma and wheeze were evaluated with questionnaires. Results from studies of this highly exposed population were used to illustrate the magnitude of the contribution of outdoor air pollution and the potential for interventions to reduce the burden of asthma.
An experimental exposure study was conducted to determine whether diesel exhaust particles (DEPs) enhance allergic responses to allergens in a manner dependent on GST genotype.25 In a crossover exposure design using a nasal challenge model with DEPs and ragweed allergen, 19 ragweed-allergic subjects, 20 to 34 years of age, were exposed randomly to 4 conditions, that is, placebo, allergen, DEPs, and DEPs plus allergen. Measured responses in nasal lavage fluid included allergen-specific immunoglobulin E (IgE), histamine, interferon-γ, and interleukin 4 levels. GST genotypes were determined, and responses were examined in the GSTM1- and GSTT1-null and -present groups and the GSTP1-variant group.
RESULTS
Asthma Prevalence
Table 1 summarizes a number of important adverse health effects of regional outdoor air pollutants on children with asthma. Key adverse effects for this discussion are new-onset asthma, asthma symptoms, exacerbations, school absences, and hospitalizations. The California Health Interview Study determined that a large population of vulnerable children in southern California is exposed to levels of air pollutants spanning the entire range found across the nation. In addition, a substantial number of children with asthma in that region reside and go to school near extremely busy highways. The lifetime prevalence of childhood asthma was 12.1%, accounting for >400 000 children in Los Angeles and San Diego counties. Of the children with asthma, 10% had severe asthma with daily or weekly symptoms, and 11% had an asthma-related emergency department visit or hospitalization in the year before the interview.16
TABLE 1.
Adverse Effects of Regional and Traffic-Related Air Pollutants on Children With Asthma
| Pollutants |
| Ozone |
| Nitrogen oxide |
| Respirable PM (<10 and <2.5 μm) |
| Vehicle exhaust (trucks, cars, off-road vehicles, trains, or ships) |
| Health effects in children with asthma |
| Respiratory symptoms |
| Wheezing (acute) |
| Bronchitis (chronic) |
| Rescue medication use |
| New-onset asthma |
| Decreased lung function |
| Emergency department visits |
| Hospitalizations |
| School absences |
Outdoor Air Pollution and New-Onset Asthma
A number of older epidemiological studies did not indicate an excess risk of asthma in geographic regions with high outdoor air pollution levels, which suggests that air pollution might not be a cause of asthma. More-recent studies, however, support an important role for air pollutants in asthma pathogenesis when key factors are considered, including air pollution dose, traffic-related pollution, and genetic susceptibility.
In the CHS, long-term average ozone levels were associated with new-onset asthma but only in children with high inhaled doses of ozone (Fig 1). Children who played ≥3 team sports (a marker for high outdoor ventilation rates) had more than threefold increased risk if they resided in communities with the highest average ozone levels. Team sports were not associated with new-onset asthma in communities with low levels of ozone. These findings indicate that inhaled doses of ozone may be an important contributor to asthma, especially in communities with high ozone levels. Ongoing studies also show that genetic variations in antioxidant and inflammatory genes play a role in ozone susceptibility.19,25,26 Strategies designed to reduce ozone exposure through decreases in ambient levels or avoidance of vigorous outdoor activity during periods of high ozone concentrations have been developed but have yet to be fully implemented.
FIGURE 1.
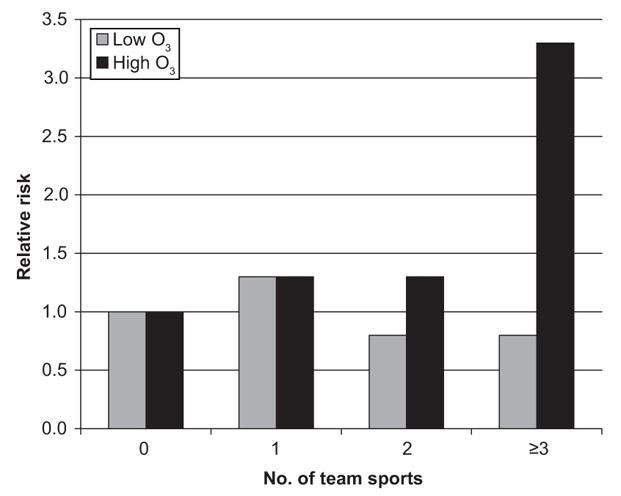
Ozone, exercise, and new-onset asthma in the CHS.28
Traffic-related air pollutants also seem to increase the risk of asthma, as shown in results from the CHS (Fig 2). Children living within 75 m of a major road with elevated estimated levels of traffic-related pollution showed an increased risk of asthma. Residence within 75 m of a major road was associated with a 1.5-fold increased risk of lifetime asthma and wheeze. This association was not explained by differences in ethnicity or other sociodemographic characteristics of children living near major roads. Ongoing work also shows a strong relationship between traffic and new-onset asthma during school years. These findings are consistent with a growing body of evidence from international studies that breathing fresh vehicle exhaust increases the risk of new-onset asthma.5
FIGURE 2.
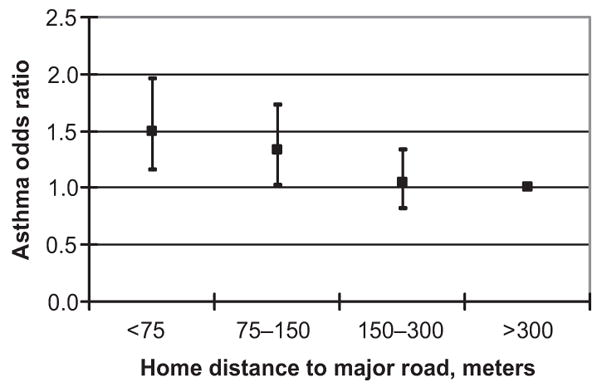
Traffic proximity and risk of asthma in the CHS.6 (Reproduced with permission from McConnell R, Berhane K, Yao L, et al. Environ Health Perspect. 2006;114(5):769).
Air Pollution Effects on Children With Asthma
Regional and local air pollutants have been implicated in producing a broad spectrum of adverse effects in children with asthma. In the CHS, children with physician-diagnosed asthma had more bronchitis and persistent phlegm production if they lived in communities with more nitrogen dioxide or particulate pollution (Fig 3).27 Children with asthma in communities with the highest PM or nitrogen dioxide levels had twice the prevalence of bronchitis as their counterparts in communities with cleaner air. These findings illustrate that a modest increase in the risk of bronchitis attributable to air pollution may result in a considerable burden of increased asthma symptoms in children living in communities with high air pollution levels.
FIGURE 3.
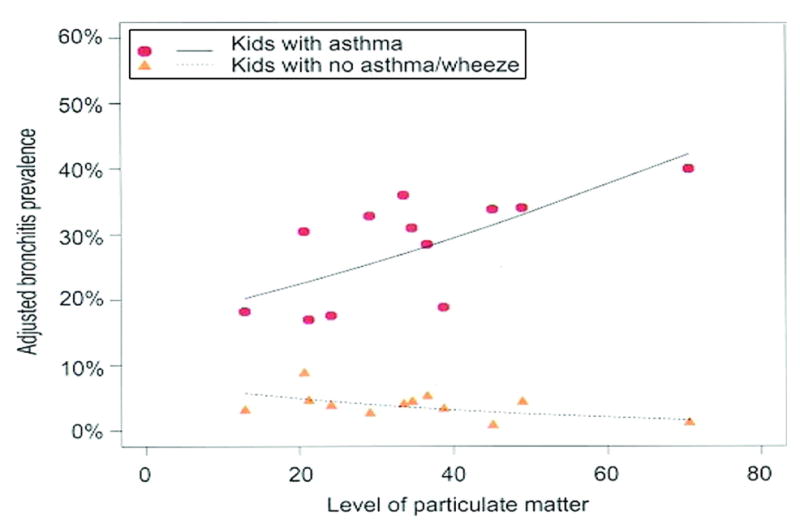
PM of <10-μm diameter (PM10) and bronchitis in children with and without asthma in the CHS.27 Reproduced with permission from McConnell R, Berhane K, Gilliand F, et al. Air pollution and bronchitic symptoms in Southern California children with asthma. Environ Health Perspect. 1999;107(9):759.
Local traffic-related pollutants also have substantial adverse effects on children with asthma. In the CHS, heavy residential traffic was associated with a 3.5-fold increase in the prevalence of emergency department visits or hospitalizations among children with asthma.16 Traffic exposures also were associated with increased wheezing, medication use, and school absences. For example, residential proximity to busy roads or high traffic volumes was associated with increased respiratory-related school absences among children with asthma, with a 300% increase in the risk of absence for children living within 75 m of a major road.
Clinical and Public Health Approaches to Reducing the Contribution of Air Pollution to the Burden of Asthma
The large population of children exposed to high levels of outdoor air pollutants and the substantial risks for adverse health effects present unexploited opportunities to reduce the burden of asthma. For the outcomes discussed, high exposure levels were associated with approximately threefold increased risk for each outcome. If air pollution levels were reduced to match levels in the cleanest community, then annual asthma-related emergency department visit and hospitalization rates would be predicted to decrease from 22% to 6%, the prevalence of bronchitis would decrease from 40% to 20%,27 and asthma-related school absences could be reduced by two thirds. New cases of asthma among the most-active children living in polluted communities would be predicted to decrease by 75%.28
Because the evidence indicates significant adverse effects of air pollution at current levels, there is clearly a need to reduce levels of criteria pollutants and tailpipe emissions from motor vehicles. Success in achieving this long-term goal requires the active involvement of physicians and medical providers to ensure that the health of children is at the top of the list of competing priorities for policymaking. Table 2 provides a list of selected options for interventions involving changes in technology, urban planning, and patient behavior.
TABLE 2.
Selected Options for Primary and Secondary Strategies to Reduce Children’s Exposure to Outdoor and Traffic-Related Air Pollution
| Policy Target | Intervention |
|---|---|
| Primary strategies | |
| Technology | Reduce emissions in new vehicles |
| Retrofit school buses and diesel trucks | |
| Inspect vehicle emissions for all engines | |
| Increase fuel economy | |
| Use clean fuels | |
| Develop zero-emission vehicles | |
| Urban design | Invest in public transport |
| Limit urban sprawl | |
| Build bicycle and walking paths | |
| Behavior | Use carpools |
| Take public transportation to work | |
| Walk/bicycle | |
| Use school buses or walk to school instead of driving | |
| Forbid idling of school buses | |
| Secondary strategies | |
| Technology | Air condition or filter air in schools |
| Urban design | Limit vehicles near schools |
| Avoid construction of schools near busy roadways | |
| Behavior | Avoid walking along streets with heavy traffic |
| Review guidelines for children with asthma | |
| Reduce outdoor activity when pollution levels are high | |
| Consider antioxidant supplementation for chemoprevention in high-pollution areas | |
Adapted from Künzli N, McConnell R, Bates D, et al. Breathless in Los Angeles: the exhausting search for clean air. Am J Public Health. 2003;93(9):1497.
Although there have been impressive improvements in emissions as a result of national, state, and regional air pollution policies and regulations, these gains have been negated by increasing vehicle-miles driven, and air pollutant levels have not decreased significantly.25 Although recent increase in fuel prices have been associated with decreased miles driven in the United States, exposures are likely to continue to increase with population growth and increasing world trade traffic, without novel approaches to target regulation of vehicles and urban planning. Given the new economic models that depend on international trade, just-in-time logistical systems, and goods movement, emission levels are likely to increase unless innovative approaches are developed to address this new challenge.
Given the time needed for regulations to be developed and implemented, outdoor pollution levels are not likely to decrease substantially in the near term, necessitating secondary approaches to reduce exposure or to decrease susceptibility. Clinical approaches include appropriate medication regimens to maintain asthma control and patient education to reduce adverse effects through avoidance of high-level exposures, such as secondhand smoke and outdoor activities during pollution advisories. Patient education should include the following elements: (1) reduce outdoor activities when the air quality index and daily ozone levels are in the unhealthy range; (2) increase peak flow checks during periods with poor air quality index values; and (3) exercise away from major roads.
Schools offer an important opportunity to reduce air pollution exposures.29 Reduction in exposures can also be approached at a policy level, through procedural and structural changes in schools and school-bus operations. Procedures to reduce exposures during transit on diesel-powered school buses or from idling buses near schools can be addressed with minimal cost. Exposures on days when air pollution levels are unhealthy can be reduced by using air conditioning and canceling outdoor recess and sports practices. On a longer-term basis, locating schools away from busy freeways and locating recess areas and sports fields away from busy roads are important considerations when new schools are being designed.
DISCUSSION
New Opportunities to Reduce the Contribution of Air Pollution to the Burden of Asthma: Genetic Susceptibility and Antioxidant Defenses
Beyond clinical and public health approaches to reducing exposure, decreasing the susceptibility of children to air pollution offers another strategy that can be used until clean air goals are met. Emerging research indicates that modifiable antioxidant defenses may be an important response to air pollution. Airborne particulate pollutants, such as DEPs, are thought to exacerbate lung and cardiovascular diseases through induction of oxidative stress.18 The role of genes involved in oxidative stress produced by xenobiotics (phase II enzymes) has been examined by using GST genotypes, including GSTM1 and GSTT1, which have a null genotype that results in no protein product, and GSTP1, which has a well-studied functional variant (Ile105Val).
In individuals with GSTM1-null or GSTP1-Ile105 genotypes, DEPs enhanced nasal responses to allergen (Table 3). Compared with subjects with a functional GSTM1 genotype, GSTM1-null subjects had significantly larger increases in IgE levels (146 vs 13.5 U/mL; P <.01) and histamine levels (13.9 vs 6.1 nmol/L; P = .03) after a DEP plus allergen challenge (Table 3). The wild-type GSTP1 genotype was associated with increased IgE levels (149 vs 29.6 U/mL; P < .01) and histamine levels (14.5 vs 6.1 nmol/L; P = .01) after the same challenge. None of the GSTs modified the response to allergen alone. Common polymorphisms in GSTM1 and GSTP1 powerfully modify the adjuvant effect of DEPs on allergic inflammation and identify a large population susceptible to adverse health effects of DEP exposure.
TABLE 3.
Effects of GSTM1, GSTT1, and GSTP1 on Nasal IgE Levels
| Genotype | Nasal IgE Level, Median, U/mL |
||
|---|---|---|---|
| Allergen +Clean Air | Allergen +DEP | Median Difference | |
| GSTM1 | |||
| Null (n = 14) | 6.9 | 106.6 | 102.5 |
| Present (n = 5) | 8.9 | 49.8 | 45.5 |
| Wilcoxon test, P | .40 | .15 | .03 |
| GSTT1 | |||
| Null (n = 9) | 7.9 | 89.5 | 84.7 |
| Present (n = 10) | 7.8 | 49.3 | 45.9 |
| Wilcoxon test, P | .57 | .35 | .35 |
| GSTP1 | |||
| Ile/Ile (n = 13) | 7.8 | 123.5 | 120.3 |
| Ile/Val (n = 6) | 8.4 | 31.5 | 27.7 |
| Wilcoxon test, P | 1.0 | .02 | .03 |
Adapted from Gilliland FD, Li YF, Saxon A, Diaz-Sanchez D. Effect of glutathione-S-transferase M1 and P1 genotypes on xenobiotic enhancement of allergic responses: randomized, placebo-controlled crossover study. Lancet. 2004;363(9403):121.
One attractive approach to reducing the effects of air pollutants such as DEPs is through induction of enzymatic antioxidant defenses, especially in individuals with at-risk genetic variants of key antioxidant enzymes. A prototype for this approach is dietary induction of phase II metabolic enzymes to protect against a model pollutant (DEPs). In a proof-of-principle study, dietary sulforaphane, a potent inducer of phase II enzymes, was shown to increases enzyme expression and to reduce inflammatory responses.30 In an in vitro cell system, Wan and Diaz-Sanchez30 investigated whether sulforaphane stimulated phase II enzyme induction and subsequently reduced the effect of diesel extracts on cytokine production. Sulforaphane increased GSTM1 and NQO1 expression, as well as GST activity, while reducing cytokine production. In primary bronchial epithelial cells, sulforaphane also blocked the increased production of interleukin 8, granulocyte-macrophage colony-stimulating factor, and interleukin 1β from primary human bronchial epithelial cells. As discussed, DEPs have been shown to increase the production of IgE in vitro and in human nasal challenges. The induction of phase II enzymes in B cells by sulforaphane has been shown to reduce the ability of DEPs to increase IgE production.30 Dietary sulforaphane also increases phase II enzyme expression in nasal cells. These results suggest that sulforaphane or other compounds that induce phase II enzymes have promise as air pollution chemopreventive agents.31 These chemopreventive effects also might be available through dietary modification. Additional research is needed to determine whether the adverse effects of air pollution can be reduced through interventions tailored to individual genetic susceptibility.
Clinical and Research Recommendations
The American Academy of Pediatrics has published a policy statement advising that discussions of air quality be integrated into patient education.4 This statement provides a framework for children’s environmental health advocacy and recommends development of effective air pollution policies to protect children’s health. Other clinical and research recommendations for reducing the burden of asthma by addressing the substantial impacts of air pollution are presented in Table 4. Physicians and other health care providers have an opportunity to use both short- and long-term strategies to reduce the effects of air pollution on their patients with asthma. This opportunity can be summarized in 3 broad recommendations, namely, (1) incorporate control of air pollution exposures as an integral component of clinical asthma management, (2) work toward creation of asthma-friendly environments with low air pollution levels, to reduce the overall burden of asthma on individuals, families, and society, and (3) recognize and promote the critical role of health care providers in clinical care, patient advocacy, policy-making, and standard-setting for air pollution, by identifying opportunities for local involvement in policy decision-making and health advocacy.
TABLE 4.
Research, Clinical, and Public Health Recommendations for Reducing the Burden of Asthma
| Research recommendation |
| Develop personalized interventions that target antioxidant defenses in genetically susceptible groups to reduce the adverse effects of air pollution, such as supplements, diet, or chemopreventive medications. |
| Clinical and public health recommendations |
| Incorporate control of air pollution exposures as an integral component of clinical asthma management. |
| Work toward creation of asthma-friendly environments to reduce the overall burden of asthma on individuals, families, and society. |
| Recognize and promote the critical role of physicians and other health care providers in clinical care, patient advocacy, policymaking, and standard- setting for air pollution; identify opportunities for local involvement, and advocate effective air pollution policies to protect children’s health. |
CONCLUSIONS
Interventions that reduce genetic susceptibility to air pollution will likely have a role in personalized asthma management. An urgent research need is development of the knowledge base for personalized interventions that target antioxidant defenses in genetically susceptible groups, to reduce the adverse effects of air pollution. Development of this knowledge base will require a multidisciplinary approach and focused research to address key gaps in the understanding of genetic susceptibility and gene regulation.
Acknowledgments
This work was supported by the Southern California Environmental Health Sciences Center (grant 5P30ES007048), funded by the National Institute of Environmental Health Sciences; the Children’s Environmental Health Center (grants 5P01ES009581, R826708-01, and RD831861-01), funded by the National Institute of Environmental Health Sciences and the Environmental Protection Agency; the National Institute of Environmental Health Sciences (grant 5P01ES011627); the National Heart, Lung, and Blood Institute (grants 5R01HL061768 and 5R01HL076647); and the Hastings Foundation.
Abbreviations
- CHS
Children’s Health Study
- DEP
diesel exhaust particle
- GST
glutathione-S-transferase
- IgE
immunoglobulin E
- PM
particulate matter
Footnotes
The author has indicated he has no financial relationships relevant to this article to disclose.
References
- 1.Bascom R, Bromberg P, Costa D, et al. State of the art: health effects of outdoor air pollution, part 1. Am J Respir Crit Care Med. 1996;153(1):3–50. doi: 10.1164/ajrccm.153.1.8542133. [DOI] [PubMed] [Google Scholar]
- 2.Bascom R, Bromberg P, Costa D, et al. State of the art: health effects of outdoor air pollution, part 2. Am J Respir Crit Care Med. 1996;153(2):477–498. doi: 10.1164/ajrccm.153.2.8564086. [DOI] [PubMed] [Google Scholar]
- 3.Kim JH, Kim JK, Son BK, et al. Effects of air pollutants on childhood asthma. Yonsei Med J. 2005;46(2):239–244. doi: 10.3349/ymj.2005.46.2.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim JJ. Ambient air pollution: health hazards to children. Pediatrics. 2004;114(6):1699–1707. doi: 10.1542/peds.2004-2166. [DOI] [PubMed] [Google Scholar]
- 5.Brauer M, Hoek G, Smit HA, et al. Air pollution and development of asthma, allergy and infections in a birth cohort. Eur Respir J. 2007;29(5):879–888. doi: 10.1183/09031936.00083406. [DOI] [PubMed] [Google Scholar]
- 6.McConnell R, Berhane K, Yao L, et al. Traffic, susceptibility, and childhood asthma. Environ Health Perspect. 2006;114(5):766–772. doi: 10.1289/ehp.8594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Künzli N, McConnell R, Bates D, et al. Breathless in Los Angeles: the exhausting search for clean air. Am J Public Health. 2003;93(9):1494–1499. doi: 10.2105/ajph.93.9.1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brunekreef B, Holgate ST. Air pollution and health. Lancet. 2002;360(9341):1233–1242. doi: 10.1016/S0140-6736(02)11274-8. [DOI] [PubMed] [Google Scholar]
- 9.Delfino RJ. Epidemiologic evidence for asthma and exposure to air toxics: linkages between occupational, indoor, and community air pollution research. Environ Health Perspect. 2002;110(suppl 4):573–589. doi: 10.1289/ehp.02110s4573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhu Y, Kuhn T, Mayo P, Hinds WC. Comparison of daytime and nighttime concentration profiles and size distributions of ultrafine particles near a major highway. Environ Sci Technol. 2006;40(8):2531–2536. doi: 10.1021/es0516514. [DOI] [PubMed] [Google Scholar]
- 11.Nel A. Atmosphere: air pollution-related illness: effects of particles. Science. 2005;308(5723):804–806. doi: 10.1126/science.1108752. [DOI] [PubMed] [Google Scholar]
- 12.Nel A, Xia T, Madler L, Li N. Toxic potential of materials at the nanolevel. Science. 2006;311(5761):622–627. doi: 10.1126/science.1114397. [DOI] [PubMed] [Google Scholar]
- 13.Polidori A, Arhami M, Sioutas C, Delfino RJ, Allen R. Indoor/outdoor relationships, trends, and carbonaceous content of fine particulate matter in retirement homes of the Los Angeles Basin. J Air Waste Manag Assoc. 2007;57(3):366–379. doi: 10.1080/10473289.2007.10465339. [DOI] [PubMed] [Google Scholar]
- 14.Brauer M, Gehring U, Brunekreef B, et al. Traffic-related air pollution and otitis media. Environ Health Perspect. 2006;114(9):1414–1418. doi: 10.1289/ehp.9089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brauer M, Hoek G, Van Vliet P, et al. Air pollution from traffic and the development of respiratory infections and asthmatic and allergic symptoms in children. Am J Respir Crit Care Med. 2002;166(8):1092–1098. doi: 10.1164/rccm.200108-007OC. [DOI] [PubMed] [Google Scholar]
- 16.Meng Y, Rull R, Wilhelm M, et al. Living Near Heavy Traffic Increases Asthma Severity. Los Angeles, CA: UCLA Center for Health Policy Research; 2006. [PubMed] [Google Scholar]
- 17.Li N, Hao M, Phalen RF, Hinds WC, Nel AE. Particulate air pollutants and asthma: a paradigm for the role of oxidative stress in PM-induced adverse health effects. Clin Immunol. 2003;109(3):250–265. doi: 10.1016/j.clim.2003.08.006. [DOI] [PubMed] [Google Scholar]
- 18.Gilliland FD, McConnell R, Peters J, Gong H., Jr A theoretical basis for investigating ambient air pollution and children’s respiratory health. Environ Health Perspect. 1999;107(suppl 3):403–407. doi: 10.1289/ehp.99107s3403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sienra-Monge JJ, Ramirez-Aguilar M, Moreno-Macias H, et al. Antioxidant supplementation and nasal inflammatory responses among young asthmatics exposed to high levels of ozone. Clin Exp Immunol. 2004;138(2):317–322. doi: 10.1111/j.1365-2249.2004.02606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Romieu I, Sienra-Monge JJ, Ramirez-Aguilar M, et al. Antioxidant supplementation and lung functions among children with asthma exposed to high levels of air pollutants. Am J Respir Crit Care Med. 2002;166(5):703–709. doi: 10.1164/rccm.2112074. [DOI] [PubMed] [Google Scholar]
- 21.Gilliland FD, Li YF, Gong H, Jr, Diaz-Sanchez D. Glutathione S-transferases M1 and P1 prevent aggravation of allergic responses by secondhand smoke. Am J Respir Crit Care Med. 2006;174(12):1335–1341. doi: 10.1164/rccm.200509-1424OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Peters J, Avol E, Gauderman W, et al. A study of twelve southern California communities with differing levels and types of air pollution, part II: effects on pulmonary function. Am J Respir Crit Care Med. 1999;159(3):768–775. doi: 10.1164/ajrccm.159.3.9804144. [DOI] [PubMed] [Google Scholar]
- 23.Peters JM, Avol E, Navidi W, et al. A study of twelve Southern California communities with differing levels and types of air pollution, part I: prevalence of respiratory morbidity. Am J Respir Crit Care Med. 1999;159(3):760–767. doi: 10.1164/ajrccm.159.3.9804143. [DOI] [PubMed] [Google Scholar]
- 24.Gilliland FD, Berhane K, Rappaport EB, et al. The effects of ambient air pollution on school absenteeism due to respiratory illnesses. Epidemiology. 2001;12(1):43–54. doi: 10.1097/00001648-200101000-00009. [DOI] [PubMed] [Google Scholar]
- 25.Li YF, Gauderman WJ, Avol E, Dubeau L, Gilliland FD. Associations of tumor necrosis factor G-308A with childhood asthma and wheezing. Am J Respir Crit Care Med. 2006;173(9):970–976. doi: 10.1164/rccm.200508-1256OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Romieu I, Ramirez-Aguilar M, Sienra-Monge JJ, et al. GSTM1 and GSTP1 and respiratory health in asthmatic children exposed to ozone. Eur Respir J. 2006;28(5):953–959. doi: 10.1183/09031936.06.00114905. [DOI] [PubMed] [Google Scholar]
- 27.McConnell R, Berhane K, Gilliland F, et al. Air pollution and bronchitic symptoms in Southern California children with asthma. Environ Health Perspect. 1999;107(9):757–760. doi: 10.1289/ehp.99107757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McConnell R, Berhane K, Gilliland F, et al. Asthma in exercising children exposed to ozone: a cohort study. Lancet. 2002;359(9304):386–391. doi: 10.1016/S0140-6736(02)07597-9. [DOI] [PubMed] [Google Scholar]
- 29.Hricko A. Outdoor air pollution: an issue for schools. In: Frumkin H, Geller R, Rubin I, Nodvin J, editors. Safe and Healthy School Environments. New York, NY: Oxford University Press; 2006. pp. 141–152. [Google Scholar]
- 30.Wan J, Diaz-Sanchez D. Phase II enzymes induction blocks the enhanced IgE production in B cells by diesel exhaust particles. J Immunol. 2006;177(5):3477–3483. doi: 10.4049/jimmunol.177.5.3477. [DOI] [PubMed] [Google Scholar]
- 31.Ritz SA, Wan J, Diaz-Sanchez D. Sulforaphane-stimulated phase II enzyme induction inhibits cytokine production by airway epithelial cells stimulated with diesel extract. Am J Physiol Lung Cell Mol Physiol. 2007;292(1):L33–L39. doi: 10.1152/ajplung.00170.2006. [DOI] [PubMed] [Google Scholar]
- 32.Gilliland FD, Li YF, Saxon A, Diaz-Sanchez D. Effect of glutathione-S-transferase M1 and P1 genotypes on xenobiotic enhancement of allergic responses: randomized, placebo-controlled crossover study. Lancet. 2004;363(9403):119–125. doi: 10.1016/S0140-6736(03)15262-2. [DOI] [PubMed] [Google Scholar]


