Abstract
Current guidelines recommend β blockers for patients after myocardial infarction (MI). Novel therapies for heart failure should be tested in combination with this medication before entering clinical trials. In this methodologic study, we sought to describe the time course of systolic and diastolic parameters of cardiac performance over a 6-wk period in closed-chest model of swine MI treated with a β blocker. Myocardial infarction in pigs (n = 10) was induced by 90-min balloon occlusion of the left anterior descending coronary artery. Echocardiography and pressure–volume data were collected before and at 1 and 6 wk after MI; histopathology was assessed at 6 wk. Left-ventricular (LV) volume increased significantly over 6 wk, with significant decreases in ejection fraction, wall motion index, stroke work, rate of pressure development (dP/dtmax), preload recruitable stroke work, and mechanical efficiency. Impairment of diastolic function was manifested by a significant increase in the exponential β coefficient of the LV end-diastolic pressure–volume relation and reduction of LV pressure decay. At 6 wk, histopathologic analysis showed that the size of the infarct area was 16.3% ± 4.4%, and the LV mass and myocyte cross-sectional area in both the infarct border and remote zones were increased compared with those of noninfarcted pigs (n = 5). These findings suggest a dynamic pattern of remodeling over time in a closed-chest ischemia–reperfusion swine model of acute MI on β-blocker therapy and may guide future studies.
Abbreviations: dP/dt, rate of LV pressure development during systole; LV, left ventricular; LVEDP, LV end-diastolic pressure; LVEF, LV ejection fraction; MI, myocardial infarction; PRSW, preload recruitable stroke work; PVA, pressure–volume area; Vd, ‘dead’ volume intercept
Acute myocardial infarction (MI) is frequently associated with left-ventricular (LV) remodeling leading to progressive heart failure. The mechanisms leading to the development of heart failure are complex, involving changes in LV chamber dimensions and geometry, as well as hemodynamic alterations resulting in overall decreased cardiac pump performance.9 The swine MI model frequently is used to assess the efficacy of novel therapies (such as stem-cell–based treatments) in preclinical studies,12,18,21 because of similarities to the human heart in terms of cardiovascular anatomy, ventricular performance, cardiac metabolism, electrophysiology, coronary artery distribution, and collateralization after MI.18
Guidelines from the American College of Cardiology, American Heart Association,2 and European Society of Cardiology25 recommend that patients with MI should be treated with β blockers, unless contraindicated, because of their proven benefits in reducing mortality.2,25 However, in animal models, especially large animals including swine, most novel therapies are tested in the absence of recommended supportive therapies.3,16,26 Whether improvements in cardiac function seen in the absence of β blockers will persist in animals treated with this agent is unknown. In addition, to our knowledge, a detailed description of changes in the systolic and diastolic parameters that occur after induced MI in swine treated with β blockers has not been published. Accordingly, we sought to use echocardiography, LV pressure–volume measurements, and histology to describe the time course of changes in systolic and diastolic parameters of cardiac performance over 6 wk after MI in a closed-chest swine model of MI with β-blocker therapy. Our data will facilitate assessment of the efficacy of novel therapies in the context of a clinically used medication.
Materials and Methods
Animal model.
Fifteen pathogen-free Yorkshire–Landrace pigs (age, 3 to 4 mo; weight, 35 to 43 kg) were obtained (Pork Power, Turlock, CA) and housed consistent with University of California San Francisco Large Animal Resource Center policy and according to a protocol approved by the institutional animal care and use committee. The study complied with institutional regulations and those of the National Institutes of Health publication the Guide for the Care and Use of Laboratory Animals.13 Before experimental use, the pigs were housed in pairs in pens with a floor area that meets federal standards [1 pig (not exceeding 50 kg) has a floor area of 15 ft2; 2 to 5 pigs (each not exceeding 50 kg) have a floor area of 10 ft2 per animal]. Once the animals underwent surgery, they were individually housed during recovery. Behavior, excreta, and attitude (alertness, responsiveness, and appetite) were monitored for the entire length of the study
Animals (n = 10) each received β blocker (atenolol, 25 mg PO) 1 d prior to infarction to prevent arrhythmia and daily thereafter, beginning 3 d after MI.2,6 The medication was put inside of a banana or mixed with applesauce, and the animal was observed to ensure that the entire dose was ingested. The agent and dose were chosen based on clinical guidelines and absence of side effects.2,25 General anesthesia at all time points was induced by intramuscular injection of ketamine (20 mg/kg), xylazine (2 mg/kg), and atropine (0.04 mg/kg) and then maintained with 2% isoflurane administered through an endotracheal tube. Continuous blood pressure, oxygen saturation, and telemetry monitoring were performed during all procedures.
Closed-chest anteroseptal MI was created by a 90-min transient occlusion of the left anterior descending artery. Briefly, the vessel was engaged by using a 6-French ‘hockey-stick’ guide catheter (diameter, 0.75 mm) through a 6-French arterial sheath in the femoral position. After systemic administration of heparin (100 U/kg), a angioplasty balloon (3.0 × 15 mm) was positioned over a guide wire in the middle of the artery just distal to the second largest diagonal branch. The balloon was inflated at 4 to 5 atm to completely occlude distal flow for 90 min. After balloon deflation, angiography was performed to confirm patency of the artery. Lidocaine (1 mg/min IV) was started before balloon occlusion, and amiodarone (75 mg IV over 10 min) and additional boluses of lidocaine (1 to 3 mg/kg IV) were given at the discretion of the cardiologist to control pronounced ventricular arrhythmias. Two animals died during coronary occlusion due to malignant arrhythmias. The 5 unmanipulated animals were included as reference (noninfarcted) controls for histology and echocardiography findings.
Blood sampling and laboratory analysis.
Whole blood was collected at baseline (before MI) and weekly after MI until euthanasia from the femoral vein (baseline, 1 wk after MI, and at euthanasia) or form the ear venous access site (week 2 to 5). Enzymatic infarct size was assessed by measuring creatine kinase (MB fraction) and troponin I at 4 time points (baseline and 2 h, 1 wk, and 6 wk after ischemia–reperfusion; IDEXX Laboratories, Sacramento, CA).
Serum samples were collected at baseline and weekly intervals, and levels of porcine brain natriuretic peptide were measured by ELISA (BNP26, Peninsula Laboratories, San Carlos, CA) according to the manufacturer's instructions.
Echocardiography.
Transthoracic echocardiography was performed at baseline and 1 and 6 wk after MI after animals were anesthetized as described. Transthoracic echocardiography (Acuson 128XP, Siemens, Malvern, PA) was performed with standard views by using S3 (1 to 3 MHz) and S8 (3 to 8 MHz) probes. Left ventricular volumes at the ends of systole and diastole and the wall motion index were calculated by using the method previously described by the American Society of Echocardiography.20 Posterior wall thickness and interventricular septal thickness were measured at the midpapillary level basal to the infarct zone.
Evaluation of ventricular function by pressure-volume catheter.
LV conductance and pressure signals were acquired using a dual-field 5-French 12-electrode pigtail pressure–volume catheter (Millar Instruments, Houston, TX) and pressure–volume data were collected at baseline (before ischemia–reperfusion) and 1 and 6 wk later. The catheter was connected to a console (CFL512, CD Leycom, Zoetermeer, Netherlands) via a pressure-control unit (TC510, Millar) and patient module (CD Leycom). Continuous data were acquired during steady state and transient balloon occlusion of the inferior vena cava.
Heart rate, maximum rate of LV pressure development during systole (dP/dtmax), decline during isovolumic relaxation (dP/dtmin), LV end-diastolic pressure (LVEDP) and volume (LVEDV) , and LV end-systolic pressure (LVESP) and volume (LVESV) were recorded. The stroke volume, cardiac output, LV ejection fraction (LVEF), and LV stroke work then were calculated.
Data from the inferior vena cava occlusion were used to calculate the linear end-systolic pressure–volume relation [characterized by the slope; also called end-systolic elastance (Ees)], ‘dead’ volume intercept (Vd), preload recruitable stroke work (PRSW),11 the myocardial oxygen consumption as estimated by the pressure–volume area (PVA), mechanical efficiency, and effective arterial elastance. Diastolic function was evaluated during steady state by monitoring the time constant of isovolumic relaxation (τ)19 and dP/dtmin5 as previously described. The end-diastolic pressure–volume relation (EDPVR) was evaluated by following changes in β, the stiffness coefficient, as described previously.15
Histology.
All animals were euthanized 6 wk after ischemia–reperfusion and their hearts excised, weighed, and analyzed for gross surface abnormalities. The ratio of LV weight to body weight was used to determine LV mass. The ventricles were sliced serially at approximately 1-cm intervals parallel to the posterior atrioventricular sulcus from the apex to the base. The thickness of each slice was measured and recorded. Digital images were obtained for morphometric analysis of LV area, infarct size, and thickness by using IPLab software (Scanalytics, Rockville, MD) to obtain total scar area as a percentage of total LV area and as the percentage of scar versus normal noninfarcted tissue. Myocardial tissue was taken from the basal, middle, and apical levels; sectioned circumferentially into 4 adjacent transmural areas to include the central area of infarction, 2 adjacent border areas, and a control (noninfarcted) area; and embedded in paraffin. Sections (4 to 5 µm) were mounted on charged slides and stained with hematoxylin and eosin and Masson trichrome to evaluate for fibrosis. Cardiomyocyte diameter was determined by staining with wheat germ agglutinin labeled with fluorescein isothiocyanate (Invitrogen, Carlsbad, CA) and measuring 50 to 60 cells by using fluorescence microscopy (LSM 5 PASCAL, version 3.2 SP2, Carl Zeiss, Thornwood, NY). Data were analyzed by using IPLab software.
Statistical analysis.
Results are expressed as mean ± SEM. The primary test for an effect was a test of the interaction in a 1-way repeated-measures ANOVA, where the factor was the echocardiographic or the pressure–volume loop parameter versus time. Data were compared by using the Holm–Sidak method (observed values compared with baseline versus each time point). The ANOVA and unadjusted Holm–Sidak P values were computed by using SigmaStat 3.5 (Systat Software, San Jose, CA). Correlations were performed by using the Pearson method. Statistical significance was determined by a P value of less than 0.05.
Results
Laboratory analysis and animal growth.
Compared with baseline levels, creatine kinase and troponin I levels in the 8 pigs evaluated were increased 2 h after occlusion and reperfusion (creatine kinase, 3.8 ± 1.8 to 32.3 ± 7.2 μg/L, P < 0.01; troponin I, 0.06 ± 0.07 to 21.9 ± 2.1 μg/L, P < 0.01) and returned to baseline 1 wk later, thus confirming acute MI. Levels of porcine brain natriuretic peptide did not change significantly at 1 wk (57 ± 7 pg/mL) and 6 wk (72 ± 6 pg/mL) after MI compared with baseline levels (64 ± 6 pg/mL), suggesting that animals compensated well. The baseline weights of noninfarcted (38.3 ± 1.3 kg) and infarcted (38.5 ± 2.9 kg) animals were similar. At 6 wk, noninfarcted swine weighed more than infarcted pigs (50.7 ± 1.8 kg versus 46.6 ± 3.9 kg, P = 0.05).
General hemodynamics.
Despite β-blocker therapy, there was a trend toward increased heart rate from baseline to 6 wk (P = 0.08; Table 1) and significant decreases in cardiac output (P = 0.006) and mean arterial pressure (P = 0.008) at 6 wk compared with baseline. The stroke volume decreased from baseline to 1 wk (P = 0.048) and from 1 wk to 6 wk (P < 0.001). Stroke work fell below baseline by 6 wk after MI (P = 0.002). The significant increase in LVEDP at 1 wk subsequently returned to an intermediate level at 6 wk [higher than baseline value (P = 0.03) but lower than at 1 wk (P = 0.004)]. The LV pressure at the end of systole was unchanged at 1 wk but decreased at 6 wk compared with values at both baseline (P = 0.048) and week 1 (P = 0.002).
Table 1.
General hemodynamics before (baseline) and 1 and 6 wk after induction of acute MI in pigs
| Baseline | Week 1 | Week 6 | |
| Heart rate (beats/min) | 79.9 ± 2.9 | 82.5 ± 2.9 | 88 ± 2.9 |
| Cardiac output (L/min) | 2.3 ± 0.1 | 2.1 ± 0.1 | 1.73 ± 0.1a |
| Mean arterial pressure (mm Hg) | 84.5 ± 3.7 | 79.6 ± 5.9 | 67.5 ± 3.2a |
| Stroke volume (mL) | 29.1 ± 1.2 | 26.3 ± 1.2a | 21.3 ± 1.2ab |
| Stroke work (mm Hg × mL) | 2224.6 ± 113.4 | 1985.4 ± 113.4 | 1450.5 ± 113.4ab |
| LVEDP (mm Hg) | 3 ± 1.8 | 9.8 ± 1.7a | 6 ± 1.9ab |
| LVESP (mm Hg) | 86 ± 2.5 | 89.7 ± 2.5 | 76.1 ± 2.5ab |
LVEDP, left ventricular end-diastolic pressure; LVESP, left ventricular end-systolic pressure.
P < 0.05 compared with baseline value.
P < 0.05 compared with value at wk 1 after MI.
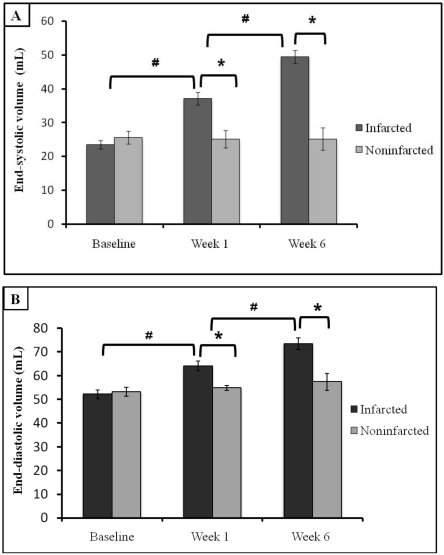
The volumes of the left ventricle at end-systole and end-diastole were increased at 1 wk (both P < 0.001) and 6 wk (both P < 0.001) compared with data obtained at baseline (Figure 1). These LV parameters did not differ from those of noninfarcted animals at baseline but were significantly higher at 1 and 6 wk after MI (both P < 0.001).
Figure 1.
Left ventricular remodeling before (baseline) and 1 and 6 wk after acute MI in swine. (A) End-systolic and (B) and end-diastolic volumes (mean ± SEM) are significantly increased when compared with values at baseline and from control (noninfarcted) animals. *, P < 0.05 between values for infarcted and noninfarcted animals at the same time point; #, P < 0.05 between values from infarcted animals at successive time points.
Left ventricular systolic properties.
Among measures of LV contractile function (Table 2), the LVEF measured by the pressure–volume catheter fell over time (baseline, 56% ± 1.4%; 1 wk, 39% ± 2%; 6 wk, 27% ± 2%; P < 0.001) and did not differ from values measured by echocardiography. Impairment of systolic performance after MI was demonstrated by statistically significant reductions in LVEF and dP/dtmax at 1 wk (P < 0.001 and P = 0.014, respectively) and 6 wk (both P < 0.001). Although Ees did not change significantly throughout the postinfarction course, rightward shift of the linear derived Vd (Figure 2) reached statistical significance (P = 0.012) at 6 wk when compared with baseline. Moreover, Vd and LVEF (but not LVEF and linear Ees) showed significant correlation at 1 wk (r = 0.65, P < 0.01) and 6 wk (r = 0.73, P < 0.01), suggesting that the pressure–volume loop measurements were sensitive to changes in LV dimensions over time. In addition, PRSW fell over time, reaching statistical significance (P = 0.022) at week 6.
Table 2.
Indices of left ventricular contractility and energetics before (baseline) and 1 and 6 wk after induction of acute MI in pigs
| Baseline | Week 1 | Week 6 | |
| LV ejection fraction (%) | 55 ± 1.4 | 41 ± 1.4a | 33 ± 1.4ab |
| dP/dtmax (mm Hg/s) | 1270 ± 35 | 1035 ± 35a | 842 ± 35a |
| Elastance at end-systole (mm Hg/mL) | 1.5 ± 0.8 | 1.6 ± 0.2 | 1.9 ± 0.2 |
| Vd (mL) | −29.6 ± 7 | −25 ± 8 | 11 ± 3ab |
| PRSW (mm Hg) | 53.2 ± 1.5 | 40.9 ± 1.5 | 34.4 ± 1.5a |
| Pressure–volume area | 4197 ± 208 | 4979 ± 436 | 2599 ± 506ab |
| Work efficiency | 0.73 ± 0.01 | 0.55 ± 0.03a | 0.41 ± 0.01ab |
| Arterial elastance (mm Hg/mL) | 3 ± 0.3 | 3.7 ± 0.3a | 4.2 ± 0.3a |
dP/dtmax, maximum rate of change of left ventricular pressure with time; PRSW, preload-recruitable stroke work; Vd, volume intercept of linear end-systolic pressure–volume relation
P < 0.05 compared with baseline value.
P < 0.05 compared with value at 1 wk after MI.
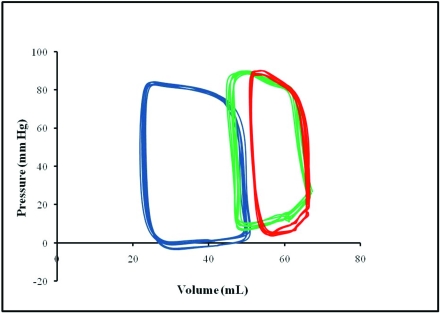
Figure 2.
Representative steady-state pressure–volume loops from 1 pig each at baseline (blue) and 1 wk (green) and 6 wk (red) after MI. Pressure–volume loops were constructed by plotting instantaneous pressure versus volume. The loop repeated with each cardiac cycle and showed how the heart transitioned from its end-diastolic state to the end-systolic state. After infarction, the pressure–volume loops narrowed, indicating reduction in stroke work, and shifted rightward due to increasing volume.
Myocardial oxygen consumption remained stable at 1 wk compared with baseline but decreased significantly (P < 0.001) by 6 wk. Work efficiency decreased significantly (P = 0.015) by 1 wk and remained lower than baseline at 6 wk (P < 0.001). The total arterial impedance faced by the left ventricle, assessed as effective arterial elastance, was significantly higher than baseline at 1 and 6 wk after MI (P = 0.038 and P = 0.026, respectively), although the levels were statistically similar between weeks 1 and 6 (Table 2).
Left ventricular diastolic properties.
Diastolic function was impaired after MI (Table 3). Compared with that at baseline, the exponential stiffness coefficient, β (related to EDPVR), was increased at 1 wk after MI (P = 0.031), indicating decreased diastolic ventricular compliance; this difference was even higher at 6 wk (P = 0.008). The maximal rate of pressure decay (dP/dtmin) was unchanged at 1 wk but significantly (P = 0.003) reduced at 6 wk, suggesting prolonged myocardial relaxation. Moreover, the relaxation time constant, τ, was significantly (P = 0.024) increased 1 wk after MI and then showed a trend (P = 0.07) toward returning to baseline at 6 wk.
Table 3.
Left ventricular diastolic properties before (baseline) and 1 and 6 wk after induction of acute MI in pigs
| Baseline | Week 1 | Week 6 | |
| EDPVR β (mm Hg/mL) | 0.03 ± 0.005 | 0.05 ± 0.01a | 0.1 ± 0.01ab |
| dP/dtmin (mm Hg/s) | −1093 ± 33 | −1024 ± 32 | −890 ± 32ab |
| τ (ms) | 56.5 ± 1.8 | 68.9 ± 1.8a | 62 ± 1.8b |
dP/dtmin, peak of pressure decay; EDPVR, end-diastolic pressure–volume relationship based on the β coefficient; τ, time constant of isovolumic relaxation
P < 0.05 compared with baseline value.
P < 0.05 compared with value at 1 wk after MI.
Echocardiography.
Transthoracic echocardiography was used to calculate the chamber volume, wall thickness, and wall motion index (Table 4). Of note, the data regarding LVEF and volumes at end-diastole and end-systole (data not presented) measured by the pressure–volume catheter strongly correlated with the ones measured by echocardiography (r = 0.85, r = 80, and r = 83, respectively; P < 0.01 for all comparisons).
Table 4.
Left ventricular wall thickness and wall motion index over time
| Baseline | Week 1 | Week 6 | |
| IVST – EDWT (mm) | 7.9 ± 0.1 | 10.2 ± 0.1a | 5.8 ± 0.1ab |
| PWT – EDWT(mm) | 6.7 ± 0.05 | 7 ± 0.05 | 13.2 ± 0.2ab |
| Wall motion index | 1 | 1.68 ± 0.24a | 1.99 ± 0.34ab |
IVST, interventricular septal thickness; EDWT, end-diastolic wall thickness; PWT, posterior wall thickness
P < 0.05 compared with baseline value.
P < 0.05 compared with value at 1 wk after MI.
Compared with the baseline value, the end-diastolic interventricular septal thickness at 1 wk was increased (P < 0.001), likely due to the presence of edema, hemorrhage, and infiltration of inflammatory cells in the infarct zone. In contrast, postinfarction remodeling was associated with reduction of interventricular septal thickness at 6 wk (P < 0.001). Meanwhile, posterior wall thickness was increased at 6 wk compared with baseline (P = 0.012) and 1 wk (P < 0.001; Table 4). In addition, posterior wall thickness at 6 wk (13.2 ± 0.2 mm) was increased compared with the noninfarction measurement (10.4 ± 0.2 mm, P < 0.05), suggesting compensatory hypertrophy. Moreover, the wall motion index increased significantly (P < 0.05) over time (Table 4).
Histology.
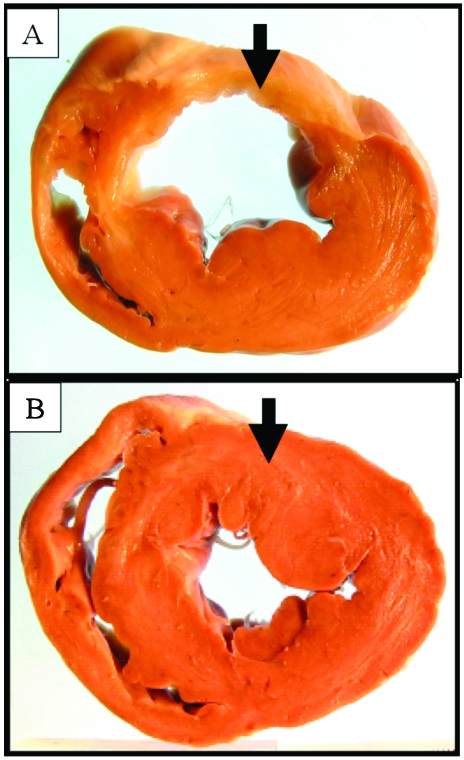
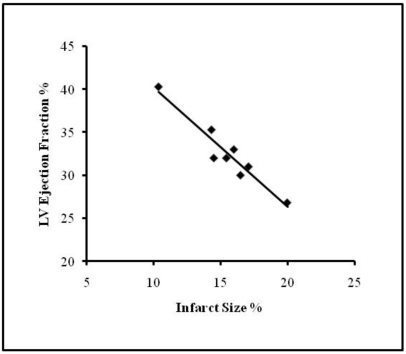
Among the 8 pigs evaluated, the total infarct area was 1822 ± 429 mm2 (mean ± SEM), which accounted for 16.3% ± 4.4% of the total free myocardial wall area (Figure 3). Masson trichrome stain revealed large areas of fibrosis accounting for 11.7% ± 1.3% of the total free myocardial wall area. Scar thickness was 8.1 ± 2.3 mm and the contralateral (posterior) wall thickness was 16.6 ± 2 mm (P < 0.001 compared with scar thickness), whereas in noninfarcted animals the posterior wall thickness was 11.3 ± 3 mm (P < 0.001 compared with scar thickness). Infarct size (%) and LVEF showed significant negative correlation (r = −0.917; P = 0.002; Figure 4).
Figure 3.
Representative pathologic sections from (A) a pig 6 wk after MI and (B) a control noninfarcted animal. Infarcted tissue is thinner and more pale compared with the equivalent region in the noninfarcted heart (black arrows).
Figure 4.
Correlation between infarct size (%) and LV ejection fraction (%) in pigs (r = – 0.917; P = 0.002); y = −1.3846 x + 54.051).
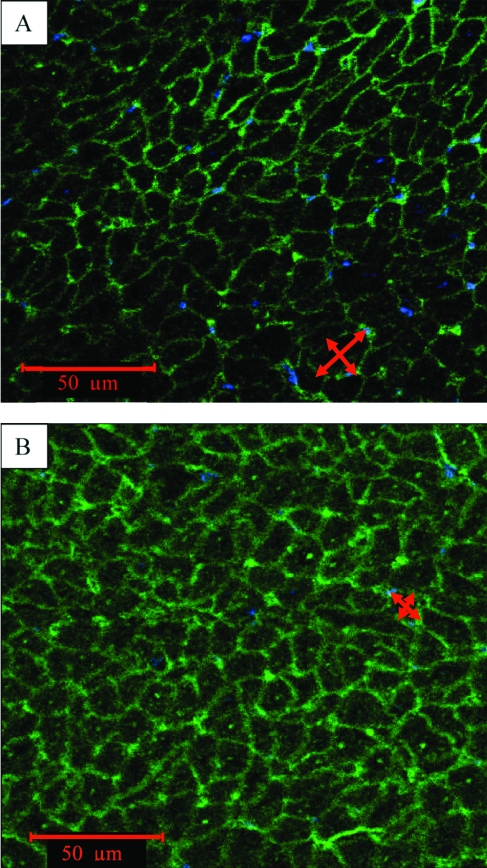
Moreover, the ratio of LV weight to body weight was significantly higher in infarcted animals (3.4 ± 0.1) compared with controls (2.6 ± 0.2, P < 0.05) and the myocyte cross-sectional area at the infarct border zone (190 ± 39 μm2) was increased compared with the remote zone (110 ± 15 μm2, P = 0.003), again suggesting the presence of compensatory hypertrophy (Figure 5).
Figure 5.
Representative immunohistochemistry of (A) infarcted border zone and (B) noninfarcted area reveals increased myocyte cross-sectional area, associated with compensatory hypertrophy, in the infarcted border zone. Red arrows indicate sites where the cross-sectional measurements were taken. Scale bar, 50 µm.
Discussion
The aim of this methodologic study was to evaluate infarct healing and remodeling during treatment with β blockers in a swine closed-chest model similar to contemporary human acute reperfused MI. We comprehensively analyzed LV systolic and diastolic hemodynamic variables over 6 wk period and assessed the utility of indices of cardiac performance and contractility.
The ischemia–reperfusion resulted in infarction of the anteroseptal and apical LV walls, with echocardiographic wall motion abnormalities, LV chamber enlargement, compensatory hypertrophy, and global functional impairment. In terms of systolic parameters, the LV volume at end-systole increased markedly with concomitant decreases in LVEF, cardiac output, and dP/dtmax. The linear Ees was unchanged over time, whereas Vd moved rightward at 1 and 6 wk, indicating increasing LV dimensions and suggesting contractility impairment over time. In our study, the nonsignificant changes in the slope of Ees might have resulted from changes in loading conditions, LV dimensions, and regional morphologic changes. These parameters are difficult to evaluate with conductance catheter method over time and actually reflect what we expect to see in clinical settings.
In line with these findings and previous reports,11,14 PRSW was decreased at 6 wk after MI and correlated with LVEF at 1 wk (r = 0.44; P < 0.01) and 6 wk (r = 0.62; P < 0.01). We also found significant changes in the myocardial oxygen consumption, as estimated by PVA, and mechanical efficiency. These parameters use PV data to incorporate ventricular preload, afterload, and contractility.22,23 Systolic PVA was significantly decreased only at 6 wk, indicating a reduction in total mechanical energy and oxygen consumption over time, which are common findings during heart failure. Moreover, the overall mechanical efficiency was significantly decreased at both 1 and 6 wk after MI, despite the decreased PVA.
Diastolic dysfunction can manifest clinically with increased LVEDP and altered filling patterns, but neither is a specific indicator of this abnormality. Our results reveal increased diastolic volume and increased slope and rightward shift of EDPVR over time, likely due to remodeling and changes in chamber distensibility. In chronic dilated cardiomyopathy, EDPVR typically is shifted to the right, as the myopathic ventricle fills to increased volumes while keeping pressure constant. Despite remodeling, patients with chronic dilated cardiomyopathy often operate at near-maximal filling, and their effective operant stiffness, determined by the slope of the EDPVR, remains significantly elevated.4 Supporting the observed changes in the EDPVR β coefficient, the relaxation time constant (τ) was significantly increased 1 wk; the trend toward an increase at 6 wk (P = 0.07) rather than a significant difference might be result of the trend toward increased heart rate. The decrease in negative dP/dtmin at 6 wk suggests a decreased rate of relaxation, perhaps due in part to the observed compensatory hypertrophy. However, the change dP/dtmin at 6 wk might indicate impaired LV systolic pressure generation, as evidenced by the significantly reduced LV pressure at end-systole at 6 wk. It is further possible that the dP/dtmin data do not agree with those regarding relaxation time 1 wk because of the dependence of dP/dtmin on afterload, represented as the increase in LV volume at end-systole and as effective arterial elastance.7
Although LVEDP was increased at 1 wk, it decreased to an intermediate value at 6 wk. A potential explanation for this finding is that the swine were in the process of adaptive remodeling, similar to what is expected during the early stages of chronic mitral regurgitation. In cases of severe mitral regurgitation, resting LVEDP may remain normal despite considerable LV contractile dysfunction.8 Similarly the relationship between LV end-diastolic pressure and volume varies during diastole;10 therefore LV end-diastolic pressure alone should not be used to diagnose chronic heart failure. Regardless of the cause of the changes in LV end-diastolic pressure that we noted, knowledge of these absolute values is critical for planning future studies to test novel therapies in conjunction with β blockers.
Despite several important strengths, our model also has some weaknesses, one of which is that we used healthy juvenile pigs. Although the anatomy of pigs is very close to that of humans, the profile of healthy juvenile animals is quite different from that in the typical human heart failure cohort. Most human patients with heart failure are middle-aged to elderly and have various cardiovascular problems including hypertension, atherosclerosis, and diabetes. However, working with adult or elderly pigs is highly challenging, because their massive weight gain greatly complicates handling, housing, and procedures such as echocardiography. The sample size in the current study was small and the follow-up duration of 6 wk may have been too short to encompass the complete evolution of cardiac remodeling and heart failure. Despite these drawbacks, we were able to identify significant differences in several parameters (that is, LVEF, relaxation time, cardiac output, SW, PRSW, Vd) as compared with baseline conditions. We did not compare endpoint measures to those of swine that received MI but not a β blocker, which is also a limitation of our study. Although this condition has been well described and characterized,1,12,17,20,23 the modulatory impact of a β blocker on the endpoints cannot be determined from our study.
Ideally, pressure–volume measurements should be performed in conscious animals. However, doing so is quite difficult because of handling constraints and the high risk of arrhythmias when infarcted animals are under stress. Even though isoflurane has far fewer deleterious effects on the myocardium than do other inhalant anesthetics, some degree of myocardial depressant effect is expected with any halogenated hydrocarbon anesthetic.
The doses of β blocker used were chosen to avoid excessive bradycardia and hypotension after MI. Due to the expense and difficulties in performing large animal studies, dose-escalation experiments could not be performed. Such studies can be considered in the future to further characterize the changes in the reported parameters under different dosing conditions.
In conclusion, we have characterized several important features after induction of acute MI with reperfusion in swine treated with β blockers. In the subacute setting, we observed LV enlargement accompanied by reduction of the LVEF, hypokinesis of the infarcted area, and hyperkinesis of the remote myocardium in response to the acute increase in preload. These results were accompanied by decreased diastolic function reflected by the increase in relaxation time. In the chronic setting, LV systolic function was impaired, characterized by depressed myocardial contractility as represented by decreased PRSW, as well as progressive chamber dilatation, thinning of the infarct, compensatory hypertrophy elsewhere, diminished LVEF and dP/dtmax, and decreased myocardial oxygen consumption and mechanical efficiency. Diastolic dysfunction was inferred through the increases in relaxation time (τ) and the stiffness coefficient (β). These data taken together with the dP/dtmin and LVEDP support the conclusion that remodeling at 6 wk is an ongoing process. Our data will guide planning of future studies of novel therapies for postinfarction heart failure tested in combination with β blockers.
Acknowledgements
Pathologic assessment was performed by CVPath Institute (Gaithersburg, MD). The authors thank Petros Minasi for administrative support. This work was supported in part by the UCSF Cardiac Stem Cell Foundation (YY and WG), a grant from the Wayne and Gladys Valley Foundation (WG, KC, and YY) and the C Breetwor Foundation (YY).
References
- 1.Amado LC, Saliaris AP, Schuleri KH, St John M, Xie JS, Cattaneo S, Durand DJ, Fitton T, Kuang JQ, Stewart G, Lehrke S, Baumgartner WW, Martin BJ, Heldman AW, Hare JM. 2005. Cardiac repair with intramyocardial injection of allogeneic mesenchymal stem cells after myocardial infarction. Proc Natl Acad Sci USA 102:11474–11479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Antman EM, Hand M, Armstrong PW, Bates ER, Green LA, Halasyamani LK, Hochman JS, Krumholz HM, Lamas GA, Mullany CJ, Pearle DL, Sloan MA, Smith SC, Jr, Anbe DT, Kushner FG, Ornato JP, Jacobs AK, Adams CD, Anderson JL, Buller CE, Creager MA, Ettinger SM, Halperin JL, Hunt SA, Lytle BW, Nishimura R, Page RL, Riegel B, Tarkington LG, Yancy CW. 2008. 2007 focused update of the ACC/AHA 2004 guidelines for the management of patients with ST-elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines: developed in collaboration with the Canadian Cardiovascular Society endorsed by the American Academy of Family Physicians: 2007 Writing Group to review new evidence and update the ACC/AHA 2004 guidelines for the management of patients with ST-elevation myocardial infarction, writing on behalf of the 2004 Writing Committee. Circulation 117:296–329 [DOI] [PubMed] [Google Scholar]
- 3.Blom AS, Pilla JJ, Gorman RC, 3rd, Gorman JH, Mukherjee R, Spinale FG, Acker MA. 2005. Infarct size reduction and attenuation of global left ventricular remodeling with the CorCap cardiac support device following acute myocardial infarction in sheep. Heart Fail Rev 10:125–139 [DOI] [PubMed] [Google Scholar]
- 4.Borlaug BA, Kass DA. 2006. Mechanisms of diastolic dysfunction in heart failure. Trends Cardiovasc Med 16:273–279 [DOI] [PubMed] [Google Scholar]
- 5.Burkhoff D, Mirsky I, Suga H. 2005. Assessment of systolic and diastolic ventricular properties via pressure-volume analysis: a guide for clinical, translational, and basic researchers. Am J Physiol Heart Circ Physiol 289:H501–H512 [DOI] [PubMed] [Google Scholar]
- 6.Chen ZM, Pan HC, Chen YP, Peto R, Collins R, Jiang LX, Xie JX, Liu LS. 2005. Early intravenous then oral metoprolol in 45,852 patients with acute myocardial infarction: randomised placebo-controlled trial. Lancet 366:1622–1632 [DOI] [PubMed] [Google Scholar]
- 7.Cohn PF, Liedtke AJ, Serur J, Sonnenblick EH, Urschel CW. 1972. Maximal rate of pressure fall (peak negative dP-dt) during ventricular relaxation. Cardiovasc Res 6:263–267 [DOI] [PubMed] [Google Scholar]
- 8.Committee on Management of Patients with Valvular Heart Disease, Task Force on Practice Guidelines 1998. ACC/AHA guidelines for the management of patients with valvular heart disease. A report of the American College of Cardiology/American Heart Association. J Am Coll Cardiol 32:1486–1588 [DOI] [PubMed] [Google Scholar]
- 9.Francis GS. 2001. Pathophysiology of chronic heart failure. Am J Med 110 Suppl 7A:37S–46S [DOI] [PubMed] [Google Scholar]
- 10.Glantz SA, Parmley WW. 1978. Factors which affect the diastolic pressure-volume curve. Circ Res 42:171–180 [DOI] [PubMed] [Google Scholar]
- 11.Glower DD, Spratt JA, Snow ND, Kabas JS, Davis JW, Olsen CO, Tyson GS, Sabiston DC, Jr, Rankin JS. 1985. Linearity of the Frank-Starling relationship in the intact heart: the concept of preload recruitable stroke work. Circulation 71:994–1009 [DOI] [PubMed] [Google Scholar]
- 12.Hansen A, Wolf D, Reinhard A, Katus HA. 2008. Dose-dependent effects of intravenous allogeneic mesenchymal stem cells in the infarcted porcine heart. Stem Cells Dev [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 13.Institute of Laboratory Animal Resources 1996. Guide for the care and use of laboratory animals. Washington (DC): National Academy Press [Google Scholar]
- 14.Karunanithi MK, Feneley MP. 2000. Single-beat determination of preload recruitable stroke work relationship: derivation and evaluation in conscious dogs. J Am Coll Cardiol 35:502–513 [DOI] [PubMed] [Google Scholar]
- 15.LaCorte JC, Cabreriza SE, Rabkin DG, Printz BF, Coku L, Weinberg A, Gersony WM, Spotnitz HM. 2003. Correlation of the Tei index with invasive measurements of ventricular function in a porcine model. J Am Soc Echocardiogr 16:442–447 [DOI] [PubMed] [Google Scholar]
- 16.Lima JA, Ferrari VA, Reichek N, Kramer CM, Palmon L, Llaneras MR, Tallant B, Young AA, Axel L. 1995. Segmental motion and deformation of transmurally infarcted myocardium in acute postinfarct period. Am J Physiol 268:H1304–H1312 [DOI] [PubMed] [Google Scholar]
- 17.Nordhaug D, Steensrud T, Korvald C, Aghajani E, Myrmel T. 2002. Preserved myocardial energetics in acute ischemic left ventricular failure–studies in an experimental pig model. Eur J Cardiothorac Surg 22:135–142 [DOI] [PubMed] [Google Scholar]
- 18.Oliveira EM, Angeli FS. 2006. Large animal models of myocardial ischemia for cardiac cell therapy. Perin EC, Silva GS, Willerson JT. An essential guide to cardiac cell therapy. London (UK): Informa Healthcare [Google Scholar]
- 19.Raff GL, Glantz SA. 1981. Volume loading slows left ventricular isovolumic relaxation rate. Evidence of load-dependent relaxation in the intact dog heart. Circ Res 48:813–824 [DOI] [PubMed] [Google Scholar]
- 20.Schiller NB, Shah PM, Crawford M, DeMaria A, Devereux R, Feigenbaum H, Gutgesell H, Reichek N, Sahn D, Schnittger I, Silverman NH, Tajik AJ. 1989. Recommendations for quantitation of the left ventricle by two-dimensional echocardiography. American Society of Echocardiography Committee on Standards, Subcommittee on Quantitation of Two-Dimensional Echocardiograms. J Am Soc Echocardiogr 2:358–367 [DOI] [PubMed] [Google Scholar]
- 21.Schuleri KH, Amado LC, Boyle AJ, Centola M, Saliaris AP, Gutman MR, Hatzistergos KE, Oskouei BN, Zimmet JM, Young RG, Heldman AW, Lardo AC, Hare JM. 2008. Early improvement in cardiac tissue perfusion due to mesenchymal stem cells. Am J Physiol Heart Circ Physiol 294:H2002–H2011 [DOI] [PubMed] [Google Scholar]
- 22.Suga H, Hayashi T, Shirahata M. 1981. Ventricular systolic pressure-volume area as predictor of cardiac oxygen consumption. Am J Physiol 240:H39–H44 [DOI] [PubMed] [Google Scholar]
- 23.Suga H, Igarashi Y, Yamada O, Goto Y. 1985. Mechanical efficiency of the left ventricle as a function of preload, afterload, and contractility. Heart Vessels 1:3–8 [DOI] [PubMed] [Google Scholar]
- 24.Tomita M, Spinale FG, Crawford FA, Zile MR. 1991. Changes in left ventricular volume, mass, and function during the development and regression of supraventricular tachycardia-induced cardiomyopathy. Disparity between recovery of systolic versus diastolic function. Circulation 83:635–644 [DOI] [PubMed] [Google Scholar]
- 25.Van de Werf F, Ardissino D, Betriu A, Cokkinos DV, Falk E, Fox KA, Julian D, Lengyel M, Neumann FJ, Ruzyllo W, Thygesen C, Underwood SR, Vahanian A, Verheugt FW, Wijns W. 2003. Management of acute myocardial infarction in patients presenting with ST-segment elevation. The Task Force on the management of acute myocardial infarction of the European Society of Cardiology. Eur Heart J 24:28–66 [DOI] [PubMed] [Google Scholar]
- 26.Wolff MR, de Tombe PP, Harasawa Y, Burkhoff D, Bier S, Hunter WC, Gerstenblith G, Kass DA. 1992. Alterations in left ventricular mechanics, energetics, and contractile reserve in experimental heart failure. Circ Res 70:516–529 [DOI] [PubMed] [Google Scholar]
- 27.Yarbrough WM, Mukherjee R, Escobar GP, Mingoia JT, Sample JA, Hendrick JW, Dowdy KB, McLean JE, Lowry AS, O'Neill TP, Spinale FG. 2003. Selective targeting and timing of matrix metalloproteinase inhibition in post-myocardial infarction remodeling. Circulation 108:1753–1759 [DOI] [PubMed] [Google Scholar]