Abstract
We established an inbred rat strain with unilateral urogenital anomalies from an incidentally identified male rat with unilateral renal agenesis and an undescended left testis. These rats were characterized by unilateral renal agenesis in both sexes, undescended testes with agenesis and hypoplasia of the accessory sex organs in male rats, and complete and partial agenesis of the uterine horn in female rats. All of these urogenital anomalies were unilateral and restricted to the left side; we named this phenotype unilateral urogenital anomalies (UUA). Breeding tests showed that these abnormalities were inherited as polygenic traits. The weight of right kidneys of affected rats was 1.7-fold higher than that of normal rats; histologically, glomerulosclerosis, tubular dilations, and tubular casts were detected at 30 wk of age. These alterations may have resulted from compensatory renal adaptation to the lack of 1 kidney. The cryptorchid left testes of affected male rats showed atrophy of seminiferous tubules and degeneration of spermatocytes and spermatids. These results indicate that the UUA rat may be a good model to study the etiology of unilateral renal agenesis accompanied by agenesis of the reproductive tract and to study compensatory alterations resulting from the congenital loss of 1 kidney.
Abbreviations: A, affected; F, female; FUBI, failure of ureteric bud invasion; M, male; N, normal; UUA, unilateral urogenital anomalies
Unilateral renal agenesis (URA) is a common malformation of the kidney,15 occurring in 1:1000 to 1:500 persons.24 This disorder often is associated with ipsilateral absence of the deferent duct in men and hypoplasia of the uterine horn in women.24 Although some cases of familial renal agenesis have been reported,2,11 the genetic etiology of this condition is almost completely unknown. Unilateral renal agenesis also occurs in other species, including dogs,12 cats,14 and guinea pigs.16 Although spontaneously mutant strains of renal agenesis in mice9 and rats6,18 have been reported, the genes responsible for renal agenesis have not yet been identified.
We identified a male rat with renal agenesis and an undescended left testis in a group of rats obtained from a laboratory animal supplier. Using this rat as a founder, we started brother–sister breeding to establish an inbred rat strain that could be used as an animal model in the study of unilateral renal agenesis. We named the rat phenotype unilateral urogenital anomalies (UUA). In the present study, we show that UUA is a congenitally genetic disorder characterized by unilateral renal agenesis with agenesis and hypoplasia of the reproductive organs restricted to the left side.
Materials and Methods
Animal care.
Rats were bred and fed as described22 and kept in a conventional animal room. Experimental procedures and care of animals were in accordance with the guidelines of the Animal Care and Use Committee of Nippon Veterinary and Life Science University.22
Establishment of UUA strain and estimation of genetic mode.
We incidentally identified a male rat with unilateral (left side) cryptorchidism in Wistar rats purchased from a laboratory animal supplier (Tokyo Jikken Doubutsu, Tokyo, Japan). In addition, the kidney ipsilateral to the undescended left testis was not palpable in this rat. To confirm the heritability of the defect, the affected founder male rat was bred twice to an unaffected female rat purchased from the same animal supplier. The 2 resultant F1 litters were crossed to produce 14 F2 litters. F1 female rats (n = 3) were bred (backcrossed) to the founder male rat, resulting 3 F1B litters. These F1B litters were brother–sister-crossed by breeding normal female rats with affected male rats, resulting in 13 F1BF1 litters (Figure 1). If the left kidney could not be detected when the rats were palpated or autopsied, they were defined as affected. Rats having normal kidneys on both sides were defined as normal. Actual incidences of affected rats were calculated in each generation and statistically compared with expected incidences by χ2 test. The hypotheses of genetic mode were single autosomal dominant, single autosomal recessive, X-linked, and Y-linked.20
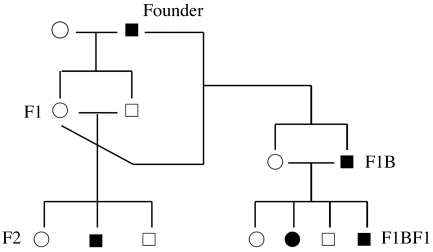
Figure 1.
Establishment of the UUA strain. A simplified schematic pedigree shows the breeding patterns of the first 3 generations during the establishment of this strain. Breeding patterns are shown in relation to left renal agenesis, with the actual incidences shown in Table 1. The founder male was bred to a female rat purchased at the same time from the same supplier. The resulting F1 male and female rats were bred to generate F2 rats. F1 female rats also were bred to the founder male to generate backcrossed progeny (F1B). F1BF1 rats were generated from brother–sister breeding between affected male and normal female rats in F1B. White circles, normal female rats; black circles, affected female rats; white squares, normal male rats; black squares, affected male rats. Not all breedings and progeny are shown.
Breeding experiment.
To examine the relation between parental phenotypes and the resulting incidences of affected progeny, breeding experiments were performed between normal (N) female (F) and normal male (M; FN × MN), affected (A) female and normal male (FA × MN), normal female and affected male (FN × MA), and affected female and affected male (FA × MA) in the F1BF5 generation. The phenotypes of these progeny were determined by palpating their left kidneys. Incidences of affected rats were reciprocally and statistically compared between different breeding patterns by χ2 test for independence.20
Necropsy, organ weights, and histologic examination.
The rats used for the establishment of the UUA strain were necropsied to examine the anatomic features of their urogenital organs. At ages 290 to 332 d, 3 normal and 2 affected male offspring in the F1B generation and 1 normal and 1 affected male and 3 normal and 4 affected female progeny in the F1BF1 generation were euthanized, and their major organs were removed and weighed by electric balance.20 Data regarding organ weight are presented as mean ± 1 SD, and the differences between normal and affected rats were determined by unpaired Student t tests; a P value of less than 0.05 was used to define statistical significance (Excel 2008 for Mac, Microsoft Corp, Redmond, WA).
For histologic examination, testes, ovaries, and kidneys isolated from normal and affected rats of the F1B, F1BF3, and F1BF4 generations were fixed in 4% phosphate-buffered formalin, dehydrated through a graded alcohol series, embedded in paraffin, and sectioned at 4 µm as described.21 After the sections were deparaffinized in xylene, hydrated through a graded alcohol series, and immersed in water, they were stained with hematoxylin and eosin or periodic acid–Schiff stain.21 Microscopic images were obtained by using an ‘all-in-one’ microscope (BZ-8000, Keyence, Osaka, Japan).
Because we found variations in the phenotypes of the reproductive organs, we necropsied a total of 119 female and 121 male affected rats from the F1BF3 through F1BF12 generations to assess the incidence of complete or partial agenesis of the reproductive organs.
Results
Estimation of genetic mode in third generation of the UUA strain.
Of the 303 rats obtained from the first 3 generations during the establishment of the UUA strain, we found that 1 F2, 2 F1B, and 6 F1BF1 male rats and 6 F1BF1 female rats were affected (Table 1). Brother–sister breeding between normal F1BF1 female rats and affected F1BF1 male rats was repeated to establish the inbred UUA strain. All 27 F1 rats had a normal left kidney. This result was inconsistent with the hypothesis that the defects in UUA might be inherited as a single autosomal dominant (expected ratio of normal:affected rats in F1, 0:1 or 1:1, χ2 = ∞ or 27.0) or Y-linked (normal:affected in F1 male rats, 0:1, χ2 = ∞) mode. The observed incidences of affected rats in F2 and F1B did not fit the expected ratios of single autosomal recessive (normal:affected in F2, 3:1, χ2 = 44.0; normal:affected in F1B, 1:1, χ2 = 27.5) or X-linked (normal:affected in F2 females, 1:0, χ2 = ∞; normal:affected in F2 male rats, 1:1, χ2 = 70.1; normal:affected in F1B, 1:1, χ2 = 27.5) mode. These results indicate that the defects in these rats were not due to mutation in a single gene.
Table 1.
Incidences of the UUA phenotype during the first 3 generations of the establishment of the UUA strain
| Normal |
Affected |
Incidence (%) |
|||||||
| Generation | Total | Female | Male | Total | Female | Male | Total | Female | Male |
| F1 | 27 | 14 | 13 | 0 | 0 | 0 | 0.0 | 0.0 | 0.0 |
| F2 | 139 | 66 | 73 | 1 | 0 | 1 | 0.7 | 0.0 | 1.4 |
| F1B | 33 | 19 | 14 | 2 | 0 | 2 | 5.7 | 0.0 | 12.5 |
| F1BF1 | 104 | 64 | 40 | 12 | 6 | 6 | 10.3 | 8.6 | 13.0 |
Genetic mode of inheritance from breeding experiments.
The incidences of affected rats in later generations due to 4 breeding schemes (FN × MN, FN × MA, FA × MN, and FA × MA) were calculated. The incidence of affected rats did not differ significantly by gender in any breeding scheme. The incidence of the affected rats due to breeding between affected parents (FA × MA) was highest (61.1%) but not 100%. The incidence resulting from normal parents (FN × MN) was lowest (20.5%), and the incidences due to breeding schemes involving normal and affected rats (FN × MA and FA × MN) were intermediate (42.6% and 33.3%, respectively; Table 2). Subsequent χ2 tests of the incidence of affected rats indicated significant deviations between the breeding patterns of FN × MN and FA × MA (P < 0.001) as well as between FN × MN and FN × MA (P < 0.01). These results indicate that the incidence of the affected progeny was related to parental phenotypes and that the genetic mode of the defects was polygenic. When we assumed that a major recessive mutation was responsible for UUA, the penetrance of the mutation causing renal agenesis was about 60%.
Table 2.
Incidences of the UUA phenotype during different parental breeding patterns
| No. of progeny |
||||||||||
| Breeding pattern | No. of litters | Normal |
Affected |
Incidence (%) |
||||||
| Total | Female | Male | Total | Female | Male | Total | Female | Male | ||
| FA x MA | 3 | 7 | 2 | 5 | 11 | 6 | 5 | 61.1 | 75.0 | 50.0 |
| FA x MN | 4 | 22 | 11 | 11 | 11 | 4 | 7 | 33.3 | 26.7 | 38.9 |
| FN x MA | 4 | 31 | 16 | 15 | 23 | 11 | 12 | 42.6 | 40.7 | 44.4 |
| FN x MN | 6 | 58 | 18 | 40 | 15 | 4 | 11 | 20.5 | 18.2 | 21.6 |
Macroscopic findings and organ weights.
Affected and normal rats had no significant differences in body weights, body lengths, and tail lengths (data not shown). Macroscopic examination of the abdominal organs of the rats used for establishment of the UUA strain showed that 14 of 15 affected rats were characterized by the absence of the left kidney (the remaining rat had an aplastic rudimentary left kidney). The right kidneys were consistently larger than those of normal rats, both in absolute (Table 3) and relative (data not shown) weight. In addition, affected male rats had unilateral cryptorchidism, and ageneses of the epididymis, deferent duct, and gland of the deferent duct, with all anomalies restricted to the left side (Figure 2 A). Left coagulating glands and left seminal vesicles were present but too small to be weighed. The undescended left testes were small and had lost resilience, and their weights were significantly lower than those of the left testes of normal rats (Table 3).
Table 3.
Absolute organ weights (mg) of normal and affected male rats
| Normal (n = 4) | Affected (n = 3) | ||
| Preputial gland | Left | 112.5 ± 21.8 | 124.7 ± 7.2 |
| Right | 116.1 ± 22.7 | 133.6 ± 13.5 | |
| Testis | Left | 1872.9 ± 60.4 | 372.6 ± 186.1c |
| Right | 1793.3 ± 143.5 | 1647.6 ± 141.5 | |
| Epididymis | Left | 683.5 ± 31.9 | not done due to partial or complete agenesis |
| Right | 634.7 ± 52.3 | 615.6 ± 47.8 | |
| Gland of deferent duct | Left | 38.0 ± 4.6 | not done due to partial or complete agenesis |
| Right | 37.0 ± 3.9 | 37.6 ± 3.1 | |
| Deferent duct | Left | 183.6 ± 15.1 | not done due to partial or complete agenesis |
| Right | 195.2 ± 10.1 | 137.3 ± 20.7b | |
| Coagulating gland | Left | 140.9 ± 53.2 | not done due to partial or complete agenesis |
| Right | 115.8 ± 41.6 | 76.8 ± 23.1 | |
| Seminal vesicle | Left | 803.8 ± 114.6 | not done due to partial or complete agenesis |
| Right | 805.0 ± 72.2 | 941.3 ± 343.8 | |
| Prostate gland | 1438.2 ± 206.1 | 1296.7 ± 142.4 | |
| Penis | 444.7 ± 21.8 | 394.4 ± 19.4a | |
| Bulbourethral gland | Left | 103.1 ± 35.9 | 80.0 ± 9.8 |
| Right | 79.6 ± 13.9 | 83.1 ± 6.2 | |
| Bulb of penis | 1248.1 ± 29.2 | 1201.7 ± 35.5 | |
| Levator ani muscle | 517.6 ± 53.0 | 452.6 ± 32.0 | |
| Kidney | Left | 1889.8 ± 156.2 | not done due to partial or complete agenesis |
| Right | 1992.6 ± 217.1 | 3406.7 ± 259.0c |
Values are presented as mean ± 1 SD.
P < 0.05 compared with value for normal male rats
P < 0.01 compared with value for normal male rats
P < 0.001 compared with value for normal male rats
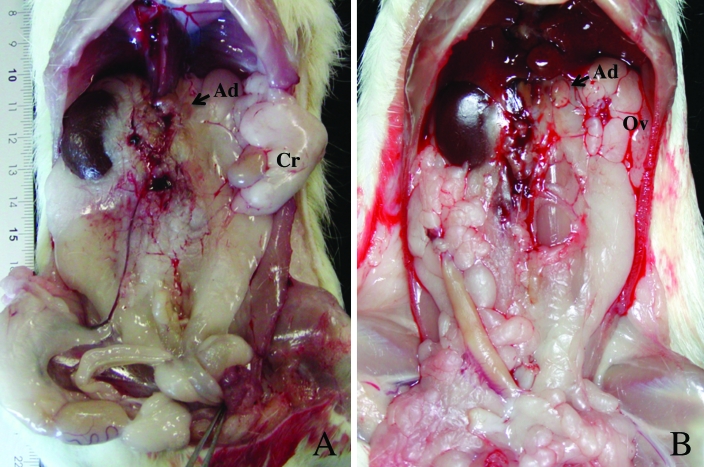
Figure 2.
Macroscopic photographs showing major type of UUA. (A) Major types of urogenital anomalies in affected male rats. Absence of the left kidney was accompanied by ipsilateral cryptorchid testis (Cr). Epididymis and deferent duct were completely absent on the left side. Both adrenal glands (Ad) were present. (B) Major types of urogenital anomalies in affected female rats. Uterine horn and kidney were completely absent on the left side, whereas both ovaries and oviducts (Ov) were present and appeared normal.
Affected female rats similarly showed absence of the left kidney and ipsilateral agenesis of the reproductive tract. In female rats, unilateral renal ageneses were associated with complete absence of the left uterine horn (Figure 2 B). However, both left and right ovaries and oviducts were present at their normal position in all affected females. The absolute and relative weights of the right kidney in affected females were significantly larger than in normal female rats, and the weights of the uterus and left oviduct were smaller in affected than in normal females (Table 4). Using the vaginal smear method, we observed a normal estrous cycle in affected female rats. When affected female rats were bred, the embryos implanted in the normal right uterine horn and were delivered normally.
Table 4.
Absolute organ weights (mg) of normal and affected female rats
| Normal (n = 3) | Affected (n = 4) | ||
| Clitoral gland | Left | 57.0 ± 2.8 | 70.3 ± 12.4 |
| Right | 52.7 ± 4.2 | 65.1 ± 15.9 | |
| Ovary | Left | 32.8 ± 3.3 | 37.6 ± 4.8 |
| Right | 33.4 ± 4.1 | 31.8 ± 8.1 | |
| Oviduct | Left | 21.9 ± 1.1 | 13.8 ± 2.3b |
| Right | 19.4 ± 2.9 | 20.3 ± 1.6 | |
| Uterus | 1449.7 ± 397.2 | 611.2 ± 182.8a | |
| Kidney | Left | 1057.2 ± 113.2 | not done due to complete agenesis |
| Right | 1021.9 ± 145.6 | 1943.3 ± 589.6a |
Values are presented as mean ± 1 SD
P < 0.05 compared with value for normal female rats
P < 0.01 compared with value for normal female rats
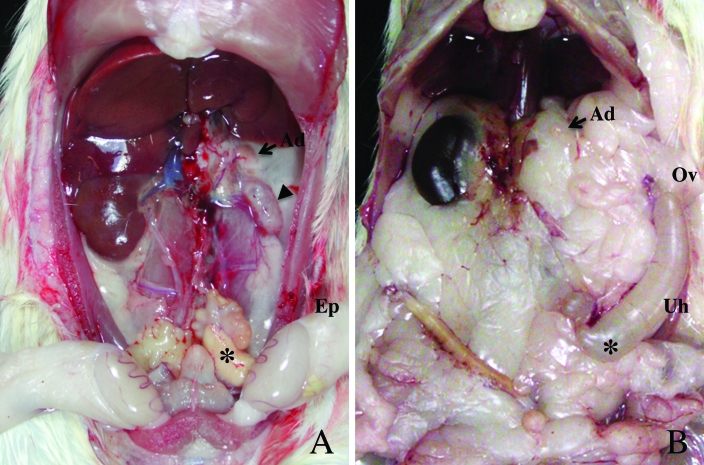
The affected male rat with a rudimentary left kidney (described in the first paragraph of this section) showed complete descent of both testes and partial ageneses of the epididymis and deferent duct on the left side (Figure 3 A). We therefore examined the variation in the agenesis of the reproductive tract in 119 female and 121 male affected rats of the F1BF3 through F1BF12 generations that composed of 269 normal males, 227 affected males, 269 normal females, and 224 affected females. In addition to 13 affected males with descended testes and partial ageneses of left reproductive organs as shown in Figure 3A, we found 14 affected female rats with partial aplasia of the left uterine horn, in which the distal portion remained and was connected to the oviduct (Figure 3 B). Consequently, 89% (213/240) of affected rats showed complete absence of the left side of the reproductive tract (epididymis and deferent duct in male rats and uterine horn in female rats), and the remaining 11% (27 of 240) of affected rats showed partial absence of these organs.
Figure 3.
Macroscopic photographs showing minor type of UUA. (A) Minor types of urogenital anomalies in affected males. A rudimentary kidney (arrowhead), a blind ending of the deferent duct (asterisk), and a descended testis were observed on the left side. The left epididymis (Ep) appeared anatomically normal. (B) Minor types of urogenital anomalies in affected female rats. The left uterine horn was partially absent near the corpus uteri (asterisk). The remaining uterine horn (Uh) showed edematous swelling but was connected to the oviduct (Ov).
Histologic findings.
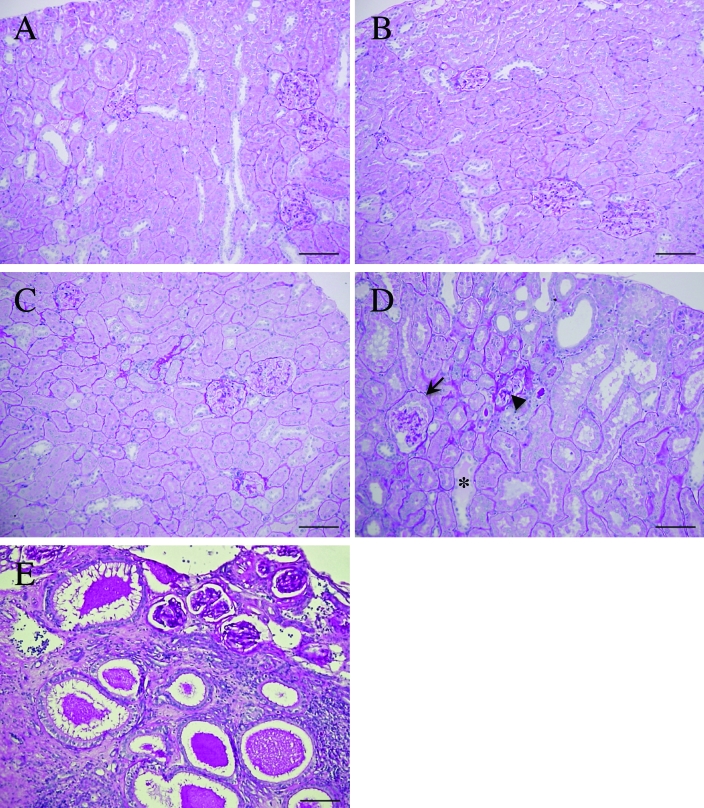
Although the right kidneys of the 10-wk-old rats had no marked pathologic alteration compared with those of normal rats of the same age (Figure 4 A, B), the right kidneys of 30-wk-old affected rats showed thickening of the walls of Bowman capsules, adhesion of the glomerular tuft to the capsular wall, tubular dilations, and tubular casts. The glomeruli of the affected rats showed increased staining with periodic acid–Schiff, indicating the progression of glomerulosclerosis (Figure 4 C, D). In the male rat with descended testes and partial agenesis of reproductive tract, the rudimentary tissue located in the normal position of the left kidney contained numerous degenerated tubules and glomeruli in abnormal fibrous tissue. The tubules were dilated with luminal cast, and the glomeruli were present in the marginal region of the tissue (Figure 4 E).
Figure 4.
Microscopic photographs showing histologic features in the right kidneys of a normal (A, C) and an affected rat (B, D) and in the left kidney of an affected rat (E). (A) Cortex of the right kidney in a 10-wk-old normal male rat. (B) Cortex of the right kidney in a 10-wk-old affected male rat. (C) Cortex of the right kidney in a 30-wk-old normal male rat. (D) Cortex of the right kidney in a 30-wk-old affected male rat. Tubular dilation and renal cast (asterisk) were present. Glomeruli demonstrated accumulation of material positively stained with periodic-acid-Schiff (arrowhead) and thickening of the Bowman capsular wall (arrow). (E) Severe alteration of renal histology in the rudimentary tissue mass remaining in the position of the left kidney in an affected rat. Numerous glomeruli and severely dilated tubules containing luminal cast were found in abnormal fibrous tissue. Periodic acid-Schiff stain; scale bar, 100 μm
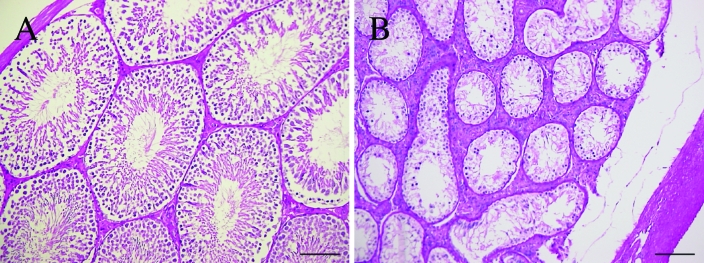
The right testes of affected rats were structurally normal, and normal spermatogenesis was present in the seminiferous tubules (Figure 5 A). In contrast, the undescended testes of affected rats showed thickening of the tunica albuginea testis and marked atrophy of seminiferous tubules (Figure 5 B). Most spermatocytes and spermatids were lost, and no normal spermatogenesis was detected in affected seminiferous tubules.
Figure 5.
Testicular histology of the (A) right and (B) left sides of a 20-wk-old affected rat. (A) Histology of the normally descended right testis of an affected rat. (B) Histology of the undescended left testis of an affected rat. The seminiferous tubules were atrophic. The seminiferous tubules showed decreased numbers of germ cells, and normal spermatogenesis was not observed. The tunica albuginea appeared thicker in the affected testis. Periodic acid–Schiff stain; scale bar, 100 µm.
The left ovaries of affected female rats contained many ovarian follicles at various developmental stages and some corpera lutea, despite the absence of an ipsilateral uterine horn, suggesting that the ovarian cycle progressed normally in these animals (data not shown).
Discussion
We have shown here that the renal agenesis incidentally discovered in a Wistar rat was a hereditary disease associated with ageneses of ipsilateral accessory reproductive organs. These defects occurred exclusively on the left side of affected animals, which we named the UUA (unilateral urogenital anomalies) strain. Our breeding experiments showed that this renal agenesis was controlled by a polygenic mechanism. To establish the inbred UUA strain, brother-sister breeding (affected male rat × phenotypically normal female rat) was continued past the F1BF1 generation; to date, inbreeding has reached generation F1BF22.
The genetic mechanism by which UUA occurs only on the left side is unknown. In general, the complete absence of metanephrons results from disruption of interactions between the metanephric mesenchyme and the ureteric bud derived from the mesonephric duct. In UUA rats, gonads were present on both sides, and agenesis was restricted to the epididymis, deferent duct, and uterine horn, all of which are derived from the mesonephric and paramesonephric ducts. Therefore, the genes primarily responsible for the UUA phenotype may be expressed in the mesonephric and paramesonephric ducts and may contribute to normal development of the urogenital tract. In human hereditary renal agenesis, an autosomal dominant trait, several patients with unilateral renal agenesis also had Müllerian anomalies, including a blind vaginal pouch and a unicornuate uterus.2,11
Hereditary unilateral renal agenesis has also been found in mice (failure of ureteric bud invasion; FUBI)9 and rats (urogenital anomalies in the ACI strain).6,18 In the FUBI strain, 50% of mice have unilateral renal agenesis on the left or right side, and 10% have bilateral renal agenesis. The renal agenesis in FUBI mice is thought to involve 2 or more genes and is accompanied by a failure of ureteric bud invasion to metanephric mesenchyme.9 In contrast to UUA rats, which show not only renal agenesis but ipsilateral defects of reproductive organs, FUBI mice with abnormalities of the reproductive organs have not been reported.9 Therefore, UUA rats might have defects in larger areas of the mesonephric tissue involved in the development of both the ureteric bud and reproductive organs, whereas FUBI mice may have defects in more restricted areas of the mesonephric duct, that is, those that support the development of the ureteric bud.
The ACI rat strain was generated from crossbreeding between August and Copenhagen rats. Unilateral renal agenesis and ipsilateral abnormalities in the reproductive organs occur frequently in this strain. Female ACI rats with unilateral renal agenesis show absence or hypoplasia of the uterine horn, and male affected rats show ipsilateral absence or hypoplasia of the deferent duct and epididymis.6,7 Unilateral urogenital anomalies in ACI rats have been regarded as due to polygenic inheritance.4 The phenotypic similarities between the UUA and ACI strains suggest that the urogenital anomalies in both strains may result from a common developmental defect in mesonephric tissue. Although the genetic defects that cause these urogenital anomalies in ACI and UUA rats have not been determined, different genes may be involved. The major determinant locus (renal agenesis 1, Renag1) for URA in the ACI rat has been mapped to chromosome 14,19 whereas the locus primarily responsible for UUA has been mapped to chromosome 20.1 In addition, unilateral renal agenesis affects either kidney of FUBI mice and ACI rats, whereas it always occurs on the left side in affected UUA rats.
Although the right kidneys of the affected UUA rats were normal in histologic appearance at 10 wk of age, those of 30-wk-old affected rats were approximately 1.7-fold heavier than the right kidneys in normal rats and contained lesions indicative of progressive glomerular sclerosis. In preliminary experiments, we found that the right kidneys of the affected rats already showed compensatory renal growth at 10 d of age and that their renal excretive function was decreased after 30 wk of age compared with normal rats having kidneys on both sides. In general, glomerular hyperfiltration and hypertrophy caused by a compensatory mechanism in response to a reduction in nephron mass is thought to result in glomerulosclerosis.23 In rats with bilateral hypoplastic kidneys containing only one-quarter of the normal number of nephrons, glomerular hyperfiltration and hypertrophy manifest during adolescence and progress to glomerulosclerosis during adulthood.22 After unilateral nephrectomy of rats at 5 d of age, increased albumin excretion in the urine at 2 mo and focal glomerulosclerosis at 6 mo have been noted.3 In addition, proteinuria and focal glomerulosclerosis were more severe in Sprague–Dawley rats uninephrectomized at 1 d than at 8 wk of age.13 These findings indicate that uninephrectomy performed during the early postnatal period may cause more severe lesions in the remaining kidney than does adult uninephrectomy. The congenital renal agenesis of affected rats in UUA may have induced lesions in the remaining kidney, similar to that observed after early postnatal uninephrectomy.
Testicular descent is controlled by 2 morphologic and hormonal steps. Transabdominal testicular descent from the abdominal cavity to the inguinal region is mediated by gubernacular swelling, which is stimulated by insulin-like hormone 3 (Isnl3), which is secreted from Leydig cells. The inguinoscrotal phase of descent from the inguinal region to the scrotum is associated with gubernacular migration, which is regulated by calcitonin gene-related peptide (CGRP) released by the genitofemoral nerve.8 In rats, the former phase begins around 16 d of gestation, and the latter phase begins around postnatal day 6.10 Because the cryptorchidism observed in affected UUA male rats is abdominal, unilateral, and accompanied by agenesis of the epididymis and deferent duct, the abnormal position of the testes in these animals may be caused by unilateral agenesis of the gubernaculum.
The left testis weight of affected UUA male rats was approximately 20% that of normal testis. Histologically, the seminiferous tubules had formed but were severely atrophic in the undescended testes. Testes surgically located in the abdominal cavity at 20 d of age failed to grow normally.5 Histologic examination of the cryptorchid testes revealed a severely reduced number of spermatocytes but almost normal appearances of Sertoli cells and spermatogonia.5 The reduced number of spermatocytes in the cryptorchid testes may be due to apoptotic cell death of spermatocytes which are most sensitive to the heat stress in the testes.17 Therefore, congenital exposure of the testis to heat stress in UUA rats may be a cause of atrophy of the seminiferous tubules. By contrast, affected female rats had histologically and functionally normal ovaries on both sides. This difference between sexes may be due to differences between testicular and ovarian development, in that the ovaries normally develop in the abdominal cavity.
In conclusion, we identified a rat with UUA and subsequently established the UUA inbred strain. UUA occurs exclusively on the left side, and its inheritance is controlled by polygenic mechanism. The high incidence of UUA in this animal model likely will facilitate studies of the pathogenesis and genetic bases involved in the occurrence of UUA. In addition, because UUA rats have a congenital reduction in renal mass, this strain may be a model for studying compensatory renal growth and associated diseases in the elderly.
Acknowledgments
We thank all the members of our laboratory for their time-consuming efforts to maintain the mutant rat strains. We also thank Mr Kei Ogasawara and Ms Junko Shiina for their cooperation in establishing the UUA strain. This work was supported in part by a Grant-in-Aid for Scientific Research to H Suzuki (Number 19580350) from the Ministry of Education, Culture, Sports, Science, and Technology of Japan.
References
- 1.Amakasu K.2006. Personal communication.
- 2.Biedel CW, Pagon RA, Zapata JO. 1984. Müllerian anomalies and renal agenesis: autosomal dominant urogenital adysplasia. J Pediatr 104:861–864 [DOI] [PubMed] [Google Scholar]
- 3.Celsi G, Bohman SO, Aperia A. 1987. Development of focal glomerulosclerosis after unilateral nephrectomy in infant rats. Pediatr Nephrol 1:290–296 [DOI] [PubMed] [Google Scholar]
- 4.Cramer DV, Gill TJ., 3rd 1975. Genetics of urogenital abnormalities in ACI inbred rats. Teratology 12:27–32 [DOI] [PubMed] [Google Scholar]
- 5.Davis JR, Firlit CF. 1966. The germinal epithelium of cryptorchid testes experimentally induced in prepubertal and adult rats. Fertil Steril 17:187–200 [DOI] [PubMed] [Google Scholar]
- 6.Deringer MK, Heston WE. 1956. Abnormalities of urogenital system in strain A × C line 9935 rats. Proc Soc Exp Biol Med 91:312–314 [DOI] [PubMed] [Google Scholar]
- 7.Fujikura T. 1970. Kidney malformations in fetuses of A × C line 9935 rats. Teratology 3:245–249 [DOI] [PubMed] [Google Scholar]
- 8.Hutson JM, Hasthorpe S. 2005. Abnormalities of testicular descent. Cell Tissue Res 322:155–158 [DOI] [PubMed] [Google Scholar]
- 9.Kamba T, Higashi S, Kamoto T, Shisa H, Yamada Y, Ogawa O, Hiai H. 2001. Failure of ureteric bud invasion: a new model of renal agenesis in mice. Am J Pathol 159:2347–2353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Klonisch T, Fowler PA, Hombach-Klonisch S. 2004. Molecular and genetic regulation of testis descent and external genitalia development. Dev Biol 270:1–18 [DOI] [PubMed] [Google Scholar]
- 11.McPherson E, Carey J, Kramer A, Hall JG, Pauli RM, Schimke RN, Tasin MH. 1987. Dominantly inherited renal adysplasia. Am J Med Genet 26:863–872 [DOI] [PubMed] [Google Scholar]
- 12.Morita T, Michimae Y, Sawada M, Uemura T, Araki Y, Haruna A, Shimada A. 2005. Renal dysplasia with unilateral renal agenesis in a dog. J Comp Pathol 133:64–67 [DOI] [PubMed] [Google Scholar]
- 13.Okuda S, Motomura K, Sanai T, Tsuruda H, Oh Y, Onoyama K, Fujishima M. 1987. Influence of age on deterioration of the remnant kidney in uninephrectomized rats. Clin Sci (Lond) 72:571–576 [DOI] [PubMed] [Google Scholar]
- 14.Robinson GW. 1965. Uterus unicornis and unilateral renal agenesis in a cat. J Am Vet Med Assoc 147:516–518 [PubMed] [Google Scholar]
- 15.Robson WL, Leung AK, Rogers RC. 1995. Unilateral renal agenesis. Adv Pediatr 42:575–592 [PubMed] [Google Scholar]
- 16.Scher S, Weisbroth SH. 1974. Unilateral renal and urogenital tract aplasia in a guinea pig (Cavia porcellus). Lab Anim Sci 24:370–371 [PubMed] [Google Scholar]
- 17.Shikone T, Billig H, Hsueh AJW. 1994. Experimentally induced cryptorchidism increases apoptosis in rat testis. Biol Reprod 51:865–872 [DOI] [PubMed] [Google Scholar]
- 18.Shoji R, Harata M. 1977. Abnormal urogenital organs occurring spontaneously in inbred ACI and Kyoto–notched rats. Lab Anim 11:247–249 [DOI] [PubMed] [Google Scholar]
- 19.Shull JD, Lachel CM, Strecker TE, Spady TJ, Tochacek M, Pennington KL, Murrin CR, Meza JL, Schaffer BS, Flood LA, Gould KA. 2006. Genetic bases of renal agenesis in the ACI rat: mapping of Renag1 to chromosome 14. Mamm Genome 17:751–759 [DOI] [PubMed] [Google Scholar]
- 20.Suzuki K, Hakamata Y, Hamada A, Kikukawa K, Wada Y, Imamichi T. 1988. Male hypogonadism as a candidate of deficiency of postnatal testicular growth or differentiating factor(s): a new autosomal recessive mutation in the rat. J Hered 79:54–58 [DOI] [PubMed] [Google Scholar]
- 21.Suzuki H, Suzuki K. 1995. Pathophysiology and postnatal pathogenesis of hypoplastic kidney (hpk/hpk) in the male hypogonadic mutant rat (hgn/hgn). J Vet Med Sci 57:891–897 [DOI] [PubMed] [Google Scholar]
- 22.Suzuki H, Tokuriki T, Saito K, Hishida A, Suzuki K. 2005. Glomerular hyperfiltration and hypertrophy in the rat hypoplastic kidney as a model of oligomeganephronic disease. Nephrol Dial Transplant 20:1362–1369 [DOI] [PubMed] [Google Scholar]
- 23.Taal MW, Luyckx VA, Brenner BM. 2004. Adaptation to nephron loss, p1955–1997 Brenner BM. The kidney. Philadelphia (PA): Saunders. [Google Scholar]
- 24.Woolf AS, Hillman KA. 2007. Unilateral renal agenesis and the congenital solitary functioning kidney: developmental, genetic, and clinical perspectives. BJU Int 99:17–21 [DOI] [PubMed] [Google Scholar]