Abstract
In the oxidative stress hypothesis of aging, the aging process is the result of cumulative damage by reactive oxygen species. Humans and chimpanzees are remarkably similar; but humans live twice as long as chimpanzees and therefore are believed to age at a slower rate. The purpose of this study was to compare biomarkers for cardiovascular disease, oxidative stress, and aging between male chimpanzees and humans. Compared with men, male chimpanzees were at increased risk for cardiovascular disease because of their significantly higher levels of fibrinogen, IGF1, insulin, lipoprotein a, and large high-density lipoproteins. Chimpanzees showed increased oxidative stress, measured as significantly higher levels of 5-hydroxymethyl-2-deoxyuridine and 8-iso-prostaglandin F2α, a higher peroxidizability index, and higher levels of the prooxidants ceruloplasmin and copper. In addition, chimpanzees had decreased levels of antioxidants, including α- and β-carotene, β-cryptoxanthin, lycopene, and tocopherols, as well as decreased levels of the cardiovascular protection factors albumin and bilirubin. As predicted by the oxidative stress hypothesis of aging, male chimpanzees exhibit higher levels of oxidative stress and a much higher risk for cardiovascular disease, particularly cardiomyopathy, compared with men of equivalent age. Given these results, we hypothesize that the longer lifespan of humans is at least in part the result of greater antioxidant capacity and lower risk of cardiovascular disease associated with lower oxidative stress.
Abbreviations: 5OHmU, 5-hydroxymethyl-2-deoxyuridine; 8isoPGF2α, 8-iso-prostaglandin F2α; HDL, high-density lipoprotein; IGF1, insulin-like growth factor 1; LDL, low-density lipoprotein; ROS, reactive oxygen species
Aging is characterized as a progressive reduction in the capacity to withstand the stresses of everyday life and a corresponding increase in risk of mortality. According to the oxidative stress hypothesis of aging, much of the aging process can be accounted for as the result of cumulative damage produced by reactive oxygen species (ROS).6,21,28,41,97 Endogenous oxygen radicals (that is, ROS) are generated as a byproduct of normal metabolic reactions in the body and subsequently can cause extensive damage to proteins, lipids, and DNA.6,41 Various prooxidant elements, in particular free transition metals, can catalyze these destructive reactions.6 The damage caused by ROS can be counteracted by antioxidant defense systems, but the imbalance between production of ROS and antioxidant defenses, over time, leads to oxidative stress and may contribute to the rate of aging.28,97
Oxidative stress has been linked to several age-related diseases including neurodegenerative diseases, ophthalmologic diseases, cancer, and cardiovascular disease.21,28,97 Of these, cardiovascular disease remains the leading cause of adult death in the United States and Europe.71 In terms of cardiovascular disease, oxidative stress has been linked to atherosclerosis, hypertension, cardiomyopathy, and chronic heart failure in humans.55,78,84 Increases in oxidant catalysts (prooxidants)—such as copper, iron, and cadmium—have been associated with hypertension, coronary artery disease, atherosclerosis, and sudden cardiac death.98,102,106 Finally, both endogenous and exogenous antioxidants have been linked to decreased risk of cardiovascular disease, although the mechanisms behind this relationship are unclear.11,52,53 However, the oxidative stress hypothesis of aging aims to explain not only the mechanism of aging and age-related diseases (such as cardiovascular disease) in humans but also the differences between aging rates and the manifestations of age-related diseases across species.
The differences in antioxidant and ROS levels between animals and humans offer promise for increasing our understanding of human aging. Additional evidence supporting the oxidative stress hypothesis of aging has come from comparative studies linking differences in aging rates across taxa with both antioxidant and ROS levels.4,17-21,58,71,86,105 In mammals, maximum lifespan potential is positively correlated with both serum and tissue antioxidant levels.17,18,21,71,105 Research has consistently demonstrated that the rate of oxidative damage varies across species and is negatively correlated with maximum lifespan potential.4,19,20,58,71,86 However, few studies involved detailed comparisons of hypothesized biochemical indicators of aging and oxidative stress between humans and animals.6 This type of interspecies comparison has great potential for directly testing the oxidative stress hypothesis of aging.
Much evolutionary and genetic evidence supports remarkable similarity between humans and chimpanzees.95,100 Despite this similarity, humans have a lifespan of almost twice that of chimpanzees.3,16,47 Most comparative primate aging research has focused on the use of a macaque model,62,81,88 and several biochemical markers of age-related diseases have been identified in both humans and macaque monkeys.9,22,28,81,93,97 Several other species of monkeys have also been used in research addressing oxidative stress, antioxidant defenses, and maximum lifespan potential.18,21,58,105 However, no study to date has examined biochemical indicators of oxidative stress and aging in chimpanzees and humans as a test of the oxidative stress hypothesis for aging. The purpose of this study is to compare biochemical markers for cardiovascular disease, oxidative stress, and aging directly between male chimpanzees and humans. Given the oxidative stress hypothesis for aging and the known role of oxidative stress in cardiovascular disease, we predict that chimpanzees will show higher levels of cardiovascular risk and oxidative stress than humans.
Materials and Methods
Subjects included 10 healthy, young men (mean age, 24 y; age range, 22 to 30 y) and 10 healthy, young male chimpanzees (Pan troglodytes; mean age, 12 y; range, 11 to 16 y). The described mean ages were chosen to reduce, as much as possible, any age-dependent changes that may occur in the biochemical parameters measured. The mean age for chimpanzees in this study represents 1 to 2 y after the median age of skeletal,32,39 dental,1,59,96 body weight,32,65 and reproductive74,119 maturity for captive chimpanzees. In addition, the age range was selected to fall below the onset of physiologic aging in chimpanzees.46,108 The age range of human subjects was chosen to equal twice that of the chimpanzee subjects, because the human lifespan is twice that of chimpanzees.3,16,47 Only male subjects were chosen to minimize hormonal variation (that is, female menstrual cycles) that could alter the biochemical parameters measured.
The chimpanzees were housed at the Primate Foundation of Arizona, an AAALAC-accredited facility, and the protocol was approved by the facility's institutional animal care and use committee. No animals were exposed to hepatitis C or HIV, nor did they have hepatitis A or B antigen. Likewise, no animals were on infectious studies at the time of blood sampling. Chimpanzees were housed in compatible social groups of 4 to 7 animals, in housing that exceeds current standards and recommendations of the US Department of Agriculture and the Guide for the Care and Use of Laboratory Animals.51 Chimps were provided water ad libitum and a varied diet of seasonal fruits, vegetables and pelleted monkey chow biscuits (Lab Diet 5045, PMI Nutrition International, Richmond, IN), in addition to vitamin supplementation (Bronson Chewable Multivitamin, Bronson Vitamins, Lindon, UT) and daily environmental enrichment and forage materials. The water source for the chimpanzees was an onsite well, and all tests showed that the water met Arizona state drinking water standards. Chimpanzees were healthy, with no sign of illness or disease at the time of the study, and body weights ranged from 51.0 to 73.6 kg.
All chimpanzees were examined while under anesthesia for routine biannual health checks. After a 12-h fast, each chimp was anesthetized by intramuscular injection of either ketamine HCl (5.0 to 7.5 mg/kg; Ketaset, Fort Dodge, IA) or tiletamine hydrochloride–zolazepam HCl (3.0 to 4.0 mg/kg; Telazol, Fort Dodge, IA). Injections were given by dart (Telinject USA, Agua Dulce, CA) or manually by syringe and hypodermic needle. Personnel used all appropriate personal protective equipment when interacting with chimpanzees, and standard precautions were taken. Blood and urine samples were collected for biochemical assays, including a cardiovascular risk factor profile and oxidative stress status profile (Tables 1 through 4). Blood was collected from the femoral vein into vacuum phlebotomy tube tubes (Vacutainer, Becton Dickinson, Rutherford, NJ). Urine was collected by using a catheter (Kendall–Sovereign, Mansfield, MA).
Table 1.
Comparison of mean (1 SD) values of serum indicators of cardiovascular risk in male chimpanzees (n = 10) and men (n = 10)
| Cardiovascular risk factor | Chimpanzee | Human | Human reference rangea | Chimpanzee: human ratio | P |
| Cholesterol (mg/dL) | 187.9 (34.1) | 180.0 (39.8) | 150.0–200.0 | 1.04 | 0.639 |
| Triglycerides (mg/dL) | 72.6 (28.1) | 75.0 (47.0 | 35.0–160.0 | 0.97 | 0.891 |
| HDL(mg/dL) | 50.0 (10.0) | 42.0 (7.2) | 40.0–70.0 | 1.19 | 0.055 |
| LDL (mg/dL) | 120.4 (28.7) | 122.3 (35.1) | 50.0–160.0 | 0.98 | 0.896 |
| LDLM (mg/dL) | 266.7 (2.9) | 274.0 (2.4) | 255.0–280.0 | 0.97 | <0.001 |
| IGF1 (ng/mL) | 519.3 (124.7) | 194.2 (60.5) | 90.0–360.0 | 2.67 | <0.001 |
| Insulin (μIU/mL) | 14.8 (9.2) | 7.7 (3.7) | 2.0–20.0 | 1.92 | 0.035 |
| Apolipoprotein A (mg/dL) | 125.6 (28.5) | 116.7 (19.9) | 90.0–170.0 | 1.08 | 0.429 |
| Apolipoprotein B (mg/dL) | 100.0 (19.1) | 92.7 (28.0) | 56.0–162.0 | 1.08 | 0.505 |
| Fibrinogen (mg/dL) | 367.9 (143.5) | 263.5 (57.9) | 200.0–400.0 | 1.40 | 0.046 |
| hs–CRP (mg/L) | 4.4 (5.4) | 2.1 (1.9) | 0.0–2.5 | 2.00 | 0.233 |
| Lipoprotein A (mg/dL) | 145.0 (38.2) | 16.7 (24.7) | 0.0–64.0 | 8.68 | <0.001 |
| Folic acid (ng/dL) | 12.7 (2.5) | 9.4 (2.5) | 3.1–17.5 | 1.35 | 0.009 |
| Vitamin B12 (pg/mL) | 1500.0 (0.0) | 519.8 (181.4) | 180.0–914.0 | 28.86 | <0.001 |
| Homocysteine (μmol/L) | 4.9 (0.9) | 9.7 (1.6) | 5.4–13.4 | 0.51 | <0.001 |
| Coenzyme Q10 (μg/mL) | 0.6 (0.2) | 0.9 (0.3) | 0.5–1.2 | 0.67 | 0.067 |
| WBC (×103/μL) | 9.5 (2.8) | 6.2 (1.8) | 4.0–11.0 | 1.53 | 0.006 |
HDL, high-density lipoproteins; hs-CRP, high-sensitivity C-reactive protein; IGF1, insulin-like growth factor 1; LDL, low-density lipoproteins; LDLM, mean low-density lipoprotein particle size
Bold type indicates chimpanzee value significantly higher than human, and italics indicate human value significantly higher than chimpanzee.
Reference ranges from the Kronos Science Laboratory.
Table 2.
Comparison of mean (1 SD) values of biomarkers of oxidative stress in male chimpanzees (n = 10) and men (n = 10)
| Oxidative stress factor | Chimpanzee | Human | Chimpanzee: human ratio | P |
| 2,3dinorPFGα (μg/g) | 2.9 (1.3) | 5.4 (1.6) | 0.54 | 0.009 |
| 5OHmU (μg/g) | 17.9 (4.6) | 7.93 (2.5) | 2.26 | <0.001 |
| 8OHdG (μg/g) | 2.6 (1.4) | 2.7 (0.7) | 0.96 | 0.851 |
| 8isoPFGα (μg/g) | 0.39 (0.29) | 0.03 (0.02) | 13.09 | 0.003 |
| Peroxidizability index | 110.1 (10.6) | 89.2 (4.8) | 1.23 | <0.001 |
2,3dinorPFGα, 2,3-dinor 8-iso-prostaglandin F2α/creatinine; 5OHmU, 5-hydroxymethy l-2-deoxyuridine/creatinine; 8OHdG, 8-hydroxy-2-deoxyguanosine/creatinine; 8isoPFGα, 8-iso-prostaglandin F2α /creatinine
Peroxidizability index = (% monenoic × 0.025) + (% dienoic × 1) + (% trienoic × 2) + (% tetraenoic × 3) + (% pentaenoic × 4) + (% hexaenoic × 5)
Bold type indicates chimpanzee value significantly higher than human, and italics indicate human value significantly higher than chimpanzee.
Table 3.
Comparison of mean (1 SD) levels of prooxidants (oxidative stress catalysts) in male chimpanzees (n = 10) and men (n = 10)
| Oxidative stress factor | Chimpanzee | Human | Human reference rangea | Chimpanzee: human ratio | P |
| Cadmium (μg/L) | 0.03 (0.04) | 0.01 (0.01) | 0.15–0.19 | 3.00 | 0.236 |
| Ceruloplasmin (mg/dL) | 44.7 (7.7) | 29.3 (6.3) | 25.0–63.0 | 1.53 | <0.001 |
| Copper (μg/L) | 1567.2 (285.5) | 963.3 (151.4) | 498.0–1945.0 | 1.63 | <0.001 |
| Ferritin (ng/mL) | 73.5 (31.6) | 96.8 (45.5) | 24.0–336.0 | 0.76 | 0.200 |
| Glucose (mg/dL) | 85.7 (11.4) | 88.6 (7.6) | 74.0–118.0 | 0.97 | 0.512 |
| Hemoglobin A1C (%) | 4.8 (0.2) | 4.9 (0.2) | 4.0–6.0 | 0.98 | 0.050 |
| Iron (μg/dL) | 111.1 (46.1) | 92.3 (25.5) | 45.0–182.0 | 1.20 | 0.274 |
| Iron saturation (%) | 33.0 (13.9) | 29.1 (8.6) | 10.0–36.0 | 1.13 | 0.506 |
| Nickel (μg/L) | 3.3 (1.0) | 3.0 (1.0) | 1.5–7.9 | 1.10 | 0.533 |
Reference ranges from the Kronos Science Laboratory.
Table 4.
Comparison of mean (1 SD) levels of factors protective against oxidative stress in male chimpanzees (n = 10) and men (n = 10)
| Protective factor | Chimpanzee | Human | Human reference rangea | Chimpanzee: human ratio | P |
| Carotenoids | |||||
| α-Carotene (ng/mL) | 8.3 2.8) | 36.3 (23.3) | 20.0-400.0 | 0.23 | 0.001 |
| β-Carotene (ng/mL) | 21.0 (42.2) | 119.8 (64.8) | 50.0-710.0 | 0.18 | <0.001 |
| β-Cryptxanthin (ng/mL) | 8.4 (3.8) | 76.7 (36.5) | 5.0-200.0 | 0.11 | <0.001 |
| Lycopene (ng/mL) | 10.3 (4.7) | 174.6 (46.3) | 42.0-435.0 | 0.06 | <0.001 |
| Lutein (ng/mL) | 138.5 (48.9) | 95.1 (36.9) | 40.0-600.0 | 1.46 | 0.038 |
| Retinol (ng/mL) | 964.9 (186.5) | 541.5 (91.5) | 400.0-1300.0 | 1.78 | <0.001 |
| Retinyl palmitate (ng/mL) | 47.4 (13.9) | 10.6 (1.0) | 5.0-27.0 | 4.47 | <0.001 |
| Zeaxanthin (ng/mL) | 30.0 (6.5) | 25.8 (4.9) | 10.0-150.0 | 1.16 | 0.121 |
| Tocopherols | |||||
| α-Tocopherols (μg/mL) | 9.7 (1.9) | 11.1 (3.2) | 7.2-22.4 | 0.87 | 0.238 |
| β-Tocopherols (μg/mL) | 0.01 (0.00) | 0.05 (0.03) | 0.05-0.20 | 0.20 | <0.001 |
| γ-Tocopherols (μg/mL) | 0.2 (0.1) | 1.7 (0.5) | 0.1-2.2 | 0.12 | <0.001 |
| Other Antioxidants | |||||
| Albumin (g/dL) | 3.2 (0.3) | 4.4 (0.2) | 3.5-4.8 | 0.73 | <0.001 |
| Ascorbate (μg/mL) | 19.0 (8.9) | 12.3 (5.4) | 5.0-28.0 | 1.54 | 0.057 |
| Direct bilirubin (mg/dL) | 0.08 (0.04) | 0.14 (0.05) | 0.0-0.5 | 0.57 | 0.011 |
| Total bilirubin (mg/dL) | 0.32 (0.09 | 0.75 (0.26) | 0.4-2.0 | 0.43 | <0.001 |
| Total thiols (μmol/L) | 193.5 (94.1) | 412.0 (0.0) | 318-578 | 0.47 | 0.309 |
| Uric acid (mg/dL) | 2.6 (0.3) | 6.1 (0.8) | 2.8-8.0 | 0.43 | <0.001 |
Bold type indicates that the chimpanzee value was significantly higher than that in men, and italics indicate that the human value was significantly higher than that in chimpanzee.
Reference ranges from the Kronos Science Laboratory.
The men were paid volunteers, recruited from the greater Phoenix community by direct mail (from a database of subjects that had previously participated in research studies) and word of mouth. The research protocol was approved by the Arizona State University Institutional Review Board, and subjects gave written informed consent to participate. Participants were asked to complete a health history questionnaire to determine health status. Subjects chosen for the study had no major medical problems or signs of illness and body mass index scores less than 30. The human subjects were examined at Kronos Science Laboratories. Blood and urine samples were collected for biochemical assays as described for the chimpanzees. Blood samples were drawn via venipuncture, after a 12-h fast. Urine samples were first-morning voided urines and were collected on the same day as the blood draw.
All biochemical assays were performed at Kronos Science Laboratories. The cardiovascular risk factor profile was run by using standard clinical methodology on an automated protein chemistry analyzer (Beckman Array, Beckman Coulter, Fullerton, CA), a clinical chemistry system (Synchron LX20, Beckman Coulter), and an immunoanalyzer (Immulite 2000, Diagnostic Products Corporation, Los Angeles, CA). High-density lipoprotein (HDL) and low-density lipoprotein (LDL) subfractions were determined manually by using a lipograph (Kronos Science Laboratories, Phoenix, AZ).26 The fatty acid profile was run by using custom (Kronos Science Laboratories) methodology on a gas chromatograph.26 Urine creatinine was measured by using standard clinical methodology (Synchron LX20, Beckman Coulter). Urinary markers of oxidative damage were measured by using custom (Kronos Science Laboratories) methodology for liquid chromatography–tandem mass spectrometry.42,67 Isoprostane levels were measured by using stable isotope dilution gas chromatography–negative ion chemical ionization mass spectrometry.87 Prooxidant metals were measured by using custom (Kronos Science Laboratories) methodology for inductively coupled plasma–mass spectrometry. Antioxidants were measured by using custom (Kronos Science Laboratories) methodology for HPLC, and additional oxidative stress protection factors were determined by using standard clinical methodology (Synchron LX20, Beckman Coulter).
Results for all cardiovascular risk factors, oxidative stress markers, and oxidative stress protection factors were compared between chimpanzees and humans by using ANOVA. Significance was set at the 0.05 level, and all statistics were conducted by using JMP 6.0 (SAS Institute, Cary, NC).
Results
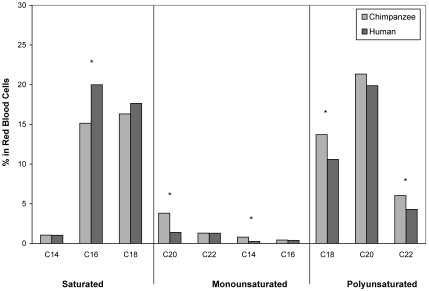
In terms of cardiovascular risk, chimpanzees had significantly higher levels of fibrinogen (F19 = 4.55, P = 0.046), insulin-like growth factor 1 (IGF1; F19 = 54.99, P < 0.001), insulin (F19 = 5.20, P = 0.035), lipoprotein a (F19 = 77.30, P < 0.001), and WBC (F19 = 9.85, P = 0.006; Table 1). All of the chimpanzees had lipoprotein a and IGF1 levels that were considerably above the human reference maximum (Table 1). Men had significantly higher levels of homocysteine (F19 = 33.43, P < 0.001) and lower levels of both folic acid (F19 = 8.60, P = 0.009) and vitamin B12 (F19 = 291.85, P < 0.001; Table 1). The fatty acid profiles (Figure 1) revealed that men had significantly higher levels of saturated (palmitic acid, C16:0, F19 = 18.49, P < 0.001) and monounsaturated fats (C18:1, F19 = 32.57, P < 0.001). Men also had significantly lower levels of unsaturated fats, both linoleic and α-linoleic (C18:2, C18:3) acid (F19 = 5.36, P = 0.035), and docosahexaenoic (C22:6) acid (F19 = 4.60, P = 0.049).
Figure 1.
Comparison of mean (± SE) saturated, monounsaturated, and polyunsaturated fatty acids in male chimpanzees (n = 10) and men (n = 10). Asterisks indicate significant (P < 0.05) difference between chimpanzee and human values.
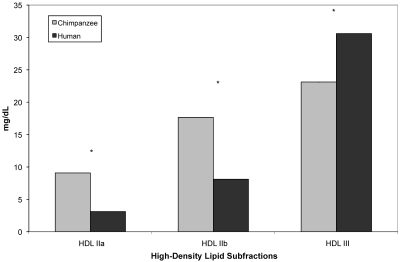
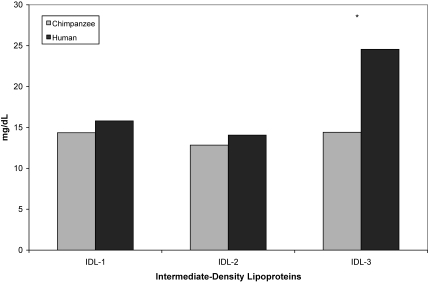
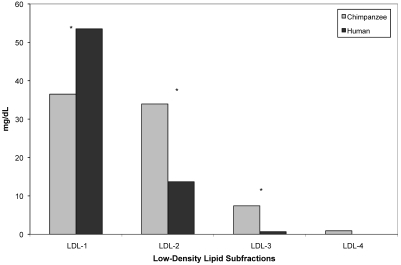
Chimpanzees and men did not differ significantly in either total HDL cholesterol (F19 = 4.20, P = 0.055) or total LDL cholesterol (F19 = 0.02, P = 0.896); however, chimpanzees had significantly smaller mean LDL particle size (F19 = 77.30, P < 0.001; Table 1). In terms of lipid subfractions, chimpanzees had significantly higher levels of both HDLIIa (F19 = 28.09, P < 0.001) and HDLIIb (F19 = 11.17, P = 0.004) and significantly lower levels of HDLIII (F19 = 12.15, P = 0.003; Figure 2). Men had significantly higher levels of IDL3 (F19 = 15.76, P = 0.001; Figure 3). Chimpanzees had significantly lower levels of LDL1 (F19 = 7.11, P = 0.016) and significantly higher levels of LDL2 (F19 = 17.25, P < 0.001) and LDL3 (F19 = 9.18, P = 0.007; Figure 4).
Figure 2.
Comparison of mean (± SE) high-density lipid subfractions in male chimpanzees (n = 10) and men (n = 10). Asterisks indicate significant (P < 0.05) difference between chimpanzee and human values.
Figure 3.
Comparison of mean (± SE) intermediate-density lipid subfractions in male chimpanzees (n = 10) and men (n = 10). Asterisks indicate significant (P < 0.05) difference between chimpanzee and human values.
Figure 4.
Comparison of mean (± SE) low-density lipid subfractions in male chimpanzees (n = 10) and men (n = 10). Asterisks indicate significant (P < 0.05) difference between chimpanzee and human values.
In terms of oxidative stress, chimpanzees had significantly higher levels of 5-hydroxymethyl-2-deoxyuridine (5OHmU; F19 = 27.76, P < 0.001) and 8-iso-prostaglandin F2α (8isoPGF2α; F19 = 13.09, P = 0.003) but significantly lower levels of 2,3-dinor-8-iso-prostaglandin F2α (F19 = 9.81, P = 0.009; Table 2). The amount of 8isoPGF2α in chimpanzees was more than 13 times that of men (Table 2). Chimpanzees also had significantly higher levels of ceruloplasmin (F19 = 23.93, P < 0.001) and copper (Cu, F19 = 31.43, P < 0.001) and a higher peroxidizability index (F19 = 23.21, P < 0.001) (Tables 2 to 3). In terms of oxidative protection, chimpanzees had significantly lower levels of albumin (F19 = 98.98, P < 0.001), uric acid (F19 = 173.53, P < 0.001), and both direct (F19 = 8.10, P = 0.011) and total bilirubin (F19 = 24.44, P < 0.001) (Table 4). In half of the chimpanzees, the amounts of total bilirubin and uric acid were below the human reference minimum, and nearly all of the chimpanzees had albumin levels that were below the human reference minimum (Table 4). The chimpanzees also had significantly lower levels of α- (F19 = 14.25, P = 0.001) and β-carotene (F19 = 16.33, P < 0.001), β-cryptoxanthin (F19 = 34.64, P < 0.001), lycopene (F19 = 124.84, P < 0.001), and tocopherols δ (F19 = 15.06, P < 0.001) and γ (F19 = 71.22, P < 0.001; Table 4). In all of the chimpanzees, the levels of α-carotene, β-carotene, lycopene, and tocopherol were below the human reference minima (Table 4). However, men had significantly lower levels of lutein (F19 = 5.01, P = 0.038), retinol (F19 = 41.59, P < 0.001), and retinyl palmitate (F19 = 69.69, P < 0.001; Table 4).
Discussion
In humans, cardiovascular disease takes many forms, including coronary artery disease, atherosclerosis, coronary heart disease, cardiomyopathy, and chronic heart failure. The chimpanzees in this study showed increased levels of lipoprotein a, fibrinogen, and IGF1, which indicate a high risk for premature cardiovascular disease, particularly coronary heart disease and cardiomyopathy.27,43,118 In particular, levels of lipoprotein a that are greater than 30 mg/dL are associated with a 200% increase in cardiovascular disease risk.35,118 Cardiomyopathy-associated congestive heart failure has been documented in chimpanzees and is the leading cause of death in captive chimpanzees.40,50,61,62,64 Elevated levels of lipoprotein a, fibrinogen, and IGF1 are all strongly associated with cardiomyopathy in humans.7,8,13,14,72 The data presented here support the observation that chimpanzees appear to be predisposed to cardiovascular disease, particularly cardiomyopathy. We hypothesize that among chimpanzees diagnosed with early cardiomyopathy, levels of lipoprotein a, fibrinogen, and IGF1 will be significantly higher than those in otherwise healthy chimpanzees. In addition, such increased levels likely are present in other great ape species, because (as in chimpanzees) cardiovascular disease is the leading cause of death among captive gorillas, bonobos, and orangutans.12,81,92,112 The predisposition for cardiovascular disease may be 1 of the factors accounting for the shorter lifespan of chimpanzees, and potentially other great ape species, compared with humans. In contrast, the men in our study showed slightly higher risk for atherosclerosis because of their increased homocysteine levels.118 The lower homocysteine levels in the chimpanzees could be due to the extremely high levels of folic acid and vitamin B12, both of which have been shown to lower homocysteine levels.68 Recent research25,54 has suggested that increased homocysteine as a causal risk factor for cardiovascular disease may be overrated. Given that the level of homocysteine in the men was below the recommended maximum (13.4 μmol/L), we do not consider that this result in any way contradicts the remaining evidence that chimpanzees are at higher risk for cardiovascular disease.
At first glance, the fatty acid profiles seem to present contradictory results suggesting a higher risk for cardiovascular disease in humans, which is due to increased levels of saturated fatty acids and decreased levels of unsaturated fatty acids. Previous human research has shown that low levels of saturated fats (that is, palmitic acid) and high levels of polyunsaturated fats (that is, linoleic acids) can result in decreased levels of fibrinogen and reduced risk of coronary heart disease.89,118 However, these results have been inconsistent, and supplementation with n3 and n6 polyunsaturated fatty acids does not consistently lower cardiovascular risk.89,91,113 That these same effects are not seen reflected in the chimpanzees' cardiovascular risk profile provides further support that the relationship between fatty acid profiles and cardiovascular risk is unclear. Additional research on fatty acid profiles and cardiovascular risk factors among chimpanzees and other great ape species is needed to address this issue.
The lipid profile indicated that the chimpanzees had higher levels of large, less-dense HDL subfractions (HDLIIa and HDLIIb), compared with humans. Increased levels of large HDL subfractions have been correlated with reduced risk of coronary artery disease.107,118 However, chimpanzees had significantly higher levels of small LDL subfractions (LDL3 and LDL4) and lower levels of the larger LDL subfractions (LDL1 and LDL2), indicating increased risk for both coronary artery disease and cardiomyopathy.2,56,57,101,107,115 Overall, these data balance out as increased risk for cardiomyopathy in chimpanzees, a pattern that matches observations of increased cardiomyopathy-associated heart failure in captive chimpanzees.40,50,61,62,64 We hypothesize that among chimpanzees diagnosed with early cardiomyopathy, levels of small LDL subfractions are significantly higher than in other chimpanzees. In addition, such elevated levels likely also are present in other great ape species, given that cardiovascular disease is the leading cause of death among captive gorillas, bonobos, and orangutans also.12,81,92,112 Finally, the chimpanzees had a smaller mean LDL particle size than did the human subjects. Among humans, members of families exhibiting extreme longevity (that is, offspring of centenarians) have larger mean LDL particle size than age-matched controls.44,104 This finding adds further support to the hypothesis that the small mean LDL particle size in chimpanzees is related to high disease risk and early death, compared with humans. All of these results support our hypothesis that chimpanzees are naturally at higher risk of cardiovascular disease compared with humans.
The chimpanzees in this study exhibited significantly increased levels of DNA oxidative damage, in the form of 5OHmU and 8isoPGF2α. In fact, half of the chimpanzees had 5OHmU levels that exceeded the recommended maximum (19.6 mg/g). DNA oxidative damage has been linked to both cardiomyopathy and heart failure, but the biomarker typically measured when evaluating cardiovascular disease risk is 8-hydroxy-2-deoxyguanosine.54,82 In addition isoprostanes (such as 8isoPGF2α) are strongly linked to oxidative damage in heart valves and the risk of both coronary heart disease and cardiomyopathy.42,78,84,116 Recent research has suggested that 5OHmU and 8isoPGF2α are better indicators of oxidative damage than 8-hydroxy-2-deoxyguanosine.42,87 We hypothesize that increased levels of DNA oxidative damage are present among other great ape species, which also show decreased longevity and increased cardiovascular risk.12,80,92,112 Increased oxidative stress among chimpanzees is further evident in their higher peroxidizability index. Because polyunsaturated fatty acids are the most sensitive to oxidative damage, an increased peroxidizability index is indicative of increased oxidative stress and a potentially decreased lifespan.19,85 In this regard, it is important to note that comparative gene expression studies between humans and chimpanzees have indicated very high levels of peroxiredoxin (PRDX2, PRDX5, PRDX6) in humans. Specifically, peroxiredoxin 6 is overexpressed 10-fold or more in humans than in chimpanzees (data not shown). The lower expression of peroxiredoxin in chimpanzees may have contributed to their higher peroxidizability index, but additional research is needed to confirm this possibility. Levels of the prooxidants ceruloplasmin and copper also were significantly higher in the chimpanzees, suggesting increased risk of cardiovascular disease and cardiac death.29,34,90,94,106 The elevated levels of 5OHmU and 8isoPGF2α, together with elevated levels of ceruloplasmin and copper and a high peroxidizability index in the chimpanzees, provide additional support for our hypothesis that chimpanzees have higher levels of oxidative stress and are at higher risk for cardiovascular disease than are humans.
The chimpanzees in this study exhibited significantly lower antioxidant levels (that is, α- and β-carotene, β-cryptoxanthin, lycopene, and tocopherols) compared with their human counterparts. This status likely is not due to dietary deficiency, because the chimpanzees daily consumed, through diet and supplementation, more than 15,000 IU of vitamin A (1000 IU as β-carotene) and more than 30 IU of vitamin E (as α-tocopherol). Therefore, the significantly lower levels of antioxidants likely do not reflect dietary deficiency but rather a decreased ability to absorb certain antioxidants or increased degradation of them. This situation has a profound effect on both oxidative stress and cardiovascular risk. Tocopherols reduce levels of biomarkers of oxidative damage and reduce the risk of coronary heart disease.11,49,53 Carotenoids, particularly α- and β-carotenes and lycopene, reduce the risk of mortality from cardiovascular disease.52 In addition, β-carotene and lycopene alter fatty acid profiles and β-cryptoxanthin reduces inflammation, thereby decreasing cardiovascular disease risk.57,117 However, results of studies examining the relationship between antioxidants and cardiovascular disease have been inconsistent.45,53,69,76,120 Further, our chimpanzees exhibited increased levels of retinol and lutein, which some may interpret as reduced risk of cardiovascular disease. These antioxidant levels are within the range published for other captive chimpanzees, but because serum antioxidant levels are unavailable for wild chimpanzees, we cannot eliminate the possibility that the levels published for captive chimpanzees are artificially inflated due to diet.15,31 These antioxidant data should be interpreted remembering that most randomized trials and meta-analyses of the effects of antioxidants on morbidity and mortality typically do not involve an integrated approach (using multiple antioxidants) and fail to examine the effects of antioxidant supplementation on measures of oxidative stress.73 In addition, some research suggests that retinol and lutein levels are not good predictors of future coronary heart disease or cardiac death.37,103,117 We hypothesize that chimpanzees are at increased risk for oxidative stress and, likely, cardiovascular disease, due to their decreased levels of tocopherols and carotenoids. However, additional research is needed.
Both albumin and bilirubin are viewed to have antioxidant properties by preventing the production of damaging free radicals and protecting free fatty acids from peroxidation.38,99 Decreased levels of serum albumin and bilirubin are related to an increased risk of coronary heart disease and heart failure.23,33,60,75 The decreased levels of albumin and bilirubin in our chimpanzees therefore add further support that chimpanzees are at high risk for oxidative stress and cardiovascular disease, and we hypothesize that chimpanzees diagnosed with early cardiomyopathy have lower levels of albumin and bilirubin than do other chimpanzees.
The lower levels of uric acid in chimpanzees could be interpreted as reducing risk of cardiovascular disease. Several studies have reported that increased uric acid levels are a risk factor for cardiovascular disease.10,30,111 However, other authors have concluded that there is no independent association between uric acid and cardiovascular disease risk.79,109,110,114 In addition, some studies suggest that uric acid has potent antioxidant properties and potential lifespan-enhancing benefits,5,18,24,36,48,83 whereas others have found no relationship between uric acid levels and maximum lifespan potential.71,77 In addition, uric acid levels are influenced heavily by nutrition and environmental circumstance109 and perhaps less so by oxidative stress. Therefore, the significance of the decreased uric acid in our chimpanzees is unclear. We hypothesize that the higher uric acid levels of humans may be unrelated to cardiovascular risk, because in studies in which levels were predictive of cardiovascular disease, uric acid levels exceeded 8 mg/dL,10,66 well above the levels in the current study. However, additional research is needed, and no definitive conclusion regarding uric acid can be made at this time.
The oxidative stress hypothesis of aging predicts that differences in aging rates between closely related species are the result of differences in both oxidative stress (that is, ROS) and antioxidant levels.4,17-21,84,96 If chimpanzees experience an aging rate twice that of humans, one would predict that chimpanzees would show correspondingly higher levels of oxidative stress and cardiovascular risk and lower levels of antioxidant capacity. Results of the current study supported this hypothesis, with chimpanzees exhibiting cardiovascular risk and oxidative stress levels twice that, on average, of humans. The data from this study generally support the idea that the extended lifespan in humans is, at least in part, the effect of genetic changes resulting in increased antioxidant capacity and decreased cardiovascular risk and oxidative stress. Further, our study suggests that throughout its lifespan, the chimpanzee may have a higher level of oxidative stress, which leads to acceleration of its aging process compared with that of humans. Fibrinogen, IGF1, lipoprotein a, 5OHmU, 8isoPGF2α, and ceruloplasmin are all good candidates for aging biomarkers in hominoids (that is, great apes and humans). Additional research is needed to test these hypotheses. Clearly, more studies are needed to explore the genetic basis for the differences observed in this study, as well as the longitudinal differences between these species in terms of oxidative stress and cardiovascular disease.
Acknowledgments
Thanks to all PFA care and research staff, especially Jo Fritz, and to all Kronos Science Laboratories staff, especially Wendy Bezotte Bennett, James Simms, and Denise Brown. This study was supported in part by Kronos Science Laboratories (Phoenix, Arizona). PFA is AAALAC-accredited. The Alamogordo Primate Facility is funded by NIH contract NO2-RR-209.
References
- 1.Anemone RL, Watts ES, Swindler DR. 1991. Dental development of known-age chimpanzees, Pan troglodytes. Am J Phys Anthropol 86:229–241 [Google Scholar]
- 2.Arosio M, Sartore G, Rossi CM, Casati G, Faglia G, Manzato EItalian multicenter octreotide study group 2000. LDL physical properties, lipoprotein and Lp(a) levels in acromegalic patients. Effects of octeotide therapy. Atherosclerosis 151:551–557 [DOI] [PubMed] [Google Scholar]
- 3.Austad SN, Fischer KE. 1992. Primate longevity: its place in the mammalian scheme. Am J Primatol 28:251–261 [DOI] [PubMed] [Google Scholar]
- 4.Barja G. 2002. Rate of generation of oxidative stress-related damage and animal longevity. Free Radic Biol Med 33:1167–1172 [DOI] [PubMed] [Google Scholar]
- 5.Becker BF. 1993. Towards the physiological-function of uric acid. Free Radic Biol Med 14:615–631 [DOI] [PubMed] [Google Scholar]
- 6.Beckman KB, Ames BN. 1998. The free radical theory of aging matures. Physiol Rev 78:547–581 [DOI] [PubMed] [Google Scholar]
- 7.Beentjes JA, van Tol A, Sluiter WJ, Dullaart RP. 2000. Low plasma lecithin: cholesterol acyltransferase and lipid transfer protein activities in growth hormone deficient and acromegalic men: role in altered high density lipoproteins. Atherosclerosis 153:491–498 [DOI] [PubMed] [Google Scholar]
- 8.Boero L, Manavelat M, Rosso LG, Insua C, Berardi V, Fornari MC, Brites F. 2009. Alterations in biomarkers of cardiovascular disease (CVD) in active acromegaly. Clin Endocrinol (Oxf) 70:88–95 [DOI] [PubMed] [Google Scholar]
- 9.Bowden DM, Short R, Williams D. 1990. Constructing an instrument to measure the rate of aging in female pigtailed macaques (Macaca nemestrina). J Gerontol 45:B59–B66 [DOI] [PubMed] [Google Scholar]
- 10.Chu NF, Wang DJ, Liou SH, Shief SM. 2000. Relationship between hyperuricemia and other cardiovascular disease risk factors among adult males in Taiwan. Eur J Epidemiol 16:13–17 [DOI] [PubMed] [Google Scholar]
- 11.Clarke R, Armitage J. 2002. Antioxidant vitamins and risk of cardiovascular disease: review of large-scale randomized trials. Cardiovasc Drugs Ther 16:411–415 [DOI] [PubMed] [Google Scholar]
- 12.Clyde VL, Roth L, Bell B, Wallace R, Slosky D, Dolan J. 2002. Cardiac and gestational ultrasound parameters in nonanesthetized bonobos (Pan paniscus). Proc Am Assoc Zoo Vet Annu Meet. 2002:365–368 [Google Scholar]
- 13.Colao A, Marzullo P, Di Somma C, Lombardi G. 2001. Growth hormone and the heart. Clin Endocrinol (Oxf) 54:137–154 [DOI] [PubMed] [Google Scholar]
- 14.Colao A, Spiezia S, Cerbone G, Pivonello R, Marzullo P, Ferone D, Di Somma C, Assanti AP, Lombardi G. 2001. Increased arterial intima-media thickness by B-M mode echodopplar ultrasonography in acromegaly. Clin Endocrinol (Oxf) 54:515–524 [DOI] [PubMed] [Google Scholar]
- 15.Crissey SD, Barr JE, Slifka KA, Bowen PE, Stacewicz-Sapuntzakis M, Langman C, Ward A, Ange K. 1999. Serum concentrations of lipids, vitamins A and E, vitamin D metabolites, and carotenoids in nine primate species at four zoos. Zoo Biol 18:551–564 [Google Scholar]
- 16.Cutler RG. 1976. Evolution of longevity in primates. J Hum Evol 5:169–202 [Google Scholar]
- 17.Cutler RG. 1984. Carotenoids and retinol: their possible importance in determining longevity of primate species. Proc Natl Acad Sci USA 81:7627–7631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cutler RG. 1984. Urate and ascorbate: their possible roles as antioxidants in determining longevity of mammalian species. Arch Gerontol Geriatr 3:321–348 [DOI] [PubMed] [Google Scholar]
- 19.Cutler RG. 1985. Peroxide-producing potential of tissues: inverse correlation with longevity in mammalian species. Proc Natl Acad Sci USA 82:4798–4802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cutler RG. 1986. Aging and oxygen radicals, p 251-285. : Taylor AE, Matalon S, Ward P. Physiology of oxygen radicals. Bethesda (MD): American Physiological Society [Google Scholar]
- 21.Cutler RG. 1991. Antioxidants and aging. Am J Clin Nutr 53:373S–379S [DOI] [PubMed] [Google Scholar]
- 22.Dalle-Donne I, Rossi R, Colombo R, Giustarini D, Milzani A. 2006. Biomarkers of oxidative damage in human disease. Clin Chem 52:601–623 [DOI] [PubMed] [Google Scholar]
- 23.Djoussé L, Levy D, Cupples LA, Evans JC, D'Agostino RB, Ellison RC. 2001. Total serum bilirubin and risk of cardiovascular disease in the Framingham offspring study. Am J Cardiol 87:1196–1200 [DOI] [PubMed] [Google Scholar]
- 24.Duan X, Ling F. 2008. Is uric acid itself a player or a bystander in the pathophysiology of chronic heart failure? Med Hypotheses 70:578–581 [DOI] [PubMed] [Google Scholar]
- 25.Dudman NPB. 1999. An alternative view of homocysteine. Lancet 354:2072–2074 [DOI] [PubMed] [Google Scholar]
- 26.Ensign W, Hill N, Heward CB. 2006. Disparate LDL phenotypic classification among 4 different methods assessing LDL particle characteristics. Clin Chem 52:1722–1727 [DOI] [PubMed] [Google Scholar]
- 27.Fang ZY, Prins JB, Marwick TH. 2004. Diabetic cardiomyopathy: evidence, mechanisms, and therapeutic implications. Endocr Rev 25:543–567 [DOI] [PubMed] [Google Scholar]
- 28.Finkel T, Holbrook NJ. 2000. Oxidants, oxidative stress and the biology of ageing. Nature 408:239–247 [DOI] [PubMed] [Google Scholar]
- 29.Ford ES. 2000. Serum copper concentrations and coronary heart disease among US adults. Am J Epidemiol 151:1182–1188 [DOI] [PubMed] [Google Scholar]
- 30.Freedman DS, Williamson DF, Gunter EW, Byers T. 1995. Relation to serum uric-acid to mortality and ischemic-heart disease – the Nhanes I epidemiologic follow-up study. Am J Epidemiol 141:637–644 [DOI] [PubMed] [Google Scholar]
- 31.Garcia AL, Raila J, Koebnick C, Eulenberger K, Schweigert FJ. 2006. Great apes show highly selective plasma carotenoids and have physiologically high plasma retinyl esters compared to humans. Am J Phys Anthropol 131:236–242 [DOI] [PubMed] [Google Scholar]
- 32.Gavan JA. 1953. Growth and development of the chimpanzee: a longitudinal and comparative study. Hum Biol 25:93–143 [PubMed] [Google Scholar]
- 33.Gillum RF, Makuc DM. 1992. Serum albumin, coronary heart disease, and death. Am Heart J 123:507–513 [DOI] [PubMed] [Google Scholar]
- 34.Giurgea N, Constantinescu MI, Stanciu R, Suclu S, Muresan A. 2005. Ceruloplasmin: acute-phase reactant or endogenous antioxidant? The case of cardiovascular disease. Med Sci Monit 11:RA48–RA51 [PubMed] [Google Scholar]
- 35.Glader CA, Birgander LS, Stetlund H, Dalén GH. 2002. Is lipoprotein(a) a predictor for survival in patients with established coronary artery disease? Results from a prospective patient cohort study in northern Sweden. J Intern Med 252:27–35 [DOI] [PubMed] [Google Scholar]
- 36.Glantzounis GK, Tsimoyiannis EC, Kappas AM, Galaris DA. 2005. Uric acid and oxidative stress. Curr Pharm Des 11:4145–4151 [DOI] [PubMed] [Google Scholar]
- 37.Hak AE, Ma J, Powell CB, Campos H, Gaziano JM, Willett WC, Stampfer MR. 2004. Prospective study of plasma carotenoids and tocopherols in relation to risk of ischemic stroke. Stroke 35:1584–1588 [DOI] [PubMed] [Google Scholar]
- 38.Halliwell B. 1988. Albumin – an important extracellular antioxidant. Biochem Pharmacol 37:569–571 [DOI] [PubMed] [Google Scholar]
- 39.Hamada Y, Chatani K, Udono T, Kikuchi Y, Gunjii H. 2003. A longitudinal study on hand and wrist skeletal maturation in chimpanzees (Pan troglodytes), with emphasis on growth in linear dimensions. Primates 44:259–271 [DOI] [PubMed] [Google Scholar]
- 40.Hansen JF, Alford PL, Keeling ME. 1984. Diffuse myocardial fibrosis and congestive heart failure in an adult male chimpanzee. Vet Pathol 21:529–531 [DOI] [PubMed] [Google Scholar]
- 41.Harman D. 1956. Aging: a theory based on free radical and radiation chemistry. J Gerontol 2:298–300 [DOI] [PubMed] [Google Scholar]
- 42.Harman SM, Liang L, Tsitouras PD, Gucciardo G, Heward CB, Reaven PD, Ping W, Ahmed A, Cutler RG. 2003. Urinary excretion of three nucleic acid oxidation adducts and isoprotane F2α measured by liquid chromatography-mass spectrometry in smokers, ex-smokers, and nonsmokers. Free Radic Biol Med 35:1301–1309 [DOI] [PubMed] [Google Scholar]
- 43.Harrela M, Qiao Q, Koistinen R, Tuomilehto J, Nissinen A, Seppălā M, Leinonen P. 2002. High serum insulin-like growth factor binding protein-1 is associated with increased cardiovascular mortality in elderly men. Horm Metab Res 34:144–149 [DOI] [PubMed] [Google Scholar]
- 44.Heijmans BT, Beekman M, Houwing-Duistermaat JJ, Cobain MR, Powell J, Blauw GJ, van der Ouderaa F, Westendorp RGJ, Slagboom PE. 2006. Lipoprotein particle profiles mark familial and sporadic human longevity. PLoS Med 3:e495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hennekens CH, Buring JE, Manson JE, Stampfer M, Rosner B, Cook NR, Belanger C, LaMotte F, Gaziano JM, Ridker PM, Willett W, Peto R. 1996. Lack of effect of long-term supplementation with beta carotene on the incidence of malignant neoplasms and cardiovascular disease. N Engl J Med 334:1145–1149 [DOI] [PubMed] [Google Scholar]
- 46.Herndon JG, Tigges J. 2001. Hematolgic and blood biochemical variables of captive chimpanzees: cross-sectional and longitudinal analyses. Comp Med 51:60–69 [PubMed] [Google Scholar]
- 47.Hill K, Boesch C, Goodall J, Pusey A, Williams J, Wrangham R. 2001. Mortality rates among wild chimpanzees. J Hum Evol 40:437–450 [DOI] [PubMed] [Google Scholar]
- 48.Hink HU, Santanam N, Dikalov S, McCann L, Nguyen AD, Parthasarathy S, Harrison DG, Fukai T. 2002. Peroxidase properties of extracellular superoxide dismutase: role of uric acid in modulating in vivo activity. Arterioscler Thromb Vasc Biol 22:1402–1408 [DOI] [PubMed] [Google Scholar]
- 49.Hu JJ, Chi CX, Frenkel K, Smith BM, Henfelt JJ, Berwick M, Mahabir S, D'Agostino RB. 1999. Alpha-tocopherol dietary supplement decreases titers of antibody against 5-hydroxymethyl-2′-deoxyurdine (HMdU). Cancer Epidemiol Biomarkers Prev 8:693–698 [PubMed] [Google Scholar]
- 50.Hubbard GB, Lee DR, Eichberg JW. 1991. Diseases and pathology of chimpanzees at the Southwest Foundation for Biomedical Research. Am J Primatol 24:273–282 [Google Scholar]
- 51.Institute of Laboratory Animal Resources 1996. Guide for the care and use of laboratory animals. Washington (DC): National Academy Press [Google Scholar]
- 52.Ito Y, Kurata M, Suzuki K, Hamajima N, Hishida H, Aoki K. 2006. Cardiovascular disease mortality and serum carotenoid levels: a Japanese population-based follow-up study. J Epidemiol 16:154–160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kaliora AC, Dedoussis GVZ, Schmidt H. 2006. Dietary antioxidants in preventing atherogensis. Atherosclerosis 187:1–17 [DOI] [PubMed] [Google Scholar]
- 54.Kaul S, Zadeh AA, Shah PK. 2006. Homocysteine hypothesis for atherothrombotic cardiovascular disease – not validated. J Am Coll Cardiol 48:914–923 [DOI] [PubMed] [Google Scholar]
- 55.Kono Y, Nakamura K, Kimura H, Nishii N, Watanabe A, Banba K, Miura A, Nagase S, Sakuragi S, Kusano KF, Matsubara H, Ohe T. 2006. Elevated levels of oxidative DNA damage in serum and myocardium of patients with heart failure. Circ J 70:1001–1005 [DOI] [PubMed] [Google Scholar]
- 56.Krauss RM. 1987. Relationship of intermediate and low-density lipoprotein subspecies to risk of coronary artery disease. Am Heart J 113:578–582 [DOI] [PubMed] [Google Scholar]
- 57.Kritchevsky SB, Bush AJ, Pahor M, Gross MD. 2000. Serum carotenoids and markers of inflammation in nonsmokers. Am J Epidemiol 152:1065–1071 [DOI] [PubMed] [Google Scholar]
- 58.Ku H-H, Brunk UT, Sohal RS. 1993. Relationship between mitochondrial superoxide and hydrogen peroxide production and longevity of mammalian species. Free Radic Biol Med 15:621–627 [DOI] [PubMed] [Google Scholar]
- 59.Kuller LH, Eichner JE, Orchard TJ, Grandits GA, McCallum L, Tracy RP. 1991. The relation between serum albumin levels and risk of coronary heart disease in the multiple risk factor intervention trial. Am J Epidemiol 134:1266–1277 [DOI] [PubMed] [Google Scholar]
- 60.Kuykendall KL, Conroy GC. 1996. Permanent tooth calcification in chimpanzees (Pan troglodytes): patterns and polymorphisms. Am J Phys Anthropol 99:159–174 [DOI] [PubMed] [Google Scholar]
- 61.Lammey ML, Baskin GB, Gigliotti AP, Lee DR, Ely JJ, Sleeper MM. 2008. Interstitial myocardial fibrosis in a captive chimpanzee (Pan troglodytes) population. Comp Med 58:389–394 [PMC free article] [PubMed] [Google Scholar]
- 62.Lammey ML, Lee DR, Ely JJ, Sleeper MM. 2008. Sudden cardiac death in 13 captive chimpanzees (Pan troglodytes). J Med Primatol 37 Suppl 1:39–43 [DOI] [PubMed] [Google Scholar]
- 63.Lane MA. 2000. Nonhuman primate models in biogerontology. Exp Gerontol 35:533–541 [DOI] [PubMed] [Google Scholar]
- 64.Lee DR, Guhad PA. 2001. Chimpanzee health care and medicine program, p 63-117. : Brent L. Special topics in primatology: the care and management of captive chimpanzees. San Antonio (TX): American Society of Primatologists [Google Scholar]
- 65.Leigh SR, Shea BT. 1996. Ontogeny of body size variation in African apes. Am J Phys Anthropol 99:43–66 [DOI] [PubMed] [Google Scholar]
- 66.Leyva F, Anker S, Swan JW, Godsland IF, Wingrove CS, Chua TP, Stevenson JC, Coats AJS. 1997. Serum uric acid as an index of impaired oxidative metabolism in chronic heart failure. Eur Heart J 18:858–865 [DOI] [PubMed] [Google Scholar]
- 67.Liang Y, Wei P, Duke RW, Reaven PD, Harman SM, Cutler RG, Heward CB. 2003. Quantification of 8-Iso-Prostaglandiin-F(2α) and 2,3-Dinor-8-Iso-Prostaglandin-F(2α) in human urine using liquid chromatography-tandem mass spectrometry. Free Radic Biol Med 34:409–418 [DOI] [PubMed] [Google Scholar]
- 68.Lonn E, Yusuf S, Arnold MJ, Sheridan P, Pogue J, Micks M, McQueen MJ, Probstfield J, Fodor G, Held C, Genest JHeart Outcomes Prevention Evaluation (HOPE) 2 Investigators 2006. Homocysteine lowering with folic acid and B vitamins in vascular disease. N Engl J Med 354:1567–1577 [DOI] [PubMed] [Google Scholar]
- 69.Lonn E, Yusuf S, Hoogwerf B, Pogue J, Yi Q, Zinman B, Bosch J, Dagenais G, Mann JF, Gerstein HC. 2002. Effects of vitamin E on cardiovascular and microvascular outcomes in high-risk patients with diabetes: results of the HOPE study and MICRO-HOPE substudy. Diabetes Care 25:1919–1927 [DOI] [PubMed] [Google Scholar]
- 70.Lopez AD, Mathers CD, Ezzati M, Jamison DT, Murray CJL. 2006. Global and regional burden of disease and risk factors, 2001: systematic analysis of population health data. Lancet 367:1747–1757 [DOI] [PubMed] [Google Scholar]
- 71.Lopez-Torres M, Perez-Campo R, Rojas C, Cadenas S, Barja G. 1993. Maximum life span in vertebrates: relationship with liver antioxidant enzymes, glutathione system, ascorbate, urate, sensitivity to peroxidation, true malondialdehyde, in vivo H2O2, and basal and maximum aerobic capacity. Mech Ageing Dev 70:177–199 [DOI] [PubMed] [Google Scholar]
- 72.Maffei P, Sicolo N, Plebani M. 1999. Lipoprotein(a) in acromegaly. Ann Intern Med 130:537–538 [DOI] [PubMed] [Google Scholar]
- 73.Mak S, Newton GE. 2004. Redox modulation of the inotropic response to dobutamine is impaired in patients with heart failure. Am J Physiol Heart Circ Physiol 286:H789–H795 [DOI] [PubMed] [Google Scholar]
- 74.Marson J, Meuris S, Cooper RW, Jouannet P. 1991. Puberty in the male chimpanzee: progressive maturation of semen characteristics. Biol Reprod 44:448–455 [DOI] [PubMed] [Google Scholar]
- 75.Mayer M. 2000. Association of serum bilirubin concentration with risk of coronary artery disease. Clin Chem 46:1723–1727 [PubMed] [Google Scholar]
- 76.Mead A, Atkinson G, Albin D, Alphey D, Baic S, Boyd O, Cadigan L, Clutton L, Craig L, Flanagan C, Greene P, Griffiths E, Lee NJ, Li M, McKechnie L, Ottaway J, Paterson K, Perrin L, Rigby P, Stone D, Vine R, Whitehead J, Wray L, Hooper L. 2006. Dietetic guidelines on food and nutrition in the secondary prevention of cardiovascular disease – evidence from systematic reviews of randomized controlled trials. J Hum Nutr Diet 19:401–419 [DOI] [PubMed] [Google Scholar]
- 77.Mecocci P, Polidori MC, Troiano L, Cherubini A, Cecchetti R, Pini G, Straatman M, Monti D, Stahl W, Sies H, Franceschi C, Senin U. 2000. Plasma antioxidants and longevity: a study on health centenarians. Free Radic Biol Med 28:1243–1248 [DOI] [PubMed] [Google Scholar]
- 78.Mehrabi MR, Serbecic N, Ekmekcioglu C, Tamaddon F, Ullrich R, Sinzinger H, Glogar HD. 2001. The isoprostane 8epiPGF (2-alpha) is a valuable indicator of oxidative injury to human heart valves. Cardiovasc Pathol 10:241–245 [DOI] [PubMed] [Google Scholar]
- 79.Moriarity JT, Folsom AR, Iribarren C, Nieto FJ, Rosamond WD. 2000. Serum uric acid and risk of coronary heart disease: atherosclerosis risk in communities (ARIC) study. Ann Epidemiol 10:136–143 [DOI] [PubMed] [Google Scholar]
- 80.Munson L, Montali RJ. 1990. Pathology and diseases of great apes at the National Zoological Park Zoo. Zoo Biol 9:99–105 [Google Scholar]
- 81.Nakamura E, Lane MA, Roth GS, Ingram DK. 1998. A strategy for identifying biomarkers of aging: further evaluation of hematology and blood chemistry data from a calorie restriction study in rhesus monkeys. Exp Gerontol 33:421–443 [DOI] [PubMed] [Google Scholar]
- 82.Nakamura K, Kushano KF, Matsubara H, Nakamura Y, Miura A, Nishii N, Banba D, Nagase S, Miyaji K, Morita H, Saito H, Emori T, Ohe T. 2005. Relationship between oxidative stress and systolic dysfunction in patients with hypertrophic cardiomyopathy. J Card Fail 11:117–123 [DOI] [PubMed] [Google Scholar]
- 83.Nieto FJ, Iribarren C, Gross MD, Comstock GW, Cutler RG. 2000. Uric acid and serum antioxidant capacity: a reaction to atherosclerosis? Athersclerosis 148:131–139 [DOI] [PubMed] [Google Scholar]
- 84.Nonaka-Sarukawa M, Yamamoto K, Aoki H, Takano H, Katsuki T, Ikeda U, Shimada K. 2003. Increased urinary 15F2t-isoprostane concentrations in patients with non-ischaemic congestive heart failure: a marker of oxidative stress. Heart 89:871–874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Pamplona R, Portero-Otin M, Riba D, Ruiz C, Prat J, Bellmunt MJ, Barja G. 1998. Mitochondiral membrane peroxidizability index is inversely correlated to maximum life span in mammals. J Lipid Res 39:1989–1994 [PubMed] [Google Scholar]
- 86.Perez-Campo R, López-Torres M, Cadenas S, Rojas C, Barja G. 1998. The rate of free-radical production as a determinant of aging: evidence from the comparative approach. J Comp Physiol [B] 168:149–158 [DOI] [PubMed] [Google Scholar]
- 87.Roberts LJ, Morrow JD. 2000. Measurement of F-2-isoprostanes as an index of oxidative stress in vivo. Free Radic Biol Med 28:505–513 [DOI] [PubMed] [Google Scholar]
- 88.Roth GS, Mattison JA, Ottinger MA, Chachich ME, Lane MA, Ingram DK. 2004. Aging in rhesus monkeys: relevance to human health interventions. Science 305:1423–1426 [DOI] [PubMed] [Google Scholar]
- 89.Salomaa VV, Salminen I, Rasi V, Vahtera E, Aro A, Myllyla G. 1997. Association of fatty acid composition of serum phospholipids with hemostatic factors. Arterioscler Thromb Vasc Biol 17:809–813 [DOI] [PubMed] [Google Scholar]
- 90.Sampietro T, Neglia D, Bionda A, Dal Pino B, Bigazzi F, Puntoni M, Startari U, Morales A, Minichilli F, Bianchi F, L'Abbate A. 2005. Inflammatory markers and serum lipids in idiopathic dilated cardiomyopathy. Am J Cardiol 96:1718–1720 [DOI] [PubMed] [Google Scholar]
- 91.Sanders TAB, Lewis F, Slaughter S, Griffin BA, Griffin M, Davies I, Millward DJ, Cooper JA, Miller GJ. 2006. Effect of varying the ratio of n-6 to n-3 fatty acids by increasing the dietary intake of alpha-linoleic, eicosapentaenoic, and docosahexaenoic acid, or both on fibrinogen and clotting factors VII and XII in persons aged 45-70 y: the OPTILIP study. Am J Clin Nutr 84:513–522 [DOI] [PubMed] [Google Scholar]
- 92.Schulman FY, Farb A, Virmani R, Montali RJ. 1995. Fibrosing cardiomyopathy in lowland gorillas (Gorilla gorilla gorilla) in the United States: a retrospective study. J Zoo Wildl Med 26:43–51 [Google Scholar]
- 93.Short R, Williams DD, Bowden DM. 1997. Circulating antioxidants as determinants of the rate of biological aging in pigtailed macaques (Macaca nemestrina). J Gerontol A Biol Sci Med Sci 52:B26–B38 [DOI] [PubMed] [Google Scholar]
- 94.Shukla N, Maher J, Masters J, D'Angelini G, Jeremy JY. 2006. Does oxidative stress change ceruloplasmin from a protective to a vasculopathic factor? Atherosclerosis 187:238–250 [DOI] [PubMed] [Google Scholar]
- 95.Sibley CG, Comstock JA, Alhquist JE. 1990. DNA hybridization evidence on hominoid phylogeny: a reanalysis of the data. J Mol Evol 30:202–236 [DOI] [PubMed] [Google Scholar]
- 96.Smith BH, Crummett TL, Brandt KL. 1994. Ages of eruption of primate teeth: a compendium of aging individuals and comparing life histories. Yearb Phys Anthropol 37:177–231 [Google Scholar]
- 97.Sorg O. 2004. Oxidative stress: a theoretical model or a biological reality? C R Biol 327:649–662 [DOI] [PubMed] [Google Scholar]
- 98.Stadler N, Lindner RA, Davies MJ. 2004. Direct detection and quantification of transition metal ions in human atherosclerotic plaques: evidence for the presence of elevated levels of iron and copper. Arterioscler Thromb Vasc Biol 24:949–954 [DOI] [PubMed] [Google Scholar]
- 99.Stocker R, Yamamoto Y, McDonagh AF, Glazer AN, Ames BN. 1987. Bilirubin is an antioxidant of possible physiological importance. Science 235:1043–1046 [DOI] [PubMed] [Google Scholar]
- 100.Takahata N, Satta Y. 1997. Evolution of the primate lineage leading to modern humans: phylogenetic and demographic inferences from DNA sequences. Proc Natl Acad Sci USA 94:4811–4815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Tan KC, Shiu SW, Janus ED, Lam KS. 1997. LDL subfractions in acromegaly: relation to growth hormone and insulin-like growth factor-1. Atherosclerosis 129:59–65 [DOI] [PubMed] [Google Scholar]
- 102.Tang YR, Zhang SQ, Xiong Y, Zhao Y, Fu H, Zhang HP, Xiong KM. 2003. Studies of five microelement contents of human serum, hair, and fingernails correlated with aged hypertension and coronary heart disease. Biol Trace Elem Res 92:97–103 [DOI] [PubMed] [Google Scholar]
- 103.Tavani A, Gallus S, Negri E, Parpinel M, La Vecchia C. 2006. Dietary intake of carotenoids and retinol and the risk of acute myocardial infarction in Italy. Free Radic Res 40:659–664 [DOI] [PubMed] [Google Scholar]
- 104.Thillet J, Doucet C, Chapman J, Herbeth B, Cohen D, Faure-Delanef L. 1998. Elevated lipoprotein(a) levels and small apo(a) isoforms are compatible with longevity: evidence from a large population of French centenarians. Atherosclerosis 136:389–394 [DOI] [PubMed] [Google Scholar]
- 105.Tolmasoff JM, Ono T, Cutler RG. 1980. Superoxide dismutase: correlation with life-span and specific metabolic rate in primate species. Proc Natl Acad Sci USA 77:2777–2781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Topuzoglu G, Erbay AR, Karul AB, Yensel N. 2003. Concentrations of copper, zinc, and magnesium in sera from patients with idiopathic dilated cardiomyopathy. Biol Trace Elem Res 95:11–17 [DOI] [PubMed] [Google Scholar]
- 107.Tulenko TN, Sumner AE. 2002. The physiology of lipoproteins. J Nucl Cardiol 9:638–649 [DOI] [PubMed] [Google Scholar]
- 108.Videan EN, Fritz J, Murphy J. 2008. Effects of aging on hematology and serum chemistry in chimpanzees (Pan troglodytes). Am J Primatol 70:327–338 [DOI] [PubMed] [Google Scholar]
- 109.Voss P, Siems W. 2006. Clinical oxidation parameters of aging. Free Radic Res 40:1339–1349 [DOI] [PubMed] [Google Scholar]
- 110.Wannamethee SG. 2005. Serum uric acid and risk of coronary heart disease. Curr Pharm Des 11:4125–4132 [DOI] [PubMed] [Google Scholar]
- 111.Waring WS, Webb DJ, Maxwell SR. 2000. Uric acid as a risk factor for cardiovascular disease. Q J Med 93:707–713 [DOI] [PubMed] [Google Scholar]
- 112.Wells SK, Sargent EL, Andrews ME, Anderson D. 1990. Medical Management of the Orangutan. New Orleans: The Audubon Institute [Google Scholar]
- 113.Wendland E, Farmer A, Glasziou P, Neil A. 2006. Effect of alpha linolenic acid on cardiovascular risk markers: a systematic review. Heart 92:166–169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Wheeler JG, Juzwishin KDM, Eiriksdottir G, Gudnason V, Danesh J. 2005. Serum uric acid and coronary heart disease in 9,458 incident cases and 155,084 controls: prospective study and meta-analysis. PLoS Med 2:e76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Williams PT, Superko HR, Haskell WL, Alderman EL, Blanche PJ, Holl LG, Krauss RM. 2003. Smallest LDL particles are most strongly correlated with coronary disease progression in men. Arterioscler Thromb Vasc Biol 23:314–321 [DOI] [PubMed] [Google Scholar]
- 116.Wolfram R, Oguogho A, Palumbo B, Sinzinger H. 2005. Enhanced oxidative stress in coronary heart disease and chronic heart failure as indicated by an increased 8epiPGF2α. Eur J Heart Fail 7:167–172 [DOI] [PubMed] [Google Scholar]
- 117.Wright AJA, Hughes DA, Bailey AL, Southon S. 1999. Beta-carotene and lycopene, but not lutein, supplementation changes the plasma fatty acid profile of healthy male non-smokers. J Lab Clin Med 134:592–598 [DOI] [PubMed] [Google Scholar]
- 118.Wu LL. 1999. Review of risk factors for cardiovascular diseases. Ann Clin Lab Sci 29:127–133 [PubMed] [Google Scholar]
- 119.Young LG, Gould KG, Smithwick EB. 1993. Selected endocrine parameters of the adult male chimpanzee. Am J Primatol 31:287–297 [DOI] [PubMed] [Google Scholar]
- 120.Yusuf S, Dagenais G, Pogue J, Bosch J, Sleight P. 2000. Vitamin E supplementation and cardiovascular events in high-risk patients. N Engl J Med 342:154–160 [DOI] [PubMed] [Google Scholar]