Abstract
A major unsolved question in cortical development is how proliferation, neurogenesis, regional growth, regional identity, and laminar fate specification are coordinated. Here we provide evidence, using loss-of-function and gain-of-function manipulations, that the COUP-TFI orphan nuclear receptor promotes ventral cortical fate, promotes cell cycle exit and neural differentiation, regulates the balance of early- and late-born neurons, and regulates the balanced production of different types of layer V cortical projection neurons. We suggest that COUP-TFI controls these processes by repressing Mapk/Erk, Akt, and β-catenin signaling.
Keywords: β-catenin, COUP-TFI, Mapk/Erk, neurogenesis, PI3K/Akt, proliferation
Introduction
The neocortex processes different types of sensory and higher order information in distinct areas located at particular anteroposterior and dorsoventral (D/V) tangential coordinates (Grove and Fukuchi-Shimogori 2003; Sur and Rubenstein 2005). The radial organization of the 6 neural layers within each area is similar, although each area has important differences in features of its molecular, cellular, and connectivity signature (Rakic 1988; Monuki and Walsh 2001; Molyneaux et al. 2007).
Ongoing studies are aimed at elucidating the developmental mechanisms that generate neocortical areas (O'Leary and Nakagawa 2002; Grove and Fukuchi-Shimogori 2003; Sur and Rubenstein 2005). Two cardinal features of cortical development have long been recognized: 1) different regions of the cortical map develop at different times, including that ventral regions develop before dorsal regions (Bayer 1991); 2) projection neurons of distinct cortical layers are generated at different times (Caviness 1982; Frantz and McConnell 1996). Thus, there is a temporal coupling of areal and laminar development. The molecular mechanisms that coordinate these processes are not known.
Recent progress has been made in demonstrating that cortical patterning is regulated by patterning centers that emanate from the telencephalic midline. In particular, Fgf signaling from the rostral patterning center, via Fgf8 and Fgf17, promotes rostral neocortical fates (Fukuchi-Shimogori and Grove 2001; Garel et al. 2003; Storm et al. 2006; Cholfin and Rubenstein 2007). Fgf signaling regulates the expression of several key transcription factors including COUP-TFI, Emx2, and Foxg1 (BFI). Fgf8 promotes the expression of Foxg1 (Shimamura and Rubenstein 1997; Ye et al. 1998). Foxg1 represses Bmp and Wnt expression and promotes the proliferative state (Monuki et al. 2001; Hanashima et al. 2002; Martynoga et al. 2005; Storm et al. 2006). Fgf8 represses COUP-TFI and Emx2 expression (Garel et al. 2003; Storm et al. 2006) and thereby contributes to their prominent caudorostral expression gradients. Emx2 controls cortical arealization through promoting caudodorsal fates (Bishop et al. 2000; Muzio and Mallamaci 2003, 2005; Hamasaki et al. 2004; Shinozaki et al. 2004). Likewise, COUP-TFI has a prominent role in cortical patterning.
COUP-TFI, an orphan nuclear receptor, is expressed in 2-dimensional gradients: caudorostral and ventrodorsal (Fig. 1B; Qiu et al. 1994; Liu et al. 2000). Mice with a null mutation of COUP-TFI have prominent defects in regional gene expression, axonal projections between the thalamus and the cortex (Qiu et al. 1997; Zhou et al. 1999), and intracortical commissural projections (Armentano et al. 2006). More recently, analysis of conditional COUP-TFI–/– mice has clearly demonstrated that frontal areas are expanded and primary sensory areas are reduced and caudally shifted (Armentano et al. 2007). How COUP-TFI regulates areal patterning, or its role in coordinating patterning with neurogenesis and laminar fate, has not been established.
Figure 1.
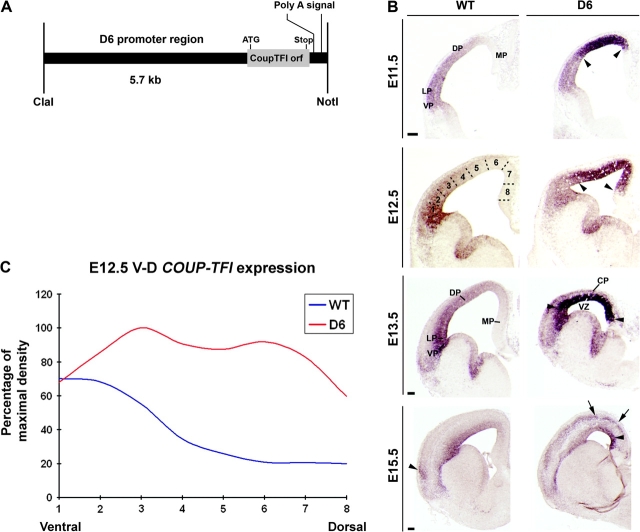
Characterization of the D6/COUP-TFI transgenic mouse line. (A) Schematic representation of the construct utilized to generate the transgenic line. (B) RNA in situ hybridization on coronal sections through the telencephalon of WT and the COUP-TFI overexpression line D6/COUP-TFI (D6). D6/COUP-TFI transgene increases COUP-TFI expression in dorsal parts of the cortical VZ (arrowheads) and CP (arrowheads in E15.5 panel). (C) Quantification of the E12.5 in situ hybridization signal intensity shown in (B): normal ventralhigh–dorsallow COUP-TFI gradient (blue line) was modified in the D6/COUP-TFI cortex (red line) due to increased VZ expression in the dorsal pallium (DP). VP, ventral pallium; LP, lateral pallium; MP, medial pallium. Scale bars: 200 μm.
COUP-TFI is expressed in a ventrodorsal gradient in cortical progenitor that correlates with the neurogenetic gradient (neurogenesis in ventral regions precedes dorsal regions). We reasoned that this expression pattern may reflect its role in coordinating several processes within the neuroepithelium: areal identity, mitosis, neurogenesis, and the fate of the neurons. Herein, we describe experiments that altered COUP-TFI dosage, using gain-of-function and loss-of-function approaches. We present evidence that COUP-TFI 1) influences D/V patterning of the cortex, 2) promotes cell cycle exit and neural differentiation, 3) regulates the balance of early- and late-born neurons, and 4) changes the balance of different types of layer V cortical projection neurons. Finally, we provide evidence that COUP-TFI coordinates these processes through modulating receptor tyrosine kinase (RTK) signaling pathways and β-catenin–mediated Wnt signaling.
Materials and Methods
Mice
To generate D6/COUP-TFI transgenic mice, a 5.7-Kb promoter region was cloned upstream of COUP-TFI open-reading frame. The D6/COUP-TFI expression cassette was purified and injected into the pronucleus of fertilized (C57BL/6 × BALB/c) F1 mouse oocytes (Hogan et al. 1994). Mice were genotyped by polymerase chain reaction using genomic DNA from the tail of postnatal and late embryonic stages or yolk sac from earlier embryos, a D6-specific sense primer (5′-GACTCGCATTTCGACTTGCGGGACA-3′), and a COUP-TFI–specific antisense primer (5′-TCTCGCCAGCTGCTAACTACCATT-3′), resulting in a transgene-specific band of 360 bp.
The mouse mutant strains with null allele of COUP-TFI (Qiu et al. 1997) and small-eye mutation of Pax6 (Hill et al. 1991) were used. The transgenic line BAT-gal (Maretto et al. 2003) was bred to the COUP-TFI–/– line to generate COUP-TFI–/–;BAT-gal animals. The D6/COUP-TFI line was mated to BAT-gal mice and to RARE-LacZ (Rossant et al. 1991) mice to obtain D6/COUP-TFI;BAT-gal embryos and D6/COUP-TFI;RARE-Lacz embryos, respectively.
In situ RNA Hybridization
In situ RNA hybridization was performed on frozen sections (20-μm thick) mounted on Fisher Superfrost/Plus slides. In situ RNA hybridization using digoxigenin-labeled RNA probes was performed according to methods described at the Rubenstein laboratory Web site (http://www.ucsf.edu/jlrrlab/protocols.html). Sections from the different genotypes (wild type [WT], D6/COUP-TFI, and COUP-TFI–/–) have been processed simultaneously. In the D6/COUP-TFI, we used basal ganglia expression as an internal control to compare results between the different experiments and between experimental and WT samples.
The probes used and their sources were as follows: COUP-TFI (M.-J Tsai), Pax6 (P. Gruss), Emx2 (A. Simeone), Cyclin D2 (A. Mallamaci), Foxg1 (E. Lai), Hes5 (F. Guillemot), Lef1 (R. Grosschedl), Ngn2 (F. Guillemot), Delta1 (G. Weinmaster), Fgfr1 (P. Lonai), Frizzled8 (S. J. Pleasure), Scip/Oct6 (M. Rosenfeld), ER81 (T. Jessel), Fezl (M. Hibi).
For quantification of in situ density, the signal along the COUP-TFI D/V axis was quantified with National Institutes of Health (NIH) Image J by plot profile. Graph was created using Microsoft Office Excel after data were normalized by defining the largest value as 100%. The colorimetric reaction is nonlinear; hence, this quantification represents the direction of the in vivo gradient but may not reflect the actual steepness.
Immunohistochemistry
Immunohistochemistry was performed on frozen sections (10- or 20-μm thick) mounted on Fisher Superfrost/Plus slides. The slices were washed in phosphate-buffered saline solution (PBS 0.1 M, pH 7.4), incubated in blocking solution (0.2% Triton X-100, 10% normal goat serum, 2% nonfat milk, 0.2% gelatin in PBS) for 1 h, and incubated 1 day at 4 °C in the primary antibody diluted in 0.2% Triton X-100, 3% normal goat serum, 0.2% gelatin in PBS. For p44/42 Map Kinase staining, Tris-buffered saline was used instead of PBS. For Ki67 and pSmad antibodies, antigen unmasking procedure was performed by briefly boiling the section in sodium citrate 10 mM pH6. The antibodies used were as follows: anti-phospho-(Ser/Thr) Akt substrate antibody (Cell Signaling, Danvers, MA), 1:100; monoclonal anti-βIII-tubulin antibody (clone TUJ1; Covance, CA), 1:1000; monoclonal anti-bromodeoxyuridine (BrdU) antibody (clone B44; Becton Dickinson, Franklin Lakes, NJ), 1:500, for Ki67/BrdU double labeling; monoclonal anti-BrdU antibody (clone BU1/75; Serotec, Raleigh, NC), 1:100, for BrdU birthdating; anti-Caspase-3 antibody (BD Pharmigen, Franklin Lakes, NJ), 1: 500; monoclonal anti-Ki67 antigen (clone TEC-3; DakoCytomation, Glostrup, Denmark), 1:50; anti-phospho-p44/42 Map Kinase (Thr202/Tyr204) antibody (Cell Signaling), 1:100; anti-phospho-Histone H3 (Ser10) (Upstate, Billerica, MA), 1:200; anti-phospho-Smad1-Smad5-Smad8 antibody (Cell Signaling), 1:100; anti-phospho-Smad2 antibody (Cell Signaling), 1:100; anti-Pax6 (Developmental Studies Hybridoma Bank, Iowa City, IA), 1:1000.
Biotinylated secondary antibodies (goat anti-rabbit, goat anti-mouse, and goat anti-rat, all from Vector Laboratories, Burlingame, CA), diluted at 1:200, followed by standard avidin–biotin–diaminobenzidine visualization procedure (Vector Laboratories), were used. For fluorescent immunohistochemistry, goat anti-rabbit Alexa-488, goat anti-mouse Alexa-594, or goat anti-rat Alexa-594 antibodies (Molecular Probes, Eugene, OR), diluted at 1:300, were used.
Terminal deoxynucleotidyl Transferase Biotin-dUTP Nick End Labeling Assay
Terminal deoxynucleotidyl Transferase Biotin-dUTP Nick End Labeling (TUNEL) apoptotic cell detection was carried out following the manufacturer's protocol (Apotag, Chemicon, Temecula, CA) on 10-μm-thick cryostat sections from WT and D6/COUP-TFI littermate. Sections were 1st permeabilized with 0.2% Triton X-100. No pretreatment with ProteinaseK was performed. Fluorescent immunohistochemistry was performed after apoptosis assay, as described above.
BrdU Birthdating Analysis
Single injections of BrdU (40 mg/kg intraperitoneally) were done following standard procedures. To quantify the number of cells born on different birth dates in controls and D6/COUP-TFI cortex, sections through the rostral cortex (level of anterior commissure) were divided in 4 or 5 (E13.5) vertical bins counting. Vertical bins (200 μm2) were positioned in deep layers for the E11.5 to P0 injections, in cortical plate (CP) for the E13.5 to P0 injections, and in upper layers for the E16.5 to P0 injections. Cells were counted in different bins in different sections (n = 4).
Results
Ectopic Expression of COUP-TFI in the Neocortex: Generation of D6/COUP-TFI Transgenic Mice
A 5.8-Kb genomic fragment upstream of mDach1 gene directs specific expression in the developing telencephalon beginning around E10.0 (van den Bout et al. 2002). We took advantage of this genomic element to generate a transgenic mouse line that overexpresses COUP-TFI in more dorsal parts of the cortical primordium (Fig. 1). Analysis of 2 independent lines (lines A and B) showed the same phenotype of cortical hypoplasia, and COUP-TFI overexpression. Moreover, we found that the changes in expression level of the markers that we studied were reproducible in different litters over many generations (Supplementary Table 1). Therefore, we focused our analysis on line A (haploid for the transgene). We found that the phenotype was extremely stable over many generations.
We began by defining the change in COUP-TFI expression at E11.5, E12.5, E13.5, E15.5, E16.5, and E18.5 (Fig. 1B and data not shown). Ordinarily, at E11.5-E13.5, COUP-TFI is expressed in cortical ventricular zone (VZ) progenitors in a ventralhigh–dorsallow and caudalhigh–rostrallow gradient (Qiu et al. 1994; Liu et al. 2000). This bidimensional gradient was disrupted in the D6/COUP-TFI cortex due to increased expression in the VZ of the dorsal pallium. We quantified the increase in expression by measuring the signal intensity using NIH Image J (see Materials and Methods). This analysis demonstrated a ∼4-fold increase in COUP-TFI expression in the VZ at E12.5 (zone 6, Fig. 1B). At E15.5, increased COUP-TFI expression was also easily detectable in the CP (Fig. 1C, arrows). We used this COUP-TFI overexpression mutant, in combination with a COUP-TFI null mutant, to dissect the role of this transcription factor in cortical development.
COUP-TFI Dosage Regulates Molecular Patterning of Cortical Progenitors
We examined the effect of modulating COUP-TFI dosage on the expression of transcription factors along the D/V dimension of the neocortical VZ (in the region of the somatosensory cortex primordium). We 1st focused on Pax6 and Emx2, both of which are known to regulate D/V patterning of the cortical VZ; Pax6 has strong effects ventrally (Yun et al. 2001; Muzio et al. 2002; Kroll and O'Leary 2005), whereas Emx2 has its strongest effect in dorsal regions (Muzio et al. 2002; Shinozaki et al. 2004).
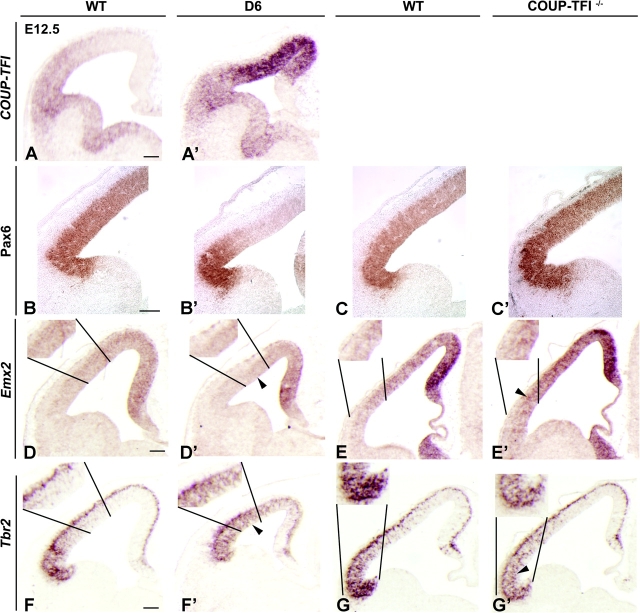
We found that overexpression of COUP-TFI began to reduce Pax6 expression by E11.5 (Fig. S1B,B′); at E12.5, Pax6 expression was nearly extinguished (Fig. 2B,B′). On the other hand, COUP-TFI loss of function led to increased Pax6 expression at E12.5, mainly in ventral regions (Fig. 2C,C′). Emx2 expression was also affected by the changes in COUP-TFI dosage. Although Emx2 expression in D6/COUP-TFI cortex was not clearly affected at E11.5 (Fig. S1C,C′), by E12.5 it was downregulated; on the other hand, it was upregulated in the COUP-TFI–/– mutant (Fig. 2D–E′). These results provide evidence that COUP-TFI is upstream of both Pax6 and Emx2 and that COUP-TFI regulates D/V patterning of the cortical progenitor zone. Both Pax6 and Emx2 are also implicated in regulating proliferation and differentiation (Warren et al. 1999; Galli et al. 2002; Estivill-Torrus et al. 2002; Bishop et al. 2003; Muzio et al. 2005; Quinn et al. 2007); thus we examined these parameters in the COUP-TFI mutants in the following sections.
Figure 2.
COUP-TFI dosage regulates molecular patterning of cortical progenitors at E12.5. (A–A′) RNA in situ hybridization for COUP-TFI on coronal sections through the telencephalon of WT (A) and D6/COUP-TFI (D6) (A′), showing the change in expression gradient due to the increase in dorsal regions. (B–C′) Immunohistochemistry and (D–G′) RNA in situ hybridizations on coronal sections through the telencephalon of D6/COUP-TFI, COUP-TFI–/–, and WT littermate embryos for Pax6 (B–C′), Emx2 (D–E′), and Tbr2 (F–G′), showing that COUP-TFI regulates expression of genes involved in patterning, proliferation, and differentiation (Pax6 and Emx2: B–E′) and in differentiating neurons (Tbr2: F–G′). For each gene marker, arrowheads show the complementary expression pattern between the genotypes. Each in situ hybridization was performed at least 3 times using different litters. Scale bars: 200 μm.
Tbr2 encodes a T-box transcription factor whose expression in the proliferative zone follows the normal ventrodorsal gradient in cortical neurogenesis (Bulfone et al. 1999) and is thought to represent an intermediate stage in neural differentiation as cells mature to become Tbr1+ (Englund et al. 2005). We compared Tbr2 expression in WT and D6/COUP-TFI brains from E11.5 to E13.5; during this interval, its expression in the mutant progenitor domains progressively increases (data not shown). At E12.5 in the D6/COUP-TFI cortex, Tbr2 was overexpressed in the dorsal proliferative zone (Fig. 2F,F′). An opposite phenotype was found in the COUP-TFI–/– mutant, where Tbr2 expression was reduced in ventral regions (Fig. 2G,G′ insets).
Increased COUP-TFI Dosage Negatively Regulates Proliferation of Cortical VZ and Subventricular Zone Progenitors
We assessed the effect of COUP-TFI dosage on proliferation using different assays. The proliferation of progenitor cells is regulated largely by the progression of cells through the restriction point in late G1, and this critical step is regulated by the D-type cyclins such as Cyclin D2 (Sherr et al. 1994). Cyclin D2 was strongly downregulated in the D6/COUP-TFI cortex, whereas it was upregulated in ventral regions of COUP-TFI–/– cortex (Fig. 3A–A″, arrowheads).
Figure 3.
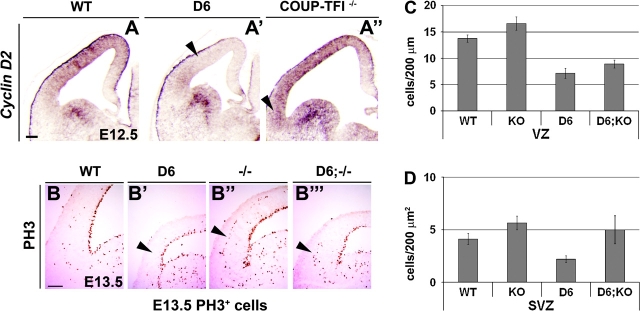
Increased COUP-TFI dosage negatively regulates proliferation of cortical VZ and SVZ progenitors. (A–A′′) RNA in situ hybridizations on coronal sections through the telencephalon of D6/COUP-TFI, COUP-TFI–/–, and WT littermate embryos for Cyclin D2, a key regulator of G1-phase progression, showing the complementary effects in the 2 genotypes (arrowheads). Staining intensity in the basal ganglia has been used to normalize Cyclin D2 expression level in the D6/COUP-TFI: Cyclin D2 was decreased of 65% in dorsal pallium. Staining intensity in the medial pallium has been used to normalize Cyclin D2 expression level in the COUP-TFI –/–: Cyclin D2 was increased of 40% in lateral/ventral pallium. (B–B′′′) PH3 immunostaining labeling cells in M-phase of the cell cycle in D6/COUP-TFI, COUP-TFI–/–, and WT at E13.5 littermate embryos. Increasing COUP-TFI dosage decreases the number of PH3+ cells in VZ (B′ and quantification in C) and in SVZ (B′, arrowhead, and quantification in D). Loss of COUP-TFI causes an increase of PH3+ cells in both VZ and SVZ (B′′ and quantification in C–D). (B′′′ and C–D) The D6/COUP-TFI proliferation defect is partially rescued at E13.5 by introducing 2 copies of the COUP-TFI null allele into the D6/COUP-TFI line. As shown in B–B′′′ and C–D, the D6/COUP-TFI-COUP-TFI KO cortex (D6;KO) shows an intermediate phenotype in the number of PH3+ cells compared with the D6 and KO cortex. Statistical analysis performed using Student's t-test. In VZ: WT-KO P < 0.001, WT-D6 P < 1.00E-07, D6-KO P < 5.00E-08, D6-D6;KO P < 0.01. In SVZ: WT-KO P < 0.05, WT-D6 P < 0.02, D6-KO P < 4.00E-05, D6-D6;KO P < 0.05. Two different litters were used.
To assess the VZ density of cells in M-phase of the cell cycle, we examined immunofluorescent labeling of phosphohistone-3 (PH3) at E11.5, E12.5, E13.5, and E15.5 (Fig. 3, 4, and S2). After quantification, D6/COUP-TFI cortex showed an ∼2-fold reduced density of PH3+ VZ cells at E13.5 (Fig. 3B,B′ and chart in C) and loss of COUP-TFI expression showed increased VZ proliferation by ∼25% in ventral cortical regions (where COUP-TFI expression is the highest) at E13.5 (Fig. 3B–B″ and chart in C). Thus, increases and decreases in COUP-TFI dosage were associated with reciprocal changes in Cyclin D2 expression and mitotic index.
Figure 4.
Increasing COUP-TFI expression promotes neurogenesis. (A–F′) D6/COUP-TFI cortex shows increasing PP/CP thickness from E11.5 through E13.5 based on expression of β-III-tubulin (A–C′, red) and Tbr1 (D–F′). At E13.5 (F–F′), the D6/COUP-TFI VZ (F′, asterisk) ectopically expresses Tbr1, a postmitotic marker. Each staining has been performed at least 3 times. (G-H) Quantification of PP and CP width during development (E11.5 to P0 for D6 and E13.5 to E18.5 for COUP-TFI–/–). In order to quantify CP thickness in the D6 line, we measured dorsal pallium (box in A′, B′, and C′). To quantify CP thickness in the COUP-TFI–/– line, we measure ventral pallium/lateral pallium (box in Fig. S2B′′). (G) The plot shows the initial (E11.5–E13.5) CP overgrowth in D6 animals, followed by the decrease after E13.5. (H) The plot shows the overall reduced ventral CP thickness in COUP-TFI–/–.
Next we examined the effect of changing COUP-TFI dosage on the secondary proliferative population, or basal progenitors, in the cortical subventricular zone (SVZ). SVZ progenitors are produced by the primary progenitors, radial glial cells (Kriegstein et al. 2006). At E13.5, SVZ progenitors are detectable by their expression of PH3. In D6/COUP-TFI animals, there was a 2-fold reduction in PH3+ in all pallial regions where COUP-TFI is overexpressed (Fig. 3B,B′ and quantification in D), whereas in the COUP-TFI–/– cortex there was an ∼30% increase in ventral regions of the pallium (Fig. 3B–B″ and quantification in D), where normally COUP-TFI expression is strongest. By E15.5 both of these phenotypes were more severe (Fig. S2); almost no SVZ cells could be detected in the D6/COUP-TFI cortex (Fig. S2 A,A′).
To test whether it was possible to rescue the hypoproliferation phenotype in the D6/COUP-TFI cortex, we lowered COUP-TFI dosage by introducing the COUP-TFI null allele into these mice. D6/COUP-TFI;COUP-TFI–/– embryos showed a partial restoration of proliferation in both the VZ and SVZ (Fig. 3: compare 3B′ with 3B′″, arrowheads). In addition, there was a partial rescue of Pax6 expression (Fig. S3A–A′″, compare 3A′ with 3A′″). These results 1) demonstrate that COUP-TFI negatively regulates cortical proliferation and 2) confirm the specificity of the transgenic line. Thus increasing COUP-TFI expression promotes cell cycle exit, thereby depleting the pool of VZ/SVZ progenitors.
Increased COUP-TFI Dosage Positively Regulates Cortical Neurogenesis
To assess whether the COUP-TFI–induced changes in proliferation were coupled with changes in neurogenesis, we studied the expression of genes that are associated with the generation of cortical neurons.
Consistent with the increase in Tbr2 expression seen in D6/COUP-TFI mice, the cortical preplate (PP) and CP were thicker than in controls at E11.5, E12.5, and E13.5 measured by expression of β-III-tubulin (TUJ1) (Fig. 4A–C′) and Tbr1 (Fig. 4D–F′). The COUP-TFI–/– mutant showed a reciprocal phenotype—the thickness of Tbr1 expression in the ventral cortex was reduced (Fig. S3B and B″); the D6/COUP-TFI;COUP-TFI–/– mutants showed an intermediate phenotype (Fig. S3B–B′″; compare S3B′ with S3B′″). Moreover, we measured the PP/CP formation in D6 (Fig. 4G, measurements performed in boxes in 3A′–C ′) and COUP-TFI–/– (Fig. 4H, measurements performed in boxes in S3), and we found an initial outgrowth in D6 animals (Fig. 4G, red line), followed by a reduced CP thickness after E13.5, showing a decrease of neuron-generating progenitors. On the other end, in COUP-TFI–/–, CP was always thinner than in the WT (Fig. 4H).
Therefore, increasing COUP-TFI dosage appears to promote the switch between proliferation and neurogenesis at early stages of cortical development. This suggests that COUP-TFI can regulate the numbers of neurons generated at different stages of corticogenesis. Thus, we tested the laminar fate of cells leaving the cell cycle at different stages using the BrdU birthdating method. We administered BrdU to pregnant mice at E11.5, E13.5, and E16.5 and then analyzed the distribution of BrdU+ cells at P0 in WT and D6/COUP-TFI siblings (Fig. 5). At E11.5, in the D6/COUP-TFI mutants, there was an ∼40% increase in the numbers of cells born (Fig. 5A,A′ and quantification in 5D, left); as in the WT, these migrated to the deep layers of the cortex. We did not observe a statistically significant change at E13.5 (Fig. 5B,B′ and quantification in 5D, middle). On the other hand, at E16.5, there was an ∼70% reduction in the number of cells generated in the D6/COUP-TFI mutants (Fig. 5C,C′ and quantification in 5D, right); as in the WT, these migrated to superficial layers of the cortex. Thus, increasing COUP-TFI dosage shifts the ratio of deep to superficial neurons. This shift in laminar fate is coupled to a corresponding reduction in expression of Scip, a marker of layer 2/3 projection neurons, at P0 (Fig. S9A,A′), whereas markers of deep layer neurons persist (Fig. 8E–F″). We did not find any changes in Reelin expression, a marker for Cajal-Reztius cells, during CP development (Fig. S9B,B′).
Figure 5.
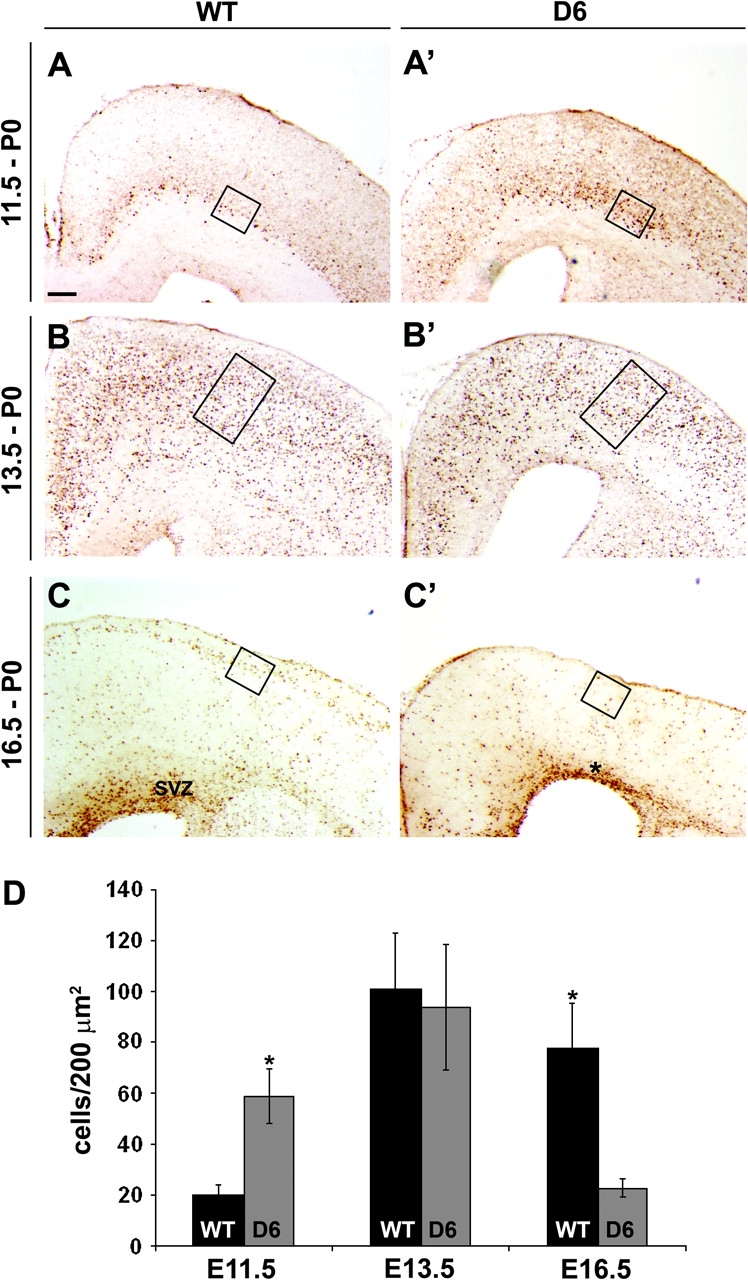
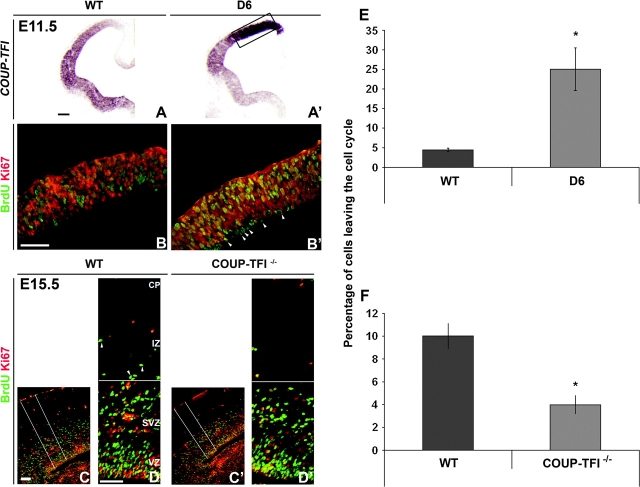
BrdU birthdating shows that increased COUP-TFI promotes generation of early-born cells and reduces late-born cells. (A–C′) BrdU injection at indicated stages, analysis at P0. BrdU immunostaining on coronal sections of WT (A–C) and D6/COUP-TFI (D6) (A′–C′) animals. The boxes show a representative region used to count the BrdU+ cells. (A–A′) In WT cortex, cells incorporating BrdU at E11.5 populated deep parts (layer VI and subplate) of the CP. In the D6/COUP-TFI cortex, there was ∼3-fold increase in the number of BrdU+ cells in deep layers (compare box in A with A′; see chart in D for quantification—cells have been counted in deep layers). (B–B′) Roughly equal numbers of BrdU+ cells were generated at E13.5 in WT and D6. Cells have been counted in CP. (C–C′) In WT cortex, cells labeled at E16.5 populated the upper layers II/III of the CP. In D6 animals, these cells were diminished (compare box in C with C′). Cells have been counted in superficial layers. Scale bar: 200 μm. (D) Quantification of BrdU+ cells per unit area (200 μm2) in the 3 experiments. Asterisks in (D), t-test statistical analysis: P < 0.0006 for E11.5, P < 0.02 for E16.5, n = 4 (see Materials and Methods).
Figure 8.
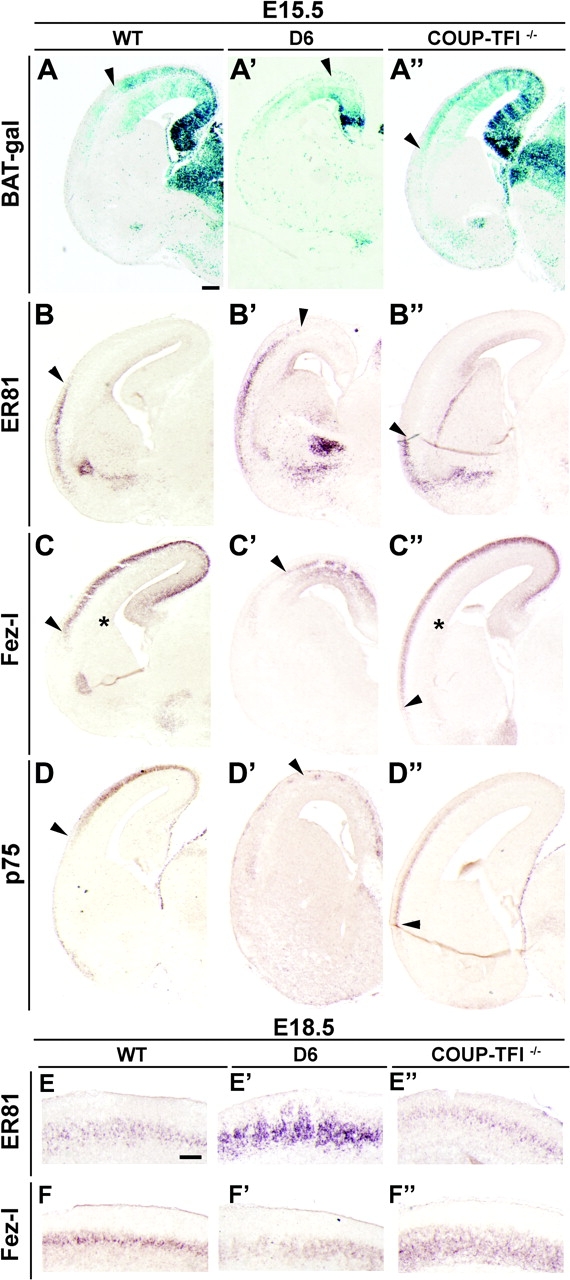
D/V patterning and layer V neurons are modified by COUP-TFI dosage. (A–A′) β-Galactosidase staining on coronal sections through the telencephalon of WT;BAT-gal (A), D6/COUP-TFI;BAT-gal (A′), and COUP-TFI–/–;BAT-gal (A′′) at E15.5. β-Catenin activation follows a dorsalhigh–ventrallow gradient that is disrupted in opposing ways in the 2 genotypes (compare arrowheads in A–A′′). (B–D′′) RNA in situ hybridizations on coronal sections through the telencephalon of WT (B–D), D6/COUP-TFI (B′–D′), and COUP-TFI–/– (B′′–D′′) for ER81 (B–B′′), Fezl (C–C′′), and p75 (D–D′′). The D/V expression pattern of these markers is affected by altering COUP-TFI gene dosage: arrowheads show the complementary D/V changes in D6 and COUP-TFI–/– animals (E-F″) RNA in situ hybridizations on coronal sections through the telencephalon of WT (E, F), D6/COUP-TFI (E′-F′), and COUP-TFI−/− (E″-F″) for ER81 (E′E″) and Fez-l (F-F″) at E18.5. COUP-TFI dosage affects milecular markers of different layer V neurons.
COUP-TFI Regulates the Fraction of Cells Leaving Cell Cycle
Our results suggest that increasing COUP-TFI dosage promotes neurogenesis of early-born neurons and the depletion of progenitors for late-born neurons. To determine whether altered COUP-TFI dosage is accompanied by altered probability of leaving the cell cycle, we measured the proportion of daughter cells that exit the proliferating population. We injected BrdU 12 h before analysis and performed double labeling for BrdU and Ki67, a marker of proliferative cells. We identified cells that had been proliferating, but had exited the cell cycle, as BrdU+;Ki67– (green); cells that had been in S-phase and remained in the cell cycle as BrdU+;Ki67+ (yellow); and cycling cells that had not been in S-phase as BrdU–;Ki67+ (red). We injected D6/COUP-TFI mice with BrdU at E11.5 (the 1st age at which we detected a reduction in proliferation). In the D6/COUP-TFI cortex, there was a clear increase in BrdU+;Ki67– cells, many of which were adjacent to the ventricle in the VZ, suggesting that they had just left the cell cycle (Fig. 6B,B′, arrowheads). In the D6/COUP-TFI dorsal cortex, the proportion of cells that had left the cell cycle increased ∼5-fold (Fig. 6E). This change was not influenced by cell death, as we did not observe a change in apoptosis using activated Caspase-3 and TUNEL assay (Fig. S4).
Figure 6.
COUP-TFI regulates the fraction of cells leaving the cell cycle. (A–A′) RNA in situ hybridization on coronal sections through the telencephalon at E11.5 showing COUP-TFI overexpression in the D6/COUP-TF cortex (box in A′): an equivalent region used for cell counting is shown in B–B′ at higher magnification. (B–B′) Twelve hours before immunofluorescence analysis at E11.5, embryos were exposed to BrdU. The cortex was stained by double labeling (arrowheads in A′) with anti-BrdU (green) and anti-Ki67 (red) antibodies. Arrowheads in B′ show BrdU+/Ki67– cells in the VZ; we suggest that these had exited the cell cycle. (C-D′) Twelve hours before immunofluorescence analysis at E15.5, embryos were exposed to BrdU. Double labeling of the E15.5 pallium with anti-BrdU (green) and anti-Ki67 (red) antibodies. The boxes in C and C′ show the region where the cells have been counted. Arrowheads in D show BrdU+/Ki67– cells that we suggest had exited the cell cycle and had started migration into the CP. (E–F) Ratio of BrdU+; Ki67–/BrdU+ cells, showing the fraction of cells that had exited the cell cycle (Q fraction). Asterisk in E: P < 0.00003, asterisk in F: P < 0.0002, Student's t-test). n = 6 sections, on 2 different brains (same result, only 1 shown) for E. n = 5 sections, on 2 different brains (same result, only 1 shown) for F. IZ, intermediate zone;. Scale bars: 200 μm.
We examined cell cycle exit in the COUP-TFI–/– mutant at E15.5 (the age where we identified increased proliferation; Fig. S2). In WT brains, cells that left the cell cycle (BrdU+/Ki67–) were visible at intermediate zone/SVZ transition (Fig. 6D, arrowheads); the number of these cells was reduced in the COUP-TFI–/– mutant (Fig. 6D′ and quantification in 6F). Together, the results show that COUP-TFI regulates the balance between proliferation and differentiation by controlling the probability that a dividing cell will exit the cell cycle and differentiate.
COUP-TFI Regulates RTK Signaling Pathways
Cortical regionalization, growth, and differentiation are regulated by secreted signals produced by patterning centers: Fgf rostroventrally and Bmp and Wnt dorsocaudally (reviewed in (Sur and Rubenstein 2005). These signals control the expression gradients of several transcription factors, including COUP-TFI and Emx2 (Fukuchi-Shimogori and Grove 2001; Theil et al. 2002; Garel et al. 2003; Storm et al. 2006). To determine how COUP-TFI promotes cell cycle exit, we investigated several signal transduction pathways implicated in this process in D6/COUP-TFI and COUP-TFI–/– cortex. To obtain information about the primary phenotype, we started our analysis on the E11.5 cortex, soon after D6/COUP-TFI expression begins; at this time point, repression of Pax6 is evident (Fig. S1B,B′). We examined the following signaling pathways that are associated with the proliferation/neurogenesis switch: Notch, Tgf-β, Bmp, Mapk/Erk, PI3-kinase/Akt, retinoid, and Wnt/β-catenin.
First, we assessed Notch signaling by examining expression of Hes5, a gene induced by this pathway. COUP-TFI positively regulates Notch signaling (Tang et al. 2006) and therefore could increase Hes5 expression. However, our analysis of Hes5 expression at E11.5 in the D6/COUP-TFI cortex showed no change in expression (Fig. S1E,E′). At E13.5, Hes5 and Delta1 (Notch ligand) expressions in the cortical VZ were maintained (Fig. S5A–D′), although the thickness of the VZ was reduced, consistent with the depletion of cortical progenitors that we previously observed. We did observe a reduction of Delta1 in the CP (Fig. S5A′); however, we interpret this to be secondary to D/V patterning defects (see below).
In the developing cerebral cortex, Foxg1 and Bmp4 show reciprocal repression (Monuki et al. 2001; Ohkubo et al. 2002). We examined expression of Foxg1, as this transcription factor promotes the proliferative state (Hanashima et al. 2002; Martynoga et al. 2005). At E11.5, we did not detect any change in Foxg1 expression in the D6/COUP-TFI cortex (Fig. S1D,–D′), suggesting that Bmp signaling may also not be affected. However, because Bmp’s and other Tgf-β proteins (i.e., activins) are implicated in promoting the switch from proliferation to differentiation (Ko et al. 1998; Seoane et al. 2004; Siegenthaler and Miller 2005), we examined activation of Bmp and Activin signaling by assaying expression of phosphorylated Smad 1-5-8 and Smad 2, respectively (pSmad). As predicted by the normal Foxg1 expression, we did not observe a change in pSmad 1-5-8 and pSmad 2 at E11.5 in the D6/COUP-TFI cortex (Fig. S5G–H′).
RTKs, such as the Fgf receptors, are important regulators of many processes including patterning, differentiation, and proliferation (for review, see (Mason 2007; Thisse and Thisse 2005). Furthermore, Fgf8 signaling represses COUP-TFI expression (Garel et al. 2003; Storm et al. 2006); thus we investigated whether COUP-TFI gene dosage modification affected Fgf transduction pathways as well. We interrogated 2 Fgf-regulated pathways: Mapk/Erk kinase and PI3 kinase/Akt.
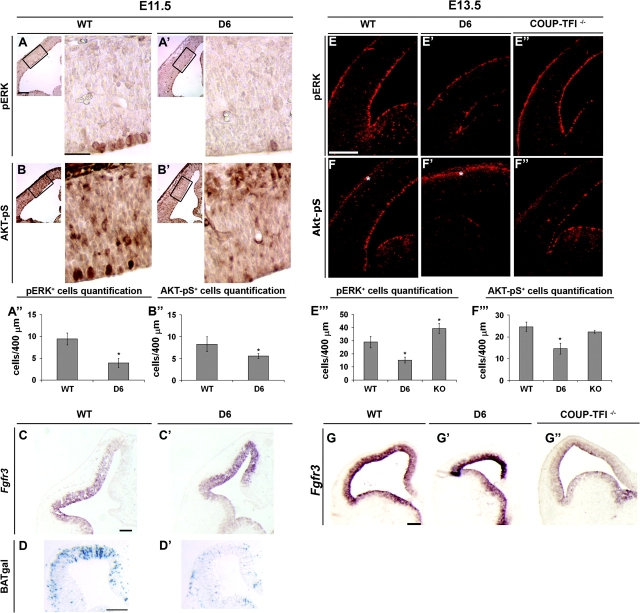
First, we examined activation of the Mapk/Erk kinase pathway. This kinase cascade culminates in phosphorylation and nuclear translocation of Erk1/2 and phosphorylates target transcription factors. We performed immunohistochemistry experiments using an antibody that detects phosphorylated forms of Erk1/2 (Corson et al. 2003). At E11.5, pErk+ cells are detectable primarily in the cortical VZ: we found a clear reduction (50%) in the number of pErk+ cells in the D6/COUP-TFI mutants (Fig. 7A,A′, boxes showing magnification on the right, and quantification in A″).
Figure 7.
Regulation of RTKs and β-catenin signaling. (A–B′) Immunohistochemistry on coronal sections at E11.5 for pErk (A–A′) and Akt-pS (B–B′), showing the early response of RTK signaling to COUP-TFI overexpression. The boxes show the area used for the high magnification picture on the right. This is the area where COUP-TFI is highly overexpressed (See Fig. 1A–A′). (A′′–B′′) Quantification of pErk+ (A′′) and Akt-pS+ (B′′) cells in A–B′. Asterisk in A′′: P < 0.00005; asterisk in B′′: P < 0.005. (C–C′) RNA in situ hybridization on coronal sections at E11.5 for Fgfr3, showing its upregulation. (D–D′) β-Galactosidase staining on coronal sections at E11.5 for β-catenin activation of the BAT-gal transgene. In D6/COUP-TFI (D6) brains β-catenin activation is reduced. (E–F′′) Immunofluorescence on coronal sections at E13.5 for pErk (E–E′′) and Akt-pS (F–F′′) in WT (E, F), D6/COUP-TFI (E′, F′), and COUP-TFI–/– (E′′, F′′), showing the complementary effects on these 2 signaling pathways by changing COUP-TFI dose. (E′′′–F′′′) Quantification of pErk+ (E′′′) and Akt-pS+ (F′′′) cells in E–F′′. Asterisks in E′′′: P < 0.0005 for D6 and KO; asterisk in F′′′: P < 0.007. (G–G′′) RNA in situ hybridization on coronal sections through the telencephalon at E13.5 for Fgfr3, showing its complementary regulation in D6/COUP-TFI (G′) and COUP-TFI–/– (G′′). Scale bars: 200 μm.
We next assayed the PI3 kinase/Akt pathway using an antibody that recognizes the substrates that are phosphorylated by Akt (Akt-pS) (Alessi et al. 1996). At E11.5, we detected Akt-pS in cells lining the ventricle (Fig. 7B,B′) and more diffusely throughout the VZ. In the D6/COUP-TFI mutants, Akt-pS immunoreactivity was diminished (30%) throughout the VZ (Fig. 7B,B′, boxes showing magnification on the right, and quantification in B″).
At E13.5, assays for pErk and Akt-pS in the D6/COUP-TFI cortex confirmed the findings at E11.5. Furthermore, COUP-TFI–/– mutants showed complementary results for pErk and Akt-pS, providing additional evidence that COUP-TFI dosage indeed regulates these signaling pathways (Fig. 7E–F′″). pErk staining prominently labeled VZ cells lining the WT ventricle (Fig. 7E). In D6/COUP-TFI, these pErk+ cells were almost absent (Fig. 7E′, quantification in 7E′″), whereas in COUP-TFI–/– there was a 25% increase (Fig. 7E″, quantification in 7E′″).
In addition to labeling of VZ cells lining the WT ventricle, Akt-pS also labeled differentiating neurons in the CP (Fig. 7F, asterisk). In the D6/COUP-TFI cortex, Akt-pS staining in the VZ was severely reduced (50%, quantification in 7F′″), whereas Akt-pS reactivity in the CP was greatly increased (Fig. 7F′, asterisk). In COUP-TFI–/–, Akt-pS staining in the VZ and CP was slightly reduced (Fig. 7F″ and quantification in 7F′″). These results suggest that loss of COUP-TFI expression increases Mapk/Erk signaling and decreases PI3K/Akt signaling. Furthermore, increasing COUP-TFI dosage suppresses pErk in the VZ and increases Akt-pS in the CP, further implying that these 2 signaling pathways are differentially regulated as COUP-TFI mediates the switch from proliferation to neural differentiation.
A link between COUP-TFI dosage and the Mapk/Erk–PI3K/Akt responses comes from analysis of Fgfr3 RNA expression; increased COUP-TFI increases Fgfr3 at E11.5 and E13.5 (Fig. 7C,C′ and 7G,G′), whereas its expression is greatly reduced in the E13.5 COUP-TFI–/– cortex (Fig. 7G–G″). These results link decreased proliferation/increased neurogenesis with Mapk/Erk–PI3K/Akt inhibition and Fgfr3 activation. Studies on Fgfr3–/– mice have associated signaling through Fgfr3 with reduced proliferation and increased differentiation of chondrocytes (Deng et al. 1996; Sahni et al. 1999) and pancreatic cells (Arnaud-Dabernat et al. 2007), consistent with the phenotypes in the 2 COUP-TFI mutants. We did not find a change in Fgfr1 RNA expression in the D6/COUP-TFI cortex at E11.5 and E13.5 (Fig. S6A–B′).
We assessed Wnt signaling through the β-catenin pathway because of its known role in promoting proliferation and delaying neurogenesis (Chenn and Walsh 2002; Soshnikova et al. 2003; Backman et al. 2005). We used the BAT-gal reporter transgene that reports canonical Wnt signaling tone (Maretto et al. 2003). At E11.5, transgene activation is prominent in dorsocaudal parts of the cortical progenitor domain, the region that is known to have high levels of Wnt expression (Maretto et al. 2003). In the D6/COUP-TFI mutant, β-catenin signaling was strongly decreased (Fig. 7D,D′). At this stage, expression of the Frizzled8 (Fzd8) Wnt receptor is slightly decreased in the region of elevated COUP-TFI expression, perhaps contributing to the reduction in β-catenin signaling (Fig. S6C–D′). Because Pax6 expression is nearly extinguished by increased COUP-TFI (Fig. 2B,B′), we tested whether the reduction in Pax6 causes the reduced β-catenin signaling. We assayed the BAT-gal reporter in Pax6Sey/Sey mice and found elevated Wnt signaling at E13.5 (Fig. S6I,–I′), contrary to the D6/COUP-TFI cortex. This demonstrated that COUP-TFI represses β-catenin signaling independent of its repression of Pax6.
Patterning Defects of the Dorsal Telencephalon
Taking into account the COUP-TFI expression gradient (ventralhigh–dorsallow) and considering the disruption of this gradient in the D6/COUP-TFI and COUP-TFI–/– mice, we tested the hypothesis that COUP-TFI also regulates cortical D/V patterning. First, we examined Wnt signaling in the CP with the BAT-gal transgene (Maretto et al. 2003). At E15.5, this reporter shows a dorsalhigh–ventrallow gradient of expression in the CP (Fig. 8A). In the D6/COUP-TFI CP, BAT-gal expression is greatly reduced (Fig. 8A′), whereas in the COUP-TFI–/– mutant the expression expands ventrally (Fig. 8A″). Thus, COUP-TFI regulates the D/V spatial distribution of Wnt/β-catenin signaling in the CP.
Figure 9.
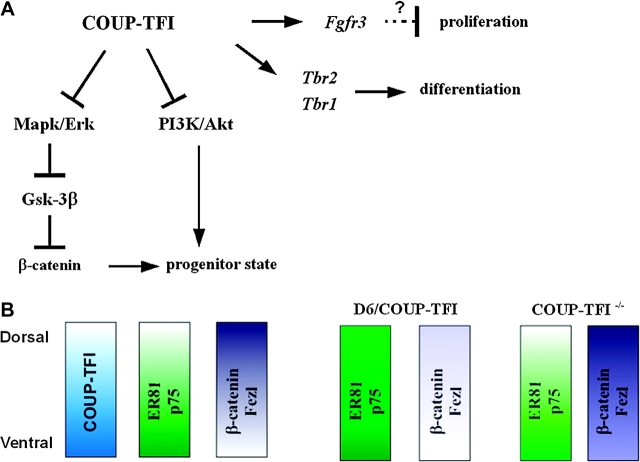
Model for COUP-TFI function in regulating proliferation, differentiation, and patterning. (A) COUP-TFI dosage regulates RTK signaling pathways through downregulation of Mapk/Erk and PI3K/Akt activity. These changes promote progenitor cells to leave the cell cycle (reducing the numbers of both VZ and SVZ cells) and to differentiate into Tbr2+ and Tbr1+ neurons. D6/COUP-TFI promotes expression of Fgfr3; we hypothesize that this Fgf receptor may participate in the proliferation-to-differentiation switch. (B) COUP-TFI promotes ventral fate in the cortical primordium. Disruption of the COUP-TFI gradient changes D/V molecular properties in the CP, as revealed by the expression of ventral (ER81 and p75, green) and dorsal (β-catenin and Fezl, violet) markers.
Next, we studied the distribution of gene expression markers of deep layers of the E15.5 CP. ER81 is a layer V marker that is expressed with a ventralhigh–dorsallow gradient in the CP (Fig. 8B). In D6/COUP-TFI cortex, ER81 was expanded into a more dorsal position, coincident with D6 overexpression (Fig. 8B′). By contrast, in COUP-TFI–/– mutants, ER81 expression shifted ventrally (Fig. 8B″). This supports the idea that the high expression of COUP-TFI in the ventral cortical progenitor domain promotes ventral cortical identity. To test this further, we assessed the effect of modifying COUP-TFI dosage on the distribution of dorsal CP markers: Fezl (layer 5) (Inoue et al. 2004; Chen, Schaevitz, et al. 2005; Chen, Rasin, et al. 2005; Molyneaux et al. 2005) and p75 (layer 6 and subplate) (Allendoerfer et al. 1990). Indeed, increased COUP-TFI expression dorsally suppressed Fezl and p75 expression, whereas reducing COUP-TFI dosage led to ventral expansion of these markers (Fig. 8C–D″). These results provide further evidence that COUP-TFI regulates cortical /V patterning.
COUP-TFI Regulates the Relative Numbers of Layer V Projection Neuron Subtypes
Layer V projection neurons have been classified according to their projection patterns: subcortically projecting (type I) and callosally projecting (type II) (Molnar and Cheung 2006). These subtypes are distinguishable by their expression of different molecular markers, including ER81 and Fezl. Fezl is expressed in type I neurons, whereas ER81 is expressed in both types (Molnar and Cheung 2006).
At E15.5, ER81+ cells appear in a ventrodorsal gradient, whereas Fezl+ cells are produced in a D/V gradient, suggesting that the type I and type II neurons are produced by distinct mechanisms. Indeed, COUP-TFI dosage differentially regulates their distributions (Fig. 8B–C″). We examined the ER81 and Fezl expression at E18.5 to determine whether COUP-TFI dosage affected the final relative numbers of these deep cortical layer cells. We found that when COUP-TFI was overexpressed, ER81 was upregulated (Fig. 8E′) and Fezl down-regulated (Fig. 8F′). In contrast, loss of COUP-TFI function resulted in ER81 downregulation and Fezl upregulation (Fig. 8E″,F″). These results suggest that COUP-TFI can control the relative numbers of type I and type II projection neurons.
Discussion
A major unsolved question in cortical development is how proliferation, neurogenesis, regional growth, regional identity, and laminar fate specification are coordinated. Here we provide evidence that the COUP-TFI transcription factor integrates these processes. By assessing the effect of increased and decreased COUP-TFI dosage, we show that it contributes to 1) patterning D/V properties of the cortex, 2) promoting cell cycle exit and neural differentiation, 3) regulating the balance of early- and late-born cells, and 4) regulating the balance of different types of cortical projection neurons. Furthermore, we provide evidence that COUP-TFI coordinates these processes through controlling signaling through Mapk/Erk, PI3K/Akt, and β-catenin signaling (Fig. 9).
COUP-TFI and Regional Patterning of the Cortex
Previous studies have demonstrated that COUP-TFI is a marker of and contributes to patterning of cortical regional and areal properties. First, this orphan nuclear receptor is expressed in prominent bidimensional gradients: caudorostral and ventrodorsal (Fig. 1B; Qiu et al. 1994; Liu et al. 2000; Zhou et al. 2001). Fgf8 signaling sculpts COUP-TFI's rostral gradient through repression (Garel et al. 2003). Loss of COUP-TFI function in mice greatly disrupts cortical areal properties (Qiu et al. 1997; Zhou et al. 1999, 2001)—Zhou et al. 2001 showed altered region-specific expression of marker genes in the cortex as well as miswired area-specific connections between the cortex and the thalamus but did not report frank alterations in Pax6 or Emx2 expression in the progenitor zone. More recently, analysis of a conditional COUP-TFI mutant showed a complementary expansion of the motor cortex and contraction of the sensory cortices associated with increased expression of Pax6 (Armentano et al. 2007). Consistent with this, we found that increased COUP-TFI represses Pax6 and Emx2 within ∼24 h (Fig. 2 and S1) and alters molecular properties of the rostral cortex (Faedo A and Rubenstein JL, unpublished data). Here, we present the 1st evidence that COUP-TFI promotes ventral cortical molecular properties (Fig. 8), consistent with its expression gradient.
At this point, it is unclear what signaling system promotes its high-ventral and low-dorsal expression. Several signaling molecules are expressed in the region of the pallial–subpallial boundary, including Fgf15, Egf, and sFrp2 that could promote RTK signaling and repress Wnt signaling (Kim et al. 2001; Assimacopoulos et al. 2003). Dorsally, Wnt’s and Bmp’s could contribute to COUP-TFI repression—for instance, in a conditional Fgf8 mutant, where Bmp4 is overexpressed, dorsal expression of COUP-TFI is further repressed (Storm et al. 2006). It is intriguing that some of the signaling systems that may control COUP-TFI's expression gradients (Fgf and Wnt) are negatively regulated by COUP-TFI (Fig. 7). We will address these points in the sections that follow.
COUP-TFI Promotes Cell Cycle Exit
Regional patterning of the cortex is coupled with maturation gradients. Within the neocortex, there is a prominent ventrodorsal gradient in which neurogenesis in ventral regions precedes that in dorsal regions (Bayer 1991). This ventrodorsal gradient correlates with COUP-TFI expression; here we demonstrate that COUP-TFI has a central role in this process. Increases in COUP-TFI dosage positively correlate with decreases in mitotic index and increased neurogenesis (Figs 3 and 4). The reduced density of neuroepithelial cells in M-phase (Figs 3 and 4) and S-phase (not shown) is associated with reduced expression of a key promoter of cell cycle progression—Cyclin D2 (Fig. 2). D-type cyclins control the G1–S transition that regulates cell cycle exit (Caviness et al. 1999). Of note, mitogen-induced signal transduction pathways promote cyclins activation at many levels, including gene transcription (Sherr and Roberts 1999). In this regard, it is interesting that the increased COUP-TFI dosage positively correlates with reduced signaling in mitogenic pathways: Mapk/Erk, PI3K/Akt, and β-catenin (Fig. 7). Consistent with this, we showed that COUP-TFI positively regulates the number of cells exiting the cell cycle (Fig. 6).
COUP-TFI also negatively regulates the number of secondary progenitors in the SVZ (Fig. 3). These cells are produced by radial glia progenitors in the VZ (Noctor et al. 2004) and are postulated to participate in generating later born neurons that populate superficial layers of the cortex (Tarabykin et al. 2001; Nieto et al. 2004). Consistent with this model, we found a 75% reduction in the number of cells born on and after E16.5 in the D6/COUP-TFI cortex (Fig. 5). However, we did not measure an increase in late-born cells in the cortex of COUP-TFI–/– mutants, as was already shown by Zhou et al. 1999. We are not certain why this does not take place, but it may reflect a bias for COUP-TFI–/– progenitor cells to remain in the cell cycle and not produce neurons; this could explain why COUP-TFI–/– have more SVZ progenitors and do not make more superficial neurons.
COUP-TFI Promotes Neurogenesis
As COUP-TFI promotes cell cycle exit, the daughter cells are specified to differentiate into neurons (Fig. 6). Within ∼24 h of COUP-TFI overexpression, the VZ accrued increasing numbers of Ngn2+ cells (Fig. S1H,H′): Ngn2 is a proneural transcription factor that promotes cortical neurogenesis (Fode et al. 1998; Ma et al. 1999). Furthermore, loss of Pax6 function results in precocious expression of neural specific markers (Estivill-Torrus et al. 2002; Quinn et al. 2007). Early functions of Pax6 prevent precocious differentiation and depletion of the progenitor pool and induce normal development of cortical basal progenitor cells. This is consistent with the reduction of Pax6 expression seen in the D6/COUP-TFI cortex (Fig. 2B,B′). COUP-TFI overexpression leads to precocious Tbr2 expression in the cortical VZ (Fig. 2F,F′, Fig. S1G,G′); this transcription factor has been reported to be expressed in early-born neurons (Englund et al. 2005). Interestingly, previous studies suggested that Pax6 is required for both Tbr2 and Ngn2 expression (Scardigli et al. 2003; Quinn et al. 2007); perhaps COUP-TFI overexpression circumvents the requirement of Tbr2 and Ngn2 for Pax6. Thus, COUP-TFI and Pax6 may together participate in regulating the balance of progenitor self-renewal and differentiation.
Consistent with the molecular changes in the VZ, neurogenesis of Tbr1+ and β-III-tubulin+ neurons is increased (Fig. 4 and S1). PP and CP width growth outpaced the WT from E11.5–E13.5 days, after which it showed reduced growth (Fig. 4G), consistent with the reduction in the number of progenitors in the VZ and SVZ. We suggest that the early coupling of cell cycle exit and neurogenesis depleted the progenitor domains of the D6/COUP-TFI cortex—this hypothesis is supported by the reduction of neurons born after E16.5 (Fig. 5C,C′) and by the reduced width of the D6/COUP-TFI cortical VZ at E13.5 (Fig. S5A–B′: see reduced Hes5 and Delta1 domains).
COUP-TFI Can Regulate the Number of Neurons Born at Different Times
Through promoting neurogenesis, COUP-TFI's dosage can regulate the number of neurons produced at a give time. For instance, increasing COUP-TFI dosage in the D6/COUP-TFI cortex leads to increased neurogenesis at early developmental stages—this has ramifications on the number of neurons produced that populate different cortical layers. Therefore, COUP-TFI can regulate cell and laminar fate: BrdU birthdating of the D6/COUP-TFI cortex shows an increase in the neurons born on E11.5 that migrate to deep cortical layer and complementary decrease in the number of neurons born on E16.5 that populate the superficial layers (Fig. 5). Furthermore, the D6/COUP-TFI cortex had a striking reduction in progenitors in the SVZ (Fig. 3); this would be consistent with the hypothesis that SVZ progenitors contribute to neurons destined for superficial layers (Tarabykin et al. 2001; Nieto et al. 2004). Thus, increasing COUP-TFI dosage has a profound effect on both the timing of neurogenesis and laminar fate of neurons.
Although COUP-TFI–/– mutants show a complementary delay in neurogenesis (Fig. 6 and S2) and increase in SVZ progenitors (Fig. 3), our BrdU birthdating analysis did not reveal increase in late-born neurons in superficial layers (not shown), consistent with the previous analysis by Zhou et al. 2001. Thus, either the model that SVZ progenitors produce neurons in superficial layers is incorrect or there is an additional problem affecting their differentiation into superficial layer neurons. Indeed, there is evidence that loss of COUP-TFI function reduces the radial migration of the late-born neurons (M. Studer unpublished results).
However, as discussed below, molecular analysis does demonstrate that both increased and decreased COUP-TFI dosage does modulate molecular correlates of laminar fate, particularly in layer V.
Evidence That COUP-TFI Regulation of D/V Patterning is Coupled with Specification of Different Types of Layer V Neurons
COUP-TFI dosage regulates the D/V gradients of Pax6 and Emx2 expression and β-catenin signaling (Figs 2B–E′ and 8A–A′″). In parallel, it regulates the D/V distribution of markers of deep layer neurons at E15.5: ER81, Fezl, and p75 (Fig. 8B–D′″). Layer V projection neurons are classified according to their projection patterns: subcortically projecting (type I) and callosally projecting (type II) (Molnar and Cheung 2006). These subtypes are distinguishable by their expression of different molecular markers, including Fezl and ER81. Fezl is expressed in type I neurons, whereas ER81 is expressed in both types (Molnar and Cheung 2006).
We suggest that specification of types I and II cells may be coupled with D/V patterning of the cortex. Thus, the number of commissural and corticofugal layer V neurons could be differentially regulated by genes that pattern the cortical progenitor domain. Indeed, at E18.5, ER81 and Fezl expression show complementary changes in presumptive layer V in the D6/COUP-TFI and COUP-TF1–/– cortices (Fig. 8E–F′″).
COUP-TFI Represses MAPK/ERK, PI3K/AKT, and β-catenin Signaling
We show that increasing COUP-TFI dosage positively correlates with reduced signaling in the Mapk/Erk, PI3K/Akt, and β-catenin pathways. We propose that these phenotypes are core mechanisms through which COUP-TFI coordinates regional patterning, proliferation, neurogenesis, and neuronal fate specification.
Alterations in COUP-TFI dosage have potent effects on Mapk/Erk and PI3K/Akt signaling in cortical progenitors. Within ∼24 h of COUP-TFI overexpression, pErk and Akt-pS immunoreactivity in VZ is reduced (Fig. 7A–B′). Although the mechanism for this is uncertain, it is intriguing that Fgfr3 expression positively correlates with COUP-TFI dosage (Fig. 7G–G′″). This Fgf receptor is known to function as a negative regulator of proliferation in other organs. Fgfr3 is expressed in the proliferative zone of chondrocytes and it is essential for reducing chondrocytes proliferation, thus inhibiting bone growth (Deng et al. 1996; Sahni et al. 1999). Similarly, Arnaud-Dabernat et al. (2007) observed in Fgfr3–/– mice an increased in the proliferation of pancreatic cells. No obvious phenotype in the cortex has been reported for the Fgfr3–/– mouse (Deng et al. 1996). Mice bearing 1 Fgfr3 allele with a constitutive activating mutation (K644E) in the kinase domain (Iwata et al. 2000) show a mild increase in progenitor cell proliferation, particularly in the caudal corex at E12.5 (Inglis-Broadgate et al. 2005; Thomson et al. 2007). Thus, so far, unlike in chondrocytes and in the pancreas, Fgfr3 function in the brain has not been associated with growth inhibition. However, the K644E mutation is ligand independent, and thus, its effects are not linked to ligand availability and may not reflect the function of the WT allele.
We speculate that COUP-TFI may negatively regulate cortical proliferation in part through promoting Fgfr3 expression. Both COUP-TFI and Fgfr3 are expressed in a caudalhigh–rostrallow gradient in the VZ; in D6/COUP-TFI animals, Fgfr3 expression is increased in the rostral cortex. Furthermore, we found no effect of COUP-TFI dosage on Fgfr1 expression (Fig. S6), highlighting the specificity of COUP-TFI regulation of Fgfr3. Although we cannot exclude that COUP-TFI is regulating other modulators of Mapk/Erk signaling, a parsimonious model is that COUP-TFI dosage negatively regulates Fgf signaling—this is an appealing idea given the primary roles of Fgf8 and Fgf17 is specifying rostral cortical identity (Fukuchi-Shimogori and Grove 2001; Garel et al. 2003; Storm et al. 2006; Cholfin and Rubenstein 2007).
The connection between Mapk/Erk signaling downregulation and the proliferation/differentiation phenotype that we found in the COUP-TFI mutants is supported by multiple studies of Erk1/2 function in many cell types (Meloche and Pouyssegur 2007). For instance, in the hematopoietic system, Mapk/Erk signaling is required for proliferation of thymocytes through different stages of their development (Fischer et al. 2005). Pkb/Akt signaling pathway is also linked to promoting proliferation; for instance, overexpression of Akt-1 in cortical progenitors promoted their retention in the VZ and SVZ, enhancing proliferation and survival and the proportion of stem cells.(Sinor and Lillien 2004).
In parallel to the modifications in Mapk/Erk and Akt signaling, COUP-TFI overexpression caused downregulation of β-catenin signaling. We suggest a connection between Mapk/Erk signaling downregulation and COUP-TFI's repression of β-catenin signaling based on the following observations. First, Erk-primed inactivation of Gsk-3β links growth factor receptor and the β-catenin signaling pathway (Ding et al. 2005); therefore, increased COUP-TFI will decrease β-catenin signaling by downregulating Mapk/Erk pathway. Second, at E11.5, increased COUP-TFI dosage reduces pErk and increases Fgfr3 levels but does not have a large effect of Fzd8 expression (Fig. S6), supporting the hypothesis that the primary effect is on Fgf signaling. At E13.5, Fzd8 expression is reduced, suggesting that decreased Wnt signaling does contribute to the reduced β-catenin signaling at later stages (Fig. S6). Nonetheless, Wnt7b expression is increased in the D6/COUP-TFI cortex (data not shown), showing that the reduced β-catenin signaling is not due to reduced ligand availability.
The link between β-catenin downregulation and the proliferation/differentiation phenotype is strengthened by the analysis of models where β-catenin activity has been reduced. For example, in a recent study of β-catenin loss of function (Machon et al. 2003), it has been shown that the absence of β-catenin decreased cell proliferation of upper layer progenitors after E15.5, reduced migration of late-born cortical neurons, and reduced superficial cortical layers,. More recently, experiments by Machon et al. 2007 showed that ectopic expression of a β-catenin–Lef1 fusion protein maintained active canonical Wnt signaling in the developing cortex and delayed the expression of the Ngn2 and Tbr2 neurogenic factors and subsequent neurogenesis.
Therefore, COUP-TFI dosage negatively controls activation of the Mapk/Erk, PI3K/Akt, and β-catenin signaling pathways. At early stages of cortical development, these pathways promote progenitor proliferation and repress neurogenesis (Vaccarino et al. 1999; Raballo et al. 2000; Chenn and Walsh 2002; Megason and McMahon 2002; Sinor and Lillien 2004), consistent with the effects seen in the D6/COUP-TFI and COUP-TFI–/– mutants. In sum, COUP-TFI has a central role in coupling regional patterning with the switch between proliferation and differentiation and the properties of layer V neurons.
The function of the Drosophila Melanogaster homologue of COUP-TFI, Seven-up (Svp), has an intriguing parallel in regulating the temporal control of neurogenesis. In Drosophila, neuroblasts (NBs) divide asymmetrically to generate an NB and a differentiating daughter cell; the latter divides symmetrically to produce 2 neurons. These neurons are generated in a specific order that is linked to a sequential expression of the transcription factors Hunchback (Hb), Kruppel (Kr), Pdm1-2, Castor (Cas), and Grainyhead (Grh). The Hb to Kr transition is mediated by Seven-up (Kanai et al. 2005); extending the time of Hb expression through loss of Seven-up function maintains NBs in an immature fully competent state (Cleary and Doe 2006). Thus, like Seven-up, we have found that COUP-TFI promotes the neural differentiation program. In Drosophila photoreceptor determination the Mapk pathway is required for Svp function (Begemann et al. 1995; Kramer et al. 1995), suggesting that the link between COUP-TFI and Mapk signaling has a fundamental role in the regulation of neurogenesis in metazoans. Thus, we propose that COUP-TFI mediates the neural differentiation program by reducing Mapk/Erk, Akt, and β-catenin signaling pathways, perhaps through upregulating Fgfr3.
Funding
Nina Ireland (to J.L.R.R.); Sandler Foundation (to J.L.R.R.); National Institute of Neurological Disorders and Stroke (NINDS) (NS34661 to J.L.R.R.); National Institute of Mental Health (K05 MH065670 to J.L.R.R.); National Alliance for Research on Schizophrenia and Depression (NARSAD) (to A.F.); National Alliance for Autism Research (NAAR) (to Y.R.); Italian Telethon Foundation (to M.S. and H.T.); European Community FP6 program under grant number LSHM-CT-2004-005139 (to M.S. and G.S.T).
Supplementary Material
Supplementary material can be found at: http://www.cercor.oxfordjournals.org/.
Acknowledgments
We thank R. Hoch, U. Borello, I. Cobos, and members of the Rubenstein laboratory for helpful discussions. Conflict of Interest: None declared.
References
- Alessi DR, Caudwell FB, Andjelkovic M, Hemmings BA, Cohen P. Molecular basis for the substrate specificity of protein kinase B; comparison with MAPKAP kinase-1 and p70 S6 kinase. FEBS Lett. 1996;399:333–338. doi: 10.1016/s0014-5793(96)01370-1. [DOI] [PubMed] [Google Scholar]
- Allendoerfer KL, Shelton DL, Shooter EM, Shatz CJ. Nerve growth factor receptor immunoreactivity is transiently associated with the subplate neurons of the mammalian cerebral cortex. Proc Natl Acad Sci USA. 1990;87:187–190. doi: 10.1073/pnas.87.1.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armentano M, Chou SJ, Srubek Tomassy G, Leingartner A, O'Leary DD, Studer M. COUP-TFI regulates the balance of cortical patterning between frontal/motor and sensory areas. Nat Neurosci. 2007;10:1277–1286. doi: 10.1038/nn1958. [DOI] [PubMed] [Google Scholar]
- Armentano M, Filosa A, Andolfi G, Studer M. COUP-TFI is required for the formation of commissural projections in the forebrain by regulating axonal growth. Development. 2006;133:4151–4162. doi: 10.1242/dev.02600. [DOI] [PubMed] [Google Scholar]
- Arnaud-Dabernat S, Kritzik M, Kayali AG, Zhang YQ, Liu G, Ungles C, Sarvetnick N. FGFR3 is a negative regulator of the expansion of pancreatic epithelial cells. Diabetes. 2007;56:96–106. doi: 10.2337/db05-1073. [DOI] [PubMed] [Google Scholar]
- Assimacopoulos S, Grove EA, Ragsdale CW. Identification of a Pax6-dependent epidermal growth factor family signaling source at the lateral edge of the embryonic cerebral cortex. J Neurosci. 2003;23:6399–6403. doi: 10.1523/JNEUROSCI.23-16-06399.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backman M, Machon O, Mygland L, van den Bout CJ, Zhong W, Taketo MM, Krauss S. Effects of canonical Wnt signaling on dorso-ventral specification of the mouse telencephalon. Dev Biol. 2005;279:155–168. doi: 10.1016/j.ydbio.2004.12.010. [DOI] [PubMed] [Google Scholar]
- Bayer SA. Neocortical development. New York: Raven Press; 1991. [Google Scholar]
- Begemann G, Michon AM, vd Voorn L, Wepf R, Mlodzik M. The Drosophila orphan nuclear receptor seven-up requires the Ras pathway for its function in photoreceptor determination. Development. 1995;121:225–235. doi: 10.1242/dev.121.1.225. [DOI] [PubMed] [Google Scholar]
- Bishop KM, Garel S, Nakagawa Y, Rubenstein JL, O'Leary DD. Emx1 and Emx2 cooperate to regulate cortical size, lamination, neuronal differentiation, development of cortical efferents, and thalamocortical pathfinding. J Comp Neurol. 2003;457:345–360. doi: 10.1002/cne.10549. [DOI] [PubMed] [Google Scholar]
- Bishop KM, Goudreau G, O'Leary DD. Regulation of area identity in the mammalian neocortex by Emx2 and Pax6. Science. 2000;288:344–349. doi: 10.1126/science.288.5464.344. [DOI] [PubMed] [Google Scholar]
- Bulfone A, Martinez S, Marigo V, Campanella M, Basile A, Quaderi N, Gattuso C, Rubenstein JL, Ballabio A. Expression pattern of the Tbr2 (Eomesodermin) gene during mouse and chick brain development. Mech Dev. 1999;84:133–138. doi: 10.1016/s0925-4773(99)00053-2. [DOI] [PubMed] [Google Scholar]
- Caviness VS., Jr Neocortical histogenesis in normal and reeler mice: a developmental study based upon [3H]thymidine autoradiography. Brain Res. 1982;256:293–302. doi: 10.1016/0165-3806(82)90141-9. [DOI] [PubMed] [Google Scholar]
- Caviness VS, Jr, Takahashi T, Nowakowski RS. The G1 restriction point as critical regulator of neocortical neuronogenesis. Neurochem Res. 1999;24:497–506. doi: 10.1023/a:1022579712262. [DOI] [PubMed] [Google Scholar]
- Chen B, Schaevitz LR, McConnell SK. Fezl regulates the differentiation and axon targeting of layer 5 subcortical projection neurons in cerebral cortex. Proc Natl Acad Sci USA. 2005;102:17184–17189. doi: 10.1073/pnas.0508732102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JG, Rasin MR, Kwan KY, Sestan N. Zfp312 is required for subcortical axonal projections and dendritic morphology of deep-layer pyramidal neurons of the cerebral cortex. Proc Natl Acad Sci USA. 2005;102:17792–17797. doi: 10.1073/pnas.0509032102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chenn A, Walsh CA. Regulation of cerebral cortical size by control of cell cycle exit in neural precursors. Science. 2002;297:365–369. doi: 10.1126/science.1074192. [DOI] [PubMed] [Google Scholar]
- Cholfin JA, Rubenstein JL. Patterning of frontal cortex subdivisions by Fgf17. Proc Natl Acad Sci USA. 2007;104:7652–7657. doi: 10.1073/pnas.0702225104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleary MD, Doe CQ. Regulation of neuroblast competence: multiple temporal identity factors specify distinct neuronal fates within a single early competence window. Genes Dev. 2006;20:429–434. doi: 10.1101/gad.1382206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corson LB, Yamanaka Y, Lai KM, Rossant J. Spatial and temporal patterns of ERK signaling during mouse embryogenesis. Development. 2003;130:4527–4537. doi: 10.1242/dev.00669. [DOI] [PubMed] [Google Scholar]
- Deng C, Wynshaw-Boris A, Zhou F, Kuo A, Leder P. Fibroblast growth factor receptor 3 is a negative regulator of bone growth. Cell. 1996;84:911–921. doi: 10.1016/s0092-8674(00)81069-7. [DOI] [PubMed] [Google Scholar]
- Ding Q, Xia W, Liu JC, Yang JY, Lee DF, Xia J, Bartholomeusz G, Li Y, Pan Y, Li Z, et al. Erk associates with and primes GSK-3beta for its inactivation resulting in upregulation of beta-catenin. Mol Cell. 2005;19:159–170. doi: 10.1016/j.molcel.2005.06.009. [DOI] [PubMed] [Google Scholar]
- Englund C, Fink A, Lau C, Pham D, Daza RA, Bulfone A, Kowalczyk T, Hevner RF. Pax6, Tbr2, and Tbr1 are expressed sequentially by radial glia, intermediate progenitor cells, and postmitotic neurons in developing neocortex. J Neurosci. 2005;25:247–251. doi: 10.1523/JNEUROSCI.2899-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estivill-Torrus G, Pearson H, van Heyningen V, Price DJ, Rashbass P. Pax6 is required to regulate the cell cycle and the rate of progression from symmetrical to asymmetrical division in mammalian cortical progenitors. Development. 2002;129:455–466. doi: 10.1242/dev.129.2.455. [DOI] [PubMed] [Google Scholar]
- Fischer AM, Katayama CD, Pages G, Pouyssegur J, Hedrick SM. The role of erk1 and erk2 in multiple stages of T cell development. Immunity. 2005;23:431–443. doi: 10.1016/j.immuni.2005.08.013. [DOI] [PubMed] [Google Scholar]
- Fode C, Gradwohl G, Morin X, Dierich A, LeMeur M, Goridis C, Guillemot F. The bHLH protein NEUROGENIN 2 is a determination factor for epibranchial placode-derived sensory neurons. Neuron. 1998;20:483–494. doi: 10.1016/s0896-6273(00)80989-7. [DOI] [PubMed] [Google Scholar]
- Frantz GD, McConnell SK. Restriction of late cerebral cortical progenitors to an upper-layer fate. Neuron. 1996;17:55–61. doi: 10.1016/s0896-6273(00)80280-9. [DOI] [PubMed] [Google Scholar]
- Fukuchi-Shimogori T, Grove EA. Neocortex patterning by the secreted signaling molecule FGF8. Science. 2001;294:1071–1074. doi: 10.1126/science.1064252. [DOI] [PubMed] [Google Scholar]
- Galli R, Fiocco R, De Filippis L, Muzio L, Gritti A, Mercurio S, Broccoli V, Pellegrini M, Mallamaci A, Vescovi AL. Emx2 regulates the proliferation of stem cells of the adult mammalian central nervous system. Development. 2002;129:1633–1644. doi: 10.1242/dev.129.7.1633. [DOI] [PubMed] [Google Scholar]
- Garel S, Huffman KJ, Rubenstein JL. Molecular regionalization of the neocortex is disrupted in Fgf8 hypomorphic mutants. Development. 2003;130:1903–1914. doi: 10.1242/dev.00416. [DOI] [PubMed] [Google Scholar]
- Grove EA, Fukuchi-Shimogori T. Generating the cerebral cortical area map. Annu Rev Neurosci. 2003;26:355–380. doi: 10.1146/annurev.neuro.26.041002.131137. [DOI] [PubMed] [Google Scholar]
- Hamasaki T, Leingartner A, Ringstedt T, O'Leary DD. EMX2 regulates sizes and positioning of the primary sensory and motor areas in neocortex by direct specification of cortical progenitors. Neuron. 2004;43:359–372. doi: 10.1016/j.neuron.2004.07.016. [DOI] [PubMed] [Google Scholar]
- Hanashima C, Shen L, Li SC, Lai E. Brain factor-1 controls the proliferation and differentiation of neocortical progenitor cells through independent mechanisms. J Neurosci. 2002;22:6526–6536. doi: 10.1523/JNEUROSCI.22-15-06526.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill RE, Favor J, Hogan BL, Ton CC, Saunders GF, Hanson IM, Prosser J, Jordan T, Hastie ND, van Heyningen V. Mouse small eye results from mutations in a paired-like homeobox-containing gene. Nature. 1991;354:522–525. doi: 10.1038/354522a0. [DOI] [PubMed] [Google Scholar]
- Hogan BL, Costantini F, Lacey E. Manipulating the mouse embryo: a laboratory manual. Cold Spring Harbor (NY): Cold Spring Harbor Laboratory Press; 1994. pp. 217–252. [Google Scholar]
- Inglis-Broadgate SL, Thomson RE, Pellicano F, Tartaglia MA, Pontikiso CC, Cooper JD, Iwata T. FGFR3 regulates brain size by controlling progenitor cell proliferation and apoptosis during embryonic development. Dev Biol. 2005;279:73–85. doi: 10.1016/j.ydbio.2004.11.035. [DOI] [PubMed] [Google Scholar]
- Inoue K, Terashima T, Nishikawa T, Takumi T. Fez1 is layer-specifically expressed in the adult mouse neocortex. Eur J Neurosci. 2004;20:2909–2916. doi: 10.1111/j.1460-9568.2004.03763.x. [DOI] [PubMed] [Google Scholar]
- Iwata T, Chen L, Li C, Ovchinnikov DA, Behringer RR, Francomano CA, Deng CX. A neonatal lethal mutation in FGFR3 uncouples proliferation and differentiation of growth plate chondrocytes in embryos. Hum Mol Genet. 2000;9:1603–1613. doi: 10.1093/hmg/9.11.1603. [DOI] [PubMed] [Google Scholar]
- Kanai MI, Okabe M, Hiromi Y. Seven-up controls switching of transcription factors that specify temporal identities of Drosophila neuroblasts. Dev Cell. 2005;8:203–213. doi: 10.1016/j.devcel.2004.12.014. [DOI] [PubMed] [Google Scholar]
- Kim AS, Anderson SA, Rubenstein JL, Lowenstein DH, Pleasure SJ. Pax-6 regulates expression of SFRP-2 and Wnt-7b in the developing CNS. J Neurosci. 2001;21:RC132. doi: 10.1523/JNEUROSCI.21-05-j0002.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko TC, Yu W, Sakai T, Sheng H, Shao J, Beauchamp RD, Thompson EA. TGF-beta1 effects on proliferation of rat intestinal epithelial cells are due to inhibition of cyclin D1 expression. Oncogene. 1998;16:3445–3454. doi: 10.1038/sj.onc.1201902. [DOI] [PubMed] [Google Scholar]
- Kramer S, West SR, Hiromi Y. Cell fate control in the Drosophila retina by the orphan receptor seven-up: its role in the decisions mediated by the ras signaling pathway. Development. 1995;121:1361–1372. doi: 10.1242/dev.121.5.1361. [DOI] [PubMed] [Google Scholar]
- Kriegstein A, Noctor S, Martinez-Cerdeno V. Patterns of neural stem and progenitor cell division may underlie evolutionary cortical expansion. Nat Rev Neurosci. 2006;7:883–890. doi: 10.1038/nrn2008. [DOI] [PubMed] [Google Scholar]
- Kroll TT, O'Leary DD. Ventralized dorsal telencephalic progenitors in Pax6 mutant mice generate GABA interneurons of a lateral ganglionic eminence fate. Proc Natl Acad Sci USA. 2005;102:7374–7379. doi: 10.1073/pnas.0500819102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Dwyer ND, O'Leary DD. Differential expression of COUP-TFI, CHL1, and two novel genes in developing neocortex identified by differential display PCR. J Neurosci. 2000;20:7682–7690. doi: 10.1523/JNEUROSCI.20-20-07682.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Q, Fode C, Guillemot F, Anderson DJ. Neurogenin1 and neurogenin2 control two distinct waves of neurogenesis in developing dorsal root ganglia. Genes Dev. 1999;13:1717–1728. doi: 10.1101/gad.13.13.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machon O, van den Bout CJ, Backman M, Kemler R, Krauss S. Role of beta-catenin in the developing cortical and hippocampal neuroepithelium. Neuroscience. 2003;122:129–143. doi: 10.1016/s0306-4522(03)00519-0. [DOI] [PubMed] [Google Scholar]
- Machon O, Backman M, Machonova O, Kozmik Z, Vacik T, Andersen L, Krauss S. A Dynamic gradient of Wnt signaling controls initiation of neurogenesis in the mammalian cortex and cellular specification in the hippocampus. Dev Biol. 2007;311:223–237. doi: 10.1016/j.ydbio.2007.08.038. [DOI] [PubMed] [Google Scholar]
- Maretto S, Cordenonsi M, Dupont S, Braghetta P, Broccoli V, Hassan AB, Volpin D, Bressan GM, Piccolo S. Mapping Wnt/beta-catenin signaling during mouse development and in colorectal tumors. Proc Natl Acad Sci USA. 2003;100:3299–3304. doi: 10.1073/pnas.0434590100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martynoga B, Morrison H, Price DJ, Mason JO. Foxg1 is required for specification of ventral telencephalon and region-specific regulation of dorsal telencephalic precursor proliferation and apoptosis. Dev Biol. 2005;283:113–127. doi: 10.1016/j.ydbio.2005.04.005. [DOI] [PubMed] [Google Scholar]
- Mason I. Initiation to end point: the multiple roles of fibroblast growth factors in neural development. Nat Rev Neurosci. 2007;8:583–596. doi: 10.1038/nrn2189. [DOI] [PubMed] [Google Scholar]
- Megason SG, McMahon AP. A mitogen gradient of dorsal midline Wnts organizes growth in the CNS. Development. 2002;129:2087–2098. doi: 10.1242/dev.129.9.2087. [DOI] [PubMed] [Google Scholar]
- Meloche S, Pouyssegur J. The ERK1/2 mitogen-activated protein kinase pathway as a master regulator of the G1- to S-phase transition. Oncogene. 2007;26:3227–3239. doi: 10.1038/sj.onc.1210414. [DOI] [PubMed] [Google Scholar]
- Molnar Z, Cheung AF. Towards the classification of subpopulations of layer V pyramidal projection neurons. Neurosci Res. 2006;55:105–115. doi: 10.1016/j.neures.2006.02.008. [DOI] [PubMed] [Google Scholar]
- Molyneaux BJ, Arlotta P, Hirata T, Hibi M, Macklis JD. Fezl is required for the birth and specification of corticospinal motor neurons. Neuron. 2005;47:817–831. doi: 10.1016/j.neuron.2005.08.030. [DOI] [PubMed] [Google Scholar]
- Molyneaux BJ, Arlotta P, Menezes JR, Macklis JD. Neuronal subtype specification in the cerebral cortex. Nat Rev Neurosci. 2007;8:427–437. doi: 10.1038/nrn2151. [DOI] [PubMed] [Google Scholar]
- Monuki ES, Porter FD, Walsh CA. Patterning of the dorsal telencephalon and cerebral cortex by a roof plate-Lhx2 pathway. Neuron. 2001;32:591–604. doi: 10.1016/s0896-6273(01)00504-9. [DOI] [PubMed] [Google Scholar]
- Monuki ES, Walsh CA. Mechanisms of cerebral cortical patterning in mice and humans. Nat Neurosci. 2001;4(Suppl):1199–1206. doi: 10.1038/nn752. [DOI] [PubMed] [Google Scholar]
- Muzio L, DiBenedetto B, Stoykova A, Boncinelli E, Gruss P, Mallamaci A. Conversion of cerebral cortex into basal ganglia in Emx2(-/-) Pax6(Sey/Sey) double-mutant mice. Nat Neurosci. 2002;5:737–745. doi: 10.1038/nn892. [DOI] [PubMed] [Google Scholar]
- Muzio L, Mallamaci A. Emx1, emx2 and pax6 in specification, regionalization and arealization of the cerebral cortex. Cereb Cortex. 2003;13:641–647. doi: 10.1093/cercor/13.6.641. [DOI] [PubMed] [Google Scholar]
- Muzio L, Mallamaci A. Foxg1 confines Cajal-Retzius neuronogenesis and hippocampal morphogenesis to the dorsomedial pallium. J Neurosci. 2005;25:4435–4441. doi: 10.1523/JNEUROSCI.4804-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muzio L, Soria JM, Pannese M, Piccolo S, Mallamaci A. A mutually stimulating loop involving emx2 and canonical wnt signalling specifically promotes expansion of occipital cortex and hippocampus. Cereb Cortex. 2005;15:2021–2028. doi: 10.1093/cercor/bhi077. [DOI] [PubMed] [Google Scholar]
- Nieto M, Monuki ES, Tang H, Imitola J, Haubst N, Khoury SJ, Cunningham J, Gotz M, Walsh CA. Expression of Cux-1 and Cux-2 in the subventricular zone and upper layers II-IV of the cerebral cortex. J Comp Neurol. 2004;479:168–180. doi: 10.1002/cne.20322. [DOI] [PubMed] [Google Scholar]
- Noctor SC, Martinez-Cerdeno V, Ivic L, Kriegstein AR. Cortical neurons arise in symmetric and asymmetric division zones and migrate through specific phases. Nat Neurosci. 2004;7:136–144. doi: 10.1038/nn1172. [DOI] [PubMed] [Google Scholar]
- Ohkubo Y, Chiang C, Rubenstein JL. Coordinate regulation and synergistic actions of BMP4, SHH and FGF8 in the rostral prosencephalon regulate morphogenesis of the telencephalic and optic vesicles. Neuroscience. 2002;111:1–17. doi: 10.1016/s0306-4522(01)00616-9. [DOI] [PubMed] [Google Scholar]
- O'Leary DD, Nakagawa Y. Patterning centers, regulatory genes and extrinsic mechanisms controlling arealization of the neocortex. Curr Opin Neurobiol. 2002;12:14–25. doi: 10.1016/s0959-4388(02)00285-4. [DOI] [PubMed] [Google Scholar]
- Qiu Y, Cooney AJ, Kuratani S, DeMayo FJ, Tsai SY, Tsai MJ. Spatiotemporal expression patterns of chicken ovalbumin upstream promoter-transcription factors in the developing mouse central nervous system: evidence for a role in segmental patterning of the diencephalon. Proc Natl Acad Sci USA. 1994;91:4451–4455. doi: 10.1073/pnas.91.10.4451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu Y, Pereira FA, DeMayo FJ, Lydon JP, Tsai SY, Tsai MJ. Null mutation of mCOUP-TFI results in defects in morphogenesis of the glossopharyngeal ganglion, axonal projection, and arborization. Genes Dev. 1997;11:1925–1937. doi: 10.1101/gad.11.15.1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn JC, Molinek M, Martynoga BS, Zaki PA, Faedo A, Bulfone A, Hevner RF, West JD, Price DJ. Pax6 controls cerebral cortical cell number by regulating exit from the cell cycle and specifies cortical cell identity by a cell autonomous mechanism. Dev Biol. 2007;302:50–65. doi: 10.1016/j.ydbio.2006.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raballo R, Rhee J, Lyn-Cook R, Leckman JF, Schwartz ML, Vaccarino FM. Basic fibroblast growth factor (Fgf2) is necessary for cell proliferation and neurogenesis in the developing cerebral cortex. J Neurosci. 2000;20:5012–5023. doi: 10.1523/JNEUROSCI.20-13-05012.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakic P. Specification of cerebral cortical areas. Science. 1988;241:170–176. doi: 10.1126/science.3291116. [DOI] [PubMed] [Google Scholar]
- Rossant J, Zirngibl R, Cado D, Shago M, Giguere V. Expression of a retinoic acid response element-hsplacZ transgene defines specific domains of transcriptional activity during mouse embryogenesis. Genes Dev. 1991;5:1333–1344. doi: 10.1101/gad.5.8.1333. [DOI] [PubMed] [Google Scholar]
- Sahni M, Ambrosetti DC, Mansukhani A, Gertner R, Levy D, Basilico C. FGF signaling inhibits chondrocyte proliferation and regulates bone development through the STAT-1 pathway. Genes Dev. 1999;13:1361–1366. doi: 10.1101/gad.13.11.1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scardigli R, Baumer N, Gruss P, Guillemot F, Le Roux I. Direct and concentration-dependent regulation of the proneural gene Neurogenin2 by Pax6. Development. 2003;130:3269–3281. doi: 10.1242/dev.00539. [DOI] [PubMed] [Google Scholar]
- Seoane J, Le HV, Shen L, Anderson SA, Massague J. Integration of Smad and forkhead pathways in the control of neuroepithelial and glioblastoma cell proliferation. Cell. 2004;117:211–223. doi: 10.1016/s0092-8674(04)00298-3. [DOI] [PubMed] [Google Scholar]
- Sherr CJ, Kato J, Quelle DE, Matsuoka M, Roussel MF. D-type cyclins and their cyclin-dependent kinases: G1 phase integrators of the mitogenic response. Cold Spring Harb Symp Quant Biol. 1994;59:11–19. doi: 10.1101/sqb.1994.059.01.004. [DOI] [PubMed] [Google Scholar]
- Sherr CJ, Roberts JM. CDK inhibitors: positive and negative regulators of G1-phase progression. Genes Dev. 1999;13:1501–1512. doi: 10.1101/gad.13.12.1501. [DOI] [PubMed] [Google Scholar]
- Shimamura K, Rubenstein JL. Inductive interactions direct early regionalization of the mouse forebrain. Development. 1997;124:2709–2718. doi: 10.1242/dev.124.14.2709. [DOI] [PubMed] [Google Scholar]
- Shinozaki K, Yoshida M, Nakamura M, Aizawa S, Suda Y. Emx1 and Emx2 cooperate in initial phase of archipallium development. Mech Dev. 2004;121:475–489. doi: 10.1016/j.mod.2004.03.013. [DOI] [PubMed] [Google Scholar]
- Siegenthaler JA, Miller MW. Transforming growth factor beta 1 promotes cell cycle exit through the cyclin-dependent kinase inhibitor p21 in the developing cerebral cortex. J Neurosci. 2005;25:8627–8636. doi: 10.1523/JNEUROSCI.1876-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinor AD, Lillien L. Akt-1 expression level regulates CNS precursors. J Neurosci. 2004;24:8531–8541. doi: 10.1523/JNEUROSCI.1470-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soshnikova N, Zechner D, Huelsken J, Mishina Y, Behringer RR, Taketo MM, Crenshaw EB, 3rd, Birchmeier W. Genetic interaction between Wnt/beta-catenin and BMP receptor signaling during formation of the AER and the dorsal-ventral axis in the limb. Genes Dev. 2003;17:1963–1968. doi: 10.1101/gad.263003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storm EE, Garel S, Borello U, Hebert JM, Martinez S, McConnell SK, Martin GR, Rubenstein JL. Dose-dependent functions of Fgf8 in regulating telencephalic patterning centers. Development. 2006;133:1831–1844. doi: 10.1242/dev.02324. [DOI] [PubMed] [Google Scholar]
- Sur M, Rubenstein JL. Patterning and plasticity of the cerebral cortex. Science. 2005;310:805–810. doi: 10.1126/science.1112070. [DOI] [PubMed] [Google Scholar]
- Tang LS, Alger HM, Pereira FA. COUP-TFI controls Notch regulation of hair cell and support cell differentiation. Development. 2006;133:3683–3693. doi: 10.1242/dev.02536. [DOI] [PubMed] [Google Scholar]
- Tarabykin V, Stoykova A, Usman N, Gruss P. Cortical upper layer neurons derive from the subventricular zone as indicated by Svet1 gene expression. Development. 2001;128:1983–1993. doi: 10.1242/dev.128.11.1983. [DOI] [PubMed] [Google Scholar]
- Theil T, Aydin S, Koch S, Grotewold L, Ruther U. Wnt and Bmp signalling cooperatively regulate graded Emx2 expression in the dorsal telencephalon. Development. 2002;129:3045–3054. doi: 10.1242/dev.129.13.3045. [DOI] [PubMed] [Google Scholar]
- Thisse B, Thisse C. Functions and regulations of fibroblast growth factor signaling during embryonic development. Dev Biol. 2005;287:390–402. doi: 10.1016/j.ydbio.2005.09.011. [DOI] [PubMed] [Google Scholar]
- Thomson RE, Pellicano F, Iwata T. Fibroblast growth factor receptor 3 kinase domain mutation increases cortical progenitor proliferation via mitogen-activated protein kinase activation. J Neurochem. 2007;100:1565–1578. doi: 10.1111/j.1471-4159.2006.04285.x. [DOI] [PubMed] [Google Scholar]
- Vaccarino FM, Schwartz ML, Raballo R, Nilsen J, Rhee J, Zhou M, Doetschman T, Coffin JD, Wyland JJ, Hung YT. Changes in cerebral cortex size are governed by fibroblast growth factor during embryogenesis. Nat Neurosci. 1999;2:848. doi: 10.1038/12226. [DOI] [PubMed] [Google Scholar]
- van den Bout CJ, Machon O, Rosok O, Backman M, Krauss S. The mouse enhancer element D6 directs Cre recombinase activity in the neocortex and the hippocampus. Mech Dev. 2002;110:179–182. doi: 10.1016/s0925-4773(01)00597-4. [DOI] [PubMed] [Google Scholar]
- Warren N, Caric D, Pratt T, Clausen JA, Asavaritikrai P, Mason JO, Hill RE, Price DJ. The transcription factor, Pax6, is required for cell proliferation and differentiation in the developing cerebral cortex. Cereb Cortex. 1999;9:627–635. doi: 10.1093/cercor/9.6.627. [DOI] [PubMed] [Google Scholar]
- Ye W, Shimamura K, Rubenstein JL, Hynes MA, Rosenthal A. FGF and Shh signals control dopaminergic and serotonergic cell fate in the anterior neural plate. Cell. 1998;93:755–766. doi: 10.1016/s0092-8674(00)81437-3. [DOI] [PubMed] [Google Scholar]
- Yun K, Potter S, Rubenstein JL. Gsh2 and Pax6 play complementary roles in dorsoventral patterning of the mammalian telencephalon. Development. 2001;128:193–205. doi: 10.1242/dev.128.2.193. [DOI] [PubMed] [Google Scholar]
- Zhou C, Qiu Y, Pereira FA, Crair MC, Tsai SY, Tsai MJ. The nuclear orphan receptor COUP-TFI is required for differentiation of subplate neurons and guidance of thalamocortical axons. Neuron. 1999;24:847–859. doi: 10.1016/s0896-6273(00)81032-6. [DOI] [PubMed] [Google Scholar]
- Zhou C, Tsai SY, Tsai MJ. COUP-TFI: an intrinsic factor for early regionalization of the neocortex. Genes Dev. 2001;15:2054–2059. doi: 10.1101/gad.913601. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.