Abstract
Williams syndrome (WS) is a rare neurodevelopmental disorder caused by a 1.6 Mb microdeletion on chromosome 7q11.23 and characterized by hypersocial personality and prominent visuospatial construction impairments. Previous WS studies have identified functional and structural abnormalities in the hippocampal formation, prefrontal regions crucial for amygdala regulation and social cognition, and the dorsal visual stream, notably the intraparietal sulcus (IPS). Although aberrant ventral stream activation has not been found in WS, object-related visual information that is processed in the ventral stream is a critical source of input into these abnormal regions. The present study, therefore, examined neural interactions of ventral stream areas in WS. Using a passive face- and house-viewing paradigm, activation and functional connectivity of stimulus-selective regions in fusiform and parahippocampal gyri, respectively, were investigated. During house viewing, significant activation differences were observed between participants with WS and a matched control group in IPS. Abnormal functional connectivity was found between parahippocampal gyrus and parietal cortex and between fusiform gyrus and a network of brain regions including amygdala and portions of prefrontal cortex. These results indicate that abnormal upstream visual object processing may contribute to the complex cognitive/behavioral phenotype in WS and provide a systems-level characterization of genetically mediated abnormalities of neural interactions.
Keywords: amygdala, fMRI, functional connectivity, fusiform gyrus, intraparietal sulcus, parahippocampal gyrus
Williams syndrome (WS) provides a model condition for understanding brain mechanisms mediating between genetic variation and cognitive–behavioral phenotypes in humans. Caused by a hemizygous microdeletion of approximately 1.6 Mb (containing some 25 genes) on chromosome 7q11.23, WS is typified by a highly specific cognitive and behavioral profile, including a gregarious, hypersocial personality combined with nonsocial anxieties, and a prominent impairment in the visuospatial construction domain (Bellugi et al. 2000; Mervis et al. 2000; Meyer-Lindenberg et al. 2006). Most individuals with WS also have mild to moderate mental retardation.
The hallmark cognitive impairment in WS is in visuospatial construction, the ability to visualize an object (or picture) as a set of parts and construct a replica from those parts (Frangiskakis et al. 1996). This is characterized neuropsychologically by poor performance on tests of block design or pattern construction (Bellugi et al. 1988, 1994; Mervis et al. 1999, 2000; Mervis and Morris 2007). Hierarchically organized and functionally specialized, the visual system is divided into 2 processing streams that emerge from the primary visual cortex: a dorsal stream extends into the parietal lobe and processes spatial information whereas a ventral stream extends into the temporal lobe and subserves object processing (Ungerleider and Mishkin 1982). The hierarchical organization and cognitive specificity of this neural system, combined with the relatively isolated visuospatial construction impairment in WS, led to the hypothesis that dorsal, but not ventral, stream function is compromised. This hypothesis is supported by behavioral studies that report relatively normal face (Tager-Flusberg et al. 2003) and object (Atkinson et al. 1997; Paul et al. 2002; Landau et al. 2006) recognition abilities, indicating a functionally intact ventral stream, and by functional neuroimaging studies that show no aberrant ventral stream activation during object-processing paradigms (Meyer-Lindenberg et al. 2004; Mobbs et al. 2004). In marked contrast, profound dorsal stream abnormalities, consistent with the observed visuospatial deficits, have been uncovered. In particular, a localized structural anomaly in the intraparietal sulcus (IPS), consisting of bilaterally reduced gray matter volume (Meyer-Lindenberg et al. 2004; Reiss et al. 2004; Boddaert et al. 2006; Eckert et al. 2006) and sulcal depth (Kippenhan et al. 2005; Van Essen et al. 2006), has been identified. This structural finding was associated with hypoactivity in directly adjacent parietal regions during spatial localization and visuospatial construction, and reduced information flow from the IPS to these later dorsal stream regions was indicated by path analysis (Meyer-Lindenberg et al. 2004).
There is, thus, clear evidence for a dorsal stream abnormality in WS in the context of relatively intact ventral stream activation during visual processing. This neurogenetic dissociation offers the unique opportunity to study how object information is further processed in brain regions known to be abnormal in WS that show well-documented and widespread neural interactions with the ventral stream. For one, the ventral stream provides input essential for an amygdala–prefrontal system that is involved in social and nonsocial fear signaling and has been shown to be dysregulated in WS (Meyer-Lindenberg, Hariri, et al. 2005; Schultz 2005) and is thought to underlie the hypersocial, gregarious behavioral profile, the lack of socially related fear, and presence of nonsocial anxieties typical in this condition (Meyer-Lindenberg, Hariri, et al. 2005). Further, during face viewing, marked hypoactivity has been observed in the anterior hippocampal formation (Meyer-Lindenberg, Mervis, et al. 2005), a region important for integrating spatial and object information from both dorsal and ventral streams (Suzuki and Amaral 1994; Janzen and van Turennout 2004). Finally, the dorsal and ventral streams themselves are extensively interconnected (Suzuki and Amaral 1994), particularly between the IPS and parahippocampal gyrus, both involved in processing aspects of the spatial environment (Epstein et al. 1999). The parahippocampal gyrus and another ventrally located region in the fusiform gyrus respond in a categorically selective manner to distinct classes of visual stimuli, such as depictions of places and images of faces, respectively (Kanwisher et al. 1997; Aguirre et al. 1998a; Epstein and Kanwisher 1998; Haxby et al. 2001). Whereas the area in the fusiform gyrus related to face processing (often referred to as the fusiform face area [FFA]) clearly resides in the ventral stream in both its anatomical and cognitive domains, the place-processing area in the parahippocampal gyrus (termed the parahippocampal place area [PPA]) appears to be functionally linked to visuospatial cognition: it is crucial for navigation and for processing information about the layout of objects in space; it receives functionally linked, convergent input from dorsal and ventral streams; and it is linked to the hippocampus.
In the present study of participants with WS and normal intelligence quotient (IQ), we used surface-based activation and functional connectivity approaches to examine the contributions of these specialized ventral areas to processing in dorsal stream and amygdala–prefrontal circuits. FFA and PPA were functionally defined for each participant with a passive face- and house-viewing functional magnetic resonance imaging (fMRI) paradigm that reliably activates these regions (Kanwisher et al. 1997; Haxby et al. 2001). We expected to find intact function in ventral structures but abnormal connectivity of these regions with key dorsal stream and social processing areas.
Methods
Participant Selection
Because the majority of individuals with WS are mildly to moderately mentally retarded, their ability to cooperate with neuroimaging procedures can be limited. Moreover, comparing WS patients with mental retardation with a normal intelligence control group presents a potential confound that impacts on the interpretation of the neuroimaging data because group differences could be related to low IQ per se. Although important information about WS has been obtained by studying individuals with WS and mental retardation, we chose to avoid this potential problem by studying extremely rare individuals with WS and normal IQs, who nonetheless have the same visuospatial construction impairment and hypersocial personality that characterize the syndrome, along with genetically confirmed typical hemideletions in the WS critical region of chromosome 7. We took this approach because 1) abnormalities found even in this high performing group are likely to be characteristic of this syndrome as a whole and 2) the neurobiological phenotype will be close to the genetic substrate of the disorder, consistent with our overall objective of using neuroimaging to forge a link between the effects of specific genes and brain mechanisms of cognitive and behavioral disorders. Our participants with WS were, therefore, matched with healthy controls not only for age, sex, and handedness, but for IQ as well (Table 1), and they were also in good physical health. Controls were carefully screened to exclude participants with histories of psychiatric or neurological disorders and of drug or alcohol abuse. All study participants provided written consent in accordance with the National Institutes of Health Internal Review Board and were compensated for their time. These individuals have participated in our previous WS studies (Kippenhan et al. 2005; Meyer-Lindenberg et al. 2004, 2006; Meyer-Lindenberg, Hariri, et al. 2005; Meyer-Lindenber, Mervis, et al. 2005).
Table 1.
Study demographics
| Gender | Age | Handedness | IQ | |
| Controls | 4 F, 6 M | 29 | 100% R | 97.5 |
| WS | 6 F, 3 M | 31.6 | 100% R | 92.4 |
| P value | 0.25a | 0.48b | 0.19b |
Chi-square test.
t-test.
Experimental Paradigm
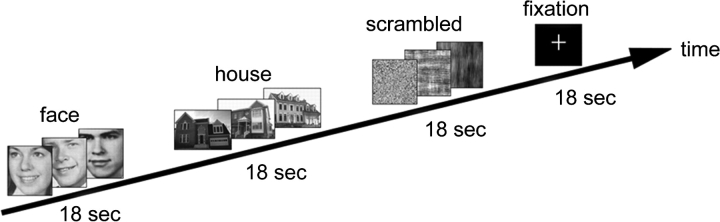
Participants viewed 18-s blocks of faces, houses, scrambled images, or a fixation cross (Fig. 1). Within the faces, houses, and scrambled blocks, each of 12 images was presented for 1.2 s followed by 0.3 s of visual fixation on a centrally located, static cross. The paradigm additionally included 18-s fixation blocks. Scrambled images controlled for luminance of face and house stimuli. Three runs of the task, each consisting of 5 blocks of 4 experimental conditions listed above, were presented during fMRI data collection. Participants were instructed to maintain visual fixation during scanning. Fixation was verified throughout each study by an observer monitoring real-time output from an eye-tracking camera (Real Eye RE-4501 eye imaging system and SV-7201 Fiber Optic Visual System; Avotec Inc., Stuart, FL).
Figure 1.
Diagram of experimental paradigm with examples of visual stimuli.
Data Acquisition and Image Processing
Six axially acquired, high-resolution, T1-weighted FSPGR structural MRI scans (echo time [TE] = 5.2 ms, repetition time [TR] = 12 ms, field of view [FOV] = 24 mm, resolution = 0.94 × 0.94 × 1.2 mm) were collected for each participant on a 1.5-T GE scanner. These images were coregistered and averaged for purposes of cortical surface-based analyses. Blood oxygen level–dependent (BOLD) fMRI T2-weighted gradient-echo echo-planar images were acquired using a 3-T GE scanner (TR = 3 s, TE = 30 ms, FOV = 24 cm, 90° flip, 64 × 64 matrix, 36 contiguous slices, voxel size = 3.75 × 3.75 × 4 mm) with a whole-head coil. Using SPM99 (http://www.fil.ion.ucl.ac.uk/spm/spm99.html), images were realigned to the middle image of the scan, spatially registered to the participant's averaged high-resolution structural scan in native space using affine mapping followed by nonlinear normalization with 7 × 8 × 7 spatial basis functions, smoothed with an 8-mm full width at half maximum Gaussian filter, and ratio normalized to the whole-brain global mean to remove global variation in fMRI signal. Brain responses to each of the 4 conditions of the paradigm (faces, houses, scrambled, fixation) were modeled as a boxcar function convolved with a synthetic hemodynamic response function and estimated using the general linear model within SPM99. For each participant, contrasts were modeled to compare conditions. The comparison of house to face viewing was used to delineate PPA and FFA across all participants in the study, within each diagnostic group and for each participant in his or her own native neuroanatomical space. To further test for between-group differences by stimulus type, the comparison of face viewing to scrambled images and of house viewing to scrambled images was examined.
Cortical Surface Generation and Activation Analysis
Activation analyses for each individual were mapped onto cortical surfaces for optimal visualization of responses in sulci as well as gyri and also to take advantage of the additional statistical power resulting from alignment of subjects based on individual gyral and sulcal patterns (Fischl et al. 1999b). Freesurfer (Dale et al. 1999; Fischl et al. 1999a) was used to segment gray and white matter and to create smooth white matter and pial surface representations from the averaged high-resolution anatomical images of each participant in native space. These surface representations consisted of large numbers of points, or nodes, typically 140 000, connected in a triangular mesh. Each individual's surface mesh was then inflated to a sphere and registered to a study-specific template that represents the average sulcal and gyral curvature across a sample of normal and WS brains. The program MapIcosahedron from the AFNI/SUMA (http://afni.nimh.nih.gov/) analysis package resampled each participant's spherically registered mesh onto a regularly sampled icosahedron to achieve a one-to-one mapping between the nodes of each person's spherically aligned surface. Each individual's modeled contrasts were coregistered with his or her mean echoplanar image and deskulled average high-resolution structural scan using FMRIB's Linear Image Registration Tool (Jenkinson et al. 2002). For each participant, these data were then mapped to this standard, regularly sampled surface, and statistics were computed for every node on the mesh. Between-groups comparisons were conducted using 2-sample t-tests in the statistical language R (Ihaka et al. 1996) and corrected for multiple comparisons via false discovery rate (FDR) (Genovese et al. 2002) using the AFNI/SUMA program 3dFDR. Significance was defined at P < 0.05, FDR corrected.
Functional Connectivity Analysis
Functional connectivity refers here to the correlation of BOLD time-series data at every location in the brain with the time-series of FFA and PPA “seed voxels.” These seeds were functionally defined for each participant using a “functional localizer” approach (Kanwisher et al. 1997) as the most significant voxel within the regions defined by the face versus house comparison and then checked to ensure appropriate anatomical locale within parahippocampal gyrus and FFA by overlaying activation maps on the individual's high-resolution MRI. Correlations were computed for each person's entire time series (across all conditions) in native space, yielding a total of 4 correlation maps (covariance with left and right PPA and FFA). These correlational maps were analyzed at the group level using a random effects model and analysis of variance in SPM99. To most effectively view results in subcortical structures, functional connectivity data are presented on axial slices rather than surface meshes. Group data are displayed on a study-specific volumetric template at a statistical threshold of P < 0.001, uncorrected. Findings that survived FDR correction at P < 0.05 are so noted below.
In addition to these voxel-wise, whole-brain analyses, in order to specifically test for abnormal connectivity of PPA and FFA with key dorsal stream and social processing areas previously defined as structurally and/or functionally abnormal in this same cohort, we compared average correlation values from each individual's data using a 5-mm radius spherical volume centered on voxels selected a priori from our previous work (Meyer-Lindenberg et al. 2004; Meyer-Lindenberg, Hariri, et al. 2005). These areas included 1) dorsal stream regions related to visuospatial processing—the IPS and parietal lobe—as well as 2) the amygdala, orbitofrontal cortex, medial prefrontal cortex, and dorsolateral prefrontal cortex, which comprise a circuit related to social processing.
Results
Functional Activation
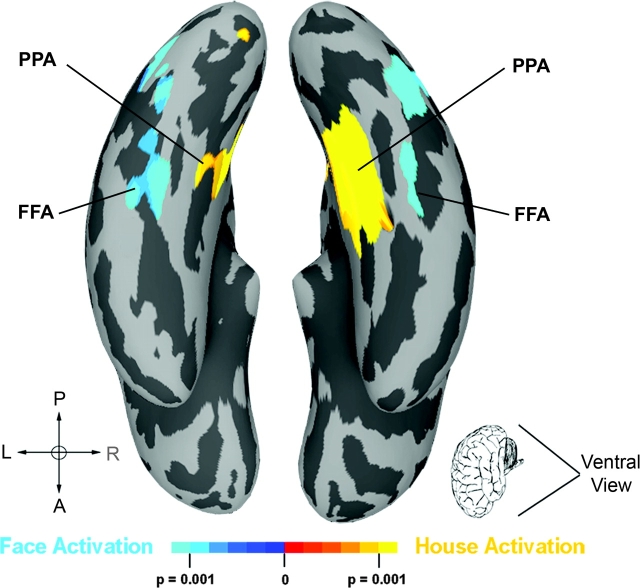
Consistent with previous studies (Haxby et al. 2001; Epstein and Kanwisher 1998), our comparison of house- versus face-viewing conditions (collapsed across WS and control groups) resulted in stimulus-related, differential activation of the ventral visual stream. Spatially normalized activation maps of the main effect of stimulus type are displayed in Figure 2. As expected, we observed bilateral activation in PPA during house viewing (house > face) and in FFA for face viewing (face > house). Each participant with WS showed activation in these regions in native space confirming previous single-subject observations in this cohort during object discrimination (Meyer-Lindenberg et al. 2004).
Figure 2.
Main effect of the face versus house comparison displayed across participant groups (P < 0.001). Face activation (face > house), indicated in blue, was observed bilaterally in fusiform gyrus (FFA), and house activation (house > face) was observed in parahippocampal gyrus (PPA).
Voxel-by-voxel between-group comparison of the face versus house contrast showed no significant differences between participants with WS and controls at the chosen threshold. However, during house viewing (house > face), we noted a trend toward decreased activity in WS (P < 0.001, uncorrected) localized bilaterally along the ventral lip of the collateral sulcus, a region that is anatomically situated between fusiform and parahippocampal gyri where we previously demonstrated abnormal cortical folding in WS (Kippenhan et al. 2005). We subsequently tested for group differences in activation during the houses and faces conditions, compared with scrambled image viewing. During the house-viewing condition (house > scrambled), BOLD signal in the right IPS of participants with WS (Fig. 3) was decreased compared with controls (P = 0.018, FDR corrected) and hypoactivity was additionally observed in Brodmann area 18, bilaterally (P < 0.05, corrected). In contrast, during the face-viewing condition (face > scrambled), no between-group differences were observed in either dorsal or ventral visual stream regions. Thus, functional activation alterations in the WS group were limited to house viewing and were highly significant in the IPS region of the dorsal stream, consistent with previous results (Meyer-Lindenberg et al. 2004).
Figure 3.
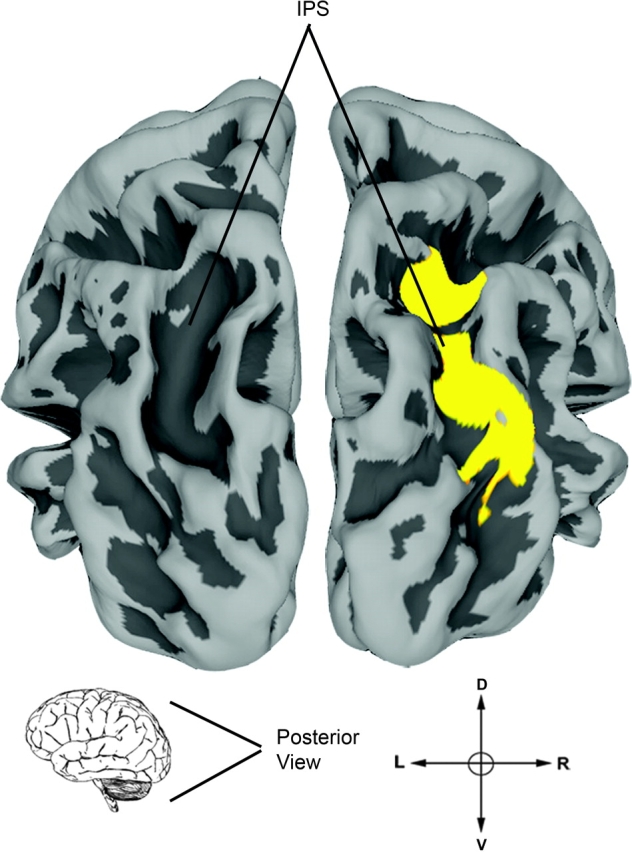
Activation difference between groups during house viewing (house > scrambled) was observed in the right IPS (P < 0.05, corrected). No differences were observed between groups during face stimuli viewing.
Functional Connectivity
Functional connectivity analyses revealed that in both participant groups, both PPA and FFA voxels exhibited similar whole-brain correlations in the left and right hemispheres, even at a liberal threshold of P < 0.05, uncorrected, and that there were no significant group-by-hemisphere interactions. Results were, therefore, collapsed across left and right hemispheres for further voxel-wise analyses.
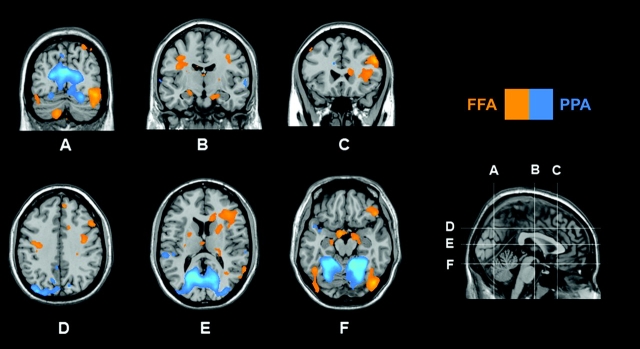
In our control group, several regions displayed differential FFA versus PPA connectivity (Fig. 4). Activity in the right inferior frontal gyrus and the right amygdala was more strongly coupled to FFA than PPA (P < 0.007, corrected), with a similar trend in the left amygdala (P < 0.001, uncorrected). Conversely, the PPA was more robustly correlated with an area extending from the parahippocampal gyrus dorsally to the IPS along the occipitoparietal junction (P < 0.01, corrected).
Figure 4.
Comparison of PPA versus FFA connectivity in control participants is displayed at P < 0.001, uncorrected. Significantly greater (P < 0.05, corrected) PPA connectivity was observed along the parietal–occipital junction. In contrast, FFA shows significantly greater (P < 0.05, corrected) functional connectivity with right amygdala (nonsignificantly in left amygdala, P < 0.001, uncorrected) (B, F), and inferior frontal gyrus (C, E).
In WS compared with controls, both ventral stream regions showed significantly less functional connectivity with prefrontal and parietal regions. Maps of group differences in connectivity derived from the group-by-region interaction analyses for each seed region (see Supplementary Table 1) are displayed and graphed in Figure 5. The FFA connectivity with inferior frontal gyrus and bilateral amygdala observed in our control group (Fig. 4) was decreased in participants with WS (P = 0.0001, corrected; P < 0.001, uncorrected; respectively; Fig. 5A,B). The IPS, bilaterally, and an area in left parietal lobe, both constituents of the dorsal visual stream, did not correlate with PPA in WS participants as robustly as in controls (P < 0.001, uncorrected; Fig. 5C). Conversely, we observed significantly greater functional connectivity between PPA and several posterior brain regions in WS participants including bilateral middle temporal gyrus (Fig. 5D), posterior hippocampus, and left superior temporal gyrus (P < 0.05, corrected).
Figure 5.
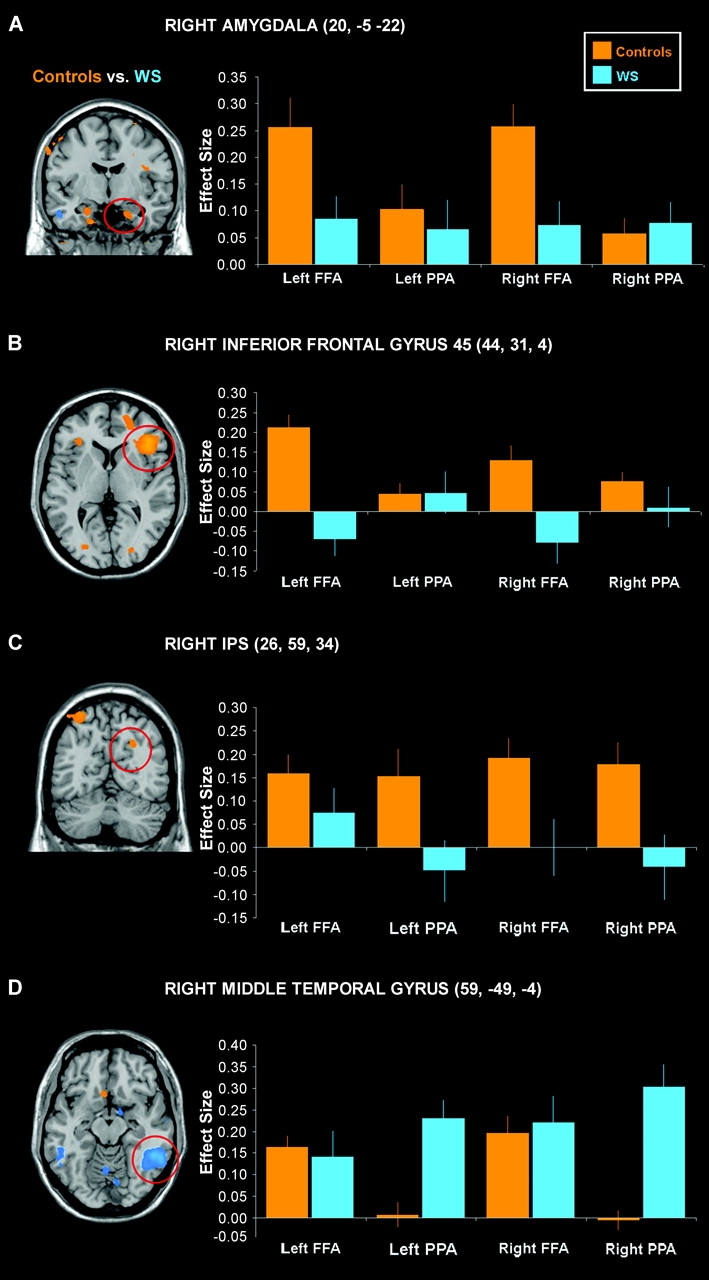
Results from the between-groups comparison of FFA and PPA connectivity are displayed here at P < 0.001, uncorrected (left). The effect size within each group for connectivity with seed region is graphed for circled regions (right). Greater FFA connectivity was observed in control than WS participants in bilateral amygdala (A, only the right side is shown) and right inferior frontal gyrus (B). The bilateral IPS (C, right side is shown) exhibited significantly greater PPA connectivity in controls than in participants with WS. Participants with WS showed significantly more PPA connectivity than controls with posterior regions, including middle temporal gyrus (D). Standard error bars and Talairach coordinates for local maxima are given.
Finally, PPA and FFA connectivity was assessed within volumes of interest selected a priori from previous work (Meyer-Lindenberg et al. 2004; Meyer-Lindenberg, Hariri, et al. 2005), including parietal and prefrontal cortices and amygdala (Table 2). Consistent with the whole-brain, voxel-wise connectivity results above, PPA showed significantly less functional connectivity with IPS bilaterally in WS, as did FFA with the right IPS. In addition, nodes in a social processing network including orbitofrontal cortex, medial prefrontal cortex, bilateral amygdala, and dorsolateral prefrontal cortex were all found to be significantly less functionally coupled with FFA in WS. Importantly, this latter finding with social processing areas was selective for FFA and was not seen for PPA connectivity.
Table 2.
Between-group (controls > WS) differences in PPA and FFA connectivity within volumes of interest (center of 5-mm sphere in Talairach coordinates provided)
| Region | x | y | z | PPA, F value | PPA, P value | FFA, F value | FFA, P value |
| Left IPS | −26 | −69 | 16 | 8.11 | 0.007 | 0.77 | 0.39 |
| Right IPS | 30 | −71 | 26 | 9.33 | 0.004 | 13.13 | 0.001 |
| Right orbitofrontal cortex | 45 | 29 | −6 | 0.62 | 0.44 | 7.51 | 0.009 |
| Right amygdala | 20 | −5 | −22 | 0.00 | 0.97 | 11.64 | 0.002 |
| Left amygdala | −22 | −9 | −18 | 0.10 | 0.92 | 17.48 | 0.0002 |
| Right dorsolateral prefrontal cortex | 49 | 27 | 26 | 0.94 | 0.34 | 4.31 | 0.05 |
| Medial prefrontal cortex | −8 | 59 | 15 | 0.14 | 0.71 | 5.85 | 0.02 |
Discussion
We examined activity and functional connectivity of anatomically and functionally distinct ventral stream object recognition areas in normal IQ individuals with WS. A passive face- and house-viewing paradigm was used to reliably activate 2 well-defined ventral stream regions, the FFA and PPA, respectively (Epstein and Kanwisher 1998; Haxby et al. 2001). We confirmed the presence of these functional areas, bilaterally, in each study participant and then performed functional connectivity analyses to address the interaction patterns of the FFA and PPA in WS and to place them within the context of the neural circuits previously discovered to be abnormal in this syndrome. No between-group activation differences in the ventral stream during face viewing were found, consistent with previous neuroimaging studies (Meyer-Lindenberg et al. 2004; Mobbs et al. 2004) and with behavioral accounts of the WS social and cognitive profile (Atkinson et al. 1997; Bellugi et al. 2000; Mervis et al. 2000; Paul et al. 2002; Tager-Flusberg et al. 2003; Landau et al. 2006). At the whole-brain level, our group interaction analyses revealed abnormalities in activation during house stimuli viewing in the IPS, a region previously found to be altered in WS (Reiss et al. 2004; Meyer-Lindenberg et al. 2004; Boddaert et al. 2006). Additionally, in the house versus face comparison, a nonsignificant decrease in BOLD response was observed along the ventral lip of the collateral sulcus, an inferior temporal structure that anatomically divides the parahippocampal and fusiform gyri, and has been functionally associated with viewing of place stimuli (O'Craven and Kanwisher 2000). This region has been previously reported to be significantly shallower in a larger study of individuals with WS and normal IQ including this same cohort (Kippenhan et al. 2005) as well as in a WS cohort with mental retardation (Van Essen et al. 2006). Therefore, the reduction in activity in this region may result from a decrease in overall size of the region recruited by house viewing in WS, possibly stemming from morphometric differences in collateral sulcal geometry. Alternatively, it may be related to the concomitant activation deficit in the IPS, to which it is functionally linked (see below), in agreement with the greater dependence of house visual stimulus processing on the dorsal stream in general (Epstein and Kanwisher 1998).
Despite the fact that ventral stream activations were confirmed to be relatively unimpaired, our functional connectivity analyses uncovered pronounced abnormalities in the interactions of these same ventral visual areas with neural systems known to be abnormal in WS, such as the IPS. Multiple WS studies (Reiss et al. 2004; Meyer-Lindenberg et al. 2004; Kippenhan et al. 2005; Thompson et al. 2005; Boddaert et al. 2006; Eckert et al. 2006) report structural abnormalities in IPS, a dorsal visual stream constituent that is linked to abnormal activation in the parietal lobe, and is likely to underlie the severe visuospatial construction deficits characteristic of this disorder (Meyer-Lindenberg et al. 2004). Primate studies have demonstrated anatomical connections between IPS and parahippocampal gyrus (Suzuki and Amaral 1994; Rushworth et al. 2005). In accordance with this, in our normal control group, we observed tight functional coupling between IPS and PPA—significantly more than with FFA—providing additional support for this anatomical link. The IPS–PPA circuit supports topographic representation of space (Epstein and Kanwisher 1998). Interestingly, the group with WS showed a significant decrease in functional connectivity between PPA and IPS, a finding in agreement with disruption of spatial orientation in this syndrome and one that might be secondary to altered interactions between IPS and collateral sulcus during development. Ontogenetic clarification of structural and functional links between the dorsal and ventral visual streams in WS, delineation of the extent to which these developmental trajectories (Golarai et al. 2007) are disturbed, and isolation of contributions of single genes in the region such as LIMK1 (Frangiskakis et al. 1996) are potential aims of future studies.
To our knowledge, even in healthy volunteers, this is the first study to directly contrast functional connectivity of FFA and PPA. However, previous work has explored effective connectivity in the visual system in preselected regions of interest (McIntosh et al. 1994) and suggested the possibility that PPA and FFA exhibit differential functional links based on descriptions of concurrent activation during object viewing (Grill-Spector 2003). In particular, both dorsal and ventral areas activate during house stimuli viewing but not during presentation of faces. Two studies have performed functional connectivity analyses using the FFA as a seed region. One reports strong correlations with amygdala (George et al. 2001), and the other reports correlations with large areas of frontal cortex, particularly in the right hemisphere (Bokde et al. 2006). Our results are consistent with these studies and go further by showing a degree of regional selectivity to this connection pattern because functional connectivity with amygdala and right frontal cortex was significantly stronger with FFA than with PPA. These data support a flow of information from FFA consistent with the high degree of salience of facial expressions for emotional processing in humans (Haxby et al. 2002), whereas PPA processing was found embedded into visual information flow from dorsal stream regions into the hippocampal formation that are critical for visuospatial function (Aguirre and D'Esposito 1997; Aguirre et al. 1998b; Kohler et al. 2002).
The group with WS displayed regional reductions in functional connectivity between ventral stream and areas supporting visuospatial constructive, executive, or emotional functions that depend on visual information input. In particular, the observed lack of connectivity between IPS and PPA in WS joins the previous demonstration of reduced effective connectivity between IPS and more dorsal parietal areas in delineating abnormal interactions of the structurally and functionally abnormal IPS as a key mechanism underlying the visuospatial constructive deficit in WS (Meyer-Lindenberg et al. 2004).
Regarding the FFA, we found significant reductions in functional connectivity with amygdala and prefrontal cortex. Previous findings for this cohort point to abnormal regulation of amygdala by prefrontal, especially orbitofrontal, regions (Meyer-Lindenberg, Hariri, et al. 2005) during presentation of threatening scenes and faces. Those data are now extended by the current observation that passively observed facial stimuli may also gain less access to amygdala and regulatory prefrontal areas from the ventral stream, a separate mechanism that could contribute to the reduced amygdala activation, and associated lack of social fear, of persons with WS. Alternatively, reduced amygdala activation might also impair coupling with FFA in top–down fashion through reduced directed attention to facial features. In either case, our results further define the circuitry underlying the striking lack of social fear and the severe spatial deficits characterizing the WS behavioral and cognitive profile and may provide a mechanistic account for the differential cognitive strategies that have been described in WS (Fossella and Casey 2006; Karmiloff-Smith 2006).
Unexpectedly, in our WS group, we found significantly stronger functional connections than in control participants between PPA and regions of the temporal lobe including bilateral middle temporal gyrus, posterior hippocampus, and left superior temporal gyrus. These incidental findings may be related to previously observed increases in sulcal depth in posterior regions reported by Van Essen et al. (2006). Interestingly, superior temporal gyrus is thought to integrate input from both dorsal and ventral visual streams (Elgar and Campbell 2001; Adolphs 2003). These findings, although not hypothesized, may therefore reflect a reorganization of neural interactions during maturation in the setting of reduced competition from afferents originating in the abnormal IPS. Because there is evidence that regions within the middle and superior temporal cortex are involved in motion processing (Beauchamp et al. 2002), this connectivity finding could be related to the preservation of motion processing, particularly of biological motion, that has been reported in previous WS studies (Jordan et al. 2002; Reiss et al. 2005).
It should be noted that the methodology of functional connectivity cannot establish the presence or absence of anatomical connections and does not provide information about the directionality, much less the causality, of the observed interactions. Future studies to extend these results should be performed with larger sample sizes, high-resolution fMRI to further refine the regionally specific and stimulus-dependent findings (Grill-Spector et al. 2006), structural imaging methods such as diffusion tensor imaging to define anatomical connectivity, and developmental neuroimaging exploring behavioral differences in the trajectories of stimulus-specific visual processing (Tottenham et al. 2006). Studies in individuals with WS and mental retardation would also be useful. The present results form a basis to study alterations of functional interactions in individuals with smaller deletions in the WS region to characterize the contributions of individual genes in the deleted region to the observed phenotype. In summary, we report stimulus-related abnormalities in functional connectivity of the ventral visual stream in WS assessed during passive viewing of faces and houses. In particular, we demonstrated abnormal interactions of the PPA with key parietal regions involved in visuospatial construction and of the FFA with amygdala and prefrontal cortex, areas involved in the hypersocial symptoms of WS. These results indicate abnormal upstream processing of visual object information in WS that may contribute to the complex phenotype and provide a systems-level characterization of genetically mediated abnormalities of neural interactions that can be probed for the identification of single-gene effects on brain maturation.
Supplementary Material
Supplementary material can be found at: http://www.cercor.odfordjournals.org/
Funding
National Institute of Mental Health Intramural Research Program; National Institute of Neurological Disorders and Stroke (NS35102 to C.B.M.).
Acknowledgments
We would like to thank the participants with WS and their families for their valuable time and effort, as well as Rosanna Olsen, Aaron Bonner-Jackson, and Paul F. Koch for their research assistance. Conflict of Interest: None declared.
References
- Adolphs R. Cognitive neuroscience of human social behaviour. Nat Rev Neurosci. 2003;4:165–178. doi: 10.1038/nrn1056. [DOI] [PubMed] [Google Scholar]
- Aguirre GK, D'Esposito M. Environmental knowledge is subserved by separable dorsal/ventral neural areas. J Neurosci. 1997;17:2512–2518. doi: 10.1523/JNEUROSCI.17-07-02512.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguirre GK, Zarahn E, D'Esposito M. An area within human ventral cortex sensitive to “building” stimuli: evidence and implications. Neuron. 1998a;21:373–383. doi: 10.1016/s0896-6273(00)80546-2. [DOI] [PubMed] [Google Scholar]
- Aguirre GK, Zarahn E, D'Esposito M. Neural components of topographical representation. Proc Natl Acad Sci USA. 1998b;95:839–846. doi: 10.1073/pnas.95.3.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkinson J, King J, Braddick O, Nokes L, Anker S, Braddick F. A specific deficit of dorsal stream function in Williams’ syndrome. Neuroreport. 1997;8:1919–1922. doi: 10.1097/00001756-199705260-00025. [DOI] [PubMed] [Google Scholar]
- Beauchamp M, Lee K, Haxby J, Martin A. Parallel visual motion processing streams for manipulable objects and human movements. Neuron. 2002;34:149–159. doi: 10.1016/s0896-6273(02)00642-6. [DOI] [PubMed] [Google Scholar]
- Bellugi U, Marks S, Bihrle A, Sabo H. Dissociation between language and cognitive functions in Williams syndrome. In: Bishop D, Mogford K, editors. London: Churchill-Livingstone; 1988. pp. 177–189. [Google Scholar]
- Bellugi U, Wang PP, Jernigan TL. Williams syndrome: an unusual neuropsychological profile. In: Broman SH, Grafman J, editors. Atypical cognitive deficits in developmental disorders: implications for brain function. Mahwah (NJ): Erlbaum; 1994. pp. 23–56. [Google Scholar]
- Bellugi U, Lichtenberger L, Jones W, Lai Z, St George M. The neurocognitive profile of Williams Syndrome: a complex pattern of strengths and weaknesses. J Cogn Neurosci. 2000;12(Suppl 1):7–29. doi: 10.1162/089892900561959. I. [DOI] [PubMed] [Google Scholar]
- Boddaert N, Mochel F, Meresse I, Seidenwurm D, Cachia A, Brunelle F, Lyonnet S, Zilbovicius M. Parieto-occipital grey matter abnormalities in children with Williams syndrome. Neuroimage. 2006;30:721–725. doi: 10.1016/j.neuroimage.2005.10.051. [DOI] [PubMed] [Google Scholar]
- Bokde AL, Lopez-Bayo P, Meindl T, Pechler S, Born C, Faltraco F, Teipel SJ, Moller HJ, Hampel H. Functional connectivity of the fusiform gyrus during a face-matching task in subjects with mild cognitive impairment. Brain. 2006;129:1113–1124. doi: 10.1093/brain/awl051. [DOI] [PubMed] [Google Scholar]
- Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage. 1999;9:179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- Eckert MA, Tenforde A, Galaburda AM, Bellugi U, Korenberg JR, Mills D, Reiss AL. To modulate or not to modulate: differing results in uniquely shaped Williams syndrome brains. Neuroimage. 2006;32:1001–1007. doi: 10.1016/j.neuroimage.2006.05.014. [DOI] [PubMed] [Google Scholar]
- Elgar K, Campbell R. Annotation: the cognitive neuroscience of face recognition: implications for developmental disorders. J Child Psychol Psychiatry. 2001;42:705–717. doi: 10.1111/1469-7610.00767. [DOI] [PubMed] [Google Scholar]
- Epstein R, Harris A, Stanley D, Kanwisher N. The parahippocampal place area: recognition, navigation, or encoding? Neuron. 1999;23:115–125. doi: 10.1016/s0896-6273(00)80758-8. [DOI] [PubMed] [Google Scholar]
- Epstein R, Kanwisher N. A cortical representation of the local visual environment. Nature. 1998;392:598–601. doi: 10.1038/33402. [DOI] [PubMed] [Google Scholar]
- Fischl B, Sereno MI, Dale AM. Cortical surface-based analysis. II: Inflation, flattening, and a surface-based coordinate system. Neuroimage. 1999b;9:195–207. doi: 10.1006/nimg.1998.0396. [DOI] [PubMed] [Google Scholar]
- Fischl B, Sereno MI, Dale AM. High-resolution intersubject averaging and a coordinate system for the cortical surface. Hum Brain Mapp. 1999a;8:272–284. doi: 10.1002/(SICI)1097-0193(1999)8:4<272::AID-HBM10>3.0.CO;2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fossella JA, Casey BJ. Genes, brain, and behavior: bridging disciplines. Cogn Affect Behav Neurosci. 2006;6:1–8. doi: 10.3758/cabn.6.1.1. [DOI] [PubMed] [Google Scholar]
- Frangiskakis JM, Ewart AK, Morris CA, Mervis CB, Bertrand J, Robinson BF, Klein BP, Ensing GJ, Everett LA, Green ED, et al. LIM-kinase1 hemizygosity implicated in impaired visuospatial constructive cognition. Cell. 1996;86:59–69. doi: 10.1016/s0092-8674(00)80077-x. [DOI] [PubMed] [Google Scholar]
- Genoves e CR, Lazar NA, Nichols T. Thresholding of statistical maps in functional neuroimaging using the false discovery rate. Neuroimage. 2002;15:870–878. doi: 10.1006/nimg.2001.1037. [DOI] [PubMed] [Google Scholar]
- George N, Driver J, Dolan RJ. Seen gaze-direction modulates fusiform activity and its coupling with other brain areas during face processing. Neuroimage. 2001;13:1102–1112. doi: 10.1006/nimg.2001.0769. [DOI] [PubMed] [Google Scholar]
- Golarai G, Ghahremani DG, Whitfield-Gabrieli S, Reiss A, Eberhardt JL, Gabrieli JD, Grill-Spector K. Differential development of high-level visual cortex correlates with category-specific recognition memory. Nat Neurosci. 2007;10:512–522. doi: 10.1038/nn1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grill-Spector K. The neural basis of object perception. Curr Opin Neurobiol. 2003;13:159–166. doi: 10.1016/s0959-4388(03)00040-0. [DOI] [PubMed] [Google Scholar]
- Grill-Spector K, Sayres R, Ress D. High-resolution imaging reveals highly selective nonface clusters in the fusiform face area. Nat Neurosci. 2006;9:1177–1185. doi: 10.1038/nn1745. [DOI] [PubMed] [Google Scholar]
- Haxby JV, Gobbini MI, Furey ML, Ishai A, Schouten JL, Pietrini P. Distributed and overlapping representations of faces and objects in ventral temporal cortex. Science. 2001;293:2425–2430. doi: 10.1126/science.1063736. [DOI] [PubMed] [Google Scholar]
- Haxby JV, Hoffman EA, Gobbini MI. Human neural systems for face recognition and social communication. Biol Psychiatry. 2002;51:59–67. doi: 10.1016/s0006-3223(01)01330-0. [DOI] [PubMed] [Google Scholar]
- Ihaka R, Gentleman R. R: a language for data analysis and graphics. J Comp Graph Stat. 1996;5:299–314. [Google Scholar]
- Janzen G, van Turennout M. Selective neural representation of objects relevant for navigation. Nat Neurosci. 2004;7:673–677. doi: 10.1038/nn1257. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17:825–841. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- Jordan H, Reiss JE, Hoffman JE, Landau B. Intact perception of biological motion in the face of profound spatial deficits: Williams syndrome. Psychol Sci. 2002;13:162–167. doi: 10.1111/1467-9280.00429. [DOI] [PubMed] [Google Scholar]
- Kanwisher N, McDermott J, Chun MM. The fusiform face area: a module in human extrastriate cortex specialized for face perception. J Neurosci. 1997;17:4302–4311. doi: 10.1523/JNEUROSCI.17-11-04302.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karmiloff-Smith A. The tortuous route from genes to behavior: a neuroconstructivist approach. Cogn Affect Behav Neurosci. 2006;6:9–17. doi: 10.3758/cabn.6.1.9. [DOI] [PubMed] [Google Scholar]
- Kippenhan JS, Olsen RK, Mervis CB, Morris CA, Kohn P, Meyer-Lindenberg A, Berman KF. Genetic contributions to human gyrification: sulcal morphometry in Williams syndrome. J Neurosci. 2005;25:7840–7846. doi: 10.1523/JNEUROSCI.1722-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohler S, Crane J, Milner B. Differential contributions of the parahippocampal place area and the anterior hippocampus to human memory for scenes. Hippocampus. 2002;12:718–723. doi: 10.1002/hipo.10077. [DOI] [PubMed] [Google Scholar]
- Landau B, Hoffman JE, Kurz N. Object recognition with severe spatial deficits in Williams syndrome: sparing and breakdown. Cognition. 2006;100:483–510. doi: 10.1016/j.cognition.2005.06.005. [DOI] [PubMed] [Google Scholar]
- McIntosh AR, Grady CL, Ungerleider LG, Haxby JV, Rapoport SI, Horwitz B. Network analysis of cortical visual pathways mapped with PET. J Neurosci. 1994;14:655–666. doi: 10.1523/JNEUROSCI.14-02-00655.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mervis CB, Morris CA. Williams syndrome. In: Mazzocco MMM, Ross J, editors. Cambridge (MA): MIT Press; 2007. pp. 199–262. [Google Scholar]
- Mervis CB, Robinson BF, Bertrand J, Morris CA, Klein-Tasman BP, Armstrong SC. The Williams syndrome cognitive profile. Brain Cogn. 2000;44:604–628. doi: 10.1006/brcg.2000.1232. [DOI] [PubMed] [Google Scholar]
- Mervis CB, Robinson BF, Pani JR. Visuospatial construction. Am J Hum Genet. 1999;65:1222–1229. doi: 10.1086/302633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer-Lindenberg A, Kohn P, Mervis CB, Kippenhan JS, Olsen RK, Morris CA, Berman KF. Neural basis of genetically determined visuospatial construction deficit in Williams syndrome. Neuron. 2004;43:623–631. doi: 10.1016/j.neuron.2004.08.014. [DOI] [PubMed] [Google Scholar]
- Meyer-Lindenberg A, Hariri AR, Munoz KE, Mervis CB, Mattay VS, Morris CA, Berman KF. Neural correlates of genetically abnormal social cognition in Williams syndrome. Nat Neurosci. 2005;8:991–993. doi: 10.1038/nn1494. [DOI] [PubMed] [Google Scholar]
- Meyer-Lindenberg A, Mervis CB, Sarpal D, Koch P, Steele S, Kohn P, Marenco S, Morris CA, Das S, Kippenhan S, et al. Functional, structural, and metabolic abnormalities of the hippocampal formation in Williams syndrome. J Clin Invest. 2005;115:1888–1895. doi: 10.1172/JCI24892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer-Lindenberg A, Mervis CB, Berman KF. Neural mechanisms in Williams syndrome: a unique window to genetic influences on cognition and behaviour. Nat Rev Neurosci. 2006;7:380–393. doi: 10.1038/nrn1906. [DOI] [PubMed] [Google Scholar]
- Mobbs D, Garrett AS, Menon V, Rose FE, Bellugi U, Reiss AL. Anomalous brain activation during face and gaze processing in Williams syndrome. Neurology. 2004;62:2070–2076. doi: 10.1212/01.wnl.0000129536.95274.dc. [DOI] [PubMed] [Google Scholar]
- O'Craven KM, Kanwisher N. Mental imagery of faces and places activates corresponding stiimulus-specific brain regions. J Cogn Neurosci. 2000;12:1013–1023. doi: 10.1162/08989290051137549. [DOI] [PubMed] [Google Scholar]
- Paul BM, Stiles J, Passarotti A, Bavar N, Bellugi U. Face and place processing in Williams syndrome: evidence for a dorsal-ventral dissociation. Neuroreport. 2002;13:1115–1119. doi: 10.1097/00001756-200207020-00009. [DOI] [PubMed] [Google Scholar]
- Reiss AL, Eckert MA, Rose FE, Karchemskiy A, Kesler S, Chang M, Reynolds MF, Kwon H, Galaburda A. An experiment of nature: brain anatomy parallels cognition and behavior in Williams syndrome. J Neurosci. 2004;24:5009–5015. doi: 10.1523/JNEUROSCI.5272-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiss JE, Hoffman JE, Landau B. Motion processing specialization in Williams syndrome. Vision Res. 2005;45:3379–90. doi: 10.1016/j.visres.2005.05.011. [DOI] [PubMed] [Google Scholar]
- Rushworth MF, Behrens TE, Johansen-Berg H. Connection patterns distinguish 3 regions of human parietal cortex. Cereb Cortex. 2005;16:1418–1430. doi: 10.1093/cercor/bhj079. [DOI] [PubMed] [Google Scholar]
- Schultz RT. Developmental deficits in social perception in autism: the role of the amygdala and fusiform face area. Int J Dev Neurosci. 2005;23:125–141. doi: 10.1016/j.ijdevneu.2004.12.012. [DOI] [PubMed] [Google Scholar]
- Suzuki WA, Amaral DG. Perirhinal and parahippocampal cortices of the macaque monkey: cortical afferents. J Comp Neurol. 1994;350:497–533. doi: 10.1002/cne.903500402. [DOI] [PubMed] [Google Scholar]
- Tager-Flusberg H, Plesa-Skwerer D, Faja S, Joseph RM. People with Williams syndrome process faces holistically. Cognition. 2003;89:11–24. doi: 10.1016/s0010-0277(03)00049-0. [DOI] [PubMed] [Google Scholar]
- Thompson PM, Lee AD, Dutton RA, Geaga JA, Hayashi KM, Eckert MA, Bellugi U, Galaburda AM, Korenberg JR, Mills DL, et al. Abnormal cortical complexity and thickness profiles mapped in Williams syndrome. J Neurosci. 2005;25:4146–4158. doi: 10.1523/JNEUROSCI.0165-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tottenham N, Leon AC, Casey BJ. The face behind the mask: a developmental study. Dev Sci. 2006;9:288–294. doi: 10.1111/j.1467-7687.2006.00491.x. [DOI] [PubMed] [Google Scholar]
- Ungerleider L, Mishkin M. Two cortical visual systems. In: Ingle D, Goodale MA, Mansfield RJW, editors. Analysis of visual behavior. Cambridge (MA): MIT Press; 1982. pp. 549–586. [Google Scholar]
- Van Essen DC, Dierker D, Snyder AZ, Raichle ME, Reiss AL, Korenberg J. Symmetry of cortical folding abnormalities in Williams syndrome revealed by surface-based analyses. J Neurosci. 2006;26:5470–5483. doi: 10.1523/JNEUROSCI.4154-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.