Abstract
Increased impulsivity caused by addictive drugs is believed to contribute to the maintenance of addiction and has been linked to hypofunction within the orbitofrontal cortex (OFC). Recent data indicate that cocaine “self-administration” induces the transcription factor ΔFosB in the OFC that alters the effects of investigator-administered cocaine on impulsivity. Here, using viral-mediated gene transfer, the effects of overexpressing ΔFosB within the OFC were assessed on the cognitive sequelae of chronic cocaine self-administration as measured by the 5-choice serial reaction time task (5CSRT). Cognitive testing occurred in the mornings, and self-administration sessions in the evenings, to enable the progressive assessment of repeated volitional drug intake on performance. Animals self-administering cocaine initially made more omissions and premature or impulsive responses on the 5CSRT but quickly developed tolerance to these disruptive effects. However, withdrawal from cocaine dramatically increased premature responding. When access to cocaine was increased, animals overexpressing ΔFosB failed to regulate their intake as effectively and were more impulsive during withdrawal. In summary, rats develop tolerance to the cognitive disruption caused by cocaine self-administration and show a deficit in impulse control that is unmasked during withdrawal. Our findings suggest that induction of ΔFosB within the OFC is one mediator of these effects and, thereby, increases vulnerability to addiction.
Keywords: addiction, 5-choice serial reaction time task, relapse, transcription factors, withdrawal
Introduction
It is increasingly recognized that impulsivity is associated with the development and maintenance of addiction and is a major risk factor for relapse to drug seeking (Jentsch and Taylor 1999; Moeller et al. 2001; Rogers and Robbins 2001; Bechara 2005). Numerous studies suggest that addicts are more impulsive, yet it is difficult to decipher from clinical data whether those who become addicts are innately more impulsive or whether increased impulsivity is caused by chronic drug abuse. Furthermore, most studies use subjects who are abstinent rather than current users, leading to the possibility that increased impulsivity is precipitated by withdrawal rather than drug use per se. Studies modeling addiction and cognitive function in rats can address this issue and suggest that intermittent cocaine “self-administration” impairs performance of the 5-choice serial reaction time test (5CSRT) of attention, impulsivity, and motivation (Dalley et al. 2005). Furthermore, animals that make more premature or impulsive responses on the 5CSRT are more likely to display escalating or “binge” patterns of drug self-administration, thought to be indicative of compulsive rather than recreational drug use (Dalley et al. 2007). However, in these experiments, 5CSRT testing was always performed during withdrawal rather than acquisition of drug self-administration. Questions therefore remain over how changes in cognitive function map on to the time course of addiction.
It has been suggested that the deficits in impulse control and decision making observed in drug addicts result in part from hypofunction within the orbitofrontal cortex (OFC) (Volkow and Fowler 2000; Rogers and Robbins 2001; Schoenbaum et al. 2006). However, the neurobiological mechanisms associated with these changes in OFC function are poorly understood. The fact that addictive drugs cause such long-lasting changes in behavior and brain function suggests that gene transcription could be altered. The transcription factor ΔFosB has been identified as a promising candidate capable of mediating some of the behavioral changes associated with addiction as it accumulates in striatal brain regions only after chronic rather than acute administration of addictive drugs. ΔFosB is also highly resistant to degradation and persists in the brain for many weeks after the last drug exposure (Nestler 2004). The majority of work investigating its function has focused on the striatum, where overexpression of ΔFosB enhances the rewarding effects of addictive drugs (Kelz et al. 1999; Colby et al. 2003; Zachariou et al. 2006). It was recently observed that chronic cocaine administration also increases ΔFosB expression in the OFC, leading to the hypothesis that it may be involved in the impulse control deficits evident in addiction as well (Winstanley et al. 2007). However, rather than impairing 5CSRT performance, overexpressing ΔFosB in the OFC prevented the ability of an acute cocaine challenge to increase omissions and impulsive responses, effects that were mimicked and potentiated by repeated intraperitoneal (i.p.) injections of cocaine (Winstanley et al. 2007). These data raise the intriguing possibility that, although withdrawal from drug can precipitate cognitive impairments, some drug-induced changes may be adaptive, potentially invoked to protect cortical function in the face of repeated drug stimulation (Kalivas and Volkow 2005), such that the cognitive disrupting effects of a drug challenge are minimized. Whether the same neurobiological changes are involved in both processes remains an open question.
The current study therefore had 2 main aims: 1) to determine the progressive changes in cognition and impulsivity that may occur during the development of persistent drug taking through allowing animals to volitionally self-administer cocaine in the afternoon and assessing their 5CSRT performance on the subsequent morning and 2) to determine whether ΔFosB induction in the OFC would affect any drug-induced deficits in impulse control.
Materials and Methods
All experiments were carried out in strict accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee at University of Texas Southwestern.
Animals
Male Long–Evans rats (n = 34, initial weight: 300–320 g; Charles River, Kingston, RI) were housed individually in a ventilated rack under a reverse light cycle (lights on from 21:00 to 09:00) in a climate-controlled colony room. Animals were food restricted to 85% of their free-feeding weight and maintained on 14 g of rat chow per day. Water was available ad libitum. Quarter-inch corncob bedding was used to minimize the risk of infection at the catheterization site. Cognitive behavioral testing took place between 09:00 and 12:00 5 days per week, Monday through Friday. Cocaine self-administration took place between 15:00 and 02.00 6 days per week (Sunday through Friday).
Experimental Design
Animals were initially trained on the 5CSRT to stable performance criteria. They were subsequently divided into 2 groups matched for baseline levels of performance, one of which received intra-OFC infusions of AAV-ΔFosB (n = 18) and the other AAV-GFP (n = 17) using standard stereotaxic techniques (see below). Animals were then tested for 4 weeks on the 5CSRT to confirm that overexpression of ΔFosB in the OFC did not alter this measure of cognitive behavior as reported previously for drug-naive rats (data not shown, see Winstanley et al. 2007). These 2 groups of rats were again carefully matched for levels of baseline performance and subdivided into 2 further groups, one of which learned to self-administer cocaine (total n = 22—AAV-ΔFosB: n = 11; AAV-GFP: n = 11) and the other saline (total n = 13—AAV-ΔFosB: n = 7; AAV-GFP: n = 6) following implantation of intrajugular catheters. In order to track any changes in cognitive function during the development of cocaine self-administration behavior, animals were tested on the 5CSRT during the morning and cocaine self-administration in the afternoon/evenings. Three weeks after the final self-administration session, the effects of an acute cocaine challenge on 5CSRT performance were determined. Animals received an i.p. injection of either cocaine (10 mg/kg/ml) or saline 10 min before the start of the task, according to a crossover design. These challenge injections were given on Tuesday and Friday, and animals were not tested the day following the injection. Animals were killed 2 weeks later and their brains processed for immunohistochemistry to confirm the extent and localization of viral infection. During the course of the experiment, 1 animal from each of the saline groups and 2 animals in the ΔFosB cocaine group were excluded from the analysis due to ill health and catheter failure.
The 5CSRT Testing
Testing took place in 8 5-hole operant chambers (Med Associates, Georgia, VT); detailed descriptions of the apparatus and training and testing procedures have been provided previously (Winstanley et al. 2003, 2007). In short, animals were trained to make nose-poke responses into the response apertures upon brief illumination (0.5 s) of the light located therein. The stimulus light could appear in any of the 5 holes, and the spatial location of the target was varied randomly from trial to trial. Each session consisted of 100 trials and lasted approximately for 30 min. Animals initiated each trial by making a nose-poke response at the food tray. There was then a 5-s intertest interval during which animals had to withhold from making a response at the array before the stimulus light was presented in one of the holes. Premature or impulsive responses made at the array during this time period were punished by a 5-s time-out period during which the houselight was turned on, and no further trials could be initiated. A correct response at the illuminated hole was rewarded with delivery of one food pellet in the food tray. Food delivery was signaled by onset of the traylight that remained on until the animal collected its reward. An incorrect or lack of response (omission) was not rewarded and was punished in the same manner as premature responses. Repeated responding at the correct hole was classified as perseverative responding and, while monitored, was not punished. Animals received 5–6 sessions per week until a high level of stable performance was reached (≥80% accuracy and ≤20% omissions) prior to surgery.
Viral Gene Transfer Surgery
Rats were anesthetized with ketamine (Ketaset, 100 mg/kg intramuscular [i.m.] injection) and xylazine (10 mg/kg i.m.; both drugs from Henry Schein, Melville, NY). Adeno-associated virus (AAV) vectors were infused into the OFC using a 31-gauge stainless steel injector (Small Parts, Miami Lakes, FL) attached to a Hamilton microinfusion pump by polyethylene tubing (Instech Solomon, Plymouth Meeting, PA). The viral vectors (AAV-GFP and AAV-ΔFosB) were infused at a rate of 0.1 μl/min according to the following coordinates taken from a stereotaxic atlas (Paxinos and Watson 1998): site 1, anteroposterior (AP) +4.0 mm, lateral (L) ±0.8, dorsoventral (DV) −3.4, 0.4 μl; site 2, AP +3.7, L ±2.0, DV −3.6, 0.6 μl; site 3, AP +3.2, L ±2.6, DV −4.4, 0.6 μl. See Hommel et al. (2003) for details of AAV preparation. AAV-GFP itself is the most appropriate condition against which to compare the effects of AAV-ΔFosB as this allows us to control for any effects of viral infection and general protein overexpression. However, the AAV-2 serotype that we used does not cause any neuronal damage or irregularities (Howard et al. 2008), and no behavioral consequences have been observed following its infusion intracerebrally as compared with vehicle infusions (e.g., Zachariou et al. 2006); therefore, we did not include an additional “sham” control group. The AP coordinate was taken from bregma, the L coordinate from the midline, and the DV coordinate from dura. Animals were allowed 1 week to recover from surgery before behavioral testing recommenced.
Cocaine Self-Administration
Acquisition
Rats were implanted with intrajugular catheters under surgical anesthesia as described previously (Self et al. 1998). Following recovery from surgery (∼1 week), animals were trained to self-administer either cocaine (0.5 mg/kg/infusion: n = 22) or saline (n = 13). In the first 2 weeks, animals were trained under a fixed ratio (FR) 1 schedule of reinforcement, after which all rats showed stable levels of responding (responses varied ≤10% of the mean of 3 consecutive sessions). Each session lasted 2 h. A single response on the active lever resulted in a 0.1-ml intravenous (i.v.) infusion delivered over 5 s, concurrent with the illumination of a circular cue light located above the lever. Each infusion was followed by a 10-s time-out period during which the house light was extinguished, and animals could not earn more cocaine reward. Throughout the session, responding on the inactive lever was recorded but had no consequences. Over the next 2 weeks, animals were switched to an FR3 and subsequently to an FR5 schedule.
Withdrawal
Following 4 weeks of self-administration training, animals underwent a 1-week withdrawal period during which 5CSRT testing continued in the mornings, but the animals remained in their home cages at all other times.
Extinction
Animals were given 4 days of extinction training in the self-administration boxes during which time responding was not reinforced by drugs or injection cues. Each extinction session lasted 2 h. Within 4 sessions, responding on the active lever was comparable in animals trained to self-administer cocaine or saline.
Reinstatement
Reinstatement of responding on the active lever in animals with a history of cocaine self-administration was induced by i.p. administration of cocaine (15 mg/kg/ml). The reinstatement session lasted 2 h. In the first hour, the animals were responding in extinction as per the previous 4 sessions, after which the animals were injected with cocaine or saline, and their response patterns monitored for 1 h. Animals in the saline self-administration group received an injection of saline to control for administration of the injection. This group of animals was not given cocaine because it was important that they maintain their drug-naive status until challenged with cocaine during performance of the 5CSRT at the end of the experiment.
Rebaseline
The following week, animals were allowed to reacquire cocaine self-administration under an FR1 schedule. Within the first session, animals showed a similar pattern of lever pressing to that observed during acquisition, and stable self-administration behavior under an FR5 schedule was established within 7 sessions.
Dose–Response
A within-session dose–response function was obtained for animals responding for cocaine under an FR5 schedule. Each session lasted 5.5 h. During the first 30 min, animals received infusions of 0.5 mg/kg cocaine in a loading phase. The dose of cocaine was then increased to 1 mg/kg for 1 h and successively reduced to 0.3, 0.1, 0.03, and 0.01 mg/kg over the next 4 h. Data were averaged over 2 sessions given on consecutive days.
Long Access to Cocaine
The self-administration paradigm was identical to that used during the acquisition and rebaseline phases, with animals responding for infusions on an FR5 schedule; only the length of the self-administration session was increased from 2 to 6 h for 4 sessions.
Final Withdrawal Period
Animals were tested on the 5CSRT as before but remained in their home cages at all other times.
Immunohistochemistry
Animals were perfused intracardially, and the tissue was processed for immunohistochemistry as described (Perrotti et al. 2004). In summary, cells expressing green fluorescent protein (GFP) or ΔFosB were detected using rabbit polyclonal antisera (ΔFosB: SC-48 [Santa Cruz Biotechnology, Santa Cruz, CA; 1:500]; GFP: ab6556 [Abcam, Cambridge, MA; 1:500]) and visualized using a CY2 fluorophore-labeled secondary antibody (1:200, Jackson Immunoresearch, West Grove, PA). As reported previously (Zachariou et al. 2006), the AAV vectors infected only neurons and did not cause detectable toxicity greater than that observed upon vehicle infusions. The antibodies used detected both endogenous FosB/ΔFosB as well as the overexpressed ΔFosB resulting from viral infection; moreover, virally overexpressed ΔFosB was not uniform throughout the OFC as would be expected. It was therefore difficult to directly quantify levels of ΔFosB in animals that had been self-administering cocaine compared with those who also received infusions of AAV-ΔFosB. The localization and extent of viral-mediated over-expression is depicted in Figure 1.
Figure 1.
Localization and extent of viral overexpression. Panel (A) shows a schematic of the area infected by the virus, based on Paxinos and Watson (1998). The largest area affected in any individual is shown in gray and the smallest in black. Viral overexpression encompassed the medial, ventral, and lateral areas of the OFC. There was minimal evidence of spread into other frontal regions, although some viral infection was occasionally detected in very anterior parts of the prelimbic cortex and the more ventral aspect of the agranular insular cortex. Photomicrographs depicting the immunohistochemical detection of ΔFosB overexpression in the ventral/lateral OFC of a representative animal are also provided, magnified 4× (panel B) and 20× (panel C). PrLC, prelimbic cortex; MO, medial orbitofrontal cortex; VO, ventral orbitofrontal cortex; LO, lateral orbitofrontal cortex; DLO, dorsolateral orbitofrontal cortex; Cg1, anterior cingulate cortex; IL, infralimbic cortex; AIV, ventral anterior insular cortex; AID, dorsal anterior insular cortex.
Data Analysis
All data were analyzed using SPSS software (SPSS, Chicago, IL) running on an IBM compatible PC (Dell, Round Rock, TX). From the 5CSRT, 7 variables were analyzed: the percent of correct responses made (number of correct responses/total correct and incorrect responses); percent of responses omitted (number of omissions/total number of correct, incorrect, and omitted responses); percent of premature responses (number of premature responses/total number of trials), latency to make a correct response, latency to collect reward, perseverative responses, and the total number of trials completed per session. Variables that were expressed as a percentage were subjected to an arcsine transformation in order to limit the effect of an artificially imposed ceiling (ie, 100%). Data were subjected to analysis of variance (ANOVA) with surgery (AAV-GFP or −ΔFosB) and i.v. group (self-administering saline or cocaine) as between-subjects factors and session as a within-subjects factor. Data from the acute cocaine challenges were analyzed using ANOVA with surgery and i.v. group as between-subjects factors and dose of cocaine (saline, cocaine) as a within-subjects factor. Significant terms were clarified using simple main effects analysis and, where appropriate, Student's t-tests.
Behavioral stability for both 5CSRT performance and self-administration behavior was set when ANOVA revealed that there was no longer a significant effect of session across 5 days. When discussing the results, the cognitive effects of a period of cocaine self-administration are assessed in the subsequent 5CSRT testing session, that is, cognitive changes observed in a Monday morning 5CSRT session would be attributed to the Sunday evening cocaine self-administration session. Data from 2 5CSRT sessions had to be excluded from the withdrawal phase due to technical failure.
Results
Acquisition of Cocaine Self-Administration
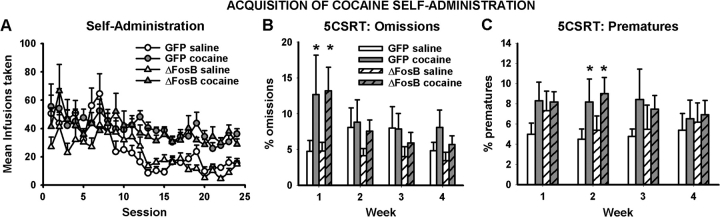
Animals that had received either intra-OFC AAV-GFP or AAV-ΔFosB learned to reliably self-administer cocaine to the same level and made significantly more responses on the active lever than those administering saline (Fig. 2A, using ANOVA with surgery and i.v. group as between-subjects factors and session as a within-subjects factor—i.v. group: F1,15 = 5.333, P < 0.036; surgery: F1,15 = 0.661, not significant [NS]). Animals taking cocaine made significantly more omissions on the 5CSRT than saline-administering controls during the first week of self-administration training, regardless of whether they had received intra-OFC AAV-GFP or AAV-ΔFosB (Fig. 2B, i.v. group: F1,26 = 4.484, P < 0.044; surgery: F1,26 = 0.179, NS). This impairment was selective in that it was not accompanied by deficits in the accuracy of target detection (i.v. group: F1,26 = 0.079, NS; surgery: F1,26 = 0.891, NS), the number of premature responses made (i.v. group: F1,26 = 1.501, NS; surgery: F1,26 = 0.042, NS), or the latency to respond correctly (i.v. group: F1,26 = 0.396, NS; surgery: F1,26 = 0.01, NS) or collect food reward (i.v. group: F1,26 = 0.045, NS; surgery: F1,26 = 0.553, NS).
Figure 2.
Acquisition of cocaine self-administration transiently increases omissions and premature responding on the 5CSRT. The number of infusions of cocaine or saline self-administered over 24 sessions (A) and the percentage of omissions (B) and premature responses (C) made during the concurrent 5CSRT sessions, averaged per week. Data shown are mean ± standard error of the mean. Asterisk denotes P < 0.05.
During the second week of self-administration training, animals self-administering cocaine appeared to have grown tolerant to the amotivational effects on the 5CSRT and no longer omitted more trials than animals self-administering saline (i.v. group: F1,26 = 0.834, NS; surgery: F1,26 = 0.558, NS). However, all animals in the cocaine group made significantly more premature responses during this time period (Fig. 2C, i.v. group: F1,26 = 5.559, P < 0.026; surgery: F1,26 = 0.029, NS). All other measures of behavioral performance were similar in both cocaine and saline groups, regardless of surgery condition. During weeks 3 and 4 of self-administration training, there were no longer any signs of either motivational disturbance or increased impulsivity attributable to repeated intake of cocaine (omissions week 3—i.v. group: F1,26 = 0.38, NS; omissions week 4—i.v. group: F1,26 = 3.204, NS; premature responses week 3—i.v. group: F1,26 = 1.108, NS; premature week 4—i.v. group: F1,26 = 0.033, NS).
Withdrawal
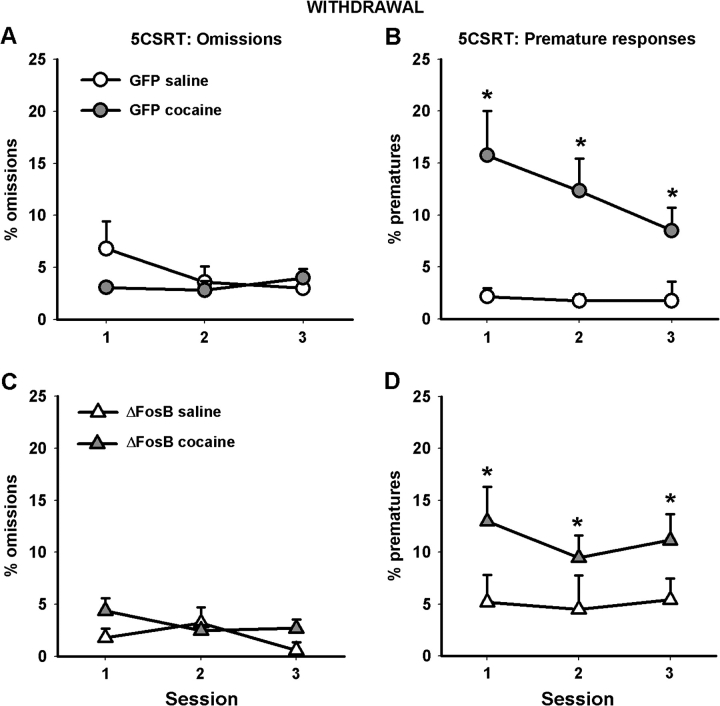
All animals that had been self-administering cocaine became more impulsive following withdrawal from the drug, regardless of whether they had been treated with AAV-GFP or AAV-ΔFosB (Fig. 3, using ANOVA with surgery and i.v. group as between-subjects factors and session as a within-subjects factor—i.v. group: F1.26 = 7.887, P < 0.009; surgery: F1,26 = 1.103, NS). This increased impulsivity was evident throughout the week of testing (session: F2,52 = 2.598, P < 0.084) and was still evident during the final test session (independent samples t-test comparing saline and cocaine self-administering animals: t(28) = −2.491, P < 0.019). No changes were observed in the number of omissions made or any other 5CSRT variable.
Figure 3.
Withdrawal from cocaine self-administration increases premature responding on the 5CSRT. The left-hand column shows the percentage of trials omitted by both GFP control animals (A) and those overexpressing ΔFosB (C). The right-hand column shows the percentage of premature responses made by both GFP control animals (B) and those overexpressing ΔFosB (D). Data shown are mean ± standard error of the mean. Asterisk denotes P < 0.05.
Extinction
During the course of extinction training, responding on the active lever declined in the cocaine self-administration group until it was statistically indistinguishable from animals that had been self-administering saline (using ANOVA with surgery and i.v. group as between-subjects factors and session as a within-subjects factor—i.v. group: F1,26 = 4.983, P < 0.033; surgery F1,26 = 1.190, NS; session: F3,90 = 4.496, P < 0.005; session 4—i.v. group: t(28) = −2.69, NS; mean responses on active lever in session 4: saline 40.92 ± 8.38, cocaine 47.71 ± 6.81). There was no longer a significant difference between the number of premature responses made by those in the cocaine or saline self-administration groups, and this was evident from the first day of testing (all 4 sessions—i.v. group: F1,26 = 1.574, NS; session 1—i.v. group: F1,26 = 0.013, NS; number of premature responses, saline 3.3 ± 0.6 and cocaine 3.8 ± 0.6). No other 5CSRT variable was significantly affected during this phase of the experiment, and there were no differences between animals overexpressing ΔFosB or GFP on any measure.
Reinstatement and Rebaseline of Cocaine Self-Administration
A noncontingent injection of cocaine significantly increased responding on the active lever in animals that had previously been self-administering cocaine, and this was not affected by the overexpression of ΔFosB (using ANOVA with surgery and i.v. group as between-subjects factors and hour as a within-subjects factor—hour × i.v. group: F1,26 = 12.455, P < 0.002; cocaine group–hour: F1,18 = 15.152, P < 0.001; number of infusions taken in hour 1, saline 27 ± 3.70 and cocaine 25.5 ± 4.7; number of infusions taken in hour 2, saline 16.5 ± 3.9 and cocaine 58.5 ± 9.7; surgery: F1,26 = 0.103, NS). No changes were observed in 5CSRT performance following this self-administration session (data not shown). In the following week, stable cocaine self-administration was reestablished at a level comparable to that observed at the end of the acquisition period. In contrast to the changes in cognitive behavior that were observed when animals initially learned to self-administer cocaine, 5CSRT performance remained remarkably stable during this phase (data not shown). The tolerance animals had developed to the cognitive sequelae of cocaine self-administration was clearly still evident.
Dose–Response Curve
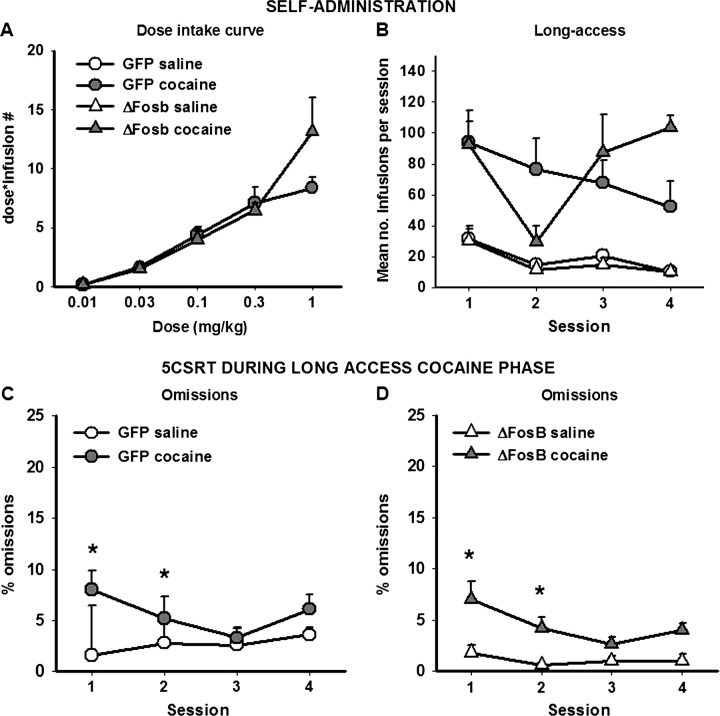
The shape of the dose–response curve was very similar for animals overexpressing ΔFosB or GFP within the OFC. However, there was a tendency for animals overexpressing ΔFosB to take more cocaine at the highest dose only (using ANOVA with surgery as a between-subjects factors and dose as a within-subjects factor: Fig. 4A, dose × surgery: F4,56 = 2.163, P < 0.085).
Figure 4.
Changes in behavior when the amount of cocaine available increases. The dose–response intake curve indicates that animals overexpressing ΔFosB tend to take more cocaine at the highest dose offered (A). Animals overexpressing ΔFosB show less stable responding and tend to increase the amount of cocaine taken when given longer access to cocaine per session (B). The number of trials omitted during the 5CSRT sessions corresponding to the long-access sessions is provided in panels (C) (GFP control animals) and (D) (animals overexpressing ΔFosB). Data shown are mean ± standard error of the mean. Asterisk denotes P < 0.05.
Long Access to Cocaine
As expected, animals self-administered considerably more cocaine during the first long-access session (Fig. 4B, using ANOVA with surgery and i.v. group as between-subjects factors and session as a within-subjects factor—i.v. group: F1,21 = 43.375, P < 0.0001; session: F3,63 = 4.586, P < 0.006; surgery × session × i.v. group: F3,63 = 2.254, P < 0.061). Restricting analysis to those animals self-administering cocaine, whereas control GFP-expressing animals tended to maintain or decrease drug intake across the sessions, animals overexpressing ΔFosB showed a different pattern of responding, dramatically decreasing their intake on day 2 but then increasing their intake on days 3 and 4 (using ANOVA with surgery as between-subjects factors and session as a within-subjects factor—session × surgery: F3,36 = 3.140, P < 0.037; day 2 vs day 3—session × surgery: F1,11 = 5.683, P < 0.036; day 4—t(10) = −2.011, P < 0.089). In conjunction with the dose–response data, these findings suggest that overexpression of ΔFosB in the OFC reduced an animal's ability to regulate its cocaine intake following an increase in the amount of drug available.
Looking at the 5CSRT data, increasing the amount of cocaine intake per session lead to an increase in the number of trials omitted in the first 2 sessions, reminiscent of the initial effects observed during acquisition of cocaine self-administration (Fig. 4C,E, using ANOVA with surgery and i.v. group as between-subjects factors and session as a within-subjects factor—session: F3,78 = 2.856, P < 0.042; comparing saline and cocaine self-administering rats, session 1: t(28) = −2.215, P < 0.035; session 2: t(28) = −1.966, P < 0.06). These effects were short lived and were no longer evident by day 3 (t(28) = −0.264, NS).
Withdrawal from Long Access to Cocaine
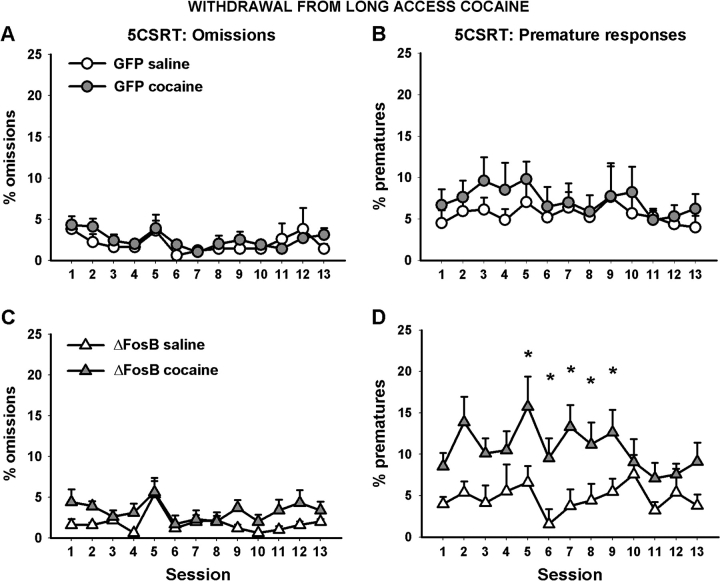
Overexpression of ΔFosB within the OFC appeared to sensitize animals to the effects of withdrawal from cocaine, in that these rats became more impulsive during this phase of the experiment (Fig. 5). This effect reached significance during the second week of testing (using ANOVA with surgery and i.v. group as between-subjects factors and session as a within-subjects factor—i.v. group × surgery: F1,26 = 4.063, P < 0.05; ΔFosB only—i.v. group: F1,12 = 5.175, P < 0.042, GFP controls only—i.v. group: F1,14 = 0.017, NS) but was absent in the third week (i.v. group × surgery: F1,26 = 0.801, NS). No other variable was significantly affected, and the 5CSRT performance of control GFP-expressing rats was generally unaltered during this second withdrawal period.
Figure 5.
The effects of withdrawal from long-access cocaine on 5CSRT performance. The left-hand column shows the percentage of trials omitted by both GFP control animals (A) and those overexpressing ΔFosB (C). The right-hand column shows the percentage of premature responses made by both GFP control animals (B) and those overexpressing ΔFosB (D). ΔFosB overexpression increased premature responses during this withdrawal period. Data shown are mean ± standard error of the mean. Asterisk denotes P < 0.05.
Effects of Acute Cocaine Challenge on 5CSRT Performance
In general concordance with previous observations (Winstanley et al. 2007), prior history of repeated cocaine exposure reduced the effects of an acute cocaine challenge on the 5CSRT task, and this tolerance-like effect was mimicked by overexpression of ΔFosB in the OFC. Cocaine increased premature responding in animals that were drug naive, but this was attenuated in animals that had a history of cocaine self-administration and in animals overexpressing ΔFosB (Fig. 6A,D, using ANOVA with surgery and i.v. group as between-subjects factors and dose as a within-subjects factor—dose × i.v. group: F1,26 = 12.644, P < 0.002; dose × surgery: F1,26 = 3.528, P < 0.044). Likewise, this dose of cocaine tended to produced a mild impairment in the accuracy of target detection, which seemed less evident in animals with a previous history of cocaine self-administration, as well as in animals overexpressing ΔFosB (Fig. 6B,E, dose × i.v. group × surgery: F1,26 = 3.296, P < 0.081; GFP only—dose × i.v. group: F1,11 = 10.770, P < 0.007; ΔFosB—dose × i.v. group: F1,14 = 0.006, NS).
Figure 6.
The effects of an acute cocaine challenge (10 mg/kg) on 5CSRT performance compared with performance after an injection of saline. The xaxis denotes the injection given to the animals on that session, whereas the color of the symbols indicates the self-administration group to which the animals had been assigned, that is, whether they had been self-administering saline (white) or cocaine (gray). The left-hand column shows the percentage of premature responses made by both GFP control animals (A) and those overexpressing ΔFosB (D). The center column shows the percentage of correct responses made by both GFP control animals (B) and those overexpressing ΔFosB (E). The right-hand column shows the percentage of trials omitted by both GFP control animals (C) and those overexpressing ΔFosB (F). ΔFosB overexpression reduced the increase in premature responding and in omissions elicited by the acute cocaine challenge in animals previously trained with saline. Data shown are mean ±standard error of the mean.
Cocaine also increased the number of omissions animals made (Fig. 6C,F, dose: F1,26 = 47.945, P < 0.0001), which tended to depend on whether animals had a cocaine history (dose × i.v. group: F1,26 = 3.735, P < 0.065). Animals that had been self-administering saline made more omissions when given cocaine on task, but this effect was blocked in animals that were overexpressing ΔFosB in the OFC (dose: F1,9 = 27.044, P < 0.001; surgery: F1,9 = 7.981, P < 0.02), again consistent with a tolerance-like effect of ΔFosB. However, animals that had been self-administering cocaine showed a pronounced response to the drug independent of whether they had been treated with AAV-GFP or AAV-ΔFosB (dose: F1,16 = 38.111, P < 0.0001; surgery: F1,16 = 0.001, NS).
Discussion
This is the first time that changes in cognitive function and impulsivity have been tracked during the progression of drug-taking behavior and indicate that the most pronounced cognitive changes occurred during initial engagement in drug seeking, during withdrawal, and when drug intake was increased. In the initial acquisition phase of cocaine self-administration, animals became transiently more impulsive and less motivated on the 5CSRT. Although tolerance soon developed to these disruptive effects of drug intake, impulsive responding increased again during withdrawal, an effect that dissipated after extinction training, and little change in 5CSRT performance was observed when cocaine self-administration resumed. However, when the amount of cocaine available was increased, animals overexpressing ΔFosB appeared less able to regulate their drug intake, and only these animals became more impulsive during the second withdrawal phase. These data suggest that increasing expression of ΔFosB in the OFC may act to potentiate the cycle of addiction by facilitating a loss of behavioral control during periods of frequent drug use and to increase vulnerability to relapse by enhancing impulsivity during subsequent withdrawal.
Regardless of the cognitive impairments caused by cocaine intake or withdrawal, all animals with a prior drug history made fewer premature responses on the 5CSRT when given an acute cocaine challenge. A similar effect was seen in drug-naive animals overexpressing ΔFosB, and we have previously observed that overexpression of ΔJunD (a dominant-negative antagonist of ΔFosB) prevents development of this tolerance-like phenomenon (Winstanley et al. 2007). These data indicate that increasing levels of ΔFosB in the OFC significantly contributes to the reduced impact of an acute drug challenge, even though this manipulation also increases impulsivity during withdrawal. Taken together, these data suggest that ΔFosB induction within the OFC may reflect an adaptive, compensatory response within the brain, intended to maintain cortical function in the face of repeated drug stimulation, but which may also lead to cognitive impairment during withdrawal. This is consistent with prior studies involving investigator-administered cocaine (Winstanley et al. 2007). Given the excitatory effects of psychostimulants, such a compensatory response may act to dampen cortical function (Kalivas and Volkow 2005; Homayoun and Moghaddam 2006). Indeed, a role for ΔFosB induction as a means to decrease cortical activation has been suggested previously (Powell et al. 2006). In support of this hypothesis, microarray analysis revealed that both increasing ΔFosB in the OFC and repeated administration of cocaine lead to a pattern of changes in gene transcription suggestive of reduced OFC function, potentially through increased activation of local inhibitory circuits (Winstanley et al. 2007).
Although this mechanism may enable the animal to function better when cocaine is on board, it may also impair normal regulation of drug intake and contribute to the hypofunction and impulse control deficits observed during cocaine withdrawal (current study, Volkow and Fowler 2000; Rogers and Robbins 2001). Lesions to the OFC have been shown to impair rats’ ability to regulate their intake during cocaine self-administration sessions and also affect tests of impulse control in both humans and laboratory animals (Bechara et al. 1999; Mobini et al. 2002; Chudasama et al. 2003; Hutcheson and Everitt 2003; Winstanley et al. 2004). The extent to which preventing tolerance from building to the cognitive effects of cocaine would also prevent increasing drug intake, and subsequent cognitive impairment is currently unclear, but data from the current study suggest that some of the same neurobiological mechanisms may influence both processes.
Numerous studies suggest that processing of reward-related information is altered during withdrawal from drug. For example, animals will work progressively harder for drug-paired cues as the withdrawal period lengthens, a phenomenon thought to capture the incubation of craving (Lu et al. 2004). Deficits in impulse control encourage relapse, and cocaine-dependent subjects with higher levels of impulsivity drop out of treatment programs more rapidly than less impulsive individuals (Moeller et al. 2001). Treatment strategies designed to reduce impulsivity, either pharmacologically or behaviorally, may therefore benefit drug users, particularly early in the withdrawal process. In support of this suggestion, the 5-HT2A receptor antagonist M100907 has been shown to both reduce premature responding on the 5CSRT and decrease drug seeking in the extinction–reinstatement model of relapse (Fletcher et al. 2002; Higgins et al. 2003; Winstanley et al. 2003).
Although previous preclinical studies using intermittent cocaine self-administration have linked elevations in impulsivity to increased drug intake (Dalley et al. 2007), this is the first demonstration that such impulsive behavior occurs as a consequence of withdrawal from chronic daily cocaine self-administration rather than cocaine exposure per se; intermittent cocaine self-administration lead to a progressive decrease in the accuracy of performance during the first 5CSRT session of each withdrawal phase, but premature responding was unaffected (Dalley et al. 2005, 2007). One interpretation of these data is that continuous daily self-administration testing is required to produce deficits in impulse control, whereas intermittent drug exposure more profoundly affects attentional function. It would appear that repeated cycling between self-administration and 5CSRT performance generates a different phenotype than concurrent testing of both behaviors, and these testing schedules may even model different patterns of drug use observed clinically. For example, given the relatively short duration of the majority of self-administration sessions used here (2 vs. 6–8 h), concurrent testing may better model the high-performing addict who uses drug frequently, rather than those engaged in repeated bingeing and withdrawal. It is also worth noting that, in studies of this kind, the animals’ prior experience with behavioral testing could theoretically affect self-administration performance. Although this is a possibility, the patterns of self-administration observed by us and by Dalley et al. (2007) are similar to other self-administration studies in terms of both the amount of cocaine administered and the time taken to acquire self-administration behavior (e.g., Kosten et al. 2007).
It has been argued that only animals that develop escalating patterns of drug intake over prolonged periods of self-administration should be classified as “addicted” (e.g., see Ahmed and Koob 1998; Piazza et al. 2000). Due to the inherent challenges involved in testing both self-administration and 5CSRT performance within the same day, it proved possible to incorporate only a limited number of long-access self-administration sessions into the experimental design. However, this brief exposure to prolonged daily access proved sufficient to produce a more sensitized reaction in animals overexpressing ΔFosB, in terms of both their increased drug intake and elevated impulsivity during withdrawal. From this perspective, induction of ΔFosB expression within the OFC, which is particularly pronounced after cocaine self-administration (Winstanley et al. 2007), may enhance vulnerability to addiction. Because not all recreational drug use leads to addiction, individual variation in the induction of ΔFosB during drug use may facilitate the development of the addicted state, particularly because increases in ΔFosB have been observed within models of chronic stress and psychosis as well (Perrotti et al. 2004; Powell et al. 2006), conditions that can exacerbate drug use.
The increased impulsivity observed during withdrawal from cocaine was no longer evident after the first session of extinction. Extinction training weakens the association between responding on the drug-paired lever and delivery of cocaine, and, thereby, reduces relapse to drug seeking (Bouton and Schwartzberg 1991). It has been suggested that these beneficial effects of extinction training arise in part through increased expression of GluR1 alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor subunits within the nucleus accumbens (NAc) (Sutton et al. 2003; Self and Choi 2004). Chronic drug use weakens frontocortical glutamatergic input to the NAc, partly due to the internalization of AMPA receptors leading to long-term depression of corticostriatal synapses (Thomas et al. 2001) but also due to hypofunction in frontal regions (Volkow and Fowler 2000). This extinction-induced upregulation of GluR1 subunits may therefore strengthen excitatory cortical control of the NAc and contribute to a reduction in impulsive responding as well as a reduction in propensity to relapse. However, it is impossible to conclude from the current study whether the decrease in premature responding observed when animals engaged in extinction training was caused by extinction of responding on the drug-paired lever or a function of increasing the duration since the last drug exposure. Analysis of this issue requires further investigation. Whether behavior-based therapeutic strategies for human addicts based on extinction training can reduce the impulsive component of relapse in humans has not been determined.
In summary, this study builds on previous reports suggesting that hypofunction and changes in gene transcription within the OFC may alter cognitive responses to cocaine. Although these changes may minimize the acute effects of the drug, they appear to sensitize animals to escalating drug intake and enhance impulsivity during drug withdrawal, a phenotype that is thought to promote the maintenance of addiction and relapse to drug use. Our data support the hypothesis that induction of ΔFosB within the OFC is one molecular mechanism underlying these phenomena. Improving our knowledge of the effects of chronic drug intake on frontal cortex function will bring new insight into the cognitive dysfunction associated with drug addiction and facilitate the design of fundamentally novel behavioral and pharmacological treatment strategies.
Acknowledgments
This work was supported by grants awarded to EJN by the National Institute of Drug Addiction (R01 DA 07359, P01 DA 08227). Conflict of Interest: None declared.
References
- Ahmed SH, Koob GF. Transition from moderate to excessive drug intake: change in hedonic set point. Science. 1998;282:298–300. doi: 10.1126/science.282.5387.298. [DOI] [PubMed] [Google Scholar]
- Bechara A. Decision making, impulse control and loss of willpower to resist drugs: a neurocognitive perspective. Nat Neurosci. 2005;8:1458–1463. doi: 10.1038/nn1584. [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio H, Damasio AR, Lee GP. Different contributions of the human amygdala and ventromedial prefrontal cortex to decision-making. J Neurosci. 1999;19:5473–5481. doi: 10.1523/JNEUROSCI.19-13-05473.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouton ME, Schwartzberg D. Sources of relapse after extinction in pavlovian and instrumental learning. Clin Psychiatry Rev. 1991;11:123–140. [Google Scholar]
- Chudasama Y, Passetti F, Rhodes SEV, Lopian D, Desai A, Robbins TW. Dissociable aspects of performance on the 5 choice serial reaction time task following lesions of the dorsal anterior cingulate, infralimbic and orbitofrontal cortex in the rat: differential effects on selectivity, impulsivity and compulsivity. Behav Brain Res. 2003;146:105–119. doi: 10.1016/j.bbr.2003.09.020. [DOI] [PubMed] [Google Scholar]
- Colby CR, Whisler K, Steffen C, Nestler EJ, Self DW. Striatal cell type-specific overexpression of DeltaFosB enhances incentive for cocaine. J Neurosci. 2003;23:2488–2493. doi: 10.1523/JNEUROSCI.23-06-02488.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalley JW, Fryer TD, Brichard L, Robinson ES, Theobald DE, Laane K, Pena Y, Murphy ER, Shah Y, Probst K, et al. Nucleus accumbens D2/3 receptors predict trait impulsivity and cocaine reinforcement. Science. 2007;315:1267–1270. doi: 10.1126/science.1137073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalley JW, Laane K, Pena Y, Theobald DE, Everitt BJ, Robbins TW. Attentional and motivational deficits in rats withdrawn from intravenous self-administration of cocaine or heroin. Psychopharmacology (Berl) 2005;182:579–587. doi: 10.1007/s00213-005-0107-3. [DOI] [PubMed] [Google Scholar]
- Fletcher PJ, Grottick AJ, Higgins GA. Differential effects of the 5-HT(2A) receptor antagonist M100907 and the 5-HT(2C) receptor antagonist SB242084 on cocaine-induced locomotor activity, cocaine self-administration and cocaine-induced reinstatement of responding. Neuropsychopharmacology. 2002;27:576–586. doi: 10.1016/S0893-133X(02)00342-1. [DOI] [PubMed] [Google Scholar]
- Higgins GA, Enderlin M, Haman M, Fletcher PJ. The 5-HT2A receptor antagonist M100,907 attenuates motor and “impulsive-like” behaviours produced by NMDA receptor antagonism. Psychopharmacology. 2003;170:309–319. doi: 10.1007/s00213-003-1549-0. [DOI] [PubMed] [Google Scholar]
- Homayoun H, Moghaddam B. Progression of cellular adaptations in medial prefrontal and orbitofrontal cortex in response to repeated amphetamine. J Neurosci. 2006;26:8025–8039. doi: 10.1523/JNEUROSCI.0842-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hommel JD, Sears RM, Georgescu D, Simmons DL, DiLeone RJ. Local gene knockdown in the brain using viral-mediated RNA interference. Nat Med. 2003;9:1539–1544. doi: 10.1038/nm964. [DOI] [PubMed] [Google Scholar]
- Howard DB, Powers K, Wang Y, Harvey BK. Tropism and toxicity of adeno-associated viral vector serotypes 1, 2, 5, 6, 7, 8, and 9 in rat neurons and glia in vitro. Virology. 2008;372:24–34. doi: 10.1016/j.virol.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutcheson DM, Everitt BJ. The effects of selective orbitofrontal cortex lesions on the acquisition and performance of cue-controlled cocaine seeking in rats. Ann N Y Acad Sci. 2003;1003:410–411. doi: 10.1196/annals.1300.038. [DOI] [PubMed] [Google Scholar]
- Jentsch JD, Taylor JR. Impulsivity resulting from frontostriatal dysfunction in drug abuse: implications for the control of behavior by reward-related stimuli. Psychopharmacology. 1999;146:373–390. doi: 10.1007/pl00005483. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Volkow ND. The neural basis of addiction: a pathology of motivation and choice. Am J Psychiatry. 2005;162:1403–1413. doi: 10.1176/appi.ajp.162.8.1403. [DOI] [PubMed] [Google Scholar]
- Kelz MB, Chen J, Carlezon WA, Jr, Whisler K, Gilden L, Beckmann AM, Steffen C, Zhang YJ, Marotti L, Self DW, et al. Expression of the transcription factor deltaFosB in the brain controls sensitivity to cocaine. Nature. 1999;401:272–276. doi: 10.1038/45790. [DOI] [PubMed] [Google Scholar]
- Kosten TA, Zhang XY, Haile CN. Strain differences in maintenance of cocaine self-administration and their relationship to novelty activity responses. Behav Neurosci. 2007;121:380–388. doi: 10.1037/0735-7044.121.2.380. [DOI] [PubMed] [Google Scholar]
- Lu L, Grimm JW, Hope BT, Shaham Y. Incubation of cocaine craving after withdrawal: a review of preclinical data. Neuropharmacology. 2004;47(Suppl 1):214–226. doi: 10.1016/j.neuropharm.2004.06.027. [DOI] [PubMed] [Google Scholar]
- Mobini S, Body S, Ho MY, Bradshaw CM, Szabadi E, Deakin JFW, Anderson IM. Effects of lesions of the orbitofrontal cortex on sensitivity to delayed and probabilistic reinforcement. Psychopharmacology. 2002;160:290–298. doi: 10.1007/s00213-001-0983-0. [DOI] [PubMed] [Google Scholar]
- Moeller FG, Dougherty DM, Barratt ES, Schmitz JM, Swann AC, Grabowski J. The impact of impulsivity on cocaine use and retention in treatment. J Subst Abuse Treat. 2001;21:193–198. doi: 10.1016/s0740-5472(01)00202-1. [DOI] [PubMed] [Google Scholar]
- Nestler EJ. Molecular mechanisms of drug addiction. Neuropharmacology. 2004;47(Suppl 1):24–32. doi: 10.1016/j.neuropharm.2004.06.031. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic co-ordinates. Sydney (Australia): Academic Press; 1998. [Google Scholar]
- Perrotti LI, Hadeishi Y, Ulery PG, Barrot M, Monteggia L, Duman RS, Nestler EJ. Induction of DeltaFosB in reward-related brain structures after chronic stress. J Neurosci. 2004;24:10594–10602. doi: 10.1523/JNEUROSCI.2542-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piazza PV, Deroche-Gamonent V, Rouge-Pont F, Le Moal M. Vertical shifts in self-administration dose-response functions predict a drug-vulnerable phenotype predisposed to addiction. J Neurosci. 2000;20:4226–4232. doi: 10.1523/JNEUROSCI.20-11-04226.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell KJ, Binder TL, Hori S, Nakabeppu Y, Weinberger DR, Lipska BK, Robertson GS. Neonatal ventral hippocampal lesions produce an elevation of DeltaFosB-like protein(s) in the rodent neocortex. Neuropsychopharmacology. 2006;31:700–711. doi: 10.1038/sj.npp.1300883. [DOI] [PubMed] [Google Scholar]
- Rogers RD, Robbins TW. Investigating the neurocognitive deficits associated with chronic drug misuse. Curr Opin Neurobiol. 2001;11:250–257. doi: 10.1016/s0959-4388(00)00204-x. [DOI] [PubMed] [Google Scholar]
- Schoenbaum G, Roesch MR, Stalnaker TA. Orbitofrontal cortex, decision-making and drug addiction. Trends Neurosci. 2006;29:116–124. doi: 10.1016/j.tins.2005.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Self DW, Choi KH. Extinction-induced neuroplasticity attenuates stress-induced cocaine seeking: a state-dependent learning hypothesis. Stress. 2004;7:145–155. doi: 10.1080/10253890400012677. [DOI] [PubMed] [Google Scholar]
- Self DW, Genova LM, Hope BT, Barnhart WJ, Spencer JJ, Nestler EJ. Involvement of cAMP-dependent protein kinase in the nucleus accumbens in cocaine self-administration and relapse of cocaine-seeking behavior. J Neurosci. 1998;18:1848–1859. doi: 10.1523/JNEUROSCI.18-05-01848.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton MA, Schmidt EF, Choi K-H, Schad CA, Whisler K, Simmons D, Karanian DA, Monteggia L, Neve RL, Self DW. Extinction-induced upregulation in AMPA receptors reduces cocaine-seeking behaviour. Nature. 2003;421:70–75. doi: 10.1038/nature01249. [DOI] [PubMed] [Google Scholar]
- Thomas MJ, Beurrier C, Bonci A, Malenka RC. Long-term depression in the nucleus accumbens: a neural correlate of behavioral sensitization to cocaine. Nat Neurosci. 2001;4:1217–1223. doi: 10.1038/nn757. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS. Addiction, a disease of compulsion and drive: involvement of the orbitofrontal cortex. Cereb Cortex. 2000;10:318–325. doi: 10.1093/cercor/10.3.318. [DOI] [PubMed] [Google Scholar]
- Winstanley CA, Chudasama Y, Dalley JW, Theobald DE, Glennon JC, Robbins TW. Intra-prefrontal 8-OH-DPAT and M100907 improve visuospatial attention and decrease impulsivity on the five-choice serial reaction time task in rats. Psychopharmacology. 2003;167:304–314. doi: 10.1007/s00213-003-1398-x. [DOI] [PubMed] [Google Scholar]
- Winstanley CA, LaPlant Q, Theobald DEH, Green TA, Bachtell RK, Perrotti LI, DiLeone RJ, Russo SJ, Garth WJ, Self DW, et al. DeltaFosB induction in orbitofrontal cortex mediates tolerance to cocaine-induced cognitive dysfunction. J Neurosci. 2007;27:10497–10507. doi: 10.1523/JNEUROSCI.2566-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winstanley CA, Theobald DE, Cardinal RN, Robbins TW. Contrasting roles for basolateral amygdala and orbitofrontal cortex in impulsive choice. J Neurosci. 2004;24:4718–4722. doi: 10.1523/JNEUROSCI.5606-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zachariou V, Bolanos CA, Selley DE, Theobald D, Cassidy MP, Kelz MB, Shaw-Lutchman T, Berton O, Sim-Selley LJ, Dileone RJ, et al. An essential role for DeltaFosB in the nucleus accumbens in morphine action. Nat Neurosci. 2006;9:205–211. doi: 10.1038/nn1636. [DOI] [PubMed] [Google Scholar]