Abstract
The goals of this study were to investigate muscle fatigue in patients with multiple sclerosis (MS), and to determine the relationships between muscle fatigue, clinical status, and perceived fatigue. The fatigability of the anterior tibial muscle was quantitated in patients and controls during 9 min of intermittent stimulation (used to eliminate central sources of muscle fatigue). During exercise, the decline in tetanic force, phosphocreatine, and intracellular pH was greater in patients than in controls. The compound muscle action potential amplitude did not decrease during exercise, indicating that there was no failure of neuromuscular transmission during fatigue. Thus, the excessive fatigue in MS developed from sources beyond the muscle memberane. Following exercise, the recovery of tetanic force was delayed in patients (a pattern that suggests abnormal excitation-contraction coupling), whereas the recovery of metabolites was complete in both groups. Muscular fatigue was correlated with clinical disability but not with perceived fatigue. These results suggests that fatigue in MS has both central (perception, upper motor neuron dysfunction) and peripheral (impaired metabolism and excitation-contraction coupling) components.
Keywords: exercise, excitation-contraction coupling, metabolism, magnetic resonance spectroscopy
Fatigue is a common and frequently disabling symptom in patients with multiple sclerosis (MS).11 However, the mechanisms of fatigue in MS remain unclear.5,28 Central sources of fatigue might well be a factor for MS patients, and could involve at least two different mechanisms. First, recruitment of alpha motor neurons is impaired because of lesions in corticospinal pathways.33 Second, reaction times are delayed and cognitive activity is abnormal in many patients with MS, suggesting that the processes involved in preparing for and initiating motor responses are also affected.34,35
Previous studies have shown that peripheral sources of fatigue are also important in spastic muscles.24,29 Lenman et al. suggested secondary changes in spastic muscles are similar to those of immobilization and disuse.24 In another study of a small number of patients with various types of spastic paraparesis, greater changes in energy metabolites accompanied the excessive muscle fatigue observed in patients compared to controls, which suggested that intramuscular factors may contribute to fatigue in spastic paraparesis.29 Recently, in patients with MS, we have observed both reduced muscular oxidative capacity,19 and evidence of impaired activation during voluntary exercise.20 These recent results provide further evidence of intramuscular dysfunction in MS.
Therefore, the main objective of this study was to investigate muscle fatigue in MS patients, and to determine the extent to which muscle fatigue was related to clinical status and perceived fatigue. We examined fatigue in a group of MS patients and in healthy control subjects by measuring muscle force, activation, and energy metabolism during electrical stimulation of the anterior tibial muscle. By using stimulated contractions to induce muscle fatigue, central factors were eliminated and the peripheral factors associated with fatigue were quantitated. To assess neuromuscular junction and muscle membrane function during fatigue, the evoked compound muscle action potential was measured. To evaluate excitation-contraction coupling function, the rates of tension development and relaxation and the pattern of force recovery were measured.10,30 To evaluate changes in muscle metabolism during fatiguing exercise, phosphocreatine (PCr), inorganic phosphate (Pi), monovalent inorganic phosphate , and intracellular pH were measured using magnetic resonance spectroscopy (MRS).28 Finally, to determine the extent to which various components of peripheral muscle fatigue (activation, metabolism) were associated with clinical symptoms of fatigue, we measured perceived fatigue and clinical neurological dysfunction.
MATERIALS AND METHODS
Subjects
A total of 28 patients and 14 controls were studied. Each patient fulfilled clinical criteria for definite MS.32 Twenty patients had a relapsing course and 8 patients had chronic progressive disease with a mean duration of 10 years (range 2-26 years). Eight patients were ambulatory, 18 walked with a cane or a walker, and 2 were wheelchair-bound. Eighteen patients were taking medication: baclofen (7), oxybutynin (7), amantadine (6), and amitriptyline (5). Informed consent, approved by the Committee on Human Research and Experimentation at the University of California at San Francisco and the California Pacific Medical Center was obtained from patients and control subjects before enrollment in the study. Neurophysiological studies were performed at California Pacific Medical Center on the anterior tibial muscle of 28 patients with MS (21 females, 7 males; mean age 44 years, range 24-60) and 14 control subjects (6 females, 8 males; mean age 34 years, range 23-74). These studies were repeated on the legs of 4 control subjects (2 females, 2 males; mean age 41, range 32-47 years) and 13 patients (10 females, 3 males; mean age 49, range 24-60 years) in the MRS system at the Veteran’s Administration Medical Center, San Francisco.
Clinical Evaluation
Each patient was given a complete neurological examination and scored on Kurtzke’s Expanded Disability Severity Scale (EDSS), ranging from 0 (normal) to 10 (death due to MS).23 Spasticity was graded according to the Ashworth scale from 1 (normal muscle tone) to 5 (completely fixed).31 To evaluate central motor drive, the maximum number of rapid foot tapping movements achieved in a 10-s period was recorded for each subject. To assess perceived fatigue, each subject completed a 28-item fatigue questionnaire (Krupp fatigue severity scale) and a 10-cm visual analogue fatigue scale.22
Neurophysiological Measurements
All studies were performed on the anterior tibial muscle. Subjects were comfortably seated with the knee flexed and foot supported on a shoe-shaped platform and strapped in a position of 120° ± 5° plantar flexion.29 Surface electrodes (circular discs 10 mm in diameter) with conducting gel were taped to the skin over the belly and tendon of the anterior tibial muscle. A pair of stimulating electrodes was placed over the peroneal nerve just distal to the fibular head. A ground plate was taped to the skin between the recording and stimulating electrodes. An adjustable strap across the metatarsal heads held the plantar surface of the foot against the platform. A force transducer secured beneath the foot platform was used to measure force and fatigue (Gould Inc. Statham Instruments Division, Oxnard, CA). A Velcro strap across the thigh was used to restrain the knees and prevent the thigh muscles from contributing to force development during maximum voluntary contractions of the anterior tibial muscle.
Electrical Stimulation
Prior to all exercise studies, electrical stimulation of the peroneal nerve was used to evoke the compound muscle action potential (CMAP), twitch tension, and tetanic force of the tibialis anterior muscle. To assess changes in neuromuscular propagation during exercise, supramaximal stimuli 0.2 ms in duration and 30% above that necessary to produce a maximum CMAP were delivered via surface electrodes (NS6, Teca Corp., Pleasantville, NY). Three patients were given fentanyl (2.5-5.0 μg/kg, intramuscularly) to lessen the discomfort of tetanic stimuli. Two patients with excessive spasma induced by supramaximal nerve stimulation were excluded from the study.
The CMAP from the anterior tibial muscle was amplified (Teca AA6MKII; bandpass filter 1.6 Hz to 16 kHz, sweep speed of 2 or 5 ms/cm) and recorded on photographic paper. For the single evoked CMAP, amplitude was measured from the baseline to the negative peak; for the tetanus, CMAP was measured from peak to peak of the first and last response in the tetanic train. Changes in CMAP amplitude during exercise were expressed as percentage of the preexercise CMAP.
Isometric Force Measurements
Two recordings of isometric twitch tension (TT) were obtained at optimal muscle length to ensure reproducibility. The optimal muscle length was achieved by adjusting the ankle to 12° ± 5° plantar flexion, a position which evoked the maximum TT. Heel cord contractures (>100°) were not present in any patient. Supramaximal stimulation of the peroneal nerve at 50 Hz for 240 ms was applied to the resting muscle and the resultant tetanic force (TF) was recorded on photographic paper. The force of the meximum voluntary isometric contraction (MVC) was determined by asking each subject to dorsiflex the foot maximally for 3 s. The signal from the force transducer was amplified by a direct current amplifier (Teca AD6M), and fed into a calibrated analogue voltmeter to provide visual feedback to the subject while being recorded on photographic paper. Three recordings of MVC at 2-min intervals were obtained. The best of three attempts with less than 10% variation was selected as the representative MVC. To evaluate the degree of voluntary activation (“added force”), a supramaximal tetanic stimulus (50 Hz for 240 ms) was delivered at the end of the third MVC.
The TT, TF, and MVC were measured from the baseline to the the peak of the respective myogram and expressed in Newtons. The twitch contraction and half-relaxation times were measured from the onset of the twitch to the peak, and from the peak to the half-maximal force level, respectively. The meximum rate of rise of the TF was determined by direct measurement of the myogram and expressed as a percentage of TF per second. Half-relaxation time of the tetanic contraction was calculated from the time of the last stimulus to the time at which the peak tension had decayed by half. During exercise, changes in force measurements (TT, TF, rate of rise of TF) were expressed as a percentage of the preexercise values.
Metabolic Measurements
To determine the role of metabolism in fatigue in MS, the same exercise protocol was carried out in a subgroup of 13 patients and in 4 controls in the MRS system. This system (2.0 T, 30-cm bore magnet; GE CSI spectrometer, Fremont, CA) was used to quantitate changes in phosphorus energy metabolites and pH during and after exercise in 13 patients and 4 controls.28,29 Gold-plated surface electrodes (10-mm diameter; Grass Medical Instruments, Quincy, MA) were used to stimulate the peroneal nerve. A 3 × 5 cm oval surface coil was also taped to the belly of the anterior tibial muscle. The leg was securely fixed in a lexan frame with the knee slightly flexed, and the foot was fastened to an adjustable foot plate with the ankle plantar-flexed to 120° ± 5°. The leg was then inserted into the magnet. TT, TF, and MVC were recorded with a nonmagnetic force transducer (West Coast Research Corp., Los Angeles, CA) in the same manner as described above.
Phosphorus spectra were collected at 34.6 MHz with a repetition time of 0.75 s. Before exercise, a 4-min rest spectrum was collected. Subsequent spectra (1-min time resolution) were acquired continulusly during the 9 min of intermittent tetanic stimulation and 15 min of recovery. To calculate metabolic concentrations, it was assumed that the sum of PCr and Pi concentration was constant and equal to 42.2 mmol/L.29,36 The chemical shift of Pi relative to that of PCr was used to calculate intracellular pH.12
Exercise Protocoi
To examine muscle fatigue independent of central factors, supramaximal tetanic stimuli (50 Hz for 240 ms) were delivered at the peroneal nerve once every 3 s for a total of 9 min in patients and controls. During exercise, the CMAP, the ratio of last of first CMAP of the tetanic train, TF, and the rate of rise of TF were measured each minute. Immediately after the cessation of the 9-min intermittent stimulation, and at 1, 5, 10, and 15 min of recovery, CMAP, TT, TF, and rate of rise of TF were determined. The metabolic data was collected continuously throughout exercise and recovery, with a time resolution of 1 min.
Statistical Analyses
Differences between patients and controls in force and metabolites at rest were tested by using Student t-tests. ANOVA for repeated measures was used to analyze changes in TF, PCr, , Pi, and pH during 9 min of intermittent, tetanic stimulation and 15 min recovery. A Pearson’s product moment correlation was used to determine the correlations between the muscle fatigue index (percentage fall of TF at the end of 9-min stimulation) and Ashworth score, EDSS, rate of rapid foot taps, fatigue severity scale, and visual analogue scale. A Pearson’s product moment correlation was also used to determine the correlations between percentage change in TF during exercise (at 7, 8, and 9 min), recovery (at 10, 14, and 24 min), and the corresponding changes in metabolites (PCr, , Pi, and pH). A P value >0.05 was considered significant; the data were not adjusted for multiple comparisons.
RESULTS
Preexercise Studies
The means and ranges of the patients’ clinical scores were as follows: Ashworth score: 2.2 (2-4); EDSS: 5.1 (2-8), Krupp fatigue severity scale: 136 (68-177); visual analogue fatigue scale; 6.5 (1.5-10); and rapid movements in 10 s: 21.2 (0-36). There were significant correlations between the rate of rapid movements and both the Ashworth (r = 0.7, P > 0.01) and the expanded disability severity scores (r = 0.6, P > 0.01), suggesting that these are concordant clinical measures of impaired motor control. Moreover, there were significant correlations between preexercise “added force” and both the rate of rapid movements (r = 0.5, P > 0.01) and the Ashworth scores (r = 0.4, P > 0.05), suggesting that all three measures reflect the central disorder of motor unit activation.
The preexercise dynamic properties of the anterior tibial muscle are shown in Table 1. The CMAP amplitude was reduced in the patients compared to controls, which suggests a reduction in the number or size of muscle fibers in the MS patients.4 TT, TF, and MVC were also significantly reduced in patients, which is consistent with the reduced CMAP and indicates muscle weakness or reduced muscle size, or both. Relative to controls, the patients’ deficits in maximal tetanic (39% lower than controls) and voluntary (38% lower) forces were approximately the same, suggesting that much of the loss of muscular force was due to peripheral rather than central alterations. However, the “added force” was higher in patients than in controls, indicating that central activation failure in patients prior to exercise may have contributed to some of the voluntary force deficit. The normal ized rate of rise of TF was lower and the TF half-relaxation time higher in patients, suggesting slowed dynamic properties of tetanic contraction in the patients prior to exercise. Twitch contraction and half-relaxation times were similar in both groups. Prexercise PCr (38.8 ± 0.5 mmol/L for patients, 37.0 ± 0.6 mmol/L for controls) and pH (7.06 ± 0.02 for patients, 7.06 ± 0.02 for controls) were also similar in both groups.
Table 1.
Preexercise muscle properties
| Single stimulus |
Voluntary |
Tetanus |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| CMAP (mV) | TT (N) | CNT (ms) | RT1/2 (ms) | MVC (N) | Added force (%) | TF (N) | RT1/2 (ms) | Rising rate (normalized, % TF/s) | TF/TT | |
| Patients(N = 28) | 5.4 ± 0.2 | 11.1 ± 1.2 | 84.1 ± 2.7 | 104.0 ± 5.0 | 223.1 ± 17.0 | 19.2 ± 8.5 | 124.7 ± 7.6 | 234.2 ± 18.7 | 846 ± 50 | 14.0 ± 1.7 |
| Controls (N = 14) | 6.5 ± 0.3 | 15.0 ± 1.9 | 88.4 ± 1.8 | 107.1 ± 5.8 | 357.9 ± 19.7 | 0.5 | 206.1 ± 17.3 | 165.8 ± 5.1 | 10175 ± 44 | 13.8 ± 1 |
| P values | <0.01 | <0.05 | 0.13 | 0.3 | <0.01 | <O.05 | <0.01 | <0.01 | <0.05 | 0.5 |
CMAP = compound muscle action potential; TT = twitch tension; CNT = twitch contraction time; RT1/2 = half-relaxation time; MVC = maximum voluntary contraction; TF = tetanic force.
Changes in Force during Exercise
Figure 1 shows the change in mean TF during exercise in patients and controls. The data for the subgroups studied using MRS are included in Figure 1. During each of the 9 min of intermittent stimulation, TF decreased more in the patients than controls, P > 0.01. In controls, the decline in TF plateaued by the second minute of exercise, whereas in patients TF continued to decline until the end of exercise. Overall, TF declined during exercise to 64.8 ± 3.6% of initial in patients (n = 28) and to 86.1 ± 2.6% in controls (n = 14), P > 0.01. During the 15 min following exercise, the recovery of TF was slower and less complete in patients compared to controls (to 73.7 ± 3.1% vs. 95.9 ± 1.4%, P >0.01) (see Fig. 1). The change in TT during fatiguing exercise also differed between the two groups (data not shown). During exercise, potentiation of TT was greater in controls (to 164.2 ± 26.1%) than in patients (to 104.14 ± 7.7%, P > 0.01). In addition, there was a tendency for TT to recover more slowly in patients (to 80.0 ± 6.8%) than in controls (to 103.7 ± 14.4%, P = 0.18). Delayed recovery of TF and TT in the patients, as well as their reduced potentiation of TT, all suggest that impaired excitation-contraction coupling may have contributed to the development of fatigue in MS.
FIGURE 1.
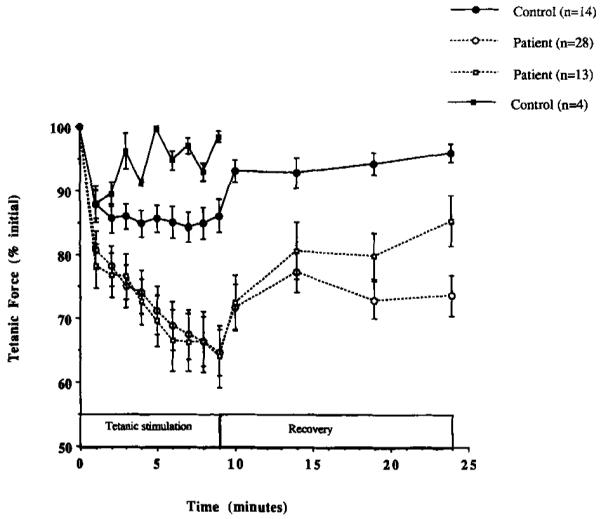
Tetanic force (TF) plotted as a function of time during 9-min intermittent, tetanic stimulation and 15-min recovery. Each datapoint represents the mean ± SE for controls (closed circle), patients (open circles), and the subgroups of controls (n = 4, closed squares) and patients (n = 13, open squares) studied using MRS. Note the significantly greater fatigue in patients compared to controls.
Before exercise, during exercise, and during recovery, the normalized rate of rise of TF was lower in patients than controls (data not shown), which suggests impaired speed of force development in MS. Before exercise the rate of rise of TF was 896.1 ± 50.0% TF/s in patients and 1017.3 ± 43.8% TF/s in controls, P > 0.05. At the end of exercise, the rate of TF was 722.8 ± 36.8 TF/s in patients and 1086.5 ± 54.3% TF/s in controls, P > 0.05; and at the end of 15 min of recovery it was 703.2 ± 42.4% TF/s in patients and 1235.0 ± 127% TF/s in controls, P > 0.05. Thus, the slowed force development observed prior to exercise in MS was further slowed during fatigue, and failed to recover following exercise. These data are consistent with imparied excitation-contraction coupling before and during fatigue in MS.
Changes in CMAP during Exercise
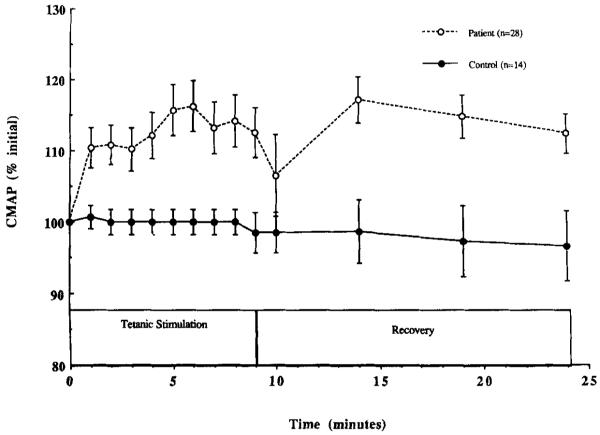
Exerciseinduced changes in the amplitude of the CMAP are shown for both groups in Figure 2. During exercise, the amplitude of the CMAP increased in patients to 112.6 ± 3.5% of initial and decreased in controls to 98.5 ± 2.8%, P = 0.06. The finding that there was not a significant fall in CMAP amplitude during exercise in either group suggests that there was no failure of transmission at the neuromuscular junction or muscle membrane during exercise. At the end of 15 min of recovery, CMAP amplitude was similar in patients (106.8 ± 2.8%) and controls (96.7 ± 55%, P = 0.60). A comparison of the ratio of the amplitudes of the first and twelfth potentials in each tetanic train disclosed enlargement (patients, 109.8 ± 2.0%; and controls, 103.7 ± 3.7% [P = 0.60]) rather than decrement (data not shown), during exercise and recovery. Thus, there was no overall decline of either the CMAP evoked by single stimuli or during a train, suggesting that fatigue cannot be attributed to a neuromuscular junction defect.
FIGURE 2.
Compound muscle action potential (CMAP) amplitude plotted as a function of time during stimulation and recovery. Each datapoint represents the mean ± SE for patients (open circles) and controls (closed circles).
There was no difference in the induced muscular fatigue (percentage fall in TF at the end of exercise) between the 7 male (to 53.1 ± 3.6%) and 7 female (to 50.1 ± 8.8%) (P = 0.80) patients matched for age and disability. Similarly, there was no difference in fatigue between the 4 male (to 83.6 ± 3.9%) and 4 female (87.4 ± 2.9%) (P = 0.50) controls. Further, there was no correlation between muscle fatigue and age for either patients (r = 0.2, P = 0.40) or control (r = 0.1, P = 0.30). Thus, there was no effect of sex or age on the magnitude of fatigue for either patients or controls.
Metabolic Changes during Exercise
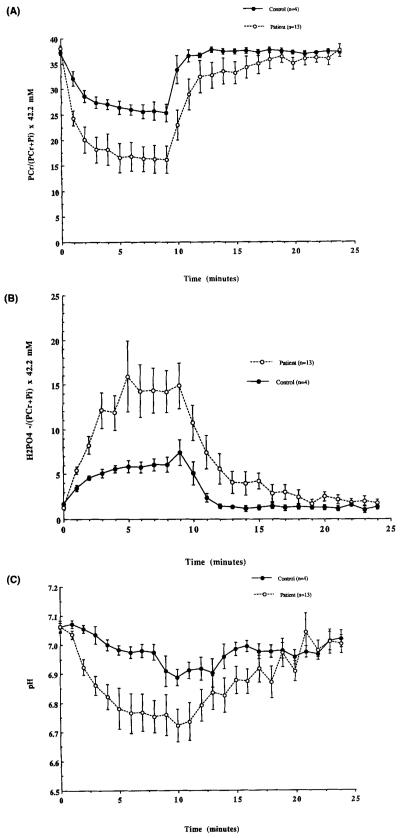
To investigate the role of metabolism in muscle fatigue in MS, measurements of energy phosphates and pH were made throughout exercise and recovery. The mean change in PCr, , and pH for the subgroup of 13 patients and 4 controls studied using 31P-MRS are shown in Figure 3A-C. As in the larger groups, the maximum decline in TF during 9 min of exercise was greater in patients than in controls (P > 0.01, Fig. 1). During exercise, PCr declined to 16.2 ± 2.7 mmol/L in patients compared with 25.3 ± 1.8 mmol/L in controls (P > 0.01) (Fig. 3A). Following exercise, PCr recovery was initially slower in the patients, but was complete in both groups after 15 min of recovery. During exercise, increased to 14.9 ± 2.6 mmol/L in patients and to 6.1 ± 0.9 mmol/L in controls (P > 0.05) (Fig. 3B). Pi also increased more in patients (to 27.9 ± 3.8 mmol/L) than in controls (to 16.4 ± 1.8 mmol/L) (p > 0.05) (same shape as Fig. 3B, data not shown). Both groups returned to normal levels after 15 min of recovery. Finally, as shown in Figure 3C, intracellular pH also decreased more in patients (to 6.76 ± 0.07) than in controls (6.91 ± 0.05) (P > 0.05). After 15 min, recovery of pH was complete in both groups. Thus, the patients demonstrated an exaggerated metabolic response to exercise compared to controls, which suggests a role for metabolism in the excessive muscle fatigue in MS.
FIGURE 3.
(A-C) Mean change of phosphocreatine (PCr) monovalent inorganic phosphate () and pH during stimulation and recovery for MS and control groups. Each datapoint represents the mean ± SE for each point. Note the significantly greater decrease in PCr and pH and the increase in in patients compared to controls.
Factors Associated with Muscle Fatigue in MS
Muscular fatigue (percentage fall in TF) was significantly correlated with two of the three preexercise clinical assessments of neurological disability. The correlations between muscle fatigue and the rate of rapid movements (r = 0.6, P > 0.01) and Ashworth score (r = 0.5, P > 0.05) were significant, while the correlation between muscle fatigue and EDSS (r = 0.3, P = 0.08) was not. The muscular fatigue index did not correlate with perceived fatigue (fatigue severity scale: r = 0.2; visual analogue fatigue scale: r = 0.1). Perceived fatigue did not correlate with clinical disability ratings. Thus, clinical assessments of disability were better associated with fatigability than were measurements of perceived fatigue.
In the patients, muscular fatigue (percentage fall in TF at the end of exercise) was significantly correlated with the changes in PCr (r = 0.6, P > 0.05), (r = 0.8, P > 0.01), Pi (r = 0.6, P > 0.05), and pH (r = 0.6, P > 0.05). During the first minutes of recovery, the changes in TF correlated with the changes in (r = 0.6, P > 0.05) and pH (r = 0.6, P > 0.05) but did not correlate to changes in PCr (r = 0.4, P = 0.50) and Pi (r = 0.30, P = 0.50). Subsequently, there was no correlation between the recovery of metabolites and TF. Thus, the data suggest a metabolic component to the muscle fatigue (resulting from 9 min of intermittent tetanic stimulation) and also in the early part of delayed recovery in MS.
DISCUSSION
The main findings of this study were: (1) there was excessive intramuscular fatigue and delayed recovery of TF in patients with MS compared to healthy controls; and (2) muscular fatigue correlated with clinical evidence of upper motor neuron dysfunction and with metabolic changes during exercise, but not with the perceived fatigue ratings of the patients. The excessive decline in TF during peripheral nerve stimulation, along with the greater metabolic changes, indicates that the source of this excessive fatigue in MS was peripheral rather than central. The significant correlation of muscular fatigue with two clinical measures of abnormal central motor control (Ashworth score and the rate of rapid foot movements) suggests that upper motor neuron lesions may be associated with secondary changes in the muscle of patients with MS. In contrast, subjective measures of perceived fatigue (fatigue severity scale questionnaire and visual analogue fatigue scale) did not correlate either with muscular fatigue or with clinical evidence of upper motor neuron dysfunction.
Preexercise Alterations in Muscle Properties
Prior to exercise, the MS group demonstrated reduced CMAP amplitude and greater weakness compared to controls (Table 1), which is consistent with previous reports.24,29,33 Because both CMAP and maximum force in part reflect muscle size, it is possible that the preexercise reductions of CMAP amplitude, MVC, and stimulated force observed in the patients may have been the result of differences in muscle size. Differences in muscle size between groups may have been due to disuse atrophy in MS or difference in sex distribution between the two groups. The fact that the patients’ relative force deficit was similar for voluntary and stimulated forces supports the hypothesis that most of the alterations in force-producing capacity were located beyond the peripheral nerve. To control for the preexercise differences between groups in CMAP amplitude and maximum force, neuromuscular junction and muscle membrane function during fatigue were evaluated by expressing the changes in CMAP amplitude and force as a percent of the preexercise values.
The patients also demonstrated slowed muscle dynamics prior to exercise compared to controls (Table 1), which is consistent with previous reports.24,29 Discue can have a significant effect on dynamic properties of mammalian muscle.3,8,9,14,16,21,26,37,38
The effects of immobilization have been studied in relation to morphological,14,37 biochemical,3 and physiological8,37 changes in animal and human muscle. Results from chronic spinal transected cats revealed muscle atrophy, reduced Tf, a shift toward faster histochemical and contractile properties, as well as increased fatigue in respose to an unfused tetanus.6 With prolonged discuse, fatigue-resistant fibers appeared to become transformed into more fatigable fibers6,9,15,18,25,27 and fibers of the soleus muscle in rats changed from slow twitch to fast twitch fibers 1 year after cordotomy.18,25 Other investigators demonstrated a transition of muscle toward faster, glycolytic metabolism in leg muscles of patients with complete spinal cord injury.15,27 Studies of immobilization of rodent limbs documented a fall in both the rate of rise of tension and rate of relaxation,16,38 as seen in our patients. The rate of tetanic tension development and the speed of relaxation are a function of the rates of crossbridge cycling and sarcoplasmic reticulum calcium pumping and the calcium saturation of parvalbumin.13 Immobilization of cat and rat muscle results in a reduced number and size of the sarcoplasmic reticulum and mitochondria, decreasd rate of ATP production, increased leakage of calcium from the sarcoplasmic reticulum, and decreased sarcoplasmic reticulum At-Pase activity.21 In summary, these findings suggest that impairment of excitation—contraction coupling occurs with disuse. The slowing of dynamic properties observed in the skeletal muscle of our MS patients suggest some impairment of calcium kinetics prior to exercise in MS. This may be an important factor in MS by contributing both to excessive muscle fatigue and delayed recovery.
Factors Associated with Muscle Fatigue
In the present study, fatigue was produced by intermittent, tetanic stimulation of the peripheral nerve, thus central fatigue was not responsible for the excessive decline in TF in MS. Furthermore, the patients demonstrated an increase in CMAP amplitude during exercise and recovery, which suggests that the origin of fatigue was not at the neuromuscular junction or the muscle membrane. In the patients, the increase of the single evoked CMAP amplitude during intermittent, tetanic stimulation may reflect increased activation of the Na-K pump in MS.17 Likewise, this would explain the patients’ increase in the ratio of the last-to-first CMAP amplitude of the tetanic train during exercise. In summary, it appears that neither central fatigue nor an impairment of the myoneural junction were primary sources of the induced muscle fatigue.
Two mechanisms appear to be important in the excessive decline in TF. First, the larger metabolite changes in patients than in controls, as previously observed in a smaller group of patients with spastic paraparesis,29 indicates that the excessive intramuscular fatigue may, in part, be due to impaired muscle metabolism. This possibility is supported by the correlation between the fatigue index and the excessive metabolic changes observed in patients.5,28
After fatiguing exercise was stopped, the metabolites recovered completely and were thus dissociated from the delayed and incomplete recovery of force. This disparity between the recovery of metabolites and TF is characteristic of the long-duration fatigue attributable to a second mechanism, impaired excitation—contraction coupling.10,30 Three further observations also suggest impaired excitation-contraction coupling. (1) During exercise, MS patients had less potentiation of twitch tension than controls, which reflects either reduced calcium released from the sarcoplasmic reticulum or decreased myosin light chain phosphorylation and light chain kinase activity at low pH.2,30 (2) The half-relaxation time of tetanic force in MS patints was longer than in controls, suggesting abnormal Ca++ pumping.7,21 (3) The rate of rise of TF was slower in MS patients than in controls at rest, during fatiguing exercise and during the delayed and incomplete recovery of TF. This slower rate of rise of TF may also indicate impaired excitation-contraction coupling1,7,13,16,38 through a reduced release of calcium from the sarcoplasmic reticulum. The fact that the normalized rising rate of TF slowed even further during exercise, and failed to recover in the 15 min following exercise, suggest that there may also be some role for impaired excitation-contraction coupling in the development of muscle fatigue in MS. Some of these altered properties of resting and exercising muscle may be due to deconditioning or disuse.
Conclusion
The present study demonstrates greater muscle fatigue and delayed force recovery in MS patients compared to controls. This fatigue was not due to central fatigue, peripheral fatigue of the motor nerve or myoneural junction, or from impaired excitability of the muscle membrane. Hence, the site of impairment during intermittent tetanic stimulation apparently lies within the muscle itself. Slowed rates of force development and greater decreases in PCr and pH were observed in MS. Therefore, we conclude that the excessive peripheral muscular fatigue observed in MS originated both from impaired excitation-contraction coupling and abnormal energy metabolism. The correlation of clinical disability ratings to intramuscular fatigue in MS suggests that individuals with upper motor neuron lesions have secondary changes in muscle that may, at least in part, be due to deconditioning.
Acknowledgments
The guidance of Professor Fritz Buchthal is gratefully acknowledged. Thanks to Regina Serrano for assistance in manuscript preparation. Support for this research was provided by the National Multiple Sclerosis Society, and NIH RO1-AG10897 (M.W.W.).
REFERENCES
- 1.Allen DG, Lee JA, Westerblad H. Intracellular calcium and tension during fatigue in isolated single muscle fibers from xenopus laevis. J. Physiol. 1989;415:433–458. doi: 10.1113/jphysiol.1989.sp017730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blumenthal DK, Stull JT. Effects of pH, ionic strength and temperature of activation by calmodulin and catalytic activity of myosin light chain kinase. Biochemistry. 1982;21:2386–2391. doi: 10.1021/bi00539a017. [DOI] [PubMed] [Google Scholar]
- 3.Booth FW, Seider MJ. Early change in skeletal muscle protein synthesis after hind limb immobilization of rats. J Appl Physiol. 1979;47:974–977. doi: 10.1152/jappl.1979.47.5.974. [DOI] [PubMed] [Google Scholar]
- 4.Brown BF. The Physiological and Technical Basis of Electromyography. Butterworths; Boston: 1984. pp. 259–264. [Google Scholar]
- 5.Cooke R, Franks K, Luciani GB, Pate E. The inhibition of rabbit skeletal muscle contraction by hydrogen ions and phosphate. J Physiol. 1988;395:77–97. doi: 10.1113/jphysiol.1988.sp016909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cope TC, Bodine SC, Fournier M, Edgerton VR. Soleus motor units in chronic spinal transected cats: physiological and morphological alterations. J Neurophysiol. 1986;55:1202–1220. doi: 10.1152/jn.1986.55.6.1202. [DOI] [PubMed] [Google Scholar]
- 7.Dawson MJ, Gadian DG, Wilkie DR. Studies of the biochemistry of contracting and relaxing muscle by the use of 31P NMR in conjunction with other techniques. Phil Trans R Soc Lond [Biol] 1980;289:445–455. doi: 10.1098/rstb.1980.0062. [DOI] [PubMed] [Google Scholar]
- 8.Duchateau J, Hainaut K. Electrical and mechanical changes in immobilized human muscle. J Appl Physiol. 1987;62:2168–2173. doi: 10.1152/jappl.1987.62.6.2168. [DOI] [PubMed] [Google Scholar]
- 9.Edstrom L. Selective atrophy of red muscle fibers in quadriceps in the long standing knee joint injuries to the anterior cruciate ligament. J Neurol Sci. 1970;11:551–558. doi: 10.1016/0022-510x(70)90105-x. [DOI] [PubMed] [Google Scholar]
- 10.Edwards RHT, Hill DK, Jones DA, Mertan PA. Fatigue of long duration in human skeletal muscle after exercise. J Physiol. 1977;272:769–777. doi: 10.1113/jphysiol.1977.sp012072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Freal JE, Kraft GH, Coryell JK. Symptomatic fatigue in multiple sclerosis. Arch Phys Med Rehabil. 1984;65:135–138. [PubMed] [Google Scholar]
- 12.Gadian DG, Radda GK, Richards RE, Seely PJ. P-31 NMR in living tissue: the road from a promising to an important tool in biology. In: Shulman RG, editor. Biochemical Applications of Magnetic Resonance. Academic Press; New York: 1979. pp. 463–535. [Google Scholar]
- 13.Gillis JM. Relaxation of vertebral skeletal muscle. A synthesis of the biochemical and physiological approaches. Biochem Biophys Acta. 1985;811:97–145. doi: 10.1016/0304-4173(85)90016-3. [DOI] [PubMed] [Google Scholar]
- 14.Goldspink G. The influence of immobilization and stretch on protein turnover of rat skeletal muscle. J Physiol. 1977;264:267–282. doi: 10.1113/jphysiol.1977.sp011667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grimby G, Broberg C, Krotiewska I, Krotkiewska M. Muscle fiber composition in patients with traumatic cord lesion. Scand J Rehabil Med. 1976;8:37–42. [PubMed] [Google Scholar]
- 16.Haida N, Fowler WM, Abresch RT, Larson DB, Sharman RB, Taylor RG, Entrikin RK. Effect of hind-limb suspension on young and adult mice skeletal muscle. Exp Neural. 1989;103:68–76. doi: 10.1016/0014-4886(89)90187-8. [DOI] [PubMed] [Google Scholar]
- 17.Hicks A, Fenton J, Garner S, McComas AJ. M wave potentiation during and after muscle activity. J Appl Physiol. 1989:2606–2610. doi: 10.1152/jappl.1989.66.6.2606. [DOI] [PubMed] [Google Scholar]
- 18.Jiang B, Roland R, Edgerten V. Expression of a fast fiber enzyme profile in the cat soleus after spinalization. Muscle Nerve. 1990;13:1037–1049. doi: 10.1002/mus.880131107. [DOI] [PubMed] [Google Scholar]
- 19.Kent-Braun JA, Sharma KR, Miller RG, Weiner MW. Postexercise phosphocreatine resynthesis is slowed in multiple sclerosis. Muscle Nerve. 1994;17:835–841. doi: 10.1002/mus.880170802. [DOI] [PubMed] [Google Scholar]
- 20.Kent-Braun JA, Sharma KR, Weiner MW, Miller RG. Effects of exercise on muscle activation and metabolism in multiple sclerosis. Muscle Nerve. 1994;17:1162–1169. doi: 10.1002/mus.880171006. [DOI] [PubMed] [Google Scholar]
- 21.Kim DH, Witzmann FA, Fitta RH. Effect of disuse on sarcoplasmic reticulum in fast and slow skeletal muscle. Am J Physiol. 1982;243:C156–C160. doi: 10.1152/ajpcell.1982.243.3.C156. [DOI] [PubMed] [Google Scholar]
- 22.Krupp LB, LaRocca NU, Muir-Nash J, Steinberg AD. The fatigue severity scale. Application to patients with multiple sclerosis and systematic lupus erythematosus. Arch Neurol. 1989;46:1121–1123. doi: 10.1001/archneur.1989.00520460115022. [DOI] [PubMed] [Google Scholar]
- 23.Kurtzke JF. Rating neurological impairment in multiple sclerosis: an expanded disability status scale (EDDS) Neurology. 1983;33:1444–1452. doi: 10.1212/wnl.33.11.1444. [DOI] [PubMed] [Google Scholar]
- 24.Lenman AJR, Tulley FM, Vrbova G, Dimitrijevic MR, Towle JA. Muscle fatigue in some neurological disorders. Muscle Nerve. 1989;12:938–941. doi: 10.1002/mus.880121111. [DOI] [PubMed] [Google Scholar]
- 25.Lieber RL. Skeletal muscle adaptability II. Muscle properties following spinal cord injury. Dev Med Child Neurol. 1986;28:533–542. doi: 10.1111/j.1469-8749.1986.tb14298.x. [DOI] [PubMed] [Google Scholar]
- 26.Maire R, Crockett JL, Simpson DR, Salibert CW, Edgerton VR. Properties of immobilized guinea pig hind limb muscles. Am J Physiol. 1976;231:1520–1526. doi: 10.1152/ajplegacy.1976.231.5.1520. [DOI] [PubMed] [Google Scholar]
- 27.Martin TP, Stein RB, Hoppner PH, Reid DC. Influence of electrical stimulation on the morphological and metabolic properties of paralyzed muscle. J Appl Physiol. 1992;72:1401–1406. doi: 10.1152/jappl.1992.72.4.1401. [DOI] [PubMed] [Google Scholar]
- 28.Miller RG, Boska MD, Moussavi RS, Carson PJ, Weiner MW. 31P nuclear magnetic resonance studies of high energy phosphates and pH in human muscle fatigue. J Clin Invest. 1988;81:1190–1998. doi: 10.1172/JCI113434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miller RG, Green AT, Moussavi RS, Carson PJ, Weiner MW. Excessive muscular fatigue in patients with spastic paraparesis. Neurology. 1990;40:1271–1274. doi: 10.1212/wnl.40.8.1271. [DOI] [PubMed] [Google Scholar]
- 30.Moussavi RS, Carson PJ, Boska MD, Weiner MW, Miller RG. Nonmetabolic fatigue in exercising human muscle. Neurology. 1989;39:1222–1226. doi: 10.1212/wnl.39.9.1222. [DOI] [PubMed] [Google Scholar]
- 31.Penn RD, Suzanne M, Savoy MNS, Corcos D, Latash M, Gottlieb G, Parke B, Kroin J. Intrathecal blacofen for severe spinal spasticity. N Engl J Med. 1989;320:1517–1521. doi: 10.1056/NEJM198906083202303. [DOI] [PubMed] [Google Scholar]
- 32.Poser CM, Paty DW, Scheinberg L, et al. New diagnostic criteria for multiple sclerosis: guidelines for research protolcols. Ann Neurol. 1983;13:227–231. doi: 10.1002/ana.410130302. [DOI] [PubMed] [Google Scholar]
- 33.Rice CL, Volmer TL, Bigland-Ritchie B. Neuromuscular responses of patients with multiple sclerosis. Muscle Nerve. 1992;15:1123–1132. doi: 10.1002/mus.880151011. [DOI] [PubMed] [Google Scholar]
- 34.Rymer WZ. Descending control of motor neuron. Pathophysiology and clinical complications. AAEE Didactic. 1989:15–21. [Google Scholar]
- 35.Sandroni P, Walker C, Starr A. `Fatigue' in patients with multiple sclerosis: motor pathway conduction and event related potentials. Arch Neurol. 1992;49:517–526. doi: 10.1001/archneur.1992.00530290105019. [DOI] [PubMed] [Google Scholar]
- 36.Taylor DJ, Styles P, Matthews PM, Arnold DA, Gadian DG, Bore P, Radda GK. Energetics of human muscle: exercise-induced ATP depletion. Magn Res Med. 1986;3:44–54. doi: 10.1002/mrm.1910030107. [DOI] [PubMed] [Google Scholar]
- 37.White MJ, Davies CT. The effects of immobilization after lower leg fracture on the contractile properties of human tricep surae. Clin Sci. 1984;66:277–282. doi: 10.1042/cs0660277. [DOI] [PubMed] [Google Scholar]
- 38.Witzmann FA, Kim DH, Fetts RH. Hind limb immobilization: length-tension and contractile properties of skeletal muscle. J Appl Physiol. 1982;53:335–345. doi: 10.1152/jappl.1982.53.2.335. [DOI] [PubMed] [Google Scholar]