Abstract
Objective
To use 1H magnetic resonance spectroscopic imaging to study differences in neuron density (N-acetylaspartate [NAA]), membrane phospholipid metabolites (choline [Cho]), and creatine-containing metabolites (creatine plus phosphocreatine [Cr]) in subjects with Alzheimer’s disease (AD), with subcortical ischemic vascular dementia (SIVD), and elderly controls.
Design
Cross-sectional, between groups.
Setting
A Veterans Affairs medical center and university memory clinic.
Participants
Forty elderly subjects with AD (n=14), with SIVD (n=8), and elderly controls (n=18).
Main Outcome Measures
We used 1H magnetic resonance spectroscopic imaging to acquire spectra from a 80 × 100 × 17-mm volume superior to the lateral ventricles. Spectra were analyzed from voxels in anterior, medial, and posterior gray and white matter using nuclear magnetic resonance-1 and the results were compared between groups using repeated measures analysis of variance (ANOVA), Tukey’s test, and individual Student’s t tests.
Results
Using ANOVA, significantly lower levels of NAA/Cho and NAA/Cr and significantly higher levels of Cho/Cr were observed across both gray and white matter voxels in subjects with AD. Using individual Student’s t tests, a significantly lower level of NAA/Cho and a higher level of Cho/Cr were observed in the posterior gray matter in subjects with AD. Using ANOVA in subjects with SIVD, significantly lower gray and white matter NAA/Cr levels were observed. Using Tukey’s test, the NAA/Cr level was significantly lower in frontal white matter voxels in subjects with SIVD compared with controls.
Conclusions
Our findings in subjects with AD suggest neuron loss in gray matter, axon loss in white matter, and altered Cho metabolism in posterior brain regions. Our findings in subjects with SIVD are consistent with higher levels of creatine-containing metabolites and/or lower levels of NAA in frontal white matter.
SUBCORTICAL ISCHEMIC vascular dementia (SIVD) and Alzheimer’s disease (AD) are conceptualized as separate disorders caused by distinct pathological processes; however, the relative roles of subcortical and cortical events in AD and SIVD are not well understood. Dementia in subjects with AD is associated with loss of larger cortical neurons in the frontal, parietal, and temporal lobes, as well as in the hippocampus.1,2 This neuron loss is accompanied by the appearance of senile plaques and neurofibrillary tangles. Studies of cerebral blood flow in patients with AD find changes in function in the parietal and temporoparietal regions.3-5 In comparison, dementia in subjects with SIVD is hypothesized to be caused by a loss or disruption of subcortical neurons leading to a disconnection of cortical neurons from subcortical structures. Subcortical ischemic vascular dementia is most commonly associated with subcortical lacunae and white matter signal hyperintensities (WSMSHs). In necropsy studies of patients with ischemic vascular disease, lacunar infarcts are most commonly found in frontal white matter adjacent to the anterior horns of the lateral ventricles, in the caudate nuclei, and in the putamen.6 These regions are believed to be vulnerable to infarction because they are supplied exclusively by small, high-resistance end arterioles. Lesions in the subcortical gray and white matter regions have been shown to cause reductions in cortical metabolism and blood flow.7-9
1H magnetic resonance spectroscopic imaging (MRSI) provides regional measures of (1) the amino acid N-acetylaspartate (NAA), a putative marker of neurons; (2) creatine (Cr) representing the sum of Cr plus phosphocreatine; and (3) choline-containing metabolites (Cho) representing primarily glycerophosphocholine and phosphocholine. We previously reported on 1H MRSI differences in eight patients with probable AD compared with 10 age-matched elderly controls.10 In that study, we found significantly lower levels of NAA/Cho and NAA/Cr in white matter in subjects with AD and significantly lower levels of NAA/Cho and significantly higher levels of Cho/Cr in posterior gray matter of subjects with AD compared with elderly controls.
The goal of our study was to extend our regional 1H MRSI findings in subjects with AD to a larger cohort and to compare these differences with findings from a group of subjects with SIVD. Our first a priori hypothesis was that gray matter and white matter NAA/Cho and NAA/Cr levels would be lower in subjects with AD compared with controls, whereas in subjects with SIVD, only differences in white matter metabolites would be observed. Our second a priori hypothesis was that metabolite differences in posterior (parietal) white matter regions would be detectable in subjects with AD compared with controls, whereas frontal white matter changes would be more prominent in subjects with SIVD. Our third a priori hypothesis was that regional or tissue metabolite differences observed in subjects with AD and subjects with SIVD would occur in proportion to degree of dementia as determined by the Mini-Mental State examination (MMSE).
SUBJECTS AND METHODS
SUBJECTS
Subjects with AD and SIVD were referred from the University of California at San Francisco Memory and Alzheimer Center. All procedures were approved by the university’s Committee on Human Research and all subjects or their guardian provided informed consent. Elderly controls were referred from the medical practices of collaborating geriatricians and recruited by posting fliers at senior citizens centers. All subjects were screened for the following: (1) major medical illnesses such as hypertension, heart disease, and diabetes; (2) major neurological illnesses, such as cortical stroke, head injury with loss of consciousness, seizure disorder, and Parkinson’s disease; (3) alcohol or drug abuse; and (4) major psychiatric illness, such as bipolar disorder or psychosis. Forty elderly subjects participated successfully in the MRSI study. They included 14 patients with AD (six men, eight women; mean age [±SD], 72±7 years; age range, 59 to 82 years), eight patients with SIVD (three men, five women; mean age [±SD], 73±9 years; age range, 51 to 80 years), and 18 elderly control subjects (11 men, seven women; mean age [±SD], 70±5 years; age range, 61 to 80 years). Data from eight of the subjects with AD and 10 of the control subjects were reported previously.10,11
All 14 subjects with AD met the National Institute of Neurological Disorders and Stroke-Alzheimer’s Disease and Related Disorders Association criteria for probable AD. Three subjects with AD were receiving thyroid replacement treatment, three had a history of hypertension, and two were receiving antihypertensive medications, although all were normotensive at the time of the procedure. One subject was taking buproprion for depression. Mean (±SD) MMSE12 score for 13 of the 14 subjects with AD was 14±9, with a range of 0 to 28 (one patient with mild probable AD had a MMSE score of 28; this patient’s diagnosis was based on a gradual, well-documented decline in psychosocial functioning). One subject with AD was too impaired to complete the test.
Eight subjects were diagnosed with SIVD using the criteria of Chui et al13 that require decline in two or more areas of intellectual performance and evidence on magnetic resonance imaging (MRI) of at least one infarct outside of the cerebellum. Four subjects with SIVD had hypertension, three had diabetes, and two had treated thyroid disease. Subjects with cortical strokes were excluded. Mean (±SD) MMSE score for the subjects with SIVD was 18.6±7, with a range of 5 to 27.
Eighteen elderly control subjects were screened as described above. One control subject was receiving thyroid replacement therapy, one had mild hypertension, and one had a prior diagnosis of lupus erythematosus that did not involve the central nervous system. All elderly controls scored 28 or more on the MMSE (mean [±SD] score, 29±0.8).
When necessary subjects were sedated with 5 to 10 mg diazepam orally or 0.5 to 2 mg lorazepam sublingually for the magnetic resonance examination. Lorazepam was the preferred agent because of its rapid onset when given sublingually and its shorter half-life. Heart rate and oxygen saturation were monitored during the magnetic resonance procedures using a pulse oximeter. Twelve of 14 subjects with AD, eight of eight subjects with SIVD, and five of 18 controls required sedation. Although the use of sedation varied among the three groups, benzodiazepines are not known to affect 1H MRSI metabolites. We previously demonstrated that 20 mg of diazepam had no effect on cerebral magnetic resonance spectroscopic metabolites radioactively labeled with phosphorus 31.14
MAGNETIC RESONANCE
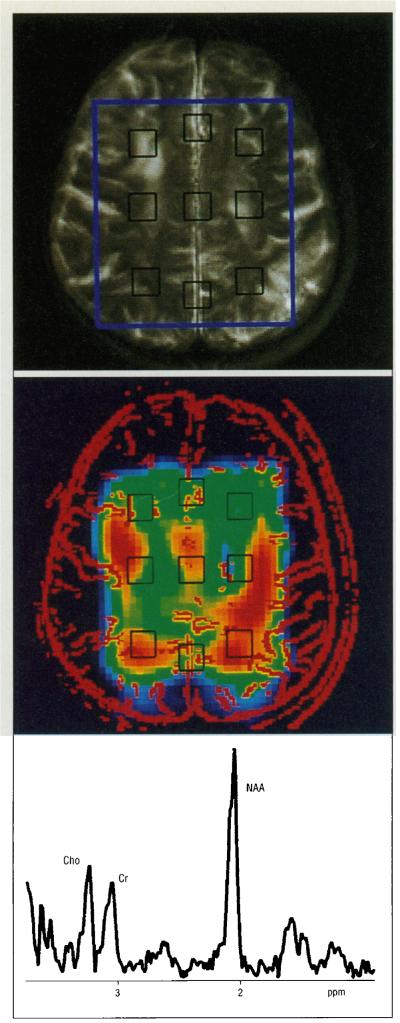
All magnetic resonance studies were performed on a whole-body 2-T MRI and magnetic resonance spectroscopic system (Philips Medical Systems, Shelton, Conn). The procedures for MRI and 1H MRSI were the same as previously described.10 Briefly, magnetic resonance slices were angulated along the canthomeatal line. Nineteen to 23 contiguous sections of 5.1-mm thickness and 0.5-mm section gap repetition time, 3000 milliseconds; echo time, 30 and 80 milliseconds were obtained to cover the entire brain from cerebellum to vertex. The magnetic resonance images were qualitatively evaluated by a board certified neuroradiologist (D.N.) blinded to each subject’s diagnosis. Ventricular dilatation and sulcal widening were each rated as follows: 0, absent; 1, mild; 2, moderate; and 3, severe. Similarly, WMSHs were rated as follows: 0, absent; 1, limited to the tips of the horns of the lateral ventricles and/or periventricular rims; 2, small focal WMSHs in subependymal or subcortical regions; and 3, large or coalescing lesions. After MRI, a 17-mm-thick volume of interest corresponding in location and thickness to the three MRI slices immediately superior to the lateral ventricles was selected for 1H MRSI. The anterior-posterior and left-right dimensions of the 1H MRSI volume of interest were adjusted for every subject according to brain size (typically about 100 by 80 mm, respectively). The position of a typical volume of interest is shown in Figure 1. The 1H MRSI parameters (field of view, 180 mm2; number of phase-encoding steps, 16×16) resulted in a nominal in-plane resolution of 11 mm, and a nominal MRSI volume element (voxel) size of approximately 2.2 mL. The total 1H MRSI acquisition time was 34 minutes. The entire MRI and 1H MRSI examination took less than 2 hours.
DATA PROCESSING
The data 1H MRSI and transverse MRI were analyzed using home-written spectroscopic imaging display software.15 The MRSI spectral dimension was zero filled to 1024 points. Both spatial dimensions were zero filled to 32 points so that 32 spectra were obtained along each of the spatial dimensions across the field of view. For display purposes only, spectroscopic images were further zero filled to 64 in each spatial dimension. A 1-Hz exponential line broadening was applied in the time domain. For both spatial domains, a mild gaussian multiplication was used corresponding to a broadening of approximately 1 mm and resulting in a final effective voxel size of approximately 2.5 mL. After Fourier transformation in spectral and spatial dimensions, two-dimensional MRSIs were created by integration over selected regions of the magnitude spectra.
For selection of the voxels to be analyzed, the spatially correlated summed MRI (composed of three thin MRI sections) was used exclusively. Spectra were extracted from nine voxels within the preselected volume of interest outlined on the MRI. The typical location and size of the analyzed voxels are indicated on the transverse summed MRI shown in Figure 1, B. Since AD primarily affects cortical gray matter, it would be desirable to select voxels largely containing superficial cortex. However, because of technical limitations, point-resolved spatially localized spectroscopy volume selection does not permit sampling of superficial cortex. To sample mesial cortex, three voxels from the midline area of the brain (one from the anterior mesial cortex, one from the posterior mesial cortex, and one from an intermediate region) were selected to include as much gray matter as possible while avoiding white matter tissue. It is recognized that these mesial voxels contain significant amounts of cerebrospinal fluid. However, since cerebrospinal fluid does not contain significant amounts of the metabolites measured by 1H MRSI, variable contribution of cerebrospinal fluid to the voxel volume should not affect metabolite ratios. Six lateral voxels were selected (two each in the anterior, medial, and posterior regions) so they contained a maximum of white matter tissue (appearing relatively dark on the T2-weighted MRI), avoiding large sulci.
The nine extracted magnitude spectra were then transferred to a workstation equipped with software (NMR1, New Methods Research Inc, Syracuse, NY) for automated line fitting and peak area determination. Following manual setting of the baseline midway through the noise, three gaussian peaks were fitted to the three major resonances in the spectra originating from Cho, from Cr, and from N-acetyl groups, predominantly NAA. The peaks were fitted with gaussian rather than lorentzian lines because they are due to multiple compounds, and spectral residuals after line fitting were smaller with gaussian line shapes. Peak areas were derived from the NMR1 software (New Methods Research Inc) in arbitrary units; no intensity standard was included in the studies. Since absolute peak areas are affected by possible long-term spectrometer instabilities and atrophy in the analyzed voxel, peak area ratios (NAA/Cho, NAA/Cr, and Cho/Cr) were used for primary data analysis.
STATISTICAL ANALYSIS
The 1H MRSI data analyses were performed by one of us (G.F.) using repeated measures analysis of variance (ANOVA) implemented in the general linear model procedure of the Statistical Analysis System software. The repeated measures were obtained from the nine spectra. Repeated measures ANOVA investigated differences between groups (ie, controls vs AD vs SIVD), between tissue types (ie, gray vs white matter), and between regions (ie, frontal vs posterior). For effects involving multiple repeated measures that were significant by ANOVA, Tukey’s test was used to compare individual metabolite ratios between groups. Although we hypothesized group by region effects (eg, differences in frontal metabolites between groups) or group by tissue-type differences (eg, differences in white matter metabolites between groups), in some analyses only between-group differences were found, meaning that, eg, metabolite differences existed between subjects with AD and controls, but they were the same in the gray matter and the white matter. These findings were reported as combined gray plus white matter differences.
Individual Student’s t tests were used when comparing posterior mesial gray matter metabolites between subjects with AD vs controls, since only two groups were compared on a single variable. The MRI findings were compared between groups using Tukey’s test. The relationship between MRSI findings and dementia scores was tested using linear regression. All values are expressed as means±SDs and a P value of less than .05 was considered statistically significant.
RESULTS
OVERALL DIFFERENCES IN GRAY AND WHITE MATTER
Our first a priori hypothesis was that, over all nine voxels, NAA/Cho and NAA/Cr levels would be lower in subjects with AD compared with controls; while in subjects with SIVD, only metabolite differences in the bilateral white matter would be observed (Table 1).
Table 1.
1H Magnetic ResonanceSpectroscopic Imaging Metabolite Ratios From WM and GM Voxels*
| Controls (n=18) |
AD (n=14)† |
SIVD (n=8) |
||||
|---|---|---|---|---|---|---|
| WM | GM | WM | GM | WM | GM | |
| NAA/Cho | 2.3±0.4 | 2.1 ±0.5 | 1.9±0.3 | 1.6±0.2 | 2.1 ±0.4 | 1.8±0.4 |
| NAA/Cr | 3.2±0.4 | 2.5±0.4 | 2.9±0.3 | 2.3±0.9 | 2.8±0.4† | 2.4±0.3† |
| Cho/Cr | 1.4±0.2 | 1.3±0.2 | 1.5±0.2 | 1.5±0.3 | 1.3±0.3 | 1.4±0.3 |
WM indicates white matter; GM, gray matter; AD, Alzheimer's disease; SIVD, subcortical ischemie vascular dementia; NAA, N-acetylaspartate; Cho, choline-containing metabolites; and Cr, sum of creatine plus phosphocreatine. Values are means±SDs.
†Significant between-group difference(WM+GM) vs controlsby repeated analysis of variance measures. In this analysis, GM and WM were combined within eachgroup.
Comparing subjects with AD with controls, there was a main effect of group indicating significantly lower levels of NAA/Cho (P=.001) and NAA/Cr (P=.02) and a significantly higher level of Cho/Cr (P=.02) in subjects with AD. We observed no group by tissue-type interaction, indicating that these differences were comparable in the gray and white matter voxels. In Table 1, these are indicated as gray matter plus white matter differences.
Comparing the SIVD group with controls, we found a main effect indicating significantly lower levels of NAA/Cr in subjects with SIVD across all nine voxels (P=.03).
An analysis of group by tissue interaction was not significant (P=.12), however, this result suggested that the difference between groups might be greater in the white matter volumes. This was supported by Tukey’s test, which found significant (P<.05) differences in levels of NAA/Cr between subjects with SIVD and controls only in the white matter.
REGIONAL WHITE MATTER EFFECTS
Our second a priori hypothesis was that metabolite differences in posterior (parietal) white matter regions would be detectable in subjects with AD compared with controls, while frontal white matter changes would be more prominent in subjects with SIVD.
Subjects With AD vs Controls
Repeated measures ANOVA of white matter metabolite ratios only revealed an effect of group that indicates lower levels of NAA/Cho (P=.005) and NAA/Cr (P=.04) in subjects with AD across all six white matter voxels, consistent with the findings described for the first hypothesis. There was no significant group by region interaction.
To further investigate the effects of individual metabolites on regional metabolite ratios in subjects with AD, we compared the relative patterns of the metabolites in frontal, medial, and posterior white matter voxels using raw signal integrals. In subjects with AD, there was a trend toward a significant NAA region by group effect (P=.07) reflecting a relative reduction in white matter NAA level in subjects with AD, primarily in lower frontal white matter. A significant between-group difference in white matter Cho level was found (P=.05), with the difference caused primarily by higher posterior Cho.
Subjects With SIVD vs Controls
A significant group by region (ie, front to back) effect was detected for NAA/Cr (P=.02), reflecting a relative reduction in the NAA/Cr level in frontal white matter voxels in subjects with SIVD vs controls (Table 2). A trend toward a group by region interaction effect was detected for Cho/Cr in subjects with SIVD vs controls (P=.07), and was a result of a lower level of Cho/Cr in frontal white matter voxels in subjects with SIVD. Taken together, these findings suggest a higher level of frontal Cr in subjects with SIVD compared with controls.
Table 2.
1H Magnetic ResonanceSpectroscopic Imaging Metabolite Ratios From Frontal and Posterior White Matter Voxels*
| Controls (n=18) |
AD (n=14) |
SIVO(n=8) |
||||
|---|---|---|---|---|---|---|
| Frontal | Posterior | Frontal | Posterior | Frontal | Posterior | |
| NAA/Cho | 1.9±0.4 | 2.6±0.4 | 1.7±0.4 | 2.1±0.4 | 1.7±0.3 | 2.6±0.6 |
| NAA/Cr | 3.3±0.6 | 3.0±0.4 | 2.9±0.3 | 2.9±0.4 | 2.5±0.5† | 2.9±0.4 |
| Cho/Cr | 1.8±0.4 | 1.2±0.2 | 1.8±0.4 | 1.4±0.4 | 1.5±0.5 | 1.2±0.3 |
*AD indicates Alzheimer's disease; SIVD, subcortical ischemie vascular dementia; NAA, ti-acetylaspartate; Cho, choline-containing metabolites; and Cr, sum of creatine plus phosphocreatine. Values are means±SDs.
Significant group by region effect vs controls by repeated analysis of variance measures.
Regional raw metabolite integrals were compared within each subject as described above. A significant group by region effect was observed for Cr (P=.04), with no significant effects detected for Cho or NAA. This suggests that the between-group differences in NAA/Cr levels are due to differences in the frontal-posterior Cr gradient. Mean regional white matter NAA/Cr results are displayed in Figure 2. A scatterplot of frontal white matter NAA/Cr levels is shown in Figure 3.
Figure 2.
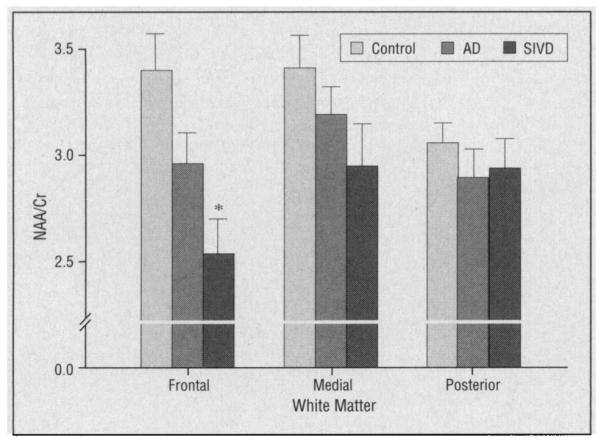
White matter N-acetylaspartate and the sum of creatine plus phosphocreatine (NAA/Cr) levels from frontal, medial, and posterior voxels of subjects with Alzheimer’s disease (AD), subjects with subcortical ischemic vascular dementia (SIVD), and controls. Values are means±SEs. Asterisk indicates P<.05 vs controls.
Figure 3.
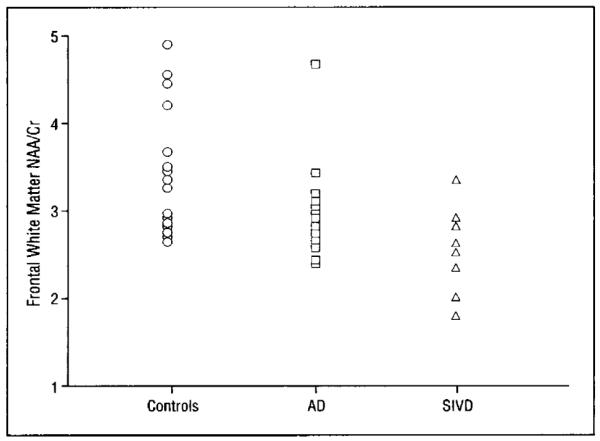
Scatterplot of individual N-acetylaspartate and sum of creatine plus phosphocreatine (NAA/Cr) values in the frontal white matter in subjects with Alzheimer’s disease (AD), subjects with subcortical ischemic vascular dementia (SIVD), and controls.
Subjects with AD vs With SIVD
A trend toward a group by region effect (P=.06) was seen for white matter NAA/Cho levels because of lower posterior whtie matter NAA/Cho levels in subjects with AD. This finding is consistent with the hypothesis that brain effects are greater in the parietal white matter in subjects with AD than in medical for frontal white matter.
POSTERIOR GRAY MATTER DIFFERENCES
A post hoc hypothesis was generated from an analysis of data from the first five subjects with AD and seven controls that was published as an abstract.11 In that report, the NAA/Cho level was significantly lower and the Cho/Cr level was significantly higher in the posterior mesial gray matter of subjects with AD vs controls. Following that analysis, an additional nine subjects with AD and 11 controls were recruited. Metabolite ratios from the posterior mesial gray matter of only these additional subjects are shown in Table 3. Significantly lower levels of NAA/Cho (P.<004) and significantly higher Cho/Cr levels (P=.004) were found i nthe posterior gray matter of subjects with AD compared with controls using Student’s t tests with Bonferroni’s correction for the two comparisons. This result confirms our earlier finding.
Table 3.
1H Magnetic ResonanceSpectroscopic Imaging Metabolite Ratios From Posterior MesialGray Matter InSubjects With AD andElderly Controls*
| Controls (n=11) | AD(n=9) | P(tTest) | |
|---|---|---|---|
| NAA/Cho | 3.6±1.0 | 2.0±0.4 | <.002 |
| NAA/Cr | 2.5±0.5 | 2.1 ±0.5 | NS |
| Cho/Cr | 0.7±0.2 | 1.1 ±0.3 | .002 |
AD indicates Alzheimer's disease: NAA, N-acetylaspartate; Cho, choline-containing metabolites: Cr, sum of creatine plus phosphocreatine; and NS, not significant. Values are means± SDs.
A similar analysis was conducted using data from our SIVD cohort. Because SIVD appears to preferentially affect frontal white matter, our hypothesis was that no significant differences in posterior mesial gray matter metabolites would be seen between subjects with SIVD and controls (Table 4). The NAA/Cho level was significantly lower in the posterior mesial gray matter voxel in subjects with SIVD vs controls (P<.05), while the Cho/Cr level was significantly higher (P<.02). Similar to the findings in subjects with AD, these results suggest a higher Cho signal in posterior gray matter in subjects with SIVD. A comparison of metabolite levels between subjects with AD and SIVD for this region found significantly lower NAA/Cho levels in subjects with AD but no differences in the ohter metabolites. Mean regional gray matter NAA/Cho levels in subjects with AD or SIVD and controls are shown in Figure 4. A scatterplot of individual values for NAA/Cho levels in the posterior mesial gray matter is shown in Figure 5.
Table 4.
1H Magnetic ResonanceSpectroscopic Imaging Metabolite Ratios From Posterior Mesial Gray Matter InSubjects With SIVD andElderly Controls*
| Controls (n=18) | SIVD (n=8) | P(t/Test) | |
|---|---|---|---|
| NAA/Cho | 3.3±1.0 | 2.5±0.6 | .05 |
| NAA/Cr | 2.5±0.5 | 2.5±0.6 | NS |
| Cho/Cr | 0.8±0.2 | 1.0±0.2 | NS |
SIVD indicates subcortical ischemie vascular dementia: NAA, H-acetylaspartate; Cho, choline-containing metabolites: Cr, sum of creatine plus phosphocreatine; and NS, not significant. Values are means±SDs.
Figure 4.
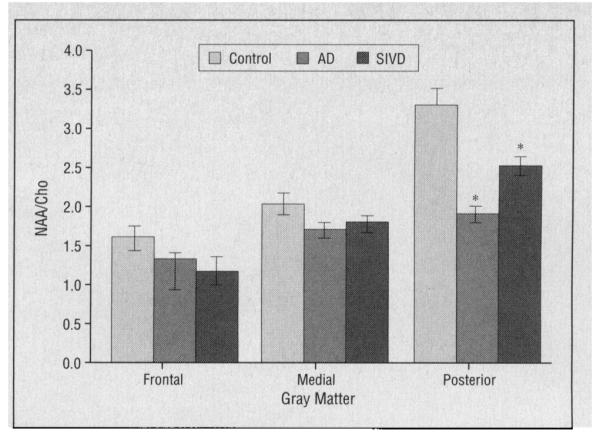
Gray matter N-acetylaspartate and choline-containing metabolite (NAA/Cho) levels from frontal, medial, and posterior voxels of subjects with Alzheimer’s disease (AD), subjects with subcortical ischemic vascular dementia (SIVD), and controls. Values are means±SEs. Asterisks indicate P<.05 vs controls.
Figure 5.
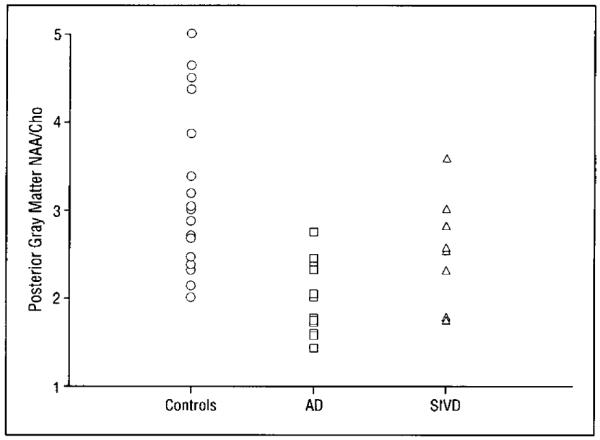
Scatterplot of individual N-acetylaspartate and choline-containing metabolite (NAA/Cho) values in the posterior mesial gray matter in subjects with Alzheimer’s disease (AD), subjects with subcortical ischemic vascular disease (SIVD), and controls.
RELATIONSHIP OF MRSI AND QUALITATIVE MRI FINDINGS
The observation of 1H MRSI abnormalities in subjects with AD or SIVD compared with controls raised the possibility that metabolite differences between groups could be related to MRI differences. Based on blinded qualitative analysis of MRI, significant differences in mean (±SD) ventricular dilatation (P<.05) were observed in subjects with AD (1.3±1.0), but not in subjects with SIVD (1.0 ±0.8) compared with controls (0.2 ±0.4) using Tukey’s test. By comparison, mean (±SD) sulcal widening was not different between subjects with AD (1.2 ±1.3) and those with SIVD (1.0±0.8) or controls (0.7±0.8). The WMSH severity was significantly greater in subjects with SIVD (2.4±0.7) compared with both subjects with AD (1.4±0.9) and controls (1.0±0.8) (P<.05 for both comparisons by Tukey’s test). No significant correlations of 1H MRSI metabolites and qualitative MRI measures were observed in subjects with AD. Comparison of frontal white matter NAA/Cr levels with qualitative MRI measures in subjects with SIVD revealed significant negative correlations with both ventricular dilatation (P=.05) and sulcal widening (P=.002), suggesting a relationship between frontal white matter NAA/Cr levels and cerebral atrophy. No relationship between regional 1H MRSI ratios and WMSH severity was observed in subjects with SIVD.
RELATIONSHIP OF MRSI AND DEMENTIA RATINGS
Our third a priori hypothesis was that regional or tissue metabolite differences observed in subjects with AD or SIVD would occur in proportion to the degree of dementia as determined by the MMSE. Analysis of the data revealed no significant correlations and no trends toward correlations of white matter or gray matter NAA/Cho, NAA/Cr, or Cho/Cr levels with MMSE scores in subjects with AD or SIVD.
COMMENT
Our primary findings were (1) lower levels of NAA/Cho and NAA/Cr and higher levels of Cho/Cr in both gray and white matter in subjects with AD and (2) lower gray and white matter NAA/Cr levels in subjects with SIVD. In subjects with AD, the differences in Cho ratios were most prominent in posterior gray matter voxels. In subjects with SIVD, differences in NAA/Cr levels were most prominent in frontal white matter voxels.
The NAA is an amino acid found exclusively in neurons and their processes and not in glia.16,17 The NAA signal appears specific for NAA in cortex, Whiel N-acetylaspartylglutamate contributes slightly to the NAA peak in white matter.18 Lower levels of NAA have been interpreted to reflect neuron loss. Recently, however, there have been reports of reversible decreases of NAA levels in multiple sclerosis19 and carbon monoxide poisoning,20 implying that neurons were not killed in these cases but rather suffered reversible injury. Our finding of lower levels of NAA/Cho and NAA/Cr in the gray and white matter of subjects with AD is consistent, therefore, with loss or injury to cortical neurons and their axons. Lower NAA ratios have been reported in the temporal lobes,21-23 temporoparietal regions,24 parietal lobes,22,25,26 and frontal lobes24,27 using in vivo proton spectroscopy in subjects with AD. Our previous report found lower levels of NAA/Cho and NAA/Cr in white matter and lower levels of NAA/Cho and higher levels of Cho/Cr in gray matter of subjects with AD compared with controls using an analysis based on individual Student’s t tests. The current findings represent an extension of those findings to a larger cohort, although this is not a replication because subjects from the earlier report were included in our analysis.
Our finding of lower NAA/Cho and higher Cho/Cr levels in posterior gray and white matter voxels in subjects with AD is consistent with alterations in levels of choline-containing metabolites that have been reported previously in postmortem studies of subjects with AD.28-31 The Cho differences in subjects with AD in the present study were most prominent in posterior gray matter. High numbers of senile plaques and neurofibrillary tangles have been observed in this region along with neurodegeneration in an autopsy study of subjects with AD.32 It has been hypothesized that “auto-cannibalism” of membrane bilayer phosphatidylcholine may occur in subjects with AD to provide Cho for production of acetylcholine.30 Phosphatidylcholine is metabolized to glycerophosphocholine (GPC) and then to phosphocholine and free choline. Both GPC and phosphocholine contribute significantly to the Cho peak in 1H MRSI and GPC contributes to the phosphodiester peak in 31P—magnetic resonance spectroscopy, while free choline and acetylcholine make much smaller contributions to the 1H MRSI Cho signal.33 Breakdown of membrane phospholipids could, therefore, cause an elevation in Cho and phosphodiester levels. Membrane bilayer phosphatidylcholine levels were reduced by 15% in a postmortem study of patients with AD.28 Increases in GPC were also reported in patients with AD.28 Enzyme kinetic studies support the idea that phosphatidylcholine catabolism rather than slowed metabolism of GPC are responsible for the higher levels of GPC in subjects with AD.31
Elevation of phosphodiester levels have been shown to correlate with the numbers of senile plaques in tissue samples taken from frontal and temporoparietal regions of subjects with AD.34 These other authors proposed that the likely sequence of events is (1) degeneration of synaptic processes; (2) elevation of membrane phospholipid breakdown products; and (3) appearance of senile plaques. Elevated Cho signal in the gray matter in subjects with dementia may, therefore, provide an in vivo marker for the apperance of senile plaques. Our findings of lower NAA/Cho and higher Cho/Cr levels in subjects with AD are consistent with an elevation of membrane phospholipid breakdown products. Because our analysis of posterior mesial gray matter metabolites excluded subjects who were reported previously,11 this result is a replication of our earlier finding.
In contrast to the findings in subjects with AD, only the NAA/Cr level was significantly lower in gray and white matter voxels in subjects with SIVD compared with controls. Regional analysis of the data suggests that the effect is strongest in the frontal white matter voxels. The frontal lobes have been shown previously to be one of the regions most affected in vascular dementia.6 The finding of a lower NAA/Cr level could be caused by a reduction in NAA or an elevation of creatine-containing metabolics. A lower level of NAA in white matter is consistent with loss or injury to axons. Lower levels of NAA/Cr in subjects with SIVD correlated with measures of both ventricular and sulcal atrophy and suggest an association with volume loss. A lower level of NAA/Cr could also be caused by higher Cr-containing metabolites in subjects with SIVD. Our analysis of the relationship between relative Cr singal in frontal, medial, and posterior white matter voxels in subjects with SIVD suggests that the slope of the anterior and posterior Cr gradient is significantly different in subjects with SIVD compared with controls. Higher levels of phosphocreatine have been reported in the frontal lobes of subjects with vascular dementia using 31P-MRSI.35 In that study, metabolite differences were detected in regions superficial to subcortical lesions such as the WMSHs. The authors speculated that this phenomenon might be due to increased glial reactivity and disconnection of cortical neurons causing accumulation of intracellular high-energy phosphates. Since creatine and phosphocreatine are in dynamic equilibrium, however, an increase in phosphocreatine would not be expected to lead an alteration in the 1H MRSI Cr signal observed in this experiment.
The severity of WMSHs was significantly greater in the SIVD group than in either the AD or control groups. The WMSHs have been shown to be associated with 1H MRSI metabolite changes in elderly controls36 and in subjects with AD or SIVD (unpublished data, 1994). Our analysis of the relationship between 1H MRSI metabolite ratios and WMSH severity in subjects with SIVD revealed no significant correlations or trends, suggesting that the observed metabolite differences are not caused by greater WMSH load in the SIVD group.
Our study has several limitations. First is the difficulty of identifying subjects with pure AD or SIVD using clinical diagnostic criteria. Without autopsy results, it is impossible to rule out the existence of degenerative changes in the SIVD cohort. Similarly, three of the subjects with AD had a history of hypertension and may have had some cerebrovascular damage, although all were normotensive at the time of study. A second limitation of our study is the failure to compare metabolite relaxation times between study groups, since the observed metabolite differences could be due solely to differences in metabolite T1- or T2-relaxation times rather than altered tissue concentrations of metabolites. Relaxation times were not measured because of the long examination time required for these studies. Another study37 found no difference in T1-relaxation times between subjects with AD and controls. A more recent study27 found a significantly longer T2-relaxation time for NAA in a mixed gray and white matter volume from the frontal lobes of subjects with AD compared with controls while the T2-relaxation times for Cho and Cr were not different between groups. A longer ANN T2-relaxation time would lead to an apparent increase in the amount of NAA in the sample. This suggests that if the T2-relaxation time for NAA was similarly increased in our AD group, the NAA signal measures presented herein would be too high, which would increase the strength of our findings.
A third limitation is that the signal for each of the voxels in our study did not come entirely from gray or white matter. Signal from subcortical white matter contributed to mesial cortical gray matter signal and signal from sulcal gray matter contributed to white matter signal. As a result, eg, it is possible that metabolite differences in the white matter could give the appearance of significant differences in the gray matter. In subjects with AD, where the effect for the NAA/Cho level is greater in white matter than in gray matter, it is possible that the observed difference in the gray matter NAA/Cho level was influenced by the white matter difference. Definitive clarification of this issue requires determination of gray and white matter contribution to each of the nine voxels of each subject in the study by tissue segmentation of the MRI. The present analysis is the focus of future work by our group.
Not withstanding these limitations, the results of these measurements indicate that 1H MRSI detects regional and tissue-specific differences in both subjects with AD and those with SIVD compared with controls. These findings support in vitro and neuropathological findings in subjects with AD and those with SIVD and provide in vivo support for theories of disease effects in these dementias.
Figure 1.
A, Transaxial T2-weighted magnetic resonance imaging (MRI) of a subject with subcortical ischemic vascular dementia showing the 1H magnetic resonance spectroscopic imaging (MRSI) field of view (red), the PRESS volume (blue), and the position of the nine voxels (black). B, 1H MRSI from the same subject, with high-pass filter of the MRI in red and of the nine voxels in black. C, Proton spectrum from the white matter of a subject with Alzheimer’s disease. Cho indicates choline-containing metabolites; Cr, creatine plus phosphocreatine; and NAA, N -acetylaspartate.
Acknowledgments
This study was supported by the Department of Veterans Affairs Biological Psychiatry Fellowship Program (Dr MacKay); a French Radiological Society Fellowship (Dr Constans); grant MHAZ5401MH45680 from the National Institute of Mental Health, Bethesda, Md (Dr Fein); grant RO1AG10897 from the National Institutes of Health, Bethesda (Dr Weiner); and the Department of Veterans Affairs Medical Research Service, San Francisco, Calif.
We thank Mary Ann Fricker, MSN, Morton Lieberman, PhD, Mariann di Minno, RN, MA, Kate Skinner, MD, and the University of California at San Francisco Memory and Alzheimer’s Center, Rex Jones, Frank Lowry, Craig Van Dyke, MD, Gerald Matson, PhD, Jay Luxenberg, MD, and William Jagust, MD.
REFERENCES
- 1.Terry RD. Structural changes in senile dementia of the Alzheimer’s type. In: Amaducci L, Davison An, Antuono P, et al., editors. Aging of the Brain and Dementia. Vol. 13. Raven Press; New York, NY: 1980. pp. 23–26. [Google Scholar]
- 2.Terry RD, Peck A, DeTeresa R, Schechter R, Horoupian DS. Some morphometric aspects of the brain in senile dementia of the Alzheimer type. Ann Neurol. 1981;10:184–192. doi: 10.1002/ana.410100209. [DOI] [PubMed] [Google Scholar]
- 3.Jagust WJ, Budinger TW, Reed BR. The diagnosis of dementia with single photon emission computed tomography. Arch Neurol. 1987;44:258–262. doi: 10.1001/archneur.1987.00520150014011. [DOI] [PubMed] [Google Scholar]
- 4.Eberling JL, Jagust WJ, Reed BR, Kwo-on-yuen PF, Martin EM. Single photon emission computed tomography studies of regional cerebral blood flow in multiple infarct dementia. J Neuroimaging. 1992;2:79–85. [Google Scholar]
- 5.Harris GJ, Links JM, Pearlson G, Camargo EE. Cortical circumferential profile of SPECT cerebral perfusion in Alzheimer’s disease. Psychiatry Res. 1991;40:167–180. doi: 10.1016/0925-4927(91)90008-e. [DOI] [PubMed] [Google Scholar]
- 6.Ishii N, Nishihara Y, Imamura T. Why do frontal lobe symptoms predominate in vascular dementia with lacunes? Neurology. 1986;36:340–345. doi: 10.1212/wnl.36.3.340. [DOI] [PubMed] [Google Scholar]
- 7.Herholz K, Heindel W, Rackl A, et al. Regional cerebral blood flow in patients with leuko-araiosis and atherosclerotic carotid artery disease. Arch Neurol. 1990;47:392–396. doi: 10.1001/archneur.1990.00530040034016. [DOI] [PubMed] [Google Scholar]
- 8.Baron JC, D’Antona R, Pantano P, Serdaru M, Samson Y, Bousser MG. Effects of thalamic stroke on energy metabolism in the cerebral cortex. Brain. 1986;109:1243–1259. doi: 10.1093/brain/109.6.1243. [DOI] [PubMed] [Google Scholar]
- 9.Szelies B, Herholtz K, Pawlik G, Karbe H, Hebold I, Heiss WD. Widespread functional effects of discrete thalamic infarction. Arch Neurol. 1991;48:178–182. doi: 10.1001/archneur.1991.00530140072019. [DOI] [PubMed] [Google Scholar]
- 10.Meyerhoff DJ, MacKay S, Constans J-M, et al. Axonal injury and membrane alterations in Alzheimer’s disease suggested by in vivo proton spectroscopic imaging. Ann Neurol. 1994;36:40–47. doi: 10.1002/ana.410360110. [DOI] [PubMed] [Google Scholar]
- 11.Meyerhoff DJ, MacKay S, Grossman N, et al. Effects of normal aging and Alzheimer’s disease on cerebral 1H metabolites. Soc Magn Reson Med. 1992;1:1931. Abstract. [Google Scholar]
- 12.Folstein MF, Folstein SE, McHugh PR. Mini-Mental State. J Psychiatr Res. 1975;12:189–193. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 13.Chui HC, Victoroff JI, Margolin D, Jagust W, Shankle R, Katzman R. Criteria for the diagnosis of ischemic vascular dementia proposed by the State of California Alzheimer’s Disease Diagnostic and Treatment Centers. Neurology. 1992;42:473–480. doi: 10.1212/wnl.42.3.473. [DOI] [PubMed] [Google Scholar]
- 14.Deicken RF, Calabrese G, Raz J, et al. A 31phosphours magnetic resonance imaging study of diazepam does not affect brain phosphorus metabolism. Biol Psychiatry. 232:628–631. doi: 10.1016/0006-3223(92)90077-d. 199. [DOI] [PubMed] [Google Scholar]
- 15.Maudsley AA, Lin E, Weiner MW. Spectroscopic imaging display and analysis. Magn Reson Imaging. 1991;10:471–485. doi: 10.1016/0730-725x(92)90520-a. [DOI] [PubMed] [Google Scholar]
- 16.Birken DL, Oldendorf WH. N-acetyl-L-aspartic acid: N-acetyl-L-aspartic acid: a literature review of a compound prominent in 1H NMR spectroscopic studies of brain. Neurosci Biobehav Rev. 1989;13:23–31. doi: 10.1016/s0149-7634(89)80048-x. [DOI] [PubMed] [Google Scholar]
- 17.Moffett JR, Aryan Namboodiri MA, Cangro CB, Neale JH. Immunohistochemical localization of N-acetylaspartate in rat brain. Neurol Rep. 1991;2:121–134. doi: 10.1097/00001756-199103000-00005. [DOI] [PubMed] [Google Scholar]
- 18.Frahm J, Michaelis T, Merboldt KD, Hanicke W, Gyngell ML, Bruhn H. On the N-acetyl methyl resonance in localized 1H NMR spectra of human brain in vivo. NMR Biomed. 1991;4:201–204. doi: 10.1002/nbm.1940040408. [DOI] [PubMed] [Google Scholar]
- 19.Davie CA, Hawkins CP, Barker GJ, et al. Serial proton magnetic resonance spectroscopy in acute multiple sclerosis lesions. Brain. 1994;117:49–58. doi: 10.1093/brain/117.1.49. [DOI] [PubMed] [Google Scholar]
- 20.Kamada K, Houkin K, Aoki T, et al. Cerebral metabolic changes in delayed carbon monoxide sequelae studied by proton MR spectroscopy. Neuroradiology. 1994;36:140–106. doi: 10.1007/BF00588070. [DOI] [PubMed] [Google Scholar]
- 21.Ide M, Naruse S, Furuya S, et al. 1H-CSI study of the Alzheimer disease. Soc Magn Res. 1994;2:599. Abstract. [Google Scholar]
- 22.Kesslak JP, Drost DJ, Naruse S, et al. Single volume proton spectroscopy in Alzheimer’s disease patients. Soc Magn Res Med. 1993;1:227. Abstract. [Google Scholar]
- 23.Jungling FD, Wakhloo AK, Stadtmüller G, Henning J. Localized 1H spectroscopy in the hippocampus of normals and patients with Alzheimer’s disease. Soc Magn Res Med. 1993;3:1555. Abstract. [Google Scholar]
- 24.Itoh S, Kimura H, Matsuda T, et al. Evaluation of neuronal changes in brain with Alzheimer’s disease using localized proton MR spectroscopy. Soc Magn Res. 1993;1:229. Abstract. [Google Scholar]
- 25.Carr CA, Guimaraes AR, Growdon JH, González RG. Combining proton MRS and MRI morphometry increases accuracy in the diagnosis of Alzheimer’s disease. Soc Magn Res Med. 1993;1:233. Abstract. [Google Scholar]
- 26.Renshaw PF, Satlin A, Johnson KA. Parietal lobe proton MRS in patients with Alzheimer’s disease. Soc Magn Res Med. 1993;1:232. Abstract. [Google Scholar]
- 27.Christiansen P, Schlosser A, Henriksen O. Reduced N-acetyl aspartate content in frontal brain of patients with Alzheimer’s disease. Soc Magn Res Med. 1993;1:226. Abstract. [Google Scholar]
- 28.Nitsch R, Pittas A, Blusztajn JK, Slack BE, Growdon JH, Wurtman RJ. Alterations of phospholipid metabolites in postmortem brain from patients with Alzheimer’s disease. Ann N Y Acad Sci. 1991;640:110–113. doi: 10.1111/j.1749-6632.1991.tb00200.x. [DOI] [PubMed] [Google Scholar]
- 29.Barany M, Chang YC, Arus C, Rustan T, Frey WH. Increased glycerol-3-phosphorylcholine in post-mortem Alzheimer’s brain. Lancet. 1985;1:517. doi: 10.1016/s0140-6736(85)92114-2. [DOI] [PubMed] [Google Scholar]
- 30.Wurtman RJ, Blusztajn JK, Maire JC. ‘Autocannibalism’ of choline-containing membrane phospholipids in the pathogenesis of Alzheimer’s disease. Neurochem Int. 1985;7:369–372. doi: 10.1016/0197-0186(85)90127-5. [DOI] [PubMed] [Google Scholar]
- 31.Nitsch RM, Blusztajn JK, Pittas AG, Slack BE, Growdon JH, Wurtman RJ. Evidence for a membrane defect in Alzheimer disease brain. Proc Natl Acad Sci U S A. 1992;89:1671–1675. doi: 10.1073/pnas.89.5.1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brun A, Englund E. A white matter disorder in dementia of the Alzheimer type. Ann Neurol. 1986;19:253–262. doi: 10.1002/ana.410190306. [DOI] [PubMed] [Google Scholar]
- 33.Miller BL. A review of chemical issues in 1H NMR spectroscopy: N-acetyl-L-aspartate, creatine and choline. NMR Biomed. 1991;4:47–52. doi: 10.1002/nbm.1940040203. [DOI] [PubMed] [Google Scholar]
- 34.Pettegrew JW, Panchalingam K, Moossy J, Martinez J, Rao G, Boller F. Correlation of phosphorus-31 magnetic resonance spectroscopy and morphological findings in Alzheimer’s disease. Arch Neurol. 1988;45:1093–1096. doi: 10.1001/archneur.1988.00520340047010. [DOI] [PubMed] [Google Scholar]
- 35.Brown GG, Garcia JH, Gdowski JW, Levine SR, Helpern JA. Altered brain energy metabolism in demented patients with multiple subcortical ischemic lesions. Arch Neurol. 1993;50:384–388. doi: 10.1001/archneur.1993.00540040046012. [DOI] [PubMed] [Google Scholar]
- 36.Sappey-Marinier D, Calabrese G, Hetherington HP, et al. Proton magnetic resonance spectroscopy of human brain. Magn Res Med. 1992;26:313–327. doi: 10.1002/mrm.1910260211. [DOI] [PubMed] [Google Scholar]
- 37.Miller BL, Moats RA, Shonk T, Ernst T, Woolley S, Ross B. Alzheimer disease. Radiology. 1993;187:433–437. doi: 10.1148/radiology.187.2.8475286. [DOI] [PubMed] [Google Scholar]