Abstract
Very-low-density lipoprotein receptor (VLDLR) is a multi ligand apolipoprotein E (apoE) receptor and is involved in brain development through Reelin signaling. Different forms of VLDLR can be generated by alternative splicing. VLDLR-I contains all exons. VLDLR-II lacks an O-linked sugar domain encoded by exon 16, while VLDLR-III lacks the third complement-type repeat in the ligand binding domain encoded by exon 4. We quantitatively compared lipoprotein binding to human VLDLR variants and analyzed their mRNA expression in both human cerebellum and mouse brain. VLDLR-III exhibited the highest capacity in binding to apoE enriched β-VLDL in vitro and was more effective in removing apoE containing lipoproteins from the circulation than other variants in vivo. In human cerebellum, the major species was VLDLR-II, while the second most abundant species was a newly identified VLDLR-IV which lacks both exon 4 and 16. VLDLR-I was present at low levels. In adult mice, exon 4 skipping varied between 30-47% in different brain regions, while exon 16 skipping ranged by 51-76%. Significantly higher levels of VLDLR proteins were found in mouse cerebellum and cerebral cortex than other regions. The deletions of exon 4 and exon 16 frequently occurred in primary neurons, indicating that newly identified variant VLDLR-IV is abundant in neurons. In contrast, VLDLR mRNA lacking exon 4 was not detectable in primary astrocytes. Such cell type-specific splicing patterns were found in both mouse cerebellum and cerebral cortex. These results suggest that a VLDLR variant lacking the third complement-type repeat is generated by neuron-specific alternative splicing. Such differential splicing may result in different lipid uptake in neurons and astrocytes.
Keywords: very-low-density-lipoprotein receptor, alternative splicing, variants, apoE
1. Introduction
The very-low-density-lipoprotein receptor (VLDLR) was first isolated as a member of the LDLR gene family that binds apolipoprotein E (apoE) containing lipoproteins. This receptor family has diverse roles beyond lipoprotein metabolism and development (Takahashi et al., 2004; Willnow et al., 2007). The phenotype of VLDLR deficiency in humans is heterogeneous. In the Hutterite population, VLDLR deficiency causes autosomal recessive cerebellar hypoplasia, a nonprogressive neurological disorder known as disequilibrium syndrome, which is characterized by moderate to profound mental retardation, delayed ambulation and ataxia (Boycott et al., 2005; Moheb et al., 2008). In humans with quadrupedal gait known as Unertan syndrome, VLDLR deficiency is associated with dysarthric speech, mental retardation, cerebrocerebellar hypoplasia as well as quadrupedal locomotion (Ozcelik et al., 2008b). However, it is debated whether VLDLR plays a key role in the transition from quadrupedal to bipedal locomotion in humans (Herz et al., 2008; Humphrey et al., 2008; Ozcelik et al., 2008a). The brain abnormality found in VLDLR deficiency is likely caused by impaired Reelin signaling. The development of the neocortex requires a coordinated migration of neurons in both radial and tangential directions to their final laminar positions. Reelin plays a critical role in the coordination of this migration via its binding to apoE receptor 2 (apoER2) and VLDLR (D'Arcangelo et al., 1999; Hiesberger et al., 1999) by providing a positional cue that determines the development of the normal cortical layering pattern (D'Arcangelo et al., 1995). The binding of Reelin to these receptors recruits the adaptor protein Dab1 through intracellular NPxY motif, which subsequently induces tyrosine phosphorylation of Dab1 and activates downstream events (Beffert et al., 2004). It has been reported that VLDLR mediates a stop signal for migrating neurons, while apoER2 plays an essential role for the migration of late generated neocortical neurons (Hack et al., 2007).
VLDLR gene is subjected to alternative splicing. The full-length VLDLR cDNA encoded by all exons is a type I VLDLR (Figure 1) (Oka et al., 1994; Sakai et al., 1994; Takahashi et al., 1992). The first variant identified in a human monocytic leukemia cell line THP-1 is a type II lacking the O-linked sugar domain that is encoded by exon 16 (VLDLR-II) (Sakai et al., 1994). The second variant lacks the third complement-type cysteine-rich repeat in the ligand binding domain (VLDLR-III) (Jokinen et al., 1994). This domain is encoded by exon 4 and has been reported to be involved in rhinovirus binding (Verdaguer et al., 2004). The VLDLR-III appears to be brain-specific (Jokinen et al., 1994). In addition, a VLDLR cDNA clone lacking exon 9 that encodes 42 amino acids in the epidermal growth factor precursor homology domain has been reported in mice (Gafvels et al., 1994). β-migrating triglyceride-rich lipoproteins (β-VLDL) are enriched by apoE and are ligands for VLDLR (Takahashi et al., 1992). The uptake of β-VLDL by VLDLR-II has been reported to be relatively low compared with VLDLR-I (Iijima et al., 1998). Rapid degradation and secretion into the culture medium has been reported for VLDLR-II (Iijima et al., 1998; Magrane et al., 1999). The VLDLR is a multi ligand receptor and binds to a variety of ligands including proteinases, proteinase-inhibitor complexes (Argraves et al., 1995; Heegaard et al., 1995), lipoprotein lipase (Argraves et al., 1995) and viruses (Marlovits et al., 1998). Although VLDLR-I and VLDLR-II bind receptor-associated protein (RAP) and serine proteinase-inhibitor complexes with similar affinity (Heegaard et al., 1995; Martensen et al., 1997), VLDLR-III displayed lower binding of RAP but similar binding of urokinase-type plasminogen activator (uPA)/plasminogen activator inhibitor-1 or uPA/protease nexin-1 (Rettenberger et al., 1999). Lipoprotein binding to VLDLR-III, however, has not been characterized.
Fig. 1.
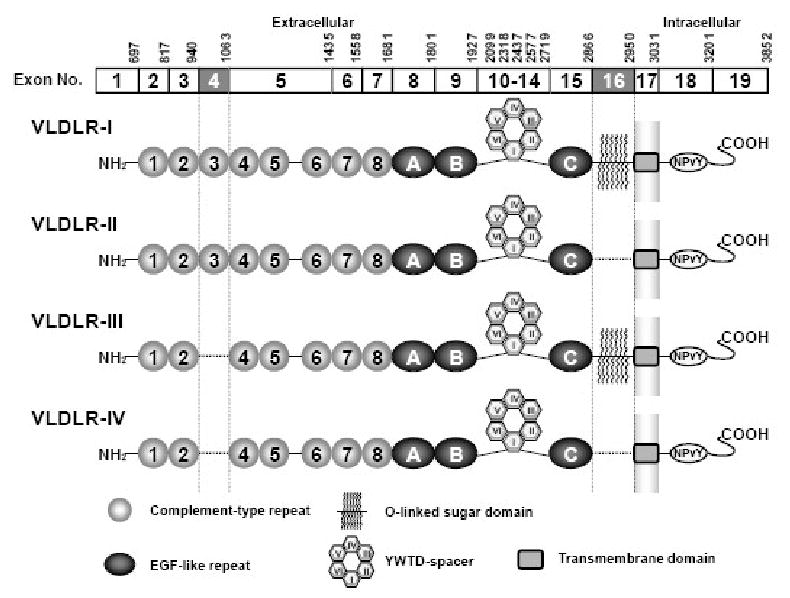
Human VLDLR variants generated by alternative splicing. Type I VLDLR (VLDLR-I) is a full length VLDLR encoded by all exons. VLDLR lacking exon 16 (VLDLR-II) or exon 4 (VLDLR-III) are generated by alternative splicing. Exon 4 skipping has been reported only in the brain. VLDLR variants lacking these exons are in frame and lack the specific functional domain. VLDLR-IV lacks both exon 4 and 16. EGF: epidermal growth factor. YWTD: Tyr-Trp-Thr-Asp β-propeller. The nucleotide number on the top of each exon indicates the last nucleotide of corresponding exon in type I VLDLR mRNA.
In this study, we analyzed the binding capacity of VLDLR variants to β-VLDL in vitro and tested their efficacy in lipoprotein uptake in mice. We found that VLDLR-III was more effective in both capacities than the other variants. These results led us to examine which variants are expressed in the human brain. We determined structures of VLDLR mRNA species in human cerebellum by RT-PCR cloning. We found that most VLDLR mRNA species in human cerebellum lacked exon 16 (VLDLR-III), but also found VLDLR mRNA species lacking exon 4 as well as exon 16 (VLDLR-IV). To determine whether alternative splicing of the VLDLR gene is brain region- or cell type-specific, we analyzed exon skipping by RNase protection assay (RPA) and protein expression by semi quantitative immunoblot analysis in mice. We prepared primary neurons and astrocytes from mouse cerebral cortex and cerebellum and analyzed exon skipping by RT-PCR. VLDLR mRNA showed both exon 4 and 16 skipping in neurons suggesting that VLDLR-II and –IV are the major species in neurons, while the majority of VLDLR mRNA species in astrocytes contained exon 4 and were therefore VLDLR-I and -II. Finally, we analyzed developmental regulation of alternative splicing of mouse VLDLR gene. Unlike in human brain, exon skipping was not highly regulated in mouse brain during development and maturity.
2. Results
2.1. VLDLR-III exhibited high capacity binding of β-VLDL
It has been reported that VLDLR-I and –II have a different binding capacity of β-VLDL (Iijima et al., 1998), but lipoprotein binding to VLDLR-III has not been examined. We constructed first generation adenoviral vectors expressing human VLDLR-I to -III (Ad-VLDLR) and examined their binding of apoE containing lipoproteins in vitro as well as in vivo. We used CHO-ldlA7 cells, which are CHO cells lacking LDLR. As LDLR is the major receptor for lipoprotein uptake in CHO cells, CHO-ldlA7 cells have only marginal lipoprotein uptake. Infection of CHO-ldlA7 cells with Ad-VLDLR variants produced VLDLR proteins of various sizes. VLDLR-II lacks 28 amino acids compared with VLDLR-I, but its size is substantially smaller than the other variants as it lacks the O-linked sugar domain (Martensen et al., 1997). VLDLR-III lacks 41 amino acids and is slightly smaller than VLDLR-I, but the difference is subtle as previously reported (Rettenberger et al., 1999) (Figure 2A). The fast migrating minor bands seen in VLDLR-I and – III infected cells are probably immature forms as previously reported (Iijima et al., 1998; Kobayashi et al., 1996). Next, we studied β-VLDL uptake using these cells. Compared to VLDLR-I, VLDLR-II showed attenuated β-VLDL uptake while VLDLR-III showed higher uptake than VLDLR-I (Figure 2B). Protein components of β-VLDL taken up by lipoprotein receptors are degraded in the endosome following endocytosis. As expected, degradation by VLDLR-III was higher than by other variants (Figure 2C). The Scatchard plot analysis indicated a single binding site with a dissociation constant of 246 ± 10 ng/ml (mean ± S.D., VLDLR-I), 228 ± 16 ng/ml (VLDLR-II) and 263 ± 4 ng/ml (VLDLR-III) and a maximal binding of 8.3 ± 0.1 ng/mg protein (VLDLR-I), 6.5 ± 0.2 ng/mg protein (VLDLR-II) and 34.5 ± 0.3 ng/mg protein (VLDLR-III).
Fig. 2.
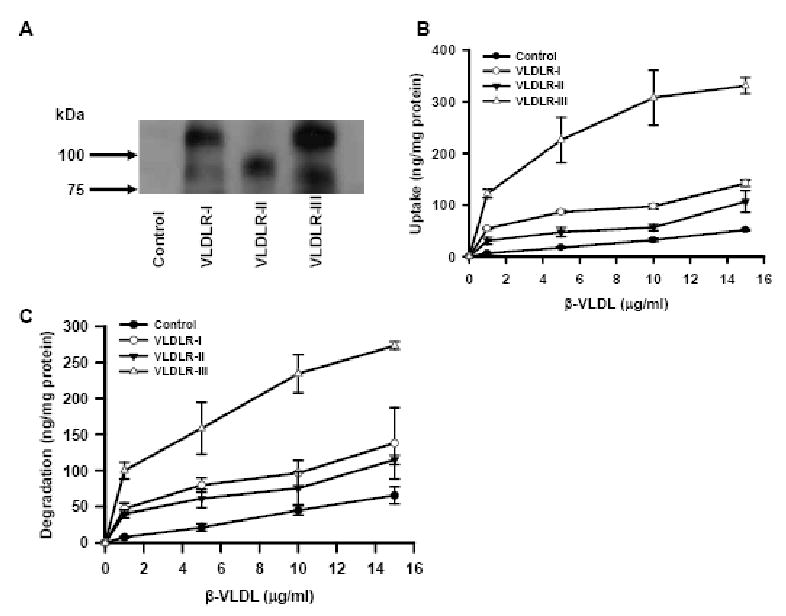
Uptake and degradation of β-VLDL by human VLDLR variants. (A) Immunoblot of VLDLR variants. Membrane fraction of CHO-ldlA7 cells infected with Ad expressing VLDLR variants were separated on a 7.5% SDS-PAGE gel and immobilized on a PVDF membrane. VLDLR protein was detected by anti-VLDLR C-terminus peptide antibody. CHO-ldlA7 cells were infected with Ad expressing VLDLR variants or control LacZ and uptake (B) or degradation (C) of β-VLDL were studied as described in Experimental Procedure. Data are expressed as mean ± SD (n=3).
2.2. VLDLR-III takes up more lipoproteins than other variants in vivo
In order to examine in vivo lipoprotein uptake by human VLDLR variants, we used LDLR-/-mice, a model of familial hypercholesterolemia. In this mouse model, plasma cholesterol is elevated due to increased LDL. The expression of VLDLR in the liver reduces plasma cholesterol by removing intermediate density lipoproteins, a precursor of LDL (Kobayashi et al., 1996). Upon intravenous injection, most Ad vectors are taken up by hepatocytes. A single injection of Ad-VLDLR variants into LDLR-/- mice led to the reduction of plasma cholesterol (Table 1). At day 4, mice treated with VLDLR-III had the lowest plasma cholesterol (Table 1). At day 9, plasma cholesterol levels in mice treated with Ad-VLDLR-II were not different from those in the control group while those in the VLDLR-I and VLDLR-III groups were still significantly lower than those in the control group. The transient reduction of cholesterol levels in LDLR-/- mice by Ad-VLDLR variants are due to host immunity against Ad transduced hepatocytes (Kozarsky et al., 1996).
Table 1.
Plasma cholesterol in Ad-VLDLR-treated LDLR-/- mice
Plasma was collected after 6 hours fasting and total plasma cholesterol was measured. Values are in mg/dl (mean ± S.D., n=6)
| Days | 0 | 4 | 9 |
|---|---|---|---|
| LacZ | 533 ±95 | 418 ±39 | 401 ± 87 |
| VLDLR-I | 503 ± 152 | 123 ±39* | 210±338* |
| VLDLR-II | 505± 116 | 155 ±47* | 338 ±70 |
| VLDLR-III | 514± 113 | 74 ±20** | 232 ±39* |
p<0.01 vs. LacZ, **p<0.001 vs. LacZ.
2.3. VLDLR variants in human cerebellum
The VLDLR deficiency in humans is associated with cerebellar ataxia. Therefore, we asked which VLDLR variants are expressed in normal adult cerebellum. We cloned VLDLR mRNA by RT-PCR and individual clones were analyzed by PCR. VLDLR-I yields a 332 bp product, whereas VLDLR lacking exon 4 yields a 209 bp product. For exon 16 deletion, the size of PCR product is 417 bp instead of 501 bp (Figure 3). The exon deletion was also verified by DNA sequence analysis. We analyzed a total of 28 clones. Of these clones, one was VLDLR-I, twenty one were VLDLR-II. To our surprise, six clones lacking exon 4 also lacked exon 16, indicating VLDLR-IV. We did not find VLDLR-III, which suggests that VLDLR-III is either absent or a minor species in the human cerebellum.
Fig. 3.
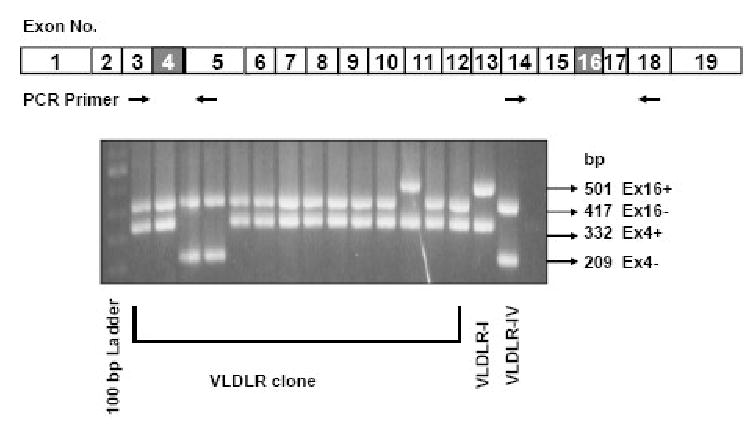
VLDLR mRNA species in human cerebellum. VLDLR cDNA was cloned by TA cloning and individual clones were picked and genotyped by PCR using primers flanking either exon 4 or 16. The size of VLDLR cDNA lacking exon 4 is 209 bp (Ex4-) while that containing exon 4 is 332 bp (Ex4+). The size of PCR products containing exon 16 is 501 bp (Ex16+) while that lacking exon 16 is 417 bp (Ex16-). VLDLR-I: VLDLR-I cDNA plasmid control; VLDLR-IV: VLDLR-IV cDNA plasmid control. The locations of PCR primers are shown for human type I VLDLR cDNA.
2.4. Analysis of VLDLR variants in adult mouse brain
The alternative splicing of VLDLR mRNA appears to be tissue-specific. Exon 4 deletion was not detectable in the heart by RPA (Figure 4A Heart, lane 1, 3, 4, 6) while the exon 16 deletion occurred at 21 ± 2% (mean ± SD, n=4) (lane 2, 3, 5, 6). This is in contrast to RNA isolated from whole brain. In adult mouse brain, exon 4 skipping occurred at 39 ± 2% (Figure 4A Brain, lane 1, 3, 4, 6) and the VLDLR mRNA lacking exon 16 was more prevalent than that containing exon 16 (73 ± 12%). Brain-specific VLDLR variant lacking exon 4 was more effective in its uptake of apoE containing lipoproteins. If lipid uptake by VLDLR plays a role in brain, this might be reflected by the specific distribution of VLDLR variants. We therefore analyzed the pattern of alternative splicing in various brain regions. Exon 4 skipping varied from 30 to 47% (Figure 4B). Thalamus had the lowest exon 4 skipping (30 ± 4%, n=4), whereas cerebral cortex had the highest exon 4 skipping (47 ± 4%), which was significantly higher than other regions (p<0.05). In contrast most VLDLR mRNA species lacked exon 16 varying 51 to 76% (Figure 4C). The lowest exon 16 skipping occurred in the pons/medulla (51 ± 4%, n=4) while the highest exon 16 skipping occurred in the cerebellum (76 ± 9%), which was significantly higher than other regions (p<0.05). We also analyzed VLDLR protein levels in various regions by semi-quantitative immunoblot. VLDLR immunoreactive proteins in the brain migrated faster than those in the heart (Figure 4D), suggesting that most brain VLDLR lacks the O-linked sugar domain. We found significantly higher levels of VLDLR proteins in cerebellum and cerebral cortex, similar levels to that found in heart. The results are consistent with their role during development in these regions.
Fig. 4.
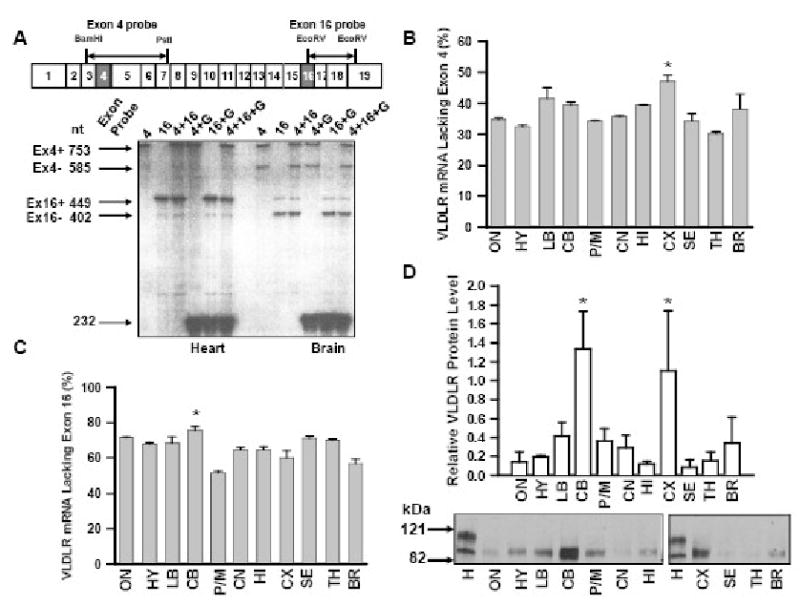
Exon skipping in VLDLR mRNA species in various mouse brain regions. (A) Representative RPA. Twenty μg of total cellular RNA isolated from the heart or the whole brain was hybridized to the RNA probes indicated in the figure. G: GAPDH RNA probe. RNA probes used for RPA are shown above the structure of mouse VLDLR cDNA. Ex4+: band containing exon 4; Ex4-: band lacking exon 4; Ex16+: band containing exon 16; Ex16-: band lacking exon 16. (B) Relative VLDLR mRNA species lacking exon 4 in various adult mouse brain regions measured by RPA. ON: olfactory nucleus; HY: hypothalamus; LB: limbic brain; CB: cerebellum; P/M: pons/medulla; CN: caudate nucleus; HI: hippocampus; CX: cerebral cortex; SE: septum; TH: thalamus; BR: brainstem. Thalamus had the lowest exon 4 skipping, whereas cerebral cortex had the highest, which was significantly higher than other regions. The data are expressed as mean relative VLDLR mRNA species lacking exon 4 to whole VLDLR mRNA in various brain regions ± SD (n=4). *p<0.05 vs. all other regions. (C) Detection of VLDLR mRNA species lacking exon 16. n=4. *p<0.05 vs. all other regions. (D) VLDLR immunoreactive protein in various brain regions. Crude membrane fractions were prepared from various brain regions and VLDLR proteins were detected by immunoblot. Mouse heart (H) was used as control. Upper panel: relative amounts of VLDLR protein. Data are expressed as mean relative to VLDLR immunoreactivity in the heart ± SD (n=4). *p<0.01 vs. all other regions. Lower panel: representative immunoblot.
2.5. Cell type specific alternative splicing in mouse brain
VLDLR deficiency in mice and humans is associated with a cerebellar hypoplasia (Boycott et al., 2005; Trommsdorff et al., 1999). Moreover, VLDLR appears to play a more important role in the development of the cerebellum while apoER2 is more important for cortical lamination (Benhayon et al., 2003; Trommsdorff et al., 1999). To determine whether this is due to the presence or lack of specific VLDLR variants, we prepared primary neuronal and astrocyte cultures from mouse cerebral cortex and cerebellum. We examined exon skipping in these cells by RT-PCR. Cortical cultures, cerebellar neurons and cerebellar astrocytes had a purity of 90%, 60% and 80%, respectively (Figure 5A). Neurons had significant exon 4 skipping and frequent exon 16 skipping (lane 2 and 4, Figure 5B). In contrast, exon 4 or exon 16 deletions were rare in astrocytes (lane 3 and 5, Figure 5B). There was no difference in the pattern of alternative splicing found between cerebellum and cerebral cortex. Therefore, different role of VLDLR in these regions may be due to other factors. We analyzed VLDLR mRNA levels in each sample by real time RT-PCR. The relative VLDLR mRNA levels to GAPDH were 0.0032 ± 0.0012 in cortical neurons (mean ± SEM), 0.0023 ± 0.0005 in cortical astrocytes, 0.0178 ± 0.0011 in cerebellar neurons, 0.0030 ± 0.0011 in cerebellar astrocytes, 0.0024 ± 0.0005 in E14.5 cortex, 0.0060 ± 0.0013 in P2 cortex and 0.0042 ± 0.0003 in P6 cerebellum. There was no significant difference in the relative amount of VLDLR mRNA between the two brain regions. Although more VLDLR mRNA appears to be present in cerebellar neurons than cortical neurons, it was inconclusive.
Fig. 5.
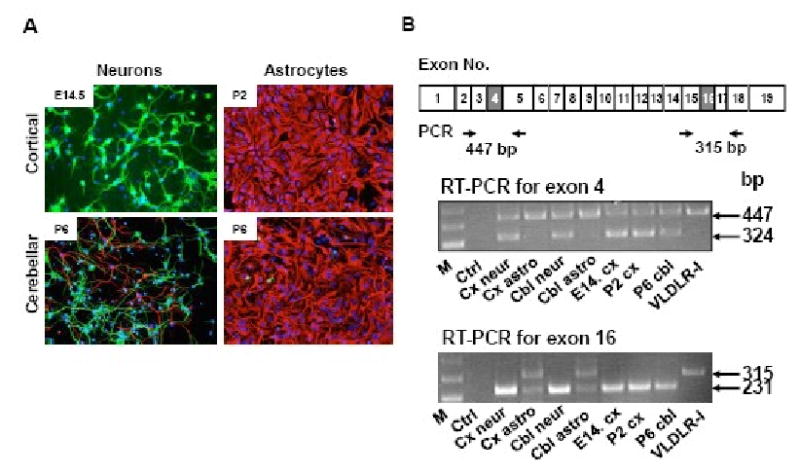
Cell type specific alternative splicing of mouse VLDLR gene. (A) Immunofluorescence of primary cultures. Neurons were isolated from E14.5 mouse cerebral cortex and P6 mouse cerebellum, while astrocytes were isolated from P2 mouse cortex and P6 mouse cerebellum. Primary cells were cultured for 7 days prior to double-staining for neurons by anti-MAP2 (green) and astrocytes by anti-GFAP (red). Blue staining is DAPI. In cortical neuronal cultures, there were few GFAP positive cells, while in cerebellar neuronal cultures there was some contamination by astrocytes. In astrocyte cultures, both cortical and cerebellar, few contaminating neurons were seen. In astrocyte cultures, a minor contaminating cell type appeared to be fibroblasts. (B) Analysis of alternative splicing. Alternative splicing was analyzed by RT-PCR using exon specific primers as described in methods. The location of PCR primers is shown below the mouse type I VLDLR cDNA. M. 1kb Plus molecular size marker (Invitrogen); Ctrl: no RNA control; Cx neur: cortical neurons; Cx astro: cortical astrocytes; Cbl neur: cerebellar neurons; Cbl astro: cerebellar astrocytes; E14 cx: embryonic day 14.5 cortex; P2 cx: postnatal day 2 cortex; P6 cbl: postnatal day 6 cerebellum, and VLDLR-I: VLDLR-I cDNA as control.
2.6. Developmental regulation of alternative splicing in mouse brain
Although exon 16 deletion has been reported to increase with aging in human brain (Deguchi et al., 2003), developmental regulation of exon 4 skipping has not been studied. We collected cerebral cortex and cerebellum at different mouse developmental stages and the exon skipping was analyzed by RT-PCR. The exon 4 skipping in cerebral cortex tends to decline with aging (Figure 6 upper panel); but this trend is not as clear as reported in human brains (Deguchi et al., 2003). In cerebellum, we analyzed postnatal exon 4 skipping and found no evidence that alternative exon 4 splicing is developmentally regulated. Most VLDLR mRNAs in cerebral cortex or cerebellum apparently lack exon 16 and we could not detect developmental regulation of exon 16 skipping by our method.
Fig. 6.
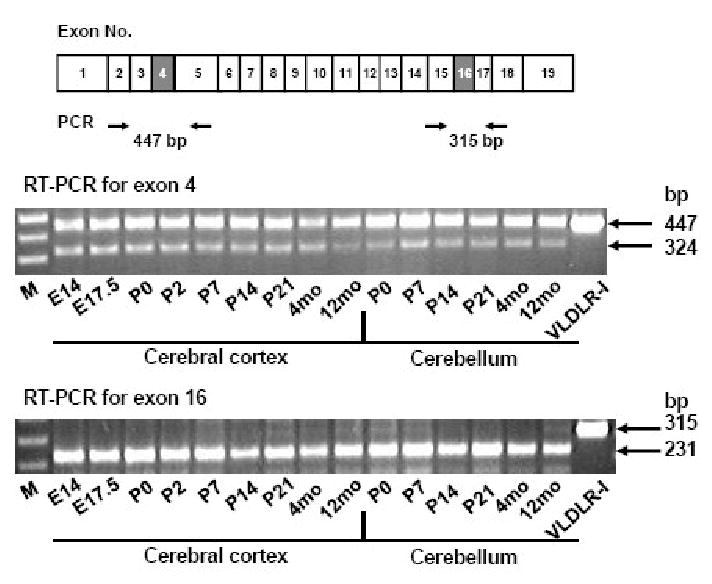
Developmental regulation of alternative splicing of mouse VLDLR gene. Mouse cerebral cortex and cerebellum were obtained at various developmental stages and the pattern of alternative splicing was analyzed by RT-PCR. The location of PCR primers is shown below the mouse type I VLDLR cDNA. M: 1kb Plus molecular size marker (Invitrogen); E14: embryonic day 14; E17.5: embryonic day 17.5; P0: postnatal day 0; P2: postnatal day 2; P7: postnatal day 7; P14: postnatal day 14; P21: postnatal day 21; 4mo: 4 months old; 12mo: 12 months old.
3. Discussion
VLDLR was initially thought to have a primary function via delivery of triglyceride-rich apoE containing lipoproteins into tissues that are active in fatty acid metabolism (Takahashi et al., 1992). However, VLDLR-/- mice displayed only a modest decrease in body mass index and adipose tissue mass in epididymal fat pads (Frykman et al., 1995), indicating a minor role of VLDLR in triglyceride metabolism. Instead, VLDLR was found to play a major role in the development of the brain via Reelin signaling (D'Arcangelo et al., 1999; Hiesberger et al., 1999). The physiological significance of the VLDLR variants in the brain is not well understood. Mouse VLDLR expression (Tiebel et al., 1999) and alternative splicing of the human VLDLR gene exon 16 (Deguchi et al., 2003) have been reported to be developmentally regulated. However, we found that alternative splicing of the VLDLR gene in developing mouse brain is not highly regulated. This is in contrast to reports for human brain in which exon 16 skipping increased with aging (Deguchi et al., 2003). The VLDLR expression patterns have been reported to be different in humans and mice (Perez-Garcia et al., 2004), therefore, it is possible that the exon 4 deletion is developmentally regulated in human brain, but not in mouse brain. VLDLR-II was found in neuroblasts, matrix cells and Cajal-Retzius cells in the early developmental stage whereas its expression was sequentially found in glioblasts, astrocytes, oligodendrocytes and myelin. In contrast, VLDLR-I was detected in primitive, classical and compact type senile plaques of normal controls and patients with Alzheimer's disease, but not in the developing human brain (Nakamura et al., 2001). Thus, regulation of alternative splicing of human VLDLR gene is apparently complex. The relationship of the structures of VLDLR variants and ligand specificity has not been fully understood. RAP and uPA/PAI-1 complexes bind to the repeats 1-4 located in the N-terminal region of the ligand binding domain (Mikhailenko et al., 1999), but apoE appears to bind to repeats 5-6 located in the C-terminal region (Ruiz et al., 2005). We found that VLDLR-III which contains 7 cystein-rich repeats in the ligand binding domain has a high binding capacity of apoE containing lipoproteins. ApoE is highly expressed in the brain and plays a pivotal role in the maintenance and repair of neurons via apoE receptor-dependent and – independent pathways (Herz and Chen, 2006; Mahley et al., 2006). Lipid-associated apoE binds to apoE receptors on neurons and modulate apoE isoform-dependent neuronal growth (Nathan et al., 1994; Nathan et al., 1995). Since exon 4 skipping occurred mainly in neurons, our results suggest that VLDLR lacking the third complement type repeat plays a role in lipid uptake for the maintenance and repairs of neurons. In support of this putative VLDLR's function in neurons, Sepp et al. have identified Drosophila Lpr2 gene, a homolog of VLDLR, as one of essential genes for neurite outgrowth using genome-wide RNAi screening (Sepp et al., 2008).
Apart from lipid uptake, a potential role of the O-linked sugar domain in ligand-mediated regulation of VLDLR activity has been proposed. VLDLR-II that lacks this domain is cleaved from the cell surface and released into the medium, whereas VLDLR-I remained attached to the cells (Iijima et al., 1998; Magrane et al., 1999). ApoE binding to VLDLR caused an increased release of the extracellular domain and the regulated proteolysis has been proposed to be the part of the regulation of VLDLR-mediated signaling (Hoe and Rebeck, 2005). It is also speculated that a soluble form of VLDLR acts as a dominant-negative receptor as shown for ApoER2 (Koch et al., 2002). It is plausible that VLDLR variants lacking an O-linked sugar domain are more sensitive to proteolysis and are therefore highly regulated by ligand binding. Interestingly, Reelin binding to VLDLR is blocked by lipid-free apoE in vitro (D'Arcangelo et al., 1999). LRP requires lipid-bound forms of apoE, whereas VLDLR-I binds lipid-bound as well as lipid-free apoE (Ruiz et al., 2005). Thus, VLDLR-mediated signaling could be regulated by binding of both lipid-associated and lipid-free apoE. Most studies on Reelin signaling have used VLDLR-I, which is a minor species in human cerebellum. It would be important to determine Reelin signaling with the VLDLR variants lacking exon 4 and/or exon 16, the major forms in cerebellum. Reelin binds to VLDLR and ApoER2, but not to LDLR (Jossin et al., 2004). The former two receptors contain 8 cystein rich repeats in the ligand binding domain while LDLR contains 7 repeats in the same functional domain. It is tempting to speculate that Reeling does not bind to VLDLR-III or VLDLR-IV as these two forms only contain 7 repeats in their ligand binding domain. While we did not determine lipid-uptake by VLDLR-IV, it is likely that VLDLR-IV has the combined properties of VLDLR-II and VLDLR-III: a high capacity apoE receptor that is sensitive to proteolytic regulation. VLDLR is a multi ligand receptor and VLDLR variants have both common and distinct ligand specificities and proteolytic regulation. Thus, neuronal VLDLR variants may have a diverse role beyond the established role for VLDLR in Reelin signaling in the central nervous system.
The association of VLDLR polymorphism with Alzheimer's disease among the Japanese population was reported in 1995 (Okuizumi et al., 1995). Recent meta-analysis revealed that this polymorphism is associated with the increased risk for late-onset Alzheimer's disease in the Asian population, while the same polymorphism is protective in the non-Asian population (Llorca et al., 2008). VLDLR could be involved by controlling synaptic function that is important for cognition, learning, memory and neuronal survival (Herz and Chen, 2006). Patients with VLDLR deficiency exhibit inferior cerebellar hypoplasia and mild cortical gyral simplification, but the hippocampi appeared to undergo normal development (Boycott et al., 2005; Ozcelik et al., 2008b). The cerebellum is a brain region that is relatively immune to Alzheimer's disease and we found high levels of VLDLR immunoreactivity in this region. Relatively high levels of VLDLR transcripts were also reported in postnatal Purkinje cells (Hack et al., 2007; Perez-Garcia et al., 2004). We asked whether specific VLDLR variants are related to the differential phenotype between the cerebral cortex and cerebellum. We did not find a difference in the pattern of exon skipping between these two regions in mouse brain. Instead, we found cell type-specific alternative splicing. Substantial exon 4 skipping was found in neurons but not in astrocytes. Moreover, exon 16 skipping occurred frequently in neurons, but less in astrocytes. This was unexpected since VLDLR-I is predominantly expressed in muscle tissues (Iijima et al., 1998). The presence of neuron-specific VLDLR variants may be related to an as yet unknown role in this cell type. Apart from its essential role in the development of the cerebellum, VLDLR has been reported to be a negative regulator of the Wnt signaling pathway through negative regulation of LRP5/6, a co-receptor for Wnt signaling (Chen et al., 2007). Genetic variation within LRP6 has been linked to late-onset Alzheimer's disease (De Ferrari et al., 2007). Furthermore, a recent report suggests that VLDLR is directly involved in amyloid β-peptide clearance across the blood brain barrier (Deane et al., 2008). Thus, it remains to be determined whether VLDLR directly or indirectly contributes to Alzheimer's disease.
In summary, our findings suggest that the alternative splicing of exon 4 in the VLDLR gene is neuron-specific and is able to generate a variant with a high binding capacity to apoE containing lipoproteins. The presence of cell type-specific variants may require consideration in future studies of VLDLR in brain.
4. Experimental Procedures
4.1. Recombinant adenoviral vector
The cDNAs for the full-length human VLDLR (type I), VLDLR lacking exon 16 (type II) or exon 4 (type III) as described previously (Rettenberger et al., 1999). VLDLR cDNAs were subcloned into the BglII/ClaI sites of pAvCvSv and first generation Ads were generated as described (Kobayashi et al., 1996).
4.2. 125I Labeling of β-VLDL
β-VLDL was isolated by ultracentrifugation from the plasma of adult female New Zealand White rabbits that were fed a diet of normal chow supplemented with 1% cholesterol (Roth et al., 1983). Blood was collected in 1% EDTA and β-VLDL was isolated by KBr density ultracentrifugation at 1.210 g/ml. The enrichment of apoE in the β-VLDL was verified by SDS-PAGE. 125I-β-VLDL was prepared by the iodine monochloride method (Bilheimer et al., 1972). The final specific activity was 450-650 cpm/ng.
4.3. Expression of VLDLR variants in CHO-ldlA7 cells
LDLR deficient CHO-ldlA7 cells were maintained in Ham's F12 containing 5% FBS. Cells were seeded in a 12-well plate. For infection, cells were washed once with serum-free medium and infection with Ad was carried out in Ham's F12 containing 2% FBS at 2,000 viral particles/cell. After 30 min infection, complete medium was added and cells were incubated for 2 days.
4.4. Uptake, binding and degradation of β-VLDL
24 hours prior to the experiment, medium was changed to Ham's F12 with 5 % lipoprotein deficient serum. On the day of experiment, cells were washed with serum-free medium and incubated with 125I β-VLDL for 5 hours. Uptake and degradation of 125I β-VLDL were measured at 37°C by the method of Goldstein et al. (Goldstein et al., 1983). For binding, cells were incubated with the same medium at 4°C for 15 min, and then replaced with the medium containing various amounts of 125I β-VLDL. After incubation for 5 hours at 4°C, cells were washed, solubilized by adding 0.1N NaOH and cell associated radioactivity was measured. Cellular protein was determined by modified Lowry method in all binding experiments and all experiments were performed in triplicate.
4.5. Animals
Female LDLR-/- or wild type mice on a C57BL/6 background were purchased from the Jackson Laboratory and maintained on a high cholesterol diet (Kobayashi et al., 1996). After 6 weeks on high cholesterol diet, mice were treated with a single intravenous injection of Ad expressing VLDLR variants (5×1012 viral particles/kg). Ad-LacZ was used as controls. FPLC analyses and plasma lipid were determined as previously described (Kobayashi et al., 1996).
4.6. Analysis of VLDLR variants in human cerebellum
Total cellular RNA from adult human cerebellum (Clontech) was reverse transcribed using random primers and the cDNA was amplified by PCR using human VLDLR specific primers: 5′ – GGAGATCCTGACTGCGAAG – 3′ (5′-upstream primer) and 5′ – GCTTTTCATGTTCTTGTGTTG – 3′ (3′-downstream primer). The PCR products were purified on a 1 % agarose gel and subcloned by TA cloning (Invitrogen). Plasmid DNA was prepared from individual clones and genotyped by PCR. The following primers were used: for exon 4 deletion, 5′ upstream primer and 5′ – GGTGCTGCACTGGAACTCATG – 3′; for exon 16 deletion, 3′ downstream primer and 5′ – GGGAAAATGAAGCAGTCTATG – 3′.
4.7. Analysis of VLDLR mRNA in mouse brain
6-8 week old C57BL/6 mice were purchased from the Jackson Laboratory and the brains were dissected on a glass plate over ice into 11 regions by the method of Carlsson and Lindqvist (Carlsson and Lindqvist, 1973) with modifications (Oka et al., 1984). Total cellular RNA was prepared using Trizol Reagent (Invitrogen) and RPA was performed as described previously (Tiebel et al., 1999). For detection of exon 4 skipping, the 0.75 kb Bam HI/Pst I of mouse VLDLR cDNA was subcloned into a KS vector (Stratagene) and the 0.45 kb EcoRV fragment was used to detect exon 16 skipping (Oka et al., 1994). Radioactive bands were quantified by Cyclone (PerkinElmer) and the results were normalized to GAPDH.
4.8. Immuno blot analysis
Crude cell membranes were prepared as described by Simonsen et al. (Simonsen et al., 1994). 5 μg of crude membrane protein was separated on a 7.5% SDS-PAGE and transferred to a PVDF membrane. VLDLR protein was detected by rabbit anti-mouse VLDLR C-terminal peptide antibody (1:500 dilution) (Kobayashi et al., 1996). The corresponding band was quantified by densitometric analysis (Epi Chemi II Darkroom, UVP laboratory) and expressed as relative intensity to the signal from crude membrane proteins of mouse heart.
4.9. Analysis of alternative splicing in primary culture of mouse neurons and astrocytes
Primary neurons were isolated from embryonic day 14.5 (E14.5) mouse (ICR) cortex and from postnatal day 6 (P6) cerebellum using a papain dissociation kit according to the manufacturer's directions (Worthington). Astrocytes were isolated using trypsin dissociation from P2 cortex and P6 cerebellum as described (Marriott et al., 1995), with the exception of cells being grown in D/ L-valine DMEM. Cells were seeded on poly-l-lysine coated 10 cm Petri dishes (for RT-PCR) or on 12 mm diameter glass coverslips (for immunofluorescence). Neurons were cultured in Neurobasal media (Invitrogen) supplemented with B27, while astrocytes were cultured in DMEM (high glucose, Invitrogen) with 10% FBS. Cells were kept for 7 days before being collected for RT-PCR or fixed in 4% paraformaldehyde for immunofluorescence. The purity of the cultures was assessed by immunofluorescence using mouse monoclonal anti-microtubule associated protein 2 (MAP2, 1:2000, Sternberger Monoclonals) and rabbit polyclonal anti-glial fibrillary protein (GFAP, 1:500, DAKO) and Alexa488 or Alexa594 labeled secondary antibodies (1:1000). Total cellular RNA was purified by a kit (5PRIME) and reverse transcribed using random primers. Exon skipping was analyzed by PCR using the specific primers; for exon 4 skipping, 5′ – ATGGCAGCGACGAGAAGAAC – 3′ and 5′ – TGAGGGGAATGCAGGAAGAG – 3′; and for exon 16 skipping, 5′ – AATATCTCTGCCTGCCAGCACC – 3′ and 5′ – TCCTCCACATCAAGTAGCCACC – 3′. For SYBR Green real time RT-PCR, we used the following primers: VLDLR, 5′ – AGTGACGAGCCCCTGAAGGA – 3′ and 5′ – TGACTGCAGATCCCCGGGTT – 3′; GAPDH, 5′- ATT GTT GCC ATC AAC GAC CC – 3′ and 5′ – CCA CGA CAT ACT CAG CAC C – 3′. Mx30000P qPCR machine (Stratagene) was used.
4.10. Developmental regulation of alternative splicing of mouse VLDLR gene
Cerebral cortex and cerebellum were dissected from C57BL/6 mice at various developmental stages [cortex: embryonic day (E) 14, E17.5, postnatal day (P) 0, P2, P7, P14, P21, 4 and 12 months; cerebellum: P0, P2, P7, P14, P21, 4 and 12 months). Cellular RNA was extracted and exon skipping was analyzed by RT-PCR as described above.
4.11. Statistical analysis
Statistical analyses were performed using the non-paired Student t-test with SIGMASTAT (Systat Software Inc.) and statistical significance was assigned at p<0.05. All results are expressed as mean ± SD.
Acknowledgments
This work was supported by HL73144 (KO), P50-HL59314, P30-DK79638 (LC) and NS04884 (HCL). We thank Monty Krieger (MIT) for providing CHO-ldlA7 cells.
Abbreviations
- Ad
adenoviral vector
- apoE
apolipoprotein E
- ApoER2
apoE receptor 2
- LRP
LDL receptor-related protein
- PAI-1
plasminogen activator inhititor-1
- RAP
receptor associated protein
- RPA
RNase protection assay
- uPA
urokinase-type plasminogen activator
- VLDLR
very-low-density-lipoprotein receptor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Argraves KM, Battey FD, MacCalman CD, McCrae KR, Gafvels M, Kozarsky KF, Chappell DA, Strauss JF, 3rd, Strickland DK. The very low density lipoprotein receptor mediates the cellular catabolism of lipoprotein lipase and urokinase-plasminogen activator inhibitor type I complexes. J Biol Chem. 1995;270:26550–26557. doi: 10.1074/jbc.270.44.26550. [DOI] [PubMed] [Google Scholar]
- Beffert U, Stolt PC, Herz J. Functions of lipoprotein receptors in neurons. J Lipid Res. 2004;45:403–409. doi: 10.1194/jlr.R300017-JLR200. [DOI] [PubMed] [Google Scholar]
- Benhayon D, Magdaleno S, Curran T. Binding of purified Reelin to ApoER2 and VLDLR mediates tyrosine phosphorylation of Disabled-1. Brain Res Mol Brain Res. 2003;112:33–45. doi: 10.1016/s0169-328x(03)00032-9. [DOI] [PubMed] [Google Scholar]
- Bilheimer DW, Eisenberg S, Levy RI. The metabolism of very low density lipoprotein proteins. I. Preliminary in vitro and in vivo observations. Biochim Biophys Acta. 1972;260:212–221. doi: 10.1016/0005-2760(72)90034-3. [DOI] [PubMed] [Google Scholar]
- Boycott KM, Flavelle S, Bureau A, Glass HC, Fujiwara TM, Wirrell E, Davey K, Chudley AE, Scott JN, McLeod DR, Parboosingh JS. Homozygous deletion of the very low density lipoprotein receptor gene causes autosomal recessive cerebellar hypoplasia with cerebral gyral simplification. Am J Hum Genet. 2005;77:477–483. doi: 10.1086/444400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsson A, Lindqvist M. Effect of ethanol on the hydroxylation of tyrosine and tryptophan in rat brain in vivo. J Pharm Pharmacol. 1973;25:437–440. doi: 10.1111/j.2042-7158.1973.tb09129.x. [DOI] [PubMed] [Google Scholar]
- Chen Y, Hu Y, Lu K, Flannery JG, Ma JX. Very low density lipoprotein receptor, a negative regulator of the wnt signaling pathway and choroidal neovascularization. J Biol Chem. 2007;282:34420–34428. doi: 10.1074/jbc.M611289200. [DOI] [PubMed] [Google Scholar]
- D'Arcangelo G, Homayouni R, Keshvara L, Rice DS, Sheldon M, Curran T. Reelin is a ligand for lipoprotein receptors. Neuron. 1999;24:471–479. doi: 10.1016/s0896-6273(00)80860-0. [DOI] [PubMed] [Google Scholar]
- D'Arcangelo G, Miao GG, Chen SC, Soares HD, Morgan JI, Curran T. A protein related to extracellular matrix proteins deleted in the mouse mutant reeler. Nature. 1995;374:719–723. doi: 10.1038/374719a0. [DOI] [PubMed] [Google Scholar]
- De Ferrari GV, Papassotiropoulos A, Biechele T, Wavrant De-Vrieze F, Avila ME, Major MB, Myers A, Saez K, Henriquez JP, Zhao A, Wollmer MA, Nitsch RM, Hock C, Morris CM, Hardy J, Moon RT. Common genetic variation within the low-density lipoprotein receptor-related protein 6 and late-onset Alzheimer's disease. Proc Natl Acad Sci U S A. 2007;104:9434–9439. doi: 10.1073/pnas.0603523104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deane R, Sagare A, Hamm K, Parisi M, Lane S, Finn MB, Holtzman DM, Zlokovic BV. apoE isoform-specific disruption of amyloid beta peptide clearance from mouse brain. J Clin Invest. 2008;118:4002–4013. doi: 10.1172/JCI36663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deguchi K, Inoue K, Avila WE, Lopez-Terrada D, Antalffy BA, Quattrocchi CC, Sheldon M, Mikoshiba K, D'Arcangelo G, Armstrong DL. Reelin and disabled-1 expression in developing and mature human cortical neurons. J Neuropathol Exp Neurol. 2003;62:676–684. doi: 10.1093/jnen/62.6.676. [DOI] [PubMed] [Google Scholar]
- Frykman PK, Brown MS, Yamamoto T, Goldstein JL, Herz J. Normal plasma lipoproteins and fertility in gene-targeted mice homozygous for a disruption in the gene encoding very low density lipoprotein receptor. Proc Natl Acad Sci U S A. 1995;92:8453–8457. doi: 10.1073/pnas.92.18.8453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gafvels ME, Paavola LG, Boyd CO, Nolan PM, Wittmaack F, Chawla A, Lazar MA, Bucan M, Angelin BO, Strauss JF., 3rd Cloning of a complementary deoxyribonucleic acid encoding the murine homolog of the very low density lipoprotein/apolipoprotein-E receptor: expression pattern and assignment of the gene to mouse chromosome 19. Endocrinology. 1994;135:387–394. doi: 10.1210/endo.135.1.8013374. [DOI] [PubMed] [Google Scholar]
- Goldstein JL, Basu SK, Brown MS. Receptor-mediated endocytosis of low-density lipoprotein in cultured cells. Methods Enzymol. 1983;98:241–260. doi: 10.1016/0076-6879(83)98152-1. [DOI] [PubMed] [Google Scholar]
- Hack I, Hellwig S, Junghans D, Brunne B, Bock HH, Zhao S, Frotscher M. Divergent roles of ApoER2 and Vldlr in the migration of cortical neurons. Development. 2007;134:3883–3891. doi: 10.1242/dev.005447. [DOI] [PubMed] [Google Scholar]
- Heegaard CW, Simonsen AC, Oka K, Kjoller L, Christensen A, Madsen B, Ellgaard L, Chan L, Andreasen PA. Very low density lipoprotein receptor binds and mediates endocytosis of urokinase-type plasminogen activator-type-1 plasminogen activator inhibitor complex. J Biol Chem. 1995;270:20855–20861. doi: 10.1074/jbc.270.35.20855. [DOI] [PubMed] [Google Scholar]
- Herz J, Boycott KM, Parboosingh JS. “Devolution” of bipedality. Proc Natl Acad Sci U S A. 2008;105:E25. doi: 10.1073/pnas.0802584105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herz J, Chen Y. Reelin, lipoprotein receptors and synaptic plasticity. Nat Rev Neurosci. 2006;7:850–859. doi: 10.1038/nrn2009. [DOI] [PubMed] [Google Scholar]
- Hiesberger T, Trommsdorff M, Howell BW, Goffinet A, Mumby MC, Cooper JA, Herz J. Direct binding of Reelin to VLDL receptor and ApoE receptor 2 induces tyrosine phosphorylation of disabled-1 and modulates tau phosphorylation. Neuron. 1999;24:481–489. doi: 10.1016/s0896-6273(00)80861-2. [DOI] [PubMed] [Google Scholar]
- Hoe HS, Rebeck GW. Regulation of ApoE receptor proteolysis by ligand binding. Brain Res Mol Brain Res. 2005;137:31–39. doi: 10.1016/j.molbrainres.2005.02.013. [DOI] [PubMed] [Google Scholar]
- Humphrey N, Mundlos S, Turkmen S. Genes and quadrupedal locomotion in humans. Proc Natl Acad Sci U S A. 2008;105:E26. doi: 10.1073/pnas.0802839105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iijima H, Miyazawa M, Sakai J, Magoori K, Ito MR, Suzuki H, Nose M, Kawarabayasi Y, Yamamoto TT. Expression and characterization of a very low density lipoprotein receptor variant lacking the O-linked sugar region generated by alternative splicing. J Biochem. 1998;124:747–755. doi: 10.1093/oxfordjournals.jbchem.a022175. [DOI] [PubMed] [Google Scholar]
- Jokinen EV, Landschulz KT, Wyne KL, Ho YK, Frykman PK, Hobbs HH. Regulation of the very low density lipoprotein receptor by thyroid hormone in rat skeletal muscle. J Biol Chem. 1994;269:26411–26418. [PubMed] [Google Scholar]
- Jossin Y, Ignatova N, Hiesberger T, Herz J, Lambert de Rouvroit C, Goffinet AM. The central fragment of Reelin, generated by proteolytic processing in vivo, is critical to its function during cortical plate development. J Neurosci. 2004;24:514–521. doi: 10.1523/JNEUROSCI.3408-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi K, Oka K, Forte T, Ishida B, Teng B, Ishimura-Oka K, Nakamuta M, Chan L. Reversal of hypercholesterolemia in low density lipoprotein receptor knockout mice by adenovirus-mediated gene transfer of the very low density lipoprotein receptor. J Biol Chem. 1996;271:6852–6860. doi: 10.1074/jbc.271.12.6852. [DOI] [PubMed] [Google Scholar]
- Koch S, Strasser V, Hauser C, Fasching D, Brandes C, Bajari TM, Schneider WJ, Nimpf J. A secreted soluble form of ApoE receptor 2 acts as a dominant-negative receptor and inhibits Reelin signaling. Embo J. 2002;21:5996–6004. doi: 10.1093/emboj/cdf599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozarsky KF, Jooss K, Donahee M, Strauss JF, 3rd, Wilson JM. Effective treatment of familial hypercholesterolaemia in the mouse model using adenovirus-mediated transfer of the VLDL receptor gene. Nat Genet. 1996;13:54–62. doi: 10.1038/ng0596-54. [DOI] [PubMed] [Google Scholar]
- Llorca J, Rodriguez-Rodriguez E, Dierssen-Sotos T, Delgado-Rodriguez M, Berciano J, Combarros O. Meta-analysis of genetic variability in the beta-amyloid production, aggregation and degradation metabolic pathways and the risk of Alzheimer's disease. Acta Neurol Scand. 2008;117:1–14. doi: 10.1111/j.1600-0404.2007.00899.x. [DOI] [PubMed] [Google Scholar]
- Magrane J, Casaroli-Marano RP, Reina M, Gafvels M, Vilaro S. The role of O-linked sugars in determining the very low density lipoprotein receptor stability or release from the cell. FEBS Lett. 1999;451:56–62. doi: 10.1016/s0014-5793(99)00494-9. [DOI] [PubMed] [Google Scholar]
- Mahley RW, Weisgraber KH, Huang Y. Apolipoprotein E4: a causative factor and therapeutic target in neuropathology, including Alzheimer's disease. Proc Natl Acad Sci U S A. 2006;103:5644–5651. doi: 10.1073/pnas.0600549103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marlovits TC, Abrahamsberg C, Blaas D. Very-low-density lipoprotein receptor fragment shed from HeLa cells inhibits human rhinovirus infection. J Virol. 1998;72:10246–10250. doi: 10.1128/jvi.72.12.10246-10250.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marriott DR, Hirst WD, Ljungberg MC. Astrocytes. In: C J, W GP, editors. Neural Cell Culture: A Practical Approach. IRL Press; 1995. pp. 85–96. [Google Scholar]
- Martensen PM, Oka K, Christensen L, Rettenberger PM, Petersen HH, Christensen A, Chan L, Heegaard CW, Andreasen PA. Breast carcinoma epithelial cells express a very low-density lipoprotein receptor variant lacking the O-linked glycosylation domain encoded by exon 16, but with full binding activity for serine proteinase/serpin complexes and Mr-40,000 receptor-associated protein. Eur J Biochem. 1997;248:583–591. doi: 10.1111/j.1432-1033.1997.00583.x. [DOI] [PubMed] [Google Scholar]
- Mikhailenko I, Considine W, Argraves KM, Loukinov D, Hyman BT, Strickland DK. Functional domains of the very low density lipoprotein receptor: molecular analysis of ligand binding and acid-dependent ligand dissociation mechanisms. J Cell Sci. 1999;112(Pt 19):3269–3281. doi: 10.1242/jcs.112.19.3269. [DOI] [PubMed] [Google Scholar]
- Moheb LA, Tzschach A, Garshasbi M, Kahrizi K, Darvish H, Heshmati Y, Kordi A, Najmabadi H, Ropers HH, Kuss AW. Identification of a nonsense mutation in the very low-density lipoprotein receptor gene (VLDLR) in an Iranian family with dysequilibrium syndrome. Eur J Hum Genet. 2008;16:270–273. doi: 10.1038/sj.ejhg.5201967. [DOI] [PubMed] [Google Scholar]
- Nakamura Y, Yamamoto M, Kumamaru E. Significance of the variant and full-length forms of the very low density lipoprotein receptor in brain. Brain Res. 2001;922:209–215. doi: 10.1016/s0006-8993(01)03170-5. [DOI] [PubMed] [Google Scholar]
- Nathan BP, Bellosta S, Sanan DA, Weisgraber KH, Mahley RW, Pitas RE. Differential effects of apolipoproteins E3 and E4 on neuronal growth in vitro. Science. 1994;264:850–852. doi: 10.1126/science.8171342. [DOI] [PubMed] [Google Scholar]
- Nathan BP, Chang KC, Bellosta S, Brisch E, Ge N, Mahley RW, Pitas RE. The inhibitory effect of apolipoprotein E4 on neurite outgrowth is associated with microtubule depolymerization. J Biol Chem. 1995;270:19791–19799. doi: 10.1074/jbc.270.34.19791. [DOI] [PubMed] [Google Scholar]
- Oka K, Ishimura-Oka K, Chu MJ, Sullivan M, Krushkal J, Li WH, Chan L. Mouse very-low-density-lipoprotein receptor (VLDLR) cDNA cloning, tissue-specific expression and evolutionary relationship with the low-density-lipoprotein receptor. Eur J Biochem. 1994;224:975–982. doi: 10.1111/j.1432-1033.1994.00975.x. [DOI] [PubMed] [Google Scholar]
- Oka K, Kojima K, Togari A, Nagatsu T, Kiss B. An integrated scheme for the simultaneous determination of biogenic amines, precursor amino acids, and related metabolites by liquid chromatography with electrochemical detection. J Chromatogr. 1984;308:43–53. doi: 10.1016/s0021-9673(01)87531-2. [DOI] [PubMed] [Google Scholar]
- Okuizumi K, Onodera O, Namba Y, Ikeda K, Yamamoto T, Seki K, Ueki A, Nanko S, Tanaka H, Takahashi H, Oyanagi K, Mizusawa H, Kanazawa I, Tsuji S. Genetic association of the very low density lipoprotein (VLDL) receptor gene with sporadic Alzheimer's disease. Nat Genet. 1995;11:207–209. doi: 10.1038/ng1095-207. [DOI] [PubMed] [Google Scholar]
- Ozcelik T, Akarsu N, Uz E, Caglayan S, Gulsuner S, Onat OE, Tan M, Tan U. Reply to Herz et al. and Humphrey et al.: Genetic heterogeneity of cerebellar hypoplasia with quadrupedal locomotion. Proc Natl Acad Sci U S A. 2008a;105:E32–33. doi: 10.1073/pnas.0804078105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozcelik T, Akarsu N, Uz E, Caglayan S, Gulsuner S, Onat SG, Tan M, Tan U. Mutations in the very low-density lipoprotein recepto VLDLR cause cerebellar hypoplasia and quadrupedal locomotion in humans. Proc Natl Acad Sci U S A. 2008b;105:4232–4236. doi: 10.1073/pnas.0710010105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Garcia CG, Tissir F, Goffinet AM, Meyer G. Reelin receptors in developing laminated brain structures of mouse and human. Eur J Neurosci. 2004;20:2827–2832. doi: 10.1111/j.1460-9568.2004.03733.x. [DOI] [PubMed] [Google Scholar]
- Rettenberger PM, Oka K, Ellgaard L, Petersen HH, Christensen A, Martensen PM, Monard D, Etzerodt M, Chan L, Andreasen PA. Ligand binding properties of the very low density lipoprotein receptor Absence of the third complement-type repeat encoded by exon 4 is associated with reduced binding of Mr 40,000 receptor-associated protein. J Biol Chem. 1999;274:8973–8980. doi: 10.1074/jbc.274.13.8973. [DOI] [PubMed] [Google Scholar]
- Roth RI, Gaubatz JW, Gotto AM, Jr, Patsch JR. Effect of cholesterol feeding on the distribution of plasma lipoproteins and on the metabolism of apolipoprotein E in the rabbit. J Lipid Res. 1983;24:1–11. [PubMed] [Google Scholar]
- Ruiz J, Kouiavskaia D, Migliorini M, Robinson S, Saenko EL, Gorlatova N, Li D, Lawrence D, Hyman BT, Weisgraber KH, Strickland DK. The apoE isoform binding properties of the VLDL receptor reveal marked differences from LRP and the LDL receptor. J Lipid Res. 2005;46:1721–1731. doi: 10.1194/jlr.M500114-JLR200. [DOI] [PubMed] [Google Scholar]
- Sakai J, Hoshino A, Takahashi S, Miura Y, Ishii H, Suzuki H, Kawarabayasi Y, Yamamoto T. Structure, chromosome location, and expression of the human very low density lipoprotein receptor gene. J Biol Chem. 1994;269:2173–2182. [PubMed] [Google Scholar]
- Sepp KJ, Hong P, Lizarraga SB, Liu JS, Mejia LA, Walsh CA, Perrimon N. Identification of neural outgrowth genes using genome-wide RNAi. PLoS Genet. 2008;4:e1000111. doi: 10.1371/journal.pgen.1000111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonsen AC, Heegaard CW, Rasmussen LK, Ellgaard L, Kjoller L, Christensen A, Etzerodt M, Andreasen PA. Very low density lipoprotein receptor from mammary gland and mammary epithelial cell lines binds and mediates endocytosis of M(r) 40,000 receptor associated protein. FEBS Lett. 1994;354:279–283. doi: 10.1016/0014-5793(94)01138-9. [DOI] [PubMed] [Google Scholar]
- Takahashi S, Kawarabayasi Y, Nakai T, Sakai J, Yamamoto T. Rabbit very low density lipoprotein receptor: a low density lipoprotein receptor-like protein with distinct ligand specificity. Proc Natl Acad Sci U S A. 1992;89:9252–9256. doi: 10.1073/pnas.89.19.9252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi S, Sakai J, Fujino T, Hattori H, Zenimaru Y, Suzuki J, Miyamori I, Yamamoto TT. The very low-density lipoprotein (VLDL) receptor: characterization and functions as a peripheral lipoprotein receptor. J Atheroscler Thromb. 2004;11:200–208. doi: 10.5551/jat.11.200. [DOI] [PubMed] [Google Scholar]
- Tiebel O, Oka K, Robinson K, Sullivan M, Martinez J, Nakamuta M, Ishimura-Oka K, Chan L. Mouse very low-density lipoprotein receptor (VLDLR): gene structure, tissue-specific expression and dietary and developmental regulation. Atherosclerosis. 1999;145:239–251. doi: 10.1016/s0021-9150(99)00068-4. [DOI] [PubMed] [Google Scholar]
- Trommsdorff M, Gotthardt M, Hiesberger T, Shelton J, Stockinger W, Nimpf J, Hammer RE, Richardson JA, Herz J. Reeler/Disabled-like disruption of neuronal migration in knockout mice lacking the VLDL receptor and ApoE receptor 2. Cell. 1999;97:689–701. doi: 10.1016/s0092-8674(00)80782-5. [DOI] [PubMed] [Google Scholar]
- Verdaguer N, Fita I, Reithmayer M, Moser R, Blaas D. X-ray structure of a minor group human rhinovirus bound to a fragment of its cellular receptor protein. Nat Struct Mol Biol. 2004;11:429–434. doi: 10.1038/nsmb753. [DOI] [PubMed] [Google Scholar]
- Willnow TE, Hammes A, Eaton S. Lipoproteins and their receptors in embryonic development: more than cholesterol clearance. Development. 2007;134:3239–3249. doi: 10.1242/dev.004408. [DOI] [PubMed] [Google Scholar]


