Abstract
Frontotemporal lobar degeneration (FTLD) is a neurodegenerative disease that dramatically alters social and emotional behavior. Recent work has suggested that self-conscious emotions (e.g., embarrassment) may be particularly vulnerable to disruption in this disease. Self-conscious emotions require the ability to monitor the self in relation to others. These abilities are thought to be subserved by brain regions (e.g., medial prefrontal, anterior cingulate, and insula) that are particularly vulnerable to damage in FTLD. This study examined emotional responding (expressive behavior, peripheral physiology, and subjective experience) in 24 FTLD patients and 16 cognitively normal control participants using a karaoke task known to elicit self-conscious emotion reliably and a nonemotional control task (isometric handgrip). Results indicated that FTLD patients showed diminished self-conscious emotional behavior (embarrassment and amusement) and diminished physiological responding while watching themselves singing. No differences were found between patients and controls in the nonemotional control task. These findings offer evidence of marked disruption of self-conscious emotional responding in FTLD. Diminished self-conscious emotional responding likely contributes significantly to social inappropriateness and other behavioral abnormalities in FTLD.
Keywords: dementia, self-conscious emotion, autonomic nervous system
Frontotemporal lobar degeneration (FTLD) is a neurodegenerative disease that selectively atrophies the frontal lobes, temporal lobes, and amygdala, regions that are important for awareness of both self and others (Craik et al., 1999; Gusnard, Akbudak, Shulman, & Raichle, 2001; Johnson et al., 2002; Kelley et al., 2002; Ochsner et al., 2004; Platek, Keenan, Gallup, & Mohamed, 2004; Zysset, Huber, Ferstl, & von Cramon, 2002). The prevalence of FTLD is higher than once thought, and it is as common as Alzheimer’s disease in individuals under the age of 65 (Ratnavalli, Brayne, Dawson, & Hodges, 2002). The category of FTLD subsumes two clinical phenotypes that are notable for social and emotional dysfunction (Neary et al., 1998): frontotemporal dementia (FTD) and semantic dementia (SD). Although the consensus diagnostic criteria proposed by Neary et al. (1998) delineate behavioral differences between the subtypes (e.g., “decline in social interpersonal conduct” in FTD and “loss of sympathy and empathy” in SD), neuroanatomical and clinical heterogeneity often make these groups difficult to dissociate (Harciarek & Jodzio, 2005; Litvan et al., 1997). Although FTD tends to have atrophy predominantly in the frontal lobes and SD has atrophy primarily in the anterior temporal lobes and amygdala, loss in the orbitofrontal cortex, insula, and anterior cingulate is often evident in both groups (Rosen, Gorno-Tempini, et al., 2002). The neuropathological features of FTLD are varied and do not easily map onto clinical symptomatology, but postmortem analyses typically reveal tau and/or ubiquitin inclusions in the frontal and temporal regions (Forman et al., 2006; McKhann et al., 2001).
FTLD patients exhibit gradual decline in self-awareness and social dexterity. Clinically, FTLD patients are often socially disinhibited (Mendez et al., 2006; Rosen et al., 2006), a symptom that may be off-putting to others and is especially stressful for caregivers (de Vugt et al., 2006). Patients may become more passive, aloof, and cold than they were before disease onset (Rankin, Kramer, Mychack, & Miller, 2003), and they typically exhibit diminished insight into and awareness of even dramatic personality changes (Rankin, Baldwin, Pace-Savitsky, Kramer, & Miller, 2005). Changes in hobbies, ideology, and aesthetic preferences (e.g., in food and dress) have also been reported in FTLD (Miller et al., 2001), prompting descriptions of the self as becoming “lost” in this disease (Levenson & Miller, 2007). Laboratory studies have consistently found that FTLD patients also fall short in their ability to recognize others’ emotions (Keane, Calder, Hodges, & Young, 2002; Lavenu, Pasquier, Lebert, Petit, & Van der Linden, 1999; Rosen, Perry, et al., 2002; Werner et al., 2007) and perspectives (Gregory et al., 2002; Lough et al., 2006; Snowden et al., 2003). These patients fail to recognize social faux pas (Gregory et al., 2002) and are unable to rate appropriately the severity of moral and social transgressions (Lough et al., 2006). These deficits suggest that FTLD patients may also have difficulty with the kinds of complicated emotions that arise in social interactions.
In the realm of emotional functioning, laboratory-based assessments (Levenson et al., 2008) have indicated that FTLD patients have intact physiological and behavioral responding in certain emotional contexts. For example, FTLD patients do not differ from neurologically healthy controls in their emotional reactions to unexpected loud noises (Sturm, Levenson, Rosen, Allison, & Miller, 2006) or to simply themed happy, sad, and fearful emotional film clips (Werner et al., 2007). Thus, there is accumulating evidence that the physiological and behavioral infrastructure that is necessary for some aspects of simple emotional responding is preserved in the early stages of FTLD.
In contrast, emotional impairment in FTLD clearly occurs in areas of socioemotional functioning that require higher order processing of the social world. FTLD patients have clear deficits in recognizing negative emotions in others (Rosen et al., 2004; Rosen, Wilson, et al., 2006; Werner et al., 2007) and in activating more complex emotions such as embarrassment (Sturm et al., 2006). Embarrassment is a member of the family of “self-conscious” emotions (others include guilt, pride, and shame) that are cognitively complex, requiring an appreciation of the self in a social context (Tangney, 1999). These self-conscious emotions are thought to emerge relatively late in phylogeny and ontogeny (Lewis, Sullivan, Stanger, & Weiss, 1989) and are likely subserved by brain regions (e.g., medial prefrontal, anterior cingulate, and insula) that are vulnerable in FTLD (Rosen, Gorno-Tempini, et al., 2002). Embarrassment occurs when one’s behavior violates social norms (Lewis, 1995). The resulting emotional state indicates that a social transgression has occurred and helps motivate attempts to correct and repair the situation (Keltner & Anderson, 2000; Keltner & Buswell, 1997; R. S. Miller & Leary, 1992). A lack of embarrassment in FTLD may help explain some of the social gaffes seen in these patients as well as their failure to correct these behaviors.
In our previous study of self-conscious emotional responding (Sturm et al., 2006), FTLD patients and controls were startled unexpectedly with a loud noise (akin to the sound of a gunshot). Typically, this startle stimulus elicits a reflexive, defensive response that includes stereotyped muscle contractions in the face and upper torso (Ekman, Friesen, & Simons, 1985). For many people, this initial response is followed by a secondary, self-conscious response that occurs as they process the fact that they have had a very large reaction to what turned out to be a harmless event. This secondary response often entails self-conscious behaviors such as nervous laughter and controlled smiling. We found that FTLD patients and controls did not differ in the magnitude of their initial behavioral or autonomic response to the startle stimulus; however, the secondary self-conscious response was markedly diminished or absent in the patients.
This work with the startle stimulus clearly suggested deficits in the behavioral aspects of self-conscious emotional responding in FTLD. However, because the startle paradigm does not allow a clear temporal delineation between the end of the primary response and the beginning of the secondary response, and because autonomic responses are slower to onset and offset than facial expressions, it was difficult to isolate the physiological concomitants of the self-conscious response. Moreover, the startle task produces a range of secondary responses, some self-conscious (e.g., embarrassment) and some not (e.g., anger and fear). For these reasons, in the present study we sought a paradigm that would isolate self-conscious emotional responding from other types of emotional reactivity and allow examination of its autonomic concomitants.
We used a karaoke task in which participants viewed themselves singing on a video monitor. This task was explicitly designed to produce a self-conscious emotional response. Thus, it allows for testing whether FTLD patients’ self-conscious emotion deficits, previously found in the startle paradigm, generalize to a quite different task. In addition, the structure of the singing task allows for isolation of the behavioral, physiological, and experiential aspects of the self-conscious emotional response. We hypothesized that FTLD patients would show less responding in both the behavioral and the physiological domains. We did not hypothesize differences in self-reported emotional response because, in our experience, FTLD patients exhibit great variation in their self-reported emotional experience.
To ensure that any physiological differences between FTLD patients and controls were not the result of more general changes in peripheral physiological systems secondary to degeneration of central control circuits, we also investigated physiological reactivity to a nonemotional control task in which participants squeezed a handgrip device. This isometric activity reliably increases cardiovascular responding (Obrist, 1981), and we have used it extensively in our past research (Levenson, 2007).
Method
Participants
Twenty-four patients diagnosed with FTLD and 16 cognitively normal control participants were studied. Patients and controls were extensively evaluated. Neurological testing and neuropsychological testing were done at the University of California, San Francisco, Memory and Aging Center. Structural MRIs were obtained at the San Francisco Veterans Administration Medical Center. All patients met diagnostic criteria (Neary et al., 1998) for FTLD (16 were diagnosed as having FTD and 8 as having SD). No controls had a previous history of neurological or psychiatric disorder.
The FTLD patients (M = 59.46 years, SD = 5.73) were significantly younger than the controls (M = 67.75 years, SD = 7.75), t(38) = −3.89, p < .05. The patient group consisted of significantly more men (83%) than the control group (44%), χ2(1, N = 40) = 6.857, p < .01. The groups had similar levels of education, t(38) = −0.53, ns (M = 16.67 years, SD = 2.46, for the FTLD patients and M = 17.06, SD = 2.02, for the controls).
Participants were paid $30 for an approximately 6-hr laboratory session in which they were exposed to a battery of tasks used to evaluate different aspects of emotional and social functioning.
Clinical evaluations and neuropsychological testing were completed within 3 months of the emotional assessments for FTLD patients and within 1 year for controls.
Mini-Mental State Examination (MMSE; Folstein, Folstein, & McHugh, 1975)
Cognitive abilities were assessed with the MMSE. Patients’ mean score was 25.96 (SD = 3.41), which places them in the mild range of impairment. Controls scored near ceiling with a mean of 29.63 (SD = 0.50).
Clinical Dementia Rating Scale
Informants were interviewed to obtain a Clinical Dementia Rating Scale score for each participant. The Clinical Dementia Rating Scale score indicates participants’ level of daily functioning. FTLD patients’ scores placed them in the mild range of functional impairment (M = .89, SD = .46). Controls were within the normal range (M = .00, SD = .00).
Neuropsychiatric Inventory (Cummings et al., 1994)
The Neuropsychiatric Inventory is an informant-based scale that assesses the frequency and severity of psychopathological symptoms in dementia patients. The FTLD patients had a mean total Neuropsychiatric Inventory score of 32.27 (SD = 18.81), which indicates a moderate level of psychopathological symptoms. Social and emotional symptoms were common among the FTLD patients: 75% were described as apathetic, 46% were described as euphoric, and 71% were described as socially disinhibited.
Medications
Medications that participants were taking on the day of emotional testing were determined. Those that were thought to have a possible effect on emotional responding (serotonin reuptake inhibitors, tricyclics, atypical antidepressants, monoamine oxidase inhibitors, lithium, antipsychotics, atypical antipsychotics, acetylcholinesterase inhibitors, glutamate agonists, benzodiazepines, dopamine agonists, barbiturates, antiepileptics, psychostimulants, anticholinergics, and beta blockers) were tallied so that their effects could be controlled for in subsequent analyses. This revealed that 83% of the patients and none of the controls were on one or more of these medications. The most common medications for the FTLD patients were serotonin reuptake inhibitors (58%).
General Procedure
Participants’ emotional functioning was assessed at the Berkeley Psychophysiology Laboratory at the University of California, Berkeley. On arrival, participants signed consent forms (approved by the Committee for the Protection of Human Subjects at the University of California, Berkeley) that delineated the laboratory tasks (including “singing a song”). An additional consent form regarding the future use of the videotapes was also presented but was not signed until the end of testing. Each participant was seated in a comfortable chair in a well-lit, 3 m × 6 m experiment room where an experimenter attached physiological sensors and briefly explained the procedures.
Laboratory Tasks
All stimuli and instructions were presented on a 21-in. (53.3-cm) color TV monitor at a distance of 1.75 m from the participant.
Singing Task
Self-conscious emotional responding was assessed with a karaoke singing task that has been shown to elicit self-conscious emotional behavior reliably (Shearn, Bergman, Hill, Abel, & Hinds, 1990). In this task, participants were asked to relax during a 60-s pretrial baseline during which an X appeared on the TV monitor. The song title (“My Girl”) was then displayed on the monitor for 9 s, followed by playing the song (the version of “My Girl” by The Temptations was used) for 2 min, 33 s (lyrics appeared on the monitor and sound was presented over headphones). Participants were asked to sing along with the song but were not told that they would later be viewing the tape of their performance.
Watch Self Singing Task
When the song ended, the experimenter returned to the room and removed the headphones. The only instruction given for the next task was to watch the TV monitor. After the experimenter left the room, the videotape that had just been recorded of the participant sitting through the baseline and then singing was played on the TV monitor (participants heard and saw themselves singing without hearing the Temptations’ version in the background).
Isometric Handgrip Task
To assess the participants physiological responding in a non-emotional control task, participants squeezed a Jamar isometric handgrip device. This task reliably increases cardiovascular arousal. Participants were instructed to squeeze the device to their maximum ability (measured in pounds of force). The experimenter noted the force they obtained and subsequently placed a mark on the device indicating 50% of this force. The trial format consisted of 60 s of prebaseline rest, 30 s of squeezing the handgrip device (to the 50% level), and 90 s of postbaseline rest.
Measures
Facial Behavior
Participants’ behavior was videotaped continuously using a remote-controlled, high-resolution video camera that was partially concealed in the experiment room. Videotape timing was synchronized to the physiological measures using a system that inserted an invisible time stamp on each video frame. The first 30 s of participants watching themselves singing was later coded by a team of trained undergraduate coders. This period captured the initial attempts at singing, which are typically the most embarrassing for participants. Coders used a modified version of the Emotional Expressive Behavior coding system (Gross & Levenson, 1993). Coders, who were unaware of participant diagnosis and the nature of the trial, coded each second for nine emotional behaviors (anger, disgust, happiness/amusement, contempt, sadness, embarrassment, fear, surprise, and confusion) on an intensity scale ranging from 0 to 3. The code for embarrassment was based on Keltner and Buswell’s (1997) description, which includes gaze aversion, smiling and laughter, smile suppression, blushing, and face touches. The code for happiness/amusement included smiling and laughing. Intercoder reliability was high (intraclass correlation coefficient = .76).
Physiological Responding
Physiological measures were monitored continuously using a Grass Model 7 polygraph (Grass Instruments, Quincy, MA), a computer with analog-to-digital capability, and an online data acquisition software package written by Robert W. Levenson. The software computed second-by-second averages for the following measures:
Heart rate: Beckman miniature electrodes with Redux paste were placed in a bipolar configuration on opposite sides of the participant’s chest; the interbeat interval was calculated as the interval, in milliseconds, between successive R waves.
Finger pulse amplitude: A UFI photoplethysmograph (UFI instruments, Morro Bay, CA) recorded the amplitude of blood volume in the finger using a photocell taped to the distal phalanx of the index finger of the nondominant hand.
Finger pulse transmission time: The time interval in milliseconds was measured between the R wave of the electrocardiogram (EKG) and the upstroke of the peripheral pulse at the finger site, recorded from the distal phalanx of the index finger of the nondominant hand.
Ear pulse transmission time: A UFI photoplethysmograph attached to the right earlobe recorded the volume of blood in the ear, and the time interval in milliseconds was measured between the R wave of the EKG and the upstroke of peripheral pulse at the ear site.
Systolic and diastolic blood pressure: A blood pressure cuff was placed on the middle phalanx of the middle finger of the nondominant hand and continuously recorded the systolic and diastolic blood pressure using an Ohmeda Finapres 2300 (Finapres Medical System, Amsterdam, The Netherlands).
Skin conductance: A constant-voltage device was used to pass a small voltage between Beckman regular electrodes (using an electrolyte of sodium chloride in unibase) attached to the palmar surface of the middle phalanges of the ring and index fingers of the nondominant hand.
General somatic activity: An electromechanical transducer attached to the platform under the participant’s chair generated an electrical signal proportional to the amount of movement in any direction.
Respiration period: A pneumatic bellows was stretched around the thoracic region, and the intercycle interval was measured in milliseconds between successive inspirations.
Respiration depth: The point of the maximum inspiration minus the point of maximum expiration was determined from respiratory tracing.
Finger temperature: A thermistor attached to the distal phalanx of the little finger of the nondominant hand recorded temperature in degrees Fahrenheit.
These measures were selected to provide a broad index of the activity of physiological systems important to emotional responding: cardiac, vascular, electrodermal, respiratory, and striate muscle.
Self-Reported Emotional Experience
After watching themselves sing, participants completed a self-report inventory that measured their subjective emotional experience of watching themselves singing. Participants were asked to rate their experience of self-conscious (i.e., embarrassment), negative (i.e., fear, anger, sadness, and disgust), and positive (i.e., happiness) emotions using a 3-point Likert-type intensity scale (i.e., not at all, a little, or a lot).
Data Reduction
Watch Self Singing Task
Facial behavior
For each emotional code, we summed the intensity scores for each occurrence during the 30-s “watch self singing” period. We computed a composite score for self-conscious emotional responding by summing the embarrassment and happiness/amusement codes and a composite score for negative emotional behavior by summing the scores for anger, disgust, contempt, sadness, fear, surprise, and confusion. Thus, there were two composite behavioral scores for each participant: self-conscious behavior and negative emotional behavior.
Physiological responding
We computed participants’ physiological reactivity for the 30-s “watch self singing” period by subtracting the average level of each physiological measure while they watched themselves during the presinging baseline period (69 s—the 60-s baseline plus the 9 s when the title was present) from the average level while they watched themselves singing (30 s). To provide a single measure of overall peripheral physiological responding, we calculated a composite score that encompassed all physiological measures. To calculate this composite, we computed standardized scores for each physiological reactivity score and reverse-scored as needed (i.e., cardiac interbeat interval, finger pulse amplitude, finger pulse transmission time, ear pulse transmission time, and respiration period) so that larger values reflected greater physiological arousal. The standardized scores were then averaged, which produced the composite physiological reactivity score for each participant.
Self-reported emotional experience
Participants’ intensity ratings were assigned numerical values (0 = not at all, 1 = a little, and 2 = a lot). An overall score for negative emotion was obtained by averaging the values for fear, anger, sadness, and disgust. The value for happiness was used as the score for positive emotion, and the value for embarrassment was used as the score for self-conscious emotion.
Isometric Handgrip Task
We computed participants’ cardiovascular reactivity during this task by subtracting the average level of each cardiovascular measure during the presqueezing baseline period (60 s) from the average level while they squeezed the handgrip device (30 s). We then computed a composite cardiovascular reactivity score for each participant in a manner similar to that described above for assessing overall physiological reactivity to the watch self singing task.
Results
Our major analyses were conducted using 2 × 2 (Diagnosis × Sex) analyses of covariance (ANCOVA) with age and MMSE scores as covariates (these were the variables that differed significantly between the patient and control groups). All F values reported below have the effects of these covariates controlled.
Watch Self Singing Task
Facial Behavior
We hypothesized that FTLD patients would show deficits in self-conscious emotional behavior compared with controls. The ANCOVA revealed a main effect for diagnosis, F(1, 34) = 4.80, p < .05. As predicted, FTLD patients showed less self-conscious behavior than controls. Overall, 46% of FTLD patients and 75% of controls exhibited a self-conscious response; a nonparametric test of proportions revealed that this difference was statistically significant (z= −1.83, p < .05, one-tailed). In addition, the main effect for sex was significant, F(1, 34) = 7.64, p < .01, with men showing less self-conscious behavior (M = 5.63, SD = 12.18) than women (M = 33.08, SD = 30.75). The Diagnosis × Sex interaction was not significant, F(1, 34) = 1.03, ns.
ANCOVAs of the negative emotional behavior composite revealed no differences between groups. The effects for diagnosis, F(1, 34) = 0.50, ns; sex, F(1, 34) = 0.09, ns; and the Diagnosis × Sex interaction, F(1, 34) = 1.53, ns, were all nonsignificant. Exploratory analyses of the seven individual negative emotion codes also revealed no differences between FTLD patients and controls in anger, F(1, 34) = 0.02, ns; disgust, F(1, 34) = 0.24, ns; contempt, F(1, 34) = 2.04, ns; sadness, F(1, 34) = 0.80, ns; fear, F(1, 34) = 0.25, ns; surprise, F(1, 34) = 0.25, ns; or confusion, F(1, 34) = 0.08, ns. Thus, in contrast to the self-conscious emotions, where FTLD patients clearly showed diminished responding, there was no diminution in negative emotional responding. Table 1 presents the group means and standard deviations for the self-conscious and negative emotional behavioral composites and for the individual negative emotional codes.
Table 1.
Group Means and Standard Deviations of the Behavioral Composites and Individual Negative Emotional Codes While Participants Watched Themselves Singing
| Behavioral composite and individual emotional codes |
M | SD | ||
|---|---|---|---|---|
| Control | FTLD | Control | FTLD | |
| Self-conscious emotional composite* | 27.81 | 5.71 | 31.60 | 9.65 |
| Happiness/amusement | 24.56 | 3.71 | 31.83 | 7.89 |
| Embarrassment | 3.25 | 2.00 | 11.96 | 6.80 |
| Negative emotional composite | 3.88 | 5.21 | 10.07 | 10.13 |
| Anger | 0.00 | 1.67 | 0.00 | 6.17 |
| Disgust | 0.00 | 0.42 | 0.00 | 1.41 |
| Contempt | 0.00 | 1.33 | 0.00 | 6.11 |
| Sadness | 0.00 | 1.08 | 0.00 | 5.31 |
| Fear | 0.00 | 0.17 | 0.00 | 0.82 |
| Surprise | 0.00 | 0.08 | 0.00 | 0.41 |
| Confusion | 3.88 | 0.46 | 10.07 | 1.86 |
Note. FTLD = frontotemporal lobar degeneration.
p < .05.
Physiological Responding
We hypothesized that FTLD patients would have a smaller physiological response to watching themselves sing than would controls. Our analyses of the physiological composite score supported this prediction. The ANCOVA revealed a main effect for diagnosis, F(1, 34) = 7.31, p < .05, with FTLD patients showing less physiological responding than controls. Neither the main effect for sex, F(1, 34) = 0.47, ns, nor the Diagnosis × Sex interaction, F(1, 34) = 0.40, ns, was significant.
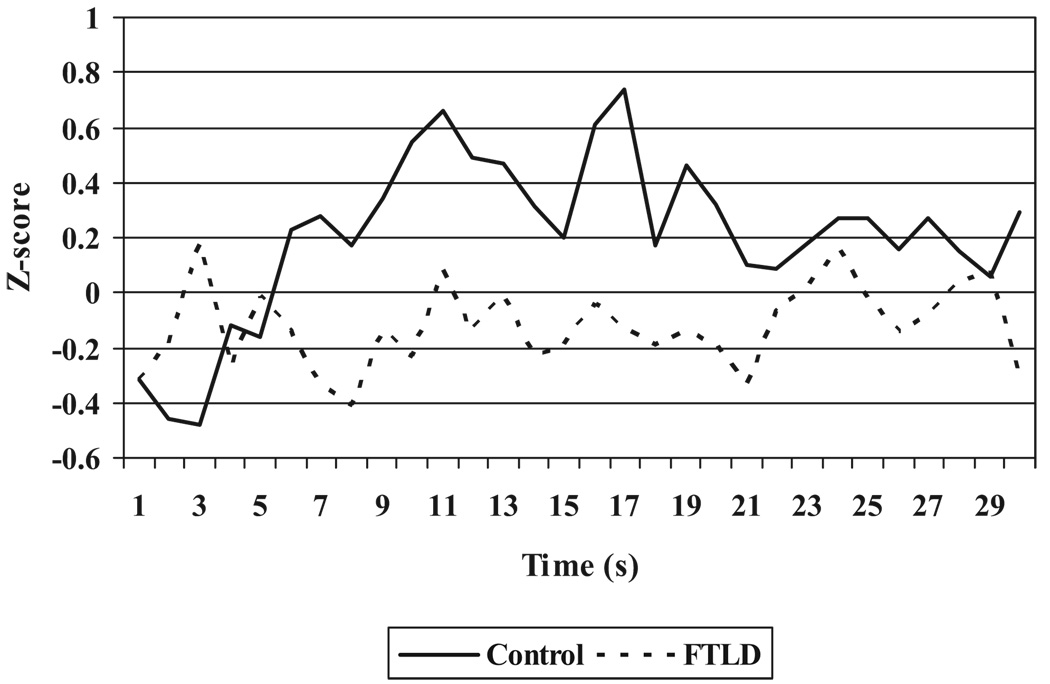
An exploratory analysis of the individual physiological measures revealed that patients responded significantly less in heart rate, F(1, 34) = 8.80, p < .01; skin conductance, F(1, 33) = 6.29, p < .05; and respiration depth, F(1, 30) = 5.77, p < .05. Table 2 presents the group means and standard deviations for the physiological composite score and individual physiological measures. To illustrate the temporal course of physiological responding for FTLD patients and controls, Figure 1 shows the second-by-second averages for the physiological composite during the first 30 s that participants watched themselves singing. This figure reveals increased physiological responding in the control participants and relatively flat physiological responding in the FTLD patients while they watched themselves singing.
Table 2.
Group Means and Standard Deviations of Physiological Responding While Participants Watched Themselves Singing
| M | SD | |||
|---|---|---|---|---|
| Measure | Control | FTLD | Control | FTLD |
| Standardized physiological composite score* (corrected for baseline) |
−0.17 | 0.24 | 0.25 | 0.69 |
| Nonstandardized individual physiological measures (not corrected for baseline) |
||||
| Cardiac interbeat interval (milliseconds)* | 852.46 | 771.29 | 155.85 | 136.34 |
| Finger pulse amplitude (units) | 7.93 | 7.01 | 6.56 | 5.58 |
| Finger pulse transit time (milliseconds) | 266.68 | 252.82 | 6.56 | 22.21 |
| Ear pulse transit time (milliseconds) | 186.71 | 174.10 | 34.59 | 24.71 |
| Systolic blood pressure (mmHg) | 137.67 | 137.08 | 21.63 | 17.75 |
| Diastolic blood pressure (mmHg)† | 78.01 | 80.96 | 14.13 | 13.22 |
| Skin conductance (µmhos)* | 3.13 | 2.63 | 1.87 | 1.85 |
| General somatic activity (units)† | 2.67 | 1.85 | 2.70 | 1.09 |
| Respiration period (milliseconds) | 3,189.56 | 3,694.85 | 446.65 | 914.70 |
| Respiration depth (units)* | 273.46 | 201.63 | 88.64 | 81.04 |
| Finger temperature (°F) | 86.24 | 90.15 | 5.67 | 5.15 |
Note. FTLD = frontotemporal lobar degeneration.
p < .05.
p < .10.
Figure 1.
Second-by-second group averages for the physiological composite (corrected for baseline levels) during the 30 s participants watched themselves singing. FTLD = frontotemporal lobar degeneration.
Self-Reported Emotional Experience
We did not expect to find differences between the FTLD patients and the controls in their self-reported levels of self-conscious, negative, or positive emotions. Consistent with this, our analyses of the self-report ratings found no group differences. For self-reported self-conscious emotion, there was no main effect of diagnosis, F(1, 33) = 1.37, ns, and there was no significant Diagnosis × Sex interaction, F(1, 33) = .30, ns. There was a significant main effect for sex, F(1, 33) = 6.69, p < .05, such that women reported more feelings of embarrassment than did men. For self-reported negative and positive emotions, the ANCOVAs revealed no main effects for diagnosis (negative emotion, F[1, 34] = 1.06, ns, and positive emotion, F[1, 33] = 0.99, ns), no main effects for sex (negative emotion, F[1, 34] = 0.34, ns, and positive emotion, F[1, 33] = .04, ns), and no Diagnosis × Sex interaction (negative emotion, F[1, 34] = 0.16, ns, and positive emotion, F[1, 33] = 3.70, ns). Table 3 presents the group means and standard deviations for the self-reported levels of self-conscious, negative, and positive emotions.
Table 3.
Group Means and Standard Deviations of Self-Reported Emotional Experience While Participants Watched Themselves Singing
| M | SD | |||
|---|---|---|---|---|
| Self-reported emotion | Control | FTLD | Control | FTLD |
| Self-conscious | 0.69 | 0.35 | 0.87 | 0.57 |
| Negative | 0.05 | 0.10 | 0.10 | 0.28 |
| Positive | 0.88 | 1.04 | 0.72 | 0.88 |
Note. FTLD = frontotemporal lobar degeneration.
Isometric Handgrip Task
Cardiovascular Responding
We expected FTLD patients and controls to show similar cardiovascular responding in the nonemotional handgrip task; analyses of the cardiovascular composite score were consistent with this prediction. The ANCOVA revealed no main effect for diagnosis, F(1, 32)= 3.48, ns; no main effect for sex, F(1, 32)=2.81, ns; and no significant Diagnosis × Sex interaction, F(1, 34) = 1.61, ns.
Additional Analyses
FTLD Subtypes
We conducted exploratory analyses to determine whether FTD and SD subtypes differed in emotional responding. Using 2 × 2 (Subtype × Sex) ANCOVAs (controlling for age and MMSE), we found no main effect for FTLD subtype on the self-conscious emotional behavior composite, F(1, 18) = 0.08, ns; the negative emotional behavior composite, F(1, 18) = 0.14, ns; or the physiological composite, F(1, 18) = 0.49, ns. There were also no main effects for FTLD subtype on levels of self-reported self-conscious, F(1, 17) = 0.31, ns; negative, F(1, 18) = 0.20, ns; or positive, F(1, 17) = 0.08, ns, emotions.
Medications
To determine whether the medications that FTLD patients were taking accounted for our results, we ran additional analyses on the self-conscious emotional behavior and physiological composites in which we used the number of medications thought to affect emotional responding (see above) as a third covariate (along with age and MMSE). These analyses revealed that FTLD patients continued to show significantly less self-conscious emotional behavior, F(1, 35) = 7.15, p<.05, and physiological responding, F(1, 35) = 6.08, p<.05, than controls even after controlling for medications and the other covariates.
Discussion
Self-conscious emotions such as shame, pride, embarrassment, and guilt are complex emotions that typically arise when the self becomes the center of social evaluation (Lewis, 1995). Thus, awareness of self in relation to others is critical for having a self-conscious emotional response. Self-conscious emotions are important because they provide signals that we have violated social norms and facilitate modification of ongoing behavior. Embarrassment is a prototypical self-conscious emotion, occurring in situations in which we make social errors and gaffes and motivating corrective behavior including apology, appeasement, and self-deprecation (Keltner & Anderson, 2000; Keltner & Buswell, 1997; R. S. Miller & Leary, 1992). Because frontotemporal brain circuitry is strongly associated with various aspects of self- and other processing (Craik et al., 1999; Gusnard et al., 2001; Johnson et al., 2002; Kelley et al., 2002; Ochsner et al., 2004; Platek et al., 2004; Zysset et al., 2002), degeneration of these networks may deleteriously affect self-conscious emotional responding.
Emotional problems are paramount in FTLD, with emerging evidence that some emotions may be more vulnerable to disruption in this disease than others. Previous work from our laboratory has found that emotional responding to simple stimuli such as loud noises (Sturm et al., 2006) and film clips that clearly portray others in the throes of an emotion (Werner et al., 2007) remain relatively intact in the early stages of FTLD. However, in the more complex realm of self-conscious emotions, FTLD patients show striking deficits (Sturm et al., 2006).
In this study, we extended this work using a karaoke task that is known to elicit embarrassment reliably in neurologically normal individuals (Shearn et al., 1990). The results clearly indicate that compared with controls, FTLD patients exhibited marked deficits in self-conscious emotional behavior and attendant physiological activation when placed in a highly embarrassing situation. These findings were obtained even after controlling for age, MMSE, and medication usage. More important, the deficit in emotional behavior was limited to the self-conscious emotions. Levels of other negative emotional behaviors (whether examined in a composite or in separate emotions) did not differ between FLTD patients and controls. Further evidence for the specificity of this deficit in FTLD derives from patients having similar levels of cardiovascular responding to controls on a nonemotional isometric handgrip task. Thus, FTLD patients’ diminished self-conscious behavioral and physiological responses during the karaoke task cannot be explained in terms of overall dampening of physiological reactivity associated with neurodegeneration. These findings, combined with prior findings of preserved behavioral and physiological responses in FTLD to simple startle and film stimuli, underscores the specific deficit in self-conscious emotional responding.
The lack of differences between FTLD patients and controls in levels of self-reported self-conscious, negative, or positive emotions requires some comment. It is not known whether FTLD patients can reflect on their emotions and accurately report on their internal states. Frontal brain regions such as the anterior cingulate and insula that are important for gauging one’s own experience (Craig, 2002; Critchley, 2004) are often compromised in FTLD (Boccardi et al., 2005; Seeley et al., 2006). Thus, it is likely that FTLD patients will have difficulty recognizing and labeling their own emotions (Lane et al., 1998). All of this suggests caution in interpreting the meaning of emotional self-reports obtained from FTLD.
The behavioral and physiological findings provide strong evidence that critical aspects of the self-conscious emotional response are diminished in FTLD. In FTLD, deficits in social behavior appear to be driven by problems with higher order processes (e.g., self-awareness, awareness of others, self-monitoring, and lack of social motivation), but our findings of diminished autonomic responding suggest that FTLD patients may also be lacking bottom-up information that could capture attention and motivate behavioral change (e.g., visceral sensations associated with blushing, heart pounding, and sweating).
Ultimately, a full understanding of the deficits in self-conscious emotions in FTLD will require a linking of specific deficits in emotional responding with specific areas of neural loss. Neural networks that include the medial prefrontal cortex (Craik et al., 1999; Johnson et al., 2002; Kelley et al., 2002; Takahashi et al., 2004; Zysset et al., 2002), anterior cingulate (Allman, Hakeem, Erwin, Nimchinsky, & Hof, 2001; Carter et al., 1998; Critchley, Tang, Glaser, Butterworth, & Dolan, 2005; Gehring & Knight, 2000), and insula (Craig, 2003; Craig, Chen, Bandy, & Reiman, 2000) are likely involved in self-conscious emotions. These brain regions have all been implicated in various aspects of self-processing (e.g., the anterior cingulate plays an important role in self-monitoring, which is necessary for a self-conscious response) and are routinely atrophied in both the FTD and SD subtypes of FTLD (Rosen, Gorno-Tempini, et al., 2002).
Several aspects of the study merit some caution. First, our coding of self-conscious behavior included both embarrassment and happiness/amusement behaviors; thus, this is not a study of embarrassment per se but rather of a wider range of self-conscious emotional responses. Second, our FTLD and control groups differed in their proportions of men and women. In this study, we found that women displayed more self-conscious emotional behavior and self-reported more feelings of self-conscious emotion than men regardless of diagnosis (Shearn et al., 1990, reported similar findings with neurologically normal participants). Although we controlled for this statistically, it would be preferable in future studies to have equal proportions of men and women in all diagnostic groups. Third, although past research from our laboratory has found that the behavioral responses of FTLD patients to simple emotional stimuli are intact, we do not have a direct test of the FTLD patients’ responses to a range of other emotional stimuli in this study. Fourth, we did not have measures of regional lobar brain volumes available to determine whether deficits in self-conscious emotional responding can be linked to neural loss in particular neural structures. This is clearly something we hope to be able to remedy in the future.
Summary
This study applied laboratory methods derived from basic affective science (Levenson, 2007) to study emotional functioning in FTLD patients. Results indicated a clear diminution of behavioral and physiological aspects of self-conscious emotional responding in FTLD patients compared with neurologically normal controls. FTLD is a disease that progressively degrades social relations with close others and strangers alike. Family members and caregivers find the social symptoms of FTLD patients to be especially problematic and stressful (de Vugt et al., 2006), and there seem to be few remedies to counter behavioral deterioration. Self-conscious emotions depend on the ability to self-monitor, self-reflect, and gauge others’ reactions to one’s behavior. These emotions function to motivate repairing social gaffes and inadequacies. It seems likely that diminished self-conscious emotional responding in FTLD patients contributes to the real-world social impairments associated with the disease. Patients who lack self-conscious emotions like embarrassment may have deficits in both visceral information (e.g., physiological responding may not signal social violations) and motivation (e.g., they may not modify their actions after acting inappropriately). Findings of diminished self-conscious emotional responding in FTLD may be useful in the early diagnosis of the disease, which will become increasingly important as treatments are developed that can slow or halt the underlying associated neural degeneration. Moreover, associating specific emotional symptoms with particular areas of neural loss can contribute greatly to our understanding of emotional processing in both the normal and the diseased brain.
Acknowledgments
We would like to acknowledge our funding sources for this work: National Institute on Aging Grants AG107766, AG19724, AG-03-006-01, and AG019724-02; National Institute of Mental Health Grant MH020006; and the State of California Alzheimer’s Disease Research Center of California Grant 03-75271.
Contributor Information
Virginia E. Sturm, Department of Psychology, University of California, Berkeley
Elizabeth A. Ascher, Department of Psychology, University of California, Berkeley
Bruce L. Miller, Department of Neurology, University of California, San Francisco
Robert W. Levenson, Department of Psychology, University of California, Berkeley
References
- Allman JM, Hakeem A, Erwin JM, Nimchinsky E, Hof P. The anterior cingulate cortex: The evolution of an interface between emotion and cognition. Annals of the New York Academy of Sciences. 2001;935:107–117. [PubMed] [Google Scholar]
- Boccardi M, Sabattoli F, Laakso MP, Testa C, Rossi R, Beltramello A, et al. Frontotemporal dementia as a neural system disease. Neurobiology of Aging. 2005;26:37–44. doi: 10.1016/j.neurobiolaging.2004.02.019. [DOI] [PubMed] [Google Scholar]
- Carter CS, Braver TS, Barch DM, Botvinick MM, Noll D, Cohen JD. Anterior cingulate cortex, error detection, and the online monitoring of performance. Science. 1998 May 1;280:747–749. doi: 10.1126/science.280.5364.747. [DOI] [PubMed] [Google Scholar]
- Craig AD. How do you feel? Interoception: The sense of the physiological condition of the body. Nature Reviews Neuroscience. 2002;3:655–666. doi: 10.1038/nrn894. [DOI] [PubMed] [Google Scholar]
- Craig AD. Interoception: The sense of the physiological condition of the body. Current Opinion in Neurobiology. 2003;13:500–505. doi: 10.1016/s0959-4388(03)00090-4. [DOI] [PubMed] [Google Scholar]
- Craig AD, Chen K, Bandy D, Reiman EM. Thermosensory activation of insular cortex. Nature Neuroscience. 2000;3:184–190. doi: 10.1038/72131. [DOI] [PubMed] [Google Scholar]
- Craik FIM, Moroz TM, Moscovitch M, Stuss DT, Winocur G, Tulving E, et al. In search of the self: A positron emission tomography study. Psychological Science. 1999;10:26–34. [Google Scholar]
- Critchley HD. The human cortex responds to an interoceptive challenge. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:6333–6334. doi: 10.1073/pnas.0401510101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Critchley HD, Tang J, Glaser D, Butterworth B, Dolan RJ. Anterior cingulate activity during error and autonomic response. NeuroImage. 2005;27:885–895. doi: 10.1016/j.neuroimage.2005.05.047. [DOI] [PubMed] [Google Scholar]
- Cummings JL, Mega M, Gray K, Rosenberg-Thompson S, Carusi DA, Gornbein J. The Neuropsychiatric Inventory: Comprehensive assessment of psychopathology in dementia. Neurology. 1994;44:2308–2314. doi: 10.1212/wnl.44.12.2308. [DOI] [PubMed] [Google Scholar]
- de Vugt ME, Riedijk SR, Aalten P, Tibben A, van Swieten JC, Verhey FR. Impact of behavioural problems on spousal caregivers: A comparison between Alzheimer’s disease and frontotemporal dementia. Dementia and Geriatric Cognitive Disorders. 2006;22:35–41. doi: 10.1159/000093102. [DOI] [PubMed] [Google Scholar]
- Ekman P, Friesen WV, Simons RC. Is the startle reaction an emotion? Journal of Personality and Social Psychology. 1985;49:1416–1426. doi: 10.1037//0022-3514.49.5.1416. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”: A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Forman MS, Farmer J, Johnson JK, Clark CM, Arnold SE, Coslett HB, et al. Frontotemporal dementia: Clinicopathological correlations. Annals of Neurology. 2006;59:952–962. doi: 10.1002/ana.20873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehring WJ, Knight RT. Prefrontal-cingulate interactions in action monitoring. Nature Neuroscience. 2000;3:516–520. doi: 10.1038/74899. [DOI] [PubMed] [Google Scholar]
- Gregory CA, Lough S, Stone V, Erzinclioglu S, Martin L, Baron-Cohen S, et al. Theory of mind in patients with frontal variant frontotemporal dementia and Alzheimer’s disease: Theoretical and practical implications. Brain. 2002;125:752–764. doi: 10.1093/brain/awf079. [DOI] [PubMed] [Google Scholar]
- Gross JJ, Levenson RW. Emotional suppression: Physiology, self-report, and expressive behavior. Journal of Personality and Social Psychology. 1993;64:970–986. doi: 10.1037//0022-3514.64.6.970. [DOI] [PubMed] [Google Scholar]
- Gusnard DA, Akbudak E, Shulman GL, Raichle ME. Medial prefrontal cortex and self-referential mental activity: Relation to a default mode of brain function. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:4259–4264. doi: 10.1073/pnas.071043098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harciarek M, Jodzio K. Neuropsychological differences between frontotemporal dementia and Alzheimer’s disease: A review. Neuropsychology Review. 2005;15:131–145. doi: 10.1007/s11065-005-7093-4. [DOI] [PubMed] [Google Scholar]
- Johnson SC, Baxter LC, Wilder LS, Pipe JG, Heiserman JE, Prigatano GP. Neural correlates of self-reflection. Brain. 2002;125:1808–1814. doi: 10.1093/brain/awf181. [DOI] [PubMed] [Google Scholar]
- Keane J, Calder AJ, Hodges JR, Young AW. Face and emotion processing in frontal variant frontotemporal dementia. Neuropsychologia. 2002;40:655–665. doi: 10.1016/s0028-3932(01)00156-7. [DOI] [PubMed] [Google Scholar]
- Kelley WM, Macrae CN, Wyland CL, Caglar S, Inati S, Heatherton TF. Finding the self? An event-related fMRI study. Journal of Cognitive Neuroscience. 2002;14:785–794. doi: 10.1162/08989290260138672. [DOI] [PubMed] [Google Scholar]
- Keltner D, Anderson C. Saving face for Darwin: The functions and uses of embarrassment. Current Directions in Psychological Science. 2000;9:187–192. [Google Scholar]
- Keltner D, Buswell BN. Embarrassment: Its distinct form and appeasement functions. Psychological Bulletin. 1997;122:250–270. doi: 10.1037/0033-2909.122.3.250. [DOI] [PubMed] [Google Scholar]
- Lane RD, Reiman EM, Axelrod B, Yun L-S, Holmes A, Schwartz GE. Neural correlates of levels of emotional awareness: Evidence of an interaction between emotion and attention in the anterior cingulated cortex. Journal of Cognitive Neuroscience. 1998;10:525–535. doi: 10.1162/089892998562924. [DOI] [PubMed] [Google Scholar]
- Lavenu I, Pasquier F, Lebert F, Petit H, Van der Linden M. Perception of emotion in frontotemporal dementia and Alzheimer disease. Alzheimer Disease and Associated Disorders. 1999;13:96–101. doi: 10.1097/00002093-199904000-00007. [DOI] [PubMed] [Google Scholar]
- Levenson RW. Emotion elicitation with neurological patients. In: Coan JA, Allen JJB, editors. Handbook of emotion elicitation and assessment. New York: Oxford University Press; 2007. pp. 158–168. [Google Scholar]
- Levenson RW, Ascher E, Goodkind M, McCarthy M, Sturm V, Werner K. Laboratory testing of emotion and frontal cortex. In: Goldenberg G, Miller BL, editors. Handbook of clinical neurology. Vol. 88: Neuropsychology and behavioral neurology. Edinburgh, Scotland: Elsevier; 2008. pp. 489–498. [DOI] [PubMed] [Google Scholar]
- Levenson RW, Miller BL. Loss of cells—loss of self: Frontotemporal lobar degeneration and human emotion. Current Directions in Psychological Science. 2007;16:289–294. [Google Scholar]
- Lewis M. Embarrassment: The emotion of self exposure and evaluation. In: Tangney JP, Fischer KW, editors. Self-conscious emotions: The psychology of shame, guilt, embarrassment, and pride. New York: Guilford Press; 1995. pp. 198–218. [Google Scholar]
- Lewis M, Sullivan MW, Stanger C, Weiss M. Self-development and self-conscious emotions. Child Development. 1989;60:146–156. [PubMed] [Google Scholar]
- Litvan I, Agid Y, Sastry N, Jankovic J, Wenning GK, Goetz CG, et al. What are the obstacles for an accurate clinical diagnosis of Pick’s disease? A clinicopathologic study. Neurology. 1997;49:62–69. doi: 10.1212/wnl.49.1.62. [DOI] [PubMed] [Google Scholar]
- Lough S, Kipps CM, Treise C, Watson P, Blair JR, Hodges JR. Social reasoning, emotion and empathy in frontotemporal dementia. Neuropsychologia. 2006;44:950–958. doi: 10.1016/j.neuropsychologia.2005.08.009. [DOI] [PubMed] [Google Scholar]
- McKhann GM, Albert MS, Grossman M, Miller BL, Dickson D, Trojanowski J. Clinical and pathological diagnosis of frontotemporal dementia. Archives of Neurology. 2001;58:1803–1809. doi: 10.1001/archneur.58.11.1803. [DOI] [PubMed] [Google Scholar]
- Mendez MF, McMurtray AM, Chen AK, Shapira JS, Mishkin F, Miller BL. Functional neuroimaging and presenting psychiatric features of frontotemporal dementia. Journal of Neurology, Neurosurgery and Psychiatry. 2006;77:4–7. doi: 10.1136/jnnp.2005.072496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller BL, Seeley WW, Mychack P, Rosen HJ, Mena I, Boone K. Neuroanatomy of the self: Evidence from patients with frontotemporal dementia. Neurology. 2001;57:817–821. doi: 10.1212/wnl.57.5.817. [DOI] [PubMed] [Google Scholar]
- Miller RS, Leary MR. Social sources and interactive functions of embarrassment. In: Clark M, editor. Emotion and social behavior. New York: Sage; 1992. pp. 202–211. [Google Scholar]
- Morris JC. The Clinical Dementia Rating (CDR): Current version and scoring rules. Neurology. 1993;43(11):2412–2414. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- Neary D, Snowden JS, Gustafson L, Passant U, Stuss D, Black S, et al. Frontotemporal lobar degeneration: A consensus on clinical diagnostic criteria. Neurology. 1998;51:1546–1554. doi: 10.1212/wnl.51.6.1546. [DOI] [PubMed] [Google Scholar]
- Obrist PA. Cardiovascular psychophysiology. New York: Plenum Press; 1981. [Google Scholar]
- Ochsner KN, Knierim K, Ludlow DH, Hanelin J, Ramachandran T, Glover G, et al. Reflecting upon feelings: An fMRI study of neural systems supporting the attribution of emotion to self and other. Journal of Cognitive Neuroscience. 2004;16:1746–1772. doi: 10.1162/0898929042947829. [DOI] [PubMed] [Google Scholar]
- Platek SM, Keenan JP, Gallup GG, Mohamed FB. Where am I? The neurological correlates of self and other. Cognitive Brain Research. 2004;19:114–122. doi: 10.1016/j.cogbrainres.2003.11.014. [DOI] [PubMed] [Google Scholar]
- Rankin KP, Baldwin E, Pace-Savitsky C, Kramer JH, Miller BL. Self-awareness and personality change in dementia. Journal of Neurology, Neurosurgery, and Psychiatry. 2005;76:632–639. doi: 10.1136/jnnp.2004.042879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rankin KP, Kramer JH, Mychack P, Miller BL. Double dissociation of social functioning in frontotemporal dementia. Neurology. 2003;60:266–271. doi: 10.1212/01.wnl.0000041497.07694.d2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratnavalli E, Brayne C, Dawson K, Hodges JR. The prevalence of frontotemporal dementia. Neurology. 2002;58:1615–1621. doi: 10.1212/wnl.58.11.1615. [DOI] [PubMed] [Google Scholar]
- Rosen HJ, Allison SC, Ogar JM, Amici S, Rose K, Dronkers N, et al. Behavioral features in semantic dementia vs other forms of progressive aphasias. Neurology. 2006;67:1752–1756. doi: 10.1212/01.wnl.0000247630.29222.34. [DOI] [PubMed] [Google Scholar]
- Rosen HJ, Gorno-Tempini ML, Goldman WP, Perry RJ, Schuff N, Weiner M, et al. Patterns of brain atrophy in frontotemporal dementia and semantic dementia. Neurology. 2002;58:198–208. doi: 10.1212/wnl.58.2.198. [DOI] [PubMed] [Google Scholar]
- Rosen HJ, Pace-Savitsky K, Perry RJ, Kramer JH, Miller BL, Levenson RW. Recognition of emotion in the frontal and temporal variants of frontotemporal dementia. Dementia and Geriatric Cognitive Disorders. 2004;17:277–281. doi: 10.1159/000077154. [DOI] [PubMed] [Google Scholar]
- Rosen HJ, Perry RJ, Murphy J, Kramer JH, Mychack P, Schuff N, et al. Emotion comprehension in the temporal variant of frontotemporal dementia. Brain. 2002;125:2286–2295. doi: 10.1093/brain/awf225. [DOI] [PubMed] [Google Scholar]
- Rosen HJ, Wilson MR, Schauer GF, Allison S, Gorno-Tempini ML, Pace-Savitsky C, et al. Neuroanatomical correlates of impaired recognition of emotion in dementia. Neuropsychologia. 2006;44:365–373. doi: 10.1016/j.neuropsychologia.2005.06.012. [DOI] [PubMed] [Google Scholar]
- Seeley WW, Carlin DA, Allman JM, Macedo MN, Bush C, Miller BL, et al. Early frontotemporal dementia targets neurons unique to apes and humans. Annals of Neurology. 2006;60:660–667. doi: 10.1002/ana.21055. [DOI] [PubMed] [Google Scholar]
- Shearn D, Bergman E, Hill K, Abel A, Hinds L. Facial coloration and temperature responses in blushing. Psychophysiology. 1990;27:687–693. doi: 10.1111/j.1469-8986.1990.tb03194.x. [DOI] [PubMed] [Google Scholar]
- Snowden JS, Gibbons ZC, Blackshaw A, Doubleday E, Thompson J, Craufurd D, et al. Social cognition in frontotemporal dementia and Huntington’s disease. Neuropsychologia. 2003;41:688–701. doi: 10.1016/s0028-3932(02)00221-x. [DOI] [PubMed] [Google Scholar]
- Sturm VE, Levenson RW, Rosen HJ, Allison SC, Miller BL. Preserved simple emotion and diminished self-conscious emotion in frontotemporal lobar degeneration. Brain. 2006;129:2508–2516. doi: 10.1093/brain/awl145. [DOI] [PubMed] [Google Scholar]
- Takahashi H, Yahata N, Koeda M, Matsuda T, Asai K, Okubo Y. Brain activation associated with evaluative processes of guilt and embarrassment: An fMRI study. NeuroImage. 2004;23:967–974. doi: 10.1016/j.neuroimage.2004.07.054. [DOI] [PubMed] [Google Scholar]
- Tangney JP. The self-conscious emotions: Shame, guilt, embarrassment, and pride. In: Dalgleish T, Power MJ, editors. Handbook of cognition and emotion. New York: Wiley; 1999. pp. 541–568. [Google Scholar]
- Werner KH, Roberts NA, Rosen HJ, Dean DL, Kramer JH, Weiner MW, et al. Emotional reactivity and emotion recognition in frontotemporal lobar degeneration. Neurology. 2007;69:148–155. doi: 10.1212/01.wnl.0000265589.32060.d3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zysset S, Huber O, Ferstl E, von Cramon DY. The anterior frontomedian cortex and evaluative judgment: An fMRI study. Neuro-Image. 2002;15:983–991. doi: 10.1006/nimg.2001.1008. [DOI] [PubMed] [Google Scholar]