Abstract
We report an investigation of P300 measures of information processing in patients with generalized epilepsy of the absence type and those with complex partial epilepsy. Studies have demonstrated that absence patients perform more poorly than complex partial patients on behavioral tests of sustained attention (the Continuous Performance Test, or CPT). Duncan (1988) reported that P300 was significantly reduced in a group of absence patients as compared with healthy controls. The present investigation was undertaken to compare the attention deficit in absence patients to that in complex partial seizure patients. Thus, ERPs were recorded while participants with absence seizure disorder, complex partial seizure disorder, and healthy controls performed auditory and visual versions of the CPT. A significant reduction in the amplitude of P300 on the visual CPT was observed in both groups of seizure patients as compared to controls. In contrast, P300 on the auditory CPT was reduced only in the group with absence seizures. These ERP data support and amplify previous behavioral findings of the impaired capacity of absence patients to mobilize and sustain attentional resources. Auditory sustained attention seems to be more affected by the pathophysiology of absence epilepsy than visual attention. Two possible factors may be involved: (a) There are separate visual and auditory attention systems in the brain, and the latter is more vulnerable than the former (Duncan et al., 2005); and (b) Auditory processing depends on intact mechanisms in the brainstem, which are dysfunctional in patients with absence seizures.
Keywords: Event-related potentials, P300, absence epilepsy, complex partial epilepsy, attention, CPT
1. Introduction
The symptom of a brief loss of attention or “absence” associated with the seizures in absence epilepsy has been the focus of research for many years (e.g., Schwab, 1939; Penfield and Jasper, 1954; Mirsky and Van Buren, 1965; Duncan, 1988; Gloor, 1988). Schwab (1939) observed that in patients suffering from absence attacks, responses to auditory or visual stimuli were delayed substantially during their characteristic paroxysmal spike-wave discharges. At times they failed to respond entirely. In 1960, Mirsky et al. published a description of the behavioral characteristics of patients with absence epilepsy. They showed that in two independent samples of patients, those with absence epilepsy performed more poorly than healthy or seizure (focal epilepsy) controls on a test of visual sustained attention—the Continuous Performance Test (CPT; Rosvold et al., 1956). The test requires the participant to attend to a visual display in which letters of the alphabet appear one at a time, with a response required to a specific target letter, i.e., the letter X. This finding, that absence patients miss significantly more targets than healthy or seizure controls, has been replicated a number of times (e.g., Lansdell and Mirsky, 1964; Fedio and Mirsky, 1969; Duncan, 1988). The significance of this finding was discussed in detail in a review of research on petit mal epilepsy (Mirsky et al., 1986).
In their theoretical treatise on absence epilepsy, which they renamed “centrencephalic” epilepsy, Penfield and Jasper (1954) accounted for observations such as Schwab’s as a disturbance in consciousness, reflecting the malfunctioning of a widespread (centrencephalic) system headquartered in the brainstem, with connections to all parts of the cerebrum. Their conception postulates the existence of a brain region (the centrencephalon) that forms the “neural substratum of consciousness.” Consciousness is defined as the highest level of functional integration of sensory and motor processes; this centrencephalic system has the responsibility for the maintenance of consciousness. This view is compatible with those of Moruzzi and Magoun (1949) and Lindsley (1950), in their description of the functions of the brainstem reticular activating system. Nevertheless, the precise neurobehavioral disturbance underlying this symptom is not well understood, and has been the subject of a number of different interpretations over the years.
On the basis of his research, Gloor (1988) questioned the wisdom of calling the absence a temporary loss of consciousness, and suggested that the absence might better be thought of as a temporary dementia. He also suggested that the term “centrencephalic” neglected the crucial participation of the cortex in the phenomena of the absence. He therefore proposed the term “cortico-reticular” to reflect more accurately the pathophysiology of the absence.
Mirsky and Van Buren (1965) speculated that altered sensory processing and/or temporary altered functioning of a central attentional mechanism might be responsible for the symptom. Orren (1974, 1978) reported smaller or absent visual evoked potentials in absence patients, when recorded during the characteristic spike-wave bursts seen in the electroencephalogram (EEG) during absence seizures.
In a later study, Duncan (1988) recorded event-related brain potentials (ERPs) in the interictal period in patients with absence seizures. ERPs were recorded while patients performed visual and auditory versions of the CPT. The visual and auditory P300 components in the absence patients were significantly smaller than in the matched healthy controls. However, Duncan’s study did not establish whether the reduction in P300 was specific to absence epilepsy as opposed to other seizure disorders. The present study was undertaken to extend the sample of participants to patients with complex partial (i.e., temporal lobe) seizures in order to explore further the specificity of the P300 finding. In addition, examination of other ERP components, reflecting both sensory (e.g., N100) and cognitive (slow wave) processing was undertaken, in order to help clarify the nature of the behavioral deficit.
2. Methods
2.1. Participants
Participants were 9 patients diagnosed with absence epilepsy, 13 patients diagnosed with complex partial epilepsy, and 10 healthy controls (see Table 1 for demographic data). Patients were referred from the National Institutes of Health Epilepsy Clinic. Healthy control participants were recruited through newspaper advertisements or from listings for research volunteers at the National Institutes of Health. There were no significant differences among the three groups on any of the matching variables (age, years of education, sex, race, handedness). Moreover, the two seizure groups did not differ in terms of time from onset of seizures. At the time of testing, all patients were on stable doses of one or more anticonvulsant medications.1
Table 1.
Demographic Data
| Group | n | Age1,2 | Education1,2 | Sex | Race3 | Handedness |
|---|---|---|---|---|---|---|
| Absence | 9 | 29.3 (8.7) | 14.2 (2.2) | 6 female | 8 1 0 | 8 right |
| Complex Partial | 13 | 34.5 (8.2) | 14.3 (2.6) | 8 female | 10 0 3 | 12 right |
| Healthy Controls | 9 | 29.8 (10.1) | 15.3 (2.0) | 6 female | 9 0 0 | 8 right |
Years
Mean (SD).
White, Hispanic, African-American.
Healthy controls were screened to exclude anyone with a significant past or current psychiatric disorder (as assessed with the lifetime version of the Schedule of Affective Disorders and Schizophrenia; Spitzer and Endicott, 1978). They reported no history of psychiatric disorder among their first-degree relatives. Controls were healthy, free of medications, and had no history of traumatic brain injury or neurologic disorders.
All participants gave written informed consent.
2.2. Stimuli and Procedure
Each participant was administered a battery of ERP tasks over the course of several testing sessions. This report focuses on the data obtained during visual and auditory versions of the CPT, a test of sustained attention. The CPT tasks comprised the first session. In the visual CPT used in this study (Mirsky and Van Buren, 1965; Rosvold et al., 1956), letters are presented for 200 ms at a constant rate of 1/s. The participant is instructed to press a button with the thumb when the letter X appeared (“targets”) and to withhold a response to other letters (“nontargets”). The letter X appeared on 25% of the trials. All letters subtended a visual angle of approximately 0.8 deg horizontally by 1.0 deg vertically.
In the auditory version of the CPT, stimuli were tone bursts, low (600 Hz), medium (1050 Hz), or high (1500 Hz) in pitch. The three tones were equated for loudness (50 dB sensation level) and rise/fall time (10 ms) and were delivered binaurally over broadband masking noise (60 dB sound pressure level). In the auditory CPT-X, the subject was instructed to press a button with the thumb when the high tone (“target”) occurred and to withhold a response to the other tones (“nontargets”). Tones occurred at the rate of 1/s and were 200 ms in duration. The high tone was presented on 25% of the trials.
While performing the CPT, the subject sat in a comfortable chair in a sound attenuated, shielded room. Prior to both tasks, the subject heard a standard set of instructions and was instructed to respond as quickly as possible without sacrificing accuracy. A series of practice trials preceded both of the tasks. Each task comprised 600 trials, lasted 10 min, and was followed by a rest period of several minutes. The order of presentation of the visual and auditory tasks was counterbalanced across participants in each group.
2.3. Data Acquisition
We recorded the EEG using Grass Ag/AgCl cup electrodes affixed with collodion at frontal (Fpz, F3, Fz, F4), central (C3, Cz, C4), parietal (P3, Pz, P4), and occipital (Oz) scalp sites according to the International 10–20 system (Jasper, 1958), referred to linked earlobes. Participants were grounded with a forehead electrode (Fp2). The EEG was sampled for 900 ms, beginning 100 ms before stimulus onset. Eye movements were recorded from above (Fp1) and 2 cm below the outer canthus of the left eye. The EEG and EOG were amplified 10,000 times with a bandpass of 0.01–100 Hz (-3 dB/octave) and sampled at 200 Hz.
2.4. Data Quantification
There were two stimulus categories in the CPT-X task, “targets” and “nontargets.” We quantified three measures of performance on each task, accuracy and reaction time to targets and commission errors to nontargets. Reaction time was defined as the time from target stimulus onset to the button press; accuracy was the percentage of targets with responses 200 to 800 ms after stimulus onset. Errors of commission were responses to nontargets. Prior to averaging, trials with incorrect responses or eye movement contamination were discarded.
For each participant, average ERPs were computed at all electrode sites for targets and nontargets in both modalities. Measures of ERP components used in the statistical analyses were derived from each participant’s ERPs elicited by the p = .25 targets in each task, using standard baseline-to-peak measurements (Picton et al., 2000). The latency of a component was defined as the time from stimulus onset to the point of peak amplitude at the electrode site with maximal amplitude. The latency windows for the visual N100, N200, and P300 components were 100–200 at Oz, 200–300 at Cz, and 300–600 ms at all sites, respectively. The latency windows for the auditory N100, N200, and P300 components were 75–175 ms at Cz, 175–275 ms at Cz, and 275–575 ms at all sites, respectively. P200 was defined as the maximum positive peak between N100 and N200 at Cz.
To reduce overlap with P200, N200 was quantified at Cz in the target minus nontarget difference waveforms. Mean slow wave activity was quantified at midline electrode sites by summing the voltages from 555 to 750 ms for visual ERPs and from 505 to 700 ms for auditory ERPs and dividing by the number of points sampled (i.e., 40)
2.5. Statistical Analysis
Repeated-measures analysis of variance (ANOVAs) was used to evaluate the effects of group on ERP and performance data. Separate ANOVAs were done for each ERP component and performance measure. Statistical significance was evaluated using an alpha level of .05 and Greenhouse-Geisser degrees of freedom were used where appropriate. When an ANOVA produced a significant main effect or interaction, the Bonferroni t-test was used for paired comparisons. We analyzed P300 and slow wave data at the midline electrodes to assess group effects, and normalized amplitudes at lateral scalp locations (F3, C3, P3, F4, C4, P4) to assess differences in distribution (McCarthy and Wood, 1985).
3. Results
3.1. Performance
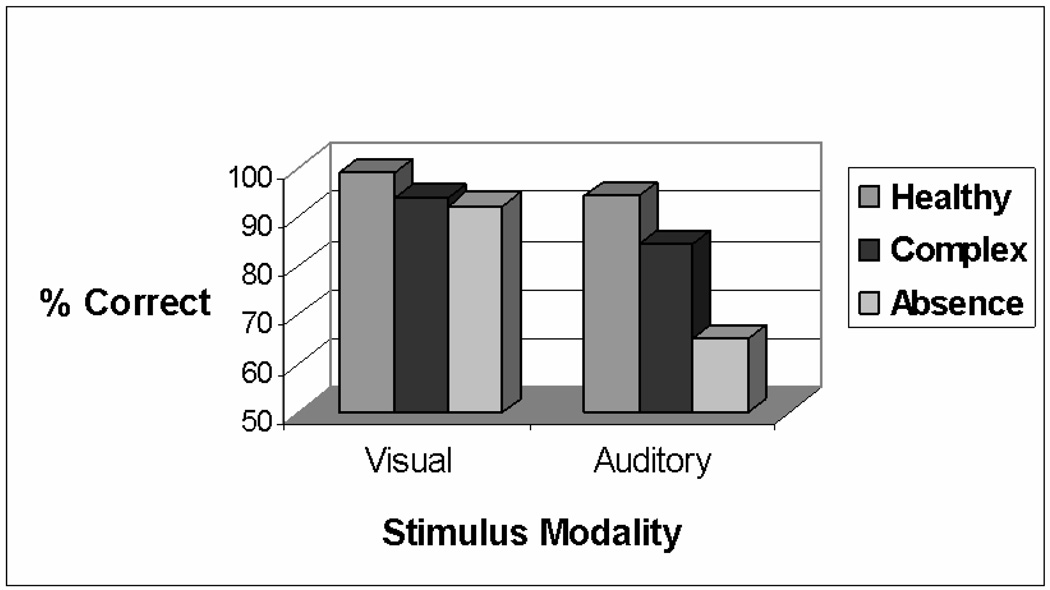
In the visual CPT, there were no significant group effects on measures of accuracy, reaction time, or commission errors (Table 2). In contrast, there was a significant group effect on the accuracy of performance in the auditory CPT, F (2,25) = 7.48, p = .0028. Post hoc tests showed that the absence patients were significantly less accurate than the controls or complex partial patients. These effects are illustrated in Figure 1. The data in Table 2 also highlight other trends indicative of impaired auditory processing in the absence group: It was the only group that did not show shorter reaction times in the auditory than in the visual CPT. In addition, the absence group had an increased rate of commission errors on the auditory CPT than the other groups, although the difference was not significant.
Table 2.
Mean1 performance data on the Visual and Auditory CPT
| Reaction Time (ms) | Accuracy (%) | Commission Errors (%) | ||||
|---|---|---|---|---|---|---|
| Visual | Auditory | Visual | Auditory | Visual | Auditory | |
| Group | ||||||
| Healthy Controls | 481 (78) | 443 (72) | 99.0 (1.0) | 94.3 (6) | 0.2 (0.3) | 1.0 (1.4) |
| Complex Partial | 460 (49) | 413 (72) | 94.0 (9.8) | 84.6 (16.9) | 1.1 (1.0) | 4.5 (5.6) |
| Absence | 476 (80) | 479 (60) | 92.2 (9.0) | 65.0 (26.6)* | 0.9 (1.3) | 6.3 (7.4) |
Mean (SD).
p =.0028.
Fig 1.
Performance on the CPT in the three groups. Percent correct refers to responses to targets. Whereas the three groups did not differ significantly on performance on the Visual CPT, the performance of the absence epilepsy group was significantly poorer on the Auditory CPT.
3.2. P300
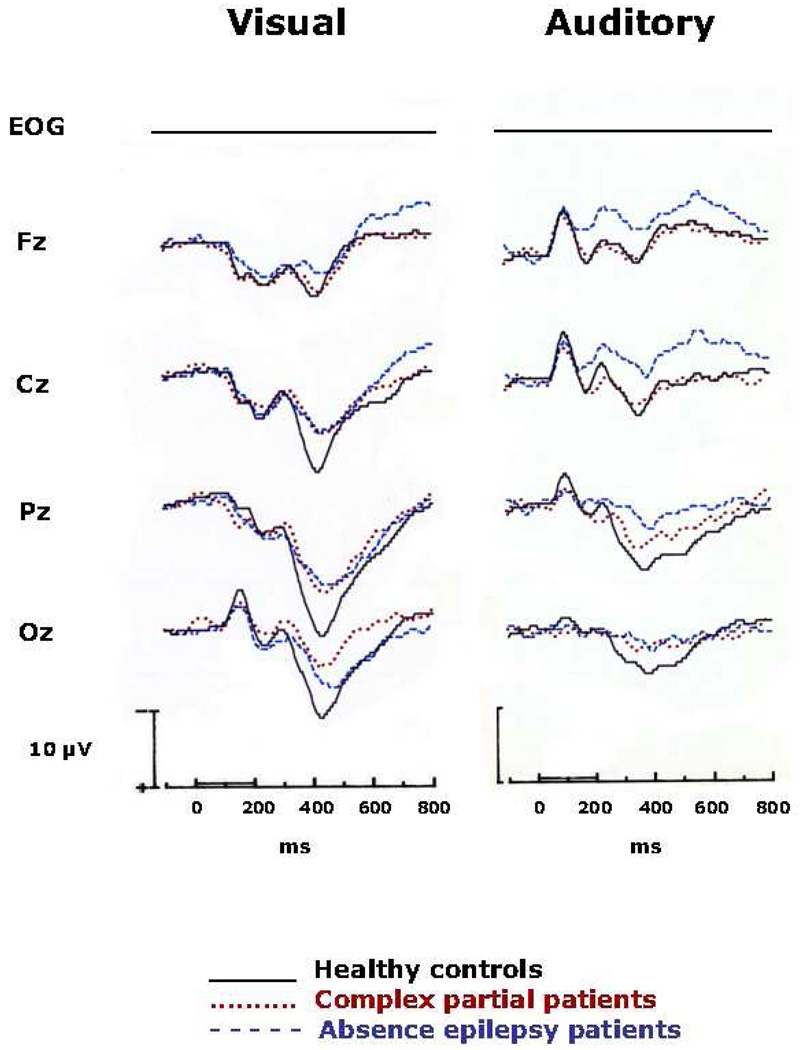
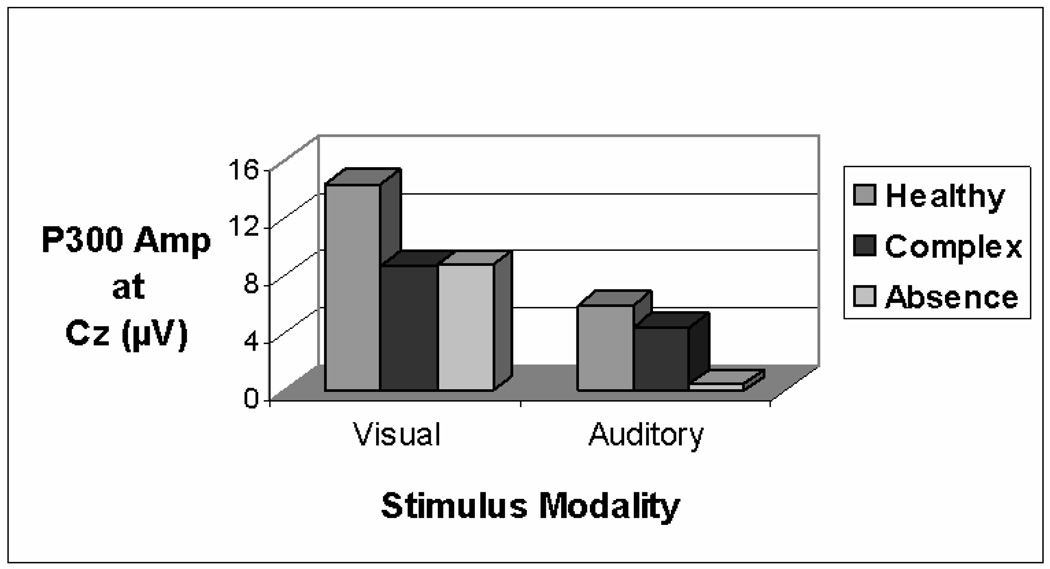
The grand average ERPs are displayed in Figure 2, in which the data for the three groups are superimposed at the four midline electrode sites and EOG. It appears that, as compared with the healthy controls, the two epilepsy groups had smaller P300s on the visual CPT. This observation was confirmed by analysis of variance on P300 amplitude at Pz, F (2,27) = 5.20, p = .0123. The two epilepsy groups did not differ. The mean amplitudes at Cz for the three groups are shown in Figure 3. There was a trend for a similar group difference at Cz, but it was not statistically significant (p = .0883).
Fig. 2.
Grand average ERPs elicited by targets (p = 0.25) in the Visual and Auditory CPT. The ERPs for the three groups are superimposed at the midline electrode sites and EOG. Stimulus onset occurred at 0 ms, and positivity is indicated by a downward deflection. Note that in the Visual CPT, P300 was smaller in the two seizure groups than in the healthy control group. In contrast, P300 and slow wave in the auditory CPT were smaller in the absence group; the seizure control and healthy control groups did not differ significantly.
Fig 3.
P300 amplitude at Cz in the Visual and Auditory CPT. It is apparent that as compared with the healthy control group, the two seizure groups had smaller P300s in the visual CPT. However, in the Auditory CPT, only the absence patients had a significantly smaller P300 in comparison to the seizure and healthy controls.
In the auditory CPT, there was a main effect of group at Cz, F (2,26) = 5.59, p < .05. Post hoc analyses confirmed that P300 was smaller in the absence group than in the complex partial and healthy control groups, which did not differ (see Figure 3). The group difference at Pz approached significance (p = .0652).
There were no significant differences in P300 latency on either task.
Analysis of variance of normalized P300 amplitudes was conducted to evaluate group differences in scalp distribution. There were no group differences in the scalp distribution of P300 elicited in the visual CPT. In contrast, the three-way interaction of Group x Hemisphere x Anterior/Posterior was statistically significant in the auditory CPT, F (4,52) = 3.05, p = .0269. Post hoc tests revealed that all groups had P300s with the typical midline distribution (frontal < central < parietal). In addition, as compared to the control and complex groups, the absence group had larger P300s at F3 and P4 but smaller P300s at C3, P3, F4, and C4.
3.3. Slow wave
There were no significant group differences in slow wave amplitude in the visual CPT. In the auditory task, there was a significant main effect of group at Cz, F (2,28) = 5.70, p = .0089. As shown in Figure 2, the absence group had slow waves at Cz that were more negative than those of the complex partial or control groups. The apparent difference at Fz failed to reach statistical significance (p = .0732).
None of the analyses of normalized slow wave amplitude revealed any significant group effects.
3.4. Other components
There were no significant differences among the groups in N100, P200, or N200. There were, however, nonsignificant trends suggesting that auditory N100 was 5–9 ms later in the absence group than in the complex partial or control groups. The mean and standard deviation (SD) of the latency of auditory N100 elicited by targets were 104 (9), 98 (18), and 98 (8), and by nontargets were 106 (9), 101 (12), and 97 (8) for the absence, complex partial, and control groups, respectively. The corresponding mean and SD of N100 latencies on the visual CPT were 144 (22), 148 (25), 143 (19) to targets and 152 (22), 146 (24), and 148 (37) to nontargets.
4. Discussion
The ERP data elicited during the CPT in this study support and amplify the previous behavioral findings of impaired attention in patients with absence epilepsy. Whereas both absence and complex partial patients had smaller P300s in the visual CPT-X, relative to healthy controls, only the absence patients showed a significantly smaller auditory P300. The specificity of impaired auditory information processing in absence epilepsy is supported further by the results of the slow wave analysis. Slow wave amplitude was significantly more negative at Cz in the absence group than in the other two groups. Additionally, some of the behavioral data shown in Table 2 are consistent with the view of impaired auditory processing in the absence group.
There have been a number of investigations in recent years of ERPs in patients with seizure disorders (e.g., Fukai et al., 1990; Zgorzalewicz and Nowak, 2000; Zgorzalewicz, 2006; Chayasirisobhon et al., 2007; Bocquillon et al., 2008). The focus and the methods used in these studies were different from the present one, thereby precluding any direct comparison of results. Nevertheless, there are a number of suggestive findings that seem to support some of the results we obtained. Thus, Zgorzalewicz (2006) reported higher N2-P3 amplitudes in patients with partial seizures than in patients with generalized seizures. Also, Chayasirisobhon et al. (2007) found no differences in P300 to auditory stimuli between temporal lobe cases and healthy controls.
The question may arise as to why the absence group was not significantly impaired on the visual CPT task, as prior studies have reported (Mirsky et al., 1960; Lansdell and Mirsky, 1964; Fedio and Mirsky, 1969). Whereas it is true that differences among groups have been observed in the visual CPT-X task, the differences between groups were typically more pronounced on the more demanding AX version of the CPT. The AX task was not used in the present ERP analysis. Moreover, there is a recent finding (Levav et al., 2002) suggesting that patients with the diagnosis of juvenile myoclonic epilepsy, a subtype of absence epilepsy, are relatively unimpaired on the visual form of the CPT, as compared with their marked impairment on the auditory version. Two other recent investigations (Sönmez et al., 2004; Kim et al., 2007) confirm the presence of impaired auditory processing in juvenile myoclonic epilepsy. The latter group of researchers reported that the largest cognitive test differences between these patients and healthy controls were in tasks dependent on auditory processing (i.e., list learning, digit span, verbal fluency).
To the extent that the current group of absence patients includes patients with the diagnosis of juvenile myoclonic epilepsy, it would be expected that their CPT deficit would be confined to the auditory task. Unfortunately, the clinical data necessary to establish the subtype of absence epilepsy is not available.
P300 and slow wave are thought to reflect the capacity to summon attentional resources (Squires et al., 1975; Duncan-Johnson and Donchin, 1977). A number of studies have pointed to the participation of brainstem structures and /or the locus coeruleus-norepinephrine system in the genesis of P300 (e.g., Duncan, 2003; Polich, 2007). Of signal importance is the fact that no differences were found among groups in the sensory components (N100, P200) elicited by the CPT stimuli. This provides convincing evidence that sensory reception and initial processing appear to be intact in absence patients. It is in the subsequent processing of this information that the cognitive capacity of the absence patient falters. It is important to recall that the CPT data in this study, indicating impaired auditory attention, were gathered during interictal epochs. This supports the contention (Mirsky and Grady, 1988) that there is a pathophysiological reticulo-cortical process in absence patients that is active continuously (i.e., between seizures), reducing attentional capacity even without observable ictal EEG phenomena. However, the process increases in intensity from time to time, possibly by recruiting more neural tissue into the growing absence seizure, and erupting from time to time in paroxysmal bursts of three-per-second, symmetrical and synchronous spike-wave activity in the EEG. The reduced P300 and slow wave findings in this study are witness to this continuous subclinical, subcortical perturbation.
There is another major point to be emphasized by these results, namely, that the deficits in attention in the absence patients are primarily in the auditory modality. This is further support for the distinction drawn by Duncan et al. (2005) between the visual and auditory attention “systems” in the brain, and points again to the greater vulnerability of the auditory attention system. The latter includes, importantly, the auditory relay nuclei of the brainstem (the superior olivary complex, the lateral lemniscus, and the inferior colliculus). This, in turn, calls to mind the “centrencephalic” (Penfield and Jasper, 1954) and/or the “cortico-reticular” (Gloor, 1988) hypotheses, which emphasize the participation of the brainstem in the manifestation of the absence phenomenon and in the pathophysiology of the disorder. Some direct support for the vulnerability of the auditory brain structures in absence epilepsy is provided by the abnormal brainstem auditory evoked responses seen in these patients (Mirsky, 1988). It will be recalled, as well, that there were suggestive, though nonsignificant, prolongations of auditory N100 latencies in the absence group in the present study. Longer N100 latencies would be consistent with delays at the level of the brainstem.
To summarize, these data illustrate the utility of ERPs, and in particular, P300, in illuminating the nature of behavioral deficits. The data also emphasize further the complexity and differentiation of attentional processing—dual attention systems in the brain, with distinct and different vulnerabilities. These results may also have implications for ultimately unraveling the pathophysiology of seizure disorders, and for tailoring educational programs for children with different forms of seizure disorders.
Acknowledgments
A preliminary report of this study was presented at the 14th World Congress of Psychophysiology, the Olympics of the Brain, International Organization of Psychophysiology, associated with the United Nations (New York), September 8–12, 2008, St. Petersburg, Russia.
The research reported here was supported in part by the Intramural Research Programs of the National Institute of Mental Health and the National Institute of Neurological Disorders and Stroke. The opinions and assertions expressed herein are those of the authors and are not to be construed as reflecting the views of the Uniformed Services University or the U.S. Department of Defense.
We thank Michele Levin for her assistance with data collection. We also acknowledge with appreciation the contributions of all of the participants in this research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Medications included Valproic Acid, Ethosuximide, Phenytoin, Mysoline, Phenylethyl Malonuria, Pentobarbital, Carbamazepine, Felbamate, Neurontin, Depakene, and Gabapentin.
References
- Bocquillon P, Dujardin K, Betrouni N, Phalempin V, Houdayer E, Bourriez JL, Derambure P, Szurhaj W. Attention impairment in temporal lobe epilepsy: a neurophysiological approach via analysis of the P300 wave. Hum Brain Mapp. 2008 Nov 25; doi: 10.1002/hbm.20666. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chayasirisobhon WV, Chayasirisobhon S, Tin SN, Leu N, Tehrani K, McGuyckin JS. Scalp-recorded auditory P300 event-related potentials in new-onset untreated temporal lobe epilepsy. Clin. EEG Neurosci. 2007;38:168–171. doi: 10.1177/155005940703800314. [DOI] [PubMed] [Google Scholar]
- Duncan CC. Application of event-related brain potentials to the analysis of interictal attention in absence epilepsy. In: Myslobodsky MS, Mirsky AF, editors. Elements of Petit Mal Epilepsy. New York: Peter Lang; 1988. pp. 341–364. [Google Scholar]
- Duncan CC. Brain potentials in normal and disordered attention: findings in search of a theory. Presidential Address presented at the annual meeting of the Society for Psychophysiological Research; Chicago. 2003. Nov, [Google Scholar]
- Duncan CC, Kosmidis MH, Mirsky AF. Closed head injury-related information processing deficits: An event-related potential analysis. Int. J. Psychophysiol. 2005;58:133–157. doi: 10.1016/j.ijpsycho.2005.05.011. [DOI] [PubMed] [Google Scholar]
- Duncan-Johnson CC, Donchin E. On quantifying surprise: The variation of event-related potentials with subjective probability. Psychophysiology. 1977;14:456–467. doi: 10.1111/j.1469-8986.1977.tb01312.x. [DOI] [PubMed] [Google Scholar]
- Fedio P, Mirsky AF. Selective intellectual deficits in children with temporal lobe or centrencephalic epilepsy. Neuropsychologia. 1969;7:287–300. [Google Scholar]
- Fukai M, Motomura N, Kobayashi S, Asaba H, Sakai T. Event-related potential (P300) in epilepsy. Acta Neurol. Scand. 1990;82:197–202. doi: 10.1111/j.1600-0404.1990.tb04488.x. [DOI] [PubMed] [Google Scholar]
- Gloor P. Neurophysiological mechanism of generalized spike-and-wave discharge and its implication for understanding absence seizures. In: Myslobodsky MS, Mirsky AF, editors. Elements of Petit Mal Epilepsy. New York: Peter Lang; pp. 159–209. [Google Scholar]
- Kim S-Y, Hwang Y-H, Lee H-W, Suh C-K, Kwon S-H, Park S-P. Cognitive impairment in juvenile myoclonic epilepsy. J. Clin. Neurol. 3:86–92. doi: 10.3988/jcn.2007.3.2.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lansdell H, Mirsky AF. Attention in focal and centrencephalic epilepsy. Exp. Neurol. 1964;9:463–469. doi: 10.1016/0014-4886(64)90054-8. [DOI] [PubMed] [Google Scholar]
- Levav M, Mirsky AF, Herault J, Xiong L, Amir N, Andermann E. Familial association of neuropsychological traits in patients with generalized and partial seizure disorders. J. Clin. Exp. Neuropsychol. 2002;24:311–326. doi: 10.1076/jcen.24.3.311.985. [DOI] [PubMed] [Google Scholar]
- Lindsley DB. Attention, consciousness, sleep and wakefulness. In: Field J, Magoun HW, Hall VE, editors. Handbook of Physiology. Washington, DC: American Physiological Society; 1960. pp. 1553–1593. [Google Scholar]
- Mirsky AF. Behavioral and psychophysiological effects of petit mal epilepsy in the light of a neuropsychologically-based theory of attention. In: Myslobodsky MS, Mirsky AF, editors. Elements of Petit Mal Epilepsy. New York: Peter Lang; 1988. pp. 311–340. [Google Scholar]
- Mirsky AF, Duncan CC, Myslobodsky MS. Petit mal epilepsy: a review and integration of recent information. J. Clin. Neurophysiol. 1986;3:179–208. [PubMed] [Google Scholar]
- Mirsky AF, Grady CL. Toward the development of alternative treatments in absence epilepsy. In: Myslobodsky MS, Mirsky AF, editors. Elements of Petit Mal Epilepsy. New York: Peter Lang; 1988. pp. 285–310. [Google Scholar]
- Mirsky AF, Primac DW, Ajmone Marsan C, Rosvold HE, Stevens JA. A comparison of the psychological test performance of patients with focal and nonfocal epilepsy. Exp. Neurol. 1960;2:75–89. doi: 10.1016/0014-4886(60)90049-2. [DOI] [PubMed] [Google Scholar]
- Mirsky AF, Van Buren JM. On the nature of the “absence” in centrencephalic epilepsy: a study of some behavioral, electroencephalographic and autonomic factors. Electroencephalogr. Clin. Neurophysiol. 1965;18:334–348. doi: 10.1016/0013-4694(65)90053-2. [DOI] [PubMed] [Google Scholar]
- Moruzzi G, Magoun HW. Brain stem reticular formation and activation of the EEG. Electroenceph. Clin. Neurophysiol. 1949;1:455–473. [PubMed] [Google Scholar]
- Orren MM. Unpublished Ph.D. dissertation. Boston: Boston University; 1974. Visuomotor behavior and visual evoked potentials during petit mal seizures. [Google Scholar]
- Orren MM. Evoked potential studies in petit mal epilepsy: visual information processing in relation to spike and wave discharges. Electroencephalogr. Clin. Neurophysiol. 1978;34:251–257. [PubMed] [Google Scholar]
- Penfield W, Jasper M, editors. Boston: Little, Brown; 1954. Epilepsy and the Functional Anatomy of the Human Brain. [Google Scholar]
- Picton TW, Bentin S, Berg P, Donchin E, Hillyard SA, Johnson R, Jr, Miller GA, Ritter W, Ruchkin DS, Rugg MD, Taylor MJ. Guidelines for using human event-related potentials to study cognition: recording standards and publication criteria. Psychophysiology. 2000;37:127–152. [PubMed] [Google Scholar]
- Polich J. Updating P300: an integrative theory of P3a and P3b. Clin. Neurophysiol. 2007;118:2128–2148. doi: 10.1016/j.clinph.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosvold HE, Mirsky AF, Sarason I, Bransome ED, Beck LM. A continuous performance test of brain damage. J. Consult. Psychol. 1956;20:343–350. doi: 10.1037/h0043220. [DOI] [PubMed] [Google Scholar]
- Schwab RS. A method of measuring consciousness in petit mal epilepsy. J. Nerv. Ment. Dis. 1939;89:690–691. [Google Scholar]
- Sönmez F, Atakli D, Sari H, Atay T, Arpaci B. Cognitive function in juvenile myoclonic epilepsy. Epilepsy Behav. 2004;5:329–336. doi: 10.1016/j.yebeh.2004.01.007. [DOI] [PubMed] [Google Scholar]
- Spitzer RL, Endicott J. 3rd ed. New York: Biometrics Research, New York State Psychiatric Insititute; 1978. Schedule for Affective Disorders and Schizophrenia (SADS) [Google Scholar]
- Squires NK, Squires KC, Hillyard SA. Two varieties of long-latency positive waves evoked by unpredictable auditory stimuli in man. Electroencephalogr. Clin. Neurophysiol. 1975;38:387–401. doi: 10.1016/0013-4694(75)90263-1. [DOI] [PubMed] [Google Scholar]
- Zgorzalewicz M. [Long latency auditory evoked potentials in schoolchildren and adolescents with epilepsy.] Przegl Lek. 2006;63(Suppl 1):8–13. [PubMed] [Google Scholar]
- Zgorzalewicz M, Nowak R. [P300 event-related potentials in epileptic children and adolescents.] Neurol. Neurochir. Pol. 2000;34(Suppl 1):109–118. [PubMed] [Google Scholar]