Abstract
Context
The endogenous cannabinoid system has been implicated in drug addiction in animal models. The cannabinoid receptor 1 (CNR1) gene is 1 of the 2 receptors expressed in the brain. It has been reported to be associated with alcoholism and multiple drug abuse and dependence.
Objective
To test the hypothesis that the CNR1 gene is associated with nicotine dependence.
Design
Genotype-phenotype association study. Ten single-nucleotide polymorphisms were genotyped in the CNR1 gene in 2 independent samples. For the first sample (n=688), a 3-group case-control design was used to test allele association with smoking initiation and nicotine dependence. For the second sample (n = 961), association was assessed with scores from the Fagerström Test for Nicotine Dependence (FTND).
Settings
Population samples selected from the Mid-Atlantic Twin Registry.
Participants
White patients aged 18 to 65 years who met the criteria of inclusion.
Main Outcome Measures
Fagerström Tolerance Questionnaire and FTND scores.
Results
Significant single-marker and haplotype associations were found in both samples, and the associations were female specific. Haplotype 1-1-2 of markers rs2023239-rs12720071-rs806368 was associated with nicotine dependence and FTND score in the 2 samples (P<.001 and P = .009, respectively).
Conclusion
Variants and haplotypes in the CNR1 gene may alter the risk for nicotine dependence, and the associations are likely sex specific.
Smoking is an addictive behavior and 1 of the leading causes of preventable deaths in developed countries.1,2 Although twin studies3-6 have established that genetic factors play a significant role in the etiology of tobacco smoking and nicotine dependence (ND), the specific genes that influence this behavior remain poorly understood. In recent years, linkage studies7-12 have found suggestive linkage peaks in several chromosomal regions. Candidate genes selected from these linkage regions and other sources were also studied, and several promising genes have been identified.13-16
It is well known that tobacco smoking coincides with the use and/or abuse of other substances. Twin studies17-19 show that smoking has high comorbidity with abuse of alcohol, marijuana, cocaine, amphetamine, and other drugs. Genetic analyses indicate that individuals who use and/or abuse these substances share common genetic factors.20 Pharmacologic and neurochemical studies in animal models suggest that the initial targets of these substances may be different,21 but they all result in dysfunction of similar neurochemical and neuroanatomical pathways.22 This finding is in agreement with human behavioral studies and implies that there may be a common liability underlying the addiction to commonly used substances of abuse.
In recent years, pharmacologic and neurochemical studies have accumulated convincing evidence that the endogenous cannabinoid system is involved in addiction to abused substances.23 Of the 2 cannabinoid receptors reported, cannabinoid receptor 1 (CNR1 [or CB1]) is largely responsible for neurophysiologic and behavioral responses to the addictive behavior. In animal models, CNR1 knockout mice display alteration in rewarding and drug-seeking behavior in response to several substances, including nicotine,24-26 ethanol,27,28 cocaine, amphetamine, and other psychostimulants.23 Cannabinoid agonists mimic the effects of abused substances, and antagonists suppress, attenuate, or block reward and drug-seeking behaviors.29 In human studies, the CNR1 -specific antagonist rimonabant helps cessation of tobacco smoking.30 Direct association studies31-37 of the CNR1 gene have been performed with substance abuse and dependence; however, the results are not always consistent.
The CNR1 gene is located on the long arm of human chromosome 6. The CNR1 protein is a G protein– coupled receptor and is widely expressed in the central nervous system.38-40 In the current version (March 2006 freeze) of the human genome browser, CNR1 spans an approximately 5.5-kilobase (kb) genomic distance. In a recent study,37 CNR1 was shown to have several transcription variants, covering approximately 35 kb of genomic DNA. In this study, we use the Haploview program41 to select 10 single-nucleotide polymorphisms (SNPs) that tagged major haplotypes (frequency >1%) spanning this 35-kb region and to test for association with smoking initiation (SI), ND, and the use and abuse of other substances.
Methods
Study Participants
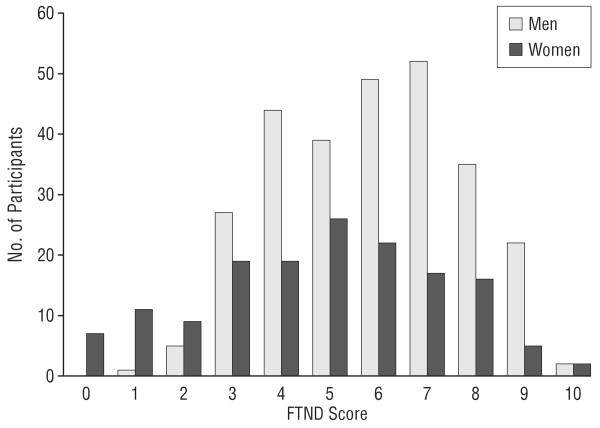
In this study, we used 2 independent samples of white patients aged 18 to 65 years, both drawn from 2 large population-based twin studies of the Mid-Atlantic Twin Registry. The sampling and ascertainment procedures for this study have been described elsewhere.5,42,43 Briefly, female-female twin pairs born between 1934 and 1974 became eligible if both members responded to a mailed questionnaire in 1987-1988. Data on smoking history and ND used in this report were collected at the fourth wave of interviews conducted in 1995-1997. Data on the male-male pairs born between 1940 and 1974 were collected at the second wave of interviews conducted in 1994-1998. The mean (SD) age and educational level of the twins were 36.3 (8.2) years and 14.3 (2.2) years, respectively, for the female-female pairs and 37.0 (9.1) years and 13.6 (2.6) years, respectively, for the male-male pairs. In this study, we used a subset of twins of European ancestry and randomly selected 1 twin from each pair. All the study participants were unrelated. All individuals were assessed with basic smoking history and the Fagerström Tolerance Questionnaire (FTQ)44 or the Fagerström Test for Nicotine Dependence (FTND).45 The FTQ was an 8-item questionnaire (score range, 0-11), and the FTND was a 6-item questionnaire (score range, 0-10). Both the FTQ and the FTND have been widely used to evaluate ND. The first sample, denoted as the Virginia Study of Nicotine Dependence (VAND), contains 688 individuals. For this sample, we used a 3-group design: nonsmokers (n=244, 164 men and 80 women), defined as those who never smoked a cigarette up to the time of the assessment; regular smokers with low ND (n=215, 151 men and 64 women), defined as those who smoked at least 5 cigarettes per week for 5 years and had FTQ scores between 0 and 2 at their lifetime maximum tobacco consumption; and regular smokers with high ND (n=229, 150 men and 79 women), defined as those who smoked for 5 years or more and had an FTQ score between 7 and 11. This 3-group design was to evaluate separately the influence of CNR1 on SI and ND, 2 measurements with overlapping but not identical genetic effects.5,46 To estimate the influence of CNR1 on SI, we compared the allele frequencies of testing SNPs between the nonsmokers and regular smokers (which included both the low -and high-ND groups). To estimate the influence of CNR1 on ND, we compared the allele frequencies between the low- and high-ND groups. Smokers with FTQ scores between 2 and 7 were not used in this dichotomized design. The second sample, denoted as the Virginia Study of Anxiety and Neuroticism (VAANX), was a sample initially selected for the study of anxiety and neuroticism. We used the software package Mx (http://www.vcu.edu/mx/) to perform a multivariate genetic analysis to identify a latent phenotype that reflected genetic covariation (ie, shared genetic susceptibility) across the 6 phenotypes (major depressive disorder, generalized anxiety disorder, panic disorder, agoraphobia, social phobia, and neuroticism [Hettema et al47 provide the details]). The inclusion criterion was the top and bottom 25th percentile of a genetic factor score—a composite index that represented several internalizing anxiety and neuroticism phenotypes.48 The cases had a mean raw neuroticism score of 6.30 (z score=1.04) and had the following frequencies of the target psychiatric illnesses: major depressive disorder (80.1%), generalized anxiety disorder (53.8%), panic disorder (20.5%), agoraphobia (14.1%), and social phobia (17.5%). The controls were free of the 5 disorders and had a mean raw neuroticism score of 0.55 (z score=−0.89). This sample contains 1128 individuals, of whom 6 were included in the VAND sample and another 161 were co-twins of the participants in the VAND. To maintain the independence of the 2 samples, these 167 overlapping individuals were removed from all analyses conducted with the VAANX sample. Because the VAANX was selected by the genetic factor score, there were not enough individuals with high and low FTQ or FTND scores for a dichotomized design, so we used a quantitative design (FTND scores) to assess ND in this sample. As defined in the VAND sample, individuals who reported never having smoked regularly and whom we, therefore, did not attempt to assess with the FTQ or FTND were classified as nonsmokers. All others were classified as smokers, including those who were not smoking at assessment but had smoked previously. The remaining 961 individuals included 532 nonsmokers (299 men and 233 women) and 429 smokers (276 men and 153 women). The distributions of FTND scores for these individuals are shown in Figure 1. For the study participants, both the FTQ and FTND scores were negatively correlated with the level of education (polychoric correlation: FTQ, r=−0.31 [P=.04]; FTND, r=−0.33 [P=.04]) and socioeconomic status as measured by yearly income (polychoric correlation: FTQ, r=−0.20 [P=.047]; FTND, r=−0.23 [P=.05]).
Figure 1.
Distribution of the Fagerström Test for Nicotine Dependence (FTND) scores in the Virginia Study of Anxiety and Neuroticism sample.
The buccal epithelial cell samples were collected using standard cytology brushes (Fisher Scientific, Fair Lawn, New Jersey). DNA was isolated from the brushes as reported previously.49 All individuals provided informed consent for participation in this study. Sixteen unlinked microsatellite markers and 33 SNPs were genotyped separately for the VAND and VAANX to assess the potential population stratification, and no evidence of stratification was found.48,49
SNP Selection and Genotyping
Ten SNPs were chosen from the SNP database (http://www.ncbi.nlm.nih.gov/SNP/index.html) and the genomics database of Applied BioScience Inc, Foster City, California, with the following criteria: (1) SNPs located in coding or regulatory regions of the gene or reported to be associated with drug dependence in the literature, (2) haplotype-tagged SNPs suggested by the Haploview program41 using the default parameters, and (3) SNPs with a minor allele frequency of 0.05 or higher in the SNP database. Table 1 lists the characteristics of the selected SNPs.
Table 1. Marker Characteristics in the VAND and VAANX Samples.
| Marker | Identification No. | Function | Chromosomal Position | Polymorphism | Minor Allele | VAND MAF | VAND HWE P Value | VAANX MAF | VAANX HWE P Value |
|---|---|---|---|---|---|---|---|---|---|
| rs2180619 | 1 | Intron | 88934671 | A/G | G | 0.38 | .24 | 0.39 | .11 |
| rs11756397 | 2 | Intron | 88930447 | C/T | C | 0.20 | .39 | 0.24 | .93 |
| rs6454674 | 3 | Intron | 88929649 | G/T | G | 0.29 | .32 | 0.33 | .60 |
| rs806380 | 4 | Intron | 88921372 | A/G | G | 0.36 | .46 | 0.37 | .60 |
| rs6928499 | 5 | Intron | 88918008 | C/G | C | 0.20 | .12 | 0.18 | >.99 |
| rs806379 | 6 | Intron | 88917986 | A/T | T | 0.50 | .18 | 0.50 | .01 |
| rs2023239 | 7 | Thr>Thr | 88917201 | C/T | C | 0.19 | .21 | 0.18 | .82 |
| rs1049353 | 8 | Thr>Thr | 88910354 | C/T | T | 0.28 | .10 | 0.29 | .60 |
| rs12720071 | 9 | Intron | 88907900 | C/T | C | 0.09 | .12 | 0.09 | .29 |
| rs806368 | 10 | Intron | 88906819 | C/T | C | 0.20 | .85 | 0.20 | .20 |
Abbreviations: HWE, Hardy-Weinberg equilibrium; MAF, minor allele frequency; VAANX, Virginia Study of Anxiety and Neuroticism; VAND, Virginia Study of Nicotine Dependence.
Genotyping was performed with the TaqMan genotyping method.50 Briefly, the polymerase chain reactions (PCRs) were conducted with 384-well microplates. To ensure the quality of genotyping, negative control samples were included in each plate. The PCRs were performed with 5 ng of genomic DNA, 0.25 μL of TaqMan assay mix (Applied Biosystems, Inc, Foster City), and 2.5 μL of TaqMan universal PCR master mix in a total reaction volume of 5 μL. After activating the polymerase and denaturizing DNA by heating at 95°C for 10 minutes, 40 cycles at 92°C for 15 seconds and 60°C for 1 minute were performed. After the reaction, the fluorescence intensities of reporter 1 and 2 (reporter 1: VIC fluorescent dye, excitation,520[10] nm; emission,550[10] nm; reporter 2: FAM fluorescent dye, excitation,490[10] nm; emission,510[10] nm) were measured (data are given as central wavelength [bandwidth] of filters used to measure fluorescence) by the Analyst fluorescence plate reader (LJL Biosytems, Sunnyvale, California). Based on the ratio of fluorescence intensities, genotypes were scored by a euclidean clustering algorithm developed in our laboratory.51
Statistical Analysis
To distinguish the effects of the CNR1 gene on SI and ND, we made 2 dichotomous comparisons. For the effect on SI, we compared the allele frequencies of nonsmokers with that of regular smokers in both the low- and high-ND groups. For the effect on ND, we compared the allele frequencies of the low-ND group with that of the high-ND group. For individual SNP association analyses, allele frequencies in the 3 groups were compared by the χ2 test or Fisher exact test when the expected counts were less than 5 in any cell of the contingency table. Sex-stratified analyses and multimarker haplotype analyses were conducted with the UNPHASED program (version 3.06),52 which uses a log-likelihood retrospective regression model. In the sex-stratified analyses, sex was used as a modifier covariate, and either the male or female was used as baseline. In these analyses, both main effect and sex effect were estimated in a single test. The SNP spectral decomposition (SNPSpD) method53 was used to evaluate the equivalent number of independent tests, and Bonferroni correction was applied for multiple testing correction. Pairwise linkage disequilibrium (LD) was estimated for all study participants by Haploview 3.2 software.41 Hardy-Weinberg equilibrium tests were conducted with the software.41
Results
LD Structure
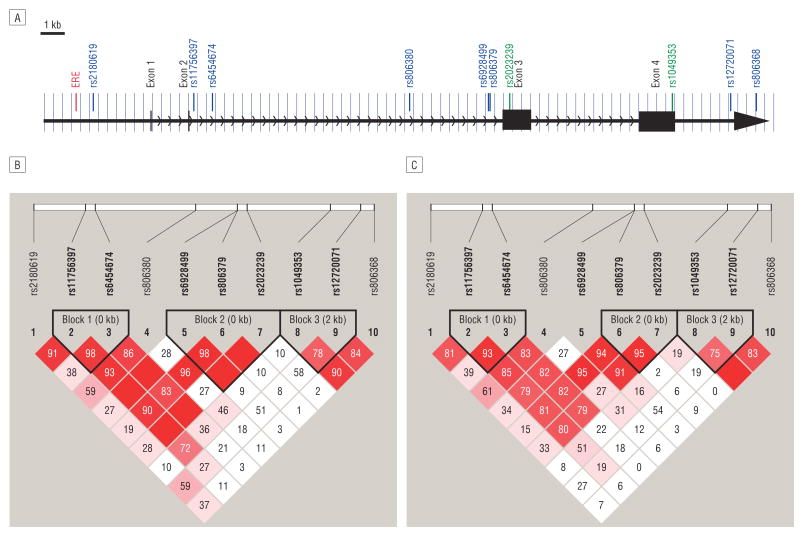
In this study, we typed 10 SNPs in the CNR1 gene (Table 1). Of these SNPs, 2 (rs2023239 and rs1049353) are synonymous coding SNPs. All the rest are located in the introns of the CNR1 gene (Figure 2A). Except for rs806379, which showed a deviation (P = .01) from the Hardy-Weinberg equilibrium in the VAANX sample, all other SNPs were in the Hardy-Weinberg equilibrium. For both the VAND and VAANX samples, the 10 SNPs were partitioned into 3 LD blocks using the default parameters, and the block boundaries were the same except for the second LD block, which included markers rs6928499, rs806379, and rs2023239 in the VAND sample but included only rs806379 and rs2023239 in the VAANX sample (Figure 2B and C). The overall LD in these 2 samples was similar.
Figure 2.
The cannabinoid receptor 1 (CNR1) gene and linkage disequilibrium (LD) structure in the Virginia Study of Nicotine Dependence (VAND) and the Virginia Study of Anxiety and Neuroticism (VAANX) samples. A, Gene structure and genotyped single-nucleotide polymorphisms. An estrogen response element (ERE) in the promoter region is also shown. B, The LD structure of the VAND sample. C, The LD structure of the VAANX sample. kb indicates kilobase.
Association with SI and ND in the Vand
To examine the effect of CNR1 genotypes on SI and ND, we compared the allele frequencies of typed SNPs between nonsmokers and smokers and between low- and high-ND smokers, respectively. Of the 10 SNPs, rs6928499 and rs2023239 showed nominally significant associations for both SI and ND (Table 2). For both markers, the major alleles, G and T, respectively, were overrepresented in cases. To evaluate whether sex plays a role in the association, we used a computer program (UNPHASED) and used sex as a modifier covariate. In these analyses, rs6928499 showed nominally significant results for both main effect and sex effect for SI (Table 3) but only a significant main effect for ND; rs2023239 showed similar association but with weaker signal strength. Another 2 SNPs, rs1049353 and rs12720071, showed similar female-specific associations with ND.
Table 2. Single-Marker Allelic Associations in the VAND Sample.
| Smoking Initiation | Nicotine Dependence | |||
|---|---|---|---|---|
| Marker | χ2 | P Value | χ2 | P Value |
| rs2180619 | 0.50 | .48 | 0.04 | .84 |
| rs11756397 | 0.51 | .48 | 1.06 | .30 |
| rs6454674 | 0.04 | .84 | 0.01 | .92 |
| rs806380 | 0.80 | .37 | 0.19 | .67 |
| rs6928499 | 3.86 | .05 | 4.85 | .03 |
| rs806379 | 2.69 | .10 | 0.34 | .56 |
| rs2023239 | 4.03 | .04 | 5.35 | .02 |
| rs1049353 | 0.35 | .55 | 3.59 | .06 |
| rs12720071 | 0.56 | .45 | 0.17 | .68 |
| rs806368 | 0.92 | .34 | 1.27 | .26 |
Abbreviation: See Table 1.
Table 3. Sex-Stratified Analyses (P Values) of the VAND Sample.
| Men | Women | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Nicotine Dependence | Smoking Initiation | Nicotine Dependence | Smoking Initiation | ||||||
| Marker | Main Effect | Sex Effect | Main Effect | Sex Effect | Main Effect | Sex Effect | Main Effect | Sex Effect | |
| rs2180619 | .90 | .60 | .30 | .47 | .58 | .60 | .82 | .47 | |
| rs11756397 | .10 | .16 | .72 | .70 | .59 | .16 | .47 | .70 | |
| rs6454674 | .41 | .18 | .58 | .48 | .29 | .18 | .66 | .48 | |
| rs806380 | .54 | .65 | .50 | .90 | .90 | .65 | .54 | .90 | |
| rs6928499 | .23 | .25 | .54 | .05 | .04 | .25 | .007 | .05 | |
| rs806379 | .62 | .14 | .41 | .37 | .10 | .14 | .09 | .37 | |
| rs2023239 | .16 | .34 | .42 | .09 | .05 | .34 | .01 | .09 | |
| rs1049353 | .85 | .02 | .53 | .07 | .002 | .02 | .07 | .07 | |
| rs12720071 | .05 | .007 | .21 | .26 | .06 | .007 | .65 | .26 | |
| rs806368 | .58 | .47 | .50 | .83 | .21 | .47 | .48 | .83 | |
Abbreviation: VAND, Virginia Study of Nicotine Dependence.
Based on the results of single-marker associations that suggest that the signals come largely from markers 5 through 10, we conducted haplotype analyses. In these analyses, marker combination 5-8 showed strong associations with both SI and ND in women; however, the associated haplotypes were different. Table 4 provides a brief summary of the haplotype analyses; complete results are given in eTable 1 (http://www.archgenpsychiatry.com). Other combinations (7-9-10 and 5-7-9-10) showed strong associations with ND but were only marginally associated with SI. As observed in the single-marker associations, these multimarker associations were also sex specific.
Table 4. Sex-Stratified Haplotype Analyses of the VAND Sample.
| Marker Combination | Global P Value | Haplotype | Case Frequency | Control Frequency | OR | Haplotype P Value |
|---|---|---|---|---|---|---|
| Smoking Initiation | ||||||
| Men | ||||||
| 5-8 | .14 | 1-2 | 0.19 | 0.17 | 1.17 | .76 |
| 7-9-10 | .83 | 1-1-1 | 0.62 | 0.59 | 1.15 | .29 |
| 5-7-9-10 | .82 | 1-1-1-1 | 0.62 | 0.59 | 1.15 | .33 |
| Women | ||||||
| 5-8 | .007 | 1-2 | 0.30 | 0.18 | 2.01 | .006 |
| 7-9-10 | .13 | 1-1-1 | 0.70 | 0.58 | 1.64 | .02 |
| 5-7-9-10 | .12 | 1-1-1-1 | 0.70 | 0.59 | 1.62 | .02 |
| Nicotine Dependence | ||||||
| Men | ||||||
| 5-8 | .40 | 1-1 | 0.63 | 0.56 | 1.32 | .18 |
| 7-9-10 | .29 | 1-1-2 | 0.10 | 0.09 | 1.14 | .74 |
| 5-7-9-10 | .37 | 1-1-1-2 | 0.10 | 0.09 | 1.19 | .67 |
| Women | ||||||
| 5-8 | <.001 | 1-1 | 0.67 | 0.42 | 2.85 | <.001 |
| 7-9-10 | .002 | 1-1-2 | 0.12 | 0.02 | 6.93 | <.001 |
| 5-7-9-10 | .001 | 1-1-1-2 | 0.12 | 0.02 | 7.99 | <.001 |
Abbreviations: OR, odds ratio; VAND, Virginia Study of Nicotine Dependence.
To correct for multiple testing, we used the SNPSpD method to estimate the number of equivalent independent tests using the genotype data from this sample. This calculation produced 8 equivalent tests for the 10 SNPs. For both SI and ND, we tested all 10 SNPs for simple χ2 tests and sex-stratified tests in addition to 3 haplotype tests. The total number of tests was 21. Using Bonferroni correction, only rs1049353 survived correction for association with ND; all other single markers failed multiple testing correction. In haplotype testing, although none of the haplotype associations with SI survived correction, all 3 haplotype associations with ND remained significant after the correction.
Replication of SI and ND in the VAANX Sample
To verify the findings in the VAND sample, we genotyped the same 10 SNPs in the VAANX sample and conducted sex-stratified analyses directly. For rs2023239, we were able to replicate the female-specific association with SI (main effect P=.01 and sex effect P=.02); rs6928499 showed a trend of association with SI in the same sex (Table 5). For both SNPs, the associated alleles were the same in the 2 samples; rs806368 was nominally significant in the women for the FTND.
Table 5. Single-Marker Associations (P Values) of the VAANX Sample.
| Men | Women | |||||||
|---|---|---|---|---|---|---|---|---|
| FTND | Smoking Initiation | FTND | Smoking Initiation | |||||
| Marker | Main Effect | Sex Effect | Main Effect | Sex Effect | Main Effect | Sex Effect | Main Effect | Sex Effect |
| rs2180619 | .46 | .13 | .07 | .03 | .12 | .13 | .14 | .03 |
| rs11756397 | .67 | .76 | .56 | .70 | .98 | .76 | .10 | .70 |
| rs6454674 | .17 | .12 | .58 | .52 | .37 | .12 | .71 | .52 |
| rs806380 | .58 | .77 | .48 | .68 | .92 | .77 | .97 | .68 |
| rs6928499 | .62 | .65 | .63 | .10 | .88 | .65 | .07 | .10 |
| rs806379 | .82 | .91 | .39 | .19 | .95 | .91 | .28 | .19 |
| rs2023239 | .42 | .76 | .49 | .02 | .66 | .76 | .01 | .02 |
| rs1049353 | .75 | .76 | .23 | .21 | .92 | .76 | .51 | .21 |
| rs12720071 | .69 | .45 | .62 | .31 | .47 | .45 | .35 | .31 |
| rs806368 | .18 | .47 | .37 | .25 | .03 | .47 | .46 | .25 |
Abbreviations: FTND, Fagerström Test for Nicotine Dependence; VAANX, Virginia Study of Anxiety and Neuroticism.
To determine whether the VAANX shares the same risk haplotypes with VAND, we conducted haplotype analyses with the VAANX sample. A brief summary of these analyses is listed in Table 6, and complete results were shown in eTable 2. In these analyses, combinations 7-9-10 and 5-7-9-10 showed female-specific associations with both SI and the FTND (Table 6). The haplotypes associated with the FTND were the same (1-1-2 and 1-1-1-2 for combinations 7-9-10 and 5-7-9-10, respectively) as those found in the VAND sample, but the haplotypes associated with SI were not. Given the 8 independent tests estimated by the SNPSpD program and the 3 haplotype tests, the corrected threshold for significance would be .05/11 = .004545. Because our replication was in the same haplotype and same sex, we arguably could use a 1-tailed test criterion (ie, the threshold of 2 × 0.004545 or 0.00909). Using this criterion, the association of the FTND with haplotype 1-1-2 of combination 7-9-10 just met this threshold. All other tests failed multiple testing correction.
Table 6. Stratified Haplotype Analyses of the VAANX Sample.
| Marker Combination | Global P Value | Haplotype | Case Frequency | Control Frequency | OR | Haplotype P Value |
|---|---|---|---|---|---|---|
| Smoking Initiation | ||||||
| Men | ||||||
| 7-9-10 | .64 | 2-1-1 | 0.13 | 0.15 | 0.89 | .45 |
| 5-7-9-10 | .86 | 2-2-1-1 | 0.13 | 0.14 | 0.89 | .49 |
| Women | ||||||
| 7-9-10 | .12 | 2-1-1 | 0.19 | 0.12 | 1.72 | .008 |
| 5-7-9-10 | .02 | 2-2-1-1 | 0.17 | 0.11 | 1.66 | .02 |
| FTND | ||||||
| Men | ||||||
| 9-10 | .60 | 1-2 | 0.13 | NA | NA | .41 |
| 7-9-10 | .56 | 1-1-2 | 0.10 | NA | NA | .55 |
| 5-7-9-10 | .50 | 1-1-1-2 | 0.11 | NA | NA | .90 |
| Women | ||||||
| 9-10 | .01 | 1-2 | 0.13 | NA | NA | .002 |
| 7-9-10 | .14 | 1-1-2 | 0.11 | NA | NA | .009 |
| 5-7-9-10 | .04 | 1-1-1-2 | 0.10 | NA | NA | .02 |
Abbreviations: FTND, Fagerström Test for Nicotine Dependence; NA, not applicable; OR, odds ratio; VAANX, Virginia Study of Anxiety and Neuroticism.
Comment
The endogenous cannabinoid system has been implicated in drug addiction in animal models.23,27,54,55 The CNR1 gene is an essential component of the endogenous cannabinoid system. It has been shown that CNR1 is widely expressed in various regions of the brain.38-40 Zhang and colleagues37 reported that CNR1 is associated with polysubstance abuse. Associations with cannabis dependence,56 alcohol dependence,31,36 and other drug dependence32,33,57 and with psychiatric disorders58-60 were also reported. In this study, we tested the association of CNR1 with SI and ND or FTND score in 2 independent samples drawn from 2 large epidemiologic studies of Virginia twins. In both samples, we found nominally significant associations in individual markers and multimarker haplotypes: rs2023239 showed sex-specific associations with SI in the same allele in the 2 samples and rs6928499 was significantly associated with SI in the VAND sample but only showed a trend in the VAANX sample. Two multimarker haplotypes showed the same sex-specific associations with ND in both samples. Haplotype 1-1-2 for combination 7-9-10 just met the cutoff of multiple testing correction (0.00909 vs 0.00909) for a 1-tailed test. Because the threshold was calculated with the Bonferroni method and the 3 haplotype tests were nested, we could argue that the association of haplotype 1-1-2 is significant after correction. In general, the results obtained from the 2 independent samples are mutually supportive.
In association studies, the evaluation of false-positive results is an important issue. In this study, VAND was used initially to identify the associations, and VAANX was used to confirm the associations. For VAND, we performed simple and sex-stratified analyses for all 10 SNPs. We also performed sex-stratified haplotype analyses for several combinations. The effective number of tests was 23. Based on this calculation, only rs1049353 survives multiple testing correction for association with ND. For haplotype tests, associations with ND remain significant for both the global and haplotype tests for all combinations. In the replication panel, we performed 11 tests (8 equivalent independent tests for sex-stratified single-marker tests and 3 sex-stratified haplotype tests). Although rs2023239 showed nominal association with SI in the same allele and same sex as observed in the VAND sample and rs6928499 showed a trend in the same allele and sex, none of them could survive correction for multiple comparison. In haplotype analyses, although combinations 7-9-10 and 5-7-9-10 had associations with the FTND score in the same haplotypes and sex, only haplotype 1-1-2 of the combination 7-9-10 could be considered significant. Considering the replication criteria proposed in the recent literature,61,62 in which a replication could be made at the level of a single marker, a haplotype, or a gene, we could argue that we have replicated the association with ND at haplotype 1-1-2 for combination 7-9-10. Based on the simulations reported by Sullivan,62 replications at the same marker, same allele, and same sex or at the same marker combination, same haplotype, and same sex had the lowest false-positive rate. Accordingly, we could conclude that CNR1 is likely associated with ND. The association with SI, however, is less clear, and additional studies will be needed to clarify this issue.
In recent years, several studies31-37,59,60 have examined the associations of CNR1 with substance abuse and dependence, but the results are not always consistent. A study by Zhang et al37 reported association of a 3-marker (rs806379-rs1535255-rs2023239) haplotype with multiple substance abuse. We typed 2 (rs806379 and rs2023239) of these 3 markers. As mentioned herein, rs2023239 showed nominal associations with SI in both the VAND and VAANX samples, but it also showed nominal association (P=.05) with ND in the VAND sample. However, in our samples, it is the major allele, T, not the minor allele, C, that is overrepresented in the affected individuals. In another report just published as this article was written, Zuo et al31 reported associations of 2 SNPs (rs6454674 and rs806368) with drug dependence and alcohol dependence. We also typed these 2 SNPs. To compare whether the reported genotypes are associated with SI and ND in our sample, we conducted post hoc analyses for genotype associations for these 2 markers. In the VAND sample, we did not find associations at these 2 markers. In the VAANX sample, the 2/2 genotype (G/G) of rs6454674 is marginally associated with the FTND score (P = .05). This is the same genotype reported in the study by Zuo et al. For rs806368, the 1/1 genotype (T/T) is associated with the FTND score (P = .01). This is also the same genotype reported in the study by Zuo et al. In the 2 marker interaction analyses, 2/2-1/1 (G/G-T/T) is significantly associated with the FTND score (P< .001); once again, this is the same interaction observed in the article by Zuo et al. These post hoc analyses support the findings by Zuo et al.
Another interesting finding in our study is the sex effect. In our analyses, sex was considered a modifier covariate. In these analyses, both main effect and sex effect were estimated simultaneously in the regression model. For both SI and ND phenotypes tested in this study, sex has a significant modifying effect. A similar effect was observed by Zuo et al.31 In the tables reported in that study, sex was used as a covariate and its effect was statistically significant for all phenotypes studied. This is consistent with our observation. This finding raises a possibility that the failure to find evidence of association in some previous studies may be due to heterogeneity in the sexes. This finding may have a molecular basis. There is an estrogen response element in the promoter region of the CNR1 gene63 (Figure 1A). There is also evidence that estrogen modulates the expression of the CNR1 gene.64
The 2 samples used in this study were selected using different criteria. In the VAND sample, the 3 groups were never smokers, low-ND smokers (FTQ score ≤2), and high-ND smokers (FTQ score ≥7). The VAANX sample is a 2-group, case-control design for studying neuroticism and anxiety.56 Because the selection was based on a composite genetic factor score, we do not have a sufficient number of participants for a dichotomized design for studying ND. The best comparable phenotype in this sample to ND is the FTND score. For this reason, we used a quantitative design to approximate the ND phenotype in the VAND sample. As reported in the literature,45,65,66 the FTND is a reliable instrument to assess ND. However, our dichotomized ND definition in the VAND sample is not the same as the FTND scores in the VAANX sample. This is a potential weakness of our study that may contribute to the difference in association signals observed between the 2 samples.
In conclusion, we present evidence that CNR1 may be associated with ND in 2 independent samples. We also find that sex may have a significant modifying effect. Given the reported associations of this gene with other substance abuse and dependence in the literature, it is likely that CNR1 has an important role in the manifestation of multiple substance abuse and dependence.
Supplementary Material
Acknowledgments
Funding/Support: This study was supported by K01DA019498 from the National Institute on Drug Abuse (Dr Chen) and grant 5100004ST from the Virginia Tobacco Settlement Foundation through the Virginia Youth Tobacco Project to Virginia Commonwealth University (subcontracted to Dr Kendler).
Footnotes
Additional Information: The eTables are available at http://www.archgenpsychiatry.com.
Additional Contributions: Linda Corey, PhD, assisted with the ascertainment of twins from the Virginia Twin Registry, now part of the Mid-Atlantic Twin Registry, currently directed by Judy Silberg, PhD. The Mid-Atlantic Twin Registry has received support from the National Institutes of Health, the Carman Trust, and the W. M. Keck, John Templeton, and Robert Wood Johnson Foundations.
References
- 1.Breslau N, Johnson EO, Hiripi E, Kessler R. Nicotine dependence in the United States: prevalence, trends, and smoking persistence. Arch Gen Psychiatry. 2001;58(9):810–816. doi: 10.1001/archpsyc.58.9.810. [DOI] [PubMed] [Google Scholar]
- 2.Murray CJ, Lopez AD. Alternative projections of mortality and disability by cause 1990-2020: Global Burden of Disease Study. Lancet. 1997;349(9064):1498–1504. doi: 10.1016/S0140-6736(96)07492-2. [DOI] [PubMed] [Google Scholar]
- 3.Heath AC, Madden PA, Slutske WS, Martin NG. Personality and the inheritance of smoking behavior: a genetic perspective. Behav Genet. 1995;25(2):103–117. doi: 10.1007/BF02196921. [DOI] [PubMed] [Google Scholar]
- 4.Heath AC, Martin NG, Lynskey MT, Todorov AA, Madden PA. Estimating two-stage models for genetic influences on alcohol, tobacco or drug use initiation and dependence vulnerability in twin and family data. Twin Res. 2002;5(2):113–124. doi: 10.1375/1369052022983. [DOI] [PubMed] [Google Scholar]
- 5.Kendler KS, Neale MC, Sullivan P, Corey LA, Gardner CO, Prescott CA. A population-based twin study in women of smoking initiation and nicotine dependence. Psychol Med. 1999;29(2):299–308. doi: 10.1017/s0033291798008022. [DOI] [PubMed] [Google Scholar]
- 6.True WR, Heath AC, Scherrer JF, Waterman B, Goldberg J, Lin N, Eisen SA, Lyons MJ, Tsuang MT. Genetic and environmental contributions to smoking. Addiction. 1997;92(10):1277–1287. [PubMed] [Google Scholar]
- 7.Straub RE, Sullivan PF, Ma Y, Myakishev MV, Harris-Kerr C, Wormley B, Kadambi B, Sadek H, Silverman MA, Webb BT, Neale MC, Bulik CM, Joyce PR, Kendler KS. Susceptibility genes for nicotine dependence: a genome scan and followup in an independent sample suggest that regions on chromosomes 2, 4, 10, 16, 17 and 18 merit further study. Mol Psychiatry. 1999;4(2):129–144. doi: 10.1038/sj.mp.4000518. [DOI] [PubMed] [Google Scholar]
- 8.Li MD, Payne TJ, Ma JZ, Lou XY, Zhang D, Dupont RT, Crews KM, Somes G, Williams NJ, Elston RC. A genomewide search finds major susceptibility loci for nicotine dependence on chromosome 10 in African Americans. Am J Hum Genet. 2006;79(4):745–751. doi: 10.1086/508208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Swan GE, Hops H, Wilhelmsen KC, Lessov-Schlaggar CN, Cheng LS, Hudmon KS, Amos CI, Feiler HS, Ring HZ, Andrews JA, Tildesley E, Benowitz N. A genome-wide screen for nicotine dependence susceptibility loci. Am J Med Genet B Neuropsychiatr Genet. 2006;141(4):354–360. doi: 10.1002/ajmg.b.30315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bergen AW, Korczak JF, Weissbecker KA, Goldstein AM. A genome-wide search for loci contributing to smoking and alcoholism. Genet Epidemiol. 1999;17(suppl 1):S55–S60. doi: 10.1002/gepi.1370170710. [DOI] [PubMed] [Google Scholar]
- 11.Gelernter J, Liu X, Hesselbrock V, Page GP, Goddard A, Zhang H. Results of a genomewide linkage scan: support for chromosomes 9 and 11 loci increasing risk for cigarette smoking. Am J Med Genet B Neuropsychiatr Genet. 2004;128(1):94–101. doi: 10.1002/ajmg.b.30019. [DOI] [PubMed] [Google Scholar]
- 12.Vink JM, Beem AL, Posthuma D, Neale MC, Willemsen G, Kendler KS, Slag-boom PE, Boomsma DI. Linkage analysis of smoking initiation and quantity in Dutch sibling pairs. Pharmacogenomics J. 2004;4(4):274–282. doi: 10.1038/sj.tpj.6500255. [DOI] [PubMed] [Google Scholar]
- 13.Yu Y, Panhuysen C, Kranzler HR, Hesselbrock V, Rounsaville B, Weiss R, Brady K, Farrer LA, Gelernter J. Intronic variants in the dopa decarboxylase (DDC) gene are associated with smoking behavior in European-Americans and African-Americans. Hum Mol Genet. 2006;15(14):2192–2199. doi: 10.1093/hmg/ddl144. [DOI] [PubMed] [Google Scholar]
- 14.Beuten J, Ma JZ, Payne TJ, Dupont RT, Crews KM, Somes G, Williams NJ, Elston RC, Li MD. Single- and multilocus allelic variants within the GABA(B) receptor subunit 2 (GABAB2) gene are significantly associated with nicotine dependence. Am J Hum Genet. 2005;76(5):859–864. doi: 10.1086/429839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ma JZ, Beuten J, Payne TJ, Dupont RT, Elston RC, Li MD. Haplotype analysis indicates an association between the DOPA decarboxylase (DDC) gene and nicotine dependence. Hum Mol Genet. 2005;14(12):1691–1698. doi: 10.1093/hmg/ddi177. [DOI] [PubMed] [Google Scholar]
- 16.Feng Y, Niu T, Xing H, Xu X, Chen C, Peng S, Wang L, Laird N, Xu X. A common haplotype of the nicotine acetylcholine receptor α4 subunit gene is associated with vulnerability to nicotine addiction in men. Am J Hum Genet. 2004;75(1):112–121. doi: 10.1086/422194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.John U, Meyer C, Rumpf HJ, Hapke U. Probabilities of alcohol high-risk drinking, abuse or dependence estimated on grounds of tobacco smoking and nicotine dependence. Addiction. 2003;98(6):805–814. doi: 10.1046/j.1360-0443.2003.00381.x. [DOI] [PubMed] [Google Scholar]
- 18.Kendler KS, Prescott CA, Myers J, Neale MC. The structure of genetic and environmental risk factors for common psychiatric and substance use disorders in men and women. Arch Gen Psychiatry. 2003;60(9):929–937. doi: 10.1001/archpsyc.60.9.929. [DOI] [PubMed] [Google Scholar]
- 19.True WR, Xian H, Scherrer JF, Madden PA, Bucholz KK, Heath AC, Eisen SA, Lyons MJ, Goldberg J, Tsuang M. Common genetic vulnerability for nicotine and alcohol dependence in men. Arch Gen Psychiatry. 1999;56(7):655–661. doi: 10.1001/archpsyc.56.7.655. [DOI] [PubMed] [Google Scholar]
- 20.Kendler KS, Jacobson KC, Prescott CA, Neale MC. Specificity of genetic and environmental risk factors for use and abuse/dependence of cannabis, cocaine, hallucinogens, sedatives, stimulants, and opiates in male twins. Am J Psychiatry. 2003;160(4):687–695. doi: 10.1176/appi.ajp.160.4.687. [DOI] [PubMed] [Google Scholar]
- 21.Hyman SE, Malenka RC. Addiction and the brain: the neurobiology of compulsion and its persistence. Nat Rev Neurosci. 2001;2(10):695–703. doi: 10.1038/35094560. [DOI] [PubMed] [Google Scholar]
- 22.Nestler EJ. Is there a common molecular pathway for addiction? Nat Neurosci. 2005;8(11):1445–1449. doi: 10.1038/nn1578. [DOI] [PubMed] [Google Scholar]
- 23.Maldonado R, Valverde O, Berrendero F. Involvement of the endocannabinoid system in drug addiction. Trends Neurosci. 2006;29(4):225–232. doi: 10.1016/j.tins.2006.01.008. [DOI] [PubMed] [Google Scholar]
- 24.De Vries TJ, Schoffelmeer AN. Cannabinoid CB1 receptors control conditioned drug seeking. Trends Pharmacol Sci. 2005;26(8):420–426. doi: 10.1016/j.tips.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 25.Forget B, Hamon M, Thiebot MH. Cannabinoid CB1 receptors are involved in motivational effects of nicotine in rats. Psychopharmacology (Berl) 2005;181(4):722–734. doi: 10.1007/s00213-005-0015-6. [DOI] [PubMed] [Google Scholar]
- 26.Valverde O, Karsak M, Zimmer A. Analysis of the endocannabinoid system by using CB1 cannabinoid receptor knockout mice. Handb Exp Pharmacol. 2005;(168):117–145. doi: 10.1007/3-540-26573-2_4. [DOI] [PubMed] [Google Scholar]
- 27.Colombo G, Serra S, Vacca G, Carai MA, Gessa GL. Endocannabinoid system and alcohol addiction: pharmacological studies. Pharmacol Biochem Behav. 2005;81(2):369–380. doi: 10.1016/j.pbb.2005.01.022. [DOI] [PubMed] [Google Scholar]
- 28.Racz I, Bilkei-Gorzo A, Toth ZE, Michel K, Palkovits M, Zimmer A. A critical role for the cannabinoid CB1 receptors in alcohol dependence and stress-stimulated ethanol drinking. J Neurosci. 2003;23(6):2453–2458. doi: 10.1523/JNEUROSCI.23-06-02453.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Beardsley PM, Thomas BF. Current evidence supporting a role of cannabinoid CB1 receptor (CB1R) antagonists as potential pharmacotherapies for drug abuse disorders. Behav Pharmacol. 2005;16(56):275–296. doi: 10.1097/00008877-200509000-00003. [DOI] [PubMed] [Google Scholar]
- 30.Fernandez JR, Allison DB. Rimonabant Sanofi-Synthelabo. Curr Opin Investig Drugs. 2004;5(4):430–435. [PubMed] [Google Scholar]
- 31.Zuo L, Kranzler HR, Luo X, Covault J, Gelernter J. CNR1 variation modulates risk for drug and alcohol dependence. Biol Psychiatry. 2007;62(6):616–626. doi: 10.1016/j.biopsych.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 32.Ballon N, Leroy S, Roy C, Bourdel MC, Charles-Nicolas A, Krebs MO, Poirier MF. (AAT)n repeat in the cannabinoid receptor gene (CNR1): association with cocaine addiction in an African-Caribbean population. Pharmacogenomics J. 2006;6(2):126–130. doi: 10.1038/sj.tpj.6500352. [DOI] [PubMed] [Google Scholar]
- 33.Comings DE, Muhleman D, Gade R, Johnson P, Verde R, Saucier G, MacMurray J. Cannabinoid receptor gene (CNR1): association with i.v. drug use. Mol Psychiatry. 1997;2(2):161–168. doi: 10.1038/sj.mp.4000247. [DOI] [PubMed] [Google Scholar]
- 34.Herman AI, Kranzler HR, Cubells JF, Gelernter J, Covault J. Association study of the CNR1 gene exon 3 alternative promoter region polymorphisms and substance dependence. Am J Med Genet B Neuropsychiatr Genet. 2006;141(5):499–503. doi: 10.1002/ajmg.b.30325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li T, Liu X, Zhu ZH, Zhao J, Hu X, Ball DM, Sham PC, Collier DA. No association between (AAT)n repeats in the cannabinoid receptor gene (CNR1) and heroin abuse in a Chinese population. Mol Psychiatry. 2000;5(2):128–130. doi: 10.1038/sj.mp.4000670. [DOI] [PubMed] [Google Scholar]
- 36.Schmidt LG, Samochowiec J, Finckh U, Fiszer-Piosik E, Horodnicki J, Wendel B, Rommelspacher H, Hoehe MR. Association of a CB1 cannabinoid receptor gene (CNR1) polymorphism with severe alcohol dependence. Drug Alcohol Depend. 2002;65(3):221–224. doi: 10.1016/s0376-8716(01)00164-8. [DOI] [PubMed] [Google Scholar]
- 37.Zhang PW, Ishiguro H, Ohtsuki T, Hess J, Carillo F, Walther D, Onaivi ES, Arinami T, Uhl GR. Human cannabinoid receptor 1: 5′ exons, candidate regulatory regions, polymorphisms, haplotypes and association with polysubstance abuse. Mol Psychiatry. 2004;9(10):916–931. doi: 10.1038/sj.mp.4001560. [DOI] [PubMed] [Google Scholar]
- 38.Fernández-Ruiz J, Gomez M, Hernandez M, de Miguel R, Ramos JA. Cannabinoids and gene expression during brain development. Neurotox Res. 2004;6(5):389–401. doi: 10.1007/BF03033314. [DOI] [PubMed] [Google Scholar]
- 39.Villares J. Chronic use of marijuana decreases cannabinoid receptor binding and mRNA expression in the human brain. Neuroscience. 2007;145(1):323–334. doi: 10.1016/j.neuroscience.2006.11.012. [DOI] [PubMed] [Google Scholar]
- 40.Herkenham M, Lynn AB, Little MD, Johnson MR, Melvin LS, de Costa BR, Rice KC. Cannabinoid receptor localization in brain. Proc Natl Acad Sci U S A. 1990;87(5):1932–1936. doi: 10.1073/pnas.87.5.1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Barrett JC, Fry B, Maller J, Daly MJ. Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics. 2005;21(2):263–265. doi: 10.1093/bioinformatics/bth457. [DOI] [PubMed] [Google Scholar]
- 42.Prescott CA, Kendler KS. Genetic and environmental contributions to alcohol abuse and dependence in a population-based sample of male twins. Am J Psychiatry. 1999;156(1):34–40. doi: 10.1176/ajp.156.1.34. [DOI] [PubMed] [Google Scholar]
- 43.Kendler KS, Prescott CA. Genes, Environment, and Psychopathology: Understanding the Causes of Psychiatric and Substance Use Disorders. New York, NY: Guilford Press; 2007. [Google Scholar]
- 44.Fagerström KO. Measuring degree of physical dependence to tobacco smoking with reference to individualization of treatment. Addict Behav. 1978;3(34):235–241. doi: 10.1016/0306-4603(78)90024-2. [DOI] [PubMed] [Google Scholar]
- 45.Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO. The Fagerstrom Test for Nicotine Dependence: a revision of the Fagerstrom Tolerance Questionnaire. Br J Addict. 1991;86(9):1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- 46.Maes x HH, Sullivan PF, Bulik CM, Neale MC, Prescott CA, Eaves LJ, Kendler KS. A twin study of genetic and environmental influences on tobacco initiation, regular tobacco use and nicotine dependence. Psychol Med. 2004;34(7):1251–1261. doi: 10.1017/s0033291704002405. [DOI] [PubMed] [Google Scholar]
- 47.Hettema JM, Prescott CA, Myers JM, Neale MC, Kendler KS. The structure of genetic and environmental risk factors for anxiety disorders in men and women. Arch Gen Psychiatry. 2005;62(2):182–189. doi: 10.1001/archpsyc.62.2.182. [DOI] [PubMed] [Google Scholar]
- 48.Hettema JM, An SS, Neale MC, Bukszar J, van den Oord EJ, Kendler KS, Chen X. Association between glutamic acid decarboxylase genes and anxiety disorders, major depression, and neuroticism. Mol Psychiatry. 2006;11(8):752–762. doi: 10.1038/sj.mp.4001845. [DOI] [PubMed] [Google Scholar]
- 49.Silverman MA, Neale MC, Sullivan PF, Harris-Kerr C, Wormley B, Sadek H, Ma Y, Kendler KS, Straub RE. Haplotypes of four novel single nucleotide polymorphisms in the nicotinic acetylcholine receptor β2-subunit (CHRNB2) gene show no association with smoking initiation or nicotine dependence. Am J Med Genet. 2000;96(5):646–653. [PubMed] [Google Scholar]
- 50.Livak KJ. Allelic discrimination using fluorogenic probes and the 5′ nuclease assay. Genet Anal. 1999;14(56):143–149. doi: 10.1016/s1050-3862(98)00019-9. [DOI] [PubMed] [Google Scholar]
- 51.van den Oord EJ, Jiang Y, Riley BP, Kendler KS, Chen X. FP-TDI SNP scoring by manual and statistical procedures: a study of error rates and types. Biotechniques. 2003;34(3):610–616. 618–620, 622. doi: 10.2144/03343dd04. [DOI] [PubMed] [Google Scholar]
- 52.Dudbridge F. Pedigree disequilibrium tests for multilocus haplotypes. Genet Epidemiol. 2003;25(2):115–121. doi: 10.1002/gepi.10252. [DOI] [PubMed] [Google Scholar]
- 53.Nyholt DR. A simple correction for multiple testing for single-nucleotide polymorphisms in linkage disequilibrium with each other. Am J Hum Genet. 2004;74(4):765–769. doi: 10.1086/383251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Arnold JC. The role of endocannabinoid transmission in cocaine addiction. Pharmacol Biochem Behav. 2005;81(2):396–406. doi: 10.1016/j.pbb.2005.02.015. [DOI] [PubMed] [Google Scholar]
- 55.Lupica CR, Riegel AC. Endocannabinoid release from midbrain dopamine neurons: a potential substrate for cannabinoid receptor antagonist treatment of addiction. Neuropharmacology. 2005;48(8):1105–1116. doi: 10.1016/j.neuropharm.2005.03.016. [DOI] [PubMed] [Google Scholar]
- 56.Hopfer CJ, Young SE, Purcell S, Crowley TJ, Stallings MC, Corley RP, Rhee SH, Smolen A, Krauter K, Hewitt JK, Ehringer MA. Cannabis receptor haplotype associated with fewer cannabis dependence symptoms in adolescents. Am J Med Genet B Neuropsychiatr Genet. 2006;141(8):895–901. doi: 10.1002/ajmg.b.30378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Johnson JP, Muhleman D, MacMurray J, Gade R, Verde R, Ask M, Kelley J, Comings DE. Association between the cannabinoid receptor gene (CNR1) and the P300 event-related potential. Mol Psychiatry. 1997;2(2):169–171. doi: 10.1038/sj.mp.4000246. [DOI] [PubMed] [Google Scholar]
- 58.Siegfried Z, Kanyas K, Latzer Y, Karni O, Bloch M, Lerer B, Berry EM. Association study of cannabinoid receptor gene (CNR1) alleles and anorexia nervosa: differences between restricting and binging/purging subtypes. Am J Med Genet B Neuropsychiatr Genet. 2004;125(1):126–130. doi: 10.1002/ajmg.b.20089. [DOI] [PubMed] [Google Scholar]
- 59.Tsai SJ, Wang YC, Hong CJ. Association study between cannabinoid receptor gene (CNR1) and pathogenesis and psychotic symptoms of mood disorders. Am J Med Genet. 2001;105(3):219–221. doi: 10.1002/ajmg.1259. [DOI] [PubMed] [Google Scholar]
- 60.Tsai SJ, Wang YC, Hong CJ. Association study of a cannabinoid receptor gene (CNR1) polymorphism and schizophrenia. Psychiatr Genet. 2000;10(3):149–151. doi: 10.1097/00041444-200010030-00008. [DOI] [PubMed] [Google Scholar]
- 61.Neale BM, Sham PC. The future of association studies: gene-based analysis and replication. Am J Hum Genet. 2004;75(3):353–362. doi: 10.1086/423901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sullivan PF. Spurious genetic associations. Biol Psychiatry. 2007;61(10):1121–1126. doi: 10.1016/j.biopsych.2006.11.010. [DOI] [PubMed] [Google Scholar]
- 63.Bourdeau V, Deschenes J, Metivier R, Nagai Y, Nguyen D, Bretschneider N, Gannon F, White JH, Mader S. Genome-wide identification of high-affinity estrogen response elements in human and mouse. Mol Endocrinol. 2004;18(6):1411–1427. doi: 10.1210/me.2003-0441. [DOI] [PubMed] [Google Scholar]
- 64.González S, Mauriello-Romanazzi G, Berrendero F, Ramos JA, Franzoni MF, Fernandez-Ruiz J. Decreased cannabinoid CB1 receptor mRNA levels and immunoreactivity in pituitary hyperplasia induced by prolonged exposure to estrogens. Pituitary. 2000;3(4):221–226. doi: 10.1023/a:1012874029689. [DOI] [PubMed] [Google Scholar]
- 65.Fagerstrom KO, Schneider NG. Measuring nicotine dependence: a review of the Fagerstrom Tolerance Questionnaire. J Behav Med. 1989;12(2):159–182. doi: 10.1007/BF00846549. [DOI] [PubMed] [Google Scholar]
- 66.Pomerleau CS, Carton SM, Lutzke ML, Flessland KA, Pomerleau OF. Reliability of the Fagerstrom Tolerance Questionnaire and the Fagerstrom Test for Nicotine Dependence. Addict Behav. 1994;19(1):33–39. doi: 10.1016/0306-4603(94)90049-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.