Abstract
In the classic retinoid cycle, 11-cis retinol is synthesized in the retinal pigment epithelium (RPE) by two enzymes: Isomerase I (RPE65) and lecithin:retinol acyltransferase (LRAT). The purpose of this study is to provide experimental evidence for two active isomerases in the cone-dominated chicken eye: an LRAT-dependent Isomerase I in the RPE and an ARAT (acyl CoA:retinol acyltransferase)-dependent isomerase (Isomerase II) in the retina. First, we show that whole chicken retina in vitro, removed from the RPE/choroid and sclera, produces 11-cis retinoids upon light exposure, indicating the existence of RPE-independent isomerase (Isomerase II) activity in the retina. RT-PCR studies show high levels of RPE65 expression in the RPE, low levels in the retina, and none in primary Müller cell cultures, indicating the presence of Isomerase I in the RPE and a minimal amount in the retina. Activities of the RPE and retina isomerases were then measured by enzyme assays with specific enzyme inhibitors. 2,2′-Bipyridine, a known Isomerase I inhibitor, and N-ethyl-maleimide (NEM), a known LRAT inhibitor, significantly reduced Isomerase I activity but not Isomerase II activity. Progesterone, a known ARAT inhibitor, completely blocked Isomerase II activity but not Isomerase I activity. Thus the present study reports novel results to distinguish the biochemical properties of Isomerase I from Isomerase II, as well a difference in their locations in the chicken eye. Based on these differences, the cone-dominated chicken eye must contain two retinoid cycles: a classic visual cycle for retinoid exchange between the RPE and the retina supported by Isomerase I in the RPE, and an additional visual cycle for retinoid processing in the retina supported by Isomerase II.
Light sensitivity in the eye requires the regeneration of 11-cis retinaldehyde through different isomers and oxidation states of vitamin A. An intimate relationship exists between photoreceptors and the retinal pigment epithelium (RPE) to regenerate this 11-cis retinaldehyde by way of the biochemical pathway known as the visual or retinoid cycle. This classic retinoid cycle has been well established for rod photoreceptors (for review see (1-7)). Attention has recently been focused on an additional vitamin A processing pathway in the retina for the supply of visual chromophore to cone photoreceptors (6;8-13). Little is known about the pathway of this novel cone cycle (6).
A key reaction in both retinoid cycles involves the isomerization of retinoids from the all-trans to the 11-cis conformation. The first (and more extensively characterized) pathway is enzymatic and driven by the protein RPE65 (Isomerase I) (14-16) where it is localized in the RPE and uses all-trans retinyl esters as the substrate for the production of 11-cis retinol (17;18). RPE65 from the RPE of the cone-dominated chicken has been shown to possess significantly higher isomerase activity than the RPE from rod-dominated species (12).
The second pathway is a photic cycle (5) driven by the retinal G protein coupled receptor (RGR). RGR is located in the RPE and Müller cells of rod-dominated species (19-22). RGR has also been shown to isomerize all-trans retinaldehyde to 11-cis retinaldehyde in a light-dependent (23;24), as well as a light-independent manner (25). More recently, RGR has been suggested to mediate the translocation of all-trans retinyl esters for the synthesis of visual chromophore in the RPE (26). The exact role of RGR in the regeneration of visual chromophore, however, has not yet been elucidated.
Recently, in the cone-dominated zebrafish model, evidence for two parallel visual cycles has been reported (27;28) along with evidence of an RPE65-independent pathway for the regeneration of 11-cis retinaldehyde for cone vision (29). The ability of cone photoreceptors to recover sensitivity in isolated salamander and mouse retinas after bleaching has also been demonstrated, suggesting an intra-retinal cone-specific cycle in these species (30).
The enzymes responsible for regeneration of cone pigment within the cone-dominated retina are yet to be determined. Isomerization of retinoids is a crucial reaction in the classic retinoid cycle and it has been suggested that in the retina, the cones acquire retinal chromophore from a different pathway (a cone-specific retinoid cycle) for pigment regeneration (8;13;30-32). The purpose of this investigation is to study the classic retinoid cycle (supported by Isomerase I in the RPE) and the novel cone cycle (supported by Isomerase II in the retina) in the cone-dominated chicken eye. We first demonstrated that significant isomerase activity exists in the RPE-free isolated chicken retina by examining the formation of 11-cis retinoids in the light-adapted retina. We then studied the expression of RPE65 in the chicken RPE, retina, and primary culture of Müller cells using real-time PCR. To study the nature of retinoid isomerization, in vitro Isomerase I and II assays were conducted in the presence of inhibitors of RPE65, ARAT and LRAT. Our results show the cone-dominated chicken eye contains two isomerases that support two visual cycles: a classic retinoid cycle for retinoid exchange between the RPE and the retina, as well as a cone cycle for retinoid processing within the retina. Our data also suggest that, as in the rod-dominated eye, Isomerase I in the cone-dominated chicken eye is also located in the RPE and its activity is LRAT-dependent. Furthermore, a second isomerase (Isomerase II) was found in the retina and has significantly different biochemical properties from Isomerase I in the RPE, i.e. Isomerase II activity is ARAT-dependent and iron-independent. The present study is the first to provide biochemical evidence for both retinoid regeneration pathways in the cone-dominated chicken eye. Our results strongly suggest that there are two functional retinoid cycles in the cone-dominated eye: a classic retinoid cycle supported by Isomerase I in the RPE (for rod and cone pigment regeneration) and an additional retinoid cycle supported by Isomerase II for cone pigment regeneration.
Experimental Procedures (Materials and Methods)
Isolated chicken retina light exposure study
Heads from freshly decapitated adult chickens (Gallus domesticus, from Tyson Foods, Inc., Seguin, TX, USA) were obtained and transported to the laboratory on ice. Upon arrival, retinas were dissected free from RPE in ice-cold minimal essential media (MEM) and suspended in 1.0 ml of MEM. Retinas were incubated at 40°C for 5 minutes prior to light exposure (≈ 3,000 lux) for the indicated times. Retinas were immediately homogenized under dim red light and retinoids were extracted using hexane for both retinyl esters and retinol, and the formaldehyde method for retinaldehydes (33).
Chicken primary Müller cell cultures
Freshly decapitated chicken heads were acquired as described above. Primary chicken Müller cell cultures were established according to Das et al. with minor modifications. Eyes were enucleated within 3 hours post-mortem and sterilized with 10% Wescodyne for 5 minutes. Eyes were sectioned at the level of the ora serrata, the vitreous was removed, and the retina dissected from the eye. After being rinsed in 1% antibiotic/antimycotic in Hank's balanced salt solution (HBSS), the retinas were incubated for 45 minutes at 37°C in HBSS containing 5.0 mg/ml papain. The retinas were then rinsed three times in HBSS, and tissue was collected by centrifugation (1500 ×g). Cells from two to three retinas were seeded into each T75 flask with 10-12 ml of minimum essential medium (MEM) (10% fetal bovine serum (FBS) and 5 mg/ml glucose) and incubated at 37°C and 5% CO2. The medium was changed 24 hours later and every 48 hours thereafter until cultured cells were confluent (approximately 14-19 days).
Polymerase Chain Reaction (PCR) expression studies
Chicken primary Müller cells were isolated and cultured as previously described (11). Freshly decapitated chicken heads were acquired as described above. Total RNA was isolated from retinal tissue, RPE tissue, and primary Müller cell cultures using Trizol reagent. Equal amounts (5 μg) of RNA were used in the reverse transcription reaction. Primers were designed using Invitrogen's Oligoperfect Designer software, based on the DNA sequence of chicken RPE65 (RPE65 forward- 5′ACA AAG GAG ACC TGG GTG TGG 3′, RPE65 reverse- 5′ GCT GGA AGA AGC CTA ACA CGG 3′). Primers were diluted according to supplier's instructions. Real-time PCR was performed using an ABI prism real-time PCR machine at the following settings: 50°C for 2 minutes (1 repetition), 95°C for 10 minutes, (1 repetition), 95°C for 15 seconds and 60°C for 1 minute (50 repetitions). All reactions were normalized with the housekeeping gene, 18s, provided as a pre-optimized control probe (ABI prism software, Applera, UK) enabling data to be expressed as delta threshold cycle (▲CT) values (where ▲CT= CT of 18s subtracted from CT of RPE gene of interest). Measurements were done in triplicate for each sample. The level of expression was analyzed from the amplification data generated from the ABI PRISM SOFTWARE in different tissues.
DNA gel electrophoresis
Agarose gel electrophoresis was performed to determine the PCR product size. A 2% agarose gel containing ethidium bromide was prepared and run in a MINICELL EC37OM Electrophoretic Gel Systems apparatus. A Promega 100 bp DNA ladder was used as the molecular weight standard. The gel was then photographed under UV light using a Polaroid electrophoresis system, photodocumentation camera Model No. FBPDC-34. The PCR product was sequenced by The University of Texas Health Science Center DNA Lab to confirm its identity.
Immunoblot/Western Analysis
Protein concentrations of samples prepared from the chicken retina, RPE, and primary Müller cell cultures were determined using Bio-Rad protein assay and bovine serum albumin (BSA) standards. Chicken RPE, retina, and primary Müller cell culture proteins were loaded in equal amounts (50 μg), separated by SDS-PAGE, and transferred onto Sequi-blot PVDF membranes using a Bio-Rad mini-transblot cell system. The membranes were then incubated with primary antibody overnight at a dilution of 1:1000 (anti-RPE65 from Dr. Jian-Xing Ma, University of Oklahoma Health Science Center). This was followed with a two hour incubation using peroxidase labeled secondary antibody at a dilution of 1:2500 (Vector Laboratories). Protein bands were visualized using Diaminobenzadine (DAB easy tablets for immunohistochemistry, ACROS Organics).
Isomerase I assay
Microsomal proteins were prepared from fresh ocular tissues (retina or RPE) obtained from bovine eyes (Bos taurus, provided by Wiatrek Meat Markets, Poth, TX) and decapitated chicken heads. Tissues were immediately placed on ice and transported to the laboratory. Retinas were dissected free from RPE. Ocular tissues were then homogenized in Tyrode's avian buffer (consisting of in g/L NaCl 8.0; KCl 0.2; MgCl2 0.05; CaCl2 0.2; NaH2CO4 0.33; NaHCO3 1.0; glucose 1.0) (34) using protease inhibitor cocktail (Sigma Aldrich Inc., product number P2714). Microsomal and homogenate fractions were then prepared, and protein concentrations were determined using Bio-Rad's standard protein assay (35). Protein samples were frozen and stored at -80°C until used.
The isomerase assays were performed as described by Winston and Rando (36) with minor modifications. Microsomal or homogenate proteins were exposed to UV light (Spectroline lamp model # EN-140L), wavelength 365 nm, for 5 minutes to destroy endogenous retinoids. 200 μg of RPE microsomal protein or 1 mg of retina homogenate was pre-incubated in reaction buffer (100 mM Tris, 2 mM CaCl2 and 2 mM MgCl2, pH 8.0). The substrates (20 mM all-trans retinol, 20 mM all-trans retinyl palmitate, or 20 mM all-trans retinol and 100 mM palmitoyl-coenzyme-A (palm-CoA)) were delivered with 1.0% BSA and incubated for 30 minutes at room temperature. Cellular retinaldehyde binding protein (CRALBP from Dr. Krzysztof Palczewski, Case Western Reserve University) was then added to the mixture after the initial incubation and incubated for an additional 60 minutes at 37°C. The total reaction volume was 500 μl. The reaction was stopped by the addition of 1 ml of ice-cold ethanol, and retinoids were extracted for HPLC analyses.
2,2′-Bipyridine inhibition studies
Chicken retina homogenate (1.0 mg) and RPE homogenate (0.5 mg) were pre-incubated, at room temperature, with reaction buffer (100 mM Tris, 2 mM CaCl2 and 2 mM MgCl2, pH 8.0) containing 20 μM all-trans retinol or 20 μM all-trans retinol + 5 mM 2,2′-bipyridine (Alfa Aesar) for 30 minutes. After the initial incubation, 30 μM CRALBP was added to each sample and incubated for 1 hour at 37°C. Total reaction volume was 500 μl. Isomerase inhibition studies were conducted using chicken retina homogenate as described above, adding 100 μM of palm-CoA. The reaction was then stopped using 1 ml of ice-cold ethanol, and the retinoids were extracted for HPLC analyses.
Progesterone and N-ethyl-maleimide (NEM) inhibition studies
It is known that Isomerase I activity requires all-trans retinyl esters as a substrate, and that their synthesis is LRAT-dependent in the RPE. To test whether the isomerase in the cone-dominated chicken retina is similar to Isomerase I in the RPE, the retinyl ester synthase inhibitors progesterone (ARAT inhibitor),(11;37-40) and NEM (LRAT inhibitor) (41) were incubated with chicken RPE and retina homogenates. Isomerase assays were conducted as described above, except that the homogenates were pre-incubated with 100 μM progesterone or 20 μM NEM for 30 minutes at room temperature prior to substrate delivery.
Retinoid Analyses
Retinoids were analyzed by HPLC. Retinaldehydes were analyzed at an absorbance wavelength of 365 nm using a Beckman System Gold 168 Detector, with a Zorbax Rx-sil 5 μm 4.6 × 250 mm column, at a flow rate of 1.0 ml/min, 3.0% tert-butyl methyl ether/hexane mobile phase. 11-cis Retinol and retinyl esters were analyzed at an absorbance wavelength of 318 nm using a Waters System equipped with a 2996 PDA Detector, Zorba Rx-sil 5 μm 4.6 × 250 mm column at a flow rate of 1.0 ml/min using 10% and 0.20% dioxane/hexane mobile phase, respectively. Isomerization in RPE samples was assessed by 11-cis retinol synthesis; isomerization in chicken retina samples was assessed by the synthesis of 11-cis retinyl esters. Retinoids were identified by comparison of retention times of authentic retinoid standards and by UV spectra from the photodiode array detector. Quantification was achieved by comparing peak areas of retinoids with those from calibration curves obtained from authentic standards.
Results
Isomerase activity in isolated chicken retina
Fully dark-adapted chicken retinas were exposed to light to study the formation of 11-cis retinoids (9). The dark-adapted chicken retina had 0.20 ± 0.01 nmol/mg of 11-cis retinaldehyde; this 11-cis retinaldehyde decreased by a factor of 10 to 0.02 ± 0.01 nmol/mg after 5 minutes of illumination, further decreasing to 0.01± 0.00 nmol/mg of retinal protein after 15 minutes of light exposure. All-trans retinaldehyde concentrations correspondingly increased by a factor of 5, from 0.01 ± 0.00 in the dark-adapted chicken retina, to 0.05 ± 0.00 nmol/mg after 5 minutes of illumination (Figure 1A). Light treatments did not produce any significant change in 11-cis retinol levels in the dark-adapted retina (0.03 ± 0.00 nmol/mg), while all-trans retinol increased from 0.05 ± 0.00 nmol/mg to 0.14 ± 0.02 nmol/mg after 5 minutes of light exposure and remained at similar levels after 15 minutes of light treatment (Figure 1B). All-trans retinyl esters also showed minimal changes upon light exposure, while 11-cis retinyl esters increased from 0.08 ± 0.00 nmol/mg to 0.18 ± 0.05 nmol/mg and remained constant after 15 minutes of light exposure (Figure 1C). Recovery of total retinoids extracted from the isolated retina remained unchanged between dark and light exposed conditions (Figure 1D).
Figure 1.
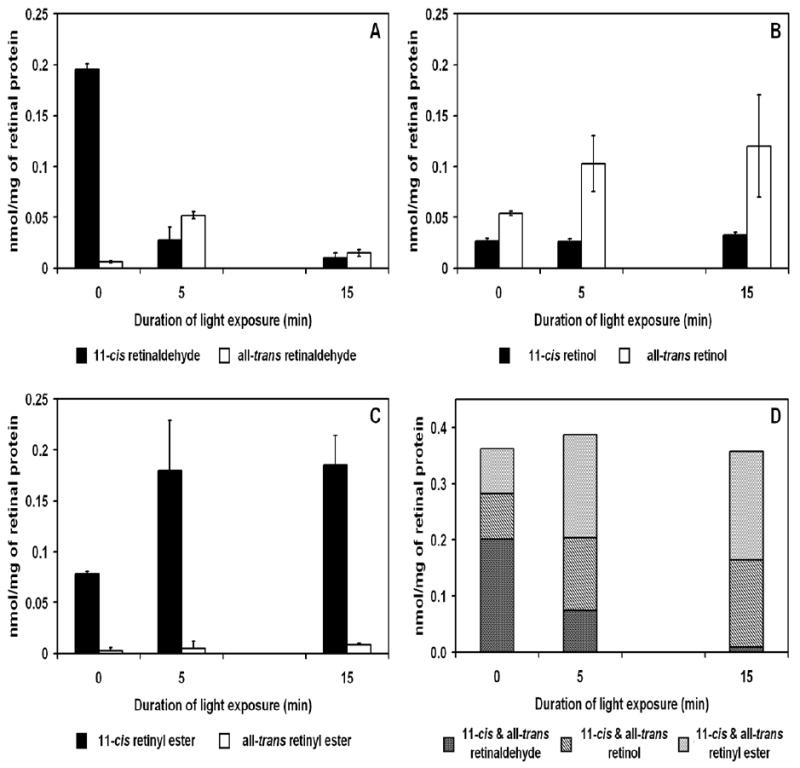
Changes in retinoid concentrations in response to light-adaptation in isolated chicken retina. Isolated dark adapted chicken retinas were light exposed (≈ 3000 lux) for indicated time intervals at 40°C. (A) Change in retinaldehyde concentrations (nmol/mg) in isolated retina when dark-adapted for 5 minutes and 15 minutes after light exposure. 11-cis Retinaldehyde decreased from 0.20 ± 0.01 to 0.01 ± 0.00 nmol/mg. All-trans Retinaldehyde increased after 5 minutes of light exposure and decreased back to near dark-adapted levels after 15 minutes of light exposure. (B) The decrease in 11-cis retinaldehyde was accompanied by a corresponding increase in all-trans retinol after light exposure while 11-cis retinol concentrations remained constant. (C) 11-cis Retinyl ester concentrations doubled from 0.08 ± 0.00 to 0.18 ± 0.03 nmol/mg after light exposure demonstrating the existence of isomerase in the isolated chicken retina. No change in all-trans retinyl ester concentrations was detected. (D) Total retinoid concentrations showed little variance under light or dark conditions. (n=3)
RPE65 expression studies
Real-time PCR results indicate that RPE65 was expressed in the chicken RPE, but only minimal RPE65 expression was found in the chicken retina and no expression in cultured Müller cells (at 6.00% and 0.00% respectively when compared to RPE65 expression in chicken RPE, normalized at 100%; Figure 2B). The real-time PCR product was confirmed by sequencing and by agarose gel electrophoresis (Figure 2A). Anti-RPE65 Western blot analyses of chicken primary Müller cell cultures, chicken RPE, and chicken retina showed strong positive staining in chicken RPE and no detectable staining in chicken retina or chicken primary Müller cell culture samples (Figure 2C).
Figure 2.
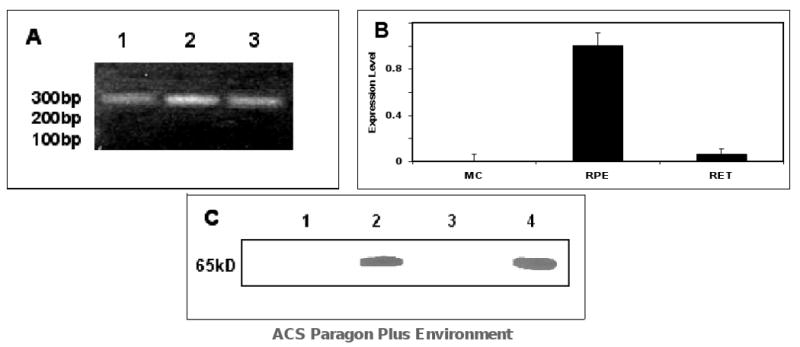
Expression of RPE65 in chicken primary Müller cell cultures, RPE, and retina by RT-PCR and Western Blot. (A) RPE65 product was confirmed by electrophoresis: Lanes: 1. PCR product from primary Müller cell cultures, 2. PCR product from chicken RPE, 3. PCR Product from chicken retina. (B) Normalized RPE65 expression levels in chicken primary Müller cell cultures, chicken RPE, and chicken retina (n=6). (C) Western blot anti-RPE65: Lane 1. chicken primary Müller cell culture homogenate, Lane 2. chicken RPE homogenate, Lane 3. chicken retina homogenate, Lane 4. Bovine RPE homogenate (positive control). 50 μg of protein was loaded in each lane. No detectable amounts of RPE65 were observed in the chicken retina or chicken primary Müller cell culture samples.
Isomerase activity in cone-dominated chicken retina
In order to study the isomerase activity in the cone-dominated chicken retina, we performed in vitro Isomerase I and II activity assays on chicken retina and RPE microsomes using bovine retina and RPE microsomes as controls for Isomerase I activity. Table 1A shows 11-cis retinol was produced in bovine RPE microsomes at a rate of 16.90 ± 0.90 pmol/min/mg; this rate is comparable with published results of 16.6 pmol/min/mg (42). 11-cis retinyl esters and all-trans retinyl esters were also produced by RPE microsomes at rates of 4.61 ± 0.30 and 328.89 ± 43.25 pmol/min/mg (Table 1A). Bovine retinal microsomes did not synthesize 11-cis retinol or 11-cis retinyl esters, but all-trans retinyl esters were synthesized at 5.55 ± 0.11 pmol/min/mg. Table 1 also shows the formation of 11-cis retinol in chicken RPE microsomes at a rate of 11.71 ± 0.55 pmol/min/mg, and 11-cis retinyl esters at a rate of 1.61 ± 0.43 pmol/min/mg. All-trans retinyl esters formed from all-trans retinol at a rate of 2.40 ± 0.28 pmol/min/mg, and 11-cis retinyl esters at a rate of 0.58 ± 0.20 pmol/min/mg in chicken retina microsomes. No 11-cis retinol was formed from all-trans retinol in chicken retina microsomes.
Table 1.
Retinoid isomerase activity in bovine and chicken retina and RPE microsomes from all-trans retinol or all-trans retinyl palmitate substrate. A. Ester synthase and Isomerase I activity after incubating the protein samples with 20 μM all-trans retinol as substrate, and Isomerase I activity after incubating protein samples with 20 μM all-trans retinyl palmitate as substrate. B. Isomerase II activity in chicken retina after incubating protein samples with 20 μM all-trans retinol as substrate in the presence of 100 μM palm-CoA and 30 μM CRALBP.
| A: Ester synthase and Isomerase I activity using all-trans retinol (AtRoL) and all-trans retinyl palmitate (AtRP) substrates in the bovine and chicken eyes. | ||||
|---|---|---|---|---|
| Microsomal Protein | Ester Synthase | Isomerase I | Isomerase I / Ester Synthase | Isomerase I |
| (AtRoL→AtRP) | (AtRoL→11-cisOL) | (AtRoL→11-cisRP) | (AtRP→11-cisOL) | |
| all-trans retinyl palmitate | 11-cis retinol | 11-cis retinyl palmitate | 11-cis retinol | |
| (pmol/min/mg) | (pmol/min/mg) | (pmol/min/mg) | (pmol/min/mg) | |
| Bovine RPE | 328.89 ± 43.25 | 16.90 ± 0.897 | 4.61 ± 0.29 | 5.66 ± 0.17 |
| Bovine Retina | 5.55 ± 0.11 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.08 ± 0.05 |
| Chicken RPE | 313.39 ± 21.60 | 11.71 ± 0.55 | 1.61 ± 0.43 | 1.13 ± 0.04 |
| Chicken Retina | 2.40 ± 0.28 | 0.00 ± 0.00 | 0.58 ± 0.20 | 0.00 ± 0.00 |
| B: Ester synthase and Isomerase II activity assayed in the presence of palm-CoA in the chicken retina. | ||||
| Ester Synthase | Isomerase II | Isomerase II / Ester Synthase | ||
| (AtRoL→AtRP) | (AtRoL→11-cisOL) | (AtRoL→11-cisRP) | ||
| all-trans retinyl palmitate | 11-cis retinol | 11-cis retinyl palmitate | ||
| (pmol/min/mg) | (pmol/min/mg) | (pmol/min/mg) | ||
| 12.33 ±1.53 | 0.00 ± 0.00 | 2.00 ± 0.19 | ||
Isomerase I activity was tested in chicken retina and RPE, as well as in bovine retina and RPE, using microsomes made from respective tissues. All-trans retinyl palmitate was used as the substrate (Table 1A). Enzymatic activity was determined by measuring the formation of 11-cis retinol. Bovine RPE microsomes produced 11-cis retinol at a rate of 5.66 ± 0.17 pmol/min/mg, while negligible amounts were produced by the bovine retina (0.08 ± 0.05 pmol/min/mg). Chicken RPE microsomes produced 11-cis retinol at a rate of 1.13 ± 0.04 pmol/min/mg. No 11-cis retinol was synthesized from all-trans retinyl palmitate using chicken retina microsomes.
Finally, we incubated chicken retina microsomes with all-trans retinol in the presence of palm-CoA and studied the formation of 11-cis retinoids (Table 1B). 11-cis and all-trans retinyl esters were synthesized at a rate of 2.00 ± 0.19 and 12.33 ± 1.53 pmol/min/mg, respectively. 11-cis retinol was not detected.
Studies using Isomerase, ARAT and LRAT Inhibitors
To further investigate the nature of the isomerase activity in the cone-dominated retina, we conducted Isomerase I inhibition studies on chicken RPE and retina homogenates using 2,2′-bipyridine which inhibits RPE65 by chelating necessary iron. Bovine RPE microsome synthesis of 11-cis retinol (control) was reduced to a non-detectable level (data not shown). Chicken RPE homogenate synthesis of 11-cis retinol was similarly reduced from 0.04 ± 0.004 nmol/mg to a non-detectable level (Figure 3A&C). The 11-cis retinyl ester synthesis in retinal homogenate was 0.12 ± 0.03 nmol/mg in the absence of 2,2′-bipyridine and remained at a steady level 0.11 ± 0.04 nmol/mg in the presence of the inhibitor (Figure 4A&C).
Figure 3.
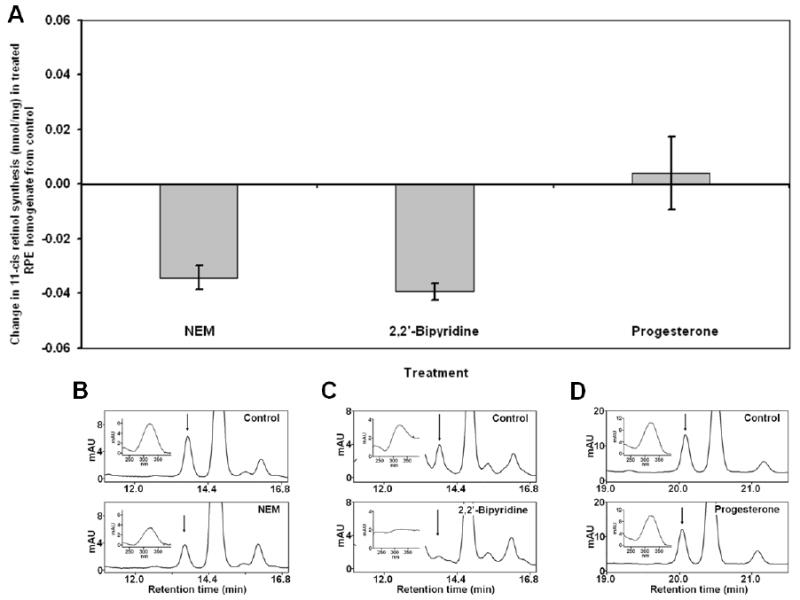
Inhibition of Isomerase activity in chicken RPE homogenate by NEM, 2,2′-bipyridine, and progesterone. Isomerase assays were performed on chicken RPE homogenates in the presence or absence of indicated inhibitors. The effect of treatment was determined by measuring the change in 11-cis retinol synthesized from treated RPE homogenate.from 11-cis retinol synthesized in control experiments. (A) Effect of NEM on RPE homogenate from control was -0.034 ± 0.004 nmol/mg. 2,2′-Bipyridine also provided an inhibitory effect on 11-cis retinol synthesis in RPE homogenate (-0.039 ± 0.003 nmol/mg). Progesterone had no significant effect on RPE retinoid isomerase activity (0.004 ± 0.013 nmol/mg). (B-D) Representative HPLC chromatograms of retinoid extracts from control and treated (NEM, 2,2′-Bipyridine, and progesterone from left to right) RPE homogenates. Insets show absorbance spectrum of HPLC peak indicated by arrow (11-cis retinol). (n = 3)
Figure 4.
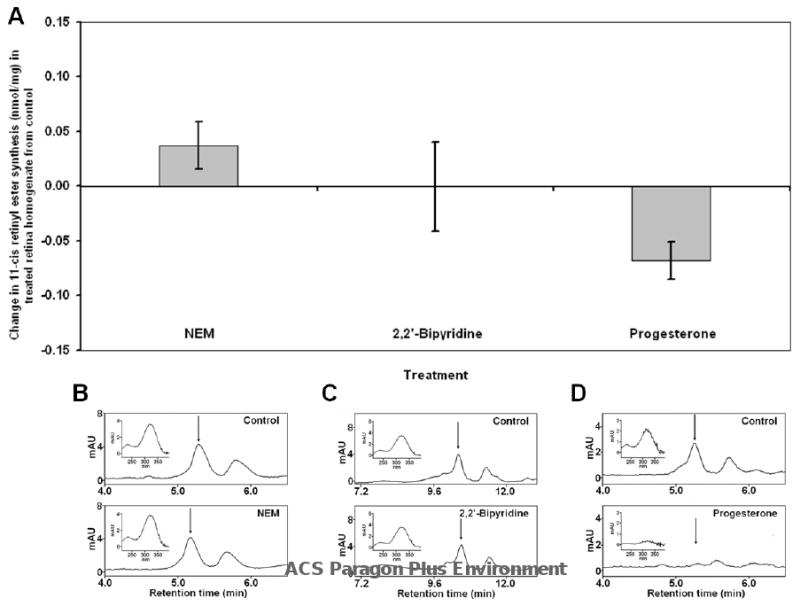
Inhibition of Isomerase activity in chicken retina homogenate by NEM, 2,2′-bipyridine, and progesterone. Isomerase assays were performed on chicken retina homogenates in the presence or absence of indicated inhibitors. The effect of treatment was determined by measuring the change in 11-cis retinyl esters synthesized from treated retina homogenate from 11-cis retinyl esters synthesized in control experiments. (A) Effect of NEM on retina homogenate from control was not significant 0.037 ± 0.021 nmol/mg. 2,2′-Bipyridine also produced no inhibitory effects on 11-cis retinyl ester synthesis in retina homogenate (-0.001 ± .041 nmol/mg). However, progesterone produced a significant inhibitory effect on the isomerase activity in the retina (-0.068 ± 0.017 nmol/mg). (B-D) Representative HPLC chromatograms of retinoid extracts from control and treated (NEM, 2,2′-bipyridine, and progesterone from left to right) retinal homogenates. Insets show absorbance spectrum of HPLC peak indicated by arrow (11-cis retinyl ester). (n = 3)
We also examined whether the isomerase activity observed in the chicken retina and RPE was ARAT-dependent. Chicken retina and RPE homogenates were incubated with progesterone prior to the isomerase assays. Results from these inhibition studies showed no significant reduction in Isomerase I activity in chicken RPE homogenate in the presence of progesterone (Figure 3A&D). In contrast, the Isomerase II activity in the chicken retina homogenate was reduced by 84.0% from 0.08 ± 0.02 nmol/mg to 0.012 ± 0.00 nmol/mg in the presence of progesterone (Figure 4A&D). Synthesis of 11-cis retinol was not observed in any of the retinal homogenate samples.
To further differentiate between Isomerase I in the RPE and Isomerase II in the cone- dominated chicken eye, we conducted isomerase assays using chicken RPE or retina homogenates in the presence of NEM, an inhibitor of LRAT; this would inhibit Isomerase I activity by depriving the enzyme of its substrate. Results from this study showed that Isomerase I activity in chicken RPE was reduced by NEM, from 0.08 ± 0.01 nmol/mg to 0.05 ± 0.00 nmol/mg (Figure 3A&B). Synthesis of 11-cis retinyl esters in chicken retina homogenate was not significantly affected by NEM (0.043 ± 0.02 vs 0.08 ± 0.01 nmol/mg; Figure (4A&B).
Apparent kinetic constants of Isomerase II in chicken retina homogenate
To demonstrate protein dependency and enzyme kinetics for the formation of 11-cis retinoids in chicken retina by Isomerase II enzyme, we conducted Isomerase II assays as described above with increasing amounts of protein and substrates. Increasing amounts of protein resulted in increased formation of 11-cis retinyl esters by a factor of 5, from 0.004 to 0.022 nmol, at 1.0 mg protein. When 2.0 mg of homogenate protein was used, the amount of 11-cis retinyl esters doubled to 0.045 nmol (data not shown).
To determine apparent kinetic constants for the formation of 11-cis retinoids, by Isomerase II, from all-trans retinol in chicken retina homogenates, homogenate proteins were incubated with fixed concentrations of palm-CoA and CRALBP; all-trans retinol was added to the reaction mixture in increasing amounts. Lineweaver-Burk plot transformation of the data yielded apparent values for 11-cis retinoid formation ie. Isomerase II activity: Vmax = 2.10 pmol/min/mg and KM = 0.10 μM (Figure 5A&B).
Figure 5.
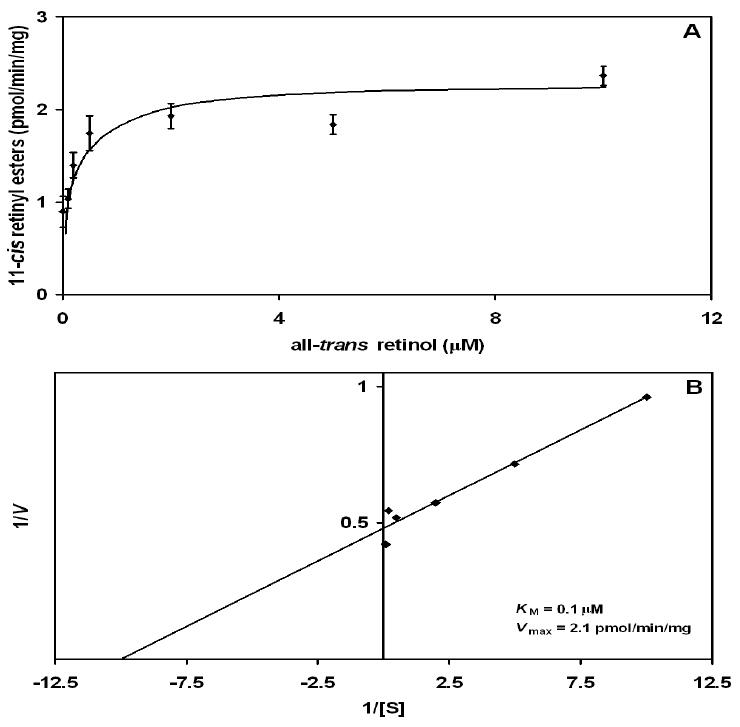
Enzyme kinetics of the formation of 11-cis retinyl esters from all-trans retinol substrate in chicken retina homogenate. (A) Saturation curve of Isomerase II in chicken retina homogenate. Protein was incubated with increasing concentrations of all-trans retinol while maintaining constant palm-CoA concentrations. (B) Lineweaver-Burk plot conversion of the substrate saturation curve yielded apparent kinetic constants: Vmax = 2.10 pmol/min/mg and KM = 0.10 μM. Protein saturation experiments were conducted from 0.0 μg to 1000.0 μg/ml of chicken retina homogenate. 1000.0 μg/ml of chicken retina homogenate was used for all kinetic studies. 11-cis Retinyl esters were undetectable at protein concentrations lower than 1000.0 μg/ml. Synthesis of 11-cis retinoids was not observed when protein was heated to 90°C for 5 minutes prior to incubation (data not shown).
Discussion
The classic retinoid cycle for rod and cone photoreceptors has been well established (1-7) but retinoid processing in support of cone pigment regeneration in the cone-dominated retina has yet to be fully elucidated. Does an RPE-independent visual cycle exist in the cone-dominated retina? One of the key reactions in retinoid processing is isomerization of vitamin A. At present, the nature of this reaction and the identification of this isomerase are not known for cone-specific retinoid regeneration. It has been suggested that the cone-dominated chicken retina possesses a novel retinoid processing pathway, supported by a novel retinol isomerase (8;13;31;32). This view was not supported by others who suggest that the retinoid cycle in the cone-dominated retina is supported by RPE65 (43). Recent reports provide new experimental evidence for an RPE65-independent pathway in the zebrafish for the regeneration of 11-cis retinaldehyde in cone-mediated vision (29). Cones, located in the retina of both salamander and mouse, have been shown to recover light sensitivity independent of the RPE (30). In this study, we investigated RPE65 expression and isomerase activities in the cone-dominated chicken eye. We have demonstrated the presence of two isomerases in the cone-dominated chicken eye [8] and have distinguished the locations and activities of Isomerase I (RPE65) and Isomerase II.
We first showed the existence of isomerase activity in the isolated chicken retina. Fully dark-adapted chicken retina, free of RPE, was exposed to light to bleach the photopigments, leading to a significant reduction of 11-cis retinaldehyde. This decrease in 11-cis retinaldehyde was accompanied by an increase and accumulation of all-trans retinol and 11-cis retinyl esters, while 11-cis retinol levels remained constant (Figure 1A-C). This increase in all-trans retinol in the isolated retina was the result of a decreased amount of all-trans retinaldehyde produced by photoisomerization of 11-cis retinaldehyde. Based on results from our in vitro isomerase assay, all-trans retinol is isomerized to 11-cis retinol by Isomerase II. 11-cis retinol is then esterified, leading to the observed accumulation of 11-cis retinyl esters. The formation and accumulation of 11-cis retinyl esters in the isolated retina can be attributed to 1) the higher rates of isomerization of 11-cis retinol from all-trans retinol, as previously published (11;13), 2) the esterification of 11-cis retinol (≈ 190 pmol/min/mg vs. 170 pmol/min/mg respectively) (11;13), and 3) the relatively lower rates of 11-cis retinyl ester hydrolysis (≈ 20 pmol/min/mg) (32) and oxidation of 11-cis retinol to 11-cis retinaldehyde (≈ 100 pmol/min/mg) (8).
RPE65 is an isomerase in the classic retinoid cycle, in rod-dominated species (14-16), which supports rod and cone pigment regeneration. Our real-time PCR results indicate that RPE65 was expressed in the chicken RPE, a cone-dominated species, but that there is little expression of this enzyme in the chicken retina and no expression in cultured Müller cells (Figure 2). These results suggest that Isomerase I activity observed in the present study is from RPE65 which supports the classic retinoid cycle. Our data also suggest that isomerase activity in the isolated retina is not attributable to RPE65, as RPE65 was not found to be expressed in the retina.
To determine how Isomerase I activity supports the isomerization reactions in the RPE and in the retina, we conducted Isomerase I assays on bovine RPE and retina, as well as chicken RPE and retina (Table 1A). Isomerase I assays, using all-trans retinol as substrate, resulted in the formation of 11-cis retinol in the bovine RPE and chicken RPE at similar rates (Table 1A), suggesting that the Isomerase I protein in the chicken RPE possesses isomerase activity for support of a classic retinoid cycle in the RPE. The rate of 11-cis retinol synthesis in chicken RPE was reported to be approximately 11 times faster than in the bovine RPE (12). This difference is likely due to the fact that, in the present study, a saturating concentration of substrate (20 μM all-trans retinol) was used instead of the 0.20 μM used (12). In comparison to the RPE, incubation of all-trans retinol with chicken or bovine retina did not yield any 11-cis retinol, suggesting the absence of Isomerase I activity in the chicken and bovine retina (Table 1A). A small amount of 11-cis retinyl esters were formed in chicken retina, which likely result from Isomerase II activity.
To further confirm Isomerase I activity in the chicken RPE, we conducted Isomerase I assays using all-trans retinyl palmitate as the substrate (36). As expected, Isomerase I activity was observed in the RPE and was not detected in chicken retina microsomes (Table 1A).
Synthesis of 11-cis retinyl esters was observed in chicken retina microsomes incubated with all-trans retinol in the presence of palm-CoA (Table 1B), suggesting an isomerase (Isomerase II) with different biochemical properties than RPE65 (Isomerase I). The requirement of palm-CoA as an acyl donor in this reaction suggests that Isomerase II is dependent on ARAT instead of LRAT (as in Isomerase I). One of the enzymes previously suggested to be located in the cone-dominated chicken retina is a novel ester synthase which is coupled with an isomerase activity (8). A novel 11-cis ARAT activity has also been recently described, and was suggested to be localized in the chicken retinal Müller cell (11). Acyl-CoA:diacylglycerol acyltransferase-1 (DGAT-1) has recently been suggested to be responsible for the ARAT activity in the cone-dominated retina (44). The identity of the enzyme responsible for synthesis of 11-cis retinyl esters requires further investigation.
To further distinguish between Isomerase I and Isomerase II activities, we employed the iron chelator, 2,2′-bipyridine, a known Isomerase I inhibitor (45). Figure 3A&C shows that in the presence of 2,2′-bipyridine, isomerization of all-trans retinol to 11-cis retinol in the chicken RPE was completely inhibited. In contrast, the presence of the Isomerase I inhibitor had no effect on the synthesis of 11-cis retinyl esters in the retinal samples (Figure 4A&C). Progesterone, a known ARAT inhibitor, was also employed to distinguish between the isomerase activity in chicken RPE and chicken retina. Results from this study show that retinoid isomerization in the RPE was not significantly affected by the presence of progesterone (Figure 3A&D). In contrast, the presence of progesterone in the chicken retina greatly decreased retinoid isomerization, suggesting that retinoid isomerization is coupled to 11-cis retinyl ester synthesis in the cone-dominated chicken retina (Figure 4A&D). It is also important to note that no 11-cis retinol was detected in any of the chicken retina samples. It is possible that the apparent lack of 11-cis retinol is due to the similar isomerization and retinyl ester synthesis rates as well as the relatively slow rate of 11-cis retinyl ester hydrolysis and 11-cis retinol oxidation, as described above.
In the classic retinoid cycle, LRAT is responsible for the conversion of all-trans retinol to all-trans retinyl palmitate, the substrate for Isomerase I. Therefore, by inhibiting synthesis of all-trans retinyl palmitate, retinoid isomerization by Isomerase I is also inhibited. To differentiate between isomerization activities in chicken RPE and retina, isomerase assays were conducted in the presence of an LRAT inhibitor. Isomerization activity in the chicken RPE was reduced by 37.5% in the presence of NEM (Figure 3A&B). The remaining 62.5% of isomerase activity may have resulted from the presence of endogenous all-trans retinyl palmitate prior to NEM delivery, or all-trans retinyl palmitate synthesized by ARAT activity in the RPE (38). When NEM was added to retinal samples, there was no significant reduction in retinoid isomerization as indicated by the levels of 11-cis retinyl esters (Figure 4A&B). This further supports the idea that the isomerase in the chicken retina is not RPE65, and that its activity is not LRAT-dependent.
In the present study, we employed three visual cycle enzyme inhibitors to distinguish between the isomerase activity in the chicken RPE and retina. Results from 2,2′- bipyridine inhibition clearly show that Isomerase II activity in the chicken retina was not affected by this inhibitor which completely inhibited Isomerase I in the RPE. Furthermore, in contrast to Isomerase I, 2,2′-bipyridine did not affect Isomerase II suggesting that this activity is not iron-dependent. Both progesterone and NEM studies demonstrated that Isomerase II activity in the retina is coupled to synthesis of 11-cis retinyl esters while, in the RPE, Isomerase I is dependent on the formation of all-trans retinyl esters. Our results agree with those from a recent study in which cone dark-adaptation was demonstrated in isolated retinas of rod-dominated mice and salamanders, suggesting the existence of an RPE-independent intra-retinal retinoid cycle in these species (30). The present study is the first to provide biochemical evidence that the chicken eye utilizes both the classic retinoid cycle, and a cone cycle in the retina. The presence of two retinoid isomerase enzymes at different locations in the chicken eye suggests the possible cooperation between two visual cycles for maintenance of visual sensitivity under different light conditions (see below and Figure 6).
Figure 6.
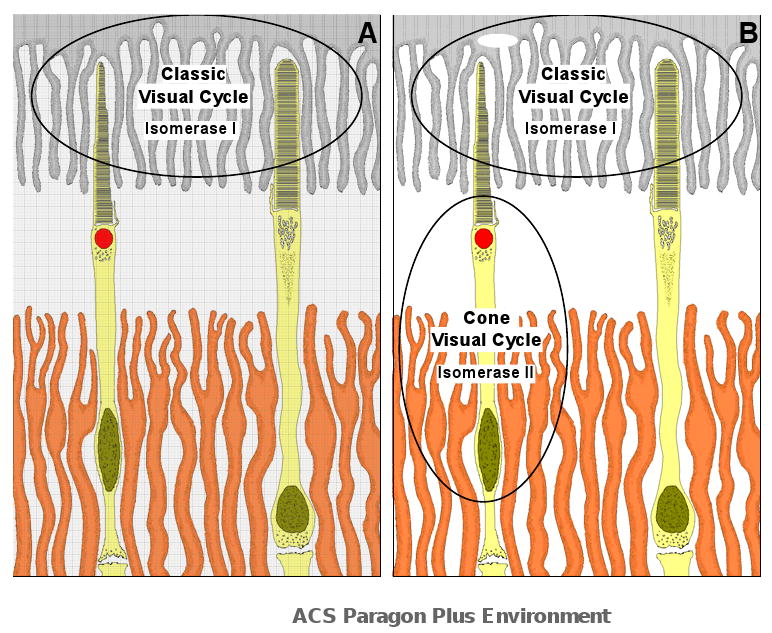
Location of two visual cycles involving exchange of retinoids between photoreceptors and RPE (classic, canonical visual cycle) or Müller cells (cone visual cycle). (A) The classic visual cycle involving both rod and cone photoreceptors and RPE (grey shade), operates under light illumination. All-trans retinaldehyde released from photopigments are reduced and transported from the outer segment to the RPE where they are processed (esterified, hydroisomerized, oxidized) to become 11-cis-retinaldehyde and returned to the photoreceptor for pigment regeneration (1-5). This visual cycle is supported by Isomerase I (RPE 65) and LRAT. Under intense light illumination, the rate of cone pigment regeneration exceeds the rate of chromophore recovery from the classic visual cycle and an additional visual cycle to supply chromophore for cone pigment regeneration occurs between cone outer segment and Müller cells (orange shade) in the retina (B). All-trans retinaldehyde released from cone pigments are transported from the cone outer segment to Müller cells (30;31) where they are processed (esterified and isomerized) to become cis-retinoids and returned to the cone photoreceptors for pigment regeneration (8;11;13). This visual cycle is supported by Isomerase II (an unidentified protein). It is not known how the cone cycle is activated by intense light illumination or how chromophores are selectively transported to the RPE and to the Müller cell (when both cycles are in operation). In addition, the relative contribution of chromophore (from the RPE and the Müller cells) for pigment regeneration is also not known. However, IRBP has been implicated as the transport protein for retinal chromophore for both visual cycles (46). Based on results in the present study, the classic visual cycle in the chicken RPE is supported by RPE65 (Isomerase I). Isomerase I was inhibited by 2,2′-bipyridine (an iron chelator), NEM (an LRAT inhibitor) but not by progesterone (an ARAT inhibitor). In contrast, Isomerase II activity is inhibited by progesterone but not by 2,2′-bipyridine and NEM. Based on results from enzyme assays, Isomerase I produced cis-retinoids at a rate 6 times faster than Isomerase II in the chicken eye, suggesting a faster recovery of visual chromophores from the classic visual cycle. This schematic diagram was adopted from a previous anatomical study of the chicken retina (47).
The relative contribution of cis retinoids (from these two cycles) for pigment regeneration is not known at this time. Can cone pigments in the retina regenerate in the absence of either one of these two visual cycles? Based on electrophysiological data, it has been shown that cone pigment regeneration can be completed in 7 minutes in the dark adapted amphibian eye cup, solely supported by retinoids from the RPE [30, Figure 5] without apparent contributions from Müller cells in the retina. If so, what is the role of the cone cycle in the retina for cone pigment regeneration? It is possible that this additional visual cycle plays an essential role to provide needed chromophore for cone pigment regeneration under intense light illumination when the demand of cis retinods far exceeds the capacity of retinoid supply from the classic visual cycle (Figure 6) [8]. Based on Isomerases I and II activities reported in the present study (see Table 1 and Figure 5), Isomerase II in the retina produces cis retinoids at a rate ≈ 17% of Isomerase I in the RPE. This suggests that the classic visual cycle may contribute six times more cis retinoids for cone pigment regeneration. However, it is important to consider that cis retinoids from Isomerase II may be more readily available for cone pigment regeneration because of the proximity of this Isomerase II enzyme in the retina to the cone photoreceptors. Furthermore, it has recently been shown, in IRBP knockout studies (46), that cone functions are significantly reduced without IRBP to transfer retinoids to cones. This constitutes an additional factor which may determine the relative availability of cis retinoids from the two visual cycles for cone pigment regeneration.
Further studies are needed to explore the nature of Isomerase II in the retina, and the location and function of the 11-cis retinyl ester storage pool in the retina, as well as interactions between the classic retinoid cycle and the cone cycle on cone pigment regeneration.
Acknowledgments
*We thank Dr. Krzysztof Palczewski for providing the CRALBP protein as well as initial review and comments on the manuscript. We would also like to thank Dr. Don Allen for review and comments on the manuscript, Dr. Joe L. Martinez for PCR assistance, Mr. Michael Anderson for assistance with statistical analysis, Mr. Bagrat Grigoryan, Ms. Eileen Kotchan Vidro for critical review of the manuscript, and Tyson Foods Inc., Seguin, TX, for donating fresh chicken tissue.
Our work was supported by a grant from NIH (GM08194), The San Antonio Area Foundation, and the NIH-MBRS-RISE program.
Abbreviations
- CRALBP
cellular retinaldehyde binding protein
- RPE
retinal pigment epithelium
- LRAT
lecithin:retinol acyl transferase
- ARAT
acyl CoA:retinol acyltransferase
- RGR
retinal G protein coupled receptor
- HBSS
Hanks Buffered Salt Solution
- MEM
minimal essential medium
- FBS
fetal bovine serum
- PBS
phosphate buffer saline
- PBST
PBS containing 0.2% Triton X-100
- BSA
bovine serum albumin
- DGAT-1
acyl CoA:diacylglycerol acyltransferase-1
- ▲CT
delta threshold cycle
- palm-CoA
palmitoyl-coenzyme-A
- NEM
N-ethyl-maleimide
- mAU
milli-absorbance units
References
- 1.Crouch RK, Chader GJ, Wiggert B, Pepperberg DR. Retinoids and the visual process. Photochem Photobiol. 1996;64(4):613–621. doi: 10.1111/j.1751-1097.1996.tb03114.x. [DOI] [PubMed] [Google Scholar]
- 2.Saari JC. Biochemistry of visual pigment regeneration: the Friedenwald lecture. Invest Ophthalmol Vis Sci. 2000;41(2):337–348. [PubMed] [Google Scholar]
- 3.McBee JK, Palczewski K, Baehr W, Pepperberg DR. Confronting complexity: the interlink of phototransduction and retinoid metabolism in the vertebrate retina. Prog Retin Eye Res. 2001;20(4):469–529. doi: 10.1016/s1350-9462(01)00002-7. [DOI] [PubMed] [Google Scholar]
- 4.Rando RR. The biochemistry of the visual cycle. Chem Rev. 2001;101(7):1881–1896. doi: 10.1021/cr960141c. [DOI] [PubMed] [Google Scholar]
- 5.Lamb TD, Pugh EN., Jr Dark adaptation and the retinoid cycle of vision. Prog Retin Eye Res. 2004;23(3):307–380. doi: 10.1016/j.preteyeres.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 6.Muniz A, Villazana-Espinoza ET, Hatch AL, Trevino SG, Allen DM, Tsin AT. A novel cone visual cycle in the cone-dominated retina. Exp Eye Res. 2007;85(2):175–184. doi: 10.1016/j.exer.2007.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Travis GH, Golczak M, Moise AR, Palczewski K. Diseases Caused by Defects in the Visual Cycle: Retinoids as Potential Therapeutic Agents. Annu Rev Pharmacol Toxicol. 2006 doi: 10.1146/annurev.pharmtox.47.120505.105225. E-published. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mata NL, Radu RA, Clemmons RC, Travis GH. Isomerization and oxidation of vitamin A in cone-dominant retinas: a novel pathway for visual-pigment regeneration in daylight. Neuron. 2002;36(1):69–80. doi: 10.1016/s0896-6273(02)00912-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Trevino SG, Villazana-Espinoza ET, Muniz A, Tsin AT. Retinoid cycles in the cone-dominated chicken retina. J Exp Biol. 2005;208(Pt 21):4151–4157. doi: 10.1242/jeb.01881. [DOI] [PubMed] [Google Scholar]
- 10.Villazana-Espinoza E, Hatch A, Tsin AT. In-vitro Conversion of All-Trans Retinol to 11-cis Retinol in Chicken Eye. Invest Ophthalmol Vis Sci. 2006;270:2036. [Google Scholar]
- 11.Muniz A, Villazana-Espinoza ET, Thackeray B, Tsin AT. 11-cis-Acyl-CoA:retinol O-acyltransferase activity in the primary culture of chicken Muller cells. Biochemistry. 2006;45(40):12265–12273. doi: 10.1021/bi060928p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moiseyev G, Takahashi Y, Chen Y, Kim S, Ma JX. RPE65 from Cone-dominant Chicken Is a More Efficient Isomerohydrolase Compared with That from Rod-dominant Species. J Biol Chem. 2008;283(13):8110–8117. doi: 10.1074/jbc.M703654200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mata NL, Ruiz A, Radu RA, Bui TV, Travis GH. Chicken retinas contain a retinoid isomerase activity that catalyzes the direct conversion of all-trans-retinol to 11-cis-retinol. Biochemistry. 2005;44(35):11715–11721. doi: 10.1021/bi050942m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jin M, Li S, Moghrabi WN, Sun H, Travis GH. Rpe65 is the retinoid isomerase in bovine retinal pigment epithelium. Cell. 2005;122(3):449–459. doi: 10.1016/j.cell.2005.06.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moiseyev G, Chen Y, Takahashi Y, Wu BX, Ma JX. RPE65 is the isomerohydrolase in the retinoid visual cycle. Proc Natl Acad Sci U S A. 2005;102(35):12413–12418. doi: 10.1073/pnas.0503460102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Redmond TM, Poliakov E, Yu S, Tsai JY, Lu Z, Gentleman S. Mutation of key residues of RPE65 abolishes its enzymatic role as isomerohydrolase in the visual cycle. Proc Natl Acad Sci U S A. 2005;102(38):13658–13663. doi: 10.1073/pnas.0504167102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gollapalli DR, Rando RR. All-trans-retinyl esters are the substrates for isomerization in the vertebrate visual cycle. Biochemistry. 2003;42(19):5809–5818. doi: 10.1021/bi0341004. [DOI] [PubMed] [Google Scholar]
- 18.Moiseyev G, Crouch RK, Goletz P, Oatis J, Jr, Redmond TM, Ma JX. Retinyl esters are the substrate for isomerohydrolase. Biochemistry. 2003;42(7):2229–2238. doi: 10.1021/bi026911y. [DOI] [PubMed] [Google Scholar]
- 19.Jiang M, Pandey S, Fong HK. An opsin homologue in the retina and pigment epithelium. Invest Ophthalmol Vis Sci. 1993;34(13):3669–3678. [PubMed] [Google Scholar]
- 20.Pandey S, Blanks JC, Spee C, Jiang M, Fong HK. Cytoplasmic retinal localization of an evolutionary homolog of the visual pigments. Exp Eye Res. 1994;58(5):605–613. doi: 10.1006/exer.1994.1055. [DOI] [PubMed] [Google Scholar]
- 21.Shen D, Jiang M, Hao W, Tao L, Salazar M, Fong HK. A human opsin-related gene that encodes a retinaldehyde-binding protein. Biochemistry. 1994;33(44):13117–13125. doi: 10.1021/bi00248a022. [DOI] [PubMed] [Google Scholar]
- 22.Chen XN, Korenberg JR, Jiang M, Shen D, Fong HK. Localization of the human RGR opsin gene to chromosome 10q23. Hum Genet. 1996;97(6):720–722. doi: 10.1007/BF02346179. [DOI] [PubMed] [Google Scholar]
- 23.Hao W, Fong HK. The endogenous chromophore of retinal G protein-coupled receptor opsin from the pigment epithelium. J Biol Chem. 1999;274(10):6085–6090. doi: 10.1074/jbc.274.10.6085. [DOI] [PubMed] [Google Scholar]
- 24.Chen P, Hao W, Rife L, Wang XP, Shen D, Chen J, Ogden T, Van Boemel GB, Wu L, Yang M, Fong HK. A photic visual cycle of rhodopsin regeneration is dependent on Rgr. Nat Genet. 2001;28(3):256–260. doi: 10.1038/90089. [DOI] [PubMed] [Google Scholar]
- 25.Wenzel A, Oberhauser V, Pugh EN, Jr, Lamb TD, Grimm C, Samardzija M, Fahl E, Seeliger MW, Reme CE, von Lintig J. The retinal G protein-coupled receptor (RGR) enhances isomerohydrolase activity independent of light. J Biol Chem. 2005;280(33):29874–29884. doi: 10.1074/jbc.M503603200. [DOI] [PubMed] [Google Scholar]
- 26.Radu RA, Hu J, Peng J, Bok D, Mata NL, Travis GH. Retinal Pigment Epithelium-Retinal G Protein Receptor-Opsin Mediates Light-dependent Translocation of All-trans-retinyl Esters for Synthesis of Visual Chromophore in Retinal Pigment Epithelial Cells. J Biol Chem. 2008;283(28):19730–8. doi: 10.1074/jbc.M801288200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fleisch VC, Schonthaler HB, von Lintig J, Neuhauss SC. Subfunctionalization of a retinoid-binding protein provides evidence for two parallel visual cycles in the cone-dominant zebrafish retina. J Neurosci. 2008;28(33):8208–8216. doi: 10.1523/JNEUROSCI.2367-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Collery R, McLoughlin S, Vendrell V, Finnegan J, Crabb JW, Saari JC, Kennedy BN. Duplication and divergence of zebrafish CRALBP genes uncovers novel role for RPE- and Muller-CRALBP in cone vision. Invest Ophthalmol Vis Sci. 2008;49(9):3812–3820. doi: 10.1167/iovs.08-1957. [DOI] [PubMed] [Google Scholar]
- 29.Schonthaler HB, Lampert JM, Isken A, Rinner O, Mader A, Gesemann M, Oberhauser V, Golczak M, Biehlmaier O, Palczewski K, Neuhauss SC, von Lintig J. Evidence for RPE65-independent vision in the cone-dominated zebrafish retina. Eur J Neurosci. 2007;26(7):1940–1949. doi: 10.1111/j.1460-9568.2007.05801.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang JS, Estevez ME, Cornwall MC, Kefalov VJ. Intra-retinal visual cycle required for rapid and complete cone dark adaptation. 2009;12(3):295–302. doi: 10.1038/nn.2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Das SR, Bhardwaj N, Kjeldbye H, Gouras P. Muller cells of chicken retina synthesize 11-cis-retinol. Biochem J. 1992;285(Pt 3):907–913. doi: 10.1042/bj2850907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bustamante JJ, Ziari S, Ramirez RD, Tsin AT. Retinyl ester hydrolase and the visual cycle in the chicken eye. Am J Physiol. 1995;269(6 Pt 2):R1346–1350. doi: 10.1152/ajpregu.1995.269.6.R1346. [DOI] [PubMed] [Google Scholar]
- 33.Suzuki T, Maeda Y, Toh Y, Eguchi E. Retinyl and 3-dehydroretinyl esters in the crayfish retina. Vision Res. 1988;28(10):1061–1070. doi: 10.1016/0042-6989(88)90132-0. [DOI] [PubMed] [Google Scholar]
- 34.Wilson JA. Principles of Animal Physiology. New York: Mcmillan Publishing; 1972. [Google Scholar]
- 35.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 36.Winston A, Rando RR. Quantitative measurements of isomerohydrolase activity. Methods Enzymol. 2000;316:324–330. doi: 10.1016/s0076-6879(00)16732-1. [DOI] [PubMed] [Google Scholar]
- 37.Ross AC. Retinol esterification by rat liver microsomes Evidence for a fatty acyl coenzyme A: retinol acyltransferase. J Biol Chem. 1982;257(5):2453–9. [PubMed] [Google Scholar]
- 38.Kaschula CH, Jin MH, Desmond-Smith NS, Travis GH. Acyl CoA:retinol acyltransferase (ARAT) activity is present in bovine retinal pigment epithelium. Exp Eye Res. 2005;82:111–121. doi: 10.1016/j.exer.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 39.Ross AC. Retinol esterification by rat liver microsomes Evidence for a fatty acyl coenzyme A: retinol acyltransferase. J Biol Chem. 1982;257(5):2453–2459. [PubMed] [Google Scholar]
- 40.Fortuna VA, Trugo LC, Borojevic R. Acyl-CoA: retinol acyltransferase (ARAT) and lecithin:retinol acyltransferase (LRAT) activation during the lipocyte phenotype induction in hepatic stellate cells. The Journal of Nutritional Biochemistry. 2001;12(11):610–621. doi: 10.1016/s0955-2863(01)00179-6. [DOI] [PubMed] [Google Scholar]
- 41.MacDonald P, Ong DE. Assay of lecithin-retinol acyltransferase. Methods Enzymol. 1988;189(1990):450–459. doi: 10.1016/0076-6879(90)89322-9. [DOI] [PubMed] [Google Scholar]
- 42.Rando RR. The Chemistry of Vitamin A and Vision. Angew Chem Int Ed Engl. 1990;29:461–480. [Google Scholar]
- 43.Gollapalli DR, Rando RR. Molecular logic of 11-cis-retinoid biosynthesis in a cone-dominated species. Biochemistry. 2003;42(50):14921–14929. doi: 10.1021/bi0356505. [DOI] [PubMed] [Google Scholar]
- 44.Kaylor J, Radu R, Miu A, Travis GH. Retinoid processing in Muller cells. Fort Luderdale, Florida: IOVS; 2008. [Google Scholar]
- 45.Moiseyev G, Takahashi Y, Chen Y, Gentleman S, Redmond TM, Crouch RK, Ma JX. RPE65 is an iron(II)-dependent isomerohydrolase in the retinoid visual cycle. J Biol Chem. 2006;281(5):2835–2840. doi: 10.1074/jbc.M508903200. [DOI] [PubMed] [Google Scholar]
- 46.Jin M, Li S, Nusinowitz S, Lloyd M, Hu J, Radu RA, Bok D, Travis GH. The Role of Interphotoreceptor Retinoid-Binding Protein on the Translocation of Visual Retinoids and Function of Cone Photoreceptors. J Neurosci. 2009;29(5):1486–1495. doi: 10.1523/JNEUROSCI.3882-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Morris VB, Shorey CD. An electron microscope study of types of receptor in the chick retina. J Comp Neurol. 1967;129(4):313–340. doi: 10.1002/cne.901290404. [DOI] [PubMed] [Google Scholar]


