Abstract
Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) is a promising cancer therapeutic because of its highly selective apoptosis-inducing action on neoplastic versus normal cells. However, some cancer cells express resistance to recombinant soluble TRAIL. To overcome this problem, we used a TRAIL adenovirus (Ad5/35-TRAIL) to induce apoptosis in a drug-sensitive and multidrug-resistant variant of HL-60 leukemia cells and determined the molecular mechanisms of Ad5/35-TRAIL-induced apoptosis. Ad5/35-TRAIL did not induce apoptosis in normal human lymphocytes, but caused massive apoptosis in acute myelocytic leukemia cells. It triggered more efficient apoptosis in drug-resistant HL-60/Vinc cells than in HL-60 cells. Treating the cells with anti-DR4 and anti-DR5 neutralizing antibodies (particularly anti-DR5) reduced, whereas anti-DcR1 antibody enhanced, the apoptosis triggered by Ad5/35-TRAIL. Whereas Ad5/35-TRAIL induced apoptosis in both cell lines through activation of caspase-3 and caspase-10, known to link the cell death receptor pathway to the mitochondrial pathway, it triggered increased mitochondrial membrane potential change (Δψm) only in HL-60/Vinc cells. Ad5/35-TRAIL also increased the production of reactive oxygen species, which play an important role in apoptosis. Therefore, using Ad5/35-TRAIL may be an effective therapeutic strategy for eliminating TRAIL-resistant malignant cells and these studies may provide clues to treat and eradicate acute myelocytic leukemias.
Introduction
Apo2L/TRAIL, a member of the tumor necrosis factor (TNF) family, has a profound apoptotic effect in neoplastic cells, but not in normal ones (Wiley et al., 1995; Pitti et al., 1996; Ashkenazi et al., 1999; K. Kim et al., 2001), and holds enormous promise for cancer therapy. TRAIL (TNF-related apoptosis-inducing ligand) induces apoptosis via two signaling pathways: (1) the extrinsic pathway, also called the receptor-mediated pathway, and (2) the intrinsic or mitochondrial pathway. The extrinsic pathway is activated by the binding of TRAIL to its cell surface receptors, TRAIL-R1/DR4 and TRAIL-R2/DR5. The downstream messengers and effectors such as FLICE-associated death domain (FADD), caspase-8, or caspase-10 form the death-inducing signal complex (DISC), initiate the TRAIL-induced apoptotic signals, and activate the subsequent downstream caspases such as caspase-3, −6, and −7 (Salvesen and Dixit, 1997, 1999). Reports have confirmed that activated and processed caspase-8 or caspase-10 can cleave the BH3 domain containing the proapoptotic molecule BID, which then translocates to the mitochondrial membrane and triggers the mitochondrially mediated events including the cytosolic release of cytochrome c and caspase-9 activation (Li et al., 1998; Luo et al., 1998; Gross et al., 1999), thereby linking the extrinsic pathway to the mitochondrial pathway of apoptosis. The intrinsic pathway of TRAIL-induced apoptosis includes the loss of mitochondrial membrane potential (Δψm), the release of mitochondrial cytochrome c, the production of reactive oxygen species (ROS), and activation of caspase-9 (Suliman et al., 2001; K. Kim et al., 2004; S.H. Kim et al., 2004). In the cytosol, cytochrome c and dATP bind to Apaf-1 and cause its oligomerization (Li et al., 1997; Zou et al., 1999). Apaf-1 in turn binds to and processes caspase-9 into an active caspase that recruits, cleaves, and activates the effector caspase-3 (Li et al., 1997; Srinivasula et al., 1999; Zou et al., 1999). Activated caspase-3 can proteolytically cleave a number of cellular proteins, for example, poly(ADP-ribose) polymerase (PARP), gelsolin, protein kinase C δ (PKCδ), lamins, fodrin, DFF45 (DNA fragmentation factor, ICAD [inhibitor of caspase-3-activated DNase]), Rb (retinoblastoma protein), DNA-dependent protein kinase (DNA-PK), and so on, resulting in apoptosis (Salvesen and Dixit, 1997; Green, 1998; Salvesen and Dixit, 1999).
Recombinant soluble TRAIL kills many cancer cell lines from various types of cancers (Zhang et al., 1999, 2004; Ozoren et al., 2000; Jia et al., 2001; Nagane et al., 2001; Ehtesham et al., 2002; Griffith et al., 2002; Poulaki et al., 2002). However, some cancer cells such as osteosarcoma, cholangiocarcinoma, and most breast cancers and leukemias are resistant to recombinant TRAIL protein (Bouralexis et al., 2003; Hasegawa et al., 2005). Any deletion, mutation, or inhibition of the proteins involved in this pathway will affect the sensitivity or resistance of the cells to TRAIL (Eggert et al., 2001; Zuzak et al., 2002; S.H. Kim et al., 2004). Furthermore, inhibition of the nuclear factor (NF)-κB pathway enhances TRAIL-mediated apoptosis in neuroblastoma and breast cancer cells (Keane et al., 2000; Karacay et al., 2004), indicating that this transcription factor plays an important role in TRAIL-induced apoptosis. For leukemias, it is believed that expression of the BCR-ABL gene (Bedi et al., 1994, 1995; McGahon et al., 1994; Nimmanapalli et al., 2001), upregulation of cellular FLICE-like inhibitory protein (c-FLIP), downregulation of TRAIL-R1 and TRAIL-R2 receptors, inactivation of caspase-8 (Shiiki et al., 2000; MacFarlane et al., 2002), NF-κB activation (Bortul et al., 2003; Ehrhardt et al., 2003), and constitutive Akt activation (Bortul et al., 2003; Martelli et al., 2003) can cause resistance to TRAIL. Furthermore, it is not clear whether the resistance of human normal cells to TRAIL is due to the lack of DR4 and DR5 expression on the cell surface (Hao et al., 2004). To conquer the problem of TRAIL resistance in cancer cells, a replication-deficient adenovirus encoding the human TRAIL gene, TNFSF10 (Ad5/35-TRAIL), was engineered as an alternative to recombinant soluble TRAIL protein (Ni et al., 2005). Adenoviral tropism is mediated largely by the fiber protein. This fiber protein encoded by the L5 gene is a homotrimeric molecule characterized by a C-terminal knob or shaft domain that mediates binding to cellular receptors during the infection process, and a tail region that attaches the fiber to the penton base (van Oostrum and Burnett, 1985; Ruigrok et al., 1990; Louis et al., 1994). Ad5, a group C respiratory adenovirus, the target fiber on which its infectivity depends, has been identified as the cox-sackie–adenovirus receptor (CAR) (Bergelson et al., 1997). Unfortunately, hematopoietic and leukemia cells contain few CARs but more CD46, a major receptor for group B human adenovirus such as adenovirus Ad35 (Gaggar et al., 2003, 2005; Segerman et al., 2003). To overcome this problem, adenoviral vector containing chimeric type 5 and type 35 fiber proteins was constructed. The chimeric fibers contain the Ad5 penton base and fiber tail plus the Ad35 fiber shaft and knobs. This adenovirus exhibits altered and expanded tropism and increases the limit on the size of foreign genes that may be carried (Seshidhar Reddy et al., 2003; Balamotis et al., 2004; Ni et al., 2005). Furthermore, hematopoietic and leukemia cells were infected about 50 times more efficiently with the chimeric Ad5/35 fiber compared with Ad5 fiber (Bernt et al., 2003; Seshidhar Reddy et al., 2003; Balamotis et al., 2004; Yotnda et al., 2004). Taken together, this Ad5/35-TRAIL adenovirus has great potential for treating leukemias. In this paper, we show that (1) Ad5/35-TRAIL induces apoptosis in the HL-60 cell line and its P-glycoprotein (P-gp)-over-expressing drug-resistant variant HL-60/Vinc, and (2) Ad5/35-TRAIL triggers more apoptosis in HL-60/Vinc cells compared with HL-60 cells; furthermore, (3) we determined the mechanisms of Ad5/35-TRAIL-triggered apoptosis and why Ad5/35-TRAIL kills the resistant variant to a greater extent than its sensitive counterpart.
Materials and Methods
Adenoviral construction and propagation
The construction system used for AdCMV-TRAIL and Ad-CMV-EGFP was developed by X. Danthinne (O.D. 260, Boise, ID) with some subsequent improvements. Briefly, the present system contains three parts: (1) a cloning vector, pAd1020SfidA, containing the adenoviral left inverted terminal repeat (ITR) and a packaging signal (bp 1–358); (2) a modified adenovirus 5 genome backbone vector, called E1bE4, which contains the Ad5 genome from the E1b TATA box to the E4 TATA box; and (3) another cloning vector, called p304Sfi, containing the right-sided ITR (from bp 35819 to 35935) of the adenoviral genome, which allows us to clone the desired genes into the right end of the adenoviral genome. The expression cassette, including the cytomegalovirus (CMV) promoter, human TRAIL (from pORFh-TRAIL; Invivogen, San Diego, CA) or enhanced green fluorescent protein (EGFP) cDNA, and the simian virus 40 (SV40) poly(A) signal, was cloned into pAd1020SfidA and then cut from the cloning vector with a kanamycin-resistant gene and a λ phage packaging signal (COS), using restriction enzyme SfiI. On the other side, a 250-bp telomerase reverse transcriptase (TERT) promoter (Shayakhmetov et al., 2000; Huang et al., 2004) and E1a gene were cloned into p304Sfi to form p304SfipTERT-E1a. To create our fiber-modified Ad5/35 hybrid vector, we amplified by polymerase chain reaction (PCR) an NdeI/AflII fragment (1261 bp) containing Ad5/35 fiber from Ad5EGFP/F35 (a gift from A. Lieber, University of Washington, Seattle, WA) (Huang and Hearing, 1989; Shayakhmetov et al., 2000), and then cloned the fragment into the E1bE4 vector to make E1bf5/35E4. These adenoviral vectors were digested with the restriction enzyme SfiI and ligated. The ligation mixture was then used to transform bacteria. After transformation, the bacteria were double-selected with ampicillin and kanamycin. Cosmids were isolated from clones containing the right construct. Recombinant adenoviral genomes were released by PacI digestion and transfected into 911E4 cells in the absence of doxycycline. AdCMV-TRAIL and AdCMV-EGFP are replication defective in all cell lines tested, indicating a failure of pTERT to control viral replication in these constructs.
As described above, the titers and TRAIL expression of Ad5/35-GFP and Ad5/35-TRAIL have been confirmed with an Adeno-X rapid titer kit (Clontech, Palo Alto, CA) and by Western blotting (Li et al., 2005). To replicate and purify the adenovirus, HEK-293 cells were infected with either Ad5/35-GFP or Ad5/35-TRAIL for 4 hr, transferred to RPMI 1640 medium with 10% fetal calf serum (FCS) and penicillin–streptomycin (100 ng/ml each) (Invitrogen, Carlsbad, CA), and cultured at 37°C under 5% CO2 for 4 to 7 days to allow for sufficient cytopathic effect. The cell lysate and supernatant were then used for the next infection from one 60-mm petri dish to one 75-cm2 flask, to triple flasks, and finally to Nunclon Δ Cell Factories (Nalge Nunc International, Rochester, NY). The adenovirus was purified by CsCl gradient centrifugation. All gradient-purified viral stocks were then dialyzed against dialysis buffer (1000 ml of dialysis buffer contained 789 ml of double-distilled water, 1 M MgCl2, 10 ml of 1 M Tris-HCl [pH 7.5], and 200 ml of 50% glycerol) for 24 hr at 4°C, with three buffer changes. Aliquots of purified and dialyzed viruses were stored at −70°C for future use. Viral titers were determined with the Adeno-X rapid titer system (Invitrogen) and TRAIL protein expression was detected by Western blotting.
Cell lines, culture conditions, and cell survival assay
Human lymphocyte buffy coats were purchased from the Indiana Blood Center (Indianapolis, IN). Lymphocytes were isolated by low-density cell enrichment based on Ficoll-Hypaque separation. Primary acute myelocytic leukemia cells from patients were kindly provided by Dr. S. Boswell (Department of Internal Medicine, Indiana University School of Medicine, Indianapolis, IN). The human acute myeloid leukemia cell line HL-60 was purchased from the American Type Culture Collection (ATCC, Manassas, VA). The MDR1-mediated variant HL-60/Vinc was developed in our laboratory (Ogretmen and Safa, 2000). Cells were maintained in the above-mentioned medium at 37°C in 5% CO2. The 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay was performed as follows: briefly, 5 × 104 cells were plated in 100 μl of the growth medium in the presence or absence of increasing concentrations of chemotherapeutic drugs in 96-well plates and cultured at 37°C in 5% CO2 for 72 hr. The cells were then incubated for 4 hr with 25 μl of MTT (5 mg/ml) at 37°C. After dissolving the crystals in 0.04 N HCl in isopropanol, plates were read in a microplate reader (Dynex Technologies, Chantilly, VA) at 570 nm. The concentration of drug that inhibited cell survival by 50% (IC50) was determined from cell survival plots.
RNA isolation, RT-PCR, and real-time PCR
Total RNA from treated and untreated HL-60 and HL-60/Vinc cells was isolated with TRI reagent (TR-118; Molecular Research Center, Cincinnati, OH) as described by the manufacturer. One microgram of total RNA was used in reverse transcription reactions with Moloney murine leukemia virus (MMLV) reverse transcriptase and oligo(dT)15 primer (Promega, Madison, WI) as described by the manufacturer. Two microliters of the resulting total cDNA was then used as the template in PCR to measure the mRNA level of interest, using designed primers: for c-FLIPlong (c-FLIPL), forward, 5′-GCTGAAGTTATCCATCAGGT-3′; reverse, 5′-CATACTGAGATGCAAGAATT-3′. These will give an 840-bp band. For c-FLIPshort (c-FLIPS), the forward primer is the same as for c-FLIPL; the reverse primer is 5′-GATCAGGACAATGGGCATAG-3′. These will give a 662-bp band. For β-actin: forward, 5′-CAGAGCAAGAGAGGCATCCT-3′; reverse, 5′-TTGAAGGTCTCAAACATGAT-3′. These will give a 200-bp band. The reactions were performed at 94°C for denaturation, 58°C for annealing, and 72°C for extension for 30 cycles. β-Actin mRNA levels were used as internal controls. Amplified fragments were separated on 1.5% agarose gels and visualized by ethidium bromide staining. For real-time PCR, total RNA was isolated as described above and the expression of c-FLIPL and c-FLIPS was quantified with an iCycler (Bio-Rad, Hercules, CA). SYBR green methods were employed according to the manufacturer's protocol. The c-FLIP primers used for real-time PCR were the same as those used for the semiquantitative RT-PCR analysis. The expression value was normalized to glyceraldehyde-3-phosphate dehydrogenase (GAPDH). Relative gene expression was determined by assigning the control a relative value of 1.0, with all other values expressed relative to the control.
Annexin V binding assay to detect apoptotic cells
After treatment as stated previously, cells (5 × 105 cells per treatment) were used to determine the translocation of phosphatidylserine to the outer surface of the plasma membrane during apoptosis, using the human phospholipid-binding protein annexin V–Cy5 (cyanine dye 5), conjugated with fluorescein isothiocyanate (FITC) (Invitrogen Molecular Probes, Eugene, OR), by flow cytometry as described by the manufacturer. Apoptosis and necrosis were analyzed by quadrant statistics on propidium iodide (PI)-negative, Cy5-positive cells and both positive cells, respectively. Annexin V–Cy5 was used instead of FITC-conjugated annexin because of the overlapping fluorescence of Ad5/35-GFP.
Flow cytometric analysis of DR4, DR5, and DcR1
Cells (5 × 106) were spun down at 500 × g, washed with phosphate-buffered saline (PBS), and resuspended in 500 μl of PBS. The cells were then incubated for 1 hr with 10 μl of IgG2a or with anti-DR4, anti-DR5, or anti-DcR1 monoclonal antibody (diluted 1:100; R&D Systems, Minneapolis, MN). After washing with PBS, FITC-conjugated rabbit anti-goat polyclonal antibody (diluted 1:200; Sigma-Aldrich, St. Louis, MO) was added to the cell suspension and incubated for 1 hr on ice followed by washing with PBS. After rinsing, the samples were analyzed by flow cytometry with a FACSCalibur flow cytometer (BD Biosciences, San Jose, CA). Data were analyzed with the provided CellQuest program.
Analysis of reactive oxygen species and apoptosis
Levels of intracellular ROS were measured with dichlorodihydrofluorescein diacetate (DCFH-DA; Invitrogen Molecular Probes). After treatment, cells were incubated for 30 min at 37°C with 5 μM DCFH-DA and analyzed with a FACSCalibur flow cytometer as described by the manufacturer. To determine whether increased ROS were generated by TRAIL, Ad5/35-GFP, or Ad5/35-TRAIL, cells were treated with or without a 10 mM concentration of the antioxidant N-acetylcysteine (NAc) 3 hr before ROS analysis.
Cytofluorimetric analysis of mitochondrial transmembrane potential
Cells were studied with 5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazolyl carbocyanine (JC-1; Invitrogen Molecular Probes) as a probe after treatment. JC-1 membrane potential-related fluorescence was recorded by a FACSCalibur flow cytometer with an FL1 photomultiplier tube (PMT).
Western blot analysis
Western blot analysis was performed with several antibodies. In short, 50 μg of protein per lane were separated by 5–15% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS–PAGE) and blotted onto a polyvinylidene difluoride (PVDF) Immobilon membrane (Millipore, Bedford, MA), and then protein levels were detected with dilutions of antibodies and peroxidase-conjugated secondary antibodies as described by the manufacturer. The membranes were then exposed to Kodak X-OMAT film (Eastman Kodak, Rochester, NY) for various times. The human MDR1 P-gp-specific polyclonal antibody MDR-7 was produced in rabbits, using a peptide sequence obtained from the deduced amino acid sequence of the MDR1 gene. The MDR-7 antibody was used at a concentration of 1:2000 (v/v). The mouse anti-caspase-10 polyclonal antibody (diluted 1:1000, v/v) was purchased from Medical and Biological Laboratories (Watertown, MA). The mouse anti-caspase-8 polyclonal antibody (diluted 1:1000, v/v) was purchased from Cell Signaling Technology (Beverly, MA). The anti-caspase-3 polyclonal antibody (diluted 1:1000, v/v) was purchased from BD Biosciences Pharmingen (San Diego, CA). The anti-caspase-9 mouse monoclonal antibody (diluted 1:1000, v/v) was purchased from BD Biosciences Pharmingen. The neutralizing polyclonal goat antibodies raised against extracellular domains of TRAIL-R1 (DR4), TRAIL-R2 (DR5), and TRAIL-R3 (DcR1) (20 μg/ml) were purchased from R&D Systems. The monoclonal anti-c-FLIP antibody (diluted 1:200, v/v) was purchased from Santa Cruz Biotechnology (Santa Cruz, CA). The anti-TRAIL mouse monoclonal antibody (diluted 1:1000, v/v) was purchased from Santa Cruz Biotechnology. The mouse monoclonal anti-β-actin antibody (diluted 1:5000, v/v) was purchased from Sigma-Aldrich.
Statistical analysis
Two-way analysis of variance (ANOVA) and the Student t test were used to determine statistical significance.
Results
Drug resistance characteristics and analysis of P-gp expression in HL-60 and HL-60/Vinc cells
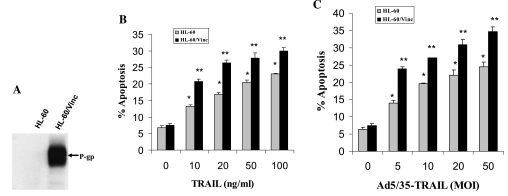
In this study, we determined the apoptotic effect of Ad5/35-TRAIL on HL-60 human myelogenic leukemia and its P-gp-overexpressing drug-resistant variant HL-60/Vinc, and elucidated the molecular mechanism of TRAIL-induced apoptosis in these leukemia cells. HL-60/Vinc cells display 8-, 620-, and 1300-fold resistance to doxorubicin (DOX), vinblastine (VBL), and vincristine (VCR), respectively, compared with HL-60 cells (Ogretmen and Safa, 2000). As seen in Fig. 1A, Western blot analysis with the monoclonal antibody for P-gp showed that HL-60/Vinc cells robustly over-expressed P-gp, whereas no P-gp was detected in the parental HL-60 cells. As shown in Fig. 1B and C, both TRAIL and Ad5/35-TRAIL treatments induced dose-dependent apoptosis. TRAIL-triggered apoptosis reached a plateau after treating the cells with TRAIL at 20–50 ng/ml (Fig. 1B). Therefore, in subsequent experiments, we used TRAIL at 20 or 50 ng/ml. Moreover, as seen in Fig. 1C, maximal apoptosis was observed when the cells were treated with 50 multiplicities of infection (MOI) of Ad5/35-TRAIL for 24 hr. Hence, subsequent experiments were performed with 50 MOI of Ad5/35-TRAIL for 24 hr.
FIG. 1.
Dose-dependent TRAIL- and Ad5/35-TRAIL-induced apoptosis in the drug-sensitive HL-60 cell line and its P-gp-overexpressing MDR variant, HL-60/Vinc. (A) Western blot analysis of endogenous P-gp levels in HL-60 and HL-60/Vinc cells. Western blotting was performed as described in Materials and Methods. (B) Effect of TRAIL on apoptotic cell death in HL-60 and HL-60/Vinc cells. Cells (5 × 105) were treated for 24 hr with or without TRAIL at 10, 20, 50, and 100 ng/ml, and apoptosis was measured by annexin V-binding assay. Significant differences between 10- and 100-ng/ml TRAIL treatments were obtained (*p < 0.003, **p < 0.001). (C) Effect of Ad5/35-TRAIL on apoptotic cell death in HL-60 and HL-60/Vinc cells. Cells (5 × 105) were treated for 24 hr with or without 5, 10, 20, and 50 MOI of Ad5/35-TRAIL, and apoptosis was measured by annexin V-binding assay. Significant differences between 5 and 50 MOI of Ad5/35-TRAIL treatments were obtained (*p < 0.002, **p < 0.001).
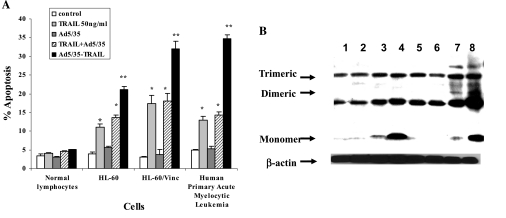
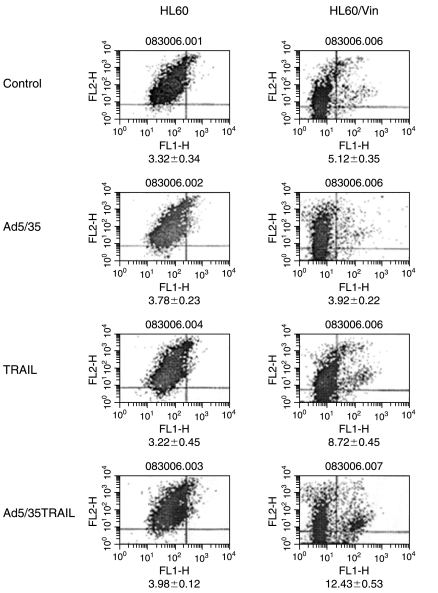
The data presented in Fig. 2A revealed that treating HL-60 and HL-60/Vinc cells with TRAIL at 20 ng/ml for 24 hr induced 13.68 and 18.02% apoptosis, respectively, whereas Ad5/35-TRAIL infection for 24 hr triggered 22.06 and 33.58% apoptosis, respectively. Similarly, soluble and Ad5/35-TRAIL caused 12.86 and 34.73% apoptosis, respectively, after 24 hr in primary acute myelocytic leukemia cells. Moreover, after 48 hr of infection with Ad5/35-TRAIL, more than 80% of these primary leukemia cells died (data not shown), whereas no significant apoptosis was observed in normal human lymphocytes treated with TRAIL, Ad5/35-TRAIL, or Ad5/35 adenoviral vector. These data indicate that Ad5/35-TRAIL kills leukemia cells more efficiently than does recombinant soluble TRAIL, and that TRAIL and Ad5/35-TRAIL induced more apoptosis in resistant HL-60/Vinc cells than in their sensitive counterpart HL-60 cells. Combined use of TRAIL and the control adenoviral vector had only a little greater apoptotic effect compared with TRAIL itself, whereas Ad5/35-TRAIL induces robust apoptosis. We have previously shown that soluble TRAIL induced significantly more apoptosis in several multidrug-resistant, P-gp-overexpressing solid tumor cell lines compared with their drug-sensitive counterparts (Park et al., 2006). We also found that, like soluble TRAIL, Ad5/35-TRAIL induced more apoptosis in the BC19 cell line (an MCF-7 breast cancer cell line transfected with the P-gp gene MDR1) and drug-resistant human colon carcinoma cell line (SW620/Ad300) compared with their drug-sensitive parental cell lines (data not shown).
FIG. 2.
TRAIL- and Ad5/35-TRAIL-induced apoptosis in the drug-sensitive HL-60 cell line and its P-gp-expressing MDR variant, HL-60/Vinc. (A) Effect of TRAIL and Ad5/35-TRAIL on apoptotic cell death in HL-60 and HL-60/Vinc cells. Cells (5 × 105) were treated with or without 50 MOI of Ad5/35, TRAIL at 20 ng/ml, and 50 MOI of Ad5/35-TRAIL for 24 hr, and apoptosis was measured by annexin V-binding assay. Significant differences between various treatments were obtained (*p < 0.004, **p < 0.0008). (B) Analysis of trimeric, dimeric, and monomeric TRAIL expression in cell lysate. HL-60 and HL-60/Vinc cells (5 × 106) were incubated with or without 50 MOI of Ad5/35, TRAIL at 20 ng/ml, or 50 MOI of Ad5/35-TRAIL at 37°C for 24 hr followed by protein extraction. Aliquots (50 μg of protein per lane) were separated by 5–15% SDS–PAGE and subjected to Western blot analysis as described in Materials and Methods. Equivalent loading was confirmed by reprobing the blot with anti-β-actin. Lanes 1–4 in each panel are HL-60/control, Ad5/35 vector, TRAIL, and Ad5/35-TRAIL, respectively. Lanes 5–8 in each panel are HL-60/Vinc control, Ad5/35 vector, TRAIL, and Ad5/35-TRAIL, respectively.
As shown in Fig. 2B, trimeric and dimeric TRAIL were detected only in HL-60/Vinc cells treated with TRAIL and Ad5/35-TRAIL. Both Ad5/35 HL-60 and HL-60/Vinc cells expressed much more monomeric TRAIL than did control and soluble TRAIL-treated cells.
DR4, DR5, and DcR1 expression and their antibody effects
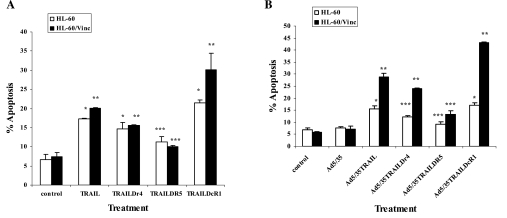
We next investigated the reasons why Ad5/35-TRAIL triggers more efficient apoptosis in HL-60 and HL-60/Vinc cell lines than does recombinant soluble TRAIL. First, we explored whether Ad5/35-TRAIL increases the expression of TRAIL receptors DR4 and DR5, and decreases DcR1. Flow cytometric analysis revealed that there is no significant change in the expression levels of DR4, DR5, and DcR1 after treating cells with Ad5/35-TRAIL or recombinant TRAIL (data not shown). Next, we pretreated cells with neutralizing anti-DR4, -DR5, or -DcR1 antibodies and measured Ad5/35-TRAIL-induced apoptosis. Data shown in Fig. 3 revealed that anti-DR4 and -DR5 antibodies reduced, whereas anti-DcR1 (decoy) antibody enhanced, TRAIL (Fig. 3A) or Ad5/35-TRAIL (Fig. 3B)-triggered apoptosis in both HL-60 and HL-60/Vinc cells. In addition, data in Fig. 2A showed that DR5 antibody inhibits apoptosis to a greater extent than DR4, revealing that DR5 plays the major role in Ad5/35-TRAIL-induced apoptosis.
FIG. 3.
Effect of anti-TRAIL receptor antibodies on (A) TRAIL (*p < 0.01, **p < 0.004, ***p < 0.09) and (B) Ad5/35-TRAIL (*p < 0.007, **p < 0.0001, ***p < 0.06)-induced apoptosis. HL-60 and HL-60/Vinc cells were incubated with anti-DR4, -DR5, or -DcR1 neutralizing antibodies for 3 hr at 37°C, and then the cells were treated with or without 50 MOI of Ad5/35 or 50 MOI of Ad5/35-TRAIL at 37°C for 24 hr; apoptosis was measured by annexin V binding assay as described in Materials and Methods.
c-FLIP expression
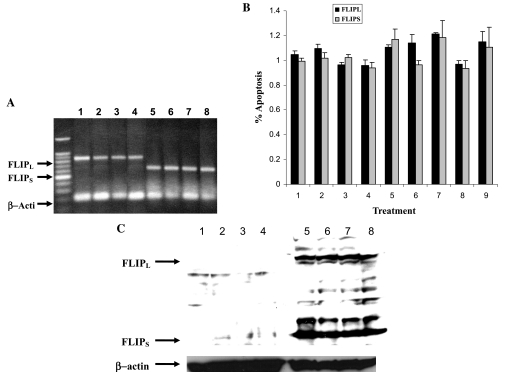
Because c-FLIP has been shown to inhibit TRAIL-induced apoptosis (Abedini et al., 2004), we investigated whether Ad5/35-TRAIL causes downregulation of c-FLIPS and c-FLIPL in HL-60 and HL-60/Vinc cells. The results in Fig. 4A and B showed that there is no difference in the mRNA levels for c-FLIPS and c-FLIPL, determined by RT-PCR and real-time PCR analysis in these cell lines. Surprisingly, Western blot analysis showed that HL-60/Vinc cells expressed significantly more c-FLIPL and c-FLIPS than did HL-60 cells (Fig. 4C).
FIG. 4.
RT-PCR, real-time PCR, and Western blot analysis of c-FLIP expression in HL-60 and HL-60/Vinc cells. (A) Expression of c-FLIPL and c-FLIPS before and after treatment. Lanes 1–4 show c-FLIPL mRNA levels in HL-60/control, HL-60/Ad5/35-TRAIL, HL-60/Vinc/control, and HL-60/Vinc/Ad5/35-TRAIL, respectively. Lanes 5–8 show c-FLIPS mRNA levels in HL-60/control, HL-60/Ad5/35-TRAIL, HL-60/Vinc/control, and HL-60/Vinc/Ad5/35-TRAIL, respectively. (B) Expression levels of c-FLIPL and c-FLIPS mRNA with or without treatments were quantified by real-time PCR. Values represent means ± SD of three independent experiments. (C) Western blot shows little c-FLIPL and c-FLIPS before and after treatment in HL-60 and HL-60/Vinc cells. Lanes 1–4 are HL-60/control, (HL-60)/Ad5/35 vector, (HL-60)/TRAIL, and (HL-60)/Ad5/35-TRAIL, respectively. Lanes 5–8 are HL-60/Vinc/control, (HL-60/Vinc)/Ad5/35 vector, (HL-60/Vinc)/TRAIL, and (HL-60/Vinc)/Ad5/35-TRAIL, respectively.
Caspase activation in Ad5/35-TRAIL-induced apoptosis
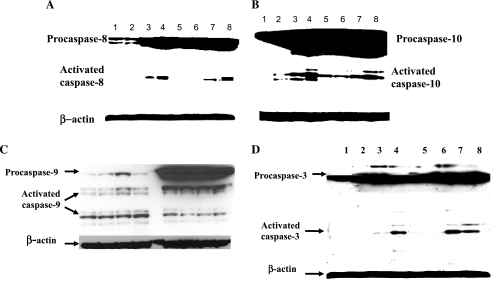
To discover the mechanisms of TRAIL- and Ad5/35-TRAIL-induced apoptosis, we performed Western blotting of the initiator caspases (caspase-8, −9, and −10) and the executioner caspases (caspase-3, −6, and −7) in control cells and cells treated with Ad5/35-TRAIL using antibodies capable of recognizing both the proforms and activated forms of these caspases. As shown in Fig. 5A, B, and D, treatment of HL-60 and HL-60/Vinc cells with TRAIL at 20 ng/ml or with 50 MOI of Ad5/35-TRAIL per cell for 24 hr caused processing and activation of procaspase-10, −8, and −3 to their active forms, which were seen to a greater degree in cells incubated with Ad5/35-TRAIL. Ad5/35-TRAIL only slightly increased caspase-9 activation (Fig. 5C). Similarly, Ad5/35-TRAIL triggered activation of caspase-6 and caspase-7 (data not shown).
FIG. 5.
TRAIL and Ad5/35-TRAIL trigger caspase activation in HL-60 and HL-60/Vinc cells: (A) caspase-8, (B) caspase-10, (C) caspase-9, and (D) caspase-3. Aliquots (50 μg of protein per lane) were separated by 10% SDS–PAGE and subjected to Western blot analysis as described in Materials and Methods. Lanes 1–4 in each panel are HL-60/control, HL-60/Ad5/35 vector, HL-60/TRAIL, and HL-60/Ad5/35-TRAIL, respectively. Lanes 5–8 in each panel are HL-60/Vinc/control, HL-60/Vinc/Ad5/35 vector, HL-60/Vinc/TRAIL, and HL-60/Vinc/Ad5/35-TRAIL, respectively.
Effects of caspase inhibitors on Ad5/35-TRAIL-induced apoptosis
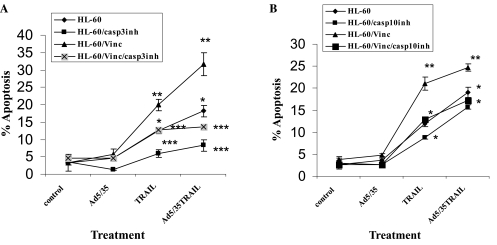
To determine whether caspase-3, −8, −9, and −10 are involved in Ad5/35-TRAIL-induced apoptosis, inhibitors of these caspases were used to pretreat cells before incubation with or without Ad5/35, TRAIL, or Ad5/35-TRAIL. Caspase-8 inhibitor Ac-DEVD-CHO and caspase-9 inhibitor Z-LEHD-FMK did not significantly inhibit TRAIL- and Ad5/35-TRAIL-induced apoptosis, whereas caspase-3 inhibitor Z-DEVD-FMK and caspase-10 inhibitor Ac-DEVD-CHO did inhibit apoptosis (Fig. 6A and B). These results showed that TRAIL-induced apoptosis in these leukemia cells is caspase-3 and −10 dependent.
FIG. 6.
Effect of (A) caspase-3 and (B) caspase-10 inhibitors on TRAIL- and Ad5/35-TRAIL-induced apoptosis. HL-60 and HL-60/Vinc cells were incubated in growth medium in the presence or absence of 50 μM caspase-3 inhibitor Ac-DEVD-CHO for 3 hr at 37°C, and then the cells were treated with or without 50 MOI of Ad5/35, TRAIL at 20 ng/ml, or 50 MOI of Ad5/35-TRAIL at 37°C for 24 hr, and apoptosis was measured by annexin V binding assay as described in Materials and Methods. (A) *p < 0.005, **p < 0.02, ***p < 0.03; (B) *p < 0.008, **p < 0.001.
Ad5/35-TRAIL triggers increase mitochondrial membrane potential preferentially in resistant HL-60/Vinc cells
To further understand why TRAIL and Ad5/35-TRAIL trigger more apoptosis in HL-60/Vinc cells than in HL-60 cells, we determined whether altered mitochondrial membrane potential (Δψm) or the release of cytochrome c from mitochondria is crucial for Ad5/35-TRAIL-induced apoptosis in these cell lines. After treating these cells with Ad5/35-TRAIL, there was no significant difference in cytochrome c release from the mitochondria of HL-60 and HL-60/Vinc cells (data not shown), but Ad5/35-TRAIL increased Δψm only in HL-60/Vinc cells (Fig. 7), indicating that Ad5/35-TRAIL triggers more efficient apoptosis in the HL-60/Vinc cell line by a different mechanism than its sensitive counterpart.
FIG. 7.
Effect of Ad5/35-TRAIL on mitochondrial potential change (Δψm). Cells (5 × 105) were treated with or without 50 MOI of Ad5/35, TRAIL at 20 ng/ml, or 50 MOI of Ad5/35-TRAIL at 37°C for 24 hr, and 5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazolyl carbocyanine (JC-1) was used as a probe as described in Materials and Methods. JC-1 membrane potential-related fluorescence was recorded with an FL1 photomultiplier tube (PMT) and a FACSCalibur flow cytometer. This experiment was performed in triplicate and results are shown as means ± SD.
Ad5/35-TRAIL enhances ROS generation and the effect of NAc
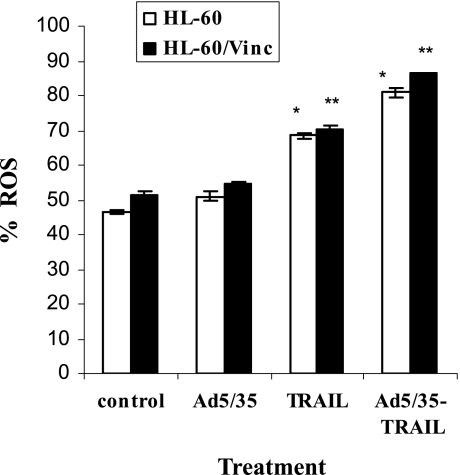
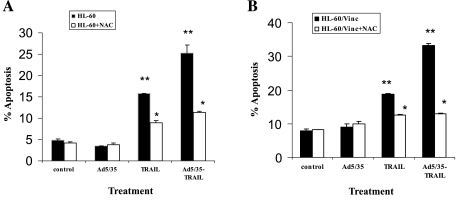
It is well known that increased ROS are likely to act as signaling intermediates in the signal transduction pathways of apoptosis. Cells treated with Ad5/35-TRAIL showed elevated levels of ROS compared with cells treated with recombinant TRAIL (Fig. 8). To determine whether the increased ROS generated by TRAIL or Ad5/35-TRAIL enhance the induced apoptosis, cells were incubated with or without a 10 mM concentration of the antioxidant N-acetylcysteine (NAc) 3 hr before treatment and the percentage of apoptosis was determined by annexin V flow cytometry. The results shown in Fig. 9 revealed that NAc significantly blocked the apoptosis triggered by TRAIL and Ad5/35-TRAIL. Therefore, ROS play an important role in enhancing the level of apoptosis.
FIG. 8.
Analysis of reactive oxygen species (ROS) in TRAIL- and Ad5/35-TRAIL-triggered apoptosis. Cells (5 × 105) were treated with or without 50 MOI of Ad5/35, TRAIL at 20 ng/ml, or 50 MOI of Ad5/35-TRAIL at 37°C for 24 hr. After treatment, the cells were incubated for 30 min at 37°C with 5 μM dichlorodihydrofluorescein diacetate (DCFH-DA) and analyzed with a FACSCalibur flow cytometer as described by the manufacturer (*p < 0.005, **p < 0.0008).
FIG. 9.
NAc effect on TRAIL- and Ad5/35-TRAIL-induced apoptosis: (A) HL-60 cells; (B) HL-60/Vinc cells. Cells (5 × 105) were incubated with 10 mM NAc for 3 hr before being treated with or without 50 MOI of Ad5/35, TRAIL at 20 ng/ml, or 50 MOI of Ad5/35-TRAIL at 37°C for 24 hr; apoptosis was determined by annexin V flow cytometry as described in text. NAc decreases Ad5/35-TRAIL-induced apoptosis: (A) *p < 0.004, **p < 0.005; (B) *p < 0.005, **p < 0.001.
Discussion
In this study we determined the levels of apoptosis triggered by TRAIL and Ad5/35-TRAIL in HL-60 cells and in drug-resistant variant HL-60/Vinc cells, but not in normal lymphocytes. It is likely that the resistance of normal human cells to TRAIL is due to the lack of DR4 and DR5 expression on the cell surface (Hao et al., 2004). We also elucidated the mechanism by which Ad5/35-TRAIL induces apoptosis in these cells. Ad5/35-TRAIL did not induce apoptosis in normal human lymphocytes, but caused robust apoptosis in HL-60 and HL-60/Vinc cells through overexpression and production of TRAIL protein including its monomeric, dimeric, and trimeric isoforms. Secretable trimeric TRAIL (stTRAIL) has shown higher tumor suppressor activity than full-length TRAIL (C.Y. Kim et al., 2006). Ad5/35-TRAIL triggered more efficient apoptosis in drug-resistant variant HL-60/Vinc cells than in HL-60 cells and the enhanced apoptosis was associated with increased expression of stTRAIL.
Although Ad5/35-TRAIL did not significantly affect the expression of TRAIL receptors DR4 and DR5, pretreating cells with neutralizing antibodies against DR4 and DR5 receptors reduced, whereas DcR1 enhanced, the apoptosis triggered by Ad5/35-TRAIL. These results indicate that increased apoptosis is not due to a change in TRAIL receptor numbers and suggests that DISC formation is required to generate apoptosis. In addition, DR5 antibody inhibited apoptosis to a greater extent than did DR4 antibody, revealing that Ad5/35-TRAIL induced apoptosis preferentially via DR5.
We found that caspase-3 and caspase-10 are involved in Ad5/35-TRAIL-triggered apoptosis, whereas caspase-8 and caspase-9 are not. Caspase-3 and caspase-10 inhibitors significantly inhibit Ad5/35-TRAIL-induced apoptosis, and data revealed that inhibitors of caspase-8 and caspase-9 neither reduced Ad5/35-TRAIL-induced apoptosis nor affected the activation of caspase-3. However, it is well documented that caspase-3 can be activated by caspase-9 or via caspase-8 or caspase-10. Because caspase-9 did not play a role in Ad5/35-TRAIL-induced apoptosis, our data suggest that caspase-3 is activated through caspase-10 activation, and that caspase-10 plays a role in Ad5/35-TRAIL-triggered apoptosis through the activation of caspase-3, the main caspase involved in Ad5/35-TRAIL-induced apoptosis.
Truncated BID (tBID) is known to link the cell death receptor pathway to the mitochondrial pathway, but our result showed that BID was not cleaved to tBID by TRAIL or Ad5/35-TRAIL treatment in HL-60 and HL-60 cells (data not shown). Therefore, the proapoptotic protein(s) linking the two pathways in Ad5/35-TRAIL-induced apoptosis remains to be found.
Persistent c-FLIP expression is necessary and sufficient to maintain resistance to TRAIL in cancer cells (Bortul et al., 2003; Abedini et al., 2004; Jin et al., 2004). Our data revealed that there is a significant difference in c-FLIP protein expression between HL-60 and HL-60/Vinc cells, but not at the mRNA level, suggesting that overexpression of c-FLIP in HL-60/Vinc cells is posttranscriptionally regulated. However, the results clearly showed that c-FLIP does not prevent Ad5/35-TRAIL-induced apoptosis in these cells.
In Ad5/35, RID protein degrades and internalizes TRAIL DR4 receptor (Tollefson et al., 2001; Lichtenstein et al., 2004b), while its E3 6.7K protein is required in conjunction with the E3–RID protein complex to internalize and degrade DR5 (Tollefson et al., 2001; Lichtenstein et al., 2002, 2004a, b). These proteins therefore sufficiently prevent infected cells from being killed by TRAIL production, prolong acute and persistent infection, and kill the surrounding cells by their bystander effects (Kagawa et al., 2001; Huang et al., 2003; He et al., 2004). It has been reported that cell-to-cell contact is required for the bystander effect of the TRAIL gene (Huang et al., 2003) and induced apoptosis supports the spread of this adenovirus in tumors (Mi et al., 2001). It is consistent with our data that Ad5/35-TRAIL generates much more membrane and cytoplasmic TRAIL than the exogenous TRAIL treatment. Our results clearly showed that Ad5/35-TRAIL increased Δψm only in HL-60/Vinc cells, revealing that mitochondria play an important role in Ad5/35-TRAIL-induced apoptosis in these cells compared with HL-60 cells. Furthermore, P-gp in drug-resistant cells may also enhance enhanced TRAIL binding to DR5 (Park et al., 2006). Moreover, NAc significantly blocks the apoptosis triggered by TRAIL and Ad5/35-TRAIL, suggesting that increased ROS production also contributed to the superiority and effectiveness of Ad5/35-TRAIL in leukemia treatment.
It was reported that TRAIL promotes metastasis of human pancreatic ductal adenocarcinoma (Trauzold et al., 2006). This shortcoming is one of the disadvantages to using recombinant TRAIL in cancer therapy. Using Ad5/35-TRAIL instead of recombinant TRAIL may overcome this problem. In support of our data, others have demonstrated that the tumor-targeted and conditionally replicating oncolytic adenoviral vector expressing TRAIL is the choice to treat liver metastases of several cancers (Li et al., 2005). Moreover, the recombinant adeno-associated virus-mediated TRAIL gene suppresses liver metastatic tumors (Ma et al., 2005). Therefore, our data provide encouraging information about the potential usefulness of Ad5/35-TRAIL in treating multidrug-resistant leukemia cells.
Acknowledgments
The authors thank Dr. Mary D. Kraeszig for her editorial assistance. This work was supported by research grants from the National Cancer Institute (CA 90878, and CA 101743) to A.R.S.
Author Disclosure Statement
No competing financial interests exist among authors.
References
- Abedini M.R. Qiu Q. Yan X. Tsang B.K. Possible role of FLICE-like inhibitory protein (FLIP) in chemoresistant ovarian cancer cells in vitro. Oncogene. 2004;23:6997–7004. doi: 10.1038/sj.onc.1207925. [DOI] [PubMed] [Google Scholar]
- Ashkenazi A. Pai R.C. Fong S. Leung S. Lawrence D.A. Marsters S.A. Blackie C. Chang L. McMurtrey A.E. Hebert A. Deforge L. Koumenis I.L. Lewis D. Harris L. Bussiere J. Koeppen H. Shahrokh Z. Schwall R.H. Safety and antitumor activity of recombinant soluble Apo2 ligand. J. Clin. Invest. 1999;104:155–162. doi: 10.1172/JCI6926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balamotis M.A. Huang K. Mitani K. Efficient delivery and stable gene expression in a hematopoietic cell line using a chimeric serotype 35 fiber pseudotyped helper-dependent adenoviral vector. Virology. 2004;324:229–237. doi: 10.1016/j.virol.2004.03.030. [DOI] [PubMed] [Google Scholar]
- Bedi A. Zehnbauer B.A. Barber J.P. Sharkis S.J. Jones R.J. Inhibition of apoptosis by BCR-ABL in chronic myeloid leukemia. Blood. 1994;83:2038–2044. [PubMed] [Google Scholar]
- Bedi A. Barber J.P. Bedi G.C. El-Deiry W.S. Sidransky D. Vala M.S. Akhtar A.J. Hilton J. Jones R.J. BCR-ABL mediated inhibition of apoptosis with delay of G2/M transition after DNA damage: A mechanism of resistance to multiple anticancer agents. Blood. 1995;86:1148–1158. [PubMed] [Google Scholar]
- Bergelson J.M. Cunningham J.A. Droguett G. Kurt-Jones E.A. Krithivas A. Hong J.S. Horwitz M.S. Crowell R.L. Finberg R.W. Isolation of a common receptor for coxsackie B viruses and adenoviruses 2 and 5. Science. 1997;275:1320–1323. doi: 10.1126/science.275.5304.1320. [DOI] [PubMed] [Google Scholar]
- Bernt K.M. Ni S. Gaggar A. Li Z.Y. Shayakhmetov D.M. Lieber A. The effect of sequestration by nontarget tissues on anti-tumor efficacy of systemically applied, conditionally replicating adenovirus vectors. Mol. Ther. 2003;8:746–755. doi: 10.1016/j.ymthe.2003.07.006. [DOI] [PubMed] [Google Scholar]
- Bortul R. Tazzari P.L. Cappellini A. Tabellini G. Billi A.M. Bareggi R. Manzoli L. Cocco L. Martelli A.M. Constitutively active Akt1 protects HL60 leukemia cells from TRAIL-induced apoptosis through a mechanism involving NF-κB activation and cFLIPL up-regulation. Leukemia. 2003;17:379–389. doi: 10.1038/sj.leu.2402793. [DOI] [PubMed] [Google Scholar]
- Bouralexis S. Findlay D.M. Atkins G.J. Labrinidis A. Hay S. Evdokiou A. Progressive resistance of BTK-143 osteosarcoma cells to Apo2L/TRAIL-induced apoptosis is mediated by acquisition of DcR2/TRAIL-R4 expression: Resensitisation with chemotherapy. Br. J. Cancer. 2003;89:206–214. doi: 10.1038/sj.bjc.6601021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggert A. Grotzer M.A. Zuzak T.J. Wiewrodt B.R. Ho R. Ikegaki N. Brodeur G.M. Resistance to tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)-induced apoptosis in neuroblastoma cells correlates with a loss of caspase-8 expression. Cancer Res. 2001;61:1314–1319. [PubMed] [Google Scholar]
- Ehrhardt H. Fulda S. Schmid I. Hiscott J. Debatin K.M. Jeremias I. TRAIL induced survival and proliferation in cancer cells resistant towards TRAIL-induced apoptosis mediated by NF-κB. Oncogene. 2003;22:3842–3852. doi: 10.1038/sj.onc.1206520. [DOI] [PubMed] [Google Scholar]
- Ehtesham M. Kabos P. Gutierrez M.A. Chung N.H. Griffith T.S. Black K.L. Yu J.S. Induction of glioblastoma apoptosis using neural stem cell-mediated delivery of tumor necrosis factor-related apoptosis inducing ligand. Cancer Res. 2002;62:7170–7174. [PubMed] [Google Scholar]
- Gaggar A. Shayakhmetov D.M. Lieber A. CD46 is a cellular receptor for group B adenoviruses. Nat. Med. 2003;9:1408–1412. doi: 10.1038/nm952. [DOI] [PubMed] [Google Scholar]
- Gaggar A. Shayakhmetov D.M. Liszewski M.K. Atkinson J.P. Lieber A. Localization of regions in CD46 that interact with adenovirus. J. Virol. 2005;79:7503–7513. doi: 10.1128/JVI.79.12.7503-7513.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green D.R. Apoptotic pathways: The roads to ruin. Cell. 1998;94:695–698. doi: 10.1016/s0092-8674(00)81728-6. [DOI] [PubMed] [Google Scholar]
- Griffith T.S. Fialkov J.M. Scott D.L. Azuhata T. Williams R.D. Wall N.R. Altieri D.C. Sandler A.D. Induction and regulation of tumor necrosis factor-related apoptosis-inducing ligand/Apo-2 ligand-mediated apoptosis in renal cell carcinoma. Cancer Res. 2002;62:3093–3099. [PubMed] [Google Scholar]
- Gross A. Yin X.M. Wang K. Wei M.C. Jockel J. Milliman C. Erdjument-Bromage H. Tempst P. Korsmeyer S.J. Caspase cleaved BID targets mitochondria and is required for cytochrome c release, while BCL-XL prevents this release but not tumor necrosis factor-R1/Fas death. J. Biol. Chem. 1999;274:1156–1163. doi: 10.1074/jbc.274.2.1156. [DOI] [PubMed] [Google Scholar]
- Hao C. Song J.H. Hsi B. Lewis J. Song D.K. Petruk K.C. Tyrrell D.L. Kneteman N.M. TRAIL inhibits tumor growth but is nontoxic to human hepatocytes in chimeric mice. Cancer Res. 2004;64:8502–8506. doi: 10.1158/0008-5472.CAN-04-2599. [DOI] [PubMed] [Google Scholar]
- Hasegawa H. Yamada Y. Harasawa H. Tsuji T. Murata K. Sugahara K. Tsuruda K. Ikeda S. Imaizumi Y. Tomonaga M. Masuda M. Takasu N. Kamihira S. Sensitivity of adult T-cell leukaemia lymphoma cells to tumour necrosis factor-related apoptosis-inducing ligand. Br. J. Haematol. 2005;128:253–265. doi: 10.1111/j.1365-2141.2004.05289.x. [DOI] [PubMed] [Google Scholar]
- He C. Lao W.F. Hu X.T. Xu X.M. Xu J. Fang B.L. Anti-liver cancer activity of TNF-related apoptosis-inducing ligand gene and its bystander effects. World J. Gastroenterol. 2004;10:654–659. doi: 10.3748/wjg.v10.i5.654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang M.M. Hearing P. Adenovirus early region 4 encodes two gene products with redundant effects in lytic infection. J. Virol. 1989;63:2605–2615. doi: 10.1128/jvi.63.6.2605-2615.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Q. Zhang X. Wang H. Yan B. Kirkpatrick J. Dewhirst M.W. Li C.Y. A novel conditionally replicative adenovirus vector targeting telomerase-positive tumor cells. Clin. Cancer Res. 2004;10:1439–1445. doi: 10.1158/1078-0432.ccr-03-0122. [DOI] [PubMed] [Google Scholar]
- Huang X. Lin T. Gu J. Zhang L. Roth J.A. Liu J. Fang B. Cell to cell contact required for bystander effect of the TNF-related apoptosis inducing ligand (TRAIL) gene. Int. J. Oncol. 2003;22:1241–1245. [PubMed] [Google Scholar]
- Jia L. Patwari Y. Kelsey S.M. Newland A.C. Trail-induced apoptosis in type I leukemic cells is not enhanced by overexpression of Bax. Biochem. Biophys. Res. Commun. 2001;283:1037–1045. doi: 10.1006/bbrc.2001.4895. [DOI] [PubMed] [Google Scholar]
- Jin T.G. Kurakin A. Benhaga N. Abe K. Mohseni M. Sandra F. Song K. Kay B.K. Khosravi-Far R. Fas-associated protein with death domain (FADD)-independent recruitment of c-FLIPL to death receptor 5. J. Biol. Chem. 2004;279:55594–55601. doi: 10.1074/jbc.M401056200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagawa S. He C. Gu J. Koch P. Rha S.J. Roth J.A. Curley S.A. Stephens L.C. Fang B. Antitumor activity and bystander effects of the tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) gene. Cancer Res. 2001;61:3330–3338. [PubMed] [Google Scholar]
- Karacay B. Sanlioglu S. Griffith T.S. Sandler A. Bonthius D.J. Inhibition of the NF-κB pathway enhances TRAIL-mediated apoptosis in neuroblastoma cells. Cancer Gene Ther. 2004;11:681–690. doi: 10.1038/sj.cgt.7700749. [DOI] [PubMed] [Google Scholar]
- Keane M.M. Rubinstein Y. Cuello M. Ettenberg S.A. Banerjee P. Nau M.M. Lipkowitz S. Inhibition of NF-κB activity enhances TRAIL mediated apoptosis in breast cancer cell lines. Breast Cancer Res. Treat. 2000;64:211–219. doi: 10.1023/a:1006458407515. [DOI] [PubMed] [Google Scholar]
- Kim C.Y. Jeong M. Mushiake H. Kim B.M. Kim W.B. Ko J.P. Kim M.H. Kim M. Kim T.H. Robbins P.D. Billiar T.R. Seol D.W. Cancer gene therapy using a novel secretable trimeric TRAIL. Gene Ther. 2006;13:330–338. doi: 10.1038/sj.gt.3302658. [DOI] [PubMed] [Google Scholar]
- Kim K. Takimoto R. Dicker D.T. Chen Y. Gazitt Y. El-Deiry W.S. Enhanced TRAIL sensitivity by p53 over-expression in human cancer but not normal cell lines. Int. J. Oncol. 2001;18:241–247. doi: 10.3892/ijo.18.2.241. [DOI] [PubMed] [Google Scholar]
- Kim K. Nakagawa H. Fei P. Rustgi A.K. El-Deiry W.S. Targeting Bcl-xL in esophageal squamous cancer to sensitize to chemotherapy plus TRAIL-induced apoptosis while normal epithelial cells are protected by blockade of caspase 9. Cell Death Differ. 2004;11:583–587. doi: 10.1038/sj.cdd.4401388. [DOI] [PubMed] [Google Scholar]
- Kim S.H. Kim K. Kwagh J.G. Dicker D.T. Herlyn M. Rustgi A.K. Chen Y. El-Deiry W.S. Death induction by recombinant native TRAIL and its prevention by a caspase 9 inhibitor in primary human esophageal epithelial cells. J. Biol. Chem. 2004;279:40044–40052. doi: 10.1074/jbc.M404541200. [DOI] [PubMed] [Google Scholar]
- Li H. Zhu H. Xu C.J. Yuan J. Cleavage of BID by caspase 8 mediates the mitochondrial damage in the Fas pathway of apoptosis. Cell. 1998;94:491–501. doi: 10.1016/s0092-8674(00)81590-1. [DOI] [PubMed] [Google Scholar]
- Li P. Nijhawan D. Budihardjo I. Srinivasula S.M. Ahmad M. Alnemri E.S. Wang X. Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell. 1997;91:479–489. doi: 10.1016/s0092-8674(00)80434-1. [DOI] [PubMed] [Google Scholar]
- Li X. Zhang Y.P. Kim H.S. Bae K.H. Stantz K.M. Lee S.J. Jung C. Jimenez J.A. Gardner T.A. Jeng M.H. Kao C. Gene therapy for prostate cancer by controlling adenovirus E1a and E4 gene expression with PSES enhancer. Cancer Res. 2005;65:1941–1951. doi: 10.1158/0008-5472.CAN-04-3666. [DOI] [PubMed] [Google Scholar]
- Lichtenstein D.L. Krajcsi P. Esteban D.J. Tollefson A.E. Wold W.S. Adenovirus RIDβ subunit contains a tyrosine residue that is critical for RID-mediated receptor internalization and inhibition of Fas- and TRAIL-induced apoptosis. J. Virol. 2002;76:11329–11342. doi: 10.1128/JVI.76.22.11329-11342.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtenstein D.L. Doronin K. Toth K. Kuppuswamy M. Wold W.S. Tollefson A.E. Adenovirus E3-6.7K protein is required in conjunction with the E3–RID protein complex for the internalization and degradation of TRAIL receptor 2. J. Virol. 2004a;78:12297–12307. doi: 10.1128/JVI.78.22.12297-12307.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtenstein D.L. Toth K. Doronin K. Tollefson A.E. Wold W.S. Functions and mechanisms of action of the adenovirus E3 proteins. Int. Rev. Immunol. 2004b;23:75–111. doi: 10.1080/08830180490265556. [DOI] [PubMed] [Google Scholar]
- Louis N. Fender P. Barge A. Kitts P. Chroboczek J. Cell binding domain of adenovirus serotype 2 fiber. J. Virol. 1994;68:4104–4106. doi: 10.1128/jvi.68.6.4104-4106.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo X. Budihardjo I. Zou H. Slaughter C. Wang X. Bid, a Bcl2 interacting protein, mediates cytochrome c release from mitochondria in response to activation of cell surface death receptors. Cell. 1998;94:481–490. doi: 10.1016/s0092-8674(00)81589-5. [DOI] [PubMed] [Google Scholar]
- Ma H. Liu Y. Liu S. Kung H.F. Sun X. Zheng D. Xu R. Recombinant adeno-associated virus-mediated TRAIL gene therapy suppresses liver metastatic tumors. Int. J. Cancer. 2005;116:314–321. doi: 10.1002/ijc.20982. [DOI] [PubMed] [Google Scholar]
- Macfarlane M. Harper N. Snowden R.T. Dyer M.J. Barnett G.A. Pringle J.H. Cohen G.M. Mechanisms of resistance to TRAIL induced apoptosis in primary B cell chronic lymphocytic leukaemia. Oncogene. 2002;21:6809–6818. doi: 10.1038/sj.onc.1205853. [DOI] [PubMed] [Google Scholar]
- Martelli A.M. Tazzari P.L. Tabellini G. Bortul R. Billi A.M. Manzoli L. Ruggeri A. Conte R. Cocco L. A new selective AKT pharmacological inhibitor reduces resistance to chemotherapeutic drugs, TRAIL, all-trans-retinoic acid, and ionizing radiation of human leukemia cells. Leukemia. 2003;17:1794–1805. doi: 10.1038/sj.leu.2403044. [DOI] [PubMed] [Google Scholar]
- McGahon A. Bissonnette R. Schmitt M. Cotter K.M. Green D.R. Cotter T.G. BCR-ABL maintains resistance of chronic myelogenous leukemia cells to apoptotic cell death. Blood. 1994;83:1179–1187. [PubMed] [Google Scholar]
- Mi J. Li Z.Y. Ni S. Steinwaerder D. Lieber A. Induced apoptosis supports spread of adenovirus vectors in tumors. Hum. Gene Ther. 2001;12:1343–1352. doi: 10.1089/104303401750270995. [DOI] [PubMed] [Google Scholar]
- Nagane M. Huang H.J. Cavenee W.K. The potential of TRAIL for cancer chemotherapy. Apoptosis. 2001;6:191–197. doi: 10.1023/a:1011336726649. [DOI] [PubMed] [Google Scholar]
- Ni S. Bernt K. Gaggar A. Li Z.Y. Kiem H.P. Lieber A. Evaluation of biodistribution and safety of adenovirus vectors containing group B fibers after intravenous injection into baboons. Hum. Gene Ther. 2005;16:664–677. doi: 10.1089/hum.2005.16.664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimmanapalli R. Porosnicu M. Nguyen D. Worthington E. O'Bryan E. Perkins C. Bhalla K. Cotreatment with STI-571 enhances tumor necrosis factor α-related apoptosis-inducing ligand (TRAIL or apo-2L)-induced apoptosis of Bcr-Abl-positive human acute leukemia cells. Clin. Cancer Res. 2001;7:350–357. [PubMed] [Google Scholar]
- Ogretmen B. Safa A.R. Identification and characterization of the MDR1 promoter-enhancing factor 1 (MEF1) in the multidrug resistant HL60/VCR human acute myeloid leukemia cell line. Biochemistry. 2000;39:194–204. doi: 10.1021/bi991943f. [DOI] [PubMed] [Google Scholar]
- Ozoren N. Kim K. Burns T.F. Dicker D.T. Moscioni A.D. Eldeiry W.S. The caspase 9 inhibitor Z-LEHD-FMK protects human liver cells while permitting death of cancer cells exposed to tumor necrosis factor related apoptosis-inducing ligand. Cancer Res. 2000;60:6259–6265. [PubMed] [Google Scholar]
- Park S.J. Wu C.H. Choi M.R. Najafi F. Emami A. Safa A.R. P-glycoprotein enhances TRAIL-triggered apoptosis in multidrug resistant cancer cells by interacting with the death receptor DR5. Biochem. Pharmacol. 2006;72:293–307. doi: 10.1016/j.bcp.2006.04.024. [DOI] [PubMed] [Google Scholar]
- Pitti R.M. Marsters S.A. Ruppert S. Donahue C.J. Moore A. Ashkenazi A. Induction of apoptosis by Apo-2 ligand, a new member of the tumor necrosis factor cytokine family. J. Biol. Chem. 1996;271:12687–12690. doi: 10.1074/jbc.271.22.12687. [DOI] [PubMed] [Google Scholar]
- Poulaki V. Mitsiades C.S. Kotoula V. Tseleni-Balafouta S. Ashkenazi A. Koutras D.A. Mitsiades N. Regulation of Apo2L/tumor necrosis factor-related apoptosis-inducing ligand-induced apoptosis in thyroid carcinoma cells. Am. J. Pathol. 2002;161:643–654. doi: 10.1016/S0002-9440(10)64220-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruigrok R.W. Barge A. Albiges-Rizo C. Dayan S. Structure of adenovirus fibre. II. Morphology of single fibres. J. Mol. Biol. 1990;215:589–596. doi: 10.1016/S0022-2836(05)80170-6. [DOI] [PubMed] [Google Scholar]
- Salvesen G.S. Dixit V.M. Caspases: Intracellular signaling by proteolysis. Cell. 1997;91:443–446. doi: 10.1016/s0092-8674(00)80430-4. [DOI] [PubMed] [Google Scholar]
- Salvesen G.S. Dixit V.M. Caspase activation: The induced-proximity model. Proc. Natl. Acad. Sci. U.S.A. 1999;96:10964–10967. doi: 10.1073/pnas.96.20.10964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segerman A. Arnberg N. Erikson A. Lindman K. Wadell G. There are two different species B adenovirus receptors: sBAR, common to species B1 and B2 adenoviruses, and sB2AR, exclusively used by species B2 adenoviruses. J. Virol. 2003;77:1157–1162. doi: 10.1128/JVI.77.2.1157-1162.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seshidhar Reddy P. Ganesh S. Limbach M.P. Brann T. Pinkstaff A. Kaloss M. Kaleko M. Connelly S. Development of adenovirus serotype 35 as a gene transfer vector. Virology. 2003;311:384–393. doi: 10.1016/s0042-6822(03)00161-2. [DOI] [PubMed] [Google Scholar]
- Shayakhmetov D.M. Papayannopoulou T. Stamatoyannopoulos G. Lieber A. Efficient gene transfer into human CD34+ cells by a retargeted adenovirus vector. J. Virol. 2000;74:2567–2583. doi: 10.1128/jvi.74.6.2567-2583.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiiki K. Yoshikawa H. Kinoshita H. Takeda M. Ueno A. Nakajima Y. Tasaka K. Potential mechanisms of resistance to TRAIL/Apo2L-induced apoptosis in human promyelocytic leukemia HL-60 cells during granulocytic differentiation. Cell Death Differ. 2000;7:939–946. doi: 10.1038/sj.cdd.4400727. [DOI] [PubMed] [Google Scholar]
- Srinivasula S.M. Ahmad M. Guo Y. Zhan Y. Lazebnik Y. Fernandes-Alnemri T. Alnemri E.S. Identification of an endogenous dominant-negative short isoform of caspase-9 that can regulate apoptosis. Cancer Res. 1999;59:999–1002. [PubMed] [Google Scholar]
- Suliman A. Lam A. Datta R. Srivastava R.K. Intracellular mechanisms of TRAIL: Apoptosis through mitochondrial-dependent and -independent pathways. Oncogene. 2001;20:2122–2133. doi: 10.1038/sj.onc.1204282. [DOI] [PubMed] [Google Scholar]
- Tollefson A.E. Toth K. Doronin K. Kuppuswamy M. Doronina O.A. Lichtenstein D.L. Hermiston T.W. Smith C.A. Wold W.S. Inhibition of TRAIL-induced apoptosis and forced internalization of TRAIL receptor 1 by adenovirus proteins. J. Virol. 2001;75:8875–8887. doi: 10.1128/JVI.75.19.8875-8887.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trauzold A. Siegmund D. Schniewind B. Sipos B. Egberts J. Zorenkov D. Emme D. Roder C. Kalthoff H. Wajant H. TRAIL promotes metastasis of human pancreatic ductal adenocarcinoma. Oncogene. 2006;25:7434–7439. doi: 10.1038/sj.onc.1209719. [DOI] [PubMed] [Google Scholar]
- van Oostrum J. Burnett R.M. Molecular composition of the adenovirus type 2 virion. J. Virol. 1985;56:439–448. doi: 10.1128/jvi.56.2.439-448.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiley S.R. Schooley K. Smolak P.J. Din W.S. Huang C.P. Nicholl J.K. Sutherland G.R. Smith T.D. Rauch C. Smith C.A. Goodwin R.G. Identification and characterization of a new member of the TNF family that induces apoptosis. Immunity. 1995;3:673–682. doi: 10.1016/1074-7613(95)90057-8. [DOI] [PubMed] [Google Scholar]
- Yotnda P. Zompeta C. Heslop H.E. Andreeff M. Brenner M.K. Marini F. Comparison of the efficiency of transduction of leukemic cells by fiber-modified adenoviruses. Hum. Gene Ther. 2004;15:1229–1242. doi: 10.1089/hum.2004.15.1229. [DOI] [PubMed] [Google Scholar]
- Zhang X. Jin T.G. Yang H. Dewolf W.C. Khosravi-Far R. Olumi A.F. Persistent c-FLIPL expression is necessary and sufficient to maintain resistance to tumor necrosis factor-related apoptosis-inducing ligand mediated apoptosis in prostate cancer. Cancer Res. 2004;64:7086–7091. doi: 10.1158/0008-5472.CAN-04-1498. [DOI] [PubMed] [Google Scholar]
- Zhang X.D. Franco A. Myers K. Gray C. Nguyen T. Hersey P. Relation of TNF-related apoptosis-inducing ligand (TRAIL) receptor and FLICE-inhibitory protein expression to TRAIL-induced apoptosis of melanoma. Cancer Res. 1999;59:2747–2753. [PubMed] [Google Scholar]
- Zou H. Li Y. Liu X. Wang X. An APAF-1.cytochrome c multimeric complex is a functional apoptosome that activates procaspase-9. J. Biol. Chem. 1999;274:11549–11556. doi: 10.1074/jbc.274.17.11549. [DOI] [PubMed] [Google Scholar]
- Zuzak T.J. Steinhoff D.F. Sutton L.N. Phillips P.C. Eggert A. Grotzer M.A. Loss of caspase-8 mRNA expression is common in childhood primitive neuroectodermal brain tumour/medulloblastoma. Eur. J. Cancer. 2002;38:83–91. doi: 10.1016/s0959-8049(01)00355-0. [DOI] [PubMed] [Google Scholar]