Abstract
Ultraviolet radiation (UVR) is an essential risk factor for the development of premalignant skin lesions as well as of melanoma and non-melanoma skin cancer. UVR exerts many effects on the skin, including tanning, carcinogenesis, immunomodulation, and production of vitamin D. Vitamin D (vit D) is important in the maintenance of healthy bones as well as other purported beneficial effects, amongst which is the potential for reducing risk of malignancy—though oral supplementation is fully capable of maintaining systemic levels. The known medical harm from UV exposure relates primarily to cancer of the skin—the most common organ in man to be affected by cancer. In this review, we summarize the knowledge about the ultraviolet (UV) response in regards to inflammation, immunosuppression, carcinogenesis and the tanning response. We also discuss vit D and UV, as well as public health implications of tanning behavior and commercial interests related to the promotion of UV exposure. As the most ubiquitous human carcinogen, UVR exposure represents both a challenge and enormous opportunity in the realm of skin cancer prevention.
Introduction
Despite public awareness campaigns, the tanning industry is growing, with at least 5 billion a year in estimated annual revenue, a five-fold increase from the level in 1992(Balk and Geller, 2008, Levine et al., 2005). About 28 million US citizens, of whom about 70% are white teenagers and women, aged between 16 and 49 years, use about 50,000 tanning facilities, amounting to about 1 million users per day(Levine et al., 2005). Prevalence of artificial tanning among white girls rises rapidly with age, more than doubling from ages 14 to 15 (7% to 15%), and doubling again at age 17 (35%)(Geller et al., 2002). Increased tanning behavior has been associated with female sex, residence in Midwest or South, rural schooling, increasing age, usage of tobacco and alcohol, and available spending money(Levine et al., 2005, Abdulla et al., 2005, Demko et al., 2003) It is widely felt that an effective lobbying effort by the tanning industry has contributed to continued growth and public use of the indoor tanning industry. Teenagers are specifically targeted by the tanning industry through methods such as advertisements placed in high school newspapers including those that offer coupons for discounts and “unlimited tanning” programs(Freeman et al., 2006). Statements about positive effects of UV lights as well as the safety and benefits of tanning in a salon are well-advertised on the Indoor Tanning Association website(ITA). Moreover, the tanning industry has fought vigorously for teenagers to have access to tanning salons, given that many states have attempted to pass laws to limit teenagers’ access to tanning facilities(Balk and Geller, 2008). While a portion of this behavior is undoubtedly predicated upon perceptions of attractiveness, additional advertising messages have touted “health benefits” of UVR (focusing primarily around vit D production in skin) while attempting to suggest that linkage of UV to melanoma is less clear than previously assumed. These advertising messages are quite broadly and vigorously disputed by scientists and physicians who cite abundant data implicating UV as the chief environmental carcinogen in skin cancers. Further, as discussed below, the medical benefits of vit D notwithstanding, physicians recommend oral vit D supplements rather than UV (carcinogen) induced vit D, since the two are chemically indistinguishable and the oral form does not entail carcinogenic risk. Efforts by the American Academy of Dermatology to request stricter regulation on indoor tanning have met with vigorous opposition from the tanning industry through paid lobbyists and a well orchestrated media campaign(NTTI, Gilchrest, 2007). Moreover recent molecular understanding of UV-induced tanning has strongly suggested that the initiating chemical event is DNA damage—thus rendering it increasingly unlikely that there exist doses or delivery strategies which could uncouple tanning from carcinogenic risk. The theoretical concept of “safe tanning” thus warrants legitimate scientifically based skepticism.
Tanning Overview
Since ancient time, the sun has been a remarkable attraction for the human race, not only for its central role in religion and mythology, but also in the practice of medicine, where sunlight was used to treat various conditions from vitiligo to lupus vulgaris to rickets (Albert and Ostheimer, 2002). Exposure to sunlight also results in a darkening of the skin which has been considered attractive in certain societies, particularly with the advent of the industrial revolution when much labor was moved indoors (Albert and Ostheimer, 2003a, Ibrahim and Brown, 2008). However, beginning in the 1900s, excessive sun exposure has been linked to skin cancer and by the second half of the century, there was overwhelming evidence implicating ultraviolet radiation (UVR) as a carcinogen (Ibrahim and Brown, 2008, Blum et al., 1941, Findlay, 1928, Hall, 1950, Levine et al., 2005). There have also been increased suggestions of purported benefits via vitamin D (vit D) in regard to bone health and, more recently, reduced risk for internal malignancy(Giovannucci, 2005, Freedman et al., 2007, Skinner, 2008, Lu et al., 2008, Stolzenberg-Solomon, 2008, Ahn et al., 2008, Khazai et al., 2008). Although vit D can easily be obtained from non-UV sources, this argument has nonetheless been used to support purposeful UV exposure, thus sparking a controversy due to concerns about skin carcinogenesis and other toxic effects of UV. Here, we will attempt to outline scientific understanding behind the UV response and the vitamin D controversy.
UV radiation
The sun emits UV radiation (UVR) that is subdivided into UVA [400-320nm], UVB [320-290nm] and UVC [290-200nm. More than 95% of the sun’s UVR that reaches the earth’s surface is UVA whereas most UVC is absorbed by the ozone layer and oxygen in the atmosphere and is thus a very small source of adverse human health effects. About 1-10% of radiation that reaches the earth is UVB, which contains the shortest wavelengths that penetrate the ozone layer. This radiation interacts with photosensitive molecules within skin which, upon receiving photons, subsequently lift electrons to a higher energy state. These chromophores may pass the excited energy to other molecules and cause chain reactions(Tyrrell, 1994). Whereas targets of UV include nucleic acids, proteins, lipids, and other macromolecules, the biological consequences for DNA structure are particularly striking, resulting in “signature” mutations which are commonly found in cutaneous malignancies in man. The depth of penetration into the skin is dependent on the wavelength, the longer the wavelength, the deeper the penetration(Ibrahim and Brown, 2008, Latonen and Laiho, 2005). When UV and visible radiation reach the skin, some of the energy is reflected while wavelengths in the UVB range are largely absorbed by epidermal cellular components (eg, proteins or DNA). UVA radiation penetrates deeply into the skin, reaching the basal layer of the epidermis and even dermal fibroblasts(Marrot and Meunier, 2008). While ultraviolet A (UV-A) has historically been implicated in skin aging, it has now been linked, along with UV-B, in the development of skin cancers in animals and in immunosuppression in humans. Although the main source of UVA exposure is from sunlight, use of UV-A emitting lamps in sunbeds for recreational tanning has raised additional concerns about artificial sources of human exposure(Gallagher and Lee, 2006).
UV and the immune system
The acute effects of UVR on human skin involves several mechanisms including direct effects on the keratinocytes to release pro-inflammatory cytokines, direct effects on the DNA, depletion of cellular antioxidants as well as generation of reactive oxygen species (ROS) such as hydrogen peroxide, hydroxyl radical, singlet oxygen and peroxyl radicals, and generation of other inflammatory mediators(Pillai et al., 2005), which induce damage of lipids, proteins and DNA. The epidermis contains antioxidant defenses including the enzymes superoxide dismutase, glutathione peroxidase and catalase, which remove ROS from the skin (Moysan et al., 1996) and are depleted with prolonged exposure to UVR(Podda et al., 1998). Free radical scavengers, such as vitamins C and E, carotenoids and glutathione are also present in the skin to reduce the damaging effects of ROS(Moysan et al., 1996, Halliday, 2005). UVR also activates neural and endothelial nitric oxide synthase, which produces nitric oxide (NO) from L-arginine. NO interacts with superoxide to form peroxynitrite, which is in equilibrium with peroxynitrous acid(Szabo et al., 1996). Peroxynitrite is a highly toxic reactive nitrogen intermediate that can react directly with nucleic acids promoting DNA strand breakage, activating the enzyme poly(ADP-ribose) polymerase (PARP) which breaks down NAD+ and reduces ATP formation, causing an energy crisis in the cells(Halliday, 2005). UVR also induces lipid peroxidation, which increases production of prostaglandins such as prostaglandin E2 (PGE2), a pro-inflammatory cytokine. Other UV induced mediators such as tumor necrosis factor (TNF) and interleukin 1α (IL-1α) also contribute to the pro-inflammatory cascade(Halliday, 2005) . UVR augments blood flow and infiltration by macrophages and neutrophils into the skin. These also produce large amounts of ROS and NO resulting in peroxynitrite formation and likely represent an important mechanism of damage to tissues by inflammatory cells(Halliday, 2005).
Whereas a sunburn exemplifies a proinflammatory effect on the skin, UV radiation also has an immunosuppressive effect, evidenced by its therapeutic effect against certain inflammatory skin disorders (albeit with carcinogenic risk). UVR also suppresses the normal pathways of immune surveillance responsible for eliminating mutant cells, shown first by Kripke and colleagues over 30 years ago by demonstrating that UV immunosuppression could be transferred by injecting T cells from UV treated mice into naïve recipients. It was subsequently established that UV-induced suppression generated a subset of T-suppressor cells that were antigen specific (first shown for contact hypersensitivity responses to hapten in 1963)(Timares et al., 2008). UVR-induced immunosuppression can also affect delayed type hypersensitivity responses, susceptibility to infections, radiation recall responses, and vaccine immunogenicity(Timares et al., 2008). Although immune-modulation by UV is beyond the scope of this review, an impressive series of cellular and cytokine perturbations are produced, with major implications to the balance of immune activation and suppression within the skin(Schwarz et al., 2004, Schwarz et al., 2005, Schwarz et al., 2006). Even systemic immunosuppression is seen following UV irradiation (Ullrich, 2005).
UV and carcinogenesis
The suspicion that UV radiation is a human carcinogen has existed in the medical literature since the late 1800s, though participation of specific genes and signaling pathways in the cellular response to UV-induced DNA damage have been more recently identified(Albert and Ostheimer, 2003b). DNA and RNA contain strongly absorbing chromophores for UVB, with the aromatic heterocyclic nitrogen bases absorbing with wavelength maxima at 260–265 nm. Photoproducts known as cyclobutane pyrimidine dimers (CPD) and pyrimidine 6-4 pyrimidones are generated upon saturation of the 5,6 double bonds and formation of a four-membered cyclobutyl ring, creating C → T and CC→ TT mutations(Brash and Haseltine, 1982, Ravanat et al., 2001). The 6,4-photoproduct is a non-cyclobutane dipyrimidine photoproduct, which is formed upon covalent linkage between the C-6 position of one pyrimidine and the C-4 position of the 3′ adjacent pyrimidine. The bulkiness of the dimer disrupts the replication and transcription of DNA. Strong evidence for the involvement of these lesions in UV-skin-carcinogenesis is provided by the high proportion of p53 mutations (TC to TT or CC to TT transitions) detected at dipyrimidine sites in skin tumors(Giglia et al., 1998, Gailani et al., 1996, Ziegler et al., 1994, Brash et al., 1996, Brash et al., 1991, Ziegler et al., 1993). T-T lesions are proposed to play a role in generating Nras mutations found in rodent UV tumors(Pierceall et al., 1992) as well as in human melanomas(Jiveskog et al., 1998). Until recently, UVA was thought to act mainly via non-DNA chromophores in the skin, transferring energy to generate reactive intermediates which include oxygen free radicals capable of causing single- and double stranded DNA breaks. Singlet oxygen and the other reactive oxygen species react predominantly with guanine and generate several DNA changes, including mutagenic 7,8-dihydro-8-oxoguanosine (8-oxoG)(Kielbassa et al., 1997). However, a recent paper by Mouret et. al (Mouret et al., 2006). provides evidence suggesting that CPD are the predominant lesions in the DNA damage induced by UVA. Regardless of which mutagenic lesions are produced by UVA, it is notable that in the past many sunscreens were formulated for UVB protection but not UVA and most tanning booths use ∼95% UVA as a tanning source. UVA has also been shown to have a role in immunosuppression and is suspected to play a role in melanoma(Halliday, 2005).
Recent studies have utilized microarray expression profiling to study the transcriptional responses of keratinocytes, melanocytes, and fibroblasts to UV. The results suggest that the different cell-type responses are overlapping but distinct, indicating different roles in the UVR response in skin(Latonen and Laiho, 2005). Furthermore, UV doses capable of causing either cell cycle arrest or apoptosis provoke transcriptionally distinct responses. An important regulator of the genotoxic response in skin is p53.
p53 is an important tumor suppressor gene that is mutated frequently in human cancer, including skin cancers, though less so in melanoma than non-melanoma skin cancers. It governs responses to numerous cellular stresses including DNA damage, hypoxia, nucleotide imbalance, oxidative stress and spindle damage. UVB induced photodamage can be repaired through a process called nucleotide excision repair (NER) which utilizes either relatively fast transcription-coupled repair for actively expressed genomic regions, and slower global genomic repair (GGR) for lesions within less frequently transcribed genomic regions(Bartek et al., 2001). p53 is thought to participate in DNA repair via multiple mechanisms, including control of cell cycle checkpoint activity as well as regulating components of the DNA repair machinery.
Apoptosis is an important tumor suppressor function of p53 (reviewed in (Vousden, 2000)). The discovery of p53 as a regulator of keratinocyte apoptosis following UV irradiation in skin represented one of the early demonstrations of p53′s important role as an apoptosis regulator(Ziegler et al., 1993, Ziegler et al., 1994) . Numerous factors have been suggested to reside downstream of p53 in its apoptotic response, and these may also vary between cell-types. For example, p53′s ability to regulate the physiologic tanning response involves its transcriptional induction of the POMC gene via a mechanism which appears to occur within keratinocytes, but not a variety of non-keratinocyte cell types(Cui et al., 2007).
The loss of p53 is thought to confer a survival advantage to UV-damaged cells. Lesions with mutant p53 are readily found in UV-exposed, hairless mouse skin(Berg et al., 1996, Jonason et al., 1996) and sun-exposed healthy human skin(Jonason et al., 1996), suggesting that UV mutagenesis is a common consequence of exposure to the sun (or other UV sources, such as indoor tanning), likely correlating with the high incidence of skin cancer among humans.
UV and tanning
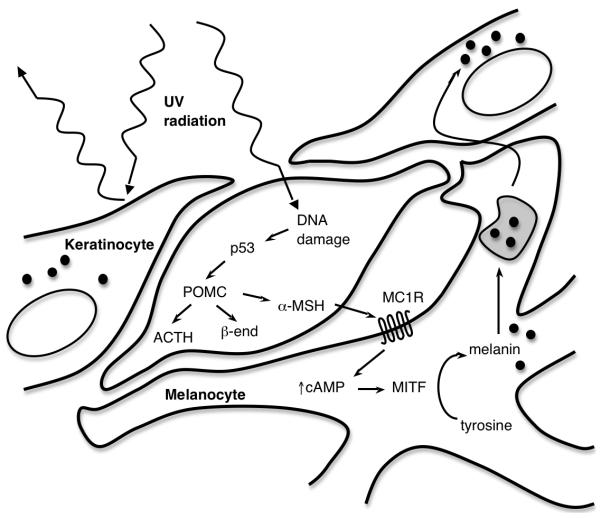
The same stimulus that commonly induces DNA damage in the skin — ultraviolet radiation — also triggers the tanning pathway, a response which may provide some degree of protection from subsequent UV exposure (though not the primary mutagenic UV exposure). This pathway appears to involve cross-talk between keratinocytes and melanocytes, and results in the transfer of melanin-containing melanosomes into the more superficially located keratinocytes, where the pigment forms a “cap” over the sun-exposed surface of the nucleus (Duval et al., 2001, Yamaguchi et al., 2006).
In keratinocytes, UV-mediated DNA damage activates p53, which then binds and activates the pro-opiomelanocortin (POMC) gene (Cui et al., 2007). The POMC polypeptide is post-translationally cleaved, producing adrenocorticotropic hormone, α-melanocyte-stimulating hormone (α-MSH), and β-endorphin. Secreted keratinocyte-derived α-MSH then signals to melanocytes via the melanocortin 1 receptor (MC1R), a G protein-coupled receptor(D’Orazio et al., 2006). If signaling through this receptor is disrupted, tanning does not occur, as in red-haired individuals who harbor loss-of-function polymorphisms of MC1R and burn in response to sun exposure without tanning(Valverde et al., 1995).
In the melanocyte, signaling through MC1R triggers a cascade of events, beginning with the activation of adenylate cyclase. The resulting increase in intracellular cAMP induces expression of microphthalmia-associated transcription factor (MITF) through the binding of a cAMP-responsive element within the MITF promoter(Bertolotto et al., 1998, Price et al., 1998). MITF itself then transcriptionally activates a set of genes which catalyzes the conversion of tyrosine into melanin(Levy et al., 2006).
It is noteworthy that the induction of POMC leads not only to elevated expression of α-MSH, but also of β-endorphin, an opioid peptide(Cui et al., 2007). Because opioid receptor activation is thought to elicit a variety of behavioral and mood-related effects, βendorphin expression in response to UV exposure may underlie, and, in fact, reinforce UV-seeking behavior. Indeed, treatment of frequent tanners with an opioid antagonist has been suggested to cause symptoms of opioid withdrawal(Kaur et al., 2006), suggesting that tanning can be motivated by both cosmetic and psychological factors, both of which relate to POMC gene transcription. It is an interesting thought then, that sun-seeking behavior mimics addictive traits in patients using controlled substances, and this may be important in prevention and education.
UV and cutaneous malignancy
There are few lingering doubts from epidemiological data as well as scientific information regarding the massive risk UVR imposes on development of skin cancer. Skin cancer is the most common malignancy in the US with well over 1 million cases of non melanoma skin cancers as well as over 60,000 cases of malignant melanoma estimated for 2007 (melanoma in situ will account for an additional 46,000 cases)(Moan et al., 2008, Dennis, 1999), with skin cancer accounting for more than 50% of all malignancies(Ibrahim and Brown, 2008). The World Health Organization has estimated that in the year 2000, up to 71,000 deaths worldwide were attributable to excessive UV exposure(WHO, 2006). The incidence of skin cancer continues to rise faster than that of any other cancer, with the lifetime risk for an American to develop melanoma estimated to have increased approximately 2000% in the past 75 years(Dennis, 1999). Although several associations have been established for skin cancer risk, such as skin phototype, immune response, viral infection, and genetic background, nonetheless solar UVR is broadly accepted to be the main initiator and promoter of skin cancer, particularly basal cell carcinoma (BCC) and squamous cell carcinoma (SCC)(Gallagher and Lee, 2006). For SCC, the weight of evidence points towards “chronic” or total exposure. Among women under 40, the rate of BCC has tripled in the past 30 years, while that of SCC has quadrupled(Marks et al., 1988). At current rates, 1 in 5 people in the United States will develop a skin cancer of some sort during their lifetime(Levine et al., 2005), with more than 1 million new cases appearing in 2008 alone. The life-time risk in the US of invasive melanoma has increased from 1:1500 in 1935 to 1 in 63 for invasive melanomas and 1 in 33 if in situ melanoma is included, in 2007(Rigel, 2008). In the USA, melanoma is the most common form of cancer in young adults 25-29 years old(SEER); and the 2nd most common cancer in adolescents and young adults 15-29 years old(AAD, SEER). One American dies from melanoma almost every hour (every 62 minutes)(AAD). Melanoma incidence has most strongly and consistently been associated with reported “intermittent sun exposure” mostly accrued through recreational activities(Walter et al., 1999, Gallagher and Lee, 2006). Although melanoma accounts for only 5% of total cutaneous malignancy, it is responsible for approximately 75-80% of skin cancer-related deaths(Ibrahim and Brown, 2008, ACS). Among other factors, risk of skin cancer increases with artificial UV exposure in tanning salons(2007), average annual UVR(Armstrong and Kricker, 2001), and latitude, with a direct correlation with BCC and SCC with latitude(Muir et al., 1987, Scotto et al., 1983). A definitive analysis was recently presented, which reviewed multiple studies on indoor tanning use and risk of melanoma as well as non-melanoma skin cancers(2007). This study revealed a 75% increased risk of melanoma for individuals who had first use of a tanning bed prior to age 35 as well as a significant increased risk of melanoma in the “ever” vs. “never” indoor tanning group (Relative risk (RR), 1.15; Confidence interval (CI), 1.00–1.31). The meta-analysis also suggested a significant effect of exposure to indoor tanning appliances for SCC, but not for BCC (RR, 2.25; CI, 1.08–4.70). The risks for BCC and SCC were also noted to be related to age of first use, with the risk increasing by 10% (Odds ratio (OR), 1.1; CI, 0.9–1.5) and 20% (OR, 1.2; CI, 0.9–1.6) respectively for each decade younger the person was at first use of indoor tanning equipment. Although the relative risk figures are lower (though still statistically significant) for melanoma as compared to non-melanoma skin cancer risk, there has been a tendency to ignore non-melanoma skin cancer as a deleterious consequence of recreational UVR. This is a dangerous omission because non-melanoma skin cancer (especially SCC) has a clear metastatic propensity, albeit lower than melanoma, and there are thousands of deaths from non-melanoma skin cancer each year in the US(Ibrahim and Brown, 2008).
Vitamin D
1,25-dihydroxyvitamin D3 [1,25(OH)(2)D(3)] is the biologically active vit D metabolite. Our body can acquire vit D both by UVB-induced conversion of 7-dehydrocholesterol in the skin to pre-vit D or through intake of food and nutritional supplements. Pre-vit D undergoes isomerization, yielding vit D, which is hydroxylated in the liver to the active agent 1,25(OH)2 vit D (Holick et al., 1980). Unfortunately, the UVB spectrum that produces sunburn, suntan, and epidermal DNA damage peaks at approximately 290-300 nm while the action spectrum for vit D synthesis is extremely similar, peaking at 300nm(Gilchrest, 2007). Whereas UV photoproducts are linearly associated with UVB dose over a wide range, vit D photosynthesis is balanced by conversion of pre-vit D to inactive photoproducts, lumisterol and tachysterol, such that the concentration of pre-vit D reaches maximum exposure after a relatively short UV exposure, less than one minimal erythemal dose (MED). Given the similar range of action, it is not possible to have vit D synthesis without being exposed to the same UVB spectrum(Gilchrest, 2007, Wolpowitz and Gilchrest, 2006). This translates to a tanning salon patron receiving 4.5-7 times the amount of UVB radiation needed for vit D production in addition to the UVA exposure in one 20 minutes tanning session(Levine et al., 2005).
Considerable interest has been expressed recently on the potential beneficial effects of vit D in relation to various malignancies(Giovannucci, 2005, Holick, 2008, Grant and Garland, 2008, Grant, 2008, Khazai et al., 2008, Skinner, 2008, Garland et al., 2006, Freedman et al., 2007). In addition, it has become increasingly clear that vit D deficiency is relatively common, particularly in the elderly and in human populations residing at relatively high latitudes. While a complete review of the evidence supporting (and refuting) the cancer-risk associations is beyond the scope of this review, it is fair to state that intriguing associations have been observed and considerably greater analysis (particularly prospective randomized trials) will be needed to draw solid conclusions.
The tanning industry has stirred up an apparent “controversy” in this area, whereas it is unclear that an important controversy exists. It has touted the benefits of tanning and vit D over the risk of cutaneous malignancy and encouraged tanning behavior, in framing the question as the need to choose the lesser of two evils: skin cancer and photoaging versus cancer of various internal organs and/or the long list of other ailments(Gilchrest, 2007, UVFoundation). While vit D may indeed have anti-cancer beneficial effects, there is no benefit to utilizing UVR as the vehicle to boost vit D levels. Oral vit D supplements are routinely (and increasingly) prescribed by internists to patients with laboratory-proven vit D deficiency. The use of unreliable dose-relationships between UV and vit D production (either from sunlight or indoor tanning) to boost skin-produced vit D in the blood is medically dangerous due to the known carcinogenic risk in skin, with several of those skin cancers producing measurable risk of cancer lethality. Whereas the highest risk group for vit D deficiency is in the elderly as well as darkly pigmented individuals, the group that is most attracted to sun bathing and is the subject of the tanning industry’s attention is healthy Caucasian teenagers and young adults, including many fair-skinned individuals who tan poorly, a group that is at the lowest risk of vit D insufficiency and yet at the greatest risk of long-term photodamage(Swerdlow and Weinstock, 1998, Gilchrest, 2007). Despite the importance of adequate vit D levels, the amount of sunlight needed to produce sufficient vit D is small and does not justify the need for tanning beds. There has been research supporting this concept, including a 6-year study of 6 patients with xeroderma pigmentosum that found mean levels of 25-hydroxyvitamin D were in the low normal range and levels of 1,25-dihydoxyvitamin D, calcium, ionized calcium, and parathyroid hormone were all in the normal range despite meticulous sun avoidance in this UV-hypersensitive population(Sollitto et al., 1997).
Conclusions
The skin is a remarkable organ, subjected to numerous insults from the environment and yet able to perform many vital functions. UVR exerts a remarkable effect on the skin, affecting the immune system as well as causing DNA damage, photoaging, cancer, and pigmentary changes through biologically complex mechanisms that still are under investigation. There has been increasing evidence supporting the mutagenic and carcinogenic effects of UVB and now also UVA in the skin, necessitating the need for public education and increased vigilance regarding the potential harmful effects of excessive UV exposure. Research in the molecular biology of UV-pigmentation has linked tanning and β-endorphin production, which may induce positive mood, possibly underlying UV-seeking behavior. The initiating lesion for tanning appears to be the same mutagenic and carcinogenic target (DNA) as for skin cancers, suggesting that it may be impossible to uncouple the two. Thus “safe tanning” with UV may be a physical impossibility. Vit D remains an intriguing aspect of modern medical research, but its blood levels may be easily maintained with oral supplementation, thereby avoiding use of a carcinogen (UV) to replace an oral vitamin. Taken together, UVR exposure represents one of the most avoidable causes of cancer risk and mortality in man. Whereas genetic and other factors undoubtedly contribute importantly to skin cancer risk, the role of UV is incontrovertible, and efforts to confuse the public, particularly for purposes of economic gain by the indoor tanning industry, should be vigorously combated for the public health.
Acknowledgement
The authors gratefully acknowledge useful comments from Drs. Marianne Berwick and Dot Bennett. DEF is Distinguished Clinical Scholar of the Doris Duke Medical Research Foundation and acknowledges research support from NIH.
References
- The association of use of sunbeds with cutaneous malignant melanoma and other skin cancers: A systematic review. Int J Cancer. 2007;120:1116–22. doi: 10.1002/ijc.22453. [DOI] [PubMed] [Google Scholar]
- AAD The American Academy of Dermatology 2008 Melanoma Fact Sheet. http://www.aad.org/media/background/factsheets/fact_melanoma.html.
- ABDULLA FR, FELDMAN SR, WILLIFORD PM, KROWCHUK D, KAUR M. Tanning and skin cancer. Pediatr Dermatol. 2005;22:501–12. doi: 10.1111/j.1525-1470.2005.00129.x. [DOI] [PubMed] [Google Scholar]
- ACS The American Cancer Society Detailed Guide: Skin Cancer - Melanoma What Are the Key Statistics About Melanoma? http://www.cancer.org/docroot/CRI/content/CRI_2_4_1X_What_are_the_key_sta tistics_for_melanoma_50.asp?sitearea=&level=
- AHN J, PETERS U, ALBANES D, PURDUE MP, ABNET CC, CHATTERJEE N, HORST RL, HOLLIS BW, HUANG WY, SHIKANY JM, et al. Serum vitamin D concentration and prostate cancer risk: a nested case-control study. J Natl Cancer Inst. 2008;100:796–804. doi: 10.1093/jnci/djn152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ALBERT MR, OSTHEIMER KG. The evolution of current medical and popular attitudes toward ultraviolet light exposure: part 1. J Am Acad Dermatol. 2002;47:930–7. doi: 10.1067/mjd.2002.127254. [DOI] [PubMed] [Google Scholar]
- ALBERT MR, OSTHEIMER KG. The evolution of current medical and popular attitudes toward ultraviolet light exposure: part 2. J Am Acad Dermatol. 2003a;48:909–18. doi: 10.1067/mjd.2003.272. [DOI] [PubMed] [Google Scholar]
- ALBERT MR, OSTHEIMER KG. The evolution of current medical and popular attitudes toward ultraviolet light exposure: part 3. J Am Acad Dermatol. 2003b;49:1096–106. doi: 10.1016/s0190-9622(03)00021-5. [DOI] [PubMed] [Google Scholar]
- ARMSTRONG BK, KRICKER A. The epidemiology of UV induced skin cancer. J Photochem Photobiol B. 2001;63:8–18. doi: 10.1016/s1011-1344(01)00198-1. [DOI] [PubMed] [Google Scholar]
- BALK SJ, GELLER AC. Teenagers and Artificial Tanning. Pediatrics. 2008;121:1040–1042. doi: 10.1542/peds.2007-2256. [DOI] [PubMed] [Google Scholar]
- BARTEK J, FALCK J, LUKAS J. CHK2 kinase--a busy messenger. Nat Rev Mol Cell Biol. 2001;2:877–86. doi: 10.1038/35103059. [DOI] [PubMed] [Google Scholar]
- BERG RJ, VAN KRANEN HJ, REBEL HG, DE VRIES A, VAN VLOTEN WA, VAN KREIJL CF, VAN DER LEUN JC, DE GRUIJL FR. Early p53 alterations in mouse skin carcinogenesis by UVB radiation: immunohistochemical detection of mutant p53 protein in clusters of preneoplastic epidermal cells. Proc Natl Acad Sci U S A. 1996;93:274–8. doi: 10.1073/pnas.93.1.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BERTOLOTTO C, ABBE P, HEMESATH TJ, BILLE K, FISHER DE, ORTONNE JP, BALLOTTI R. Microphthalmia gene product as a signal transducer in cAMP-induced differentiation of melanocytes. J Cell Biol. 1998;142:827–35. doi: 10.1083/jcb.142.3.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BLUM H, KIRBY-SMITH J, HG G. Quantitative induction of tumors in mice with ultraviolet radiation. J Natl Cancer Inst. 1941;2:259–268. [Google Scholar]
- BRASH DE, HASELTINE WA. UV-induced mutation hotspots occur at DNA damage hotspots. Nature. 1982;298:189–92. doi: 10.1038/298189a0. [DOI] [PubMed] [Google Scholar]
- BRASH DE, RUDOLPH JA, SIMON JA, LIN A, MCKENNA GJ, BADEN HP, HALPERIN AJ, PONTEN J. A role for sunlight in skin cancer: UV-induced p53 mutations in squamous cell carcinoma. Proc Natl Acad Sci U S A. 1991;88:10124–8. doi: 10.1073/pnas.88.22.10124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRASH DE, ZIEGLER A, JONASON AS, SIMON JA, KUNALA S, LEFFELL DJ. Sunlight and sunburn in human skin cancer: p53, apoptosis, and tumor promotion. J Investig Dermatol Symp Proc. 1996;1:136–42. [PubMed] [Google Scholar]
- CUI R, WIDLUND HR, FEIGE E, LIN JY, WILENSKY DL, IGRAS VE, D’ORAZIO J, FUNG CY, SCHANBACHER CF, GRANTER SR, et al. Central role of p53 in the suntan response and pathologic hyperpigmentation. Cell. 2007;128:853–64. doi: 10.1016/j.cell.2006.12.045. [DOI] [PubMed] [Google Scholar]
- D’ORAZIO JA, NOBUHISA T, CUI R, ARYA M, SPRY M, WAKAMATSU K, IGRAS V, KUNISADA T, GRANTER SR, NISHIMURA EK, et al. Topical drug rescue strategy and skin protection based on the role of Mc1r in UV-induced tanning. Nature. 2006;443:340–4. doi: 10.1038/nature05098. [DOI] [PubMed] [Google Scholar]
- DEMKO CA, BORAWSKI EA, DEBANNE SM, COOPER KD, STANGE KC. Use of Indoor Tanning Facilities by White Adolescents in the United States. Arch Pediatr Adolesc Med. 2003;157:854–860. doi: 10.1001/archpedi.157.9.854. [DOI] [PubMed] [Google Scholar]
- DENNIS LK. Increasing risk of melanoma with increasing age. Jama. 1999;282:1037–8. doi: 10.1001/jama.282.11.1037-a. [DOI] [PubMed] [Google Scholar]
- DUVAL C, REGNIER M, SCHMIDT R. Distinct melanogenic response of human melanocytes in mono-culture, in co-culture with keratinocytes and in reconstructed epidermis, to UV exposure. Pigment Cell Res. 2001;14:348–55. doi: 10.1034/j.1600-0749.2001.140506.x. [DOI] [PubMed] [Google Scholar]
- FINDLAY GM. Ultra-violet Light and Skin Cancer. The Lancet. 1928;212:1070–1073. [Google Scholar]
- FREEDMAN DM, LOOKER AC, CHANG SC, GRAUBARD BI. Prospective study of serum vitamin D and cancer mortality in the United States. J Natl Cancer Inst. 2007;99:1594–602. doi: 10.1093/jnci/djm204. [DOI] [PubMed] [Google Scholar]
- FREEMAN S, FRANCIS S, LUNDAHL K, BOWLAND T, DELLAVALLE RP. UV Tanning Advertisements in High School Newspapers. Arch Dermatol. 2006;142:460–462. doi: 10.1001/archderm.142.4.460. [DOI] [PubMed] [Google Scholar]
- GAILANI MR, LEFFELL DJ, ZIEGLER A, GROSS EG, BRASH DE, BALE AE. Relationship between sunlight exposure and a key genetic alteration in basal cell carcinoma. J Natl Cancer Inst. 1996;88:349–54. doi: 10.1093/jnci/88.6.349. [DOI] [PubMed] [Google Scholar]
- GALLAGHER RP, LEE TK. Adverse effects of ultraviolet radiation: a brief review. Prog Biophys Mol Biol. 2006;92:119–31. doi: 10.1016/j.pbiomolbio.2006.02.011. [DOI] [PubMed] [Google Scholar]
- GARLAND CF, GARLAND FC, GORHAM ED, LIPKIN M, NEWMARK H, MOHR SB, HOLICK MF. The role of vitamin D in cancer prevention. Am J Public Health. 2006;96:252–61. doi: 10.2105/AJPH.2004.045260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GELLER AC, COLDITZ G, OLIVERIA S, EMMONS K, JORGENSEN C, AWEH GN, FRAZIER AL. Use of Sunscreen, Sunburning Rates, and Tanning Bed Use Among More Than 10 000 US Children and Adolescents. Pediatrics. 2002;109:1009–1014. doi: 10.1542/peds.109.6.1009. [DOI] [PubMed] [Google Scholar]
- GIGLIA G, DUMAZ N, DROUGARD C, AVRIL MF, DAYA-GROSJEAN L, SARASIN A. p53 mutations in skin and internal tumors of xeroderma pigmentosum patients belonging to the complementation group C. Cancer Res. 1998;58:4402–9. [PubMed] [Google Scholar]
- GILCHREST BA. Sun protection and Vitamin D: three dimensions of obfuscation. J Steroid Biochem Mol Biol. 2007;103:655–63. doi: 10.1016/j.jsbmb.2006.12.028. [DOI] [PubMed] [Google Scholar]
- GIOVANNUCCI E. The epidemiology of vitamin D and cancer incidence and mortality: a review (United States) Cancer Causes Control. 2005;16:83–95. doi: 10.1007/s10552-004-1661-4. [DOI] [PubMed] [Google Scholar]
- GRANT WB. Solar ultraviolet irradiance and cancer incidence and mortality. Adv Exp Med Biol. 2008;624:16–30. doi: 10.1007/978-0-387-77574-6_2. [DOI] [PubMed] [Google Scholar]
- GRANT WB, GARLAND CF. The health benefits of vitamin D greatly outweigh the health risks. Bioessays. 2008;30:506–7. doi: 10.1002/bies.20753. author reply 510-1. [DOI] [PubMed] [Google Scholar]
- HALL A. Relationships of sunlight, complexion and heredity to skin carcinogenesis. Arch Dermatol Syph. 1950;61:589–610. [Google Scholar]
- HALLIDAY GM. Inflammation, gene mutation and photoimmunosuppression in response to UVR-induced oxidative damage contributes to photocarcinogenesis. Mutat Res. 2005;571:107–20. doi: 10.1016/j.mrfmmm.2004.09.013. [DOI] [PubMed] [Google Scholar]
- HOLICK MF. Vitamin D and Sunlight: Strategies for Cancer Prevention and Other Health Benefits. Clin J Am Soc Nephrol. 2008 doi: 10.2215/CJN.01350308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOLICK MF, MACLAUGHLIN JA, CLARK MB, HOLICK SA, POTTS JT, JR., ANDERSON RR, BLANK IH, PARRISH JA, ELIAS P. Photosynthesis of previtamin D3 in human skin and the physiologic consequences. Science. 1980;210:203–5. doi: 10.1126/science.6251551. [DOI] [PubMed] [Google Scholar]
- IBRAHIM SF, BROWN MD. Tanning and cutaneous malignancy. Dermatol Surg. 2008;34:460–74. doi: 10.1111/j.1524-4725.2007.34092.x. [DOI] [PubMed] [Google Scholar]
- ITA The Indoor Tanning Association http://www.theita.com/indoor/PositiveEffects_ofUV.cfm.
- JIVESKOG S, RAGNARSSON-OLDING B, PLATZ A, RINGBORG U. N-ras mutations are common in melanomas from sun-exposed skin of humans but rare in mucosal membranes or unexposed skin. J Invest Dermatol. 1998;111:757–61. doi: 10.1046/j.1523-1747.1998.00376.x. [DOI] [PubMed] [Google Scholar]
- JONASON AS, KUNALA S, PRICE GJ, RESTIFO RJ, SPINELLI HM, PERSING JA, LEFFELL DJ, TARONE RE, BRASH DE. Frequent clones of p53-mutated keratinocytes in normal human skin. Proc Natl Acad Sci U S A. 1996;93:14025–9. doi: 10.1073/pnas.93.24.14025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAUR M, LIGUORI A, LANG W, RAPP SR, FLEISCHER AB, JR., FELDMAN SR. Induction of withdrawal-like symptoms in a small randomized, controlled trial of opioid blockade in frequent tanners. J Am Acad Dermatol. 2006;54:709–11. doi: 10.1016/j.jaad.2005.11.1059. [DOI] [PubMed] [Google Scholar]
- KHAZAI N, JUDD SE, TANGPRICHA V. Calcium and vitamin D: skeletal and extraskeletal health. Curr Rheumatol Rep. 2008;10:110–7. doi: 10.1007/s11926-008-0020-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KIELBASSA C, ROZA L, EPE B. Wavelength dependence of oxidative DNA damage induced by UV and visible light. Carcinogenesis. 1997;18:811–6. doi: 10.1093/carcin/18.4.811. [DOI] [PubMed] [Google Scholar]
- LATONEN L, LAIHO M. Cellular UV damage responses--functions of tumor suppressor p53. Biochim Biophys Acta. 2005;1755:71–89. doi: 10.1016/j.bbcan.2005.04.003. [DOI] [PubMed] [Google Scholar]
- LEVINE JA, SORACE M, SPENCER J, SIEGEL DM. The indoor UV tanning industry: a review of skin cancer risk, health benefit claims, and regulation. J Am Acad Dermatol. 2005;53:1038–44. doi: 10.1016/j.jaad.2005.07.066. [DOI] [PubMed] [Google Scholar]
- LEVY C, KHALED M, FISHER DE. MITF: master regulator of melanocyte development and melanoma oncogene. Trends Mol Med. 2006;12:406–14. doi: 10.1016/j.molmed.2006.07.008. [DOI] [PubMed] [Google Scholar]
- LU L, QIU J, LIU S, LUO W. Vitamin D3 analogue EB1089 inhibits the proliferation of human laryngeal squamous carcinoma cells via p57. Mol Cancer Ther. 2008;7:1268–74. doi: 10.1158/1535-7163.MCT-07-2222. [DOI] [PubMed] [Google Scholar]
- MARKS R, RENNIE G, SELWOOD T. The relationship of basal cell carcinomas and squamous cell carcinomas to solar keratoses. Arch Dermatol. 1988;124:1039–42. [PubMed] [Google Scholar]
- MARROT L, MEUNIER JR. Skin DNA photodamage and its biological consequences. J Am Acad Dermatol. 2008;58:S139–48. doi: 10.1016/j.jaad.2007.12.007. [DOI] [PubMed] [Google Scholar]
- MOAN J, POROJNICU AC, DAHLBACK A. Ultraviolet radiation and malignant melanoma. Adv Exp Med Biol. 2008;624:104–16. doi: 10.1007/978-0-387-77574-6_9. [DOI] [PubMed] [Google Scholar]
- MOURET S, BAUDOUIN C, CHARVERON M, FAVIER A, CADET J, DOUKI T. Cyclobutane pyrimidine dimers are predominant DNA lesions in whole human skin exposed to UVA radiation. Proc Natl Acad Sci U S A. 2006;103:13765–70. doi: 10.1073/pnas.0604213103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOYSAN A, CLEMENT-LACROIX P, MICHEL L, DUBERTRET L, MORLIERE P. Effects of ultraviolet A and antioxidant defense in cultured fibroblasts and keratinocytes. Photodermatol Photoimmunol Photomed. 1996;11:192–7. doi: 10.1111/j.1600-0781.1995.tb00168.x. [DOI] [PubMed] [Google Scholar]
- MUIR C, WATERHOUSE J, MACK T, POWELL J, WHELAN S. Cancer incidence in five continents. International Agency for Research on Cancer; Lyon: 1987. [Google Scholar]
- NTTI The National Tanning Training Institute ITA responds to flawed study on links between indoor tanning, skin cancer. http://www.tanningtraining.com/hotnews/61h181519848010.html.
- PIERCEALL WE, KRIPKE ML, ANANTHASWAMY HN. N-ras mutation in ultraviolet radiation-induced murine skin cancers. Cancer Res. 1992;52:3946–51. [PubMed] [Google Scholar]
- PILLAI S, ORESAJO C, HAYWARD J. Ultraviolet radiation and skin aging: roles of reactive oxygen species, inflammation and protease activation, and strategies for prevention of inflammation-induced matrix degradation - a review. Int J Cosmet Sci. 2005;27:17–34. doi: 10.1111/j.1467-2494.2004.00241.x. [DOI] [PubMed] [Google Scholar]
- PODDA M, TRABER MG, WEBER C, YAN LJ, PACKER L. UV-irradiation depletes antioxidants and causes oxidative damage in a model of human skin. Free Radic Biol Med. 1998;24:55–65. doi: 10.1016/s0891-5849(97)00142-1. [DOI] [PubMed] [Google Scholar]
- PRICE ER, HORSTMANN MA, WELLS AG, WEILBAECHER KN, TAKEMOTO CM, LANDIS MW, FISHER DE. alpha-Melanocyte-stimulating hormone signaling regulates expression of microphthalmia, a gene deficient in Waardenburg syndrome. J Biol Chem. 1998;273:33042–7. doi: 10.1074/jbc.273.49.33042. [DOI] [PubMed] [Google Scholar]
- RAVANAT JL, DOUKI T, CADET J. Direct and indirect effects of UV radiation on DNA and its components. J Photochem Photobiol B. 2001;63:88–102. doi: 10.1016/s1011-1344(01)00206-8. [DOI] [PubMed] [Google Scholar]
- RIGEL DS. Cutaneous ultraviolet exposure and its relationship to the development of skin cancer. J Am Acad Dermatol. 2008;58:S129–32. doi: 10.1016/j.jaad.2007.04.034. [DOI] [PubMed] [Google Scholar]
- SCHWARZ A, MAEDA A, KERNEBECK K, VAN STEEG H, BEISSERT S, SCHWARZ T. Prevention of UV radiation-induced immunosuppression by IL-12 is dependent on DNA repair. J Exp Med. 2005;201:173–9. doi: 10.1084/jem.20041212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHWARZ A, MAEDA A, STANDER S, VAN STEEG H, SCHWARZ T. IL-18 reduces ultraviolet radiation-induced DNA damage and thereby affects photoimmunosuppression. J Immunol. 2006;176:2896–901. doi: 10.4049/jimmunol.176.5.2896. [DOI] [PubMed] [Google Scholar]
- SCHWARZ A, MAEDA A, WILD MK, KERNEBECK K, GROSS N, ARAGANE Y, BEISSERT S, VESTWEBER D, SCHWARZ T. Ultraviolet radiation-induced regulatory T cells not only inhibit the induction but can suppress the effector phase of contact hypersensitivity. J Immunol. 2004;172:1036–43. doi: 10.4049/jimmunol.172.2.1036. [DOI] [PubMed] [Google Scholar]
- SCOTTO J, FEARS TR, FARAUMENI JF. In: Incidence of nonmelanoma skin cancer in the United States. US DEPARTMENT OF HEALTH AND HUMAN SERVICES; W. D., editor. 1983. [Google Scholar]
- SEER Cancer Epidemiology in Older Adolescents and Young Adults 15 to 29 Years of Age, Including SEER Incidence and Survival: 1975-2000. Melanoma. http://seer.cancer.gov/publications/aya/5_melanoma.pdf.
- SKINNER HG. Vitamin D for the treatment and prevention of pancreatic cancer. Cancer Biol Ther. 2008;7 doi: 10.4161/cbt.7.3.5585. [DOI] [PubMed] [Google Scholar]
- SOLLITTO RB, KRAEMER KH, DIGIOVANNA JJ. Normal vitamin D levels can be maintained despite rigorous photoprotection: six years’ experience with xeroderma pigmentosum. J Am Acad Dermatol. 1997;37:942–7. doi: 10.1016/s0190-9622(97)70069-0. [DOI] [PubMed] [Google Scholar]
- STOLZENBERG-SOLOMON RZ. Vitamin D and Pancreatic Cancer. Ann Epidemiol. 2008 doi: 10.1016/j.annepidem.2008.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SWERDLOW AJ, WEINSTOCK MA. Do tanning lamps cause melanoma? An epidemiologic assessment. J Am Acad Dermatol. 1998;38:89–98. doi: 10.1016/s0190-9622(98)70544-4. [DOI] [PubMed] [Google Scholar]
- SZABO C, ZINGARELLI B, O’CONNOR M, SALZMAN AL. DNA strand breakage, activation of poly (ADP-ribose) synthetase, and cellular energy depletion are involved in the cytotoxicity of macrophages and smooth muscle cells exposed to peroxynitrite. Proc Natl Acad Sci U S A. 1996;93:1753–8. doi: 10.1073/pnas.93.5.1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TIMARES L, KATIYAR SK, ELMETS CA. DNA damage, apoptosis and langerhans cells--Activators of UV-induced immune tolerance. Photochem Photobiol. 2008;84:422–36. doi: 10.1111/j.1751-1097.2007.00284.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TYRRELL RM. The molecular and cellular pathology of solar ultraviolet radiation. Mol Aspects Med. 1994;15:1–77. [PubMed] [Google Scholar]
- ULLRICH SE. Mechanisms underlying UV-induced immune suppression. Mutat Res. 2005;571:185–205. doi: 10.1016/j.mrfmmm.2004.06.059. [DOI] [PubMed] [Google Scholar]
- UVFOUNDATION The UV Foundation is committed to funding educational efforts designed to increase the public awareness of the biologic effects of ultraviolet light. http://www.uvfoundation.org/
- VALVERDE P, HEALY E, JACKSON I, REES JL, THODY AJ. Variants of the melanocyte-stimulating hormone receptor gene are associated with red hair and fair skin in humans. Nat Genet. 1995;11:328–30. doi: 10.1038/ng1195-328. [DOI] [PubMed] [Google Scholar]
- VOUSDEN KH. p53: death star. Cell. 2000;103:691–4. doi: 10.1016/s0092-8674(00)00171-9. [DOI] [PubMed] [Google Scholar]
- WALTER SD, KING WD, MARRETT LD. Association of cutaneous malignant melanoma with intermittent exposure to ultraviolet radiation: results of a case-control study in Ontario, Canada. Int J Epidemiol. 1999;28:418–27. doi: 10.1093/ije/28.3.418. [DOI] [PubMed] [Google Scholar]
- WHO Global disease burden from solar ultraviolet radiation [monograph on the Internet] 2006 http://www.who.int/mediacentre/factsheets/fs305/en/index.html)
- WOLPOWITZ D, GILCHREST BA. The vitamin D questions: how much do you need and how should you get it? J Am Acad Dermatol. 2006;54:301–17. doi: 10.1016/j.jaad.2005.11.1057. [DOI] [PubMed] [Google Scholar]
- YAMAGUCHI Y, TAKAHASHI K, ZMUDZKA BZ, KORNHAUSER A, MILLER SA, TADOKORO T, BERENS W, BEER JZ, HEARING VJ. Human skin responses to UV radiation: pigment in the upper epidermis protects against DNA damage in the lower epidermis and facilitates apoptosis. Faseb J. 2006;20:1486–8. doi: 10.1096/fj.06-5725fje. [DOI] [PubMed] [Google Scholar]
- ZIEGLER A, JONASON AS, LEFFELL DJ, SIMON JA, SHARMA HW, KIMMELMAN J, REMINGTON L, JACKS T, BRASH DE. Sunburn and p53 in the onset of skin cancer. Nature. 1994;372:773–6. doi: 10.1038/372773a0. [DOI] [PubMed] [Google Scholar]
- ZIEGLER A, LEFFELL DJ, KUNALA S, SHARMA HW, GAILANI M, SIMON JA, HALPERIN AJ, BADEN HP, SHAPIRO PE, BALE AE, et al. Mutation hotspots due to sunlight in the p53 gene of nonmelanoma skin cancers. Proc Natl Acad Sci U S A. 1993;90:4216–20. doi: 10.1073/pnas.90.9.4216. [DOI] [PMC free article] [PubMed] [Google Scholar]