Abstract
Objectives
To evaluate the comparative efficacy and tolerability of topical calcipotriol in the treatment of mild to moderate chronic plaque psoriasis.
Design
Quantitative systematic review of randomised controlled trials.
Subjects
6038 patients with plaque psoriasis reported in 37 trials.
Main outcome measures
Mean difference in percentage change in scores on psoriasis area and severity index, and response rate ratios for both patients' and investigators' overall assessments of marked improvement or better. Adverse effects were estimated with the rate ratio, rate difference, and number needed to treat.
Results
Calcipotriol was at least as effective as potent topical corticosteroids, calcitriol, short contact dithranol, tacalcitol, coal tar, and combined coal tar 5%, allantoin 2%, and hydrocortisone 0.5%. Calcipotriol caused significantly more skin irritation than potent topical corticosteroids (number needed to treat to harm for irritation 10, 95% confidence interval 6 to 34). Calcipotriol monotherapy also caused more irritation than calcipotriol combined with a potent topical corticosteroid (6, 4 to 8). However, the number needed to treat for dithranol to produce lesional or perilesional irritation was 4 (3 to 5). On average, treating 23 patients with short contact dithranol led to one more patient dropping out of treatment owing to adverse effects than if they were treated with calcipotriol.
Conclusions
Calcipotriol is an effective treatment for mild to moderate chronic plaque psoriasis, more so than calcitriol, tacalcitol, coal tar, and short contact dithranol. Only potent topical corticosteroids seem to have comparable efficacy at eight weeks. Although calcipotriol caused more skin irritation than topical corticosteroids this has to be balanced against the potential long term effects of corticosteroids. Skin irritation rarely led to withdrawal of calcipotriol treatment. Longer term comparative trials of calcipotriol versus dithranol and topical corticosteroids are needed to see whether these short term benefits are mirrored by long term outcomes such as duration of remission and improvement in quality of life.
Introduction
Psoriasis affects 1%-2% of the population in the United Kingdom.1,2 Despite the availability of several treatments, psoriasis is often difficult to treat owing to its sporadic course, variable response to treatments, and adverse effects. In the absence of a permanent cure the goal of treatment is to minimise the extent and severity of the condition so that it no longer disrupts substantially the patient's quality of life.3
In recent years calcipotriol, a synthetic vitamin D3 analogue, has become one of the most widely prescribed treatments for psoriasis in the United Kingdom. To assess its usefulness compared with the more traditional topical treatments for psoriasis we undertook a systematic review of trials of topical calcipotriol in the treatment of mild to moderate chronic plaque psoriasis.
Methods
Criteria for considering studies for review
We included only randomised controlled trials of calcipotriol. Patients with chronic plaque psoriasis treated with calcipotriol 0.005% cream or ointment were eligible for inclusion.
The primary efficacy criteria were the proportion of patients showing “marked improvement” or better in the patients' overall assessments and the mean percentage change in scores from baseline on the psoriasis area and severity index.4 The proportion of patients graded as marked improvement or better in the investigators' overall assessments of response was used as a secondary outcome measure. Adverse events were also recorded regarding lesional or perilesional irritation, facial or scalp irritation, exacerbation of psoriasis, and the number of withdrawals due to adverse effects.
Search strategy
We systematically searched (1987 to January 1999) Medline, Embase, the Cochrane controlled trials register, and BIDS index to scientific and technical proceedings using the textwords calcipotriol, MC903, calcipotriene, Dovonex, Daivonex, and Psorcutan. Reference lists of all retrieved randomised controlled trials were also searched and the manufacturer of calcipotriol contacted. Trial eligibility was determined by two authors, who also independently extracted the data. Any disagreements were resolved by discussion. Abstracts were considered; relevant information not included in the published reports was obtained by either contacting the principal author of the trial or the manufacturer.
Methods of the review
We grouped the topical corticosteroids on the basis of their potencies: moderate (clobetasone butyrate 0.05%), potent (betamethasone valerate 0.1%, betamethasone dipropionate 0.1%, desoxymethasone 0.25%, fluocinonide 0.05%, halobetasol 0.05%), and very potent (clobetasol propionate 0.05%, diflorasone diacetate 0.05%).
Outcomes
Dichotomous outcomes—Efficacy was estimated with the rate ratio, defined as the proportion of patients achieving at least marked improvement in the calcipotriol group compared with the control group. Adverse effects were also estimated with the rate ratio, the rate difference, and the number needed to treat. We performed an intention to treat analysis. In all cases we used the Mantel-Haenszel method for interval estimation of the individual rate ratio and rate difference.5
Continuous outcomes—We calculated the mean difference in effect (di) and 95% confidence interval for each trial. When the standard errors or standard deviations and sample sizes were not provided or could not be calculated on the basis of the data reported, we used the pooled interstudy standard error from studies reporting variances.6 In estimating the weighted pooled difference in effect (d), the inverse of the squared standard error (sampling variance) of the difference in response was used as the weight (ωi). For interval estimation and calculation of the 95% confidence interval of the pooled estimates, we used the inverse variance method.7,8
We examined heterogeneity between trials with χ2 tests. Provided no significant heterogeneity was identified, we pooled the summary estimates for the effect from each trial using a fixed effects model. We used the DerSimonian random effects model if P≤0.05 for the test for heterogeneity.7
Results
We identified 62 reports of randomised controlled trials. We included 37 trials, randomising 6038 patients in the analysis (table A on website).w1–37 Of the remaining 25 trials, 12 duplicated results from reports already included9–20 and eight failed to meet our inclusion criteria (non-relevant outcomes,21–24 different concentrations or regimens,25–27 and scalp psoriasis28). A further five reports were excluded: four were abstracts for which we were unable to obtain the necessary patient data29–32 and one had insufficient primary data for analysis.33 In all cases we attempted to obtain the data by contacting the manufacturer and the principal author of the trial.
Comparative efficacy of topical calcipotriol
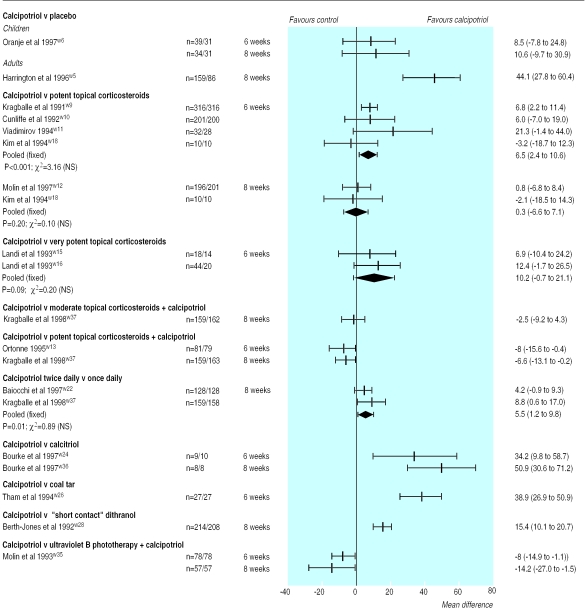
Placebo—Overall, 1185 patients participated in eight placebo controlled trials of calcipotriol. At six and eight weeks calcipotriol was more effective than placebo in adults (figure and figs A and B on website). At eight weeks in one trial the rate ratio was 2.9 (95% confidence interval 1.7 to 5.0) in the patients' overall assessment of response, and the mean difference in the percentage change in scores on the psoriasis area and severity index was 44.1% (27.8% to 60.4%).w5 Based on the results of one trial calcipotriol was no better than placebo in children.w6
Topical corticosteroids—Calcipotriol was significantly more effective than potent topical corticosteroids at six weeks but not at eight weeks. In two trials the pooled mean difference in the percentage change in scores on the psoriasis area and severity index at six weeks was 6.5% (2.4% to 10.6%) (figure).w9 w10 Calcipotriol was also as effective as very potent topical corticosteroids. In two trials the pooled mean difference in the percentage change in psoriasis area and severity index scores was 10.2% (−0.7% to 21.1%).w15 w16 A combination regimen of a potent topical corticosteroid plus calcipotriol, however, proved more effective than calcipotriol monotherapy. In one trial the rate ratio for marked improvement or better in the patients' overall assessment at eight weeks was 0.8 (0.6 to 1.0).w37
Once daily calcipotriol—Two trials compared twice daily and once daily regimens with calcipotriol in 480 patients.w22 w37 Efficacy based on the percentage change in psoriasis area and severity index scores showed superiority for the twice daily regimen; the pooled mean difference in effect was 5.5% (1.2% to 9.8%).
Calcitriol and calcipotriol had a greater effect over twice daily topical calcitriol (figure). At six and eight weeks in one parallel trial, the mean difference in percentage change in psoriasis area and severity index scores was 34.2% (9.8% to 58.7%) and 50.9% (30.6% to 71.2%) respectively.w24
Coal tar—One trial showed that at six weeks topical calcipotriol was superior to coal tar.w26 The results were consistent with those obtained using the patients' and the investigators' overall assessments of at least marked improvement; the corresponding rate ratios were 5.5 (1.3 to 22.7) and 4.3 (1.4 to 13.7).
Coal tar 5%, allantoin 2%, and hydrocortisone 0.5%—From the investigators' overall assessment in a trial of 122 patients the rate ratio for marked improvement or better was 1.5 (1.1 to 2.1), indicating that calcipotriol was significantly better than coal tar 5%, allantoin 2%, and hydrocortisone 0.5% (fig B on website).
“Short contact” dithranol—Calcipotriol was significantly more effective than short contact dithranol on the basis of all three response measures. At eight and 12 weeks the rate ratios for marked improvement or better in the patients' overall assessment were 1.5 (1.3 to 1.7) and 1.3 (1.0 to 1.6) and for the investigators' overall assessment were 1.6 (1.4 to 1.8) and 1.2 (1.0 to 1.5).
Tacalcitol—From the patients' and investigators' overall assessments in one trial, twice daily calcipotriol was more effective than once daily tacalcitol at eight weeks of treatmentw32; both rate ratios were 1.4 (1.1 to 1.8).
Ultraviolet B phototherapy and calcipotriol—No statistical difference was found between the treatments except for scores on the psoriasis area and severity index (figure).
Withdrawal from treatment
Significantly more patients withdrew from placebo compared with calcipotriol (table B on website). Surprisingly, more patients withdrew from treatment with very potent topical corticosteroids. Most patients (32 of 48) dropped out as a result of resolution of their psoriasis. In contrast, short contact dithranol resulted in a significantly higher overall withdrawal rate but also a higher withdrawal rate due to adverse effects of treatment.
Adverse effects
The most common adverse effects were lesional or perilesional irritation, facial or scalp irritation, or exacerbation of psoriasis (table B on website). For every 10 patients treated with calcipotriol one more patient experienced lesional or perilesional irritation than if they were treated with a potent topical corticosteroid. For every six patients treated with calcipotriol alone, one more patient experienced lesional or perilesional irritation than if they were treated with calcipotriol and a moderate or potent topical corticosteroid.
In contrast, short contact dithranol was significantly more irritant than calcipotriol. On average, treating four patients with dithranol led to one more patient experiencing lesional or perilesional irritation than if they were using calcipotriol. Facial or scalp irritation, however, occurred less frequently with dithranol than with calcipotriol.
Sensitivity analysis
Intention to treat and remaining patient analyses led to the same conclusions. With placebo controlled trials we also examined the effect of including one trial of only children treatedw6 and of excluding one trial of a once daily regimen of calcipotriol.w7 No qualitative differences in results were identified except that inclusion of the only paediatric trial showed a significant difference in efficacy on the basis of the investigators' overall assessment of response (table). Exclusion of trials using half body comparisons or those with imputed variances did not change the results qualitatively.
Discussion
Calcipotriol is effective in the treatment of mild to moderate chronic plaque psoriasis. Overall, calcipotriol was superior to calcitriol, coal tar, combined coal tar 5%, allantoin 2%, and hydrocortisone 0.5%, short contact dithranol, and tacalcitol. Comparable effects were seen with potent topical corticosteroids after eight weeks of treatment.
What is already known on this topic
Calcipotriol has become one of the most commonly prescribed antipsoriatic treatments, but until now there has not been any systematic assessment of its usefulness compared with other topical agents for psoriasis
What this study adds
Calcipotriol is a useful drug for treating mild to moderate psoriasis. It seems to be more effective than calcitriol, tacalcitol, coal tar, and short contact dithranol but it is associated with a high frequency of skin irritation
Potent topical steroids are as effective as calcipotriol but this has to be balanced against possible long term systemic effects. The trials have generally been short term, and the advantage of calcipotriol in longer term trials over the cheaper alternatives, particularly regarding improved quality of life and lower relapse rates, has yet to be shown
Implications
Once daily calcipotriol and a potent topical corticosteroid may be more effective and better tolerated than twice daily calcipotriol. One explanation might be that corticosteroids suppress the occurrence of irritation induced by calcipotriol. With long term use, however, topical corticosteroids have the potential to cause side effects such as atrophy, striae, telangiectasia, and masking of local infections.3 Systemic effects cannot be completely ignored.
Lesional or perilesional irritation is common but rarely requires withdrawal of calcipotriol. In contrast, short contact dithranol caused significantly more skin irritation. Importantly this contributed to a statistically higher withdrawal rate from dithranol than from calcipotriol. We did not have sufficient data to determine whether the newer dithranol formulations are any less irritant than the established products.
Limitations
Only one trial included quality of life assessments.w31 European investigators favoured a modified psoriasis area and severity index as the primary measure of efficacy whereas trials conducted in the United States tended to use an investigators' overall assessment of improvement. Previously, we proposed that a minimum core set of outcome measures should be included in all psoriasis clinical trials, and we have acknowledged major limitations with the psoriasis area and severity index.w34
Potential sources of heterogeneity in the results may include the formulation, choice of topical corticosteroid, and patient population (children or adults). Unfortunately, there were too few studies for further exploring these issues. However, calcipotriol seemed more effective in adults than in children, an observation that needs further confirmation.
Future trials should examine efficacy over a much longer period than six to eight weeks (for example, up to six months) to capture the relapsing and remitting nature of the disease. Adverse effects may also not be detected during the short durations of the published randomised controlled trials. Number needed to treat values for adverse reactions were based on data pooled from trials lasting four to 12 weeks. Caution in their interpretation is obviously required.
Conclusions
Pooled data from randomised controlled trials show that calcipotriol is an effective and well tolerated treatment for mild to moderate chronic plaque psoriasis. Although skin irritation is comparatively common, this rarely requires withdrawal of calcipotriol treatment. Potent topical corticosteroids have also emerged as equally effective agents when assessed at eight weeks, with less short term side effects than calcipotriol. Future trials should focus on examining the risk to benefit ratios from combined regimens of calcipotriol with other antipsoriatic agents and include information on quality of life and disease remission.
Supplementary Material
Figure.
Mean (95% confidence interval) differences in percentage change in scores on psoriasis area and severity index from baseline between treatments
Table.
Sensitivity analysis (95% confidence intervals) of type of patients treated in placebo controlled trials at eight weeks
| Patients treated | Mean difference in % change in psoriasis area and severity index | Rate ratio of overall assessment
|
|
|---|---|---|---|
| Patients | Investigators | ||
| Adults | 44.1 (27.8 to 60.4) | 2.9 (1.7 to 5.0) | 4.7 (3.5 to 6.4) |
| Children | 10.6 (−10.1 to 31.3) | 0.8 (0.5 to 1.4) | 1.1 (0.7 to 1.8) |
| Overall | 28.0 (−4.8 to 60.8) | 1.6 (0.5 to 5.3) | 3.6 (1.7 to 7.6) |
Acknowledgments
We thank the following for information and unpublished data: Dr N K Veien, Professor J P Ortonne, Dr J F Bourke, Professor G Landi, Dr L Cresti and Dr D Crippa (Schering), Dr S Lovati, and Dr L Naldi; Mrs I Müller (Leo Pharmaceutical Products); Mr Q D Clarke, Mr P Menday, and Ms C Michell (Leo Pharmaceuticals), Dr A Herxheimer, Dr T F Poyner, Dr W Y Zhang, Dr R T Plott, and Dr S F Levy (Dermik Laboratories), and Dr S B Siskin (Bristol-Myers Squibb).
Footnotes
Funding: A research grant from Boots Healthcare.
Competing interests: DMA is a recipient of a research grant from E Merck Pharmaceuticals who initially marketed tacalcitol in the United Kingdom. ALWP is the recipient of funding for studentships from Leo Pharmaceuticals (manufacturer of calcipotriol) and Merck Lipha. CEMG's department is the beneficiary of research grants from both Leo and E Merck. CEMG has also acted as a consultant to both companies on an ad hoc basis.
Additional figures and tables appear on the BMJ's website
References
- 1.Rea JN, Newhouse ML, Halil T. Skin disease in Lambeth. A community study of prevalence and use of medical care. Br J Prev Soc Med. 1976;30:107–114. doi: 10.1136/jech.30.2.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nevitt GJ, Hutchinson PE. Psoriasis in the community: prevalence, severity and patients' beliefs and attitudes towards the disease. Br J Dermatol. 1996;135:533–537. [PubMed] [Google Scholar]
- 3.Gawkrodger DJ.on behalf of the Therapy Guidelines and Audit Subcommittee of the British Association of Dermatologists. Current management of psoriasis J Dermatol Treat 1997827–55. [Google Scholar]
- 4.Fredriksson T, Pettersson U. Severe psoriasis—oral therapy with a new retinoid. Dermatologica. 1978;157:238–244. doi: 10.1159/000250839. [DOI] [PubMed] [Google Scholar]
- 5.Rothman KJ. Modern epidemiology. Boston: Little, Brown; 1986. pp. 177–233. [Google Scholar]
- 6.Li Wan Po A, Zhang WY. Systematic overview of co-proxamol to assess analgesic effects of addition of dextropropoxyphene to paracetamol. BMJ. 1997;315:1565–1571. doi: 10.1136/bmj.315.7122.1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 8.Whitehead A, Whitehead J. A general parametric approach to the meta-analysis of randomised clinical trials. Stat Med. 1991;10:1665–1677. doi: 10.1002/sim.4780101105. [DOI] [PubMed] [Google Scholar]
- 9.Wehr R, Gibson J, Epinette W, Pincus S, Chesnut C, Goffe B, et al. The safety of topically applied calcipotriene ointment 0.005% (a vitamin D3 analogue) as measured by blood, urine and bone density analysis in psoriatic patients [abstract] Skin Pharmacol. 1992;5:206–207. [Google Scholar]
- 10.Griffiths WAD. Comparison of calcipotriol ointment and betamethasone ointment in the treatment of psoriasis vulgaris (parallel group). Presented at Daivonex, calcipotriol. A totally new treatment in psoriasis [abstract]. Athens, Greece, 1991.
- 11.RT Plott. A double-blind study comparing the efficacy of diflorasone diacetate ointment 0.05% vs calcipotriene ointment 0.05% in the treatment of psoriasis [abstract]. Presented at the 54th annual meeting of the American Academy of Dermatology. Washington, DC;996:P-317.
- 12.Bourke JF, Featherstone S, Iqbal SJ, Hutchinson PE. A double-blind comparison of topical calcitriol (3 μg/g) and calcipotriol (50 μg/g) in the treatment of chronic plaque psoriasis vulgaris [abstract] Br J Dermatol. 1995;133(suppl 45):17. [Google Scholar]
- 13.Scarpa C. Calcipotriol: clinical trial versus betamethasone dipropionate + salicylic acid. Acta Derm Venereol (Stockh) 1994;186(suppl):47. [PubMed] [Google Scholar]
- 14.Hutchinson PE, Berth-Jones J, Chalmers RJG, Chu AC, Griffiths WAD, Klaber MR. A comparison of calcipotriol ointment and short contact dithranol in the treatment of chronic plaque psoriasis [abstract] Br J Dermatol. 1992;127(suppl 40):17. doi: 10.1111/j.1365-2133.1992.tb00126.x. [DOI] [PubMed] [Google Scholar]
- 15.Jurgensen HJ. A comparative study of Dovonex ointment and Dithrocream in treating psoriasis vulgaris [abstract]. Presented at the Dovonex Satellite symposium, 2nd Congress of the European Academy of Dermatology and Venereology. Athens, Greece 1991.
- 16.Lehmann P, Kerscher M. Combination phototherapy of psoriasis with calcipotriene and narrow-band (311 nm) UVB [letter] J Am Acad Dermatol. 1997;36:501–502. doi: 10.1016/s0190-9622(97)80250-2. [DOI] [PubMed] [Google Scholar]
- 17.Kerscher M, Volkenandt M, Plewig G, Lehmann P. Combination phototherapy of psoriasis with calcipotriol and narrow-band UVB [letter] Lancet. 1993;342:923. doi: 10.1016/0140-6736(93)91968-r. [DOI] [PubMed] [Google Scholar]
- 18.Kerscher M, Lehmann P, Plewig G. Combination phototherapy of psoriasis with calcipotriol and narrow-band UVB-light [abstract] J Invest Dermatol. 1996;1:107. doi: 10.1016/0140-6736(93)91968-r. [DOI] [PubMed] [Google Scholar]
- 19.Austad J. A comparative study of calcipotriol ointment in combination with UVB phototherapy and calcipotriol ointment alone in the treatment of psoriasis vulgaris [abstract]. Presented at the 27th Nordic Dermatology Congress. Finland, 1995.
- 20.van de Kerkhof PCM. Calcipotriol cream and concurrent corticosteroids in psoriasis [abstract] J Eur Acad Dermatol Venereol. 1995;5(suppl 1):S184. [Google Scholar]
- 21.Bourke JF, Berth-Jones J, Hutchinson PE. Occlusion enhances the efficacy of topical calcipotriol in the treatment of psoriasis vulgaris. Clin Exp Dermatol. 1993;18:504–506. doi: 10.1111/j.1365-2230.1993.tb01018.x. [DOI] [PubMed] [Google Scholar]
- 22.Berardesca E, Vignoli GP, Farinelli N, Vignini M, Distante F, Rabbiosi G. Non-invasive evaluation of topical calcipotriol versus clobetasol in the treatment of psoriasis. Acta Derm Venereol (Stockh) 1994;74:302–304. doi: 10.2340/0001555574302304. [DOI] [PubMed] [Google Scholar]
- 23.Baadsgaard O, Traulsen J, Roed-Petersen J, Jakobsen HB. Optimal concentration of tacalcitol in once-daily treatment of psoriasis. J Dermatol Treat. 1995;6:145–150. [Google Scholar]
- 24.Austad J. Clobetasol propionate followed by calcipotriol is superior to calcipotriol alone in topical treatment of psoriasis [abstract] Aust J Dermatol. 1997;38(suppl 2):45. [PubMed] [Google Scholar]
- 25.Kragballe K, Beck HI, Søgaard H. Improvement of psoriasis by a topical vitamin D3 analogue (MC903) in a double-blind study. Br J Dermatol. 1988;119:223–230. doi: 10.1111/j.1365-2133.1988.tb03204.x. [DOI] [PubMed] [Google Scholar]
- 26.Staberg B, Roed-Petersen J, Menné T. Efficacy of topical treatment in psoriasis with MC903, a new vitamin D analogue. Acta Derm Venereol (Stockh) 1989;69:147–150. [PubMed] [Google Scholar]
- 27.Schwartzel E, Blum R, Siskin S, Epinette W. A parallel group study evaluating the safety of 30 gram per day dosing of calcipotriene (BMS181161/MC903) ointment and its vehicle in patients with plaque psoriasis [abstract]. Presented at the 54th annual meeting of the American Academy of Dermatology. Washington, DC;1996:P-287.
- 28.Köse O. Calcipotriol ointment vs clobetasol solution in scalp psoriasis [letter] J Dermatol Treat. 1997;8:287. [Google Scholar]
- 29.Mallett RB, Coulson IH, Purkis PE, Leigh IM, Holden CA. An immunohistochemical analysis of the changes in the immune infiltrate and keratin expression in psoriasis treated with calcipotriol compared with betamethasone ointment. Br J Dermatol. 1990;123:837. [Google Scholar]
- 30.Arevalo A, Vega-Lopez F. Calcipotriol versus coal tar in Mexican patients with psoriasis [abstract] J Eur Acad Dermatol Venereol. 1995;5(suppl 1):S92. [Google Scholar]
- 31.Katz HI, Lindholm JS, Wilde M, Siskin S, Wehr RW, Koeowald M, et al. A pilot, double-blind, bilateral, paired comparison of the efficacy, safety, and atrophogenic potential of calcipotriene ointment 0.005% versus its vehicle in the treatment of plaque psoriasis utilizing clinical evaluations and bioinstrumentation [abstract]. Presented at the 54th annual meeting of the American Academy of Dermatology. Washington, DC;1996:P-293.
- 32.Munro CS and study group. Calcipotriol soft cream in treatment of body psoriasis [abstract] J Invest Dermatol. 1996;1:111. [Google Scholar]
- 33.Van der Vleuten CJM, de Jong EMGJ, Rulo EHFC, Gerritsen MP, van de Kerkhof PCM. In-patient treatment with calcipotriol versus dithranol in refractory psoriasis. Eur J Dermatol. 1995;5:676–679. [Google Scholar]
- 34.Ashcroft DM, Li Wan Po A, Williams HC, Griffiths CEM. Clinical measures of disease severity and outcome in psoriasis: a critical appraisal of their quality. Br J Dermatol. 1999;141:185–191. doi: 10.1046/j.1365-2133.1999.02963.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.