Abstract
Few studies have examined underlying mechanisms linking social behavior, motivated behavior, and reward and punishment systems. The current study was designed to investigate these mechanisms by examining responses to both rewarding and punishing non-social stimuli in shy and non-shy adults. Ninety-three participants, comprising three social behavior groups (Shy, Non-shy, Control) completed the Monetary Incentive Delay task. Consistent with previous research, all participants were sensitive to incentive manipulations. There were also significant individual differences in response. Non-shy participants demonstrated sensitivity to both reward and punishment stimuli, and behavior indicative of high levels of arousal in approach motivation. Shy individuals demonstrated a large discrepancy in sensitivity to reward compared to punishment, with this discrepancy being driven by enhanced sensitivity to reward. Their behavior suggested conflict generated by increased arousal in both approach and withdrawal motivation systems. Current findings contribute to theoretical accounts of relations between social behavior and behavior modulated by reward and punishment. These findings carry implications for the study of psychopathology and neuroimaging research designed to examine relationships between social behavior, motivated behavior, and underlying reward and punishment systems.
Keywords: Motivation, Reward, Punishment, Social behavior, Shyness, Anxiety, Behavioral inhibition
1. Introduction
Converging evidence from many disciplines suggests relations between social behavior and behavior modulation by reward or punishment. While different terminology is used across disciplines to describe individuals who exhibit individual differences in social behavior (i.e., extraversion or exuberance versus introversion, shyness, or social anxiety), these individuals generally demonstrate different patterns of sensitivity to potentially rewarding or punishing stimuli. Personality theories suggest that socially outgoing individuals (extraverts) and socially withdrawn individuals (introverts) differ in conditionability and reward sensitivity, reflecting reward dependence effects on social behavior Cloninger, 1987; Eysenck, 1967; Gray, 1970. Reward expectancy is typically high in extraverts and low in introverts, while individuals scoring high in Neuroticism manifest anxiety and sensitivity to punishment Zuckerman, 1991; (Zuckerman, Joireman, Kraft, & Kuhlman, 1999).
Behavioral studies demonstrate that introverts and extraverts differ in response to appetitive compared to aversive stimuli (Corr, 2004). For example, extraverts and introverts demonstrate different patterns of responsivity in the contexts of reward compared to punishment (Nichols & Newman, 1986; Patterson, Kosson, & Newman, 1987). Individuals with social anxiety show both attention and memory biases for threatening, potentially punishing stimuli, particularly when stimuli are social in nature (Monk & Pine, 2004). Similarly, extraverts show biased attention toward rewarding stimuli, while introverts show biases toward punishing stimuli (Derryberry, 1987; Derryberry & Reed, 1994).
Cognitive neuroscience research implicates a number of neural structures that overlap in processing socially relevant, and potentially rewarding and/or punishing stimuli. Human neuroimaging, lesion, and animal studies recognize the amygdala (Baxter & Murray, 2002), ventromedial cortex (Adolphs, 1999), orbitofrontal cortex (Kringelbach & Rolls, 2004), and ventral striatum (Ernst et al., 2004; Knutson, Adams, Fong, & Hommer, 2001; Robbins & Everitt, 1996) as playing roles in processing both reward and social stimuli, and generating behavior in response to these stimuli.
Most neural structures implicated in processing both socially relevant and potentially rewarding or punishing stimuli are likewise implicated in models of neural systems regulating approach or withdrawal motivated behavior in response to appetitive or aversive stimuli (Davidson, Jackson, & Kalin, 2000). The presentation of salient appetitive or aversive stimuli modulate activation of these systems, which subsequently results in approach or withdrawal motivated behavior (Lang, Bradley, & Cuthbert, 1998). Appetitive stimuli will activate approach motivation systems and facilitate approach behavior, whereas aversive stimuli will activate withdrawal motivation systems and facilitate withdrawal behavior. Anxiety and inhibited behavior occur when conflict exists between motivational incentive (i.e., appetitive or aversive) of salient stimuli and behavioral output (i.e., approach or withdrawal) required by the stimuli (McNaughton & Corr, 2004). For example, a stimulus that is interpreted as potentially punishing/threatening (thus energizing the withdrawal system) but requiring an approach behavior, will result in anxiety and delayed response.
Despite the wealth of evidence indicating relations among social behavior, motivated behavior, and reward or punishment systems, few studies examine mechanisms underlying these relationships. Previous work has focused selectively on the role of reward in extraverted, outgoing, exuberant social behavior, or on the role of punishment in introverted, withdrawn, shy social behavior. Most studies designed to examine neural correlates of reward in social behavior have selectively utilized socially relevant stimuli, preventing findings from being interpreted in terms of more general reward processes. The aim of the current study was to begin filling these gaps by examining response to both rewarding and punishing non-social stimuli in socially shy, anxious and non-shy, non-anxious individuals.
The present study utilizes the Monetary Incentive Delay (MID) task to probe reward processing systems. The task is modeled after similar work with non-human primates (Schultz, Apicella, Scarnati, & Ljungberg, 1992; Schultz, Dayan, & Montague, 1997) and has been used in a number of human neuroimaging studies (Knutson et al., 2001; Knutson, Fong, Bennett, Adams, & Hommer, 2003). These studies provide a distinct advantage for the present work, as inferences about neural reward systems can be made from performance characteristics. Levels of activation in the approach or withdrawal systems are indexed by reaction time. In this task, both potentially rewarding and potentially punishing stimuli need to be rapidly approached by button press to obtain a positive outcome.
Based on theoretical and empirical work demonstrating high reward sensitivity in socially outgoing, non-anxious individuals, and high punishment sensitivity in socially withdrawn, anxious individuals, we hypothesized that: (1) As a result of high arousal in response to salient stimuli, shy individuals will show short latencies to potential reward. Because the task paradigm creates an approach—withdrawal conflict for shy individuals by requiring an approach behavior toward a negatively laden stimulus, shy individuals will show a longer latency to potential punishment. Thus, a substantial difference in latency between reward and punishment conditions will exist for shy individuals; (2) As a result of high arousal in approach motivation systems, non-shy individuals will show short latencies (relative to no-incentive) to both potential reward and potential punishment.
2. Methods
2.1. Participants
Participants were 93 (M = 22.3 years, SD = 4.8 years) students at the University of Maryland. Sixty-five (69.9%) participants were female. The study was approved by the University of Maryland Institutional Review Board, and all participants enrolled on a voluntary basis. Prior to participation, each subject met with a research assistant who provided a study description and conducted informed consent. Compensation was provided as class research credit. To increase motivation during the MID task, participants were informed that the top three money winners would receive an additional $20 compensation.
2.2. Procedures
Participants were asked to complete self-report questionnaires and perform the 15 min computer-based MID task. Participants took part in a training and practice session prior to data collection in a quiet lab room on a laptop computer. Participants then completed two 54 trial runs of the MID task. Following the task, participants completed a series of self-report personality assessments.
2.3. Measures
2.3.1. MID task
We used a non-parameterized version of the MID task developed by Knutson et al. (2003). The MID task is a reaction time task, in which participants are required to make a button press during the presentation of a specific target. Motivational contingency (potential reward, potential punishment, or no potential incentive) is manipulated by presenting a cue before target onset. Cues signal the potential for reward ($1 gain), punishment ($1 loss), or no incentive. If the participant succeeds in making a button press during the short time the target is presented, they either receive reward (win money) or avoid punishment (lose money).
The MID task consists of two runs of 54 six-second trials. Each trial consists of one of three possible cues (Cue; 250 ms duration). Cues are followed by a cross-hair fixation point for a variable length of time (Anticipation Period; duration 2000-2500 ms). A solid white square is then presented (Target; duration 160-260 ms). Participants respond to the Target by pressing the spacebar on a computer keyboard. Trial feedback is then presented (Feedback; 1650 ms duration). Feedback informs participants: (1) if they win or lose money based on their preceding response; (2) how much total money they have accumulated to that point in the task. A successful trial (Hit) means the participant is fast enough to respond while the Target is on the computer screen. An unsuccessful trial (Miss) means the participant is not fast enough to respond while the Target is on the computer screen. All task symbols (Cue, Target, Feedback) are presented in the center of the computer screen.
Cues signal three incentive conditions: circles signify potential reward (Reward condition); squares signify potential punishment (Punishment condition); and triangles signify no potential monetary outcome (No-Incentive condition). Each run includes 18 trials of each condition, and trials are randomly ordered. Participants receive $1 for a Hit following a Reward cue, lose $1 for a Miss following a Punishment cue, and neither win nor lose money for a Hit or a Miss following a No-Incentive cue.
Two behavioral dependent variables reflect performance. Accuracy rate (Accuracy) is the percent of trials for which a Hit is recorded. Response reaction time (RT) is the length of time between onset of Target and participant button press. Because of the continuous nature of response reaction time (compared to the dichotomous nature of Hit/Miss accuracy score), RT scores are more sensitive measures of motivation than accuracy rate.
2.3.2. Revised Cheek and Buss Shyness and Sociability Scales (RCBSS)
The CBSS (Cheek & Buss, 1981) is an 18 item self-report questionnaire assessing shy and social behavior. Shyness is measured using the 13-item Revised Cheek and Buss Shyness Scale (Cheek, 1983). A sample item includes “I find it hard to talk to strangers.” Sociability is assessed using the 5-item Cheek and Buss Sociability Scale (Cheek & Buss, 1981). A sample item includes “I like to be with people.” Items are rated on a 5-point scale from 0 (not at all characteristic) to 4 (extremely characteristic). Reliability and validity data for both scales have been previously reported (Leary, 1991).
2.3.3. Eysenck Personality Questionnaire—Revised Short Form (EPQ-RS)
The EPQ-RS (Eysenck & Eysenck, 1991) is a 48 item forced choice (true/false) personality questionnaire assessing three personality dimensions: Extraversion, Neuroticism, and Psychoticism. A sample Extraversion dimension item includes “Do you usually take the initiative in making new friends?” A sample Neuroticism dimension item includes “Do you worry too long after an embarrassing experience?”.
2.3.4. Adult Temperament Questionnaire—Short Form (ATQ)
The ATQ-short form (Rothbart, Ahadi, & Evans, 2000) consists of 77 items assessing a self-report based model of temperament. Five general factors are assessed, including: effortful control, negative affect, extraversion/surgency, and orienting sensitivity. Items from all factors are rated on a scale from 1 (extremely untrue) to 7 (extremely true). Validity data have been reported else-where (Rothbart et al., 2000).
2.4. Social behavior classification
A social behavior composite score was constructed from the self-report personality assessments. Because we were particularly interested in withdrawn social behavior that coincides with high anxiety and/or high perceived social threat (as opposed to the non-anxious desire to be in solitary, non-social environments), social composite scores included factors reflecting worry and anxiety. Social composite scores were not calculated for nine participants because of incomplete self-report data. To construct the social composite scores, six factors were selected from the three original self-report personality assessments. These included: Shyness and Sociability from the RCBSS; Extraversion and Neuroticism from the EPQ-RS; and Sociability and Fear from the ATQ. These factors were included because of theoretical and empirically based relationships with social behavior (Cheek & Buss, 1981; Rothbart et al., 2000; Turner, Johnson, Beidel, Heiser, & Lydiard, 2003), and the ecologically valid nature of individual items constituting each factor. The Neuroticism factor from the EPQ-RS and Fear factor from the ATQ were included because they relate to anxiety, worry, and mood and anxiety disorders (Clark & Watson, 1991; Heiser, Turner, & Beidel, 2003; Zuckerman, Kuhlman, Joireman, & Teta, 1993).
Raw scores from each factor contributing to the composite score were z-normalized. Resulting factor z-scores were summed to form the final social composite score (CBSS Sociability, EPQ-RS Extraversion, and ATQ Sociability contributed negatively to total composite score). Composite scores ranged from -6.88 to +12.45 (M = -.141, SD = 3.39). Greater positive scores reflected more self-reported shyness and anxiety, while greater negative scores reflected more self-reported social exuberance and low anxiety. A relationship with shy, withdrawn social behavior was confirmed by significant correlations between the Shyness factor from the CBSS and the remaining five factors comprising the social behavior composite score (Table 1).
Table 1.
Correlations between self-report personality factors comprising social behavior composite score
| Measure | Shyness | Sociability | ATQ Sociability | ATQ Fear | Neuroticism |
|---|---|---|---|---|---|
| Extraversion | -.43** | +.55** | +.50** | -.15 | -.20 |
| Neuroticism | +.45** | -.03 | +.01 | +.56** | |
| ATQ fear | +.37** | -.11 | +.01 | ||
| ATQ sociability | -.27* | +.54** | |||
| Sociability | -.36** |
p < .05.
p < .001.
Three groups were formed from the social composite scores. A high shy, anxious group (Shy) was comprised of participants whose social composite scores fell in approximately the highest 33% of all scores. A socially outgoing, low anxiety group (Non-shy) was comprised of participants whose social composite scores fell in approximately the lowest 33% of all scores. The remaining 33% of participants formed a control group (Control), as they did not report extreme shy or exuberant behavior (Table 2).
Table 2.
Mean social behavior composite and self-report scores by social behavior group
| Measure | Social grouping |
||
|---|---|---|---|
| Shy | Non-shy | Control | |
| Shyness composite | 3.61** | -3.40 | -.61 |
| CBSS Shyness | 20.91** | 8.75 | 14.25 |
| CBSS Sociability | 12.55** | 17.07 | 15.44 |
| EPQ-RS Extraversion | 19.76** | 23.29 | 22.22 |
| EPQ-RS Neuroticism | 19.94** | 14.64 | 16.89 |
| ATQ Fear | 4.53** | 3.22 | 3.92 |
| ATQ Sociability | 5.19 | 5.61 | 5.78 |
p < .001.
3. Results
3.1. Reaction time
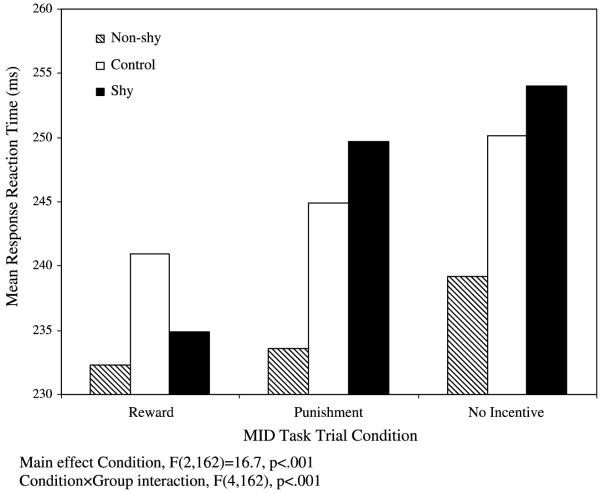
A repeated-measures analysis of variance (ANOVA) on reaction time, with group (Shy, Non-shy, Control) as the between-subjects factor, and condition (Reward, Punishment, Neutral) as the within-subjects factor, revealed a main effect of condition, F(2, 162) = 16.7, p < .001 (Fig. 1). Subjects responded fastest during Reward condition trials (mean RT: Reward = 236.03 ms), slower during Punishment condition trials (mean RT: Threat = 242.74 ms) and slowest during No-Incentive trials (mean RT: No-Incentive = 247.80) (Table 3).
Fig. 1.
Mean RT (ms) for social behavior group (shy, Non-shy, Control) by MID condition (Reward, Punishment, No-incentive).
Table 3.
MID task RT descriptive data by contingency condition
| Group | Mean (SD) reaction time (ms) by contingency condition |
|||
|---|---|---|---|---|
| Total | Reward | Punishment | No incentive | |
| Overall (n = 84) | 242.46 (20.29) | 236.86 (24.97) | 242.80 (24.19) | 249.10 (20.44) |
| Non-shy (n = 28) | 235.04 (25.73) | 232.28 (29.53) | 233.62 (29.40) | 239.23 (27.20) |
| Control (n = 27) | 245.33 (13.12) | 240.91 (13.68) | 244.90 (16.81) | 250.19 (14.76) |
| Shy (n = 29) | 245.75 (19.24) | 234.90 (27.49) | 249.69 (21.44) | 253.99 (16.12) |
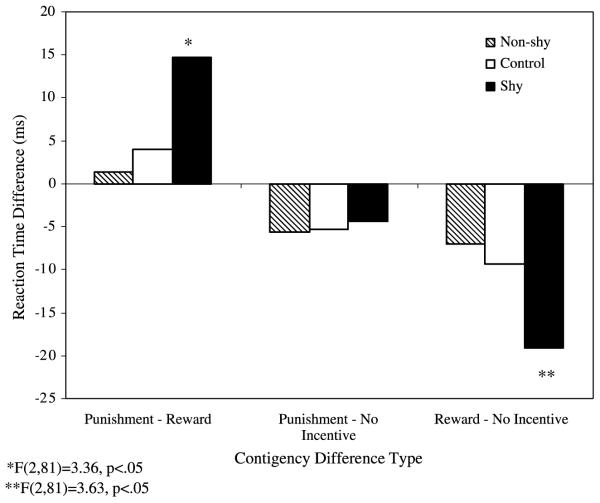
In addition, the Condition × Group interaction was statistically significant, F(4,162) = 2.52, p < .05 (Fig. 1), reflecting a different modulation of RT among groups as a function of condition. As illustrated in Fig. 2, the Shy group showed the strongest modulation by contingencies, particularly for reward trials, with the largest differences in RT between Reward and Punishment conditions. The Non-shy group showed the smallest modulation by contingencies (i.e., smallest RT difference between the Reward and Punishment condition). This group difference in RT between Reward and Punishment conditions was statistically significant among the three groups, F(2, 81) = 3.36, p < .05, and between Shy and Non-shy, F(1,55) = -2.20, p < .05. Further examination of these data, using the individual No-Incentive condition as a baseline, showed that, compared to the Control and Non-shy groups, the Shy group was particularly fast during the Reward condition, F(2, 81) = 3.63, p < .05, and RT did not differ significantly among the three groups during the Punishment condition, F(2, 81) = .05, p =ns (Fig. 2).
Fig. 2.
Mean RT differences (ms) for social behavior group (Shy, Non-shy, Control) by MID condition differences (Reward-Punishment, Punishment-No-Incentive, Reward-No-Incentive).
3.2. Accuracy
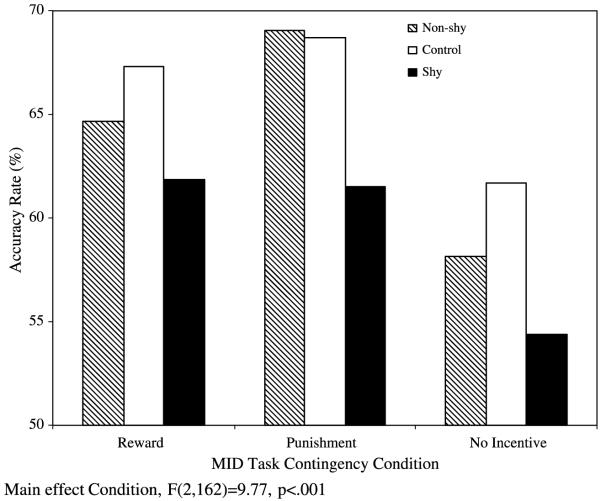
Analyses of group differences in accuracy during the MID task revealed a main effect of condition, F(2, 162) = 9.77, p < .001 (Fig. 3), indicating participants were most accurate during Punishment condition (mean accuracy 66.4%), less accurate during Reward condition (mean accuracy 64.6%), and least accurate during No-Incentive condition (mean accuracy 58.1%). There was no interaction between Group and Condition.
Fig. 3.
Mean accuracy rate (%) for social groups (Shy, Non-shy, Control) by MID condition (Reward, Punishment, No-Incentive).
4. Discussion
Overall, our data showed that the MID performance was sensitive to contingencies in all three groups. A faster response to obtain a reward ($1) or to prevent a loss ($1) reflects enhanced vigilance energized by higher levels of motivation. Accuracy of performance was improved by contingencies, but the dichotomous nature of this variable, together with individual variability, reduced the power to detect significant group differences, and group by condition interactions.
With respect to reaction time, findings were consistent with predictions. Non-shy individuals demonstrated sensitivity to potential incentive by adapting their behavior to effectively both obtain reward and avoid punishment. Specifically, Reward and Punishment conditions in the Non-shy group elicited similarly enhanced performance relative to No-Incentive condition. The Non-shy group was also faster in the No-Incentive condition compared to the Shy and Control groups. Given the approach oriented nature of the MID task, and previous findings demonstrating activity in neural substrates of the reward system during the task, these findings suggest an overactive approach motivation system in this population that is either poorly modulated by contingencies, or functioning at a ceiling level. Non-shy individuals differed significantly from the shy individuals in their response to Punishment cues rather than Reward cues. This is consistent with previous work demonstrating shorter latencies following punishment in extraverted compared to introverted (shy-like) subjects, when the potential for both reward and punishment are present (Nichols & Newman, 1986; Patterson et al., 1987).
Shy individuals showed a large discrepancy between response to reward and response to punishment, as indexed by a significant reaction time difference between the two conditions. This discrepancy resulted from enhanced sensitivity to potential reward (shortest reaction time during Reward condition) rather than sensitivity to potential punishment (only slight reduction of reaction time during Punishment than during No-Incentive condition). Given prior data linking shyness to responses to socially rewarding stimuli (Davidson, Marshall, Tomarken, & Henriques, 2000), the current and previous data suggest that various forms of rewards may be particularly powerful modifiers of behavior in shy individuals.
In the Punishment condition for our paradigm, subjects needed to approach stimuli signaling potential punishment in order to avoid the negative outcome. We hypothesized that shy individuals experience conflict from their predisposition to withdraw from negatively valenced stimuli, coupled with task demands requiring approach behavior (press button) to avoid negative consequences. Accordingly, we expected reaction time for the Shy group, but not other groups, to be slower during the Punishment compared to the Reward condition. Whereas performance scores are consistent with this idea, this interpretation remains speculative and cannot be clearly tested in the present paradigm. However, given that imaging data are available for the current task, we chose to begin our studies with the established MID task. Overall, the shy group showed a unique pattern of response to contingencies, characterized by a substantial discrepancy in strength of approach behavior in response to positive cues vs. negative cues, and a strong modulation of behavior by positive reinforcers.
This pattern of responses in shy individuals is consistent with proposals of an approach-withdrawal conflict in the social domain. It has been suggested that shy individuals exhibit normal motivation to approach and engage socially with others, while simultaneously finding themselves too fearful and withdrawal motivated to actually do so (Asendorpf, 1990; Cheek & Buss, 1981; Neal & Edelmann, 2003). In a consistent line of study, Schmidt and Fox (1994) reported patterns of electrophysiological and autonomic arousal in shy and sociable individuals just prior to a social encounter that support the idea of an approach-withdrawal conflict. While our current findings are consistent with these previous reports, they suggest that this conflict for shy individuals may not be specific to social contexts, and may generalize to less context specific types of rewarding or punishing stimuli.
These data may also carry implications for research on psychopathology. Considerable data document an association between perturbed reward-system function in the form of major depression, and perturbed regulation of anxiety in the form of extreme shyness. This includes data from both longitudinal and family-genetic studies documenting associations between social phobia and major depression (Lieb, Isensee, Hofler, Pfister, & Wittchen, 2002; Stein et al., 2001), as well as data from brain imaging studies implicating perturbed dopamine function specifically in both social phobia and major depression (Drevets, 2001; Drevets et al., 2001; Schneier et al., 2000). The current data provide some of the first evidence to suggest that extreme shyness is associated with perturbed reward system function, as assessed using a task previously shown to engage relevant underlying neural circuitry.
Our findings extend theoretical accounts of personality that discuss outgoing social behavior only in terms of reward reactivity, and shy, anxious social behavior only in terms of punishment reactivity. Our findings suggest differences in the function or modulation of reward systems in shy and non-shy individuals, which can be tested using functional neuroimaging tools. Because of an increased sensitivity to potential reward in the shy and non-shy groups, a disproportionate involvement of the ventral striatum might be anticipated. The ventral striatum has been associated with reward processing (Di Chiara, 2002) and therefore may be an area where both non-shy and shy individuals demonstrate differences during the processing of potential reward. Additionally, the amygdala has been particularly implicated in the processing of both potentially aversive stimuli and socially relevant stimuli (Adolphs, 1999), and in stimulus-value associative learning (Baxter & Murray, 2002). These roles suggest that the amygdala may be an area of functional divergence between non-shy and shy individuals during the processing of potential punishment, as well as, the processing of potential reward.
Results of the current study should be considered in light of some limitations. The sample for this study consisted of undergraduate university students that were not screened for psychiatric disorders. Given the nature of our social composite score, it is possible that cases of social anxiety or other anxiety disorders were included in the shy group. Additionally, this study did not have an objective measure of social behavior, such as those reported in previous studies (for example, Davidson, Marshall, et al., 2000. Future studies exploring the relationship between social behavior and motivation processes would benefit from inclusion of psychiatric assessment by psychiatric interview, and objective measures confirming participant social behavior.
The current study serves as a valuable starting point for future research on the relationship between general motivation systems, and social behavior. Because the behavioral task employed in this study was initially developed for use in neuroimaging environments, the current findings can provide very useful guidance for future neuroimaging studies designed to explore neurophysiology involved in processing socially relevant stimuli and production of social behavior, and interaction with reward systems.
References
- Adolphs R. Social cognition and the human brain. Trends in Cognitive Science. 1999;3(12):469–479. doi: 10.1016/s1364-6613(99)01399-6. [DOI] [PubMed] [Google Scholar]
- Asendorpf JB. Beyond social withdrawal: Shyness, unsociability, and peer avoidance. Human Development. 1990;33(4):250–259. [Google Scholar]
- Baxter MG, Murray EA. The amygdala and reward. Nature Reviews Neuroscience. 2002;3(7):563–573. doi: 10.1038/nrn875. [DOI] [PubMed] [Google Scholar]
- Cheek JM. The Revised Cheek and Buss Shyness and Sociability Scale (RCBS) Wellesley College; Wellesley, MA: 1983. Unpublished. [Google Scholar]
- Cheek JM, Buss AH. Shyness and sociability. Journal of Personality and Social Psychology. 1981;41:330–339. [Google Scholar]
- Clark LA, Watson D. Tripartite model of anxiety and depression: Psychometric evidence and taxonomic implications. Journal of Abnormal Psychology. 1991;100(3):316–336. doi: 10.1037//0021-843x.100.3.316. [DOI] [PubMed] [Google Scholar]
- Cloninger CR. A systematic method for clinical description and classification of personality variants. A proposal. Achieves of General Psychiatry. 1987;44(6):573–588. doi: 10.1001/archpsyc.1987.01800180093014. [DOI] [PubMed] [Google Scholar]
- Corr PJ. Reinforcement sensitivity theory and personality. Neuroscience Biobehavioral Review. 2004;28(3):317–332. doi: 10.1016/j.neubiorev.2004.01.005. [DOI] [PubMed] [Google Scholar]
- Davidson RJ, Jackson DC, Kalin NH. Emotion, plasticity, context, and regulation: Perspectives from affective neuroscience. Psychological Bulletin. 2000;126(6):890–909. doi: 10.1037/0033-2909.126.6.890. [DOI] [PubMed] [Google Scholar]
- Davidson RJ, Marshall JR, Tomarken AJ, Henriques JB. While a phobic waits: Regional brain electrical and autonomic activity in social phobics during anticipation of public speaking. Biological Psychiatry. 2000;47(2):85–95. doi: 10.1016/s0006-3223(99)00222-x. [DOI] [PubMed] [Google Scholar]
- Derryberry D. Incentive and feedback effects on target detection: A chronometric analysis of Gray’s model of temperament. Personality and Individual Differences. 1987;8(6):855–865. [Google Scholar]
- Derryberry D, Reed MA. Temperament and attention: Orienting toward and away from positive and negative signals. Journal of Personality and Social Psychology. 1994;66(6):1128–1139. doi: 10.1037//0022-3514.66.6.1128. [DOI] [PubMed] [Google Scholar]
- Di Chiara G. Nucleus accumbens shell and core dopamine: Differential role in behavior and addiction. Behavioural Brain Research. 2002;137(12):75–114. doi: 10.1016/s0166-4328(02)00286-3. [DOI] [PubMed] [Google Scholar]
- Drevets WC. Neuroimaging and neuropathological studies of depression: Implications for the cognitive-emotional features of mood disorders. Current Opinion in Neurobiology. 2001;11(2):240–249. doi: 10.1016/s0959-4388(00)00203-8. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Gautier C, Price JC, Kupfer DJ, Kinahan PE, Grace AA, et al. Amphetamine-induced dopamine release in human ventral striatum correlates with euphoria. Biological Psychiatry. 2001;49(2):81–96. doi: 10.1016/s0006-3223(00)01038-6. [DOI] [PubMed] [Google Scholar]
- Ernst M, Nelson EE, McClure EB, Monk CS, Munson S, Eshel N, et al. Choice selection and reward anticipation: An fMRI study. Neuropsychologia. 2004;42(12):1585–1597. doi: 10.1016/j.neuropsychologia.2004.05.011. [DOI] [PubMed] [Google Scholar]
- Eysenck HJ. The biological basis of personality. Thomas; Spring-field, IL: 1967. [Google Scholar]
- Eysenck HJ, Eysenck SBG. Manual of the Eysenck Personality Scales. Hodder and Stoughton; London: 1991. [Google Scholar]
- Gray JA. The psychophysiological basis of introversion-extraversion. Vol. 8. Elsevier Science; Amsterdam: 1970. [DOI] [PubMed] [Google Scholar]
- Heiser NA, Turner SM, Beidel DC. Shyness: Relationship to social phobia and other psychiatric disorders. Behaviour Research and Therapy. 2003;41(2):209–221. doi: 10.1016/s0005-7967(02)00003-7. [DOI] [PubMed] [Google Scholar]
- Knutson B, Adams CM, Fong GW, Hommer D. Anticipation of increasing monetary reward selectively recruits nucleus accumbens. Journal of Neuroscience. 2001;21(16):RC159. doi: 10.1523/JNEUROSCI.21-16-j0002.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson B, Fong GW, Bennett SM, Adams CM, Hommer D. A region of mesial prefrontal cortex tracks monetarily rewarding outcomes: Characterization with rapid event-related fMRI. Neuroimage. 2003;18(2):263–272. doi: 10.1016/s1053-8119(02)00057-5. [DOI] [PubMed] [Google Scholar]
- Kringelbach ML, Rolls ET. The functional neuroanatomy of the human orbitofrontal cortex: Evidence from neuroimaging and neuropsychology. Progress in Neurobiology. 2004;72(5):341–372. doi: 10.1016/j.pneurobio.2004.03.006. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. Emotion, motivation, and anxiety: Brain mechanisms and psychophysiology. Biological Psychiatry. 1998;44(12):1248–1263. doi: 10.1016/s0006-3223(98)00275-3. [DOI] [PubMed] [Google Scholar]
- Leary MR. Social anxiety, shyness, and related constructs. In: Robinson JP, Shaver PR, editors. Measures of personality and social psychological attitudes. Academic Press, Inc.; New York: 1991. pp. 161–194. [Google Scholar]
- Lieb R, Isensee B, Hofler M, Pfister H, Wittchen HU. Parental major depression and the risk of depression and other mental disorders in offspring: A prospective-longitudinal community study. Archives of General Psychiatry. 2002;59(4):365–374. doi: 10.1001/archpsyc.59.4.365. [DOI] [PubMed] [Google Scholar]
- McNaughton N, Corr PJ. A two-dimensional neuropsychology of defense: Fear/anxiety and defensive distance. Neuroscience and Biobehavioral Reviews. 2004;28(3):285–305. doi: 10.1016/j.neubiorev.2004.03.005. [DOI] [PubMed] [Google Scholar]
- Monk CS, Pine DS. Childhood anxiety disorders: A cognitive neurobiological perspective. In: Charney DS, Nester E, editors. Neurobiology of mental illness. 2nd ed. Oxford University Press; Oxford: 2004. pp. 1022–1046. [Google Scholar]
- Neal JA, Edelmann RJ. The etiology of social phobia: Toward a developmental profile. Clinical Psychology Review. 2003;23(6):761–786. doi: 10.1016/s0272-7358(03)00076-x. [DOI] [PubMed] [Google Scholar]
- Nichols SL, Newman JP. Effects of punishment on response latency in extraverts. Journal of Personality and Social Psychology. 1986;50(3):624–630. doi: 10.1037//0022-3514.50.3.624. [DOI] [PubMed] [Google Scholar]
- Patterson CM, Kosson DS, Newman JP. Reaction to punishment, reflectivity, and passive avoidance learning in extraverts. Journal of Personality and Social Psychology. 1987;52(3):565–575. doi: 10.1037//0022-3514.52.3.565. [DOI] [PubMed] [Google Scholar]
- Robbins TW, Everitt BJ. Neurobehavioural mechanisms of reward and motivation. Current Opinion in Neurobiology. 1996;6(2):228–236. doi: 10.1016/s0959-4388(96)80077-8. [DOI] [PubMed] [Google Scholar]
- Rothbart MK, Ahadi SA, Evans DE. Temperament and personality: Origins and outcomes. Journal of Personality and Social Psychology. 2000;78(1):122–135. doi: 10.1037//0022-3514.78.1.122. [DOI] [PubMed] [Google Scholar]
- Schmidt LA, Fox NA. Patterns of cortical electrophysiology and autonomic activity in adults’ shyness and sociability. Biological Psychology. 1994;38(23):183–198. doi: 10.1016/0301-0511(94)90038-8. [DOI] [PubMed] [Google Scholar]
- Schneier FR, Liebowitz MR, Abi-Dargham A, Zea-Ponce Y, Lin SH, Laruelle M. Low dopamine D(2) receptor binding potential in social phobia. American Journal of Psychiatry. 2000;157(3):457–459. doi: 10.1176/appi.ajp.157.3.457. [DOI] [PubMed] [Google Scholar]
- Schultz W, Apicella P, Scarnati E, Ljungberg T. Neuronal activity in monkey ventral striatum related to the expectation of reward. Journal of Neuroscience. 1992;12(12):4595–4610. doi: 10.1523/JNEUROSCI.12-12-04595.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W, Dayan P, Montague PR. A neural substrate of prediction and reward. Science. 1997;275(5306):1593–1599. doi: 10.1126/science.275.5306.1593. [DOI] [PubMed] [Google Scholar]
- Stein MB, Fuetsch M, Muller N, Hofler M, Lieb R, Wittchen HU. Social anxiety disorder and the risk of depression: A prospective community study of adolescents and young adults. Archives of General Psychiatry. 2001;58(3):251–256. doi: 10.1001/archpsyc.58.3.251. [DOI] [PubMed] [Google Scholar]
- Turner SM, Johnson MR, Beidel DC, Heiser NA, Lydiard RB. The Social Thoughts and Beliefs Scale: A new inventory for assessing cognitions in social phobia. Psychological Assessment. 2003;15(3):384–391. doi: 10.1037/1040-3590.15.3.384. [DOI] [PubMed] [Google Scholar]
- Zuckerman M. Psychobiology of Personality. Cambridge University Press; 1991. [Google Scholar]
- Zuckerman M, Kuhlman DM, Joireman J, Teta P. A comparison of three structural models for personality: The Big Three, the Big Five, and the Alternative Five. Journal of Personality and Social Psychology. 1993;65(4):757–768. [Google Scholar]
- Zuckerman M, Joireman J, Kraft M, Kuhlman DM. Where do motivational and emotional traits fit within three factor models of personality? Personality and Individual Differences. 1999;26(3):487–504. [Google Scholar]