Abstract
Background
GLP-1 treatment leads to short term improvements in myocardial function in ischemic and non-ischemic cardiomyopathy. It is unknown whether GLP-1 improves survival when administered over a longer time period. Spontaneously hypertensive, heart failure prone rats (SHHF) progress to advanced heart failure and death over a 15 month period. We sought to determine whether a continuous infusion of GLP-1 would reduce mortality in this model.
Methods and Results
At 9 months of age, 50 SHHF rats were randomized to receive a 3-month continuous infusion of either GLP-1 or saline. Metabolic parameters were measured and cardiac ultrasounds performed at study initiation and completion of treatment. Surviving rats were euthanized at 12 months. Hearts were perfused in an isolated, isovolumic heart preparation and Tunel staining of myocardial samples was performed. Baseline metabolic and cardiac functional parameters were comparable. GLP-1 treated SHHF had greater survival (72% vs. 44%, p =0.008) at 12 months of age. In addition, GLP-1 treatment led to higher plasma insulin, lower plasma triglycerides, and preserved LV function. GLP-1 treated rats demonstrated decreased myocyte apoptosis by Tunel staining, as well as reduced caspase-3 activation. No increase in p-BAD expression was seen. In isolated hearts, the LV systolic pressure and developed pressure were greater in the GLP-1 group. Myocardial glucose uptake was also increased in GLP-1 treated SHHF.
Conclusions
Chronic GLP-1 treatment prolongs survival in obese, hypertensive SHHF. This is associated with preserved LV function and LV mass index, increased myocardial glucose uptake and reduced myocyte apoptosis.
Keywords: apoptosis, glucose, heart failure, mortality
Introduction
Glucagon like peptide-1 (GLP-1 7-36 amide) is a member of the pro-glucagon family of incretin peptides, implicated in the control of appetite and satiety. These agents have been shown to have significant clinical efficacy in the treatment of type 2 diabetes mellitus 1,2,3. Prior studies have demonstrated that GLP-1 infusion has salutary cardiovascular effects in the presence or absence of diabetes, suggesting that GLP-1 may have cardioprotective effects independent of those attributable to tight glycemic control4. Acute infusions of GLP-1 were associated with enhanced recovery following brief ischemic insults in conscious dogs5 and in intact6 and isolated rat hearts6,7. Seventy-two hour infusions of GLP-1 have been associated with increased myocardial glucose uptake and improved left ventricular (LV) function in conscious dogs with dilated cardiomyopathy induced by rapid pacing8. Similar benefits have been seen in patients following acute MI9. However, to date these studies have involved relatively brief infusions (days) and consequently have assessed only short-term improvements in cardiac performance in post ischemic or cardiomyopathic states. There have been no longer-term studies to determine whether these acute cardiovascular benefits of GLP-1 are sustainable. Moreover, there are no studies to determine whether chronic GLP-1 infusion improves survival in experimental or human models predisposed to cardiovascular mortality.
The spontaneously hypertensive, heart failure prone rat is a model of obesity, insulin resistance, and hypertension that progresses to a dilated cardiomyopathy over a 12–15 month period10. The progressive development of the cardiac phenotype is associated with the onset of glucose intolerance. Treatment with both standard and novel heart failure therapies has been shown to moderate the development of LV dysfunction11,12,13,14. As such, the model constitutes and attractive opportunity to study the long-term effects of GLP-1 on myocardial glucose uptake, cardiac structure and function, and survival. Accordingly, the purpose of the present study was to determine the effects of a chronic (3 month), continuous infusion of GLP-1 (7-36) amide on metabolic parameters in mature SHHF rats. A second goal was to determine the effects of chronic GLP-1 infusion on LV systolic function and myocardial structure. Last, we sought to determine whether and by what mechanism GLP-1 may increase survival in this model vulnerable to premature cardiac mortality from progressive LV systolic dysfunction.
Methods
Seventy SHHF were purchased from Charles River Laboratories at 6 months of age and acclimated to the laboratory on a standard rat chow limited to 15 pellets per day to standardize caloric intake for three months prior to study. At 9 months of age, the rats were divided randomly into two cohorts; control and GLP-1. Ten rats in each cohort underwent baseline studies to define the metabolic, morphometric and hemodynamic parameters at the time point. The remaining rats were randomized to receive either GLP-1 (1.5 pmol/kg/min, n=25) or an equivolume of saline as control (n=25) administered by intraperitoneal infusion using a catheter attached to an Alzet mini-pump inserted into the subcutaneous fat. Pumps were replenished every 21 days. The rats were followed for 3 months and those that survived underwent similar analysis at 12 months. The care and use of animals were conducted under the Guidelines on Human Use and Care of Laboratory Animals for Biomedical Research published by the National Institutes of Health and according to experimental protocol approved by the Institutional Animal Care Use Committee of Allegheny General Hospital.
Metabolic Parameters
Fasting plasma insulin, glucose, NEFA, adiponectin, leptin and triglyceride levels were obtained at the time of euthanasia at either 9 (baseline) or 12 months. Blood was drawn in ice-cold tubes containing EDTA as an anticoagulant and Trasylol (Bayer Corp., West Haven, CT) and Diprotin A (Bachem Bioscience, King of Prussia, PA) as protease inhibitors. The samples were kept on ice and centrifuged within 30 minutes of collection at 1000×g for 10 minutes. The plasma was removed and stored at -80°C. The metabolic parameters were assayed as described previously8. Plasma leptin and adiponectin were assayed using the Linco leptin assay kit (Linco Research immunoassay, St. Charles, MO) on plasma stored at -20°C. The plasma concentration of total GLP-1 (including both the (7-36) and (9-36) amides) was determined in plasma collected in ice-cooled tubes containing EDTA and a DPP-IV inhibitor using an antibody directed against the COOH-terminal portion of the peptides (Linco Research, St. Charles, MO).
Echocardiography
M-mode and 2-D echocardiography was performed under light isoflurane anesthesia in 10 rats in each group at baseline (9 months) and in the surviving animals at 12 months. Measurements of left ventricular (LV) dimensions in end-diastole (LVEDD) and end-systole (LVESD), as well as respective measurements of the inter-ventricular septum (IVS) and posterior wall (PW) in systole and diastole were recorded. The modified Quinones15 and Deveraux16 equations were utilized to calculate left ventricular ejection fraction (LVEF) and left ventricular mass (LVM) respectively. The volumetric and LV indices were normalized to body mass to account for the effect of obesity in these rats. The echo reader was blinded to the treatment groups.
Isolated Isovolumic Heart Preparations
The Langendorff methodology of isolated perfused rat hearts has been described previously in detail7. Briefly, hearts were cannulated via ascending aorta for retrograde perfusion at 37 °C under constant pressure (74 mmHg) using KH buffer containing (in mM) 119 NaCI, 5.4 KCl, 1.2 MgSO4, 1.2 KH2PO4, 2.5 CaCl2, 25 NaHCO3, 0.25 EDTA, 5.0 mM glucose and 40 μU/ml of insulin. All buffer components were obtained from Sigma (St. Louis,MO). A left atrial incision was made to expose the mitral annulus through which a water-filled latex balloon was passed into the left ventricle (LV). The balloon was attached via polyethylene tubing to a pressure transducer (Baxter, Model Px 272) that was connected to Triton System I. The initial balloon volume was set to generate left ventricular end diastolic pressure (LVEDP) ≈5 mmHg. Myocardial function was measured including left ventricular developed pressure (LV Dev P), LVEDP, and LV dP/dt. LV Dev P was calculated by subtracting LVEDP from the peak systolic pressure. Coronary flow was calculated by a timed collection of the effluent.
For the measurement of myocardial glucose uptake during steady state, the perfusate leaving the heart was collected over 1 minute every 10-15 minutes. Glucose concentration in the effluent was measured using YSI Glucose Analyzer. Glucose uptake was calculated as described previously7,17.
Myocyte Apoptosis
TUNEL staining was performed on sections of LV myocardium to quantify myocyte and non-myocyte apoptosis. DAPI staining was used to identify nuclei as a control. The percent of apoptotic cells was calculated using the Metamorph system. Western blot analysis of whole heart homogenate was performed as described previously18 to determine protein levels of the activated form of caspase -3 and phosphorylated form of BAD at serine136. The anti-caspase 3, anti-BAD and anti p-BAD serine136 antibodies were obtained from and Cell Signaling Technology and Santa Cruz Biotechnology, respectively.
Insulin Signaling
At baseline (9 months) and in surviving rats at 12 months, subsets of rats were euthanized, the myocardium was removed and LV samples were snap frozen in liquid nitrogen. Components of the myocardial insulin signaling cascade were assayed by Western blot using rat-specific antibodies in purified sarcolemmal membrane preparations generated by density gradient centrifugation, as previously described from our laboratory18. In half of the rats within each group, insulin (100 U SQ) was administered 5 minutes prior to removing the heart, in order to assess the functional response of insulin signaling proteins under physiological stimulation. The other half of the rats in each group were used to investigate the integrity of these cellular pathways under basal metabolic conditions. Immunoblotting for IR-β, IRS-1, Akt-1 total protein expression, serine (Ser 472, 473) and threonine (Thr 308) phosphorylated Akt-1, and serine (307) phosphorylated IRS-1 was conducted using specific antibodies as described previously18. All Western blots were performed using 10 μg protein/lane. Adjustment for protein loading was accomplished by normalizing bands based on Coomassie staining of the blot.
For the measurement of GLUT-4 translocation, purified light (sarcolemmal) and heavy (intracellular) membrane vesicles were isolated from LV myocardium using a sucrose gradient7,18. Membranes were probed with rabbit anti-GLUT4 polyclonal antibody (1:1000) (Santa Cruz Biotechnology, Inc., Santa Cruz, CA) overnight at 4°C. Densitometric analysis of the bands was carried out using a Personal Densitometer SI and ImageQuaNT™ Software (Molecular Dynamics, Sunnyvale, CA) and expressed as the % sarcolemmal membrane to total GLUT-418. The signaling data were expressed as the % insulin stimulated in each group at the two time points.
Statistical analysis
All measurements are expressed as means ± SEM. The data were analyzed by unpaired Student’s t-test with a Bonferroni correction applied for multiple comparisons. The survival response with respect to time between groups was analyzed by two-way ANOVA. P values were provided for all comparisons. Statistical significance was indicated when p<0.05.
The authors had full access to and take full responsibility for the integrity of the data. All authors have read and agree to the manuscript as written.
Results
LV weight, body weight and metabolic parameters
There was no difference in body weight, body mass index, LV mass or the LV mass index between the groups at baseline, nor were there differences in baseline metabolic parameters, with both groups demonstrating significant fasting hyperglycemia at 9 months of age (Table 1). GLP-1 treatment was associated with a significant reduction in body weight and body mass index and a trend (p=0.061) toward an increase in LV mass index over the three month period of treatment. These responses were significantly different compared to the response over time in the saline control group. There were significant declines in plasma glucose and triglyceride levels, as well as significant increases in plasma insulin and adiponectin levels in the GLP-1 group during the three month period of treatment. These responses as well as the glucagon response over time were significantly different than those observed in the saline control group. As expected, plasma GLP-1 levels were significantly higher in the GLP-1 treatment group compared to the saline controls (Table 1). Myocardial glucose uptake was also greater in the GLP-1 treated rats and the response was significantly greater than that observed in the saline control (Table 2).
Table 1.
Morphometrics and Metabolic Parameters
| Saline Treatment | GLP-1 Treatment | ||||||
|---|---|---|---|---|---|---|---|
| n=10 | n=11 | p* | n=10 | n=18 | p* | p† | |
| Age (months) | 9 | 12 | 9 | 12 | |||
| Body Weight (gm) | 733±16 | 731±21 | 0.94 | 744±19 | 641±35*† | 0.046 | 0.01 |
| Body Mass Index (g/m2) | 11.6±0.3 | 11.4±0.7 | 0.80 | 11.7±0.3 | 9.7±0.5*† | 0.009 | 0.0001 |
| LV mass (gm) | 1.98±0.07 | 1.97±0.11 | 0.94 | 2.10±0.11 | 2.31±0.09 | 0.158 | 0.151 |
| LV mass index (gm/m2) | 28±1 | 27±3 | 0.76 | 30±2 | 36±2† | 0.061 | 0.023 |
| Glucose (mg/dl) | 199±15 | 180±10 | 0.29 | 242±15 | 145±5*† | 0.0001 | 0.0001 |
| NEFA (μmol/L) | 523±105 | 587±61 | 0.59 | 541±61 | 585 ±58 | 0.630 | 0.558 |
| Insulin (pmol/L) | 1439±189 | 1679±210 | 0.049 | 1736 ±164 | 2309±178*† | 0.038 | 0.0001 |
| Glucagon (pg/ml) | 297±50 | 406±51* | 0.14 | 312 ±25 | 265 ±31† | 0.310 | 0.0001 |
| Triglycerides (μmol/L) | 1687±345 | 1567±213 | 0.77 | 1701±222 | 945±178*† | 0.032 | 0.0001 |
| Adiponectin (μg/ml) | 4.8±1.2 | 4.7±0.8 | 0.94 | 3.9±0.8 | 6.0±0.6*† | 0.044 | 0.0001 |
| Leptin (ng/ml) | 91±12 | 75±22 | 0.54 | 85±5 | 75 ±11 | 0.512 | 0.326 |
| GLP-1 (pmol/L) | 16±2 | 21±3 | 0.18 | 12±2 | 61±10*† | 0.001 | 0.001 |
All values expressed as mean±SEM.
p value: 12 months compared to 9 months in each group
p value: response over time in GLP-1 group compared to saline control
Table 2.
In–vitro Hemodynamic Parameters and Glucose Uptake: Isolated Perfused Heart Preparations
| Saline Treatment | GLP-1 Treatment | ||||||
|---|---|---|---|---|---|---|---|
| n=10 | n=11 | p* | n=10 | n=18 | p* | p† | |
| Age (months) | 9 | 12 | 9 | 12 | |||
| LV Systolic Pressure (mmHg) | 136±6 | 114±7* | 0.025 | 141±9 | 144±5† | 0.909 | 0.005 |
| LV Developed Pressure (mmHg) | 131±8 | 108±4* | 0.011 | 138±10 | 137±10 | 0.936 | 0.053 |
| LV (+)dP/dt (mmHg/sec) | 3,471±261 | 3,312±213 | 0.638 | 3,399±136 | 3,491±136 | 0.754 | 0.911 |
| LV (-) dP/dt (mmHg/sec) | 2,401±161 | 1,512±113* | 0.001 | 2,699±111 | 2,401±111† | 0.210 | 0.015 |
| Heart Rate (min-1) | 241±28 | 225±31 | 0.701 | 252±32 | 231±32 | 0.637 | 0.081 |
| Coronary Flow (ml/mm) | 26±2 | 24±1 | 0.647 | 25±2 | 24±2 | 0.627 | 0.913 |
| Glucose Uptake (μm/ min/g) | 1.7±0.06 | 1.3±0.05* | 0.0002 | 1.6 ±0.06 | 2.1±0.07*† | 0.001 | 0.001 |
All values expressed as mean ± SEM.
p value: 12 months compared to 9 months in each group
p value: response over time in GLP-1 group compared to saline control
LV hemodynamic parameters
At baseline, there was no significant difference between groups in mean arterial pressure, heart rate, stroke volume, LV dimensions or LV ejection fraction (Table 3). Notably, both groups had significant baseline hypertension with mean arterial pressures in excess of 130 mmHg.
Table 3.
In-vivo Hemodynamic Parameters
| Saline Treatment | GLP-1 Treatment | ||||||
|---|---|---|---|---|---|---|---|
| n=10 | n=11 | p* | n=10 | n=18 | p* | p† | |
| Age (months) | 9 | 12 | 9 | 12 | |||
| Mean Arterial Pressure (mmHg) | 133±16 | 127±21 | 0.826 | 141±19 | 134±15 | 0.776 | 0.903 |
| Heart Rate (min-1) | 376±28 | 401±27* | 0.527 | 363±10 | 371±20 | 0.771 | 0.135 |
| Stroke Volume (ml) | 1.5±0.1 | 1.0±0.1* | 0.001 | 1.3±0.1 | 1.4±0.1*† | 0.497 | 0.001 |
| Cardiac Output (ml/min) | 564±38 | 402±43* | 0.295 | 468±22 | 543±22*† | 0.051 | 0.001 |
| LVEDD (mm) | 9.61±0.2 | 9.80±0.1 | 0.357 | 9.20±0.2 | 9.21±5† | 0.944 | 0.582 |
| LVESD (mm) | 1.73±0.23 | 2.94±0.18* | 0.0004 | 1.84±0.20 | 1.66 ±0.28 | 0.631 | 0.001 |
| LV Ejection (%) | 82±4 | 70±4 | 0.033 | 80±4 | 82±3 | 0.661 | 0.016 |
All values expressed as mean±SEM.
p value: 12 months compared to 9 months in each group
p value: response over time in GLP-1 group compared to saline control
Following the 3-month treatment period, significant differences were observed between the groups. The saline control group demonstrated decreased stroke volume and LV ejection fraction and increased LV end-systolic dimension over three months. By contrast, cardiac output increased in the GLP-1 treated group, and LV end-systolic dimensions, LV ejection fraction, and heart rate remained unchanged (Table 3). These findings were corroborated by in vitro data, which showed that LV systolic pressure and LV developed pressures were significantly greater in the GLP-1 treated rats (Table 2). The decline in LV systolic and developed pressure in the isolated hearts of the saline treated group reflects a decline in LV contractile performance under isovolumic conditions. Thus, GLP-1 treatment preserved LV size and function, preventing the deterioration observed in the saline control group.
Survival
SHHF rats treated with GLP-1 had significantly greater survival (72%) after three months compared with saline controls (44%) (Figure 1). This was associated with significantly reduced myocyte apoptosis as assessed by Tunel staining. Notably, there was no difference in apoptotic cell death among non-myocyte cells in either treatment group (Figure 2). The reduction in myocyte apoptosis in the GLP-1 treatment group was accompanied by reduced myocardial caspase-3 activation (Figure 3). In addition, GLP-1 treated rats had increased Akt phosphorylation and GLUT-4 translocation, consistent with the increase in plasma insulin. However, Akt activation was not associated with inactivation of the pro-apoptotic protein BAD as assessed by the ratio of ser136 p-BAD / total BAD (Con: 0.49±0.03; GLP-1: 0.45±0.03). GLP-1 receptor (GLP-1R) expression was increased in GLP-treated rats (Figure 4).
Figure 1.
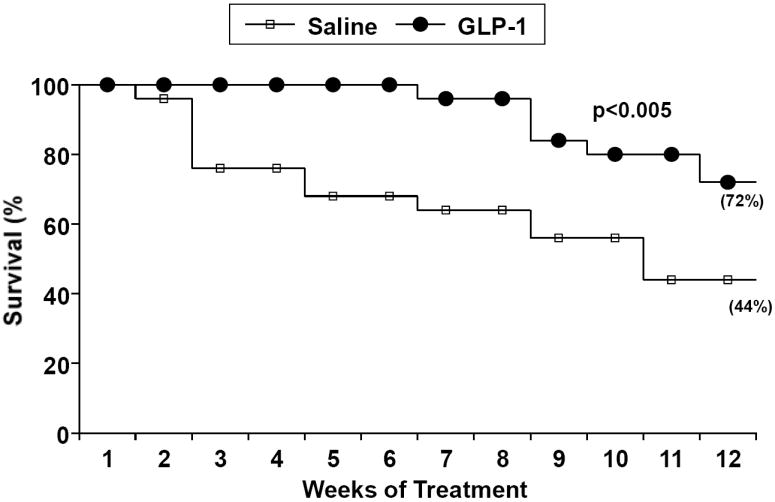
The survival of two groups of SHHF randomized to receive intraperitoneal continuous infusion of GLP-1 or saline. GLP treated animals had better survival compared to control (p =0.008).
Figure 2.
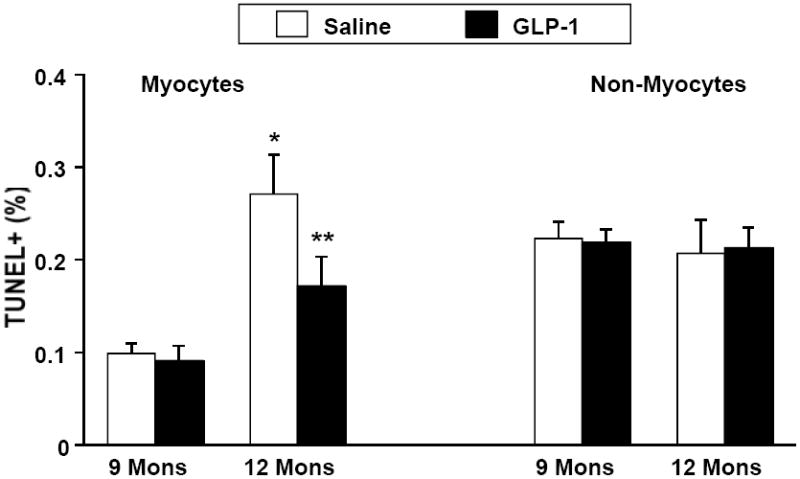
Percent of myocyte and non myocyte cells staining positive for TUNEL at 9 months (n=5 in each group) and 12 months of in a group treated with GLP-1 (n=6) compared to saline control (n=5). There was significantly less myocyte apoptosis in the GLP-1 group at 12 months. There was no difference in non-myocyte apoptosis. *p = 0.0002 saline control at 9 versus 12 months. ** p = 0.03 GLP-1 compared to control at 12 months.
Figure 3.
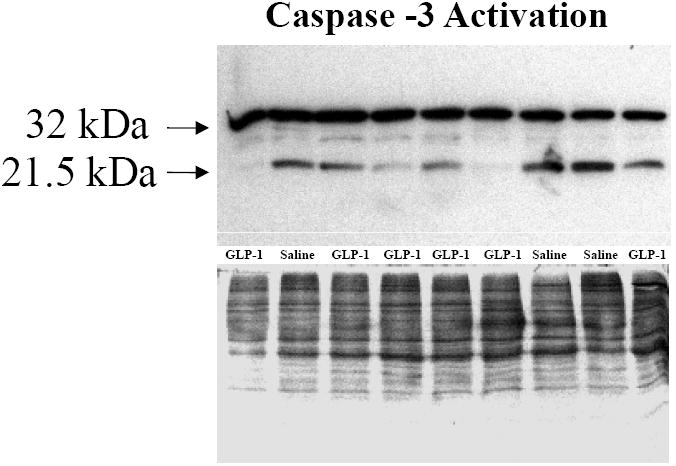
Western blots for caspase-3 protein (32 kDa) and activated, cleaved form of caspase-3 (21 kDa). GLP-1 treatment was associated with decreased caspase-3 activation.
Figure 4.
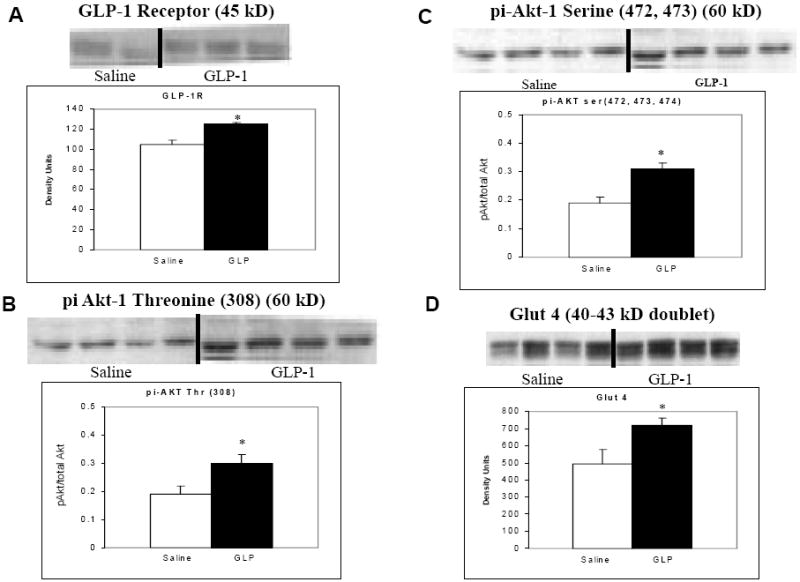
The effects of GLP-1 on myocardial GLP-1 receptor expression (A), threonine phosphorylation of Akt (B), serine phosphorylation of Akt (C) and myocardial GLUT-4 translocation (D). GLP-1 receptor expression was upregulated in the GLP-1 treated group at 12 months (p = 0.043). There was increased Akt phosphorylation and activation in the GLP-1 treated group (p=0.025 for serine and p = 0.018 for threonine) with resultant increase in GLUT-4 translocation (p =0.043).
Discussion
Obesity, hypertension, and diabetes are well-established risk factors for cardiovascular mortality in humans, but strategies designed to modify these risks have been largely elusive. In the present study, we demonstrate that a 3-month, continuous infusion of GLP-1 is associated with improvements in myocardial functional and systemic metabolic parameters, as well as with prolonged survival in an obese, hypertensive rat model predisposed to accelerated cardiovascular disease and premature mortality. To our knowledge, there are no previous studies of the effects of GLP-1 on cardiovascular mortality in this type of high-risk animal model.
The current observations are consistent with our prior study in human heart failure subjects, where a 5-week infusion of GLP-1 led to improvements in LV function, functional capacity, and systemic metabolic parameters4. In both the current and referenced study, better glycemic control in the GLP-1-treated group may have contributed to improvement in cardiac function, since elevated glucose levels have been shown to depress LV function in both in-vitro19,20 and in-vivo studies of normal subjects and diabetics21,22. However, non-diabetic human subjects demonstrated similar improvements in myocardial function as diabetics, suggesting an effect of GLP-1 that is independent of glycemic control. Nevertheless, the inclusion of a positive control group receiving an anti-hyperglycemic agent other than GLP-1 would be required to clarify this issue further, although is beyond the scope of the present investigation.
This is also the first study to demonstrate the ability of GLP-1 to reduce cardiac myocyte apoptosis. Notably, reduced programmed cell death was not seen in non-myocyte cellular constituents. The ability of GLP-1 to protect cardiac myocytes from accelerated death was suggested in a study showing that infusion of GLP-1 is associated with improved left ventricular function in patients following successful reperfusion for myocardial infarction9. To date, the anti-apoptotic effects of GLP-1 have been examined most extensively in pancreas, where reduced programmed cell death has been observed in diabetic rodents, islet cell lines, purified rat ß-cells, heterologous cells expressing the GLP-1 receptor, and human islet cells23,24,25,26,27. Liraglutide, a long-acting GLP-1 analogue, has also been shown to inhibit apoptosis in primary neonatal rat islets28. In addition to pancreatic cells, GLP-1 receptor activation enhances neuronal survival in cellular and animal models of neuronal toxicity29,30.
The mechanism of the anti-apoptotic action of GLP-1 remains the subject of intense investigation. Nevertheless, evidence has emerged for the involvement of increased phosphatidyl inositol-3 kinase (PI3K)/Akt signaling, leading to enhanced nuclear factor-kappa B DNA binding activity and stimulation of inhibitors of apoptosis protein-2, Bcl-XL and Bcl-2 expression24,25,26,31. Activation of PI3K/Akt also functions to block BAD-mediated death by phosphorylating BAD at Ser-136, and increased levels of inactivated p-BAD have been demonstrated in rat hearts in response to treatment with GLP-16. In the present study, we observed increases in phosphorylation of Akt associated with reduced levels of the activated form of caspase-3. However, protein levels of pBAD (ser 136) were not different between the GLP-1 treated and control groups. Taken together, these data suggest that GLP-1 treatment favors survival through reduced apoptosis, but additional work will be required to determine the precise mechanism.
In addition to the reduction in myocyte apoptosis, treatment with GLP-1 was associated with increased LV mass index. The ability of the left ventricle to hypertrophy in the presence of increased afterload is an adaptive response that acts to normalize wall stress. As seen in the saline treated group, failure of the ventricle to hypertrophy adequately leads to ventricular dilation and dysfunction. It is noteworthy that GLP-1 is known to promote hyperplasia in pancreatic ß-cells32,33. Several potential mediators of growth signaling by GLP-1 have been proposed, including protein kinase Cζ32, epidermal growth factor receptor and c-src33, as well as PDX-134. The mouse model of GLP-1 receptor knock out was associated with modest cardiac phenotype characterized by decreased cardiac mass and impaired responses to superimposed metabolic stresses37. Whether our observation of increased LV mass index is due to direct effects of GLP-1, or whether this is an indirect result of the increase in plasma insulin remains uncertain. Increased activation of Akt is known to stimulate cardiac hypertrophy through both the activation of the mTOR pathway35 and through the inactivation of GSK-3β36. The inclusion of a control group receiving an alternative insulin secretagogue may help elucidate this issue in future studies.
We have shown previously that GLP-1 treatment in normal isolated, isovolumic hearts has a distinctly different physiological and signaling profile compared to insulin, with stimulation of GLUT-1 translocation via a non-Akt dependent mechanism7. In the present study, GLP-1 infusion resulted in increased plasma insulin levels, and associated phosphorylation and activation of Akt leading to GLUT-4 translocation. In vitro experiments confirmed that this was associated with increased myocardial glucose uptake. These apparently discrepant findings in these two rat models are largely due to the fact that in the current experiments, the administration of GLP-1 occurred in the setting of hyperglycemia. Under these circumstances, the insulinotropic actions of GLP-1 to stimulate pancreatic insulin release predominate over the insulinomimetic effects seen in isolated normal rat hearts under euglycemic conditons.
We also observed that GLP-1 infusion was associated with increased adiponectin expression. Both of these pathways may have contributed to increased myocardial glucose uptake and normalization of plasma glucose. The relationship between adiponectin and LV mass is not completely elucidated, although epidemiologic data in humans38,39 and experimental data in mice40 has suggested that adiponectin inhibits hypertrophic signaling in the myocardium in the setting of pressure overload. In this case, the inhibitory effect of increased adiponectin must have been outweighed by other, more potent, growth stimulating pathways.
GLP-1R expression was more abundant in cardiac myocytes from GLP-1 treated rats. Very little is known about the regulation of the GLP-1R in the heart, and existing data from other tissues do not provide insight into our findings. High glucose concentrations upregulate receptor expression in pancreatic islets41,42,43,44, but serum glucose was lower in the GLP-1 treated group than in controls in the current study (as would be expected with GLP-1 treatment). Leptin enhances GLP-1R expression in certain areas of the rat hypothalamus43 but our GLP-1 and control rats had similar serum leptin levels. Increased GLP-1R expression cannot be explained by treatment with GLP-1 itself, as this was shown to downregulate the GLP-1R gene in a rat medullary thyroid carcinoma cell line45. Additional studies will be needed to identify other determinants of GLP-1R expression that could explain our current observations.
GLP-1 (7-36) amide undergoes rapid enzymatic cleavage by the enzyme dipeptidyl peptidase-4 (DPP-4), resulting in the formation of GLP-1 (9-36), the predominant plasma metabolite. We showed previously in a canine model of pacing-induced cardiomyopathy that infusion of the metabolite GLP-1-(9-36) mimics the effects of GLP-1-(7-36) in stimulating myocardial glucose uptake and improving LV and systemic hemodynamics6. The relative contribution of each of these peptides to our current observations cannot be determined. Future studies using co-administration of GLP-1 (7-36) with a DPP-4 inhibitor or Exendin-4, which is resistant to degradation by DPP-4, are warranted.
In conclusion, a three-month continuous infusion of GLP-1 resulted in preservation of LV function and prolonged survival in an experimental animal model of obesity, insulin resistance, and hypertension that was otherwise predisposed to premature cardiac failure and death.
Acknowledgments
Funding: These studies were supported by NIH RO-1 AG 0125.
Footnotes
Disclosures: Dr. Shannon holds a patent on the use of GLP-1 in the treatment of LV systolic dysfunction
References
- 1.Baggio LL, Drucker DJ. Harnessing the therapeutic potential of glucagon-like peptide-1: A critical review. Treat Endocrinol. 2002;1:117–125. doi: 10.2165/00024677-200201020-00005. [DOI] [PubMed] [Google Scholar]
- 2.Todd JF, Wilding JP, Edwards CM, Khan FA, Ghatei MA, Bloom SR. Glucagon-like peptide-1 (GLP-1): A trial of treatment in non-insulin-dependent diabetes mellitus. Eur J Clin Invest. 1997;27:533–536. doi: 10.1046/j.1365-2362.1997.1490691.x. [DOI] [PubMed] [Google Scholar]
- 3.Zander M, Madsbad S, Madsen JL, Holst JJ. Effect of 6-week course of glucagon-like peptide 1 on glycaemic control, insulin sensitivity, and beta-cell function in type 2 diabetes: A parallel-group study. Lancet. 2002;359:824–830. doi: 10.1016/S0140-6736(02)07952-7. [DOI] [PubMed] [Google Scholar]
- 4.Sokos GG, Nikolaidis LA, Mankad S, Elahi D, Shannon RP. Glucagon-like peptide-1 infusion improves left ventricular ejection fraction and functional status in patients with chronic heart failure. J Card Fail. 2006;12:694–699. doi: 10.1016/j.cardfail.2006.08.211. [DOI] [PubMed] [Google Scholar]
- 5.Nikolaidis LA, Doverspike A, Hentosz T, Zourelias L, Shen YT, Elahi D, Shannon RP. Glucagon-like peptide-1 limits myocardial stunning following brief coronary occlusion and reperfusion in conscious canines. J Pharmacol Exp Ther. 2005;312:303–308. doi: 10.1124/jpet.104.073890. [DOI] [PubMed] [Google Scholar]
- 6.Bose AK, Mocanu MM, Carr RD, Brand CL, Yellon DM. Glucagon-like peptide 1 can directly protect the heart against ischemia/reperfusion injury. Diabetes. 2005;54:146–151. doi: 10.2337/diabetes.54.1.146. [DOI] [PubMed] [Google Scholar]
- 7.Zhao T, Parikh P, Bhashyam S, Bolukoglu H, Poornima I, Shen YT, Shannon RP. Direct effects of glucagon-like peptide-1 on myocardial contractility and glucose uptake in normal and postischemic isolated rat hearts. J Pharmacol Exp Ther. 2006;317:1106–1113. doi: 10.1124/jpet.106.100982. [DOI] [PubMed] [Google Scholar]
- 8.Nikolaidis LA, Elahi D, Hentosz T, Doverspike A, Huerbin R, Zourelias L, Stolarski C, Shen YT, Shannon RP. Recombinant glucagon-like peptide-1 increases myocardial glucose uptake and improves left ventricular performance in conscious dogs with pacing-induced dilated cardiomyopathy. Circulation. 2004;110:955–961. doi: 10.1161/01.CIR.0000139339.85840.DD. [DOI] [PubMed] [Google Scholar]
- 9.Nikolaidis LA, Mankad S, Sokos GG, Miske G, Shah A, Elahi D, Shannon RP. Effects of glucagon-like peptide-1 in patients with acute myocardial infarction and left ventricular dysfunction after successful reperfusion. Circulation. 2004;109:962–965. doi: 10.1161/01.CIR.0000120505.91348.58. [DOI] [PubMed] [Google Scholar]
- 10.Emter CA, McCune SA, Sparagna GC, Radin MJ, Moore RL. Low-intensity exercise training delays onset of decompensated heart failure in spontaneously hypertensive heart failure rats. Am J Physiol Heart Circ Physiol. 2005;289:H2030–8. doi: 10.1152/ajpheart.00526.2005. [DOI] [PubMed] [Google Scholar]
- 11.Liang Q, Elson AC, Gerdes AM. p38 MAP kinase activity is correlated with angiotensin II type 1 receptor blocker-induced left ventricular reverse remodeling in spontaneously hypertensive heart failure rats. J Card Fail. 2006;12:479–486. doi: 10.1016/j.cardfail.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 12.Minhas KM, Saraiva RM, Schuleri KH, Lehrke S, Zheng M, Saliaris AP, Berry CE, Barouch LA, Vandegaer KM, Li D, Hare JM. Xanthine oxidoreductase inhibition causes reverse remodeling in rats with dilated cardiomyopathy. Circ Res. 2006;98:271–279. doi: 10.1161/01.RES.0000200181.59551.71. [DOI] [PubMed] [Google Scholar]
- 13.Kambara A, Holycross BJ, Wung P, Schanbacher B, Ghos S, McCune SA, Bauer JA, Kwiatkowski P. Combined effects of low-dose oral spironolactone and captopril therapy in a rat model of spontaneous hypertension and heart failure. J Cardiovasc Pharmacol. 2003;41:830–837. doi: 10.1097/00005344-200306000-00002. [DOI] [PubMed] [Google Scholar]
- 14.Li H, Simon H, Bocan TM, Peterson JT. MMP/TIMP expression in spontaneously hypertensive heart failure rats: The effect of ACE-and MMP-inhibition. Cardiovasc Res. 2000;46:298–306. doi: 10.1016/s0008-6363(00)00028-6. [DOI] [PubMed] [Google Scholar]
- 15.Quinones MA, Waggoner AD, Reduto LA, Nelson JG, Young JB, Winters WL, Ribeiro LG, Miller RR. A new, simplified and accurate method for determining ejection fraction with two-dimensional echocardiography. Circulation. 1981;64:744–753. doi: 10.1161/01.cir.64.4.744. [DOI] [PubMed] [Google Scholar]
- 16.Devereux RB, Reichek N. Echocardiographic determination of left ventricular mass in man. anatomic validation of the method. Circulation. 1977;55:613–618. doi: 10.1161/01.cir.55.4.613. [DOI] [PubMed] [Google Scholar]
- 17.Bolukoglu H, Goodwin GW, Guthrie PH, Carmical SG, Chen TM, Taegtmeyer H. Metabolic fate of glucose in reversible low-flow ischemia of the isolated working rat heart. Am J Physiol. 1996;270:H817–26. doi: 10.1152/ajpheart.1996.270.3.H817. [DOI] [PubMed] [Google Scholar]
- 18.Nikolaidis LA, Sturzu A, Stolarski C, Elahi D, Shen YT, Shannon RP. The development of myocardial insulin resistance in conscious dogs with advanced dilated cardiomyopathy. Cardiovasc Res. 2004;61:297–306. doi: 10.1016/j.cardiores.2003.11.027. [DOI] [PubMed] [Google Scholar]
- 19.Ren J, Davidoff AJ. Diabetes rapidly induces contractile dysfunctions in isolated ventricular myocytes. Am J Physiol. 1997;272:H148–58. doi: 10.1152/ajpheart.1997.272.1.H148. [DOI] [PubMed] [Google Scholar]
- 20.Davidoff AJ, Ren J. Low insulin and high glucose induce abnormal relaxation in cultured adult rat ventricular myocytes. Am J Physiol. 1997;272:H159–67. doi: 10.1152/ajpheart.1997.272.1.H159. [DOI] [PubMed] [Google Scholar]
- 21.Andersen NH, Bojesen A, Christiansen JS, Gravholt CH. Glycemia, lipidemia and systolic left ventricular function evaluated by myocardial strain rate: A tissue doppler echocardiographic study. Ultrasound Med Biol. 2008;34:151–154. doi: 10.1016/j.ultrasmedbio.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 22.Andersen NH, Hansen TK, Christiansen JS. Changes in glycaemic control are related to the systolic function in type 1 diabetes mellitus. Scand Cardiovasc J. 2007;41:85–88. doi: 10.1080/14017430601156384. [DOI] [PubMed] [Google Scholar]
- 23.Farilla L, Hui H, Bertolotto C, Kang E, Bulotta A, Di Mario U, Perfetti R. Glucagon-like peptide-1 promotes islet cell growth and inhibits apoptosis in zucker diabetic rats. Endocrinology. 2002;143:4397–4408. doi: 10.1210/en.2002-220405. [DOI] [PubMed] [Google Scholar]
- 24.Li Y, Hansotia T, Yusta B, Ris F, Halban PA, Drucker DJ. Glucagon-like peptide-1 receptor signaling modulates beta cell apoptosis. J Biol Chem. 2003;278:471–478. doi: 10.1074/jbc.M209423200. [DOI] [PubMed] [Google Scholar]
- 25.Hui H, Nourparvar A, Zhao X, Perfetti R. Glucagon-like peptide-1 inhibits apoptosis of insulin-secreting cells via a cyclic 5’-adenosine monophosphate-dependent protein kinase A-and a phosphatidylinositol 3-kinase-dependent pathway. Endocrinology. 2003;144:1444–1455. doi: 10.1210/en.2002-220897. [DOI] [PubMed] [Google Scholar]
- 26.Wang Q, Brubaker PL. Glucagon-like peptide-1 treatment delays the onset of diabetes in 8 week-old db/db mice. Diabetologia. 2002;45:1263–1273. doi: 10.1007/s00125-002-0828-3. [DOI] [PubMed] [Google Scholar]
- 27.Farilla L, Bulotta A, Hirshberg B, Li Calzi S, Khoury N, Noushmehr H, Bertolotto C, Di Mario U, Harlan DM, Perfetti R. Glucagon-like peptide 1 inhibits cell apoptosis and improves glucose responsiveness of freshly isolated human islets. Endocrinology. 2003;144:5149–5158. doi: 10.1210/en.2003-0323. [DOI] [PubMed] [Google Scholar]
- 28.Bregenholt S, Moldrup A, Blume N, Karlsen AE, Nissen Friedrichsen B, Tornhave D, Knudsen LB, Petersen JS. The long-acting glucagon-like peptide-1 analogue, liraglutide, inhibits beta-cell apoptosis in vitro. Biochem Biophys Res Commun. 2005;330:577–584. doi: 10.1016/j.bbrc.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 29.During MJ, Cao L, Zuzga DS, Francis JS, Fitzsimons HL, Jiao X, Bland RJ, Klugmann M, Banks WA, Drucker DJ, Haile CN. Glucagon-like peptide-1 receptor is involved in learning and neuroprotection. Nat Med. 2003;9:1173–1179. doi: 10.1038/nm919. [DOI] [PubMed] [Google Scholar]
- 30.Perry T, Haughey NJ, Mattson MP, Egan JM, Greig NH. Protection and reversal of excitotoxic neuronal damage by glucagon-like peptide-1 and exendin-4. J Pharmacol Exp Ther. 2002;302:881–888. doi: 10.1124/jpet.102.037481. [DOI] [PubMed] [Google Scholar]
- 31.Buteau J, El-Assaad W, Rhodes CJ, Rosenberg L, Joly E, Prentki M. Glucagon-like peptide-1 prevents beta cell glucolipotoxicity. Diabetologia. 2004;47:806–815. doi: 10.1007/s00125-004-1379-6. [DOI] [PubMed] [Google Scholar]
- 32.Buteau J, Foisy S, Rhodes CJ, Carpenter L, Biden TJ, Prentki M. Protein kinase czeta activation mediates glucagon-like peptide-1-induced pancreatic beta-cell proliferation. Diabetes. 2001;50:2237–2243. doi: 10.2337/diabetes.50.10.2237. [DOI] [PubMed] [Google Scholar]
- 33.Buteau J, Foisy S, Joly E, Prentki M. Glucagon-like peptide 1 induces pancreatic beta-cell proliferation via transactivation of the epidermal growth factor receptor. Diabetes. 2003;52:124–132. doi: 10.2337/diabetes.52.1.124. [DOI] [PubMed] [Google Scholar]
- 34.Movassat J, Beattie GM, Lopez AD, Hayek A. Exendin 4 up-regulates expression of PDX 1 and hastens differentiation and maturation of human fetal pancreatic cells. J Clin Endocrinol Metab. 2002;87:4775–4781. doi: 10.1210/jc.2002-020137. [DOI] [PubMed] [Google Scholar]
- 35.Bodine SC, Stitt TN, Gonzalez M, Kline WO, Stover GL, Bauerlein R, Zlotchenko E, Scrimgeour A, Lawrence JC, Glass DJ, Yancopoulos GD. Akt/mTOR pathway is a crucial regulator of skeletal muscle hypertrophy and can prevent muscle atrophy in vivo. Nat Cell Biol. 2001;3:1014–1019. doi: 10.1038/ncb1101-1014. [DOI] [PubMed] [Google Scholar]
- 36.Hardt SE, Sadoshima J. Glycogen synthase kinase-3beta: A novel regulator of cardiac hypertrophy and development. Circ Res. 2002;90:1055–1063. doi: 10.1161/01.res.0000018952.70505.f1. [DOI] [PubMed] [Google Scholar]
- 37.Gros R, You X, Baggio LL, Kabir MG, Sadi AM, Mungrue IN, Parker TG, Huang Q, Drucker DJ, Husain M. Cardiac function in mice lacking the glucagon-like peptide-1 receptor. Endocrinology. 2003;144:2242–2252. doi: 10.1210/en.2003-0007. [DOI] [PubMed] [Google Scholar]
- 38.Mitsuhashi H, Yatsuya H, Tamakoshi K, Matsushita K, Otsuka R, Wada K, Sugiura K, Takefuji S, Hotta Y, Kondo T, Murohara T, Toyoshima H. Adiponectin level and left ventricular hypertrophy in japanese men. Hypertension. 2007;49:1448–1454. doi: 10.1161/HYPERTENSIONAHA.106.079509. [DOI] [PubMed] [Google Scholar]
- 39.Hong SJ, Park CG, Seo HS, Oh DJ, Ro YM. Associations among plasma adiponectin, hypertension, left ventricular diastolic function and left ventricular mass index. Blood Press. 2004;13:236–242. doi: 10.1080/08037050410021397. [DOI] [PubMed] [Google Scholar]
- 40.Shibata R, Ouchi N, Ito M, Kihara S, Shiojima I, Pimentel DR, Kumada M, Sato K, Schiekofer S, Ohashi K, Funahashi T, Colucci WS, Walsh K. Adiponectin-mediated modulation of hypertrophic signals in the heart. Nat Med. 2004;10:1384–1389. doi: 10.1038/nm1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Abrahamsen N, Nishimura E. Regulation of glucagon and glucagon-like peptide-1 receptor messenger ribonucleic acid expression in cultured rat pancreatic islets by glucose, cyclic adenosine 3’,5’-monophosphate, and glucocorticoids. Endocrinology. 1995;136:1572–1578. doi: 10.1210/endo.136.4.7534705. [DOI] [PubMed] [Google Scholar]
- 42.Yamato E, Ikegami H, Takekawa K, Fujisawa T, Nakagawa Y, Hamada Y, Ueda H, Ogihara T. Tissue-specific and glucose-dependent expression of receptor genes for glucagon and glucagon-like peptide-1 (GLP-1) Horm Metab Res. 1997;29:56–59. doi: 10.1055/s-2007-978985. [DOI] [PubMed] [Google Scholar]
- 43.Sanz C, Vazquez P, Navas MA, Alvarez E, Blazquez E. Leptin but not neuropeptide Y up-regulated glucagon-like peptide 1 receptor expression in GT1-7 cells and rat hypothalamic slices. Metabolism. 2008;57:40–48. doi: 10.1016/j.metabol.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 44.Xu G, Kaneto H, Laybutt DR, Duvivier-Kali VF, Trivedi N, Suzuma K, King GL, Weir GC, Bonner-Weir S. Downregulation of GLP-1 and GIP receptor expression by hyperglycemia: Possible contribution to impaired incretin effects in diabetes. Diabetes. 2007;56:1551–1558. doi: 10.2337/db06-1033. [DOI] [PubMed] [Google Scholar]
- 45.Lamari Y, Boissard C, Moukhtar MS, Jullienne A, Rosselin G, Garel JM. Expression of glucagon-like peptide 1 receptor in a murine C cell line: Regulation of calcitonin gene by glucagon-like peptide 1. FEBS Lett. 1996;393:248–252. doi: 10.1016/0014-5793(96)00895-2. [DOI] [PubMed] [Google Scholar]


