Abstract
Background
Previous magnetic resonance imaging studies of posttraumatic stress disorder reported hippocampal volume loss. The goals of this study were 1) to determine the relationship between hippocampal atrophy and posttraumatic stress disorder in the absence of alcohol abuse, and 2) to test if loss of N-acetylaspartate (a neuron marker) in the hippocampus of posttraumatic stress disorder occurs separate from atrophy. In addition, volume changes in the entorhinal cortex were also explored.
Methods
Eighteen male patients with combat-related posttraumatic stress disorder (mean age 51.2 ± 2.5 years) and 19 male control subjects (mean age 51.8 ± 3.2 years) were studied using magnetic resonance imaging and Proton magnetic resonance spectroscopic imaging. Both groups had no alcohol and drug abuse during the past 5 years.
Results
Posttraumatic stress disorder and control subjects had similar volumes of hippocampus and entorhinal cortex. In contrast to volume, N-acetylaspartate was significantly reduced by about 23% bilaterally in the hippocampus of posttraumatic stress disorder when compared with control subjects, and creatine-containing compounds were reduced by 26% in the right hippocampus of posttraumatic stress disorder.
Conclusions
N-acetyl asparate and creatine reductions imply that there are hippocampal abnormalities in posttraumatic stress disorder. Furthermore, these metabolite changes seem to be better indicators of posttraumatic stress disorder pathology than volume losses.
Keywords: Posttraumatic stress disorder, brain imaging, spectroscopy, hippocampus, brain atrophy, N-acetylaspartate
Introduction
Posttraumatic stress disorder (PTSD) is characterized by exposure to markedly distressing traumatic event(s), re-experiencing symptoms, emotional numbing, and increased arousal. Biological alterations include adrenergic hyperresponsiveness (Southwick et al 1999), increased thyroid activity (Wang et al 1995), low cortisol levels, and increased negative feedback sensitivity of the hypothalamic-pituitary-adrenal (HPA) axis following low-dose dexamethasone administration (Yehuda et al 1993). In addition, magnetic resonance imaging (MRI) studies reported decreased volumes of the hippocampus in both Vietnam combat veterans (Bremner et al 1995; Gurvits et al 1996) and noncombat trauma victims (Bremner et al 1997; Stein et al 1997) with PTSD; however, laterality was inconsistent across these MRI studies, with volume decreases being reported in the right, the left, and both hippocampi. Because alcohol dependence and abuse are common in subjects with PTSD and are also associated with hippocampal atrophy (Laakso et al 2000), the possibility that hippocampal volume loss in PTSD might be at least in part alcohol related cannot be ruled out. This possibility is also supported by another MRI study (Wilkins et al 1996) that found no difference in hippocampal volumes between alcohol abusing PTSD patients and alcohol abusers without PTSD. Therefore, the first goal of this study was to determine the relationship between PTSD and hippocampal volume in the absence of alcohol abuse.
Proton Magnetic Resonance Spectroscopic Imaging (1H MRSI) is a technique based on physical principles similar to MRI that measures the regional distribution of certain chemicals in the brain in vivo. As performed here, 1H MRSI detects the amino acid N-acetyl asparate (NAA), which occurs in high concentrations in neurons and is virtually undetectable in other tissue types (Birken and Oldendorf 1989), therefore reflecting neuronal density or metabolism. In addition to NAA, 1H MRSI detects creatine (Cr, which reflects high-energy phosphate metabolism) and the choline moieties (Cho, which presumably reflect integrity of cell membranes). In a preliminary 1H MRSI study (Schuff et al 1997), we found decreased hippocampal NAA in a small number of veterans with PTSD, many of whom had been recently abusing alcohol, compared with healthy controls without a history of alcohol abuse. Another 1H MRSI study reported NAA reductions in medial temporal lobe structures of veteran PTSD subjects (Freeman et al 1998). Therefore, the second goal of this study was to determine if 1H MRSI measurements could detect NAA changes in the hippocampus of PTSD, separate from volume changes.
This study was designed to test the following hypotheses: 1) PTSD without recent history (past 5 years) of alcohol dependence is associated with reductions in hippocampal volume and NAA, and 2) NAA loss in the hippocampus of PTSD is disproportionately greater than volume loss. In addition, we explored whether PTSD is also associated with volume loss in the entorhinal cortex (ERC), which has strong neuronal connections to the hippocampus.
Methods and Materials
Subject Recruitment and Clinical Assessment
Subjects were recruited from the outpatient clinics of the San Francisco Veterans Affairs Medical Center and from the community by advertisement. After a complete description of the study procedures, all subjects provided written informed consent that had been approved by the committee of human research at the University of California, San Francisco. There were 18 male PTSD patients (mean age 51.2 ± 2.5 years) and 19 male control subjects (mean age 51.8 ± 3.2 years) in this study. The Structured Clinical Interview for DSM-IV Diagnosis (SCID) (Spitzer et al 1992) was used to rule out neurologic and psychiatric disorders other than PTSD. Initially, an attempt was made to identify PTSD subjects without a lifetime history of alcohol abuse or dependence, but few such subjects could be identified. Therefore, entry criteria were modified to exclude subjects with alcohol abuse or dependence during the previous 5 years. The number of lifetime cumulative alcohol drinks were obtained from all but two subjects. Also excluded where subjects with abuse of illicit drugs during the previous 5 years, major depression in the past 3 months, or with a history of loss of consciousness after head trauma. Four patients and four control subjects had a history of drug abuse and ten patients and three control subjects had some depression during lifetime. Posttraumatic stress disorder patients with a history of using antipsychotic medications within the past 6 months before this study were excluded. Six patients used antidepressant medications and two patients used mood-stabilizing agents. Control subjects were free of all psychiatric medication. The Clinician Administered PTSD Scale (CAPS) (Blake et al 1995) was used to diagnose PTSD, as well as to assess current severity. Subjects with PTSD had a mean current CAPS score of 63.1 ± 11.5 and controls had a mean CAPS score of 4.6 ± 4.0.
MRI and 1H MRSI Acquisition
The subjects were scanned on a 1.5T VISION™ magnetic resonance (MR) system (Siemens Inc., Iselin, NJ), equipped with a standard head coil. Structural MRI data were acquired using a double spin echo sequence (DSE) with TR/TE1/TE2 = 2500/20/80 msec timing, 1.0 × 1.4 mm2 inplane resolution, and about 50 contiguous 3 mm thick axial slices, oriented along the optic nerve as seen from a midsection scout MRI. In addition, a volumetric magnetization-prepared rapid gradient echo (MPRAGE) sequence was acquired with TR/TE/TI = 10/4/300 msec timing, 15 degree flip angle, 1.0 × 1.0 mm2 inplane resolution, and 1.4 mm thick coronal partitions, oriented orthogonal to the DSE image plane. A Point Resolved Spectroscopy (PRESS) 1H MRSI (Bottomley 1987) sequence was used to acquire water suppressed 1H MR spectra simultaneously from both hippocampi with TR/TE = 1800/135 msec timing. K-space sampling was accomplished with 24 × 24 circularly bounded encoding steps across 210 × 210 mm2 field of view and a 15 mm thick slice was selected, aligned along the long axis of the hippocampus, yielding about 1.1 milliliter sized MRSI voxels.
Volume Measurements of Hippocampus and ERC
Volumes of the left and right hippocampus and ERC were measured by manually drawing the boundaries of these structures on the coronal T1-weighted MP-RAGE images. Boundaries of the hippocampus were drawn following the guidelines of Watson et al (1992), including hippocampal proper, dentate gyrus, subiculum, fimbria, and alveus. Boundaries of the ERC were drawn according to the protocol developed by Insausti et al (1998). The raters were blinded to all clinical information. Variability of volume measurements was 1.8% for the hippocampus and 3% for ERC. To account for variations in head size, hippocampal and ERC volumes were normalized to the total intracranial volume, which was determined based on MRI segmentation data. Segmentation of MRI data into gray matter, white matter, and cerebrospinal fluid (CSF) was achieved automatically with software developed in-house (Tanabe et al 1997). The masks from manual tracing of the left and right hippocampus were also incorporated into the segmentation data as separate tissue classes.
1H MRSI Spectral Processing
The 1H MRSI data were zero-padded to 1024 points in the spectral domain and to 32 × 32 points in the spatial domain before Fourier reconstruction. Four Hz Gaussian line broadening was used in the spectral direction, and mild Gaussian apodization was applied along the spatial directions to reduce Gibbs ringing effects, resulting in an effective voxel size of approximately 1.6 mL. Peak areas of NAA and creatine- (Cr) and choline- (Cho) containing compounds were estimated using fully automated spectral fitting software developed in-house (Soher et al 1998). Quality control was accomplished by rejecting peaks with a linewidth at half peak height of more than 10 Hz or with residual sum squares of the fits outside the .05 and .95 percentile distribution. To obtain metabolite concentrations, the amounts of gray matter, white matter, CSF, and left and right hippocampal tissue in each MRSI voxel were estimated using information from the segmented MRI data. This was accomplished first by aligning the segmented MRI with the MRSI data, assuming that there was no head movement between MRI and MRSI scans, and second by blurring the segmented MRI data to the spatial resolution of MRSI, as described in detail by Schuff et al (2001). In addition, MRSI voxels that best covered the right or left hippocampus in each subject was selected, based on the estimated amount of hippocampal tissue in the voxel. Finally, the metabolite values were corrected for the amount of CSF in the MRSI voxels to obtain concentrations (in arbitrary units) and also normalized to the median ventricular CSF intensity from each subject, as measured with proton density MRI to compensate for instrumental variations.
Statistical Analysis
Effects of group and hemisphere on volume changes in the left and right hippocampus or ERC were tested using multivariate analysis of variance (MANOVA). Similarly, effects of group and hemisphere on metabolite changes in the left and right hippocampus were tested using MANOVA. Relationships of PTSD severity (measured by CAPS) with volume or metabolite changes were tested using Pearson correlation coefficients.
Results
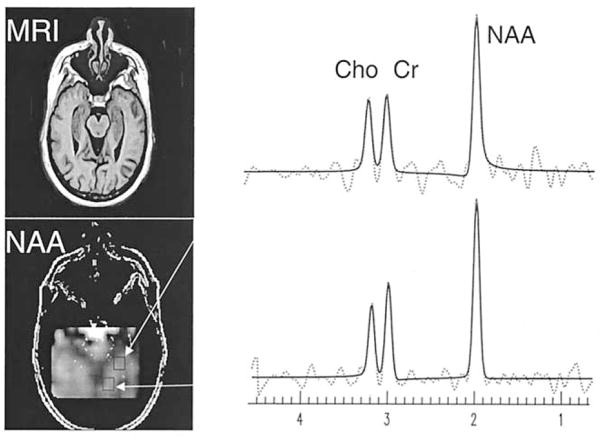
In Figure 1 is shown a representative NAA metabolite image from the hippocampal region of a 49-year-old man with PTSD and 1H MR spectra from two locations in the hippocampus. Results from automatic spectral fitting are superimposed (solid line) onto the raw spectral data (dashed line). Also shown is the corresponding MR image for better anatomical orientation.
Figure 1.
Representative oblique axial image of N-acetyl asparte (NAA) from the hippocampal region of a 49-year-old posttraumatic stress disorder (PTSD) patient. Also shown is the corresponding magnetic resonance (MR) image for better anatomical orientation. On the right are depicted representative proton magnetic resonance (1H MR) spectra from two locations in the hippocampus. The dashed lines represent the raw spectral data, the solid lines are the spectral estimations using the automated fitting software (Soher et al 1998).
Table 1 lists the volumes of the left and right hippocampus and the left and right ERC in PTSD and control subjects. There were no significant differences between PTSD and control subjects in volumes of the hippocampus [right: F(1,36) = .1, p > .7; left: F(1,36) = .4, p > .5] and the ERC [right: F(1,36) = .2, p > .6; left: F(1,36) = 1.2, p > .2].
Table 1.
Volumesa of the Hippocampus (HP) and Entorhinal Cortex (ERC)
| Region | PTSD | Control | Differenceb |
|---|---|---|---|
| Left HP | 3240 ± 417 | 3292 ± 399 | −2c |
| Right HP | 3460 ± 424 | 3364 ± 384 | +3c |
| Left ERC | 1564 ± 377 | 1604 ± 332 | −2c |
| Right ERC | 1381 ± 280 | 1453 ± 262 | −5c |
PTSD, posttraumatic stress disorder.
mm3.
Difference in percent compared to controls.
No significant group effects.
Table 2 lists the concentrations of NAA, Cr, and Cho in the hippocampal region of PTSD and control subjects. N-acetyl asparate concentrations were significantly lower by about 23% in both, the left [F(1,34) = 7.0, p = .002] and right [F(1,34) = 8.4, p = .007] hippocampus of PTSD when compared with controls. There were no significant effects for antidepressant or mood stabilizing medications on NAA concentrations within PTSD subjects (p > .2 by t test). Similarly, history of drug abuse had no significant effect on NAA differences between PTSD and control subjects (p > .4, by t test). Lifetime alcohol abuse, as measured by the number of lifetime cumulative alcoholic drinks was significantly higher in PTSD patients (34.5*103 ± 45.8*103 drinks) than in control subjects (15,1*103 ± 19,7*103 drinks, p < .001 by Wilcoxon rank test); however, lifetime alcohol abuse did not contribute significantly to the NAA differences between patients and controls (p > .2 by MANCOVA) and NAA reduction in PTSD remained significant after accounting for variations in alcohol use. Cr concentrations were also significantly lower in PTSD when compared with controls by 26% in the right hippocampus [F(1,34) = 12.4, p < .002] and 11% lower in the left hippocampus [F(1,34) = 1.5, p > .1], which, however, was not significant. There were also no significant effects of medications on Cr in PTSD (p > .8) and history of drug abuse (p > .2) on low Cr in PTSD. Furthermore, lifetime alcohol abuse did not contribute significantly to Cr differences between patients and controls (p > .4 by MANCOVA). In contrast to NAA and Cr, Cho concentrations were not significantly different between the groups [both sides: F(1,34) < .7, p > .5].
Table 2.
Concentrationsa of N-acetyl Aspartate-(NAA) and Creatine-(Cr) and Choline-(Cho) Containing Compounds from Left and Right Hippocampus Regions
| Metabolites | PTSD | Control | Differenceb | Effect Sizec |
|---|---|---|---|---|
| Left NAA | 2.8 ± .8 | 3.7 ± .8 | −24e | 1.12 |
| Right NAA | 2.9 ± .9 | 3.8 ± .7 | −23e | 1.12 |
| Left Cr | 2.3 ± .7 | 2.6 ± .9 | −11 | .37 |
| Right Cr | 2.2 ± .6 | 3.0 ± .7 | −26e | 1.23 |
| Left Cho | 2.8 ± 1.2 | 2.5 ± 1.3 | 12 | .24 |
| Right Cho | 2.6 ± 1.2 | 2.8 ± 1.2 | −7 | .17 |
| Hippocampal tissued | .4 ± .7 | .4 ± .9 | 0 |
PTSD, posttramatic stress disorder; MRSI, magnetic resonance spectroscopic imaging.
Concentrations in arbitrary units.
Difference in percent compared to controls.
Effect size, 2 × (MeanPTSD − MeanControls)/(StdevPTSD + StdevControls), where Stdev is the standard deviation.
Fraction of hippocampal to total gray and white matter tissue within a MRSI voxel.
p < .01.
Also listed in Table 2 is the fraction of hippocampal tissue within MRSI voxels from the hippocampal region. This shows that these voxels contained on average 40% tissue from the hippocampus without a significant difference between PTSD and control subjects (p < .8, two-sided t test).
Finally, there were no significant correlations between PTSD severity measured by CAPS total scores and volumes of the hippocampus and ERC. Similarly, there were no significant correlations between PTSD severity and hippocampal NAA or Cr concentrations.
Discussion
The major findings of this report are 1) NAA and Cr concentrations were significantly reduced in the hippocampus of PTSD patients when compared with control subjects, implying that there are hippocampal abnormalities in PTSD; 2) however, there were no significant differences in hippocampal or ERC volumes between PTSD patients without recent history of alcohol abuse and control subjects. This contrasts to previous reports of hippocampal atrophy in PTSD, suggesting that alcohol abuse may have been at least in part responsible for these previous findings.
Changes in NAA have been widely attributed to changes in neuron number, density, or neuronal metabolism (Birken and Oldendorf 1989). The finding of 23% to 24% reduction of hippocampal NAA in PTSD subjects of this study, in the absence of hippocampal volume loss, were surprising for two reasons. First, the magnitude of the NAA reduction is very similar to that, which we previously reported for patients with Alzheimer’s dementia (Schuff et al 1997). Notwithstanding this similarity of NAA reductions, our PTSD patients showed no gross cognitive memory impairments. Second, hippocampal NAA reductions in Alzheimer’s disease were accompanied by substantial hippocampal volume losses in the range from 20% to 40% (Schuff et al 1997). Reduced NAA of the anterior cingulate and no apparent atrophy on MRI was also reported from a 1H MRS study of children with PTSD after sexual abuse (De Bellis et al 2000). It is possible that neuron loss in PTSD is accompanied by sufficient glial proliferation that attenuates atrophy but does not affect NAA loss because there is no NAA in nonneuronal tissue (Birken and Oldendorf 1989). Single photon emission computer tomography (SPECT) studies of PTSD (Sachinvala et al 2000) showed increased blood perfusion in limbic areas, including the hippocampus, consistent with glial proliferation. We have observed reduced NAA with minimal hippocampal atrophy also in elderly patients with mild cognitive impairment, presumably due to gliosis in the early stages of neuronal degeneration (Schuff et al 1997). Another explanation for NAA decrease in PTSD is impairment of neuronal processes, resulting in secondary NAA loss. Reversible NAA losses have been found in amyotrophic lateral sclerosis (Kalra et al 1998), epilepsy after surgery (Hugg et al 1996), in multiple sclerosis (De Stefano et al 1995), and more recently in schizophrenic patients after treatment with antipsychotics (Bertolino et al 2001; Heimberg et al 1998). These changes have been attributed to reversible impairment of oxidative metabolism from which NAA production is dependent. Therefore, we cautiously interpret the finding of decreased hippocampal NAA in PTSD to reflect either neuron loss in the presence of gliosis and/or neuronal metabolic impairments. In this regard, NAA changes would be expected to be more sensitive to neuronal damage in PTSD than volume loss. The effect size in this study of hippocampal NAA changes was 1.2 (see Table 2), which is markedly higher than the effect sizes in most (albeit not all) MRI studies of hippocampal volume losses in PTSD (see Table 3).
Table 3.
Other MRI Studies of Hippocampus in PTSD
| Trauma/Publications | Combat Bremner | Combat Gurvitz | Sex Abuse Bremner | Sex Abuse Stein |
|---|---|---|---|---|
| (PTSD/Control) | (22/26) | (7/7) | (17/17) | (21/21) |
| PTSD: | ||||
| Right | 1184 ± 142 | 3200 ± 600 | 1062 ± 169 | 2099 ± 165 |
| Left | 1186 ± 138 | 3200 ± 600 | 1050 ± 152 | 2192 ± 177 |
| Control: | ||||
| Right | 1286 ± 175 | 4100 ± 400 | 1116 ± 190 | 2160 ± 210 |
| Left | 1233 ± 163 | 4300 ± 300 | 1193 ± 134 | 2307 ± 193 |
| Differencea and Significance | ||||
| Right | −8; p ± .03 | −22; p ± .001 | −4; p ± .4 | −3; ns |
| Left | −4; p ± .28 | −26; p ± .001 | −12; p ± .008 | −5; p < .05 |
| Effect Sizeb | ||||
| Right | .64 | 1.8 | .3 | .33 |
| Left | .31 | 2.4 | 1.0 | .62 |
MRI, magnetic resonance imaging; PTSD, posttraumatic stress disorder.
In percentage compared to controls.
See Table 2 for definition.
The resonance of Cr, detected by 1H MRSI, reflects tissue composition of phosphocreatine (high energy phosphate reservoir) and free creatine. Acute ischemia, or other factors, which impair oxidative metabolism will reduce the ratio of phosphocreatine to creatine, but should not necessarily alter total creatine content. Creatine concentrations are usually higher in gray matter than in white matter (Schuff et al 2001), presumably due to the higher metabolic rate of gray matter. Replacement of neurons in the hippocampus of PTSD by glial cells might explain the decrease of Cr in PTSD.
In contrast to substantial changes of NAA and Cr, we found no Cho changes in PTSD. The resonance of Cho, detected by 1H MRSI, reflects primarily phosphocholine and glycerophosphocholine, which are both constituents of cell membranes. Cho increases have been reported in multiple sclerosis lesions (Bitsch et al 1999) and in tumor cells (Wilken et al 2000), presumably indicating membrane degradation and glial proliferation. On the other hand, an increase of Cho might not be expected in chronic illnesses like PTSD, unless there is continuing gliosis of sufficient magnitude following neuronal damage.
The second major finding of this study was no significant atrophy in the hippocampus and ERC of PTSD. Previous MRI findings of hippocampal atrophy in PTSD are summarized in Table 3. Bremner et al (1995) found an 8% decrease of hippocampal volume in 26 Vietnam veterans with PTSD compared with 22 control subjects (matched for years of lifetime alcohol abuse and other factors). Furthermore, the decrease of hippocampal volume in PTSD was associated with deficits in short term verbal memory; however, only a midsection of the hippocampus was measured and volumes were not adjusted for head size that together, may have resulted in spurious findings. In another study of Vietnam veterans with PTSD, Gurvits et al (1996) compared the hippocampal volumes of seven combat veterans with PTSD, seven combat veterans without a lifetime history of PTSD, and eight normal controls. The authors found a significant bilateral 22–26% reduction in hippocampal volume (adjusted for whole brain volume) of PTSD. Smaller hippocampal volumes were positively associated with greater combat exposure, greater PTSD severity, and greater impairment on neuropsychological measures; however, this study had a small sample size (including one PTSD subject with bipolar disorder) and groups were not matched for age. Furthermore, the findings differed in magnitude and laterality from the report by Bremner (Bremner et al 1995). In a study of 17 adult victims of childhood sexual abuse, Bremner and colleagues (Bremner et al 1997) found a 12% volume reduction in the left hippocampus (not in the right as in the study of Vietnam veterans) when compared with 17 control subjects matched for years of alcohol abuse; however, there was no matching for major depression and similar to their earlier report, only a midsection of the hippocampus was measured. In another study on 21 women with a history of child sexual abuse, Stein and colleagues (Stein et al 1997) reported a significant 5% volume reduction in the left hippocampus when compared with 21 women without abuse history. In addition, hippocampal volume decrease correlated with dissociative symptom severity, but not with indices of explicit memory functioning; however, alcohol abuse, depression, and substance abuse were greater in the PTSD patients than the control group. Recently, Wikins et al (1996) found no difference in hippocampal volumes between 10 PTSD alcohol abusing patients and eight alcohol abusers without PTSD.
Despite the importance of these reports, there were some limitations: first, laterality was inconsistent across the studies, with changes being reported in the right, the left, and/or both hippocampi. Second, most studies included PTSD subjects with current alcohol dependence and/or depression. Alcoholism is common in PTSD (Fontanaet al 1990) and is associated with hippocampal atrophy (Laakso et al 2000). Depression is also common in PTSD (Fontana et al 1990), impairs memory performance, and has opposite effects on HPA axis function from PTSD (Axelson et al 1993a). The relationship between depression and hippocampal size, however, is controversial. While some MRI studies (Nurnberger et al 1994; Sheline et al 1996) reported significantly reduced hippocampal volumes in major depression, other studies found no reduction (Axelson et al 1993b; Vakili et al 2000), although depression severity and treatment response correlated with hippocampal size (Vakili et al 2000). In conclusion, there remain questions concerning the role of potential confounding variables in the previously reported MRI data in PTSD.
The current finding of no hippocampal volume difference between PTSD and controls strongly suggests that PTSD by itself is not associated with hippocampal atrophy. Given the means and SE of our measurements, we had the power to detect a 9% reduction of hippocampal volumes at .05 alpha, while previous MRI studies of PTSD reported significant hippocampal volume losses between 5% and 26%. This implies that previous reports may have been confounded by alcohol and/or depression. Whether there is an interaction between PTSD and alcoholism, and/or depression to accentuate hippocampal atrophy has not been determined.
Volumes of the ERC were also not significantly different between PTSD and controls. We had hypothesized that PTSD would be associated with reduced ERC volumes, because the ERC is part of the limbic-hippocampal complex with a high density of glucocorticoid receptors (Turner et al 1998). The results show that, similar to the hippocampus, there is no association between ERC size and PTSD.
Possible mechanisms of hippocampal injury in PTSD
Elevated levels of glucocorticoids, the adrenal steroids that are secreted during stress, are known to affect specifically hippocampal neurons and/or glial cells by inhibiting glucose metabolism (Horner et al 1990) and antioxidant enzyme activity (Sapolsky et al 1988), besides several other processes. Furthermore, excessive exposure to glucocorticoids seems to affect the ability of hippocampal neurons to survive (Sapolsky et al 1990) and in previous PTSD studies the evidence of this phenomenon was decreased hippocampal volume. The finding of decreased NAA and Cr in the absence of decreased volume of the hippocampus of PTSD is still consistent with metabolic impairment and/or loss of neurons. Furthermore, the results of this study add support to the view that the hippocampus may participate in the pathophysiology of PTSD, as a preexposure condition increasing vulnerability to PTSD, as a result of glucocorticoid bursts during acute traumatic stress or as a contributor to symptomatology. Damage to neurons in the hippocampus could begin during the exposure to the traumatic stressor(s), a view that is consistent with the large body of research about the acute effects of stress; however, MRI findings indicate that exposure alone does not impact the hippocampus (Gurvits et al 1996). On the other hand, if the impact of acute stress on the hippocampus is repeatedly encountered, as a result of the characteristic symptoms of re-experiencing for example, a more parsimonious mechanism emerges that fits the existing data about both acute stress and PTSD. Alternatively, reduced NAA and Cr levels could be related to a premorbid condition of increased vulnerability of hippocampal neurons to insults, possibly predisposing the individual for the development of PTSD. Whether these interpretations are accurate can only be addressed in future prospective studies.
There are several limitations to this study. Firstly, we did not control for lifetime burden of depression that may have contributed to hippocampal atrophy. Secondly, a technical limitation is that the coarse spatial resolution of 1H MRSI sampled also some tissue outside the hippocampus that may have introduced spurious NAA variations. Furthermore, T1/T2 relaxations of the metabolite resonances were not measured due to prohibitively long acquisition times, prohibiting determination of absolute metabolite concentrations.
In conclusion, this study showed substantial NAA and Cr losses in the absence of volume losses in the hippocampal region of PTSD patients without alcohol abuse during the last five years, a finding that is consistent with the view that the hippocampus participates in the pathophysiology of PTSD. Further research will be necessary to determine the significance of these findings.
Acknowledgments
We are very grateful to Dawn Hardin for rating of hippocampal volumes and to Marybeth Kedzior and Camilla Johnson for scanning and processing MRSI and MRI data. We thank Tom Metzler for help with data analysis. This study was supported by a VA Merit Award Grant (M. W. Weiner and C. R. Marmar).
References
- Axelson DA, Doraiswamy PM, McDonald WM, Boyko OB, Tupler LA, Patterson LJ, et al. Hypercortisolemia and hippocampal changes in depression. Psychiatry Res. 1993a;47:163–173. doi: 10.1016/0165-1781(93)90046-j. [DOI] [PubMed] [Google Scholar]
- Axelson DA, Doraiswamy PM, McDonald WM, Boyko OB, Tupler LA, Patterson LJ, et al. Hypercortisolemia and hippocampal changes in depression. Psychiatry Res. 1993b;47:163–173. doi: 10.1016/0165-1781(93)90046-j. [DOI] [PubMed] [Google Scholar]
- Bertolino A, Callicott JH, Mattay VS, Weidenhammer KM, Rakow R, Egan MF, et al. The effect of treatment with antipsychiatric drugs on brain N-acetylaspartate measures in patients with schizophrenia. Biol Psychiatry. 2001;49:39–46. doi: 10.1016/s0006-3223(00)00997-5. [DOI] [PubMed] [Google Scholar]
- Birken DL, Oldendorf WH. N-acetyl-L-aspartic acid: A literature review of a compound prominent in 1H-NMR spectroscopic studies of brain. Neurosci Biobehav Rev. 1989;13:23–31. doi: 10.1016/s0149-7634(89)80048-x. [DOI] [PubMed] [Google Scholar]
- Bitsch A, Bruhn H, Vougioukas V, Stringaris A, Lassmann H, Frahm J, et al. Inflammatory CNS demyelination: Histopathologic correlation with in vivo quantitative proton MR spectroscopy. AJNR Am J Neuroradiol. 1999;20:1619–1627. [PMC free article] [PubMed] [Google Scholar]
- Blake DD, Weathers FW, Nagy LM, Kaloupek DG, Gusman FD, Charney DS, et al. The development of a Clinician-Administered PTSD Scale. J Trauma Stress. 1995;8:75–90. doi: 10.1007/BF02105408. [DOI] [PubMed] [Google Scholar]
- Bottomley PA. Spatial localization in NMR spectroscopy in vivo. Ann N Y Acad Sci. 1987;508:333–348. doi: 10.1111/j.1749-6632.1987.tb32915.x. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Randall P, Scott TM, Bronen RA, Seibyl JP, Southwick SM, et al. MRI-based measurement of hippocampal volume in patients with combat-related posttraumatic stress disorder. Am J Psychiatry. 1995;152:973–981. doi: 10.1176/ajp.152.7.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner JD, Randall P, Vermetten E, Staib L, Bronen RA, Mazure C, et al. MRI-based measurement of hippocampal volume in posttraumatic stress disorder related to childhood physical and sexual abuse-A preliminary report. Biol Psychiatry. 1997;41:23–32. doi: 10.1016/s0006-3223(96)00162-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Bellis MD, Keshavan MS, Spencer S, Hall J. N-Acetylaspartate concentration in the anterior cingulate of maltreated children and adolescents with PTSD. Am J Psychiatry. 2000;157:1175–1177. doi: 10.1176/appi.ajp.157.7.1175. [DOI] [PubMed] [Google Scholar]
- De Stefano N, Matthews PM, Arnold DL. Reversible decreases in N-acetylaspartate after acute brain injury. Magn Reson Med. 1995;34:721–727. doi: 10.1002/mrm.1910340511. [DOI] [PubMed] [Google Scholar]
- Fontana A, Rosenheck R, Spencer H. The First Progress Report on the Department of Veterans Affairs PTSD Clinical Teams Program. West Haven, CT: Northeast Program Evaluation Center; 1990. [Google Scholar]
- Freeman TW, Cardwell D, Karson CN, Komoroski RA. In vivo proton magnetic resonance spectroscopy of the medial temporal lobes of subjects with combat-related posttraumatic stress disorder. Magn Reson Med. 1998;40:66–71. doi: 10.1002/mrm.1910400110. [DOI] [PubMed] [Google Scholar]
- Gurvits TV, Shenton ME, Hokama H, Ohta H, Orr SP, Lasko NB, et al. Magnetic resonance imaging study of hippocampal volume in chronic, combat-related posttraumatic stress disorder. Biol Psychiatry. 1996;40:1091–1099. doi: 10.1016/S0006-3223(96)00229-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heimberg C, Komoroski RA, Lawson WB, Cardwell D, Karson CN. Regional proton magnetic resonance spectroscopy in schizophrenia and exploration of drug effect. Psychiatry Res. 1998;83:105–115. doi: 10.1016/s0925-4927(98)00034-1. [DOI] [PubMed] [Google Scholar]
- Horner HC, Packan DR, Sapolsky RM. Glucocorticoids inhibit glucose transport in cultured hippocampal neurons and glia. Neuroendocrinology. 1990;52:57–64. doi: 10.1159/000125539. [DOI] [PubMed] [Google Scholar]
- Hugg JW, Kuzniecky RI, Gilliam FG, Morawetz RB, Faught RE, Hetherington HP. Normalization of contralateral metabolic function following temporal lobectomy demonstrated by h-1 magnetic resonance spectroscopic imaging. Ann Neurol. 1996;V40:236–239. doi: 10.1002/ana.410400215. [DOI] [PubMed] [Google Scholar]
- Insausti R, Juottonen K, Soininen H, Insausti AM, Partanen K, Vainio P, et al. MR volumetric analysis of the human entorhinal, perirhinal, and temporopolar cortices. AJNR Am J Neuroradiol. 1998;19:659–671. [PMC free article] [PubMed] [Google Scholar]
- Kalra S, Cashman NR, Genge A, Arnold DL. Recovery of N-acetylaspartate in corticomotor neurons of patients with ALS after riluzole therapy. Neuroreport. 1998;9:1757–1761. doi: 10.1097/00001756-199806010-00016. [DOI] [PubMed] [Google Scholar]
- Laakso MP, Vaurio O, Savolainen L, Repo E, Soininen H, Aronen HJ, et al. A volumetric MRI study of the hippocampus in type 1 and 2 alcoholism. Behav Brain Res. 2000;109:177–186. doi: 10.1016/s0166-4328(99)00172-2. [DOI] [PubMed] [Google Scholar]
- Nurnberger JI, Blehar MC, Kaufmann CA, York-Cooler C, Simpson SG, Harkavy-Friedman J, et al. Diagnostic interview for genetic studies. Arch General Psychiatry. 1994;51:849–859. doi: 10.1001/archpsyc.1994.03950110009002. [DOI] [PubMed] [Google Scholar]
- Sachinvala N, Kling A, Suffin S, Lake R, Cohen M. Increased regional cerebral perfusion by 99 mTc hexamethyl propylene amine oxime single photon emission computed tomography in posttraumatic stress disorder. Mil Med. 2000;165:473–479. [PubMed] [Google Scholar]
- Sapolsky RM, Packan DR, Vale WW. Glucocorticoid toxicity in the hippocampus: In vitro demonstration. Brain Res. 1988;453:367–371. doi: 10.1016/0006-8993(88)90180-1. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM, Uno H, Rebert CS, Finch CE. Hippocampal damage associated with prolonged glucocorticoid exposure in primates. J Neurosci. 1990;10:2897–2902. doi: 10.1523/JNEUROSCI.10-09-02897.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuff N, Amend D, El Din N, Norman D, Fein G, Weiner MW. Reduced hippocampal NAA and volume in elderly with mild cognitive impairments. Proceedings of the 5th International Society for Magnetic Resonance in Medicine; Vancouver, Canada. 1997. p. 1207. [Google Scholar]
- Schuff N, Amend D, Ezekiel F, Steinman S, Tanabe JL, Norman D, et al. Changes of hippocampal n-acetyl aspartate and volume in Alzheimer’s disease: A proton MR spectroscopic imaging and MRI study. Neurology. 1997;49:1513–1521. doi: 10.1212/wnl.49.6.1513. [DOI] [PubMed] [Google Scholar]
- Schuff N, Ezekiel F, Gamst A, Amend D, Capizzano A, Maudsley AA, et al. Region and tissue differences of metabolites in normally aged brain using 1H magnetic resonance spectroscopic imaging. Magn Reson Med. 2001;45:899 –907. doi: 10.1002/mrm.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuff N, Marmar CR, Weiss DS, Neylan TC, Schoenfeld F, Fein G, et al. Reduced hippocampal volume and n-acetylaspartate in post traumatic stress disorder. Ann N Y Acad Sci Vol. 1997;821:516–520. doi: 10.1111/j.1749-6632.1997.tb48319.x. [DOI] [PubMed] [Google Scholar]
- Sheline YI, Wang PW, Gado MH, Csernansky JG, Vannier MW. Hippocampal atrophy in recurrent major depression. Proc Natl Acad Sci USA. 1996;93:3908–3913. doi: 10.1073/pnas.93.9.3908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soher BJ, Young K, Govindaraju V, Maudsley AA. Automated spectral analysis III: Application to in vivo proton MR spectroscopy and spectroscopic imaging. Magn Reson Med. 1998;40:822–831. doi: 10.1002/mrm.1910400607. [DOI] [PubMed] [Google Scholar]
- Southwick SM, Paige S, Morgan CA, Bremner JD, Krystal JH, Charney DS. Neurotransmitter alterations in PTSD: Catecholamines and serotonin. Semin Clin Neuropsychiatry. 1999;4:242–248. doi: 10.153/SCNP00400242. [DOI] [PubMed] [Google Scholar]
- Spitzer RL, Williams JB, Gibbon M, First MB. The Structured Clinical Interview for DSM-III-R (SCID). I: History, rationale, and description. Arch General Psychiatry. 1992;49:624–629. doi: 10.1001/archpsyc.1992.01820080032005. [DOI] [PubMed] [Google Scholar]
- Stein MB, Koverola C, Hanna C, Torchia MG, McClarty B. Hippocampal volume in women victimized by childhood sexual abuse. Psychol Med. 1997;27:951–959. doi: 10.1017/s0033291797005242. [DOI] [PubMed] [Google Scholar]
- Tanabe JL, Amend D, Schuff N, DiSclafani V, Ezekiel F, Norman D, et al. Tissue segmentation of the brain in Alzheimer disease. AJNR Am J Neuroradiol. 1997;18:115–123. [PMC free article] [PubMed] [Google Scholar]
- Turner DA, Buhl EH, Hailer NP, Nitsch R. Morphological features of the entorhinal-hippocampal connection. Prog Neurobiol. 1998;55:537–562. doi: 10.1016/s0301-0082(98)00019-7. [DOI] [PubMed] [Google Scholar]
- Vakili K, Pillay SS, Lafer B, Fava M, Renshaw PF, Bonello-Cintron CM, et al. Hippocampal Volume in Primary Unipolar Major Deppression A magnetic Resonance Imaging Study. Biol Psychiatry. 2000;47:1087–1090. doi: 10.1016/s0006-3223(99)00296-6. [DOI] [PubMed] [Google Scholar]
- Wang S, Mason J, Southwick S, Johnson D, Lubin H, Charney D. Relationships between thyroid hormones and symptoms in combat-related posttraumatic stress disorder. Psychosom Med. 1995;57:398–402. doi: 10.1097/00006842-199507000-00012. [DOI] [PubMed] [Google Scholar]
- Watson C, Andermann F, Gloor P, Jones-Gotman M, Peters T, Evans A, et al. Anatomic basis of amygdaloid and hippocampal volume measurement by magnetic resonance imaging. Neurology. 1992;42:1743–1750. doi: 10.1212/wnl.42.9.1743. [DOI] [PubMed] [Google Scholar]
- Wilken B, Dechent P, Herms J, Maxton C, Markakis E, Hanefeld F, et al. Quantitative proton magnetic resonance spectroscopy of focal brain lesions. Pediatr Neurol. 2000;23:22–31. doi: 10.1016/s0887-8994(00)00141-7. [DOI] [PubMed] [Google Scholar]
- Wilkins RT, Wahlberg LJ, Marquardt JE, Sandberg EJ, Aasal R, Teale P, et al. Hippocampal volume and neuropsychological functioning in veterans with combat-related PTSd. 12th Annual Meeting of the Intl. Society for Traumatic Stress Studies; Denver. 1996. p. 43. [Google Scholar]
- Yehuda R, Southwick SM, Krystal JH, Bremner D, Charney DS, Mason JW. Enhanced suppression of cortisol following dexamethasone administration in posttraumatic stress disorder. Am J Psychiatry. 1993;150:83–86. doi: 10.1176/ajp.150.1.83. [DOI] [PubMed] [Google Scholar]