Abstract
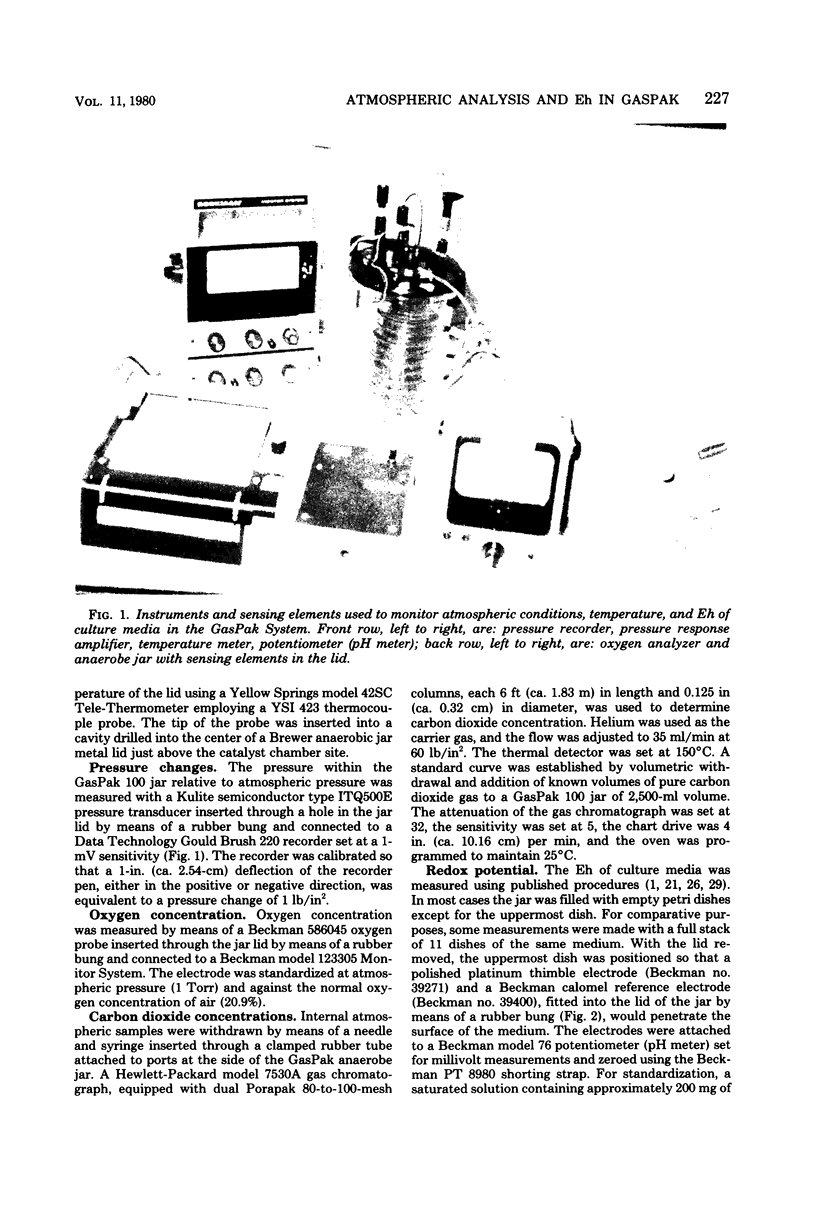
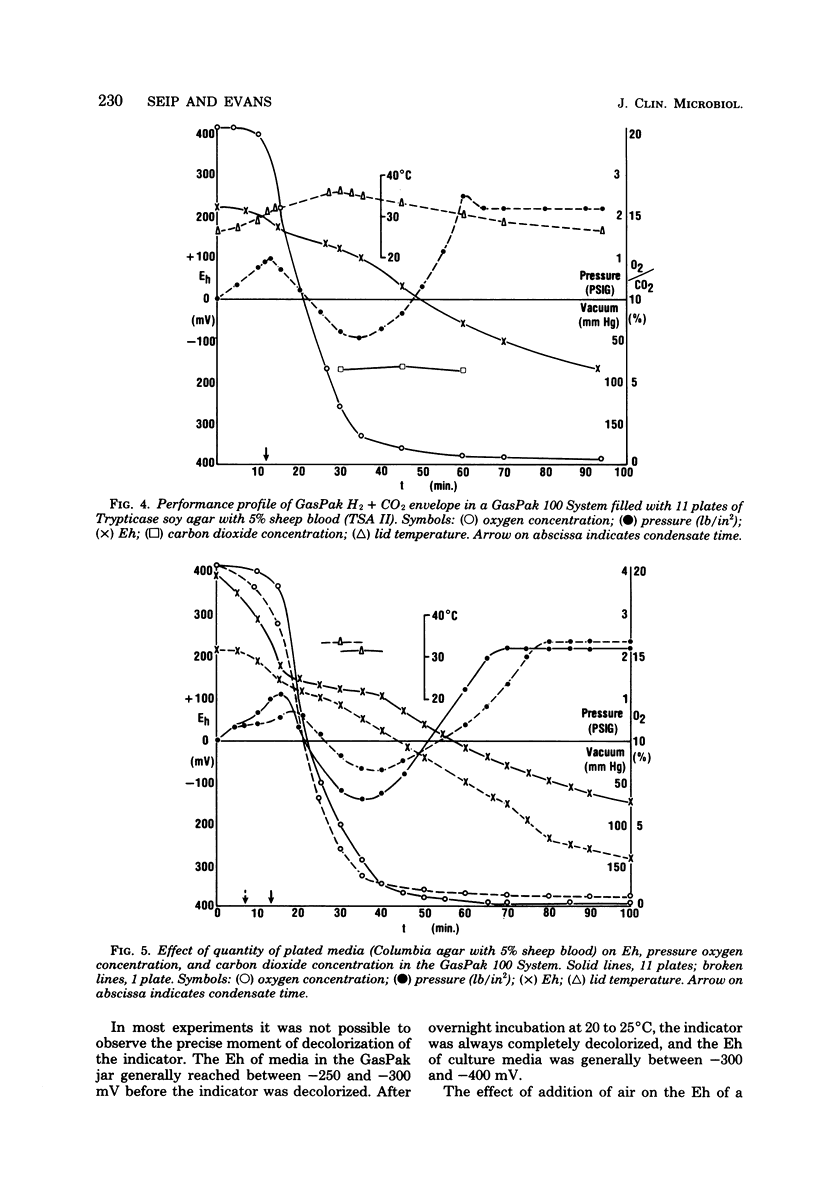
Oxygen and carbon dioxide concentrations, internal atmospheric pressure, catalyst temperature, and time of appearance of water condensate were monitored for various time intervals at ambient (20 to 25 degrees C) temperature in a GasPak 100 Anaerobic System (BBL Microbiology Systems, Cockeysville, Md). Simultaneously, the redox potential (Eh) of various plated culture media in the system was also measured. The oxygen concentration was reduced to less than 0.4% in 100 min. The Eh of the media, corrected for hydrogen ion, reached -100 mV within 60 to 100 min, and the carbon dioxide concentration increased to between 4 and 7% in 60 min, depending on the number of plates of media present. Condensate appeared generally between 10 and 15 min, and the temperature of the lid reached a maximum between 20 and 40 min. Condensate time and lid temperature increase are important early indicators of a correctly functioning GasPak System. A characteristic pressure-vacuum-pressure profile is produced as a result of controlled release of hydrogen and carbon dioxide gases and the reaction of hydrogen and oxygen to produce water. Anaerobic conditions were achieved well before the methylene blue anaerobic indicator became decolorized, which required more than 6 h at 20 to 25 degrees C. At this time the Eh of media in the jar was well below -200 mV. Since the indicator is reduced within 5 h at 35 degrees C, the Eh of media in the jar would also be expected to decrease more rapidly at the higher temperature.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arank A., Syed S. A., Kenney E. B., Freter R. Isolation of anaerobic bacteria from human gingiva and mouse cecum by means of a simplified glove box procedure. Appl Microbiol. 1969 Apr;17(4):568–576. doi: 10.1128/am.17.4.568-576.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bokkenheuser V., Richardson N. J. Simple anaerobic technique for the treponema pallidum immobilization test. Health Lab Sci. 1969 Jul;6(3):162–163. [PubMed] [Google Scholar]
- Brewer J. H., Allgeier D. L. Disposable Hydrogen Generator. Science. 1965 Feb 26;147(3661):1033–1034. doi: 10.1126/science.147.3661.1033-a. [DOI] [PubMed] [Google Scholar]
- Brewer J. H., Allgeier D. L., McLaughlin C. B. Improved anaerobic indicator. Appl Microbiol. 1966 Jan;14(1):135–136. doi: 10.1128/am.14.1.135-136.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer J. H., Allgeier D. L. Safe Self-contained Carbon Dioxide-Hydrogen Anaerobic System. Appl Microbiol. 1966 Nov;14(6):985–988. doi: 10.1128/am.14.6.985-988.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsson J., Nyberg G., Wrethén J. Hydrogen peroxide and superoxide radical formation in anaerobic broth media exposed to atmospheric oxygen. Appl Environ Microbiol. 1978 Aug;36(2):223–229. doi: 10.1128/aem.36.2.223-229.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collee J. G., Watt B., Fowler E. B., Brown R. An evaluation of the Gaspak system in the culture of anaerobic bacteria. J Appl Bacteriol. 1972 Mar;35(1):71–82. doi: 10.1111/j.1365-2672.1972.tb03675.x. [DOI] [PubMed] [Google Scholar]
- Ellner P. D., Granato P. A., May C. B. Recovery and identification of anaerobes: a system suitable for the routine clinical laboratory. Appl Microbiol. 1973 Dec;26(6):904–913. doi: 10.1128/am.26.6.904-913.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ericsson H. M., Sherris J. C. Antibiotic sensitivity testing. Report of an international collaborative study. Acta Pathol Microbiol Scand B Microbiol Immunol. 1971;217(Suppl):1+–1+. [PubMed] [Google Scholar]
- Ferguson I. R., Phillips K. D., Tearle P. V. An evaluation of the carbon dioxide component in the GasPak anaerobic system. J Appl Bacteriol. 1975 Oct;39(2):167–173. doi: 10.1111/j.1365-2672.1975.tb00558.x. [DOI] [PubMed] [Google Scholar]
- Gillespie R. W., Rettger L. F. Bacterial Oxidation-Reduction Studies: I. Differentiation of Species of the Spore-Forming Anaerobes by Potentiometric Technique. J Bacteriol. 1938 Dec;36(6):605–620. doi: 10.1128/jb.36.6.605-620.1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killgore G. E., Starr S. E., Del Bene V. E., Whaley D. N., Dowell V. R., Jr Comparison of three anaerobic systems for the isolation of anaerobic bacteria from clinical specimens. Am J Clin Pathol. 1973 Apr;59(4):552–559. doi: 10.1093/ajcp/59.4.552. [DOI] [PubMed] [Google Scholar]
- Loesche W. J. Oxygen sensitivity of various anaerobic bacteria. Appl Microbiol. 1969 Nov;18(5):723–727. doi: 10.1128/am.18.5.723-727.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGarrity G. J., Coriell L. L. Detection of anaerobic mycoplasmas in cell cultures. In Vitro. 1973 Jul-Aug;9(1):17–18. doi: 10.1007/BF02615983. [DOI] [PubMed] [Google Scholar]
- McMinn M. T., Crawford J. J. Recovery of anaerobic microorganisms from clinical specimens in prereduced media versus recovery by routine clinical laboratory methods. Appl Microbiol. 1970 Feb;19(2):207–213. doi: 10.1128/am.19.2.207-213.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore W. E., Cato E. P., Holdeman L. V. Anaerobic bacteria of the gastrointestinal flora and their occurrence in clinical infections. J Infect Dis. 1969 Jun;119(6):641–649. doi: 10.1093/infdis/119.6.641. [DOI] [PubMed] [Google Scholar]
- Onderdonk A. B., Johnston J., Mayhew J. W., Gorbach S. L. Effect of dissolved oxygen and Eh and Bacteroides fragilis during continuous culture. Appl Environ Microbiol. 1976 Feb;31(2):168–172. doi: 10.1128/aem.31.2.168-172.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolfe R. D., Hentges D. J., Campbell B. J., Barrett J. T. Factors related to the oxygen tolerance of anaerobic bacteria. Appl Environ Microbiol. 1978 Aug;36(2):306–313. doi: 10.1128/aem.36.2.306-313.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stalons D. R., Thornsberry C., Dowell V. R., Jr Effect of culture medium and carbon dioxide concentration on growth of anaerobic bacteria commonly encountered in clinical specimens. Appl Microbiol. 1974 Jun;27(6):1098–1104. doi: 10.1128/am.27.6.1098-1104.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutter V. L., Finegold S. M. Anaerobic bacteria: their recognition and significance in the clinical laboratory. Prog Clin Pathol. 1973;5:219–238. [PubMed] [Google Scholar]
- Tabatabai L. B., Walker H. W. Oxidation-reduction potential and growth of Clostridium perfringens and Pseudomonas fluorescens. Appl Microbiol. 1970 Sep;20(3):441–446. doi: 10.1128/am.20.3.441-446.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walden W. C., Hentges D. J. Differential effects of oxygen and oxidation-reduction potential on the multiplication of three species of anaerobic intestinal bacteria. Appl Microbiol. 1975 Nov;30(5):781–785. doi: 10.1128/am.30.5.781-785.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watt B. The influence of carbon dioxide on the growth of obligate and facultative anaerobes on solid media. J Med Microbiol. 1973 Aug;6(3):307–314. doi: 10.1099/00222615-6-3-307. [DOI] [PubMed] [Google Scholar]




