Abstract
Human perception of speed declines with age. Much of the decline is probably mediated by changes in the middle temporal (MT) area, an extrastriate area whose neural activity is linked to the perception of speed. In the present study, we used random-dot patterns to study the effects of aging on speed-tuning curves in cortical area MT of macaque visual cortex. Our results provide evidence for a significant degradation of speed selectivity in MT. Cells in old animals preferred lower speeds than did those in young animals. Response modulation and discriminative capacity for speed in old monkeys were also significantly weaker than those in young ones. Concurrently, MT cells in old monkeys showed increased baseline responses, peak responses and response variability, and these changes were accompanied by decreased signal-to-noise ratios. We also found that speed discrimination thresholds in old animals were higher than in young ones. The foregoing neural changes may mediate the declines in visual motion perception that occur during senescence.
Keywords: aging, area MT, degradation, inhibition, macaque, speed
Introduction
Psychophysical studies have indicated that aged humans exhibit deficits in visual motion sensitivity, motion discrimination and detection and the perception of direction and speed (Norman et al. 2003; Snowden and Kavanagh 2006; Bennett et al. 2007). All these deficits cannot be accounted for by optical factors alone and most likely reflect an aged-related degeneration in visual cortical areas responsible for motion processing (Spear 1993; Schmolesky et al. 2000; Norman et al. 2003). A number of studies in recent years have investigated the degradation of visual cortical functions during aging (Spear 1993; Spear et al. 1994; Schmolesky et al. 2000; Wang et al. 2005; Hua et al. 2006; Yu et al. 2006). To date, there have been no studies of the effects of senescence upon the cortical mechanism mediating the perception of speed in primates.
The perception of speed is important for vision and plays a crucial role in perceptual judgments and motion actions. Humans as well as nonhuman primates benefit from speed information when estimating the 3-dimensional structure of objects from motion cues (Braunstein 1962; Todd et al. 1988; Norman and Lappin 1992; Andersen 1997; Xiao et al. 1997). Psychophysical findings indicate that one can segment images based solely on gradients of speed signals (Masson et al. 1999; Grzywacz and Merwine 2003). Saccades and smooth pursuit eye movements also require the precise estimation of speed information (Lisberger et al. 1987; Groh et al. 1997).
Intimately involved in cortical encoding and decoding of speed information is visual area middle temporal (MT), a key cortical area in the magnocellular stream in primates (Newsome and Pare 1988; Maunsell et al. 1990; Born and Bradley 2005). Many neurons in MT area are tuned to speed of a moving visual stimulus (Maunsell and Van Essen 1983; Mikami et al. 1986; Perrone and Thiele 2001; DeAngelis and Uka 2003; Priebe et al. 2003). These cells are thought to play a direct role in the perception of visual speed (Liu and Newsome 2005). Lesions of MT adversely affect performance on speed discrimination and pursuit tasks (Newsome et al. 1985; Pasternak and Merigan 1994; Orban et al. 1995). Microstimulation of MT can bias speed perception in monkeys (Liu and Newsome 2005). Neuroimaging research has revealed that human area MT+ activity of the normal human brain is much higher during speed discrimination tasks than during other visual discrimination tasks (Corbetta et al. 1991; Beauchamp et al. 1997; Huk and Heeger 2000; but also see Sunaert et al. 2000). Lisberger and colleagues (Churchland and Lisberger 2001; Priebe and Lisberger 2004) have reported that speed perception in the macaque is consistent with the firing rate-weighted average of preferred speed of each MT neuron. It has been reported that the correlation between neural activity and speed discrimination could be predicted by the speed-tuning properties of MT cells (Liu and Newsome 2005). Previous studies also suggest that speed sensitivity of MT neurons may be used to generate acceleration sensitivity for the subsegment stages of visual motion processing (Price et al. 2005; Schlack et al. 2007). The visual system may use speed-tuned MT neurons for high-level motion processing tasks such as self-motion and depth estimation (Perrone and Stone 1994, 1998; Perrone and Thiele 2002). In view of the foregoing, any functional abnormality of speed-selective neurons in area MT seems to have a potential to affect speed perception adversely. It is, therefore, tempting to speculate that age-related deficits in human perception of speed are due to degeneration and/or dysfunction in area MT.
In this study, we used single-unit extracellular recording techniques to examine the speed-tuning MT neurons in very old rhesus monkeys. We found that speed tuning in macaque area MT is severely affected by normal aging.
Methods
Animal Preparation and Electrophysiology
All experimental protocols were consistent with the Society for Neuroscience and National Institute of Health guidelines for the humane use and care of animals. Experiments were performed at the Kunming Institute of Zoology (KIZ), Chinese Academy of Sciences, and were approved by the Institutional Animal Care and Use Committees at KIZ and the University of Utah. Subjects for this study were 2 groups of rhesus monkeys (Macaca mulatta). Young adult monkeys (n = 3, male) were 5–9 years old and weighed 3.6–6.2 kg. Old monkeys (n = 3, male) were 23–31 years old and weighed 5.2–8.7 kg. According to a life-span analysis of rhesus macaques housed at the Yerkes Primate Center (Tigges et al. 1988), our 23- to 31-year-old monkeys can be considered old, whereas 5- to 9-year-old monkeys are at an age that is considered sexually mature. Monkeys were examined ophthalmoscopically and had no apparent optical or retinal problems that would impair visual function. Retinal blood vessels, lens clarity and the maculae all appeared to be within normal limits.
The techniques used in our laboratory have been reported in detail elsewhere (Schmolesky et al. 2000; Leventhal et al. 2003). Subjects were sedated with ketamine HCl (10 mg/kg, i.m., Ketalar, Parke-Davis, Morris Plains, NJ) and then anesthetized with halothane (5%, Halocarbon Laboratories, River Edge, NJ) in a 70:30 mixture of N2O:O2. Intravenous and tracheal cannulae were inserted. Animals were placed in a stereotaxic apparatus, and all pressure points and incisions were infiltrated with lidocaine HCl (2%). A mixture of D-tubocurarine (0.4 mg/kg/h, Sigma, St Louis, MO) and gallamine trithiodide (7 mg/kg/h, Sigma) was infused intravenously to induce and maintain paralysis. Monkeys were ventilated, and anesthesia was maintained with a mixture of N2O (70%) and O2 (30%) and halothane (0.25–1.0%, as needed). Expired pCO2 was maintained at approximately 4%. Body temperature was maintained at 38 °C with a heating pad. Heart rate, electrocardiogram, and cortical electrical activity were monitored throughout the experiment to assess the level of anesthesia.
After the animal was placed on life support, the level of anesthesia was adjusted so that all vital signs were comparable in young and old animals. The eyes were protected from desiccation with contact lenses. Spectacle lenses and artificial pupils were used when needed. The locations of the optic discs and foveae were determined repeatedly during the course of each recording session. No visible deterioration in optics occurred during the experimental period (2–5 days). Electrode penetrations were advanced using a hydraulic microdrive (David Kopf Instruments, Tujunga, CA) through a craniotomy centered 16 mm lateral to the middle and 4 mm posterior to the lunate sulcus. The receptive fields were determined by hand using hand-held stimuli projected onto a tangent screen. Action potentials of isolated MT units were recorded using glass or glass-coated tungsten microelectrodes with impedances of 1–3 MΩ. The signals were then amplified and converted to standard pulses that were collected by computer.
Visual Stimulation
All visual stimuli were displayed at a resolution of 1024 × 768 pixels and frame rate of 100 Hz on a Sony Multiscan G220 monitor (Tokyo, Japan). The center of the video monitor was placed 57 cm away from the animals’ eyes. The program to generate the stimulus was written in MATLAB, using the extensions provided by the high-level Psychophysics Toolbox (Brainard 1997) and low-level Video Toolbox (Pelli 1997). The stimuli consisted of random-dot fields moving coherently in one direction were presented on a dim ground (<0.01 cd/m2). Dot luminance was 73.68 cd/m2 and dots were 0.15° in diameter. Dot density was 1 dot/sq. deg on average. The random-dot pattern was matched to the dimensions of the classical receptive field of an isolated MT neuron, and there was no blurring along the edges. For each neuron, we first collected a direction-tuning curve to adjust the direction of motion to match the neuron's preferred direction. To measure the speed-tuning curve, each neuron was tested with random-dot patterns having speeds of 0.5, 1, 2, 4, 8, 12, 16, 24, 32, 40, 52, and 64°/s. One hundred twenty-eight degrees per second was sometimes also used for neurons preferring very fast speeds. It is noteworthy that coherent motion was degraded at this speed because of the limited refresh rate of the monitor. All neurons we recorded in these experiments preferred speeds lower than 40 °/s and responded very weakly at a speed of 128 °/s. Thus, virtually, we always used a maximum speed of 64°/s. For all trials, textures appeared and were stationary for 500 ms before moving for 1 s. Each different speed was repeated 2–5 times in random order.
Data Collection and Analysis
After the response of an isolated cell was amplified with a microelectrode amplifier (×1000; DAGAN 2400A, Dagan Corporation, Minneapolis, MN), the amplified response was fed into an oscilloscope, an audio monitor, and was digitized using an acquisition board (National Instruments, Austin, TX) controlled by IGOR software (WaveMetrics, Portland, OR). The responses of the cells to the moving pattern were stored in the computer for offline analysis. At the time of each presentation, spontaneous activities (baseline) were obtained during a 0.5–1 s “null condition” period (no visual stimulus presented). All baseline values below 1 spike/s were set equal to 1 spike/s for peak-to-baseline analyses (Schmolesky et al. 2000; Leventhal et al. 2003). A cell's signal-to-noise ratio was defined as the ratio of the cell's response to the optimal stimulus and the cell's spontaneous activity (Schmolesky et al. 2000; Leventhal et al. 2003).
In the present study, we fitted the speed-tuning data of MT neurons with a log-Gaussian function described by the following using the constrained minimization tool, “fmincon,” in Matlab (Mathworks, Novi, MI):
| (1) |
where r is the firing rate and s corresponds to the stimulus speed in degrees per second. The function has 5 free parameters. R0 is the spontaneous firing of the cell, A is the peak amplitude, σ determines the (logarithmic) tuning width, sp represents the preferred speed and s0 is necessary to keep the logarithm from becoming undefined as stimulus speed is close to zero (Nover et al. 2005; Krekelberg et al. 2006). Here, we only consider R0, A, σ, and sp. The median R2 values are 0.971 for young group and 0.932 for old group. And the difference between the 2 populations is not significant (Mann–Whitney U test; P = 0.274). To evaluate the quality of the fit using a χ2 goodness-of-fit test, we fit all of the single-trial responses for each stimulus condition not just the mean response (DeAngelis and Uka 2003). We also fit the square root of the function to the square root of data, which helps to homogenize the variance of the neuronal response (Prince et al. 2002). Eighty-three percent of MT neurons in the young group and 78% in the old group pass this test for the tuning function. Moreover, most of the neurons that failed the χ2 test still had R2 values more than 0.9. Removal of these neurons from the sample does not affect our population analyses, so we did not exclude neurons from study based on the χ2 test alone.
For each speed-tuning curve, a modulation index was calculated to qualify speed tuning according to the formula
| (2) |
where Rmax is the mean response to the most effective stimuli, Rmin is the lowest mean response and S is the spontaneous activity (DeAngelis and Uka 2003; Liu and Newsome 2003). We also computed a discrimination index, which quantifies how well a neuron discriminates between its preferred and nonpreferred stimulus
| (3) |
where SSE is the sum of squared error around the mean response, N is the number of trials, and M is the number of tested speed values (Prince et al. 2002; DeAngelis and Uka 2003). Both the modulation and discrimination indices were calculated from the square root of the firing rate.
To relate the changes in speed tuning of MT neurons to changes in speed discrimination seen in humans, we also computed the Fisher information from the derivative of the log-Gaussian function fit to the tuning curve and a linear to the relation between response variance and mean response (Yang and Maunsell 2004; Nover et al. 2005; Purushothaman and Bradley 2005). The details of the calculating methods are well described in the paper of Nover et al. (2005). First, we calculated the absolute value of the derivative of log-Gaussian function fit to the tuning curve of 1 neuron . gives a quantitative estimate of the steepness of the tuning curve at each speed. From the best linear fit to response variance and mean response for the same neuron, we could then calculate the variance, σ2, of the response of the neuron at each speed along the tuning curve. Thus, for each reference speed, serf, the ratio of characterizes how well this neuron discriminate small differences in speed. The Fisher information is given by the sum of the square of in the population of MT neurons. Thus, the predicted threshold for speed discrimination could be simply given by the reciprocal of square root of the Fisher information (Nover et al. 2005). Notice that the Fisher information value grows with the size of the population due to summation. Nover et al. (2005) computed the Fisher information from a population of 501 MT neurons. In order to compare our data with the results of Nover et al., a bootstrap analysis was performed when we calculated the Fisher information values for young and old populations. First, we sampled neurons randomly with replacement from 1 population of MT neurons. Each sample was the same size as the original population. Then we calculated the mean of of each sample: . We repeated the process 501 times respectively for both young and old populations. Thus, for both young and old groups, IF (sref) which denotes the Fisher information was given by the following:
| (4) |
where N represents the number of neurons in the population (here for both young and old groups, N = 501). The way we compute the Fisher information is somewhat different from the way Nover et al. (2005) have done, but it gives a reasonably good approximation.
Statistical comparisons between young and old monkeys’ data were carried out using unpaired t tests, chi-square tests, 1-way ANOVAs, Mann–Whitney U tests and Kruskal–Wallis tests.
Results
Speed-tuning curves were obtained for 88 MT cells in 3 old monkeys and 107 MT cells in 3 young monkeys. The data were collected from 3 to 5 penetrations in each monkey. The recording depths and eccentricities (less than 8°) of the cells studied were comparable in the young and old groups. To quantify the effects of senescence on the response properties of MT neurons, we fitted the speed-tuning data with a log-Gaussian function (see Methods). The fitting tuning curve provides a good estimate of peak firing rate, preferred speed, width of the curve (in log speed) and spontaneous activity. Speed-tuning curves obtained from 1 young MT cell and 1 old MT cell are shown in Figure 1.
Figure 1.
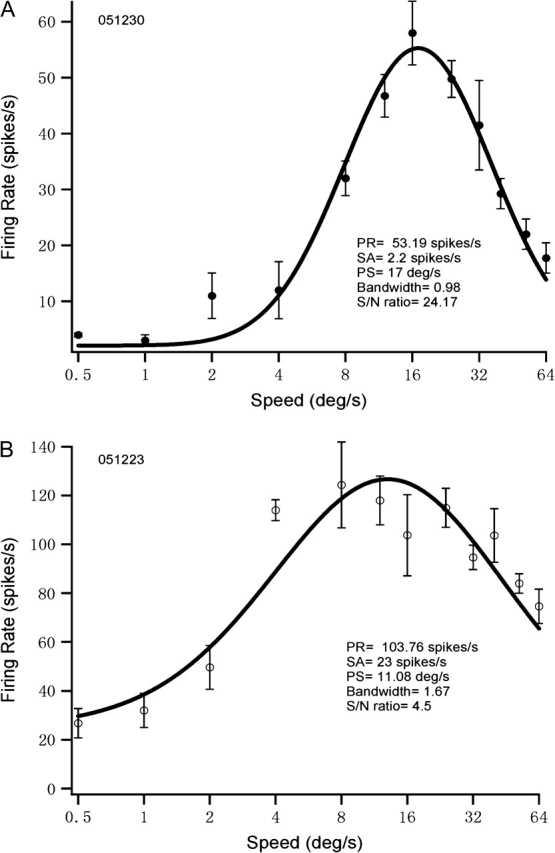
Speed-tuning curves for 1 young monkey MT cell (A) and 1 old monkey MT cell (B) are shown. Horizontal axis is plotted logarithmically. The responses to systematically varied speed were averaged. Error bars mean SEM. PR, SA, PS, and S/N represent peak response, spontaneous activity, preferred speed, and signal-to-noise ratio, respectively.
Preferred Speed
Our results showed that aging changed the distribution of preferred speeds in MT and that MT neurons in old monkeys exhibited significantly reduced preferred speeds (Table 1, Fig. 2). Specifically, the percentage of MT neurons exhibiting high speed preferences (≥10 °/s) was smaller in old animals (45%; 40 of 88) than in young ones (82%; 87 of 107; Chi-square test, P < 0.01). The percentage of cells showing even higher speed preferences (≥20 °/s) also decreased in old monkeys (16%; 14 of 88) compared with young controls (28%; 30 of 107; chi-square test, P < 0.01). Also, the mean preferred speed of MT neurons found in old monkeys (9.21 °/s, geometric mean) was lower than in young controls (14.09 °/s, geometric mean; unpaired t-test, P < 0.001). However, the range of preferred speeds in 2 groups overlapped (old group, 0.5–37.79 °/s; young group, 1.03–40 °/s) (Fig. 2).
Table 1.
Descriptive statistics of visual response properties of MT cells between young (YM) and old monkey (OM) groups
| Properties | YM cells (n = 107) | OM cells (n = 88) | Mann–Whitney U test | Kruskal–Wallis test |
| Peak response | 60.7 ± 2.75 | 84.8 ± 3.13 | P < 0.001 | P < 0.001 |
| Baseline | 5.67 ± 0.38 | 18.6 ± 0.82 | P < 0.001 | P < 0.001 |
| Preferred speed | 15.07 ± 0.74 | 11.19 ± 0.89 | P < 0.001 | P < 0.001 |
| Tuning width | 0.99 ± 0.031 | 1.69 ± 0.06 | P < 0.001 | P < 0.001 |
| Signal-to-noise ratio | 21.36 ± 2.87 | 7.0 ± 0.94 | P < 0.001 | P < 0.001 |
Note: Two group comparisons of peak response (spikes/s), baseline (spikes/s), preferred speed (°/s), and tuning width (in log speed) and signal-to-noise ratio were performed between young and old monkeys using Kruskal–Wallis test or Mann–Whitney U test. Data are presented as mean ± SEM.
Figure 2.
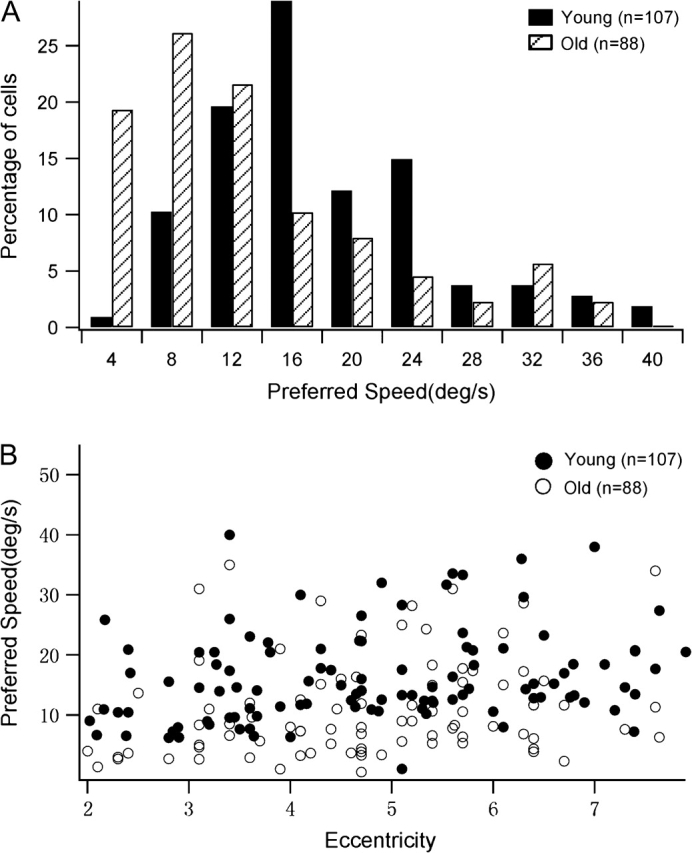
Distribution of preferred speeds for old and young monkeys. The total number of neurons studied was 88 for old monkeys and 107 for young monkeys. Cells in old monkeys exhibit significantly reduced preferred speeds compared with cells in young monkeys (P < 0.001). (A) Percentage of cells with different preferred speeds in old and young monkeys. (B) Preferred speeds of cells in old and young animals subserving different eccentricities. Notice that the ranges of the eccentricity tested in 2 populations do not differ (t-test, P = 0.286).
Figure 2B shows the distribution of preferred speeds of cells subserving different eccentricities in young and old monkeys. At all eccentricities studied, neurons in old monkeys exhibited lower preferred speeds than did cells in young ones. There was a weak correlation between preferred speed and eccentricity (Spearman rank correlation; young group: r = 0.21, P = 0.031; old group: r = 0.29, P = 0.019). This is consistent with previous studies (Maunsell and Van Essen 1983; Lagae et al. 1993).
Speed Selectivity
Bandwidth describes the breadth of the speed-tuning curve of a single MT neuron. It is a local measure of selectivity around the peak of the tuning curve (Ringach et al. 2002). Smaller bandwidths indicate stronger speed selectivity. In the present study, bandwidth (in log speed) was calculated from Equation 1 (see Methods) to estimate the speed selectivity of MT neurons. Our results showed that the tuning bandwidths of old MT neurons were significantly broader than those of young ones (Table 1, Figs 1 and 3).The percentage of cells showing significant speed selectivity (σ < 1.0) in old monkeys was smaller (7%, 6 of 88) than in young monkeys (63%, 67 of 107; Chi-square test, P < 0.001). The distribution of bandwidth values is shown in Figure 3A. Overall, MT cells in old animals exhibited significantly broader tuning than did cells in young monkeys. The results provide evidence for a significant degradation of speed selectivity in area MT of senescent animals. Figure 3B shows the tuning widths of cells in old and young animals as a function of eccentricity. There was a weak correlation between tuning width and eccentricity (young group: r = −0.193, P = 0.040; old group: r = −0.23, P = 0.031). Notice MT neurons in old monkeys exhibited broader tuning for speed than in young monkeys at the eccentricities we studied.
Figure 3.
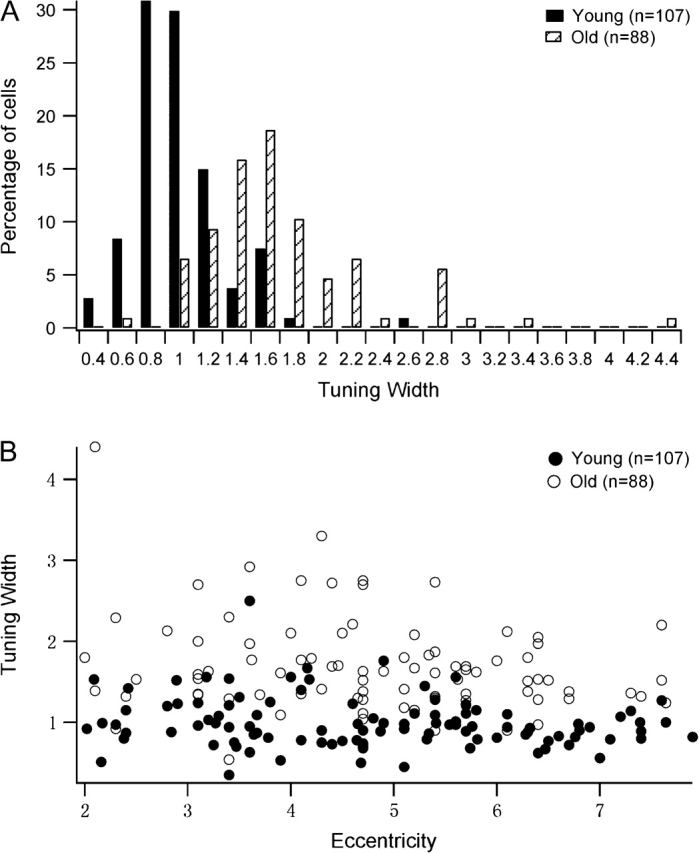
Distribution of speed-tuning widths in old and young monkeys. Cells in old monkeys showed increased (logarithmic) tuning bandwidths compared with cells in young monkeys (P < 0.001). (A) Percentage of cells with different tuning widths in old and young monkeys. (B) Tuning width as a function of eccentricity of MT neurons in young and old groups.
We also compared the bandwidths of cells with low-preferred (≤10 °/s), middle-preferred (>10 and <20 °/s), and high-preferred speeds (≥20 °/s) in both young and old animals. MT neurons in old animals had significantly wider bandwidths than did cells in young animals across all speed ranges. Thus, aging affects speed selectivity across all preferred speeds. These results are illustrated in Table 2.
Table 2.
Descriptive statistics of (logarithmic) bandwidth of the tuning curve of MT cells between young and old groups in 3 preferred-speed ranges: low-preferred speed (≤10 °/s), middle-preferred speed (>10 and <20 °/s), and high-preferred speed (≥20 °/s)
| Young | Old | Mann–Whitney U test | |||||
| Mean | SEM | n | Mean | SEM | n | ||
| Low-preferred speed | 1.23 | 0.09 | 19 | 1.76 | 0.10 | 48 | P < 0.01 |
| Middle-preferred speed | 0.96 | 0.04 | 58 | 1.68 | 0.11 | 27 | P < 0.001 |
| High-preferred speed | 0.89 | 0.05 | 30 | 1.27 | 0.09 | 13 | P < 0.01 |
Modulation and Discrimination Index
We assessed the effects of aging on the strength of tuning for speed using both modulation index (Equation 2) and discrimination index (Equation 3). Modulation index characterizes the amount of response modulation due to stimulus variations, relative to the maximal response (DeAngelis and Uka 2003; Nguyenkim and DeAngelis 2003). Larger modulation index represents stronger speed tuning. Figure 4A shows the distribution of modulation indices for speed across the 2 populations. Consistent with previous studies (DeAngelis and Uka 2003), the distribution of modulation indices for speed tuning in young monkeys was distributed approximately around 1 (mean = 0.94). In contrast, the modulation indices in the old group were not distributed around 1.0 (mean = 0.72) and were significantly lower than in the young group (Mann–Whitney U test, P < 0.001). These results indicate that response modulations elicited by varying speed were significantly weaker in old monkeys than in young monkeys.
Figure 4.
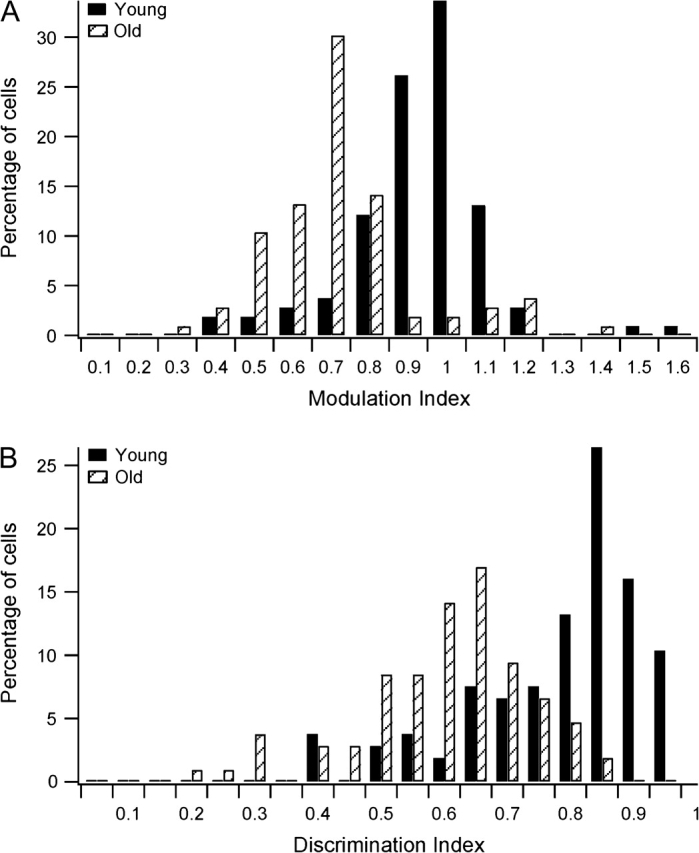
Comparison of tuning indices for speed across old and young MT neurons. (A) Distributions of the modulation indices for young and old groups, as defined by Equation 2 (see Methods). (B) Distributions of the discrimination indices for young and old groups, as defined by Equation 3 (see Methods).
Discrimination index quantifies the ability of one neuron to discriminate changes in the stimulus relative to its intrinsic level of variability (DeAngelis and Uka 2003). This metric is well correlated with neurometric thresholds in coarse discrimination tasks (Uka and DeAngelis 2003). Figure 4B shows the distribution of discrimination indices for the 2 populations. Notice that the discrimination indices in the old group (mean = 0.61) were significantly lower than that in the young group (mean = 0.79; Mann–Whitney U test, P < 0.001). This shows that MT neurons in old monkeys had substantially worse discriminative capacity for speed than MT cells in young monkeys.
Spontaneous Activity, Peak-to-Baseline Ratios, and Response Variability
We also analyzed the spontaneous activities and peak responses of cells in old and young animals. In line with our previous findings in V1 and V2 of old macaque monkeys (Schmolesky et al. 2000; Leventhal et al. 2003; Wang et al. 2005; Yu et al. 2006), in old monkeys there were significant increases in both spontaneous activities and peak responses of MT cells (Table 1). Baseline activity was affected more severely than peak response. Taken together, the increases in peak and baseline activities in old monkeys resulted in decreased peak-to-baseline (signal-to-noise) ratios in old animals. These results are illustrated in Table 1 and Figure 5.
Figure 5.
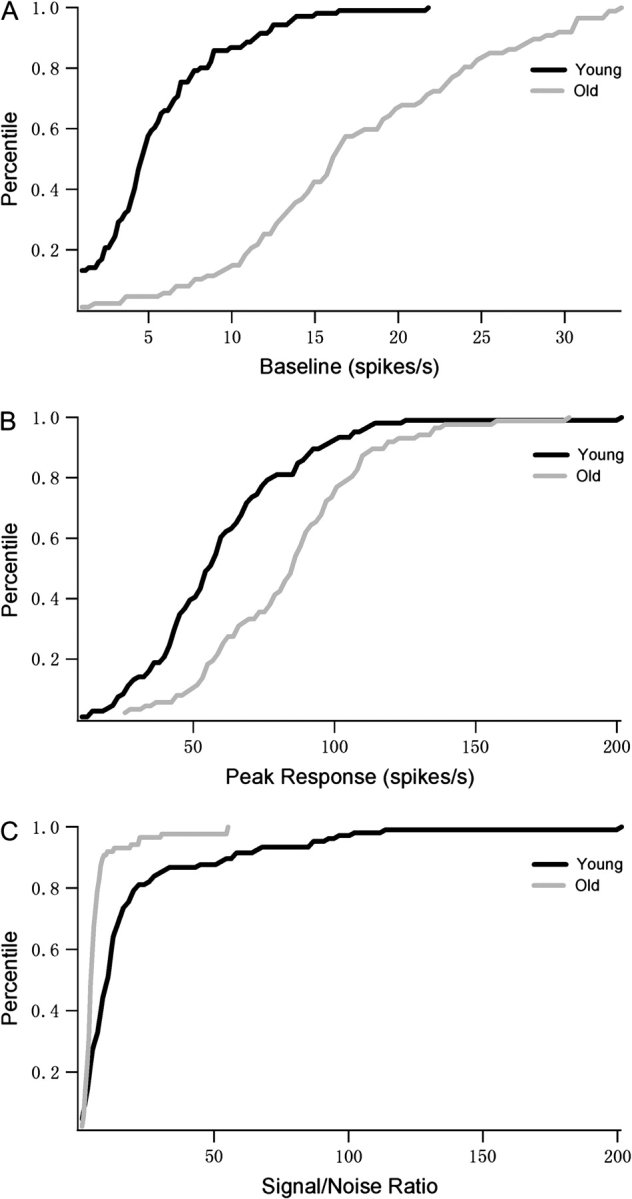
Spontaneous activities, peak responses and peak-to-noise (signal-to-noise) ratios of cells in young and old monkeys. The percentage of young (n = 107) and old (n = 88) monkey cells with different baselines (A), peak responses (B), and signal-to-noise ratios (C) are shown in cumulative distribution plots. Solid black and gray lines represent the data for young and old monkeys, respectively. Old monkey cells had increased baseline and peak responses but decreased signal-to-noise ratios compared with young monkey MT cells.
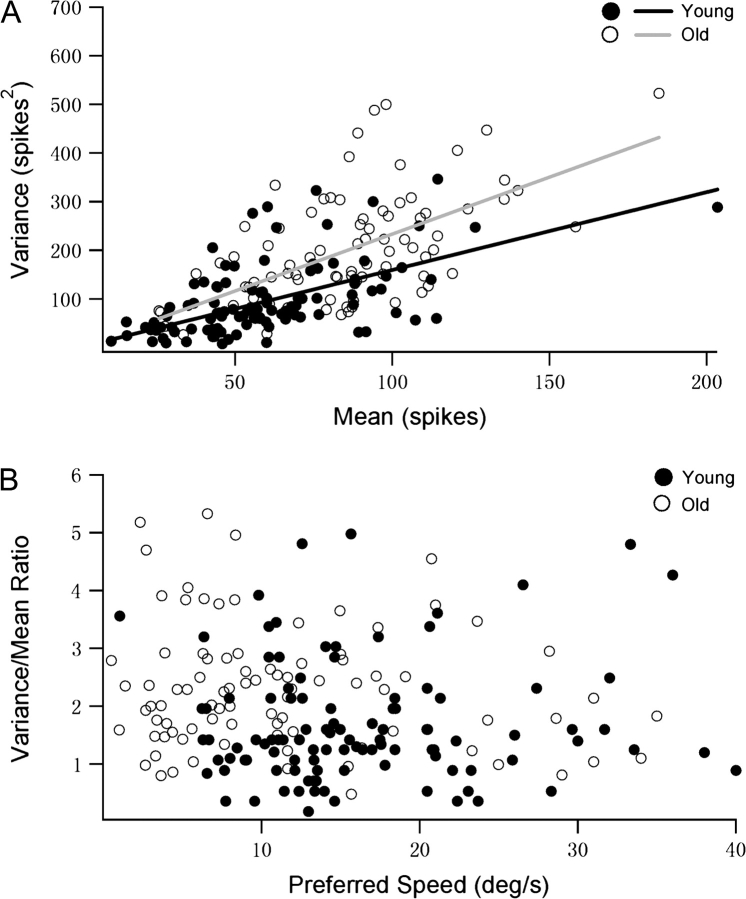
The variability of evoked neural discharges is of central importance in the study of sensory systems and should have a profound influence on cognitive functions of humans (Glimcher 2005). Response variability may reflect noise and limit the nature of the neural code (Mainen and Sejnowski 1995; Shadlen and Newsome 1998). In this study, we compared the response variability of MT neurons in young and old monkeys. The individual means and variances at the cells’ maximum responses measured in young and old monkeys are scattered in Figure 6A. MT neurons in old animals exhibited more variable responses than did cells in young ones (linear slope for young group: 1.54; linear slope for old group: 2.34). Figure 6B examines the relationship between response variability and speed preference. For each neuron, we computed the mean and variance of firing rate at each tested speed. In Figure 6B, the average variance/mean ratio (averaged across tested speeds) of each neuron in young and old groups is plotted as a function of its preferred speed. MT neurons in old animals always exhibited significantly higher average variance/mean ratio (mean = 2.3) than did cells in young ones (mean = 1.67; 1-way ANOVA, P < 0.001). It is notable that there was no correlation between response variability and preferred speed in either group (young group: r = 0.064, P = 0.512; old group: r = −0.147, P = 0.275). This is consistent with previous studies (Nover et al. 2005).
Figure 6.
Variability of MT neurons in young and old macaque monkeys studied using random-dot patterns. (A) Response variance is plotted against mean response. Data collected from 107 neurons in young monkeys and 88 in old monkeys. (B) The variance/mean ratio for each MT neurons in young and old animals is plotted as a function of its preferred speed.
Predicted Speed Discrimination Threshold
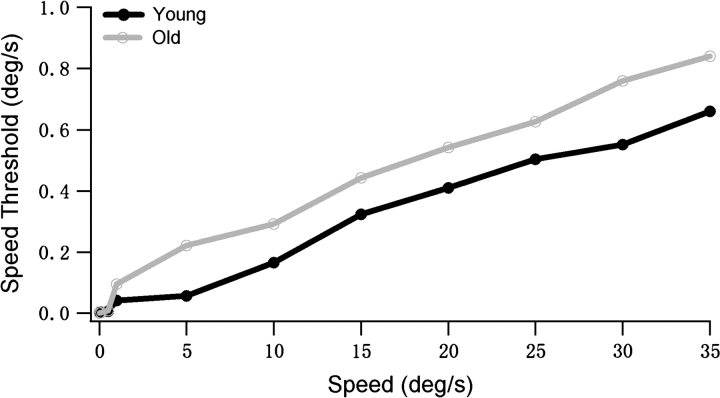
Nover et al. (2005) used the Fisher information to compute speed discrimination thresholds for MT cells and found that these predictions agree pretty well with human data. We used a similar analysis to compare the predicted speed discrimination thresholds in young and old MT populations. The Fisher information value grows with the size of the population due to the summation. Thus, in order to compare our data with the results of Nover et al., a bootstrap analysis was performed to normalize the Fisher information by the number of neurons (see Methods). The speed discrimination thresholds for young and old groups were calculated. Figure 7 shows the predicted speed discrimination threshold as a function of pedestal speed. Consistent with previous results (Nover et al. 2005), predicted speed discrimination thresholds increased approximately linearly with pedestal speed in the 2 populations, and there was an initial steep rise at very low speeds. Notice that the thresholds in the old population were higher than in the young population, although there was some overlap at very low speeds. Some factors, such as broad tuning width and high response variability, are likely to contribute to the higher speed discrimination thresholds in the old group. Figure 7 is analogous to psychophysical speed discrimination data from the studies of Snowden and Kavanagh (2006) on aging humans (see their Fig. 3). In their study, they showed that older observers exhibit a loss of the ability to discrimination different speeds (Snowden and Kavanagh 2006). Notice that the speed discrimination threshold in the young group we studied was higher than the result of Nover et al. This difference may be due to the different recording conditions. Nover et al. did the experiments on alert animals, whereas we used anesthetized animals.
Figure 7.
Predicted speed discrimination threshold for the MT population is plotted as a function of pedestal speed in young and old monkeys. Estimated threshold values increased approximately linearly with pedestal speed. There was a steep rise at low speeds in both groups. The thresholds in old population were higher than in young population, despite some overlap at very low speeds.
Relationship between Speed-Tuning Metrics and Spontaneous Activity
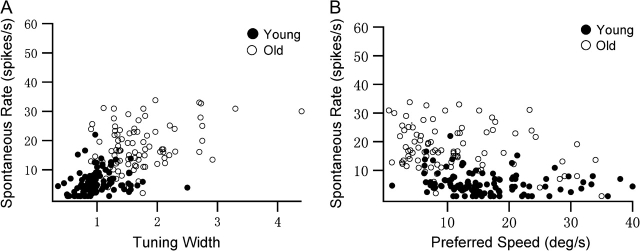
In order to understand the relationship between the speed tuning and spontaneous rate, we constructed scatter plots of preferred speed and tuning width versus spontaneous activity (Fig. 8). Figure 8A illustrates the relationship between tuning width and spontaneous activity in our samples. There was a weak positive correlation between tuning width and spontaneous activity in the young group (r = 0.206, P = 0.037). However, a strong correlation between tuning width and spontaneous activity was found in the old group (r = 0.397, P = 0.001). Compared with cells in young monkeys, cells in old animals tended to exhibit poor speed selectivity as well as increased spontaneous activity. Similar results were observed when preferred speed and spontaneous activity were compared (Fig. 8B). There was a weak negative correlation between preferred speed and the level of spontaneous activity in the young group (r = −0.190, P = 0.049), but a strong correlation in the old group (r = −0.2560, P = 0.007). Cells in old animals exhibited slower preferred speed and much higher levels of spontaneous activity than do cells in young ones. These results raise the possibility that some degeneration of intracortical inhibition may contribute to the age-related difference in speed tuning. Note that Figure 8A is similar to Figure 3A of Palanca and DeAngelis in which stationary/moving response ratios are plotted against the preferred speeds for each MT unit (Palanca and DeAngelis 2003).
Figure 8.
(A) Relationship between speed-tuning widths and spontaneous firing rates of old and young MT cells. (B) Relationship between preferred speeds and spontaneous firing rates of old and young MT cells. Cells with spontaneous rates below 1 are plotted at 1.
Effects of Anesthesia
It is possible that differential effects of anesthesia upon MT function in young and old monkeys have impacted our results. In the present study, this possibility has been tested by studying the properties of individual cells while systematically varying anesthesia levels. Peak response was affected by anesthesia similarly for both young (n = 18) and old groups (n = 14) (Fig. 9A). As the level of halothane was increased from 0.3% to 0.6%, responsiveness in the old group declined by 42% in comparison to 38% in the young group (P = 0.572). When the level of halothane was increased from 0.3% to 0.9%, responsiveness in the old group was reduced by 81% compared with 76% in the young group (P = 0.439). In addition, as shown in Figure 9B, an increase in anesthesia did not significantly affect the level of spontaneous activity in either group (young group: F2,53 = 2.859, P = 0.16, n = 18; old group: F2,44 = 1.814, P = 0.276, n = 15). Anesthetic concentration also did not affect speed selectivity (width of speed-tuning curves) in young or old groups (young group: F2,53 = 0.701, P = 0.501, n = 18; old group: F2,41 = 0.755, P = 0.477, n = 14) (Fig. 9C). Preferred speed was also not modified by changing the level of halothane (young group: P = 0.713, n = 18; old group: P = 0.583, n = 14).These results are similar to those in cats which suggest that direction selectivity and spontaneous activity are barely dependent on the level of anesthesia (Villeneuve and Casanova 2003). Thus, problems with anesthesia in old animals are not a concern.
Figure 9.
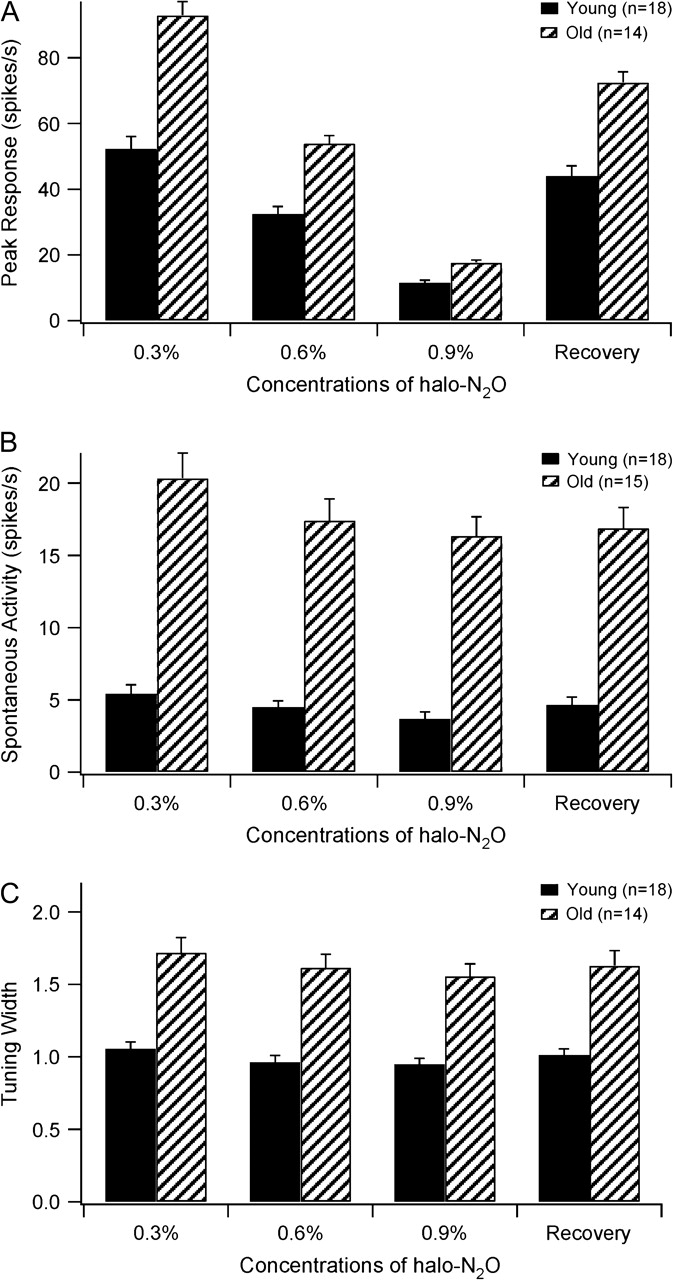
Peak responses (A), spontaneous activities (B) and tuning widths (C) (mean and SEM) of young and old monkey cells under different conditions of anesthesia. The recovery condition was set at 0.3% halothane.
Discussion
In this study, we have identified the effects of aging on MT tuning curves for speed. Our main findings are as follows. 1) Aging altered the distribution of the preferred speeds of MT cells. Neurons in old animals preferred lower speeds than do cells in young ones. 2) MT neurons in the aged macaque were more widely tuned for speed than in young ones. 3) Response modulation and discrimination capacity for speed in the old group were significantly weaker than in the young group. 4) Old MT neurons exhibited increased spontaneous and visually evoked activities. Spontaneous activity was affected more severely than peak response. As a result, the signal-to-noise ratios of old cells were reduced. Response variability in old monkeys was also higher than in young ones. 5) We also estimated the speed discrimination thresholds in the 2 groups and found that thresholds in the old group were higher than in the young group.
Speed Tuning and the log-Gaussian Function
Perrone (2006) reported that the log-Gaussian function is not a reliable method for assessing the extent of speed tuning in MT when sine-wave gratings are employed to map out the spatiotemporal frequency response profiles of MT neurons (Perrone 2006). This raises a possibility that the broader tuning curves in old monkeys are causing the peaks to be shifted to lower speed values compared with the narrower tuning curves of cells in young monkeys. However, Nover et al. (2005) have established that the speed-tuning curves of MT neurons are well described by the log-Gaussian function when random-dot patterns are employed as stimuli. These authors also concluded that the tuning width of the log-Gaussian function depends little upon the speed preference of MT neurons. Two subsequent studies have confirmed this point (Krekelberg et al. 2006; Pack et al. 2005). Thus, our use of the log-Gaussian function is not a problem in the present study.
Implication for Visual Motion Deficits in Aged Human
Older drivers are thought to be involved in more automobile accidents than younger adults and senior citizens tend to have significant impairments in their visual function (OECD 2001; NHTSA 2004; Gorrie et al. 2007). They usually underestimate or cannot accurately judge the speed of oncoming vehicles (Scialfa et al. 1991). Psychological studies showed that aged humans exhibit decreased performances at task based on speed perception (Norman et al. 2003; Snowden and Kavanagh 2006). Performance of eye-movement tasks that rely in part on speed and acceleration estimation also suffers with aging (Moschner and Baloh 1994; Ross et al. 1999). These declines could be attributed to some aged-related differences in perception of target speed. It has been well established that MT is a key area in the M-pathway and plays an important role in the analysis of object speed (Perrone and Thiele 2001; Priebe et al. 2003; Born and Bradley 2005). So losses at this site may underlie the perceptual declines above. Our findings that there is a significant functional degradation in the neural representation of speed information in MT of old monkeys may provide physiological evidence for these deficits.
Many age-related declines in cognitive function may be due to older adults’ relatively higher levels of neural noise (Welford 1984; Bennett et al. 2007). Slowed reaction times to the onset of motion in aged human are also thought to be derived from the fact that the signals within central nervous systems have to be distinguished against a background of greater noise (Welford 1984). Recent theoretical work has also suggested that noisy responses across the active population of neurons would bias human perception toward low speed estimates (Weiss et al. 2002; Priebe and Lisberger 2004). Consistent with these suggestions, we observed that MT neurons in aged monkeys exhibited increased spontaneous activities, reduced signal-to-noise ratios and extremely variable responses.
Cortical and Subcortical Contributions to Aging-Related Degradation
It has been shown that age-related deficits in speed perception are not a consequence of ocular declines present in the elderly (Norman et al. 2003). Researchers have mimicked optical loss by reducing retinal illuminance in normal subjects and found that this manipulation does not alter speed discrimination thresholds for random-dot patterns. It was concluded that deficits occur within the central visual system responsible for the estimation of speed.
Previous studies have revealed that dorsal lateral geniculate cells and possibly even the geniculorecipient cells of V1 are relatively unaffected by aging (Spear 1993; Spear et al. 1994; Schmolesky et al. 2000). It has been suggested that declines in primary visual cortex (V1) and second visual cortex (V2) do occur with age (Schmolesky et al. 2000; Leventhal et al. 2003; Yu et al. 2006). Recent evidence indicates that certain aspects of speed tuning in MT could arise from inputs from earlier visual areas and sharp speed tuning in MT can be created from broadly tuned V1 inputs (Perrone and Thiele 2001, 2002; Priebe et al. 2003, 2006; Perrone 2004). Perrone has also showed that MT neurons tuned to high speeds could be generated from V1 inputs that are themselves tuned to quite low temporal frequencies (Perrone 2005). Thus, degradation of the afferents inputs to MT would seem 1 possible candidate to explain some of the changes observed among aged monkeys, though there is still no evidence that aging affects neural representation of speed in these areas.
Previous studies on animals have suggested that the efficacy of cortical inhibitory function declines with age (Schmolesky et al. 2000; Leventhal et al. 2003; Caspary et al. 2005; Hua et al. 2006; Yu et al. 2006). Our findings that MT cells in old monkeys showed increased spontaneous and visually driven activity are consistent with this hypothesis. Previous studies have suggested that speed tuning for random-dot textures is enhanced by a nonlinear processing within MT itself (Mikami et al. 1986; Priebe et al. 2006) and suppression may play an important role in establishing speed tuning in MT (Mikami et al. 1986; Simoncelli and Heeger 1998). Suppression is to a large extent interpreted as evidence for intracortical inhibition (DeAngelis et al. 1992; Carandini et al. 2002; Bair et al. 2003). Therefore, we suggest that an age-related degeneration of intracortical inhibition within MT itself may provide another possible mechanism underlying the severe effects of aging on the speed tuning we observed. Age-related deterioration of cortical inhibitory functions could also account for the extremely variable responses in old monkeys, because the level of response variability is thought to be mediated by the balance of excitation and inhibition in the output layers (Tolhurst et al. 1983; Shadlen and Newsome 1998; Gur and Snodderly 2006).
Studies of human visual cortex showed that L-glutamic acid decarboxylase, an enzyme needed to synthesize the inhibitory transmitter gamma-aminobutyric acid (GABA) (an important inhibitory interneuron), is reduced during aging (McGeer and McGeer 1976). Other studies also suggested that there is a weakness of GABAergic inhibition in the cortex of old animals (Post-Munson et al. 1994; Dustman et al. 1996). It is known that MT neurons are susceptible to GABAergic manipulation (Thiele et al. 2004). Therefore, decreased GABAergic inhibition may contribute to functional degradation of MT cells in old monkeys. In addition, other inhibitory pathways, such as cholinergic and dopaminergic system, may also be involved (Amenta et al. 1991; Muir 1997).
The age-associated degradation reported here might be thought of as a consequence of age-associated cellular alterations. Previous studies have proved that the number of cortical neurons in macaque monkeys and humans is largely preserved during aging (Morrison and Hof 1997, 2007; Peters et al. 1998). Specifically, studies in the superior temporal sulcus, on the posterior bank of which MT is located, failed to detect an age-related loss in humans and macaques (Duan et al. 2003; Morrison and Hof 2007), however statistically significant decreases in spine numbers and densities on both apical and basal dendritic arbors in old brains were observed (Duan et al. 2003). In view of these findings, age-related pre- and postsynaptic changes might have contributed to the functional degradation of MT cells observed here.
It is worth noting that many of the effects of aging on speed tuning appear similar to those that would be obtained by lowering the contrast of the stimulus in young monkeys (Pack et al. 2005; Krekelberg et al. 2006). Two previous studies have revealed a reduction in the preferred speeds of MT neurons at low contrast. Pack et al. (2005) reported that lowering the contrast decreased the bandwidth for slow-tuned neurons and increased the bandwidth for fast-tuned neurons. Krekelberg et al. (2006) found that the width of the log-Gaussian tuning function was essentially independent of contrast. In contrast to the foregoing studies, we found that aging significantly affects both preferred speed and tuning width. Despite this, we still cannot rule out the possibility that some age-related low-level deficit that reduces stimulus contrast could contribute to the changes we observed in old monkeys. It has been reported that the influence of contrast on MT tuning curves is mostly due to contrast-dependent changes in the spatial and temporal properties of receptive properties (Pack et al. 2005; Krekelberg et al. 2006). Similarly, aging may also affect spatial–temporal properties of cortical neurons, which has been indicated in psychological studies (Scialfa et al. 1991; Salthouse 2000). Pack et al. (2005) suggested that contrast-dependent changes in speed tuning of MT neurons are due to interactions within MT and surround suppression may play an important role in this processing. Our results suggest that old age may affect speed tuning of MT neurons in the same way. Indeed, center-surround antagonism in visual motion processing degrades during normal aging. This might be caused by an abnormal GABAergic system in old brains, perhaps in MT (Betts et al. 2005).
Visual motion processing is crucial for primates’ successful interaction with their visual environments. Sensory–motor systems must use speed signals to guide accurate movements. The findings of this study have significant implications for visual motion processing deficits in aged humans. However, given the constraints of MT speed tuning, MT neurons cannot directly output speed information on their own. Some sort of population code must also be in place at later stages of the motion pathway beyond MT, such as the middle superior temporal cortex, superior temporal polysensory area, and ventral intraparietal area (Anderson and Siegel 1999; Lisberger and Movshon 1999; Bremmer et al. 2002; Priebe and Lisberger 2004; Perrone 2005). Therefore, future studies parsing age-related changes upon higher visual cortex should help in better understanding of physiological mechanisms underlying visual motion processing deficits that accompany normal aging.
Funding
National Basic Research Program of China (2009CB941303 and 2005CB522803); Natural Science Foundation of China (30520120072), to Y.Z.; and the National Institute of Health (R01 AG 17922) to A.G.L.
Acknowledgments
We thank Pinglei Bao for help developing parts of the computer programs. We are also thankful for the anonymous reviewers’ very helpful comments. Conflict of Interest: None declared.
References
- Amenta F, Zaccheo D, Collier WL. Neurotransmitters, neuroreceptors and aging. Mech Ageing Dev. 1991;61:249–273. doi: 10.1016/0047-6374(91)90059-9. [DOI] [PubMed] [Google Scholar]
- Andersen RA. Neural mechanisms of visual motion perception in primates. Neuron. 1997;18:865–872. doi: 10.1016/s0896-6273(00)80326-8. [DOI] [PubMed] [Google Scholar]
- Anderson KC, Siegel RM. Optic flow selectivity in the anterior superior temporal polysensory area, STPa, of the behaving monkey. J Neurosci. 1999;19:2681–2692. doi: 10.1523/JNEUROSCI.19-07-02681.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bair W, Cavanaugh JR, Movshon JA. Time course and time-distance relationships for surround suppression in macaque V1 neurons. J Neurosci. 2003;23:7690–7701. doi: 10.1523/JNEUROSCI.23-20-07690.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchamp MS, Cox RW, DeYoe EA. Graded effects of spatial and featural attention on human area MT and associated motion processing areas. J Neurophysiol. 1997;78:516–520. doi: 10.1152/jn.1997.78.1.516. [DOI] [PubMed] [Google Scholar]
- Bennett PJ, Sekuler R, Sekuler AB. The effects of aging on motion detection and direction identification. Vision Res. 2007;47:799–809. doi: 10.1016/j.visres.2007.01.001. [DOI] [PubMed] [Google Scholar]
- Betts LR, Taylor CP, Sekuler AB, Bennett PJ. Aging reduces center-surround antagonism in visual motion processing. Neuron. 2005;45:361–366. doi: 10.1016/j.neuron.2004.12.041. [DOI] [PubMed] [Google Scholar]
- Born RT, Bradley DC. Structure and function of visual area MT. Annu Rev Neurosci. 2005;28:157–189. doi: 10.1146/annurev.neuro.26.041002.131052. [DOI] [PubMed] [Google Scholar]
- Brainard DH. The psychophysics toolbox. Spat Vis. 1997;10:433–436. [PubMed] [Google Scholar]
- Braunstein ML. Depth perception in rotating dot patterns: effects of numerosity and perspective. J Exp Psychol. 1962;64:415–420. doi: 10.1037/h0048140. [DOI] [PubMed] [Google Scholar]
- Bremmer F, Duhamel JR, Ben Hamed S, Graf W. Heading encoding in the macaque ventral intraparietal area (VIP) Eur J Neurosci. 2002;16:1554–1568. doi: 10.1046/j.1460-9568.2002.02207.x. [DOI] [PubMed] [Google Scholar]
- Carandini M, Heeger DJ, Senn W. A synaptic explanation of suppression in visual cortex. J Neurosci. 2002;22:10053–10065. doi: 10.1523/JNEUROSCI.22-22-10053.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspary DM, Schatteman TA, Hughes LF. Age-related changes in the inhibitory response properties of dorsal cochlear nucleus output neurons: role of inhibitory inputs. J Neurosci. 2005;25:10952–10959. doi: 10.1523/JNEUROSCI.2451-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchland MM, Lisberger SG. Shifts in the population response in the middle temporal visual area parallel perceptual and motor illusions produced by apparent motion. J Neurosci. 2001;21:9387–9402. doi: 10.1523/JNEUROSCI.21-23-09387.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M, Miezin FM, Dobmeyer S, Shulman GL, Petersen SE. Selective and divided attention during visual discriminations of shape, color, and speed: functional anatomy by positron emission tomography. J Neurosci. 1991;11:2383–2402. doi: 10.1523/JNEUROSCI.11-08-02383.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeAngelis GC, Robson JG, Ohzawa I, Freeman RD. Organization of suppression in receptive-fields of neurons in cat visual-cortex. J Neurophysiol. 1992;68:144–163. doi: 10.1152/jn.1992.68.1.144. [DOI] [PubMed] [Google Scholar]
- DeAngelis GC, Uka T. Coding of horizontal disparity and velocity by MT neurons in the alert macaque. J Neurophysiol. 2003;89:1094–1111. doi: 10.1152/jn.00717.2002. [DOI] [PubMed] [Google Scholar]
- Duan H, Wearne SL, Rocher AB, Macedo A, Morrison JH, Hof PR. Age-related dendritic and spine changes in corticocortically projecting neurons in macaque monkeys. Cereb Cortex. 2003;13:950–961. doi: 10.1093/cercor/13.9.950. [DOI] [PubMed] [Google Scholar]
- Dustman RE, Emmerson RY, Shearer DE. Life span changes in electrophysiological measures of inhibition. Brain Cogn. 1996;30:109–126. doi: 10.1006/brcg.1996.0007. [DOI] [PubMed] [Google Scholar]
- Glimcher PW. Indeterminacy in brain and behavior. Annu Rev Psychol. 2005;56:25–56. doi: 10.1146/annurev.psych.55.090902.141429. [DOI] [PubMed] [Google Scholar]
- Gorrie CA, Rodriguez M, Sachdev P, Duflou J, Waite PM. Mild neuritic changes are increased in the brains of fatally injured older motor vehicle drivers. Accid Anal Prev. 2007;39:1114–1120. doi: 10.1016/j.aap.2007.02.008. [DOI] [PubMed] [Google Scholar]
- Groh JM, Born RT, Newsome WT. How is a sensory map read out? Effects of microstimulation in visual area MT on saccades and smooth pursuit eye movements. J Neurosci. 1997;17:4312–4330. doi: 10.1523/JNEUROSCI.17-11-04312.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grzywacz NM, Merwine DK. Neural basis of motion perception. Encyclopedia Cogn Sci. 2003;3:86–98. [Google Scholar]
- Gur M, Snodderly DM. High response reliability of neurons in primary visual cortex (V1) of alert, trained monkeys. Cereb Cortex. 2006;16:888–895. doi: 10.1093/cercor/bhj032. [DOI] [PubMed] [Google Scholar]
- Hua T, Li X, He L, Zhou Y, Wang Y, Leventhal AG. Functional degradation of visual cortical cells in old cats. Neurobiol Aging. 2006;27:155–162. doi: 10.1016/j.neurobiolaging.2004.11.012. [DOI] [PubMed] [Google Scholar]
- Huk AC, Heeger DJ. Task-related modulation of visual cortex. J Neurophysiol. 2000;83:3525–3536. doi: 10.1152/jn.2000.83.6.3525. [DOI] [PubMed] [Google Scholar]
- Krekelberg B, van Wezel RJA, Albright TD. Interactions between speed and contrast tuning in the middle temporal area: implications for the neural code for speed. J Neurosci. 2006;26:8988–8998. doi: 10.1523/JNEUROSCI.1983-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagae L, Raiguel S, Orban GA. Speed and direction selectivity of macaque middle temporal neurons. J Neurophysiol. 1993;69:19–39. doi: 10.1152/jn.1993.69.1.19. [DOI] [PubMed] [Google Scholar]
- Leventhal AG, Wang YC, Pu ML, Zhou YF, Ma YY. GABA and its agonists improved visual cortical function in senescent monkeys. Science. 2003;300:812–815. doi: 10.1126/science.1082874. [DOI] [PubMed] [Google Scholar]
- Lisberger SG, Morris EJ, Tychsen L. Visual motion processing and sensory-motor integration for smooth pursuit eye movements. Annu Rev Neurosci. 1987;10:97–129. doi: 10.1146/annurev.ne.10.030187.000525. [DOI] [PubMed] [Google Scholar]
- Lisberger SG, Movshon JA. Visual motion analysis for pursuit eye movements in area MT of macaque monkeys. J Neurosci. 1999;19:2224–2246. doi: 10.1523/JNEUROSCI.19-06-02224.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Newsome WT. Functional organization of speed tuned neurons in visual area MT. J Neurophysiol. 2003;89:246–256. doi: 10.1152/jn.00097.2002. [DOI] [PubMed] [Google Scholar]
- Liu J, Newsome WT. Correlation between speed perception and neural activity in the middle temporal visual area. J Neurosci. 2005;25:711–722. doi: 10.1523/JNEUROSCI.4034-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mainen ZF, Sejnowski TJ. Reliability of spike timing in neocortical neurons. Science. 1995;268:1503–1506. doi: 10.1126/science.7770778. [DOI] [PubMed] [Google Scholar]
- Masson GS, Mestre DR, Stone LS. Speed tuning of motion segmentation and discrimination. Vision Res. 1999;39:4297–4308. doi: 10.1016/s0042-6989(99)00143-1. [DOI] [PubMed] [Google Scholar]
- Maunsell JH, Nealey TA, DePriest DD. Magnocellular and parvocellular contributions to responses in the middle temporal visual area (MT) of the macaque monkey. J Neurosci. 1990;10:323–3334. doi: 10.1523/JNEUROSCI.10-10-03323.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maunsell JH, Van Essen DC. Functional properties of neurons in middle temporal visual area of the macaque monkey 1: Selectivity for stimulus direction, speed, and orientation. J Neurophysiol. 1983;49:1127–1147. doi: 10.1152/jn.1983.49.5.1127. [DOI] [PubMed] [Google Scholar]
- McGeer E, McGeer P. Neurotransmitter metabolism in the aging brain. In: Terry RD, Gershon S, editors. Neurobiology of aging. New York: Raven; 1976. pp. 389–403. [Google Scholar]
- Mikami A, Newsome WT, Wurtz RH. Motion selectivity in macaque visual cortex 1: Mechanisms of direction and speed selectivity in extrastriate area MT. J Neurophysiol. 1986;55:1308–1327. doi: 10.1152/jn.1986.55.6.1308. [DOI] [PubMed] [Google Scholar]
- Morrison JH, Hof PR. Life and death of neurons in the aging brain. Science. 1997;278:412–419. doi: 10.1126/science.278.5337.412. [DOI] [PubMed] [Google Scholar]
- Morrison JH, Hof PR. Life and death of neurons in the aging cerebral cortex. Int Rev Neurobiol. 2007;81:41–57. doi: 10.1016/S0074-7742(06)81004-4. [DOI] [PubMed] [Google Scholar]
- Moschner C, Baloh RW. Age-related-changes in visual tracking. J Gerontol. 1994;49:M235–M238. doi: 10.1093/geronj/49.5.m235. [DOI] [PubMed] [Google Scholar]
- Muir JL. Acetylcholine, aging, and Alzheimer's disease. Pharmacol Biochem Behav. 1997;56:687–696. doi: 10.1016/s0091-3057(96)00431-5. [DOI] [PubMed] [Google Scholar]
- Newsome WT, Pare EB. A selective impairment of motion perception following lesions of the middle temporal visual area (MT) J Neurosci. 1988;8:2201–2211. doi: 10.1523/JNEUROSCI.08-06-02201.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newsome WT, Wurtz RH, Dursteler MR, Mikami A. Deficits in visual motion processing following ibotenic acid lesions of the middle temporal visual area of the macaque monkey. J Neurosci. 1985;5:825–840. doi: 10.1523/JNEUROSCI.05-03-00825.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyenkim JD, DeAngelis GC. Disparity-based coding of three-dimensional surface orientation by macaque middle temporal neurons. J Neurosci. 2003;23:7117–7128. doi: 10.1523/JNEUROSCI.23-18-07117.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NHTSA. 2004. Traffic Safety facts 2004 data—older population. National Highway Traffic Safety Administration, US Dept of Transport, Washington, DC, DOT HS, 809–910. [Google Scholar]
- Norman JF, Lappin JS. The detection of surface curvatures defined by optical motion. Percept Psychophys. 1992;51:386–396. doi: 10.3758/bf03211632. [DOI] [PubMed] [Google Scholar]
- Norman JF, Ross HE, Hawkes LM, Long JR. Aging and the perception of speed. Perception. 2003;32:85–96. doi: 10.1068/p3478. [DOI] [PubMed] [Google Scholar]
- Nover H, Anderson CH, DeAngelis GC. A logarithmic, scale-invariant representation of speed in macaque middle temporal area accounts for speed discrimination performance. J Neurosci. 2005;25:10049–10060. doi: 10.1523/JNEUROSCI.1661-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OECD. Ageing and transport. Mobility needs and safety issues. Paris: OECD; 2001. [Google Scholar]
- Orban GA, Saunders RC, Vandenbussche E. Lesions of the superior temporal cortical motion areas impair speed discrimination in the macaque monkey. Eur J Neurosci. 1995;7:2261–2276. doi: 10.1111/j.1460-9568.1995.tb00647.x. [DOI] [PubMed] [Google Scholar]
- Pack CC, Hunter JN, Born RT. Contrast dependence of suppressive influences in cortical area MT of alert macaque. J Neurophysiol. 2005;93:1809–1815. doi: 10.1152/jn.00629.2004. [DOI] [PubMed] [Google Scholar]
- Palanca BJ, DeAngelis GC. Macaque middle temporal neurons signal depth in the absence of motion. J Neurosci. 2003;23:7647–7658. doi: 10.1523/JNEUROSCI.23-20-07647.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasternak T, Merigan WH. Motion perception following lesions of the superior temporal sulcus in the monkey. Cereb Cortex. 1994;4:247–259. doi: 10.1093/cercor/4.3.247. [DOI] [PubMed] [Google Scholar]
- Pelli DG. The VideoToolbox software for visual psychophysics: transforming numbers into movies. Spat Vis. 1997;10:437–442. [PubMed] [Google Scholar]
- Perrone JA. A visual motion sensor based on the properties of V1 and MT neurons. Vision Res. 2004;44:1733–1755. doi: 10.1016/j.visres.2004.03.003. [DOI] [PubMed] [Google Scholar]
- Perrone JA. Economy of scale: a motion sensor with variable speed tuning. J Vis. 2005;5:28–33. doi: 10.1167/5.1.3. [DOI] [PubMed] [Google Scholar]
- Perrone JA. A single mechanism can explain the speed tuning properties of MT and V1 complex neurons. J Neurosci. 2006;26:11987–11991. doi: 10.1523/JNEUROSCI.4024-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrone JA, Stone LS. A model of self-motion estimation within primate extrastriate visual cortex. Vision Res. 1994;34:2917–2938. doi: 10.1016/0042-6989(94)90060-4. [DOI] [PubMed] [Google Scholar]
- Perrone JA, Stone LS. Emulating the visual receptive-field properties of MST neurons with a template model of heading estimation. J Neurosci. 1998;18:5958–5975. doi: 10.1523/JNEUROSCI.18-15-05958.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrone JA, Thiele A. Speed skills: measuring the visual speed analyzing properties of primate MT neurons. Nat Neurosci. 2001;4:526–532. doi: 10.1038/87480. [DOI] [PubMed] [Google Scholar]
- Perrone JA, Thiele A. A model of speed tuning in MT neurons. Vision Res. 2002;42:1035–1051. doi: 10.1016/s0042-6989(02)00029-9. [DOI] [PubMed] [Google Scholar]
- Peters A, Morrison JH, Rosene DL, Hyman BT. Feature article: are neurons lost from the primate cerebral cortex during normal aging? Cereb Cortex. 1998;8:295–300. doi: 10.1093/cercor/8.4.295. [DOI] [PubMed] [Google Scholar]
- Post-Munson DJ, Lum-Ragan JT, Mahle CD, Gribkoff VK. Reduced bicuculline response and GABAA agonist binding in aged rat hippocampus. Neurobiol Aging. 1994;15:629–633. doi: 10.1016/0197-4580(94)00057-3. [DOI] [PubMed] [Google Scholar]
- Price NSC, Ono S, Mustari MJ, Ibbotson MR. Comparing acceleration and speed tuning in macaque MT: Physiology and modeling. J Neurophysiol. 2005;94:3451–3464. doi: 10.1152/jn.00564.2005. [DOI] [PubMed] [Google Scholar]
- Priebe NJ, Cassanello CR, Lisberger SG. The neural representation of speed in macaque area MT/V5. J Neurosci. 2003;23:5650–5661. doi: 10.1523/JNEUROSCI.23-13-05650.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priebe NJ, Lisberger SG. Estimating target speed from the population response in visual area MT. J Neurosci. 2004;24:1907–1916. doi: 10.1523/JNEUROSCI.4233-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priebe NJ, Lisberger SG, Movshon JA. Tuning for spatiotemporal frequency and speed in directionally selective neurons of macaque striate cortex. J Neurosci. 2006;26:2941–2950. doi: 10.1523/JNEUROSCI.3936-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prince SJ, Pointon AD, Cumming BG, Parker AJ. Quantitative analysis of the responses of V1 neurons to horizontal disparity in dynamic random-dot stereograms. J Neurophysiol. 2002;87:191–208. doi: 10.1152/jn.00465.2000. [DOI] [PubMed] [Google Scholar]
- Purushothaman G, Bradley DC. Neural population code for fine perceptual decisions in area MT. Nat Neurosci. 2005;8:99–106. doi: 10.1038/nn1373. [DOI] [PubMed] [Google Scholar]
- Ringach DL, Bredfeldt CE, Shapley RM, Hawken MJ. Suppression of neural responses to nonoptimal stimuli correlates with tuning selectivity in macaque V1. J Neurophysiol. 2002;87:1018–1027. doi: 10.1152/jn.00614.2001. [DOI] [PubMed] [Google Scholar]
- Ross RG, Olincy A, Harris JG, Radant A, Adler LE, Compagnon N, Freedman R. The effects of age on a smooth pursuit tracking task in adults with schizophrenia and normal subjects. Biol Psychiatry. 1999;46:383–391. doi: 10.1016/s0006-3223(98)00369-2. [DOI] [PubMed] [Google Scholar]
- Salthouse TA. Aging and measures of processing speed. Biol Psychology. 2000;54:35–54. doi: 10.1016/s0301-0511(00)00052-1. [DOI] [PubMed] [Google Scholar]
- Schlack A, Krekelberg B, Albright TD. Recent history of stimulus speeds affects the speed tuning of neurons in area MT. J Neurosci. 2007;27:11009–11018. doi: 10.1523/JNEUROSCI.3165-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmolesky MT, Wang Y, Pu M, Leventhal AG. Degradation of stimulus selectivity of visual cortical cells in senescent rhesus monkeys. Nat Neurosci. 2000;3:384–390. doi: 10.1038/73957. [DOI] [PubMed] [Google Scholar]
- Scialfa CT, Guzy LT, Leibowitz HW, Garvey PM, Tyrrell RA. Age differences in estimating vehicle velocity. Psychol Aging. 1991;6:60–66. doi: 10.1037//0882-7974.6.1.60. [DOI] [PubMed] [Google Scholar]
- Shadlen MN, Newsome WT. The variable discharge of cortical neurons: implications for connectivity, computation, and information coding. J Neurosci. 1998;18:3870–3896. doi: 10.1523/JNEUROSCI.18-10-03870.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simoncelli EP, Heeger DJ. A model of neuronal responses in visual area MT. Vision Res. 1998;38:743–761. doi: 10.1016/s0042-6989(97)00183-1. [DOI] [PubMed] [Google Scholar]
- Snowden RJ, Kavanagh E. Motion perception in the ageing visual system: Minimum motion, motion coherence, and speed discrimination thresholds. Perception. 2006;35:9–24. doi: 10.1068/p5399. [DOI] [PubMed] [Google Scholar]
- Spear PD. Neural bases of visual deficits during aging. Vision Res. 1993;33:2589–2609. doi: 10.1016/0042-6989(93)90218-l. [DOI] [PubMed] [Google Scholar]
- Spear PD, Moore RJ, Kim CB, Xue JT, Tumosa N. Effects of aging on the primate visual system: spatial and temporal processing by lateral geniculate neurons in young adult and old rhesus monkeys. J Neurophysiol. 1994;72:402–420. doi: 10.1152/jn.1994.72.1.402. [DOI] [PubMed] [Google Scholar]
- Sunaert S, Van Hecke P, Marchal G, Orban GA. Attention to speed of motion, speed discrimination, and task difficulty: an fMRI study. Neuroimage. 2000;11:612–623. doi: 10.1006/nimg.2000.0587. [DOI] [PubMed] [Google Scholar]
- Thiele A, Distler C, Korbmacher H, Hoffmann KP. Contribution of inhibitory mechanisms to direction selectivity and response normalization in macaque middle temporal area. Proc Natl Acad Sci USA. 2004;101:9810–9815. doi: 10.1073/pnas.0307754101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tigges J, Gordon T, McClure H, Hall E, Peters A. Survival rate and life span of rhesus monkeys at the Yerkes Regional Primate Research Center. Am J Primatol. 1988;15:263–273. doi: 10.1002/ajp.1350150308. [DOI] [PubMed] [Google Scholar]
- Todd JT, Akerstrom RA, Reichel FD, Hayes W. Apparent rotation in three-dimensional space: effects of temporal, spatial, and structural factors. Percept Psychophys. 1988;43:179–188. doi: 10.3758/bf03214196. [DOI] [PubMed] [Google Scholar]
- Tolhurst DJ, Movshon JA, Dean AF. The statistical reliability of signals in single neurons in cat and monkey visual cortex. Vision Res. 1983;23:775–785. doi: 10.1016/0042-6989(83)90200-6. [DOI] [PubMed] [Google Scholar]
- Uka T, DeAngelis GC. Contribution of middle temporal area to coarse depth discrimination: comparison of neuronal and psychophysical sensitivity. J Neurosci. 2003;23:3515–3530. doi: 10.1523/JNEUROSCI.23-08-03515.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villeneuve MY, Casanova C. On the use of isoflurane versus halothane in the study of visual response properties of single cells in the primary visual cortex. J Neurosci Methods. 2003;129:19–31. doi: 10.1016/s0165-0270(03)00198-5. [DOI] [PubMed] [Google Scholar]
- Wang Y, Zhou Y, Ma Y, Leventhal AG. Degradation of signal timing in cortical areas V1 and V2 of senescent monkeys. Cereb Cortex. 2005;15:403–408. doi: 10.1093/cercor/bhh143. [DOI] [PubMed] [Google Scholar]
- Weiss Y, Simoncelli EP, Adelson EH. Motion illusions as optimal percepts. Nat Neurosci. 2002;5:598–604. doi: 10.1038/nn0602-858. [DOI] [PubMed] [Google Scholar]
- Welford AT. Between bodily changes and performance: some possible reasons for slowing with age. Exp Aging Res. 1984;10:73–88. doi: 10.1080/03610738408258548. [DOI] [PubMed] [Google Scholar]
- Xiao DK, Marcar VL, Raiguel SE, Orban GA. Selectivity of macaque MT/V5 neurons for surface orientation in depth specified by motion. Eur J Neurosci. 1997;9:956–964. doi: 10.1111/j.1460-9568.1997.tb01446.x. [DOI] [PubMed] [Google Scholar]
- Yang T, Maunsell JH. The effect of perceptual learning on neuronal responses in monkey visual area V4. J Neurosci. 2004;24:1617–1626. doi: 10.1523/JNEUROSCI.4442-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu S, Wang Y, Li X, Zhou Y, Leventhal AG. Functional degradation of extrastriate visual cortex in senescent rhesus monkeys. Neuroscience. 2006;140:1023–1029. doi: 10.1016/j.neuroscience.2006.01.015. [DOI] [PubMed] [Google Scholar]