Abstract
Attention-enhancing effects of nicotine appear to depend on the nature of the attentional function. Underlying neuroanatomical mechanisms, too, may vary depending on the function modulated. This functional magnetic resonance imaging study recorded blood oxygen level–dependent (BOLD) activity in minimally deprived smokers during tasks of simple stimulus detection, selective attention, or divided attention after single-blind application of a transdermal nicotine (21 mg) or placebo patch. Smokers’ performance in the placebo condition was unimpaired as compared with matched nonsmokers. Nicotine reduced reaction time (RT) in the stimulus detection and selective attention but not divided attention condition. Across all task conditions, nicotine reduced activation in frontal, temporal, thalamic, and visual regions and enhanced deactivation in so-called “default” regions. Thalamic effects correlated with RT reduction selectively during stimulus detection. An interaction with task condition was observed in middle and superior frontal gyri, where nicotine reduced activation only during stimulus detection. A visuomotor control experiment provided evidence against nonspecific effects of nicotine. In conclusion, although prefrontal activity partly displayed differential modulation by nicotine, most BOLD effects were identical across tasks, despite differential performance effects, suggesting that common neuronal mechanisms can selectively benefit different attentional functions. Overall, the effects of nicotine may be explained by increased functional efficiency and downregulated task-independent “default” functions.
Keywords: deactivation, default, fMRI, reaction time, skin patch, smokers
Introduction
There is ample evidence across species that nicotine possesses performance-enhancing properties (Wesnes and Warburton 1983; Heishman et al. 1994; Rezvani and Levin 2001), with improvements in attention particularly robust (Stolerman et al. 1995; Newhouse et al. 2004). The therapeutic potential of these effects motivates investigation of the precise attentional functions affected and their neuronal mediators.
With regard to the type of attentional function, nicotine consistently improves performance in tasks of vigilance and simple stimulus detection (e.g., Wesnes and Warburton 1984; Koelega 1993; Foulds et al. 1996; Mancuso et al. 1999). These findings speak toward a generalized drug effect on alertness and intensity aspects of attention that could enhance performance across different paradigms. However, even if less robust, several findings also suggest improvements specific to processes of selective attention, such that the performance-enhancing effects of nicotine are relatively greater in mitigating the effects of distractors. For example, in both smokers and nonsmokers, nicotine or cigarette smoking reduced the Stroop effect, that is, performance costs of naming the ink color of an incongruent color word, in about half the studies investigating such effects (Wesnes and Warburton 1983; Provost and Woodward 1991; Hasenfratz and Battig 1992; Parrott and Craig 1992; Foulds et al. 1996; Poltavski and Petros 2006; Domier et al. 2007). Nicotine also reduced the Garner effect, that is, performance costs due to changes in the irrelevant stimulus dimension per se (Waters 1998). Furthermore, introducing sensory distractor stimuli helped reveal performance-enhancing effects of nicotine in humans (Grobe et al. 1998), monkeys (Prendergast et al. 1998), and rats (Hahn et al. 2002; Hahn and Stolerman 2002).
This reduced interference from irrelevant stimuli may reflect enhanced attentional filtering or an enhancement in control processes of attentional resource allocation. Evidence for the former can be deduced from findings that nicotine impaired incidental memory of material that subjects had not been instructed to remember and to which attention had presumably not been directed, while improving recall of attended material (Andersson and Hockey 1977). Furthermore, improvements occurred mainly in those portions of a word list that were better recalled in the placebo condition and had probably been predominantly attended to (Warburton et al. 1992). Thus, nicotine appeared to increase attentional resources allocated to attended material and enhance the filtering of unattended material.
In addition to selective attention, or attention to individual stimuli, there is the question of divided attention. Concepts of divided attention, too, are concerned with selectivity aspects of attention but more specifically with the optimal allocation of resources between different sets of input (Parasuraman 1998). Attention can be divided between locations in space, different features of one or more objects, and stimuli in one or more sensory modalities (Braun 1998). Nicotine has been reported to either improve (Leigh et al. 1977) or have no effect (Trimmel and Wittberger 2004) on dual-task performance. Effects on selective and divided attention have never been directly compared.
Neuroimaging studies of the attention-enhancing effects of nicotine have mostly focused on nonselective alertness components of attention (with the exception of studies on specific spatial reorienting functions; Thiel et al. 2005; Giessing et al. 2006). Improvement in vigilance performance was accompanied by thalamic and parietal activation and enhanced insula and medial temporal deactivation (Lawrence et al. 2002). Nicotine also modulated cue-induced alerting-related activity in frontal, parietal, and superior temporal regions (Thiel and Fink 2007). Furthermore, in a study of visuospatial attention (Hahn et al. 2007), performance enhancement was associated with nicotine-induced deactivation of default regions of resting brain function (Gusnard and Raichle 2001), suggesting that nicotine improved performance by aiding the downregulation of task-independent thought processes. Thus, insight has been gained regarding mechanisms mediating effects on global intensity aspects of attention. However, such generalized alerting functions do not preclude the existence of mechanisms via which nicotine may specifically enhance selectivity aspects of attention, in line with its behavioral profile described above.
The present study aimed at identifying and dissociating neuroanatomical substrates of nicotine's performance effects under conditions that tax processes of selective or divided attention and in a simple stimulus detection condition that does not create any particular demands on attentional selection. A task setting was developed in which a single foveally presented stimulus accommodated tasks related to decisions about each of 2 stimulus dimensions or about both dimensions combined. A third stimulus detection task presented similar stimuli but required responses based on subsequently presented signals. We hypothesized that some of the neuronal effects of nicotine would be specific to task conditions with a selectivity component.
Materials and Methods
Participants
Eighteen right-handed smokers (9 females) participated in the study. Smokers were aged 19–49 years (mean ± standard deviation [SD] 30.1 ± 7.9 years) and smoked 21 ± 5 (range 15–30) cigarettes per day for 12.9 ± 6.6 years (range 4–27 years). Smoker's Intelligence Quotient (IQ), determined by the Wechsler Abbreviated Scale of Intelligence (Wechsler 1999), was 109 ± 10. Eighteen right-handed nonsmokers (7 females), who reported no nicotine use within the past 12 months, were matched (no significant difference in independent-samples t-tests) for age (29.6 ± 6.7 years, range 18–44) and IQ (116 ± 11). Neuroimaging and performance data from these nonsmoking participants, forming part of a larger group of control subjects, were reported in detail previously (Hahn et al. 2008).
Subjects were recruited from the general population through newspaper advertising, flyers, and referrals and gave written informed consent for a protocol approved by the National Institute on Drug Abuse (NIDA)–IRP Institutional Review Board. Subjects were screened for major medical illnesses, claustrophobia, history of neurological or psychiatric disorders, drug and alcohol abuse, and pregnancy. A urine sample was collected and assessed for common drugs of abuse (TRIAGE).
Procedure
The protocol required 3 visits. During the first visit, participants gave informed consent and were trained on 2 cognitive tasks (1 reported elsewhere), initially on a bench computer and then for 30 min in a mock scanner that mimicked all properties of the magnetic resonance imaging (MRI) scanner without the magnetic field. Participants were also familiarized with the computerized questionnaires to be completed in the scanner and with the wheel response device used for their completion. Sessions 2 and 3 were identical; however, for smokers, a Nicoderm patch (21 mg/24 h; GlaxoSmithKline, Moon Township, PA) was applied to the upper back in one session, 2–2.5 h prior to being loaded into the MR scanner, and a placebo patch in the other. The task paradigm reported here began approximately 3–3.5 h following patch application. The sequence of test sessions was counterbalanced such that 9 smokers received nicotine in the first and 9 in the second session. Order of patch application was single blind.
Nonsmokers completed both sessions without any skin patches. The purpose was to compare performance and regional activity between groups at baseline and examine potential abstinence-related differences in smokers. Nicotine was not administered to nonsmokers because initial exposure of drug-naive individuals typically leads to aversive side effects that can overshadow and interfere with the measurement of nicotine's cognitive effects (Heishman et al. 1993; Perkins et al. 1994; Heishman and Henningfield 2000). The 2 test sessions were scheduled 2–16 days apart in all except 2 subjects (both smokers) who were tested 24 and 28 days apart due to scheduling availability.
Smokers smoked a cigarette within 1 h prior to entering the NIDA–IRP research facilities, with MR scans starting approximately 3 h following their last cigarette. Participants were told not to ingest any alcohol or over-the-counter medication in the 24 h preceding each session and not to consume more than a half cup of coffee within the preceding 12 h. Prior to patch application, participants were tested for recent drug use (TRIAGE) and for alcohol intake via breath analysis (Alco-Sensor IV, Intoximeters, Inc., St. Louis, MO). Shortly after patch application, subjects received a 9-min reminder task training on a bench computer and practiced a finger-tapping procedure. In MR scans, three 8:42-min runs of the selective/divided attention task (see below) were performed, separated by 1-min rest periods. A 30-min visuospatial cueing task (Stein et al. 2004) was performed within the same session; the sequence of tasks was counterbalanced across subjects. Anatomical scans were performed between tasks. At the end of each session, a perfusion MRI scan was obtained during performance of a 6.5-min finger-tapping task described below.
For all but one smoker, a venous blood specimen (5 mL) was drawn from a forearm vein within 10 min following each scan session. Specimens were stored on ice, centrifuged within 2 h of collection, and plasma frozen at −20 °C until analysis. Plasma specimens (1 mL) from 10 participants were assayed for nicotine concentration via solid phase extraction and liquid chromatography–atmospheric pressure chemical ionization–mass spectrometry with selected ion monitoring (Kim and Huestis 2006). The assay was linear from 2.5 to 500 ng/mL with a weighting factor of 1/x; correlation coefficients for calibration curves were >0.99. Intra- and interassay precision and accuracy were <15.0%. Nicotine recovery ranged from 108.2% to 110.8% at 3 concentrations across the dynamic range. For the remaining specimens, nicotine was isolated and concentrated from 200 μL plasma by solid phase extraction with preconditioned CleanScreen DAU columns. Eluates were evaporated to dryness under nitrogen, reconstituted with 200 μL of mobile phase, and analyzed by a validated liquid chromatography–tandem mass spectrometry method with electrospray ionization. Identification and quantification of nicotine were based on selected reaction monitoring with 2 transitions. The limit of quantification was 1 ng/mL with a linear dynamic range to 500 ng/mL. Extraction efficiency was greater than 90% with inter- and intraday imprecision <20%.
Subjective state was measured by computerized versions of 2 self-report instruments while lying inside the MR scanner—once just before and once just after the scan session. One instrument, given to both smokers and control participants, consisted of a list of bidirectional visual analog scales sensitive to mood changes induced by tobacco deprivation (Parrott et al. 1996): tense/relaxed, nervous/calm, energetic/tired, alert/drowsy, contented/irritated, satisfied/dissatisfied. Additional scales added to cover further nicotine withdrawal symptoms were distracted/focused, depressed/happy, and satiated/hungry. Data are not available for 6 of the nonsmoking controls. Smokers also completed the 12-item version of the tobacco craving questionnaire (TCQ) (Heishman et al. 2003). For both scales, participants used a wheel response device to move a cursor on the screen to the desired position on a horizontal bar relative to 2 anchors.
Measurement of Selective and Divided Attention
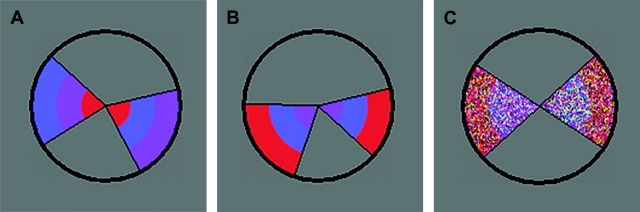
The task stimulus consisted of a circle containing 2 wedges, displayed against a gray background in the center of the screen (Fig. 1). The diameter of the circle, based on a viewing distance of 80 cm, was 3.6° of visual angle, thus allowing foveal stimulus processing without significant eye movement. In the selective and divided attention task conditions, each wedge was divided into 3 sections of an inner, middle, and outer ring of color (Fig. 1A,B). Within each wedge, each segment was always of a different color from the others (red, blue, and purple). In 3 different forced choice tasks, participants decided whether specific features of the 2 wedges were the same or different. In the 2 selective attention conditions, they were instructed to attend either to the color order of the rings (selective color, SEL-C) or to the angles of the wedges (selective angle, SEL-A) and decide whether they were the same or different. The third was a divided attention (DIV) condition, during which subjects attended to both of these features and decided whether or not the wedges were identical in both features. A button for same was pressed with the right index finger and a button for different with the left.
Figure 1.
Examples of the task stimuli. Participants were instructed to detect a difference in either the angles of the 2 wedges, in the sequence of color across the 3 rings, or in either aspect. In (A), there is a difference in the color dimension. In (B), there is a difference in the angle dimension. (C) Represents a stimulus presented during SDT, where responses did not depend on any stimulus aspects.
In the SEL-A and SEL-C tasks, the wedges differed on the task-relevant feature in 50% of trials. The task-irrelevant feature also differed in 50% of trials, independent of the status of the task-relevant feature. Thus, stimulus characteristics remained constant and only task demands defined the 2 different conditions. In the DIV task, the wedges differed on either one of the 2 stimulus features in 50% of trials, that is, 25% on the angle and 25% on the color feature; in the other half of the trials, neither feature differed.
The fourth was a simple stimulus detection task (SDT) designed not to place any particular demands on selectivity aspects of attention. The wedged circle was presented for a fixed length of time that equaled the display time (DT) entered at the beginning of the session (see below). The visual stimulus properties were the same as in the other tasks except that only 2 rings of color were presented and the color hues were composed of multicolored dots (Fig. 1C). These changes to the stimulus appearance were made to prevent potential habitual focusing on either of the 2 stimulus dimensions. The circle stimulus was followed by presentation of a letter on the left or on the right. Participants were instructed to respond not to any property of the wedges but to the side on which a letter was presented.
Each task trial started with a 500-ms central fixation cross, followed by 500 ms of blank screen. The wedged circle was then presented for the duration of DT (see below), followed by 48 ms of a back mask consisting of the circle filled with colored dots, to eliminate any persisting afterimage of the task stimulus. The letters “d” and “s” for “different” and “same” then appeared on the left and right, respectively, of where the circle had been presented (on the left or right for SDT) and stayed on display until a response was made for a maximum duration of 2 s. Trials where no response was recorded within this time were excluded from analysis (1.1% of all trials). Trials were separated by a variable interstimulus interval (ISI) of 0, 2, 4, or 6 s duration. The ISI was extended by the length of time needed to complete the preceding repetition time (TR).
For Sel-A, Sel-C, and DIV, performance accuracy was held at 75% by manipulating the stimulus DT. The purpose of this manipulation was to minimize differences in error processing and response uncertainty between the selective and divided attention conditions and to eliminate such confounds when interpreting any differential effects of nicotine. Adjustments were made in 16 ms units. Initial DT was determined during training for each individual subject. Early during the training procedure, the wedge angle difference was determined such that DT for SEL-A was identical to SEL-C at 75% accuracy. This difference value was then adopted for all 3 tasks. Angle difference values ranged from 6° to 12° across participants (mean ± SD 7.1 ± 1.8). Throughout, DT was dynamically adjusted after every 4 trials. If a correct response was made in 3 out of the 4 preceding trials, DT stayed the same. If 2 or fewer trials were correct, DT increased by 16 ms, and if all 4 trials were correct, DT decreased by 16 ms. During scan sessions, DT was adjusted in this manner independently for SEL-A, SEL-C, and DIV, starting with the values obtained at completion of the training. The same starting values were used for both scans. In this manner, response accuracy was successfully adjusted to vary around or just above 75% for each task (Hahn et al. 2008). Accuracy during SDT approached 100%.
In each scan session, three 8:42-min task runs were completed. Each run started with one 8-trial block of SDT. One 16-trial block each of SEL-A, SEL-C, and DIV was then performed in a randomized sequence, followed by 8 more trials of SDT. Each block began with the task instruction, displayed for 4 s, followed by a 6-s epoch where participants performed a forced choice test (“press the button on the side that names this task”). Blocks preceded by an incorrect answer were excluded from further analyses (7 out of a total of 432 blocks across subjects and sessions).
Controls for Nonspecific Effects of Nicotine on Blood Flow and Coupling
To test for potential nonspecific effects of nicotine on cerebral blood flow (CBF) or coupling between neuronal and hemodynamic response dynamics, perfusion functional magnetic resonance imaging (fMRI) scans were acquired from slices covering primary motor and visual cortices while subjects performed cyclic (30 s on, 30 s off) bilateral finger tapping. During on-periods, a checkerboard of black and white squares that filled the entire screen (spatial frequency ∼0.26 cycles/degree), and whose contrast reversed 3 times per second, served as a visual metronome. During off-periods, participants fixated a central cross. The scan started and ended on an off-period. Thirteen 30-s periods were presented in total.
Magnetic Resonance Imaging
Scanning was performed on a 3 Tesla Siemens Allegra scanner (Erlangen, Germany). Whole-brain functional EPI images were acquired for measurement of T2*-weighted blood oxygen level–dependent (BOLD) effects (4 mm sagittal slices, 64 × 64 matrix, field of view [FOV] = 22 × 22 cm, TR = 2 s, time echo [TE] = 27 ms, FA = 75°). In each scanning session, a whole-brain sagittal T1-weighted structural image (MPRAGE) was acquired for anatomical reference (1 mm3 isotropic voxels, TR = 2.5 s, TE = 4.38 ms, FA = 8°). Perfusion fMRI scans were acquired in six 7-mm transaxial slices using a QUIPPS II (Wong et al. 1998) arterial spin labeling (ASL) imaging sequence (FOV = 220 cm, matrix = 64 × 64, TR = 3 s, TE = 27 ms, FA = 90°, TI1 = 700 ms, TI2 = 1400 ms, gap = 10 mm). Four subjects were scanned with a Flow-sensitive Alternating Inversion Recovery (FAIR)-based sequence (Kim 1995; TI = 1400, inversion slab thickness = 58 mm).
Analysis of Subjective Self-Reports
Individual “Parrott” subscales (Parrott et al. 1996) were analyzed by 3-factor analysis of variance (ANOVA) with GROUP (smokers, controls) as a between-subject factor and SESSION (nicotine vs. placebo for smokers; no-drug vs. no-drug for controls) and PRE–POST (pre- vs. postscan) as within-subject factors. TCQ craving scores were obtained only in smokers and were analyzed by 2-factor ANOVA (SESSION × PRE–POST).
Analysis of Behavioral Data
Data from the 2 scan sessions were analyzed. DT and reaction time (RT) were expressed as averages for each task condition and analyzed, separately, by 3-factor ANOVA for repeated measures with DRUG (nicotine, placebo) and TASK (SDT, Sel-A, Sel-C, DIV) as within-subject factors and SEQUENCE OF TESTING (nicotine followed by placebo, placebo followed by nicotine) as between-subject factor. ANOVAs were followed by paired t-tests where indicated. To compare performance in the absence of nicotine between smokers and nonsmokers, data from nondrug days were analyzed by 2-factor ANOVA with GROUP (smokers, controls) as between-subject factor and TASK as within-subject factor. Session 1 data were included from 9 of the 18 controls, selected randomly, and session 2 data from the other 9, thus matching the amount of task preexposure to that of smokers’ placebo sessions.
Analysis of fMRI Data
Data were processed using the AFNI software package (Cox 1996). Motion correction was performed by registering each 3D volume to a base volume. The time series was then analyzed as an event-related design by voxel-wise multiple regression. Regressors were expressed as a delta function, time locked to the onset of each circle stimulus, and convolved with a model hemodynamic response function and its temporal derivative. Regressors corresponded to the 4 different task conditions (SDT, Sel-A, Sel-C, and DIV) and to the 6 motion parameters as nuisance regressors. Further nuisance regressors corresponded to the display and retention test of the task instruction, and if applicable, to trials in which no response was registered and blocks in which the task instruction was not correctly repeated. For each subject and test session, the voxel-wise average amplitude of signal change (β value) produced by each task condition was determined relative to baseline. The resulting activation maps were resampled to a higher (1 μL) resolution, converted to a standard stereotaxic coordinate system (Talairach and Tournoux 1988) and spatially blurred using a Gaussian 5-mm rms isotropic kernel.
Second-level random-effects analysis across smokers consisted of voxel-wise 2-factor ANOVA for repeated measures (DRUG × TASK) performed on the β values produced by each task condition. A voxel-wise threshold of P < 0.01 was applied to the activation maps and combined with a minimum cluster volume size of 450 μL. Based on Monte Carlo simulations, taking account of spatial covariation in the output dataset, this yielded an overall false positive P < 0.005. To test whether the effects of nicotine may have served to restore a normal functional state aberrant in smokers in the absence of nicotine, for example, due to neural adaptations with chronic nicotine exposure, average levels of activity in nonsmokers were determined within functional Regions of Interest (ROIs) that displayed effects of nicotine. Activations in the drug-free state were compared between groups by independent-samples t-tests. Nine smokers received placebo in session 1 and 9 in session 2; accordingly, session 1 data were used from 9 and session 2 data from the other 9 nonsmoking controls. Also, to test for group differences in brain regions not necessarily modulated by nicotine, whole-brain voxel-wise ANOVA (GROUP × TASK) was performed on the no-drug data, using the same significance criteria as for the DRUG × TASK ANOVA.
To examine the effects of nicotine on BOLD and CBF responses to visuomotor stimulation during smokers’ ASL scan, BOLD- (derived from untagged images) and flow-weighted (derived by voxel-wise subtraction of untagged from tagged images) time series were analyzed with a boxcar regressor following the 30-s on- and off-periods convolved with a model hemodynamic response function. Data from 3 subjects were corrupted and were excluded. BOLD contrast values (on- vs. off-periods) were normalized and underwent a random-effects 1-sample t-test against 0. Voxel-wise P < 0.001 combined with a minimum cluster volume of 368 μL yielded an overall false positive P < 0.05 as determined by Monte Carlo simulation. Flow- and BOLD-weighted contrast values were averaged across voxels within each identified region. For each participant, only voxels with anatomical coverage in both sessions were included. Average regional BOLD contrast values were compared between the placebo and nicotine session by paired t-tests. Flow-weighted values displayed large variability in this dataset. Although no effects of nicotine were seen, the large error variance would most likely preclude their detection. Flow-weighted values are thus excluded from this report.
Head motion during the attention task was compared between test sessions by calculating a composite motion index from the 3 translational and the 3 rotational parameters as described by Yang et al. (2005). This index reflects a subject's average head motion between 2 consecutive TRs. Values did not differ between the nicotine and the placebo session (t17 = 1.68, not significant [NS]; paired t-test).
Correlations
Each smoker's RT in the placebo session was subtracted from that in the nicotine session. Similarly, for each brain area modulated by nicotine, average regional activation under placebo was subtracted from that under nicotine. The difference values in RT and regional activation underwent partial correlation controlling for nicotine plasma concentrations in both the nicotine and placebo sessions. Plasma concentrations were controlled for because they may underlie interindividual variation in both performance and BOLD effects of nicotine and may thus enhance correlations by acting as a common antecedent. For correlations, P < 0.005 was considered significant.
Results
Nicotine Plasma Levels
Smokers’ plasma nicotine levels were 5.7 ± 2.8 ng/mL at completion of the placebo scan and 37.7 ± 9.9 ng/mL after the nicotine scan (t16 = 13.8, P < 0.001), comparable with results obtained previously under the same experimental conditions (Hahn et al. 2007).
Subjective State
Parrott Scale
Main effects of PRE–POST for 5 variables (F1,28 > 4.80, P < 0.05) indicated that all participants felt more tired, drowsy, dissatisfied, distracted, and hungry after than before scan sessions. A main effect of GROUP (F1,28 = 4.79, P < 0.05) for the “energetic–tired” subscale reflected higher reports of tiredness in the smokers than nonsmokers. A GROUP × SESSION interaction occurred on “alert–drowsy” and “focused–distracted” (F1,28 > 5.42, P < 0.05). Effects of SESSION on these scales were seen in smokers (t17 > 2.73, P < 0.05), who were more focused and alert in the nicotine than placebo session, but not in nonsmokers, who were never administered any drug. Ratings never differed significantly between groups, but numerically, smokers felt more alert and focused than nonsmokers in the nicotine session and drowsier and more distracted than nonsmokers in the placebo session. Thus, the drug effect may represent a combination of alerting effects of nicotine and impairment in the absence of nicotine.
Tobacco Craving Questionnaire
Smokers’ craving ratings were higher in the placebo than nicotine session (main effect of DRUG: F1,17 = 7.25, P < 0.05) and higher after than before scan sessions (PRE–POST: F1,17 = 8.74, P < 0.01). No DRUG × PRE–POST interaction was observed.
Effects of Nicotine on Smokers’ Performance
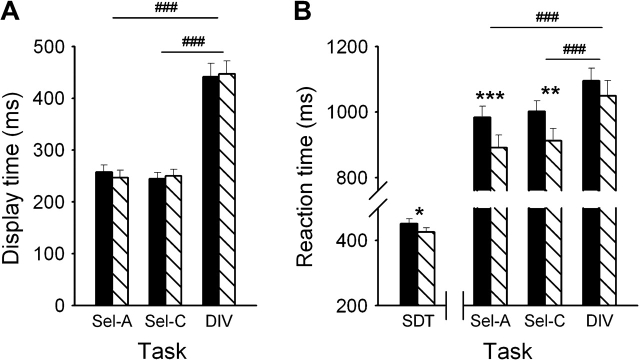
Figure 2A shows DT during Sel-A, Sel-C, and DIV for each of the 2 drug conditions. A main effect of TASK (F2,32 = 145.0, P < 0.001) reflects longer DT during DIV than during Sel-A or Sel-C. No difference between the nicotine and placebo condition was seen, as confirmed by the absence of a DRUG main effect or DRUG × TASK interaction. No main effect or interactions involving SEQUENCE OF TESTING were identified. DT during SDT was fixed and not included in the analysis.
Figure 2.
Average (±standard error of the mean) DT (A) and RT (B) of 18 smokers performing the SDT, the angle discrimination (Sel-A), color discrimination (Sel-C), or combined angle and color discrimination task (divided attention, DIV) while wearing a nicotine or placebo patch. Significant differences between the nicotine and placebo session (**P < 0.01, ***P < 0.001, paired t-test) and between task conditions (###P < 0.001, paired t-test) are indicated.
Figure 2B shows that RT was fastest during SDT and slowest during DIV, as confirmed by a main effect of TASK (F3,48 = 259.2, P < 0.001). Both the main effect of DRUG (F1,16 = 11.9, P < 0.01) and the DRUG × TASK interaction (F3,48 = 4.28, P < 0.01) were significant. Faster RT in the presence of nicotine was seen during Sel-A, Sel-C, and to a smaller degree also during SDT, but not during DIV. A SEQUENCE × DRUG × TASK interaction was observed (F3,48 = 3.34, P < 0.05). Two-factor ANOVA in each task condition revealed a DRUG × SEQUENCE interaction in SDT, where nicotine reduced RT only in participants who received placebo first. Thus, for SDT, session effects weakened the nicotine effect in participants who were tested with nicotine first, while enhancing it in those receiving nicotine second. The counterbalancing of the sequence of testing canceled these effects.
Comparison of Drug-Free Performance between Smokers and Nonsmokers
DT and RT were compared between groups in the absence of nicotine. In 2-factor ANOVA, there was no main effect of GROUP on either performance measure (F1,34 < 1). GROUP interacted with TASK on RT (F3,102 = 4.12, P < 0.01); smokers displayed somewhat slower RT in Sel-A and faster RT in Sel-C and DIV than nonsmokers (data not shown), but independent-samples t-tests did not reveal any significant group difference in any of the 4 task conditions (P > 0.2). No interaction was seen on DT (F3,102 < 1). The wedge angle difference adopted for Sel-A, Sel-C, and DIV did not differ between groups (t34 < 1). It is concluded that performance of smokers and nonsmokers in the absence of nicotine was approximately equal.
Functional Magnetic Resonance Imaging
In smokers, voxel-wise 2-factor ANOVA (DRUG × TASK) identified 15 regions displaying a main effect of DRUG (Table 1, Fig. 3). These included the medial frontal/rostral anterior cingulate cortex (ACC), left middle and inferior frontal gyrus (MFG, IFG), middle/inferior temporal gyrus (MTG/ITG), right pre-/postcentral gyrus, fusiform and parahippocampal gyrus, striate and extrastriate occipital regions, and bilateral thalamus. In each region, nicotine either reduced activation (regions 1–11 in Table 1 and Fig. 3) or induced or enhanced existing deactivation (regions 12–15). Three of the 4 regions where nicotine induced deactivation (rostral ACC, left MFG, and parahippocampal gyrus) were located in areas typically deactivated by attention-demanding tasks, termed the default network of resting brain function (Gusnard and Raichle 2001). In the absence of nicotine, the average BOLD signal did not differ between smokers and nonsmokers in any of the regions in independent-samples t-tests. Post hoc ANOVA of regional averages (DRUG × TASK × SEQUENCE) identified no effects involving the sequence of testing.
Table 1.
Main effect of nicotine
| Brain region | Side | Center of mass (mm) |
Brodmann areas | Number of 1-μL voxels | |||
| x | y | z | |||||
| Nicotine reduced activation | |||||||
| 1 | MFG and IFG | L | −46.1 | 37 | 15.9 | 46 | 1002 |
| 2 | MFG | L | −43.9 | 8.9 | 34.8 | 9 | 662 |
| 3 | Pre- and postcentral gyrus | R | 32.7 | −26.8 | 52.2 | 3, 4 | 511 |
| 4 | MTG and ITG | L | −53.8 | −41.5 | −10 | 20, 21, 37 | 676 |
| 5 | Fusiform gyrus | L | −30 | −36 | −13.8 | 37 | 510 |
| 6 | Primary visual cortex | R | 13.6 | −89 | 6 | 17 | 1855 |
| 7 | Middle occipital gyrus, cuneus | L | −21.7 | −97.5 | 5.1 | 18 | 1144 |
| 8 | Middle and inferior occipital gyrus | R | 30.3 | −78.3 | −3.1 | 18, 19 | 605 |
| 9 | Middle and inferior occipital gyrus | L | −31.9 | −86.9 | −6.6 | 18 | 588 |
| 10 | Thalamus | L | −12.2 | −13.1 | 13.8 | — | 712 |
| 11 | Thalamus | R | 8.3 | −8.4 | 7.1 | — | 461 |
| Nicotine reduced deactivation | |||||||
| 12 | Rostral anterior cingulate and medial frontal gyrus | L | −10 | 44.9 | 1 | 10, 32 | 827 |
| 13 | MTG | L | −37.9 | 16.2 | 50.2 | 6, 8 | 916 |
| 14 | Parahippocampal gyrus | L | −22.9 | −12.9 | −22.4 | 35 | 828 |
| 15 | White matter, superior to insula | R | 34.8 | −18.2 | 25.5 | — | 480 |
Note: The numbering corresponds to ROIs in Figure 3. L, left; R, right.
Figure 3.
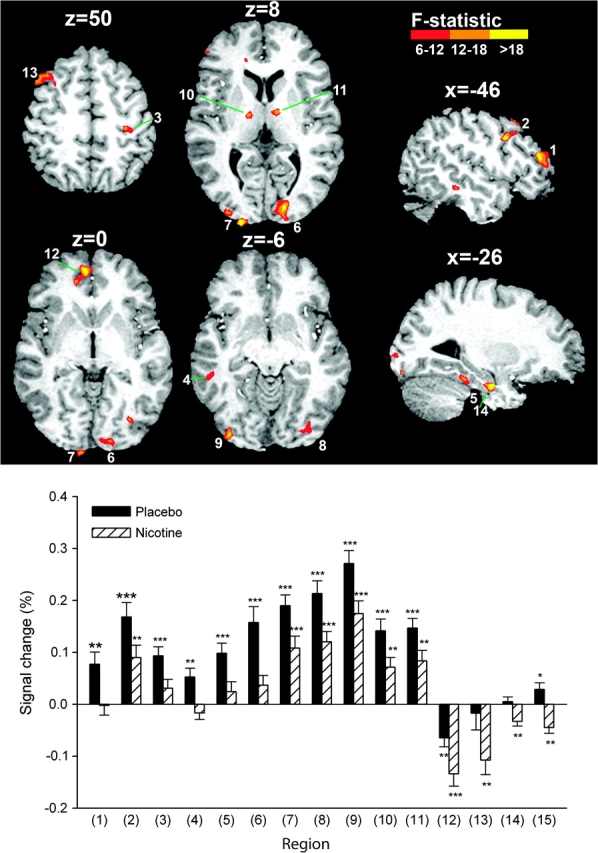
Brain regions displaying a main effect of nicotine across all 4 task conditions. Group activation maps are overlaid onto an individual anatomical scan in Talairach space. Slices are displayed in neurological view (left is on the viewer's left). In all regions, nicotine decreased the BOLD signal, causing either reductions in activation or significant deactivations. Regional BOLD activity is presented in the graph as averages ± standard error of the mean (n = 18). Significant differences from zero in 1-sample t-tests are indicated (*P < 0.05, **P < 0.01, ***P < 0.001). The difference between the nicotine and placebo session was always significant in paired t-tests (P < 0.003 in all regions). The numbering corresponds to ROIs in Table 1.
Two right frontal regions were identified as displaying a DRUG × TASK interaction (Fig. 4): 1 located in MFG (Brodmann area [BA] 9; 999 μL; x, y, z: 40.2, 23.4, 36.2) and 1 in superior frontal gyrus (SFG) extending into MFG (BA 6; 650 μL; x, y, z: 32.6, −8.9, 63.5). In both regions, nicotine reduced activation only during SDT, whereas the other 3 task conditions displayed trends toward increases. When comparing average regional BOLD signal in the absence of nicotine between smokers and nonsmokers, a significant difference was observed for SDT in SFG. In the placebo condition, smokers’ SFG activation (0.33 ± 0.06) was elevated as compared with nonsmokers (0.17 ± 0.05), which was reversed by nicotine (0.18 ± 0.04). Post hoc ANOVA of regional averages identified no effects involving the sequence of testing.
Figure 4.
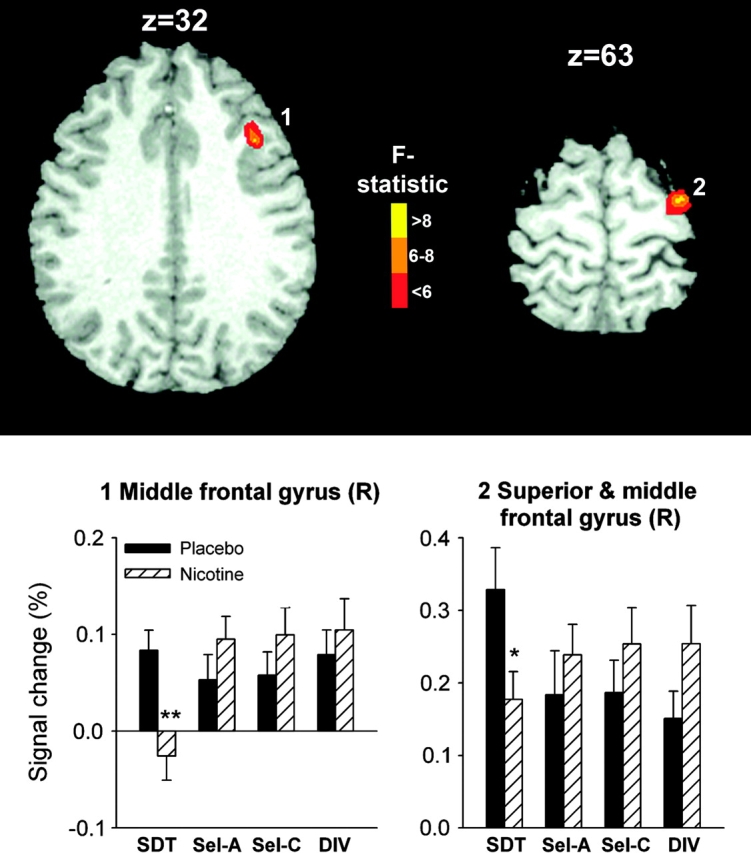
Brain regions displaying an interaction of the effects of nicotine with task condition. Nicotine induced deactivation in right (R) MFG and SFG only in the SDT task. Significant differences between the nicotine and placebo session are indicated (*P < 0.05, **P < 0.01, paired t-test).
To explore the relationship between nicotine's effects on BOLD activity and on performance, smokers’ RT and regional BOLD difference values between the nicotine and placebo sessions were correlated within each task condition. Nicotine-induced signal reductions in the left thalamus (region 10 in Table 1 and Fig. 3) correlated with RT reductions during SDT (r = 0.69, P < 0.005) but not in any of the other task conditions (P > 0.4 in each case). At a lower P threshold, the right thalamus (region 11) also displayed a correlation during SDT (r = 0.58, P < 0.05).
To test whether task-related activity in the drug-free state differed between smokers and nonsmokers in regions other than those modulated by nicotine, we performed voxel-wise ANOVA (GROUP × TASK) on BOLD signal in the absence of nicotine. Only a small region in posterior MTG/middle occipital gyrus displayed a main effect of GROUP and was hypoactivated across tasks in smokers (% signal change: 0.05 ± 0.13) as compared with nonsmokers (0.21 ± 0.14). However, several regions displayed a GROUP × TASK interaction: dorsal anterior cingulate sulcus (probably the motor region of ACC)/medial frontal gyrus, left IFG, precuneus, and cerebellum (Supplementary Table 1 and Supplementary Fig. 1). Activation in nonsmokers was generally: SDT, Sel-A < Sel-C < DIV. In the low-activation task conditions, smokers displayed greater activity and in the high-activation conditions less activity than nonsmokers, resulting in relatively even activation levels across conditions (Supplementary Fig. 2).
Lastly, we determined the effects of nicotine on BOLD responses to visuomotor stimulation. Ten regions were identified as responding to the flashing checkerboard and finger-tapping manipulation (Table 2). The occipital and thalamic regions had no coverage in one participant. BOLD contrast values (on- vs. off-periods) never differed between the nicotine and placebo session (t13,14 < 1.74, NS for each region, paired t-tests), indicating that nicotine did not alter BOLD responses to neuronal stimulation in a nonspecific manner.
Table 2.
Brain regions activated by visuomotor stimulation
| Brain region | Side | Center of mass (mm) |
Brodmann areas | Volume (μL) | ||
| x | y | z | ||||
| Pre- and postcentral gyrus, inferior parietal lobule | R | 44 | −26.3 | 41.8 | 1, 2, 3, 4, 40 | 9606 |
| Pre- and postcentral gyrus, inferior parietal lobule | L | −49.4 | −24.8 | 35.5 | 1, 2, 3, 4, 40 | 24 121 |
| Supplementary motor area, anterior cingulate sulcus | B | −0.6 | 6.2 | 42.6 | 6, 24 | 3045 |
| Middle/superior occipital gyrus, cuneus | R | 30.4 | −79.4 | 22.6 | 19 | 1246 |
| Middle/superior occipital gyrus, cuneus | L | −30.2 | −75.2 | 24 | 19 | 629 |
| Precuneus | L | −24.9 | −69.5 | 40.4 | 7 | 620 |
| Cingulate gyrus, precuneus | L | −11.5 | −27.4 | 41.5 | 31 | 409 |
| Inferior parietal lobule | R | 60.4 | −25.4 | 23 | 40 | 372 |
| Thalamus | B | 1 | −9.3 | 16.8 | — | 942 |
| Thalamus | L | −13.8 | −16 | 17.9 | — | 662 |
Note: L, left; R, right; and B, bilateral.
Discussion
The aim of the present investigation was to test the hypothesis that nicotine would exert qualitatively distinct neuronal effects when selectivity aspects of attention were taxed, consistent with its behavioral profile to differentially alter such aspects. We employed a novel paradigm designed to explore the neural substrates of nicotine's performance-enhancing effects in tasks of selective attention, divided attention, and simple stimulus detection.
Behaviorally, nicotine displayed a profile of action that suggested task selectivity. First, nicotine-induced RT reductions were more prevalent during the 2 selective attention tasks than during the stimulus detection condition (SDT), suggesting that processes of selective attention are particularly sensitive to modulation by nicotine. However, given the substantially lower average RT during SDT, floor effects cannot be excluded. Second, nicotine did not reduce RT during DIV, in stark contrast to the robust reductions seen during Sel-A and Sel-C. Both single- and dual-task conditions tax selectivity aspects of attention, but nicotine clearly did not enhance the additional mental operations engaged specifically when attention was divided between 2 stimulus dimensions. The additional task requirements by DIV may indeed mask the effects of nicotine on components shared with Sel-A and Sel-C. For example, the presence of higher cognitive control demands may diminish improvements by nicotine (see also Parrott and Craig 1992; Spilich et al. 1992; Rusted and Trawley 2006). That nicotine caused less performance enhancement in the presence of greater control demands suggests that improvements in selective attention were not due to enhanced control of attentional resource allocation but probably due to enhanced attentional focusing and filtering, as outlined in the Introduction.
The effects of nicotine on neural activity mostly consisted of main effects across task conditions that can be subdivided into 2 patterns: 1) nicotine reduced activation in frontal, temporal, thalamic, and visual regions and 2) nicotine induced or enhanced existing deactivation in areas of the default network of resting brain function. The latter effect has been suggested to reflect an aided downregulation of task-independent mental operations and shifts to externally oriented information processing (Hahn et al. 2007). Such an interpretation would be consistent with a cholinergically mediated shift from intracortical associational processing to enhanced cortical processing of external sensory stimuli (Sarter et al. 2005). Specifically, nicotine, via presynaptic nicotinic receptors, strengthens thalamo-cortical but not cortico-cortical neurotransmission (Gil et al. 1997).
Areas where nicotine-enhanced default deactivation converged between Hahn et al. (2007) and the present study in rostral ACC/medial frontal gyrus and left MFG. However, Hahn et al. (2007) also found deactivation in the posterior cingulate cortex and precuneus, which correlated with nicotine-induced improvements in visuospatial attention. Because these later regions were not altered in the present study, their engagement may constitute a mechanism specific to that subtype of attention. Modulation of frontal default regions, in contrast, appears to reflect more global effects of nicotine across different attentional functions. This apparent subdivision of nicotine's effects on the default network resonates with suggested functional subdivisions of this network (Gusnard and Raichle 2001) and deserves exploration in future studies.
Nicotine-induced deactivation of the thalamus was correlated with RT reduction selectively during SDT. Thus, although BOLD effects of nicotine did not differ between task conditions, modulation of this region appears to benefit simple stimulus detection but not more demanding selectivity aspects of attention. This agrees with a role of the thalamus in global external information processing and alerting (Coull 1998). Although required in all task conditions, enhanced general alertness was probably of particular benefit when performance primarily depended on stimulus detection and not on more involved processing.
DRUG × TASK interactions in the BOLD signal reflected differences in the effects of nicotine between SDT and task conditions with a selectivity component (Sel-A, Sel-C, DIV). Nicotine decreased activity in right MFG and SFG during SDT, while causing trends toward increases during the other tasks. The different-sized RT effects of nicotine in SDT versus Sel-A and Sel-C would be consistent with a difference in underlying neural mechanisms. Given that BOLD and performance effects of nicotine did not significantly correlate, it is not clear whether the signal decrease may account for the improvement in SDT RT or for its smaller effect size as compared with the other task conditions. A trend-level correlation (r = 0.51, P = 0.053) between MFG deactivation and RT reduction during SDT is suggestive of the former alternative. If activity decreases with nicotine tended to be associated with greater performance benefits, could the trends toward activity increases in the other task conditions be associated with smaller benefits? Findings by Hahn et al. (2007) suggest that this may be the case: right MFG activation by nicotine was identified in the vicinity of the current region in a condition requiring attentional selection, and this activation was associated with smaller RT benefits. Overall, our current findings indicate that prefrontal functions are differentially modulated by nicotine when performing simple stimulus detection versus more involved processes of attention selection.
Surprisingly, BOLD effects of nicotine did not differ between DIV versus Sel-A and Sel-C corresponding to the behavioral selectivity. It is conceivable that the same effects on brain activity benefited performance of selective but not divided attention, that is, the neuronal effects of nicotine may converge with neural mechanisms that determine performance of Sel-A and Sel-C but not with those that are of specific importance for DIV. Alternatively, the distinction may be quantitative in nature, such that nicotine-induced regional activity levels that are optimal for selective but not for dual-task conditions. The finding that activation differences between Sel-A, Sel-C, and DIV are mostly quantitative rather than qualitative in nature (Hahn et al. 2008) supports this explanation.
The fact that all effects of nicotine consisted of reductions in activity is of concern due to potential nonspecific effects on CBF and coupling between neuronal responses and brain hemodynamics. Nicotine has sympathomimetic properties (e.g., Perkins et al. 2004; Yugar-Toledo et al. 2005) and can exert direct vascular effects (Toda 1975; Boyajian and Otis 2000; Sabha et al. 2000). Notably, cerebral blood vessels express nicotinic receptors (Kalaria et al. 1994; Macklin et al. 1998). The current effects of nicotine do not reflect absolute changes but modulation of task-induced BOLD responses. Thus, given that vascular effects can alter BOLD responses (Bruhn et al. 1994, 2001; Wang et al. 2006), the relevant question is whether BOLD responses to task stimuli were affected by direct vascular effects of nicotine. The lack of a nicotine effect in our visuomotor control experiment indicated that nicotine did not modulate BOLD responses to neuronal stimulation in a nonspecific manner. Robust activation in visual and motor regions was observed, but, as in previous studies (Jacobsen et al. 2002; Hahn et al. 2007), the presence of nicotine had no effect on these responses.
Reports of decreased BOLD or rCBF responses following nicotine administration are not uncommon and are often accompanied by activation in other brain regions (Ghatan et al. 1998; Thiel et al. 2005; Giessing et al. 2006; Hahn et al. 2007). A common explanation of activity decreases that accompany equal or improved performance is that of enhanced functional efficiency, such that the same cognitive operation requires less energy. This could reflect a greater ease or automaticity with which the operation is performed. A possible link to the concomitantly observed downregulation of default activity is that a reduction in task-independent thought processes may have facilitated the execution of task-related operations, making them less effortful and resource demanding. On a cellular level, enhanced neuronal efficiency may be related to a neuromodulatory potentiation of transmitter release via presynaptic receptors (MacDermott et al. 1999; Wonnacott et al. 2006). Thus, nicotine can facilitate synaptic release of acetylcholine, dopamine, noradrenalin, serotonin, γ-aminobutyric acid, and glutamate in various cortical and subcortical structures in a manner that does not depend on increased firing of the presynaptic cell (Nisell et al. 1994; Summers and Giacobini 1995; Lambe et al. 2003; Mansvelder et al. 2006). Via autoregulatory mechanisms, cells may thus maintain the same or enhanced output with reduced firing and energy expenditure.
A question, then, would be why another well-controlled study identified predominantly increased activation by nicotine (Lawrence et al. 2002). A major difference between that report and studies identifying nicotine-induced deactivations lies in the task demands. The rapid visual information processing (RVIP) task used by Lawrence et al. creates densely spaced information processing requirements, and the major performance-limiting factor appears to be the sheer load of these processing demands in the face of limited available processing resources. The other studies including the present required responses to more widely spaced stimuli. Thus, it is possible that enhanced functional efficiency by nicotine is observable in task conditions that do not engage maximal processing capacity, whereas in conditions where capacity may be “maxed out,” as in the RVIP task, nicotine may enable recruitment of additional resources.
Comparing results from smokers and nonsmokers in the absence of nicotine overall supported the concept of net effects of nicotine, rather than a restoration of a normal state. This may not be surprising as the length of pretest abstinence was chosen to keep deprivation minimal. Subjective self-reports gave some evidence of an impaired attentional state in smokers, but this was not reflected by objective measures of performance. This raises the possibility that smokers assessed their subjective state relative to a different reference point than nonsmokers, given that subjective alerting effects of nicotine are likely to form part of a normal baseline state. On BOLD activity, only one regional effect of nicotine appeared to reflect the restoration of a normal functional state, namely activity reduction in right SFG during SDT. Hyperactivity in prefrontal regions including right SFG has been reported in deprived smokers during working memory performance (Jacobsen et al. 2007). In one report (Xu et al. 2005), this was observed only under low task load, consistent with the current selectivity for SDT, and nicotine reduced this hyperactivity (Xu et al. 2006). Our result in SFG may thus reflect the beginning of a reduced functional efficiency in abstinent smokers that was remedied by nicotine.
Baseline differences between smokers and nonsmokers were detected in regions not modulated by nicotine, such as posterior MTG, the motor area of ACC, left IFG, precuneus, and cerebellum. Here, smokers differed from nonsmokers in complex ways. Increased activity in smokers in low-load task conditions is consistent with our findings in SFG. Decreased activity in high-load task conditions agrees with findings by Lawrence et al. (2002), who employed a high-level processing task. Overall, the data suggest that chronic tobacco exposure may blunt task-adaptive changes in regional activity. In conclusion, although most of the observed effects of nicotine did not depend on baseline shifts in smokers, there were differences in task-related brain function between smokers and nonsmokers, as observed previously (e.g., Ernst et al. 2001; Lawrence et al. 2002). Clearly, it is desirable to replicate the observed effects of nicotine in a nonsmoking population, employing low doses to minimize aversive side effects.
The present study provides evidence for global neuroanatomical mechanisms of nicotine-induced attentional enhancement that span different attentional functions. Namely, the neural effects of nicotine did not, by and large, differ with task demands, although they appeared to benefit some functions more than others. However, the study also suggests that some mechanisms contribute specifically to effects of nicotine on simple stimulus detection but not on more cognitively involved tasks that tax selectivity aspects of attention. This conclusion is based on the findings that 1) prefrontal regions displayed modulation by nicotine selectively during SDT and 2) thalamic effects of nicotine correlated with performance effects only during SDT. Furthermore, comparing the present with a parallel experiment (Hahn et al. 2007) suggests that nicotine modulates specific parts of the default network depending on the attentional functions taxed.
Considering the wide distribution of nicotinic receptors throughout the brain and the variety of distinct structures and pathways nicotine interacts with via multiple secondary neurotransmitter systems (Gotti et al. 1997; Wonnacott et al. 2006), it may not be surprising to find different mechanisms associated with different performance effects. Thus, it may be time to replace the search for the neuroanatomical mechanism of nicotine-induced attentional enhancement by a broader characterization of effects on diverse task-induced neuronal states. This will enable more targeted attempts to match the neurobehavioral profile of nicotinic compounds with clinical conditions characterized by distinct attentional dysfunction and functional brain abnormalities.
Supplementary Material
Supplementary figures 1 and 2 and table 1 can be found at: http://www.cercor.oxford.journals.org/.
Funding
Intramural Research Program of the National Institutes of Health, National Institute on Drug Abuse.
Acknowledgments
We thank William Rea and Loretta Spurgeon for their assistance in the conduct of the study. Conflict of Interest: None declared.
References
- Andersson K, Hockey GR. Effects of cigarette smoking on incidental memory. Psychopharmacology. 1977;52:223–226. doi: 10.1007/BF00426703. [DOI] [PubMed] [Google Scholar]
- Boyajian RA, Otis SM. Acute effects of smoking on human cerebral blood flow: a transcranial Doppler ultrasonography study. J Neuroimaging. 2000;10:204–208. doi: 10.1111/jon2000104204. [DOI] [PubMed] [Google Scholar]
- Braun J. Divided attention: narrowing the gap between brain and behavior. In: Parasuraman R, editor. The attentive brain. Cambridge (MA): MIT Press; 1998. pp. 327–351. [Google Scholar]
- Bruhn H, Fransson P, Frahm J. Modulation of cerebral blood oxygenation by indomethacin: MRI at rest and functional brain activation. J Magn Reson Imaging. 2001;13:325–334. doi: 10.1002/jmri.1047. [DOI] [PubMed] [Google Scholar]
- Bruhn H, Kleinschmidt A, Boecker H, Merboldt KD, Hanicke W, Frahm J. The effect of acetazolamide on regional cerebral blood oxygenation at rest and under stimulation as assessed by MRI. J Cereb Blood Flow Metab. 1994;14:742–748. doi: 10.1038/jcbfm.1994.95. [DOI] [PubMed] [Google Scholar]
- Coull JT. Neural correlates of attention and arousal: insights from electrophysiology, functional neuroimaging and psychopharmacology. Prog Neurobiol. 1998;55:343–361. doi: 10.1016/s0301-0082(98)00011-2. [DOI] [PubMed] [Google Scholar]
- Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Domier CP, Monterosso JR, Brody AL, Simon SL, Mendrek A, Olmstead R, Jarvik ME, Cohen MS, London ED. Effects of cigarette smoking and abstinence on Stroop task performance. Psychopharmacology. 2007;195:1–9. doi: 10.1007/s00213-007-0869-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst M, Matochik JA, Heishman SJ, Van Horn JD, Jons PH, Henningfield JE, London ED. Effect of nicotine on brain activation during performance of a working memory task. Proc Natl Acad Sci USA. 2001;98:4728–4733. doi: 10.1073/pnas.061369098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foulds J, Stapleton J, Swettenham J, Bell N, McSorley K, Russell MAH. Cognitive performance effects of subcutaneous nicotine in smokers and never-smokers. Psychopharmacology. 1996;127:31–38. doi: 10.1007/BF02805972. [DOI] [PubMed] [Google Scholar]
- Ghatan PH, Ingvar M, Eriksson L, Stone-Elander S, Serrander M, Ekberg K, Wahren J. Cerebral effects of nicotine during cognition in smokers and non-smokers. Psychopharmacology. 1998;136:179–189. doi: 10.1007/s002130050554. [DOI] [PubMed] [Google Scholar]
- Giessing C, Thiel CM, Rosler F, Fink GR. The modulatory effects of nicotine on parietal cortex activity in a cued target detection task depend on cue reliability. Neuroscience. 2006;137:853–864. doi: 10.1016/j.neuroscience.2005.10.005. [DOI] [PubMed] [Google Scholar]
- Gil Z, Connors BW, Amitai Y. Differential regulation of neocortical synapses by neuromodulators and activity. Neuron. 1997;19:679–686. doi: 10.1016/s0896-6273(00)80380-3. [DOI] [PubMed] [Google Scholar]
- Gotti C, Fornasari D, Clementi F. Human neuronal nicotinic receptors. Prog Neurobiol. 1997;53:199–237. doi: 10.1016/s0301-0082(97)00034-8. [DOI] [PubMed] [Google Scholar]
- Grobe JE, Perkins KA, Goettler-Good J, Wilson A. Importance of environmental distractors in the effects of nicotine on short-term memory. Exp Clin Psychopharmacol. 1998;6:209–216. doi: 10.1037//1064-1297.6.2.209. [DOI] [PubMed] [Google Scholar]
- Gusnard DA, Raichle ME. Searching for a baseline: functional imaging and the resting human brain. Nat Rev Neurosci. 2001;2:685–694. doi: 10.1038/35094500. [DOI] [PubMed] [Google Scholar]
- Hahn B, Ross TJ, Yang Y, Kim I, Huestis MA, Stein EA. Nicotine enhances visuospatial attention by deactivating areas of the resting brain default network. J Neurosci. 2007;27:3477–3489. doi: 10.1523/JNEUROSCI.5129-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn B, Shoaib M, Stolerman IP. Nicotine-induced enhancement of attention in the five-choice serial reaction time task: the influence of task-demands. Psychopharmacology. 2002;162:129–137. doi: 10.1007/s00213-002-1005-6. [DOI] [PubMed] [Google Scholar]
- Hahn B, Stolerman IP. Nicotine-induced attentional enhancement in rats: effects of chronic exposure to nicotine. Neuropsychopharmacology. 2002;27:712–722. doi: 10.1016/S0893-133X(02)00348-2. [DOI] [PubMed] [Google Scholar]
- Hahn B, Wolkenberg FA, Ross TJ, Myers CS, Heishman SJ, Stein DJ, Kurup P, Stein EA. Divided versus selective attention: evidence for common processing mechanisms. Brain Res. 2008;1215:137–146. doi: 10.1016/j.brainres.2008.03.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasenfratz M, Battig K. Action profiles of smoking and caffeine: Stroop effect, EEG, and peripheral physiology. Pharmacol Biochem Behav. 1992;42:155–161. doi: 10.1016/0091-3057(92)90459-s. [DOI] [PubMed] [Google Scholar]
- Heishman SJ, Henningfield JE. Tolerance to repeated nicotine administration on performance, subjective, and physiological responses in nonsmokers. Psychopharmacology. 2000;152:321–333. doi: 10.1007/s002130000541. [DOI] [PubMed] [Google Scholar]
- Heishman SJ, Singleton EG, Moolchan ET. Tobacco craving questionnaire: reliability and validity of a new multifactorial instrument. Nicotine Tob Res. 2003;5:645–654. doi: 10.1080/1462220031000158681. [DOI] [PubMed] [Google Scholar]
- Heishman SJ, Snyder FR, Henningfield JE. Performance, subjective, and physiological effects of nicotine in non-smokers. Drug Alcohol Depend. 1993;34:11–18. doi: 10.1016/0376-8716(93)90041-n. [DOI] [PubMed] [Google Scholar]
- Heishman SJ, Taylor RC, Henningfield JE. Nicotine and smoking: a review of effects on human performance. Exp Clin Psychopharmacol. 1994;2:345–395. [Google Scholar]
- Jacobsen LK, Gore JC, Skudlarski P, Lacadie CM, Jatlow P, Krystal JH. Impact of intravenous nicotine on BOLD signal response to photic stimulation. Magn Reson Imaging. 2002;20:141–145. doi: 10.1016/s0730-725x(02)00494-0. [DOI] [PubMed] [Google Scholar]
- Jacobsen LK, Mencl WE, Constable RT, Westerveld M, Pugh KR. Impact of smoking abstinence on working memory neurocircuitry in adolescent daily tobacco smokers. Psychopharmacology. 2007;193:557–566. doi: 10.1007/s00213-007-0797-9. [DOI] [PubMed] [Google Scholar]
- Kalaria RN, Homayoun P, Whitehouse PJ. Nicotinic cholinergic receptors associated with mammalian cerebral vessels. J Auton Nerv Syst. 1994;49(Suppl):S3–S7. doi: 10.1016/0165-1838(94)90078-7. [DOI] [PubMed] [Google Scholar]
- Kim SG. Quantification of relative cerebral blood flow change by flow-sensitive alternating inversion recovery (FAIR) technique: application to functional mapping. Magn Reson Med. 1995;34:293–301. doi: 10.1002/mrm.1910340303. [DOI] [PubMed] [Google Scholar]
- Kim I, Huestis MA. A validated method for the determination of nicotine, cotinine, trans-3-hydroxycotinine, and norcotinine in human plasma using solid-phase extraction and liquid chromatography-atmospheric pressure chemical ionization-mass spectrometry. J Mass Spectrom. 2006;41:815–821. doi: 10.1002/jms.1039. [DOI] [PubMed] [Google Scholar]
- Koelega HS. Stimulant drugs and vigilance performance: a review. Psychopharmacology. 1993;111:1–16. doi: 10.1007/BF02257400. [DOI] [PubMed] [Google Scholar]
- Lambe EK, Picciotto MR, Aghajanian GK. Nicotine induces glutamate release from thalamocortical terminals in prefrontal cortex. Neuropsychopharmacology. 2003;28:216–225. doi: 10.1038/sj.npp.1300032. [DOI] [PubMed] [Google Scholar]
- Lawrence NS, Ross TJ, Stein EA. Cognitive mechanisms of nicotine on visual attention. Neuron. 2002;36:539–548. doi: 10.1016/s0896-6273(02)01004-8. [DOI] [PubMed] [Google Scholar]
- Leigh G, Tong JE, Campbell JA. Effects of ethanol and tobacco on divided attention. J Stud Alcohol. 1977;38:1233–1239. doi: 10.15288/jsa.1977.38.1233. [DOI] [PubMed] [Google Scholar]
- MacDermott AB, Role LW, Siegelbaum SA. Presynaptic ionotropic receptors and the control of transmitter release. Annu Rev Neurosci. 1999;22:443–485. doi: 10.1146/annurev.neuro.22.1.443. [DOI] [PubMed] [Google Scholar]
- Macklin KD, Maus AD, Pereira EF, Albuquerque EX, Conti-Fine BM. Human vascular endothelial cells express functional nicotinic acetylcholine receptors. J Pharmacol Exp Ther. 1998;287:435–439. [PubMed] [Google Scholar]
- Mancuso G, Andres P, Ansseau M, Tirelli E. Effects of nicotine administered via a transdermal delivery system on vigilance: a repeated measure study. Psychopharmacology. 1999;142:18–23. doi: 10.1007/s002130050857. [DOI] [PubMed] [Google Scholar]
- Mansvelder HD, van Aerde KI, Couey JJ, Brussaard AB. Nicotinic modulation of neuronal networks: from receptors to cognition. Psychopharmacology. 2006;184:292–305. doi: 10.1007/s00213-005-0070-z. [DOI] [PubMed] [Google Scholar]
- Newhouse PA, Potter A, Singh A. Effects of nicotinic stimulation on cognitive performance. Curr Opin Pharmacol. 2004;4:36–46. doi: 10.1016/j.coph.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Nisell M, Nomikos GG, Svensson TH. Infusion of nicotine in the ventral tegmental area or the nucleus accumbens of the rat differentially affects accumbal dopamine release. Pharmacol Toxicol. 1994;75:348–352. doi: 10.1111/j.1600-0773.1994.tb00373.x. [DOI] [PubMed] [Google Scholar]
- Parasuraman R. The attentive brain: issues and prospects. In: Parasuraman R, editor. The attentive brain. Cambridge (MA): MIT Press; 1998. pp. 3–15. [Google Scholar]
- Parrott AC, Craig D. Cigarette smoking and nicotine gum (0, 2 and 4 mg): effects upon four visual attention tasks. Neuropsychobiology. 1992;25:34–43. doi: 10.1159/000118807. [DOI] [PubMed] [Google Scholar]
- Parrott AC, Garnham NJ, Wesnes K, Pincock C. Cigarette smoking and abstinence: comparative effects upon cognitive task performance and mood state over 24 hours. Hum Psychopharmacol. 1996;11:391–400. [Google Scholar]
- Perkins KA, Grobe JE, Fonte C, Goettler J, Caggiula AR, Reynolds WA, Stiller RL, Scierka A, Jacob RG. Chronic and acute tolerance to subjective, behavioral and cardiovascular effects of nicotine in humans. J Pharmacol Exp Ther. 1994;270:628–638. [PubMed] [Google Scholar]
- Perkins KA, Lerman C, Keenan J, Fonte C, Coddington S. Rate of nicotine onset from nicotine replacement therapy and acute responses in smokers. Nicotine Tob Res. 2004;6:501–507. doi: 10.1080/14622200410001696547. [DOI] [PubMed] [Google Scholar]
- Poltavski DV, Petros T. Effects of transdermal nicotine on attention in adult non-smokers with and without attentional deficits. Physiol Behav. 2006;87:614–624. doi: 10.1016/j.physbeh.2005.12.011. [DOI] [PubMed] [Google Scholar]
- Prendergast MA, Jackson WJ, Terry AV, Jr, Decker MW, Arneric SP, Buccafusco JJ. Central nicotinic receptor agonists ABT-418, ABT-089, and (–)-nicotine reduce distractibility in adult monkeys. Psychopharmacology. 1998;136:50–58. doi: 10.1007/s002130050538. [DOI] [PubMed] [Google Scholar]
- Provost SC, Woodward R. Effects of nicotine gum on repeated administration of the Stroop test. Psychopharmacology. 1991;104:536–540. doi: 10.1007/BF02245662. [DOI] [PubMed] [Google Scholar]
- Rezvani AH, Levin ED. Cognitive effects of nicotine. Biol Psychiatry. 2001;49:258–267. doi: 10.1016/s0006-3223(00)01094-5. [DOI] [PubMed] [Google Scholar]
- Rusted JM, Trawley S. Comparable effects of nicotine in smokers and nonsmokers on a prospective memory task. Neuropsychopharmacology. 2006;31:1545–1549. doi: 10.1038/sj.npp.1300965. [DOI] [PubMed] [Google Scholar]
- Sabha M, Tanus-Santos JE, Toledo JC, Cittadino M, Rocha JC, Moreno H., Jr Transdermal nicotine mimics the smoking-induced endothelial dysfunction. Clin Pharmacol Ther. 2000;68:167–174. doi: 10.1067/mcp.2000.108851. [DOI] [PubMed] [Google Scholar]
- Sarter M, Hasselmo ME, Bruno JP, Givens B. Unraveling the attentional functions of cortical cholinergic inputs: interactions between signal-driven and cognitive modulation of signal detection. Brain Res Brain Res Rev. 2005;48:98–111. doi: 10.1016/j.brainresrev.2004.08.006. [DOI] [PubMed] [Google Scholar]
- Spilich GJ, June L, Renner J. Cigarette smoking and cognitive performance. Br J Addict. 1992;87:1313–1326. doi: 10.1111/j.1360-0443.1992.tb02740.x. [DOI] [PubMed] [Google Scholar]
- Stein EA, Ross TJ, Zhang YQ, Wolkenberg FA. 34th annual meeting of the society for neuroscience. San Diego (CA): 2004. Differential neural processing of selective attention vs. intention. [abstract] [Google Scholar]
- Stolerman IP, Mirza NR, Shoaib M. Nicotine psychopharmacology: addiction, cognition and neuroadaptation. Med Res Rev. 1995;15:47–72. doi: 10.1002/med.2610150105. [DOI] [PubMed] [Google Scholar]
- Summers KL, Giacobini E. Effects of local and repeated systemic administration of (-)nicotine on extracellular levels of acetylcholine, norepinephrine, dopamine, and serotonin in rat cortex. Neurochem Res. 1995;20:753–759. doi: 10.1007/BF01705545. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain. New York: Thieme; 1988. [Google Scholar]
- Thiel CM, Fink GR. Visual and auditory alertness: modality-specific and supramodal neural mechanisms and their modulation by nicotine. J Neurophysiol. 2007;97:2758–2768. doi: 10.1152/jn.00017.2007. [DOI] [PubMed] [Google Scholar]
- Thiel CM, Zilles K, Fink GR. Nicotine modulates reorienting of visuospatial attention and neural activity in human parietal cortex. Neuropsychopharmacology. 2005;30:810–820. doi: 10.1038/sj.npp.1300633. [DOI] [PubMed] [Google Scholar]
- Toda N. Nicotine-induced relaxation in isolated canine cerebral arteries. J Pharmacol Exp Ther. 1975;193:376–384. [PubMed] [Google Scholar]
- Trimmel M, Wittberger S. Effects of transdermally administered nicotine on aspects of attention, task load, and mood in women and men. Pharmacol Biochem Behav. 2004;78:639–645. doi: 10.1016/j.pbb.2004.04.030. [DOI] [PubMed] [Google Scholar]
- Wang R, Foniok T, Wamsteeker JI, Qiao M, Tomanek B, Vivanco RA, Tuor UI. Transient blood pressure changes affect the functional magnetic resonance imaging detection of cerebral activation. Neuroimage. 2006;31:1–11. doi: 10.1016/j.neuroimage.2005.12.004. [DOI] [PubMed] [Google Scholar]
- Warburton DM, Rusted JM, Muller C. Patterns of facilitation of memory by nicotine. Behav Pharmacol. 1992;3:375–378. [PubMed] [Google Scholar]
- Waters AJ. The effects of smoking on performance on the Garner speeded classification task. Hum Psychopharmacol. 1998;13:477–491. [Google Scholar]
- Wechsler D. Wechsler Abbreviated Scale of Intelligence (WASI) San Antonio, TX: Harcourt Assessment; 1999. [Google Scholar]
- Wesnes K, Warburton DM. Smoking, nicotine and human performance. Pharmacol Ther. 1983;21:189–208. doi: 10.1016/0163-7258(83)90072-4. [DOI] [PubMed] [Google Scholar]
- Wesnes K, Warburton DM. Effects of scopolamine and nicotine on human rapid information processing performance. Psychopharmacology. 1984;82:147–150. doi: 10.1007/BF00427761. [DOI] [PubMed] [Google Scholar]
- Wong EC, Buxton RB, Frank LR. Quantitative imaging of perfusion using a single subtraction (QUIPSS and QUIPSS II) Magn Reson Med. 1998;39:702–708. doi: 10.1002/mrm.1910390506. [DOI] [PubMed] [Google Scholar]
- Wonnacott S, Barik J, Dickinson J, Jones IW. Nicotinic receptors modulate transmitter cross talk in the CNS: nicotinic modulation of transmitters. J Mol Neurosci. 2006;30:137–140. doi: 10.1385/JMN:30:1:137. [DOI] [PubMed] [Google Scholar]
- Xu J, Mendrek A, Cohen MS, Monterosso J, Rodriguez P, Simon SL, Brody A, Jarvik M, Domier CP, Olmstead R, et al. Brain activity in cigarette smokers performing a working memory task: effect of smoking abstinence. Biol Psychiatry. 2005;58:143–150. doi: 10.1016/j.biopsych.2005.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Mendrek A, Cohen MS, Monterosso J, Simon S, Brody AL, Jarvik M, Rodriguez P, Ernst M, London ED. Effects of acute smoking on brain activity vary with abstinence in smokers performing the N-back task: a preliminary study. Psychiatry Res. 2006;148:103–109. doi: 10.1016/j.pscychresns.2006.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S, Ross TJ, Zhang Y, Stein EA, Yang Y. Head motion suppression using real-time feedback of motion information and its effects on task performance in fMRI. Neuroimage. 2005;27:153–162. doi: 10.1016/j.neuroimage.2005.02.050. [DOI] [PubMed] [Google Scholar]
- Yugar-Toledo JC, Ferreira-Melo SE, Sabha M, Nogueira EA, Coelho OR, Consolin Colombo FM, Irigoyen MC, Moreno H., Jr Blood pressure circadian rhythm and endothelial function in heavy smokers: acute effects of transdermal nicotine. J Clin Hypertens. 2005;7:721–728. doi: 10.1111/j.1524-6175.2005.04597.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.