Abstract
Aims
Cardiac biomarkers are routinely elevated after uncomplicated cardiac surgery to levels considered diagnostic of myocardial infarction in ambulatory populations. We investigated the diagnostic power of electrocardiogram (ECG) and cardiac biomarker criteria to predict clinically relevant myocardial injury using benchmarks of mortality and increased hospital length of stay (HLOS) in patients undergoing coronary artery bypass graft (CABG) surgery.
Methods and results
Perioperative ECGs, creatinine kinase MB fraction, and cardiac troponin I (cTnI) were assessed in 545 primary CABG patients. None of the ECG criteria for myocardial injury predicted mortality or HLOS. However, post-operative day (POD) 1 cTnI levels independently predicted 5-year mortality (hazard ratio = 1.42; 95% CI 1.14–1.76 for each 10 µg/L increase; P = 0.009), while adjusting for baseline demographic characteristics and perioperative risk factors. Moreover, cTnI was the only biomarker that significantly improved the prediction of 5-year mortality estimated by the logistic Euroscore (P = 0.02). Furthermore, the predictive value of cTnI for 5-year mortality was replicated in a separately collected cohort of 1031 CABG patients using cardiac troponin T.
Conclusion
Electrocardiogram diagnosis of post-operative myocardial injury after CABG does not independently predict an increased risk of 5-year mortality or HLOS. Conversely, cTnI is independently associated with an increased risk of mortality and prolonged HLOS.
Keywords: Cardiopulmonary bypass, Electrocardiography, Surgery, Mortality, Enzymes, Troponin
Introduction
Perioperative myocardial infarction (PMI) occurs in 7–15% of patients after cardiac surgery and is associated with increased hospital length of stay (HLOS), costs, and reduced short- and long-term survival.1–4 In non-surgical populations, myocardial infarction (MI) is diagnosed with a combination of clinical symptoms, elevation of cardiac-specific biomarkers, and electrocardiographic (ECG) pattern. However, the determination of PMI after cardiac surgery is problematic because of the absence of a diagnostic ‘gold standard’. Even after uncomplicated cardiac surgery, the high frequency of indeterminate diagnostic ECG criteria and routine elevation of cardiac-specific biomarkers to levels considered diagnostic of MI in ambulatory populations makes the diagnosis of PMI challenging.1
In 2007, the Joint Task Force of the European Society of Cardiology/American College of Cardiology for the ‘Redefinition of Myocardial Infarction’ defined PMI using a combination of biomarker elevation and ECG, imaging, or angiography.5 Despite a lack of supportive evidence, the Task Force chose a cut-off for biomarker diagnosis of PMI at five times the upper limit of laboratory normal (ULN) within the first 72 h after surgery, when associated with the appearance of new pathological Q-waves or new LBBB. However, the recommendations of the consensus committee have not been consistently validated,1,6,7 are unproven in cardiac surgical populations,8 and have been the controversial subject of numerous editorials.9–11 Therefore, utilizing a primary test cohort and a validation cohort of patients undergoing primary coronary artery bypass grafting (CABG), we assessed the predictive value of cardiac troponins I and T (cTnI and cTnT), the MB fraction of creatinine kinase (CKMB), and ECG criteria to predict the risk of 5-year mortality and increased HLOS.
Methods
Primary test cohort
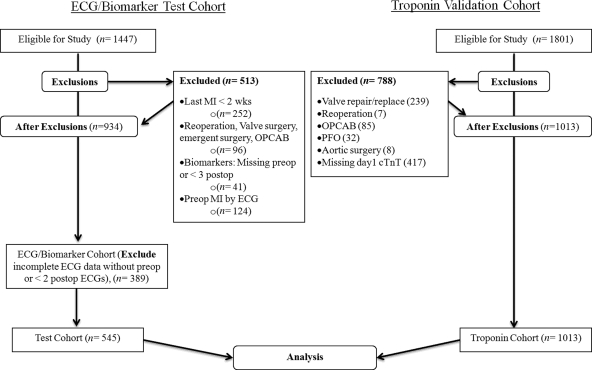
The primary test cohort was obtained from a prospective longitudinal parent study of 1447 patients undergoing primary CABG surgery with cardiopulmonary bypass (CPB) between August 2001 and May 2006 at Brigham and Women’s Hospital, Boston, MA, USA (Table 1) (CABG Genomics Program; website: http://clinicaltrials.gov/show/NCT00281164). With Institutional Review Board approval (IRB), written informed consent was obtained from each patient. Patients were excluded from the parent study if they were less than 20 years old; underwent repeat, off-pump CABG, concomitant valve or other cardiac surgery; had a pre-operative haematocrit < 25%, or if they had received leucocyte rich blood products within 30 days prior to surgery. Patients were further excluded from analysis if they had a pre-operative MI within 2 weeks of surgery or had missing perioperative ECG or biomarker data (CONSORT Diagram Figure 1). Patients underwent CPB with extracorporeal circulation using a single aortic cross-clamp technique and cold-blood cardioplegia.
Table 1.
Demographics, operative characteristics, and clinical outcomes of patients in the test and validation cohorts
| Demographics | Test cohort (n = 545) | Validation cohort (n = 1013) |
|---|---|---|
| Gender (male) | 80% | 79% |
| Age (years) | 66 (58–74) | 70 (62–77)* |
| BMI (kg/m2) | 28 (26–32) | |
| Caucasian race (%) | 91 | 90 |
| Past medical history | ||
| LVEF preoperative (%) | 55 (50–60) | |
| Diabetes (insulin or non-insulin dependent; %) | 29 | |
| Pulmonary disease (COPD, Asthma; %) | 4.0 | |
| Creatinine (mg/dL) | 1.0 (0.9–1.2) | |
| Haematocrit (%) | 39.8 (36.9–43.0) | |
| Hypertension (treated or by history; %) | 74 | |
| Hypercholesterolemia (treated or by history; %) | 79 | |
| Previous myocardial infarction (%) | 25 | |
| Medications—pre-operative (%) | ||
| ACE-inhibitor | 46 | |
| β-Blocker | 81 | |
| Ca2+ antagonist | 13 | |
| Aspirin | 80 | |
| HMG CoA reductase inhibitor | 82 | |
| Biomarkers—pre-operative | ||
| CKMB (µg/L) | 0.5 (0.2–1.1) | |
| cTnI (µg/L) | 0 (0–0.02) | |
| cTnT (µg/L) (n = 494) | 0.04 (0.01–0.62) | |
| Surgery (no. grafts; %) | ||
| 1 | 2 | |
| 2 | 13 | |
| 3 | 47 | |
| ≥4 | 38 | |
| CPB duration (min) | 102 (76–125) | |
| Aortic cross-clamp duration (min) | 77 (59–96) | |
| Post-operative data | ||
| HLOS (days) | 7 (6–9) | 7 (6–9) |
| Mortality % (n) up to 5 years | 6.1% (33) | 8.2% (83) |
Data are shown as percentage for dichotomous variables and median [25th, 75th percentiles for inter-quartile range (IQR)] for continuous variables. BMI, body mass index; LVEF, left ventricular ejection fraction; ACE, angiotensin converting enzyme; HMG, 3-hydroxy-3-methyl-glutaryl-CoA reductase; CKMB, creatinine kinase MB fraction; cTnI or cTnT, cardiac troponin I or T; CPB, cardiopulmonary bypass; HLOS, hospital length of stay. P-values are Kruskal–Wallis one-way analysis of variance by ranks for continuous data and χ2 distribution or Fisher’s exact for nominal and ordinal data.
*P < 0.0001.
Figure 1.
CONSORT diagram. ECG indicates electrocardiogram; MI, myocardial infarction; OPCAB, off-pump coronary artery bypass grafting; PFO, patent foramen ovale; cTnT, cardiac troponin T.
Demographic data, past medical and surgical history, and medications were recorded by trained research staff using defined protocols in a purpose-built case report form. Patients were followed for up to 5 years from the date of surgery (mean follow-up ± SD; 3.3 ± 1.4 years).
Biomarker assays
Blood samples were drawn prior to the induction of general anaesthesia, after administration of post-CPB protamine, and on the mornings of POD1–5. Patient caregivers were not aware of the results. Serum and plasma were stored in vapor-phase liquid nitrogen until analysis for cTnI and CKMB with a sandwich immunoassay on a Triage® platform using monoclonal and polyclonal antibodies (Biosite Inc., San Diego, CA) at a single core facility.
ECG data
Machine-generated ECG interpretations (Marquette 12SL, Mac 5500; GE Healthcare, Waukesha, MI, USA) were over-read by a board certified cardiologist taking into consideration prior ECGs when encountering new findings (bundle branch blocks, Q-waves) during the patient’s hospital admission. Cardiologist over-reading has been shown to have high sensitivity and specificity.12,13 ECG interpretations were assigned to five differing grades of injury/infarction. Machine-generated ECG interpretation of ‘Definite myocardial infarction’ was coded as Grade 4 injury/infarction, ‘Injury’ or ‘Probable myocardial infarction’ was coded as Grade 3, ‘Possible’, ‘Consider’ or ‘Cannot rule out’ infarction was coded as Grade 2, ‘Myocardial ischaemia’ was coded as Grade 1, and no change was coded as Grade 0. Patients were included in the ECG analysis if they had a pre-operative ECG with Grade 0–2 injury/infarction criteria and had ECGs performed on two or more PODs. When two or more ECGs were recorded on a single day, the ECG with the lowest grade was used. ECG grades were subsequently assigned to eight categories of PMI by the authors using the following criteria: Grade 3 or 4 or Grade 4 injury/infarction criteria on the last recorded ECG of the hospitalization; Grade 3 or 4 or Grade 4 injury/infarction criteria of the lowest postoperative grade observed during hospitalization; Grade 3 or 4 or Grade 4 injury/infarction criteria for the median postoperative grade observed during hospitalization; and Grade 3 or 4 or Grade 4 injury/infarction criteria for the highest postoperative grade observed during hospitalization.
Clinical endpoints for the test cohort
The primary clinical endpoint was all-cause mortality occurring up to 5 years after surgery. Mortality data was obtained from the social security death index. The secondary endpoint was HLOS measured in days, and included the date of surgery and date of discharge as complete days of stay. In order to establish the value of adding biomarkers to an established risk prediction model, an individual risk score was calculated for each patient using the Euroscore logistic model.14 Each biomarker, as well as each ECG criterion, was then added individually to assess improvement of model performance.
Validation cohort
To validate both the primary and secondary endpoints in the primary cohort using a different population not included in the CABG Genomics Program, the Research Patient Data Registry (RPDR) of the Massachusetts General Hospital (MGH), Boston, MA was accessed retrospectively after IRB approval, to identify all patients who underwent primary CABG from July 2001 to August 2007. A total of 1801 patients with Current Procedural Terminology codes for CABG using either vein or artery were identified. Of those, 1013 patients met eligibility criteria (CONSORT Diagram Figure 1) which included primary CABG only surgery (i.e. no concomitant procedures), and available POD1 cTnT values, the standard and only troponin measured at MGH, analyzed by electrochemiluminescence immunoassay using Elecsys 2010 (Roche Diagnostics, Indianapolis, IN, USA).
Statistical methods
Statistical analyses were performed using SAS, version 9.1.3 and JMP 7.0 (SAS Institute Inc., Cary, NC, USA). Data are presented as median with inter-quartile range (25–75%) unless otherwise stated. Continuous variables were compared using analysis of variance (ANOVA) and Wilcoxon–Mann–Whitney rank-sum test. Categorical variables were compared with the χ2 test or Fisher's exact test when appropriate.
For multivariate analysis of 5-year mortality and HLOS, covariates with a two-tailed nominal P < 0.1 in univariate analyses and additional, clinically relevant demographic variables were entered into a combined stepwise Cox proportional hazards model. Age, gender, and race were forced into the model. Pre-operative biomarker values were initially included in the model, however they were neither significant nor confounded any other variables and were consequently excluded from further analysis. Nagelkerke generalized r2 and likelihood ratio test were used to determine the additional predictive value of biomarkers and ECG criteria for HLOS and mortality developed in this study.15 cTnI and cTnT levels were examined as continuous and as dichotomous variables. The optimal dichotomization point was determined in a Cox proportional hazard model for mortality by examining the troponin level which resulted in maximal improvement in model performance shown by the negative two log-likelihood ratio. Additionally, area under the curve (AUC) was examined in receiver operating characteristic (ROC) curves in a multivariable model for mortality to confirm the optimal cut-point selection. F-tests were used to compare generalized r2. A two-sided P < 0.05 was considered significant.
The validation cohort was separately acquired after the original prospective trial and utilized only the clinical information routinely recorded by caregivers. Therefore, we performed a Cox proportional hazard model for mortality and HLOS using a minimized clinical model of age, gender, and race.
Results
Demographics and post-operative clinical outcomes were similar for the primary test and validation cohorts, except for a significantly younger population in the primary test population (Table 1). After applying exclusion criteria, 545 patients were analyzed in the test cohort and 1013 in the validation cohort.
Test cohort
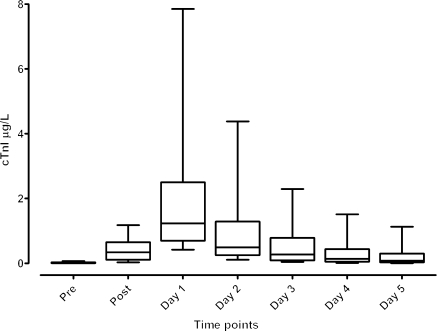
The highest mean concentrations of CKMB and cTnI (Figure 2) were measured on POD1 for 94% and 92% of patients, respectively, as has been previously shown.16 The percentages of ECGs in each of the grades for PMI varied widely and are listed in Supplementary material online, Table S1. Levels of CKMB and cTnI were different between patients diagnosed with PMI by ECG for the majority of the eight ECG criteria (Supplementary material online, Table S2).
Figure 2.
Distribution of troponin levels across all seven perioperative time points. Whisker plot showing box with median cTnI levels with edge of boxes at 25–75% and whiskers extending to the 10–90th percentile. cTnI indicates cardiac troponin I; Pre, pre-operative; Post, immediately post-protamine.
Mortality
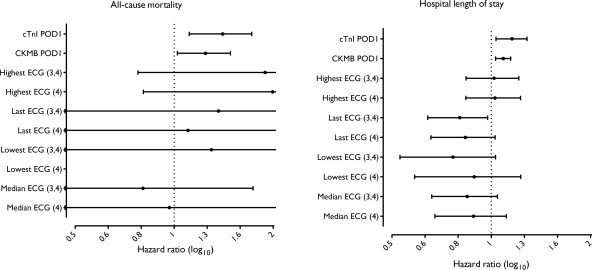
Pre-operative CKMB and cTnI did not independently predict mortality (Table 2). None of the eight ECG criteria independently predicted mortality (Table 2). The additional predictive value of POD1 biomarkers, over a clinical model that included demographics, pre-operative, and intraoperative variables were compared with the clinical model alone (Table 3). cTnI had the strongest association with 5-year mortality (P = 0.039 compared with CKMB) and was significantly more robust than POD1 CKMB (Figure 3 and Table 3). Similarly, when we accounted for the patients’ clinical condition using the well-validated logistic Euroscore, cTnI improved prediction of 5-year mortality (P = 0.023 compared with CKMB) (Table 3). Inclusion of POD2 or POD3 biomarker data did not improve the prediction of mortality. Addition of POD1 CKMB to the Cox multivariable models for mortality, while independently significant, did not improve prediction over the model with POD1 cTnI alone. Furthermore, none of the eight ECG criteria for myocardial injury predicted mortality or improved prediction of 5-year mortality when added to cohort-derived model or the logistic Euroscore (only last ECG is shown in Table 3) alone, or in combination with any biomarker.
Table 2.
Predictors of hospital length of stay and mortality in the test cohort
| All-cause mortality |
Hospital length of stay |
|||||||
|---|---|---|---|---|---|---|---|---|
| Univariate Cox |
Multivariate Cox |
Univariate Cox |
Multivariate Cox |
|||||
| Demographics and past medical history | HR (95% CI) | P-value | HR (95% CI) | P-value | HR (95% CI) | P-value | HR (95% CI) | P-value |
| Age (decade) | 1.87 (1.31–2.68) | <0.001 | 1.83 (1.25–2.68) | 0.002 | 1.19 (1.11–1.28) | <0.001 | 1.17 (1.08–1.27) | <0.001 |
| Gender (female) | 0.55 (0.19–1.57) | 0.267 | 0.39 (0.13–1.17) | 0.095 | 1.04 (0.84–1.29) | 0.683 | 0.95 (0.76–1.19) | 0.674 |
| Race (Caucasian) | N/A | 0.989 | N/A | 0.988 | 1.08 (0.81–1.45) | 0.565 | 1.20 (0.88–1.64) | 0.226 |
| Diabetes (treated) | 0.82 (0.37–1.83) | 0.632 | 0.96 (0.80–1.16) | 0.749 | ||||
| Pulmonary disease | 3.76 (1.32–10.7) | 0.013 | 1.21 (0.78–1.85) | 0.382 | ||||
| Hypercholesterolemia | 0.37 (0.18–0.75) | 0.005 | 0.74 (0.60–0.92) | 0.007 | ||||
| Previous MI | 1.17 (0.56–2.47) | 0.674 | 1.20 (0.99–1.46) | 0.061 | ||||
| Hypertension | 0.42 (0.21–0.84) | 0.014 | 0.33 (0.16–0.67) | 0.002 | 1.28 (1.05–1.55) | 0.013 | 1.27 (1.04–1.56) | 0.019 |
| LV ejection fraction (5% increment) | 0.85 (0.75–0.96) | 0.015 | 0.86 (0.75–0.98) | 0.024 | 0.94 (0.91–0.97) | 0.002 | 0.96 (0.93–0.99) | 0.034 |
| Pre-operative laboratory results | ||||||||
| Creatinine (mg/dL) | 2.70 (0.11–65.6) | 0.542 | 3.38 (1.49–7.63) | 0.003 | ||||
| Haematocrit (%) | 0.94 (0.87–1.02) | 0.112 | 0.91 (0.84–0.99) | 0.045 | 0.97 (0.95–0.99) | 0.015 | ||
| Pre-operative drugs | ||||||||
| ACE-inhibitor | 0.68 (0.34–1.39) | 0.291 | 1.11 (0.94–1.32) | 0.196 | ||||
| Antiarrhythmic | 1.77 (0.42–7.42) | 0.433 | 2.06 (1.23–3.46) | 0.006 | 1.75 (1.01–3.05) | 0.046 | ||
| Digoxin | 7.30 (2.82–18.9) | <0.001 | 2.35 (1.35–4.09) | 0.002 | 2.15 (1.20–3.87) | 0.01 | ||
| β-Blocker | 0.57 (0.27–1.20) | 0.14 | 0.93 (0.75–1.16) | 0.578 | ||||
| Ca2+ Channel inhibitor | 0.83 (0.29–2.37) | 0.729 | 1.30 (1.02–1.67) | 0.034 | 1.37 (1.06–1.79) | 0.016 | ||
| Platelet-inhibitor (non-ASA) | 0.30 (0.07–1.27) | 0.102 | 1.42 (1.14–1.78) | 0.002 | 1.58 (1.25–2.01) | <0.001 | ||
| Diuretic | 1.45 (0.67–3.12) | 0.345 | 1.31 (1.07–1.62) | 0.009 | ||||
| HMG Co-A reductase inhibitor | 0.37 (0.18–0.74) | 0.005 | 0.68 (0.54–0.85) | <0.001 | 0.59 (0.47–0.75) | <0.001 | ||
| Intraoperative characteristics | ||||||||
| Aortic cross-clamp duration (30 min increment) | 0.74 (0.50–1.10) | 0.15 | 1.12 (1.02–1.22) | 0.009 | ||||
| CPB duration (30 min increment) | 0.88 (0.65–1.19) | 0.461 | 1.19 (1.11–1.28) | <0.001 | 1.19 (1.10–1.29) | <0.001 | ||
| Euroscore (logistic, 10% increment) | 1.76 (1.06–2.91) | 0.027 | 1.75 (1.37–2.23) | <0.001 | ||||
| Post-operative ECG | ||||||||
| Last ECG-MI3, 4 | 0.61 (0.25–1.5) | 0.288 | 0.84 (0.69–1.03) | 0.1 | ||||
| Last ECG-MI4 | 0.48 (0.16–1.36) | 0.17 | 0.87 (0.71–1.08) | 0.223 | ||||
| Highest ECG-MI3, 4 | 1.13 (0.57–2.24) | 0.724 | 1.08 (0.91–1.29) | 0.335 | ||||
| Highest ECG-MI4 | 1.21 (0.60–2.43) | 0.587 | 1.12 (0.94–1.35) | 0.189 | ||||
| Lowest ECG-MI3, 4 | 0.31 (0.04–2.29) | 0.254 | 0.86 (0.63–1.18) | 0.374 | ||||
| Lowest ECG-MI4 | N/A | 0.984 | 0.90 (0.64–1.28) | 0.589 | ||||
| Median ECG-MI3, 4 | 0.37 (0.11–1.22) | 0.105 | 0.83 (0.67–1.03) | 0.108 | ||||
| Median ECG-MI4 | 0.49 (0.15–1.63) | 0.251 | 0.90 (0.71–1.14) | 0.411 | ||||
| Cardiac biomarkers | ||||||||
| CKMB Preoperative (25 µg/L increment) | 3.82 (0.03–412) | 0.574 | 2.40 (0.51–11.2) | 0.264 | ||||
| CKMB POD1 (25 µg/L increment) | 1.17 (0.99–1.37) | 0.056 | 1.12 (1.07–1.18) | <0.001 | ||||
| cTnI Preoperative (1 µg/L increment) | 0.20 (0.00–23.7) | 0.513 | 1.05 (0.77–1.44) | 0.717 | ||||
| cTnI POD1 (10 µg/L increment) | 1.35 (1.10–1.66) | 0.003 | 1.21 (1.08–1.36) | <0.001 | ||||
Variables shown in multivariable model columns are included in Cox proportional hazards analysis. Data are shown in respective increments to show better inter-variable distinction. HR, hazard ratio; CI, confidence interval; MI, myocardial infarction; LV, left ventricle; Ca2+, calcium; ASA, acetylsalicylic acid; HMG, 3-hydroxy-3-methyl-glutaryl-CoA reductase; CPB, cardiopulmonary bypass; ECG, electrocardiogram; CKMB creatinine kinase MB fraction; cTnI, cardiac troponin I; POD, post-operative day.
Table 3.
The value of adding cardiac-specific biomarkers or electrocardiogram myocardial infarction criteria to a clinical model or the logistic Euroscore
| Mortality |
Hospital length of stay |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Selection |
Nagelkerke |
Selection |
Nagelkerke |
|||||||
| Model | n | Δχ2 (df) | Marker P* | r2 | P-value† | n | Δχ2 (df) | Marker P* | r2 | P-value† |
| Clinical model only | 524 | 39.6 | — | 0.121 | — | 526 | 78.2 | — | 0.072 | — |
| Clinical model + POD1 cTnI | 516 | 45.9 | 0.009 | 0.140 | 0.001 | 518 | 84.6 | 0.011 | 0.079 | 0.052 |
| Clinical model + POD1 CKMB | 516 | 43.5 | 0.034 | 0.133 | 0.007 | 518 | 84.7 | 0.010 | 0.079 | 0.050 |
| Clinical model + last ECG3, 4 | 524 | 39.6 | 0.952 | 0.121 | 0.936 | 526 | 81.4 | 0.076 | 0.075 | 0.210 |
| Clinical model + last ECG4 | 524 | 39.8 | 0.644 | 0.121 | 0.538 | 526 | 80.1 | 0.170 | 0.074 | 0.332 |
| Euroscore only | 545 | 3.6 | — | 0.011 | — | 545 | 19.8 | — | 0.018 | — |
| Euroscore + POD1 cTnI | 537 | 9.5 | 0.017 | 0.028 | 0.002 | 537 | 30.2 | 0.002 | 0.028 | 0.021 |
| Euroscore + POD1 CKMB | 537 | 6.3 | 0.112 | 0.019 | 0.037 | 537 | 34.0 | 0.000 | 0.031 | 0.007 |
| Euroscore + last ECG3, 4 | 545 | 4.6 | 0.308 | 0.014 | 0.193 | 545 | 21.3 | 0.229 | 0.019 | 0.396 |
| Euroscore + last ECG4 | 545 | 5.6 | 0.155 | 0.017 | 0.069 | 545 | 20.6 | 0.383 | 0.019 | 0.538 |
The clinical model contains only clinical predictors which vary for mortality (age, gender, race, hypertension, left ventricular ejection fraction, pre-operative haematocrit) and for HLOS (age, gender, race, pre-operative therapy with antiarrhythmics, digoxin, calcium channel blockers, platelet inhibitors, HMG Co-A reductase inhibitors, CPB duration). Δχ2 is comparable between models of the same patient number. The biomarker P-value denotes significance of the added variable, whereas the Nagelkerke r2denotes model performance. The last ECG during hospitalization graded either as definite perioperative myocardial infarction (Grade 4) or probable myocardial infarction along with definite infarction (Grade 3 or 4). ECG, electrocardiogram; MI, myocardial infarction; HR, hazard ratio; CI, confidence interval; MI, myocardial infarction; CKMB creatinine kinase MB fraction; cTnI, cardiac troponin I; Δχ2, delta chi-squared.
*P-value is the significance of the individual variable in the multivariable model compared with the baseline clinical model.
†P-value compares the value of adding cTnI compared with CKMB when added to either the clinical model or the Euroscore.
Figure 3.
Forest plot demonstrating predictive value of biomarkers and ECG for all-cause mortality and hospital length of stay. Cox multivariable proportional hazards model, adjusted for demographic and clinical covariates, is shown for each individual variable. HRs and 95% CIs are shown. All biomarkers were entered as continuous variables. Significances for mortality: POD1 cTnI (HR 1.42 for each 10 µg/L increase; 95% CI 1.14–1.76; P = 0.009), POD1 CKMB (HR 1.23 for each 25 µg/L increase; 95% CI 1.02–1.48; P = 0.034) Significances for HLOS: POD1 cTnI (HR 1.13 for each 10 µg/L increase, 95% CI 1.02–1.26; P = 0.011), POD1 CKMB (HR 1.06 for each 25 µg/L increase, 95% CI 1.01–1.12; P = 0.01) HR indicates hazard ratio; CI, confidence interval; CKMB creatinine kinase MB fraction; cTnI, cardiac troponin I; POD, post-operative day; ECG, electrocardiogram.
Hospital length of stay
In the primary test cohort, pre-operative CKMB and cTnI did not independently predict HLOS (Table 2). In contrast, POD1 cTnI and CKMB independently predicted an increased HLOS (Figure 3), even after adjusting for demographics, patient risk factors, and perioperative variables (Figure 3 and Table 3). Addition of POD1 CKMB or cTnI to the logistic Euroscore significantly improved prediction of HLOS (Table 3). Inclusion of POD2 or POD3 biomarker data did not improve prediction of HLOS, when added to the logistic Euroscore. None of the eight ECG criteria predicted HLOS or improved prediction of HLOS by either model (Figure 3).
Validation cohort
To confirm the positive association between cardiac troponin and both 5-year mortality or HLOS in the primary test cohort, we examined the value of cardiac cTnT in an independently collected cardiac surgical population. In the validation cohort, POD1 cTnT was independently associated with 5-year mortality, even after adjusting for a minimized clinical model consisting of age, gender, and race (Supplementary material online, Table S3). Similarly, POD1 cTnT independently predicted HLOS (Supplementary material online, Table S3).
To confirm the utility of the minimized clinical model used in the validation cohort, the effect of biomarkers and ECG criteria were examined in the primary test cohort while adjusting for the minimized model. POD1 cTnI was significantly associated with an increase in mortality. Furthermore, inclusion of cTnI significantly improved overall model performance when predicting mortality (P < 0.001).
Dichotomization
A criterion of five times the ULN for biomarker assays, as proposed by the Joint Task Force as a measure of MI,5 would have identified PMI in 96% of patients using cTnI, and 52% using CKMB in our primary test cohort. Similarly, 86% would have achieved a diagnosis of PMI using cTnT in the validation cohort.
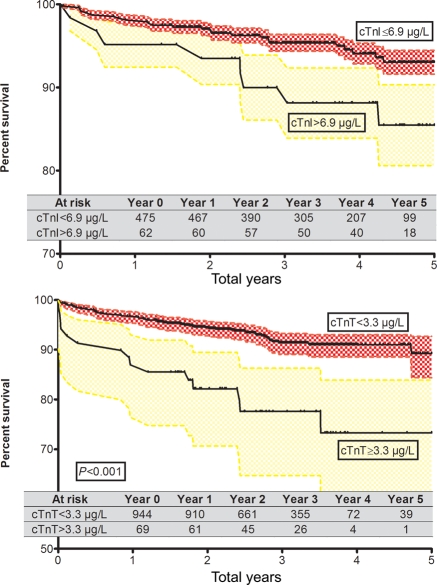
The dichotomization at the optimal ROC corresponds to a cTnI of 6.9 µg/L (61 patients) in the test cohort and a cTnT of 3.3 µg/L (69 patients) in the validation cohort. The unadjusted Kaplan–Meier significantly differentiates survival between those patients with a cTnI and cTnT below and above the cut-off in the test cohort [Hazard ratio (HR) 2.8, 95% confidence interval (CI) 1.01–7.9; P < 0.05] as well as the validation cohort (HR 7.2, 95% CI 3.0–17.3; P < 0.001) (Figure 4).
Figure 4.
Kaplan–Meier survival for troponin dichotomized at optimal ROC. Shown are unadjusted all-cause survival curves for troponin at respective cut-offs. The optimal dichotomization point was determined in a Cox proportional hazard model for mortality by examining the troponin level which resulted in maximal improvement in model performance shown by the negative two log-likelihood ratio. Additionally, area under the curve (AUC) was examined in receiver operating characteristic (ROC) curves in a multivariable model for mortality to confirm the optimal cut-point selection. cTnT and cTnI indicate cardiac troponin T and I.
When the dichotomized cTnI and cTnT are added to the multivariable clinical model unadjusted for length of survival, the AUC of the ROC improves from 0.807 to 0.815 in the test cohort and from 0.705 to 0.734 in the validation cohort. For patients above the cut-off, the mortality risk within 5 years of the operation is 1.7 (HR, 95% CI 1.1–2.5; P = 0.03) in the test cohort and 1.9 (HR, 95% CI 1.4–2.5; P < 0.001) in the validation cohort, adjusted for length of survival.
Discussion
The definition of PMI after cardiac surgery remains controversial. We now demonstrate that: (i) the ECG diagnosis of perioperative myocardial injury or PMI after cardiac surgery does not predict post-operative mortality or HLOS; (ii) use of cTnI is a more robust predictor of mortality than CKMB; (iii) cTnI improves prediction of mortality and HLOS even when added to the well-validated and commonly used logistic Euroscore; and (iv) there is no incremental benefit when predicting 5-year mortality or HLOS by measuring cTnI after POD1. Together, these findings identify POD1 troponin as an important independent predictor of the clinical consequences of perioperative myocardial injury in patients undergoing CABG surgery. Moreover, these data suggest that the definition of PMI after cardiac surgery should be re-evaluated.
Q-waves and myocardial infarction
Electrocardiographic criteria considered to be diagnostic for MI, including non-specific non-Q-wave changes, are insensitive and not specific for PMI after CABG surgery. Aetiologies of ST-segment and T-wave changes can be due to electrolyte and conduction abnormalities, external cardiac pacing, and post-surgical pericarditis.17–19 New Q-waves were once considered the best measure of PMI after CABG surgery.20,21 However, recent studies have shown poor correlation between post-cardiac surgery Q-wave development and adverse cardiac outcomes.1,6,22,23 Notably, in a study of 785 primary CABG patients, Q-wave PMI was not an independent predictor of 30-day mortality, severe LV dysfunction, or both outcomes combined (P = 0.17).1 Others have also demonstrated that only 32% of patients in the upper quartile of cTnI actually have post-operative ischaemic ECG changes, and only 6% have new onset Q-waves.2
Biomarker elevation during cardiac surgery
After uncomplicated cardiac surgery, cardiac-specific biomarkers are routinely elevated to levels considered diagnostic of MI in ambulatory populations.1 Perioperative elevation of cardiac-specific biomarkers may be due to PMI, but may also be associated with routine cardiac surgical procedures including transient myocardial ischaemia due to aortic occlusion or cardiotomy required for valve surgery.
Our findings challenge the recommendations of the 2007 Joint Task Force of the ESC/ACCF/AHA/WHF for the Redefinition of Myocardial Infarction. These recommendations included a requirement for an elevation of cardiac enzymes, dichotomized at five times the ULN within the first 72 h after surgery, when associated with the appearance of new pathological Q-waves or new LBBB.5 A criterion of five times the ULN for biomarker assays as a measure of significant myocardial injury would have identified PMI in 96% of patients using cTnI, and 52% using CKMB in our primary test cohort. Similarly, 86% would have achieved a diagnosis of PMI using cTnT in the validation cohort. With ever increasing sensitivity of the biomarker assays, the ULN cut-offs would by definition include more patients and require constant readjustment.
We therefore determined the optimal cut-off for cTnI and cTnT using a Cox proportional hazard model for mortality as well as ROC curves. In our cohorts, a cTnI of 6.9 µg/L and a cTnT of 3.3 µg/L are optimal for differentiating between patients who survive and those at increased risk of dying within 5 years. These values are significantly higher than the recommendations by the Task Force,5 similar to values that have been quoted in some studies,2 and much less than those in other similar studies.8,24,25 This illustrates the dilemma of determining a single biomarker cut-off. Peak post-operative troponin will vary depending on the population at risk, patient comorbidities, invasiveness of the procedure, underlying genetic factors, and the laboratory assay used. Restricting the definition of PMI to dichotomized biomarker data, ignores the advantage of a quantitative assessment using a continuous measurement that examines the wide range of perioperative myocardial injury from transient and reversible, to clinically significant. Consequently, we propose that increasing levels of circulating cTnI reflect a spectrum of increasing myocardial injury, with short and long-term consequences importantly described by HLOS and mortality. Finally, although the Joint Task Force recommends the measurement of biomarker levels for 72 h post-operatively, few patients in our study (8%) had a peak cTnI occurring after POD1. There was no observed incremental value for the prediction of mortality or HLOS by measuring cTnI beyond POD1. Thus, establishing a diagnosis of PMI using only POD1 cTnI may permit more efficient use of therapeutic interventions, and thereby reduce associated morbidity and mortality. Finally, we observed no predictive value of any ECG criterion alone, or in combination with biomarker level, in contradistinction to the Task Force’s recommendations.
Limitations
This study has several limitations. First, the test and validation cohorts used different components of the troponin protein complex. Despite extensive work evaluating the performance of both troponin assays in assessing the severity of myocardial injury, consensus is lacking as to the ‘superior’ marker.26 Secondly, generation cTnT assays have overcome a lack of specificity with skeletal muscle cross-reactivity and higher concentrations in renal failure.27,28 However, the failure to calibrate platforms with standard assays for cTnI remains a significant limitation for inter-laboratory studies and clinical recommendations which rely on a dichotomous cut-off for defining significant perioperative myocardial injury.29,30 Thirdly, we used the well-known logistic Euroscore as an externally valid assessment of individual risk of 5-year mortality. However, the Euroscore was originally constructed to predict 1-year mortality. Fourthly, our analysis of ECG criteria was limited to the association of conventional ECG signs of PMI with mortality and HLOS, whereas the impact of arrhythmias and their relationship to the primary and secondary endpoints was not evaluated. ST-elevation was controlled for by the Marquette ECG interpretation and by the over-reading cardiologists. Although acute ST-elevation may indicate PMI for an individual patient, no ECG criteria of PMI were significant on a population basis. Fifthly, our validation cohort was not constructed prospectively and thus was limited to a smaller number of perioperative covariates which therefore could not be controlled for, and may have affected our final analysis of this population. Finally, our primary endpoint of all-cause mortality does not account for cardiac-specific causes of death. However, prior studies have demonstrated that use of all-cause mortality is equally robust to cardiac mortality, and further removes interpretation of limited death certificate information.31,32
Conclusion
POD1 cTnI independently predicts clinically significant perioperative myocardial injury after CABG surgery, while accounting for clinical risk. ECG criteria of PMI do not independently predict mortality or HLOS, and therefore should not be included in the diagnosis of PMI after CABG surgery.
Funding
This work was supported by the Bayer® Fellowship in Blood Conservation, Biosite Inc., San Diego, CA, USA (J.D.M.); NIH (HL-068774 to S.C.B., NCRR M01 02558 to C.D.C. and A.A.F.); Society of Cardiovascular Anaesthesiologists Research Starter Grant; and Siemens Medical Solutions Diagnostics, Tarrytown, NY (J.D.M.).
Conflict of interest: J.D.M. received a Bayer® Fellowship in Blood Conservation; S.K.S. received funding from Siemens Medical Solutions Diagnostics, Tarrytown, NY, USA; J.D.M and A.A.F. received a Society of Cardiovascular Anaesthesiologists Research Starter Grant; A.A.F. received a Foundation in Anaesthesia Education and Research Starter Grant; C.D.C. and A.A.F. received a grant from NIH (NCRR M01 02558); and S.C.B. received a grant from NIH (NHLBI HL-068774).
Supplementary Material
Acknowledgements
We acknowledge the outstanding contributory efforts of the CABG Genomics research staff: James Gosnell, Kujtim Bodinaku, Jai Madan, Svetlana Gorbatov, James Chen, and Isabella Candelaria. We thank all study subjects who participated in the CABG Genomics Program and surgeons who identified their patients. We thank John Orav for assistance with methodological and statistical questions.
References
- 1.Ramsay J, Shernan S, Fitch J, Finnegan P, Todaro T, Filloon T, Nussmeier NA. Increased creatine kinase MB level predicts postoperative mortality after cardiac surgery independent of new Q waves. J Thorac Cardiovasc Surg. 2005;129:300–306. doi: 10.1016/j.jtcvs.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 2.Croal BL, Hillis GS, Gibson PH, Fazal MT, El-Shafei H, Gibson G, Jeffrey RR, Buchan KG, West D, Cuthbertson BH. Relationship between postoperative cardiac troponin I levels and outcome of cardiac surgery. Circulation. 2006;114:1468–1475. doi: 10.1161/CIRCULATIONAHA.105.602370. [DOI] [PubMed] [Google Scholar]
- 3.Chen JC, Kaul P, Levy JH, Haverich A, Menasche P, Smith PK, Carrier M, Verrier ED, Van de Werf F, Burge R, Finnegan P, Mark DB, Shernan SK. Myocardial infarction following coronary artery bypass graft surgery increases healthcare resource utilization. Crit Care Med. 2007;35:1296–1301. doi: 10.1097/01.CCM.0000262403.08546.A2. [DOI] [PubMed] [Google Scholar]
- 4.Mangano DT. Effects of acadesine on myocardial infarction, stroke, and death following surgery. A meta-analysis of the 5 international randomized trials. The Multicenter Study of Perioperative Ischemia (McSPI) Research Group. J Am Med Assoc. 1997;277:325–332. doi: 10.1001/jama.277.4.325. [DOI] [PubMed] [Google Scholar]
- 5.Thygesen K, Alpert JS, White HD on behalf of the Joint ESC/ACCF/AHA/WHF. Task Force for the Redefinition of Myocardial Infarction. Eur Heart J. 2007;28:2525–2538. doi: 10.1093/eurheartj/ehm355. [DOI] [PubMed] [Google Scholar]
- 6.Hodakowski GT, Craver JM, Jones EL, King SB, III, Guyton RA. Clinical significance of perioperative Q-wave myocardial infarction: the Emory Angioplasty versus Surgery Trial. J Thorac Cardiovasc Surg. 1996;112:1447–1453. doi: 10.1016/S0022-5223(96)70002-8. discussion 1453–1444. [DOI] [PubMed] [Google Scholar]
- 7.Svedjeholm R, Dahlin LG, Lundberg C, Szabo Z, Kagedal B, Nylander E, Olin C, Rutberg H. Are electrocardiographic Q-wave criteria reliable for diagnosis of perioperative myocardial infarction after coronary surgery? Eur J Cardiothorac Surg. 1998;13:655–661. doi: 10.1016/s1010-7940(98)00091-8. [DOI] [PubMed] [Google Scholar]
- 8.Adabag AS, Rector T, Mithani S, Harmala J, Ward HB, Kelly RF, Nguyen JT, McFalls EO, Bloomfield HE. Prognostic significance of elevated cardiac troponin I after heart surgery. Ann Thorac Surg. 2007;83:1744–1750. doi: 10.1016/j.athoracsur.2006.12.049. [DOI] [PubMed] [Google Scholar]
- 9.Armstrong PW. Defining myocardial infarction: a work in progress: ischaemic heart disease. Heart. 2008;94:1076–1079. doi: 10.1136/hrt.2007.135202. [DOI] [PubMed] [Google Scholar]
- 10.White HD. Evolution of the definition of myocardial infarction: what are the implications of a new universal definition? Heart. 2008;94:679–684. doi: 10.1136/hrt.2007.130955. [DOI] [PubMed] [Google Scholar]
- 11.Lanza GA. The universal definition of myocardial infarction: some issues and concerns. Eur Heart J. 2008;29:1209. doi: 10.1093/eurheartj/ehn130. author reply 1209–1210. [DOI] [PubMed] [Google Scholar]
- 12.Poon K, Okin PM, Kligfield P. Diagnostic performance of a computer-based ECG rhythm algorithm. J Electrocardiol. 2005;38:235–238. doi: 10.1016/j.jelectrocard.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 13.Shah AP, Rubin SA. Errors in the computerized electrocardiogram interpretation of cardiac rhythm. J Electrocardiol. 2007;40:385–390. doi: 10.1016/j.jelectrocard.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 14.Roques F, Michel P, Goldstone AR, Nashef SA. The logistic EuroSCORE. Eur Heart J. 2003;24:881–882. doi: 10.1016/s0195-668x(02)00799-6. [DOI] [PubMed] [Google Scholar]
- 15.Ash A, Shwartz M. R2: a useful measure of model performance when predicting a dichotomous outcome. Stat Med. 1999;18:375–384. doi: 10.1002/(sici)1097-0258(19990228)18:4<375::aid-sim20>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 16.Greenson N, Macoviak J, Krishnaswamy P, Morrisey R, James C, Clopton P, Fitzgerald R, Maisel AS. Usefulness of cardiac troponin I in patients undergoing open heart surgery. Am Heart J. 2001;141:447–455. doi: 10.1067/mhj.2001.113071. [DOI] [PubMed] [Google Scholar]
- 17.Barron JT. Cardiac troponin I and non-Q-wave myocardial infarction: how useful is it after coronary artery bypass surgery? Crit Care Med. 1998;26:1936–1937. doi: 10.1097/00003246-199812000-00005. [DOI] [PubMed] [Google Scholar]
- 18.Spodick DH. Acute pericarditis: current concepts and practice. J Am Med Assoc. 2003;289:1150–1153. doi: 10.1001/jama.289.9.1150. [DOI] [PubMed] [Google Scholar]
- 19.Loeb HS, Gunnar WP, Thomas DD. Is new ST-segment elevation after coronary artery bypass of clinical importance in the absence of perioperative myocardial infarction? J Electrocardiol. 2007;40:276–281. doi: 10.1016/j.jelectrocard.2006.08.098. [DOI] [PubMed] [Google Scholar]
- 20.Force T, Hibberd P, Weeks G, Kemper AJ, Bloomfield P, Tow D, Josa M, Khuri S, Parisi AF. Perioperative myocardial infarction after coronary artery bypass surgery. Clinical significance and approach to risk stratification. Circulation. 1990;82:903–912. doi: 10.1161/01.cir.82.3.903. [DOI] [PubMed] [Google Scholar]
- 21.Chaitman BR, Alderman EL, Sheffield LT, Tong T, Fisher L, Mock MB, Weins RD, Kaiser GC, Roitman D, Berger R, Gersh B, Schaff H, Bourassa MG, Killip T. Use of survival analysis to determine the clinical significance of new Q waves after coronary bypass surgery. Circulation. 1983;67:302–309. doi: 10.1161/01.cir.67.2.302. [DOI] [PubMed] [Google Scholar]
- 22.Jain U, Laflamme CJ, Aggarwal A, Ramsay JG, Comunale ME, Ghoshal S, Ngo L, Ziola K, Hollenberg M, Mangano DT. Electrocardiographic and hemodynamic changes and their association with myocardial infarction during coronary artery bypass surgery. A multicenter study. Multicenter Study of Perioperative Ischemia (McSPI) Research Group. Anesthesiology. 1997;86:576–591. doi: 10.1097/00000542-199703000-00009. [DOI] [PubMed] [Google Scholar]
- 23.Flores NA, Goulielmos NV, Seghatchian MJ, Sheridan DJ. Myocardial ischaemia induces platelet activation with adverse electrophysiological and arrhythmogenic effects. Cardiovasc Res. 1994;28:1662–1671. doi: 10.1093/cvr/28.11.1662. [DOI] [PubMed] [Google Scholar]
- 24.Thielmann M, Massoudy P, Schmermund A, Neuhauser M, Marggraf G, Kamler M, Herold U, Aleksic I, Mann K, Haude M, Heusch G, Erbel R, Jakob H. Diagnostic discrimination between graft-related and non-graft-related perioperative myocardial infarction with cardiac troponin I after coronary artery bypass surgery. Eur Heart J. 2005;26:2440–2447. doi: 10.1093/eurheartj/ehi437. [DOI] [PubMed] [Google Scholar]
- 25.Paparella D, Cappabianca G, Visicchio G, Galeone A, Marzovillo A, Gallo N, Memmola C, Schinosa Lde L. Cardiac troponin I release after coronary artery bypass grafting operation: effects on operative and midterm survival. Ann Thorac Surg. 2005;80:1758–1764. doi: 10.1016/j.athoracsur.2005.04.040. [DOI] [PubMed] [Google Scholar]
- 26.Nageh T, Sherwood RA, Harris BM, Byrne JA, Thomas MR. Cardiac troponin T and I and creatine kinase-MB as markers of myocardial injury and predictors of outcome following percutaneous coronary intervention. Int J Cardiol. 2003;92:285–293. doi: 10.1016/s0167-5273(03)00105-0. [DOI] [PubMed] [Google Scholar]
- 27.Apple FS, Ricchiuti V, Voss EM, Anderson PA, Ney A, Odland M. Expression of cardiac troponin T isoforms in skeletal muscle of renal disease patients will not cause false-positive serum results by the second generation cardiac troponin T assay. Eur Heart J. 1998;19(Suppl. N):N30–N33. [PubMed] [Google Scholar]
- 28.Baum H, Braun S, Gerhardt W, Gilson G, Hafner G, Muller-Bardorff M, Stein W, Klein G, Ebert C, Hallermayer K, Katus HA. Multicenter evaluation of a second-generation assay for cardiac troponin T. Clin Chem. 1997;43:1877–1884. [PubMed] [Google Scholar]
- 29.Apple FS. Standardization of cardiac markers. Scand J Clin Lab Invest Suppl. 2005;240:107–111. doi: 10.1080/00365510500236242. [DOI] [PubMed] [Google Scholar]
- 30.Martin CB, Shaw AD, Gal J, Aravindan N, Murphy F, Royston D, Riedel BJ. The comparison and validity of troponin I assay systems in diagnosing myocardial ischemic injury after surgical coronary revascularization. J Cardiothorac Vasc Anesth. 2005;19:288–293. doi: 10.1053/j.jvca.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 31.Daniels LB, Laughlin GA, Clopton P, Maisel AS, Barrett-Connor E. Minimally elevated cardiac troponin T and elevated N-terminal pro-B-type natriuretic peptide predict mortality in older adults: results from the Rancho Bernardo Study. J Am Coll Cardiol. 2008;52:450–459. doi: 10.1016/j.jacc.2008.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Swaminathan M, Morris RW, De Meyts DD, Podgoreanu MV, Jollis JG, Grocott HP, Milano CA, Newman MF, Mathew JP. Deterioration of regional wall motion immediately after coronary artery bypass graft surgery is associated with long-term major adverse cardiac events. Anesthesiology. 2007;107:739–745. doi: 10.1097/01.anes.0000287008.70453.81. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.