Abstract
Aims
Obesity has been shown to be a risk factor for first atrial fibrillation (AF), but whether it is associated with progression from paroxysmal to permanent AF is unknown.
Methods and results
In this longitudinal cohort study, Olmsted County, MN residents confirmed to have developed paroxysmal AF during 1980–2000 were identified and followed passively to 2006. The interrelationships of body mass index (BMI), left atrial (LA) size, and progression to permanent AF were analysed. Of a total of 3248 patients (mean age 71 ± 15 years; 54% men) diagnosed with paroxysmal AF, 557 (17%) progressed to permanent AF (unadjusted incidence, 36/1000 person-years) over a median follow-up period of 5.1 years (interquartile range 1.2–9.4). Adjusting for age and sex, BMI independently predicted the progression to permanent AF (hazard ratio, HR 1.04, CI 1.03–1.06; P < 0.0001). Compared with normal BMI (18.5–24.9 kg/m2), obesity (30–34.9 kg/m2) and severe obesity (≥35 kg/m2) were associated with increased risk for progression [HR 1.54 (CI 1.2–2.0; P = 0.0004) and 1.87 (CI 1.4–2.5; P < 0.0001, respectively)]. BMI remained highly significant even after multiple adjustments. In the subgroup with echocardiographic assessment (n = 744), LA volume was incremental to BMI for independent prediction of progression after multiple adjustments, and did not weaken the association between BMI and progression to permanent AF (HR 1.04; CI 1.02–1.05; P < 0.0001).
Conclusion
There was a graded risk relationship between BMI and progression from paroxysmal to permanent AF. This relationship was not weakened by LA volume, which was independent of and incremental to BMI for the prediction of progression to permanent AF.
Keywords: Obesity, Left atrial volume, Atrial fibrillation
Introduction
Obesity and atrial fibrillation (AF) are growing public health problems.1,2 The prevalence of both has increased over time.3–8 Prevalence of obesity has increased across all age segments, including the oldest (>70 years)4,9 who are at greatest risk of AF. Data demonstrating an association between AF and obesity have been discordant. Although a number of studies have demonstrated that greater body mass index (BMI), or obesity, is a risk factor for AF,10–12 an earlier report from Framingham Heart Study13 and data from the Cardiovascular Health Study14 did not support this association. More recently, the Framingham investigators provided convincing data that obesity is indeed associated with excess risk of AF, and that the risk is likely mediated through left atrial (LA) enlargement.15 As an extension to these studies, we evaluated, as a primary aim, whether obesity is a risk factor for the progression of paroxysmal to permanent AF. We tested the hypothesis that there is a ‘dose–response’ relationship between BMI and the risk of progression from paroxysmal to permanent AF. Our secondary aim was to evaluate the significance of LA volume, and its interaction with BMI, for the prediction of such progression to permanent AF.
Methods
Study design and population
This community-based cohort study was approved by the Mayo Foundation Institutional Review Board. Olmsted County, MN, is well suited for the conduction of studies with long-term follow-up because of a number of unique features.16 Geographically, the community is relatively isolated from other urban centres, and medical care is delivered by only a few health care providers. Most of the Olmsted County residents return to the Mayo Clinic regularly, allowing capture of events. A previous study has shown that 96% of Olmsted County residents aged 65–74 years returned to the Mayo Clinic within a 3-year period.16 For each patient at the Mayo Clinic, a unified medical record containing details of all inpatient and outpatient encounters is maintained. Within each medical record, diagnoses made during office visits, clinic consultations, emergency room visits, hospital admissions, nursing home care, and autopsy examinations, as well as surgical procedures, are listed on a master sheet and coded. Coded diagnoses are then transferred to a central diagnostic index. This diagnostic index allows all patients with a diagnosis of interest to be readily identified.
Paroxysmal atrial fibrillation cohort
The medical records of Olmsted County, MN adult residents who had AF documented between January 1, 1980 and December 31, 2000, in any of the Mayo administrative and clinical databases [medical index, surgical index, electrocardiographic (ECG), and echocardiographic databases] were reviewed and followed in medical records to March 2004. All diagnoses, covariates, and outcomes were defined a priori, and the same definitions were applied to all patients throughout the 21-year study period. Final inclusion in the study population required ECG confirmation of AF, verification of the AF episode being the first recognized AF event for the person, and that the AF type was paroxysmal.
Definitions
Subjects were categorized as underweight (BMI <18.5 kg/m2), normal weight (BMI 18.5–24.9 kg/m2), overweight (BMI 25.0–29.9 kg/m2), and obese (BMI ≥30 kg/m2) according to the World Health Organization’s definition.17 In terms of the definition of the subtypes of AF, we followed the criteria as recommended by the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines and Policy Conferences.18 Specifically, paroxysmal AF referred to recurrent, intermittent AF that was self-limiting and terminated without specific therapy. Persistent AF was defined by recurrent, sustained AF that had been previously terminated by pharmacological or electrical intervention. Permanent AF referred to continuous AF that could not be converted to normal sinus rhythm by pharmacological or electrical techniques.
Persons who could not be classified according to the definitions above were considered indeterminate, and were excluded from the analyses (n = 12). The adjudication of AF types was independently performed by two cardiologists (T.S.M.T. and Y.M.).
Coronary artery disease was defined by angiographic findings of lesions ≥50% in any of the three main arterial distributions, angina, or history of myocardial infarction. Myocardial infarction was defined by two or all of the three diagnostic criteria: compatible clinical presentation, diagnostic cardiac enzymes, and consistent ECG changes. Valvular heart disease was defined by echocardiographic confirmation of more than mild degree of valvular stenosis or regurgitation. Carotid artery disease referred to the presence of ≥50% stenosis in the carotid arteries by neurovascular imaging, or prior intervention. Stroke was defined by clinical documentation of the diagnosis with or without confirmatory findings on imaging studies. Systemic hypertension was defined by a physician’s diagnosis, need for antihypertensive therapy, systolic blood pressure >140 mmHg, or diastolic blood pressure >90 mmHg on two or more occasions that were not associated with acute illness or injury. Diabetes mellitus was defined by physician’s diagnosis, and treatment with insulin or oral hypoglycaemic agents. Dyslipidaemia was defined by a total cholesterol ≥200 mg/dL, triglycerides ≥150 mg/dL, LDL cholesterol ≥130 mg/dL, or HDL cholesterol <40 mg/dL on two or more occasions, or treatment with lipid lowering agents. Smoking history was classified as past (>6 months prior) or current smoker. Self-reported use of alcohol of >1 drink per day on a regular basis was recorded. Chronic obstructive pulmonary disease and hyperthyroidism were defined by documented clinical diagnoses. Congestive heart failure was based on the classic Framingham criteria.19
Outcome ascertainment
Progression to permanent AF was considered to have occurred if, during follow-up, AF had become continuous and could not be converted to normal sinus rhythm by pharmacological or electrical techniques.18 We abstracted the data regarding electrical and pharmacological cardioversion from medical records for all persons, and reviewed the ECG series for each. The Mayo ECG database contains all ECGs performed since 1976. Each ECG was read and signed off by a board certified cardiologist. The verified diagnoses were then electronically coded, allowing easy retrieval of the diagnoses for each person. As such, the identification of the rhythm status at the time of each ECG was simple. In addition, for the purpose of this study, we examined each ECG for confirmation of rhythm status for all patients. Since nearly 100% of Olmsted County residents return to the Mayo Clinic over a 3-year period,16 and the master record documents any diagnoses that have incurred, the review process involving the use of the ECG database and comprehensive evaluation of clinical documentation allowed us to classify AF status. Additionally, we cross-referenced the echocardiographic database which registered the rhythm at the time of any echocardiography study. Ascertainment of death was accomplished through comprehensive review of medical records and the use of the following resources: death certificates, vital status information from Mayo Registration, Minnesota State death tapes, and social security death index.
Echocardiographic data
Two-dimensional left ventricular (LV) dimensions were used for calculation of ejection fraction [LV ejection fraction (%) = (LV end-diastolic dimension2−LV end-systolic dimension2)/LV end-diastolic dimension2].20 We calculated LA volume using a validated formula:21 (LA volume) = 3.7 (LA dimension)1.8. Indexed LA volume was calculated by dividing LA volume by body surface area.
Statistical methods
Baseline characteristics were summarized using frequencies and percentages for categorical data, and mean ± SD for continuous data. Cox proportional hazards regression models were used to examine the relation between each risk factor and progression to permanent AF, where age and sex remained in the models throughout. BMI was modelled both as a continuous and a categorical variable. Since increased BMI might exert its effect on progression to permanent AF via its association with multiple other comorbidities, we performed multivariable analyses, which adjusted for relevant baseline covariates in addition to age and sex. Specifically, we included the risk factors that emerged as significant (P < 0.05) after age and sex adjustment: history of hypertension, valvular heart disease, myocardial infarction, congestive heart failure, smoking, chronic pulmonary disease, systolic and diastolic blood pressures at baseline AF diagnosis. In addition, we adjusted for interim myocardial infarction or congestive heart failure, which were considered as time-dependent covariates. Persons who were underweight (n = 146, 4%) or did not have BMI documented (n = 34, 1%) were excluded from the primary analyses. We did, however, perform supplemental analyses of the relationship between BMI and risk of progression to permanent AF without excluding the underweight group.
The development of multivariable adjustment models for the prediction of permanent AF was done first, for all patients; and second, for the subset with echocardiographic assessment. Using all patients, a set of baseline clinical variables were identified by fitting the Cox proportional model with backward elimination process. The process was repeated in the subgroup analyses of those who had echocardiogram performed (n = 744) within 30 days of AF diagnosis. LA volume and LV ejection fraction were entered into these models and evaluated for their incremental value for the prediction of permanent AF. The effect of LA size on prediction was assessed in three ways: absolute LA volume, indexed LA volume, as well as by M-mode LA dimension. The possibility of multiplicative interaction effect between indexed LA volume and BMI was tested by adding the product term to the model with indexed LA volume and BMI.
Assumptions of linearity were assessed by adding a quadratic term for each continuous variable in the model. Although age demonstrated non-linearity, the coefficient for BMI remained unchanged after these additions. To assess proportional hazards assumptions, the model was adjusted to include each variable and log follow-up time term in a time-dependent proportional hazards model. Of all the continuous variables, none showed any significance and the coefficient for BMI remained unchanged.
We also plotted the hazards of progression by BMI categories. Kaplan–Meier curves showing cumulative risk for progression by BMI categories were provided. Finally, the age- and sex-adjusted hazards of progression by BMI and LA volume were depicted. In all cases, statistical significance was defined as 2-tailed P < 0.05. Age- and sex-adjusted hazard ratios (HR) by BMI and LA volume categories were displayed in graphics.
Results
Baseline characteristics of patients by body mass index
Of the 3248 persons (1745 men; 1503 women) whose paroxysmal AF was first documented in 1980–2000, 492 (15%) had become persistent and 557 (17%) permanent AF over a median follow-up time of 5.1 years (interquartile range 1.2–9.4 years). Mean age of the cohort was 71 ± 15 years. Electrical cardioversion was performed in 445 persons (initially successful in 418), with or without attempt at pharmacological cardioversion; while only pharmacological conversion was used in 276 (initially successful in 259). The majority of those who initially were cardioverted to sinus rhythm had progressed to permanent AF by last follow-up (513/677; 75%). The unadjusted incidence of progression from paroxysmal to permanent AF was 36 per 1000 person-years overall. The age- and sex-adjusted incidence of progression per 1000 person-years was as follows: normal BMI 33; overweight 35; obesity 43; and severe obesity 47 (P < 0.0001). The baseline characteristics for the entire study population, and for the subgroup with echocardiography studies, are shown in Table 1.
Table 1.
Baseline characteristics and associated age- and sex-adjusted risks for progression to permanent atrial fibrillation
| Variables | Overall | Cox age and sex adjusted P-value | Hazard ratio (95% CI) |
|---|---|---|---|
| Entire study population (n = 3248) | |||
| Age, years | 71 ± 15 | <0.0001 | 1.034 (1.027–1.041) |
| Male, n (%) | 1745 (54) | 0.3489 | 1.087 (0.913–1.293) |
| BMI, kg/m2 | 27±6 | 0.0268 | 1.005 (1.001–1.009) |
| Normal BMI, n (%) | 1233 (38) | 0.0030 | 0.756 (0.629–0.909) |
| Overweight, n (%) | 1211 (37) | 0.2245 | 0.898 (0.755–1.068) |
| Obesity, n (%) | 521 (16) | 0.0051 | 1.337 (1.091–1.639) |
| Severe obesity, n (%) | 283 (9) | 0.0004 | 1.608 (1.236–2.093) |
| Systolic blood pressure, mm Hg | 138 ± 21 | 0.0002 | 1.016 (1.007–1.024) |
| Diastolic blood pressure, mm Hg | 77 ± 11 | <0.0001 | 1.042 (1.028–1.057) |
| History of CHF, n (%) | 866 (27) | <0.0001 | 1.568 (1.286–1.911) |
| History of hypertension, n (%) | 2577 (79) | 0.0026 | 1.439 (1.136–1.824) |
| History of valvular heart disease, n (%) | 598 (18) | <0.0001 | 1.590 (1.296–1.951) |
| Current or past smoker, n (%) | 1907 (59) | 0.0174 | 1.250 (1.040–1.503) |
| Chronic pulmonary disease, n (%) | 713 (22) | 0.0463 | 1.231 (1.003–1.511) |
| Angina/any CAD/prior MI, n (%) | 1377 (42) | 0.6919 | 0.965 (0.812–1.149) |
| History of coronary revascularization, n (%) | 491 (15) | 0.5109 | 0.924 (0.730–1.169) |
| Peripheral vascular disease, n (%) | 434 (13) | 0.6712 | 1.060 (0.810–1.388) |
| History of diabetes mellitus, n (%) | 621 (19) | 0.1660 | 1.172 (0.936–1.468) |
| History of dyslipidaemia, n (%) | 1349 (42) | 0.3197 | 1.090 (0.920–1.293) |
| Regular alcohol use, n (%) | 389 (12) | 0.3330 | 1.135 (0.878–1.468) |
| Renal disease, n (%) | 591 (18) | 0.1549 | 1.193 (0.935–1.522) |
| History of obstructive sleep apnoea, n (%) | 73 (2) | 0.1598 | 1.512 (0.849–2.693) |
| History of malignant disease, n (%) | 910 (28) | 0.5451 | 0.938 (0.763–1.154) |
| Antiarrhythmic use (Class I and Class III) | 61 (2) | 0.1738 | 1.514 (0.833–2.751) |
| Betablocker/calcium channel blocker use | 923 (28) | 0.1413 | 1.149 (0.955–1.381) |
| Echo subgroup (n = 744) | |||
| Age, years | 69 ± 15 | <0.0001 | 1.049 (1.033–1.066) |
| Male, n (%) | 411 (55) | 0.0167 | 1.549 (1.083–2.217) |
| Systolic blood pressure, mm Hg | 136 ± 19 | 0.0944 | 1.008 (0.999–1.017) |
| Diastolic blood pressure, mm Hg | 76 ± 11 | 0.0147 | 1.020 (1.004–1.037) |
| History of hypertension, n (%) | 585 (79) | 0.0256 | 1.840 (1.077–3.143) |
| History of valvular heart disease, n (%) | 216 (29) | 0.0361 | 1.48 (1.026–2.137) |
| History of obstructive sleep aponea, n (%) | 28 (4) | 0.0123 | 2.568 (1.228–5.373) |
| Current or past smoker, n (%) | 431 (58) | 0.0278 | 1.512 (1.046–2.185) |
| Chronic pulmonary disease, n (%) | 139 (19) | 0.0403 | 1.534 (1.019–2.309) |
| Left atrial diameter, mm | 45 ± 8 | <0.0001 | 1.056 (1.031–1.08) |
| Left atrial volume, mL/m2 | 36 ± 11 | <0.0001 | 1.001 (1.000–1.002) |
| Left ventricular ejection fraction | 55 ± 14 | 0.9529 | 1.000 (0.987–1.012) |
| Angina/any CAD/prior MI, n (%) | 270 (36) | 0.5242 | 0.888 (0.615–1.281) |
| History of coronary revascularization, n (%) | 137 (18) | 0.5379 | 0.865 (0.545–1.373) |
| History of CHF, n (%) | 182 (24) | 0.4189 | 1.190 (0.780–1.816) |
| Peripheral vascular disease, n (%) | 92 (12) | 0.2837 | 0.721 (0.397–1.311) |
| History of diabetes mellitus, n (%) | 137 (18) | 0.5157 | 0.845 (0.507–1.406) |
| History of dyslipidaemia, n (%) | 403 (54) | 0.2679 | 1.216 (0.861–1.717) |
| Regular alcohol use, n (%) | 85 (11) | 0.3745 | 1.276 (0.745–2.184) |
| Renal disease, n (%) | 126 (17) | 0.3520 | 1.252 (0.780–2.010) |
| History of malignant disease, n (%) | 204 (27) | 0.3820 | 1.183 (0.812–1.725) |
| Antiarrhythmic use (Class I and Class III) | 9 (1) | 0.9402 | 1.078 (0.150–7.744) |
| Beta-blocker/calcium channel blocker use | 229 (31) | 0.3364 | 1.196 (0.831–1.721) |
Relationship between body mass index and progression from paroxysmal to permanent atrial fibrillation
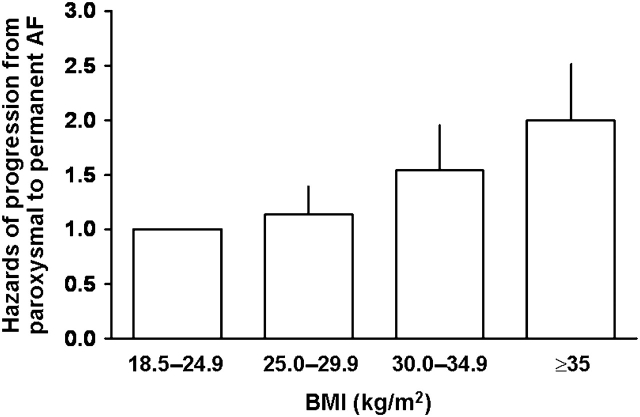
There was a graded risk relationship between BMI and progression to permanent AF: the greater the BMI at baseline, the higher the likelihood of progression from paroxysmal to permanent AF (Figure 1, Table 1). Age- and sex-adjusted Cox models showed that BMI, as a categorical or continuous variable, was strongly associated with progression to permanent AF, even after adjusting for multiple baseline clinical risk factors, and additionally for interim myocardial infarction and congestive heart failure (Table 2). Relative to normal BMI, the hazards for age- and sex-adjusted risk for progression from paroxysmal to permanent AF for overweight, obesity, and severe obesity were 1.1 (P = 0.24), 1.54 (P = 0.0004), and 1.87 (P < 0.0001), respectively.
Figure 1.
Age- and sex-adjusted hazards of progression from paroxysmal to permanent atrial fibrillation by body mass index.
Table 2.
Models for prediction of progression to permanent atrial fibrillation: role of body mass index and left atrial size
| Entire population (n = 3,248) | HR (95% CI) | P-Value |
|---|---|---|
| BMI as continuous variable (per kg/m2 increase) | ||
| Adjusted for age and sex | 1.04 (1.03-1.06) | <0.0001 |
| Adjusted for clinical variables | 1.04 (1.02-1.05) | <0.0001 |
| Adjusted for clinical variablesa and interim MI/CHF | 1.03 (1.01-1.04) | 0.0003 |
| BMI as a categorical variable (per level increase) | ||
| Adjusted for age and sex | ||
| Normal BMI | 1.000 | |
| Overweight | 1.13 (0.92–1.39) | 0.2415 |
| Obese | 1.54 (1.21–1.95) | 0.0004 |
| Severely obese | 1.87 (1.40–2.51) | <0.0001 |
| Adjusted for age, sex, and other clinical variablesa | ||
| Normal | 1.000 | |
| Overweight | 1.11 (0.90–1.37) | 0.3482 |
| Obese | 1.39 (1.09–1.79) | 0.0084 |
| Severely obese | 1.66 (1.21–2.24) | 0.0015 |
| Adjusted for clinical variablesa and interim MI/CHF | ||
| Normal | 1.000 | |
| Overweight | 1.09 (0.88–1.35) | 0.4147 |
| Obese | 1.30 (1.01–1.66) | 0.0408 |
| Severely Obese | 1.48 (1.08–2.01) | 0.0134 |
| Subgroup with echo parameters (n = 744) | HR (95% CI) | P-Value |
| BMI as continuous variable (per kg/m2 increase) | ||
| Adjusted for age, sex and LA volume | 1.05 (1.02–1.07) | <0.0001 |
| Adjusted for age, sex, other clinical variablesa, and LA volume | 1.05 (1.02–1.08) | 0.0015 |
| Adjusted for age, sex, other clinical variablesa, and interim MI/CHF | 1.03 (1.01–1.06) | 0.0206 |
| BMI as a categorical variable (per level increase) | ||
| Age, sex, and LA volume adjusted | ||
| Normal | 1.000 | |
| Overweight | 0.77 (0.50–1.19) | 0.2416 |
| Obese | 1.42 (0.89–2.26) | 0.1370 |
| Severely obese | 1.89 (1.11–3.21) | 0.0193 |
| Adjusted for age, sex, other clinical variablesa, and LA volume | ||
| Normal | 1.000 | |
| Overweight | 0.71 (0.45–1.12) | 0.1413 |
| Obese | 1.15 (0.70–1.88) | 0.5915 |
| Severely obese | 1.59 (0.89–2.83) | 0.1152 |
| Adjusted for age, sex, other clinical variablesa, interim MI/CHF and LA volume | ||
| Normal | 1.000 | |
| Overweight | 0.68 (0.42–1.08) | 0.0985 |
| Obese | 1.04 (0.64–1.71) | 0.8679 |
| Severely obese | 1.39 (0.78–2.49) | 0.2659 |
aClinical variables adjusted for include history of hypertension, valvular heart disease, myocardial infarction, congestive heart failure, smoking, chronic pulmonary disease, systolic and diastolic blood pressures at baseline AF diagnosis (see section Methods for details). History of CHF, valvular heart disease, hypertension, and diastolic blood pressure remained significant as predictors in all the above multivariable models adjusting for clinical covariates.
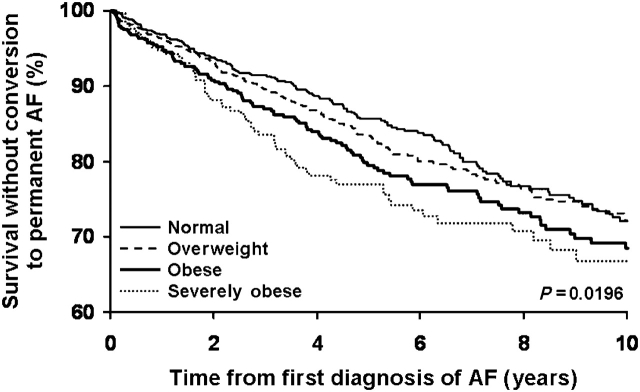
In a multivariable model, BMI ≥30 kg/m2 was associated with a >1.5 fold increase in risk of converting to permanent AF (Table 2). As a continuous variable, BMI (per kg/m2), was associated with progression to permanent AF [HR 1.03 (1.01–1.04), P < 0.0001], adjusting age, sex, and multiple clinical risk factors. Neither the use of beta-blockers nor calcium channel blockers nor antiarrhythmic drugs was associated with status of permanent AF at follow-up. Kaplan–Meier analyses showed that the progression to permanent AF progressively increased with time for all groups, with the gradient of risk predicted by BMI categories (Figure 2).
Figure 2.
Survival without conversion to permanent atrial fibrillation.
In the supplemental analysis without exclusion of the underweight group, the hazard for risk of progression for BMI <18.5 kg/m2, relative to normal BMI, was 0.96 (P = 0.68) when age- and sex-adjusted. When further adjusted for other covariates, the risk of progression remained non-significant.
Effects of left artrial size and obesity on the progression to permanent atrial fibrillation
Among those who had an echocardiogram performed within 30 days of the diagnosis of paroxysmal AF (n = 744), baseline characteristics are detailed in Table 1. The significance of BMI as a predictor of progression did not appear attenuated when adjusted for LA size, measured as LA volume, indexed LA volume, or M-mode LA dimension.
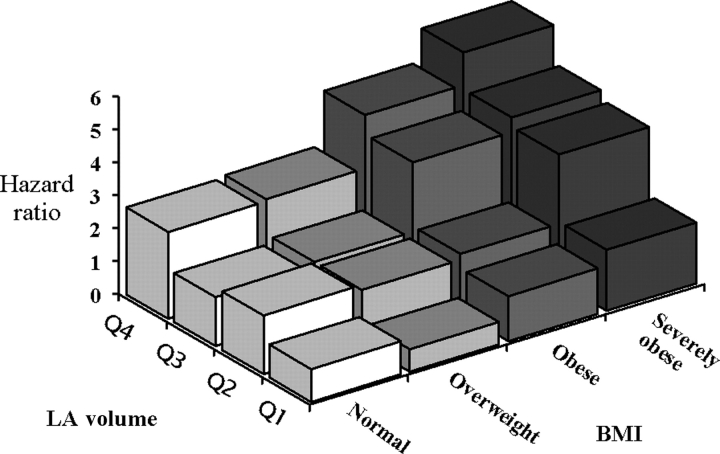
LA size was independently predictive of progression to permanent AF. LA size remained independently predictive of progression to permanent AF, after adjusting for age, sex, BMI, other baseline clinical factors, and interim myocardial infarction and congestive heart failure. When adjusted for all these factors in a multivariable model, LA volume remained highly predictive of progression to permanent AF (HR 1.04 (1.02–1.05; P < 0.0001). The level of significance was similar whether LA size was measured as LA volume or M-mode LA dimension. Age- and sex-adjusted risk of progression increased with higher BMI and larger LA volume. The risk was greatest for those with both BMI and LA volume in the highest tertile (Figure 3). We also assessed for the possibility of a joint effect of LA size and obesity by adding a multiplicative interaction term to the model with indexed LA volume and BMI as continuous variables, and no significant interaction was evident.
Figure 3.
Age- and sex-adjusted hazards of progression to permanent atrial fibrillation stratified by body mass index categories and LA volume quartiles. LA, left atrial.
Discussion
This study represents an extension of prior studies showing a relationship between obesity and AF. Unlike previous studies demonstrating an association between obesity and first AF, we report the new finding of obesity being a risk factor for progression of paroxysmal to permanent AF. The data also suggested a dose–response relationship between BMI and progression. When age- and sex-adjusted, or adjusted for other factors including interim myocardial infarction and congestive heart failure, obesity remained associated with a >1.5 fold increase in risk of progression. This level of hazard was similar to that observed for the relationship between obesity and development of first AF.15
LA enlargement was proposed as the mediating factor for obesity being a risk factor for incident AF in the Framingham Study.15 In our study, obesity remained significantly associated with progression to permanent AF, after adjusting for LA size. Neither LA volume nor M-mode LA dimension significantly attenuated the hazard of progression associated with BMI. In our study, the presence of a larger sized left atrium augmented the independent risk of obesity for the progression to permanent AF.
After accounting for LA size, the residual, minimally attenuated risk of obesity may reflect unmeasured effects frequently associated with obesity which could have an impact on LA structural and electrical remodelling. For instance, obesity is associated with systemic inflammation, as is evident by the increased levels of inflammatory markers, such as C-reactive protein, and interleukins in obese persons.22–25 Inflammation has in fact been touted as a causative factor for AF development and recurrence.26–29 Obesity is also associated with changes in neurohormonal activation,30–33 which in turn affect sodium and fluid homeostasis, culminating in increased LV diastolic filling pressures. Larger BMI is associated with increased arterial stiffness34 which correlates with levels of free oxygen radicals35 that may perpetuate atrial arrhythmia.36 Finally, obesity is a well-known risk factor for obstructive sleep apnoea, which has been increasingly linked to incident AF and its recurrence.37 We were not able to precisely delineate the contribution from these various factors to the excess risk of progression associated with obesity.
The retrospective nature of the study was a limitation in terms of classification of AF status. However, we took precautions to assure that the adjudication of the AF type followed the criteria as defined by the most recent consensus guideline.18 Despite BMI was found to be an independent risk factor for progression to permanent AF in this study, we did not have data regarding the modifying effects of diet, physical activity, or interim weight changes. Data from the subgroup analyses of patients who underwent a clinically indicated echocardiogram within 30 days of AF diagnosis cannot be extrapolated to AF patients in general. The lack of ethnic diversity is typical of studies confined to Olmsted County, MN, residents; and whether the interaction of BMI, LA size, and AF progression occurs in the same direction and magnitude in other racial ethnic groups could not be determined.
Conclusion
This study extends our understanding of the relationship between obesity/LA size and AF. Specifically, there was a graded risk relationship between BMI and progression from paroxysmal to permanent AF, and larger LA size augmented the risk of such progression. BMI and LA volume were independent predictors of progression to permanent AF. Studies of weight reduction, and of reversal of LA enlargement, may provide insight as to whether these interventions may lower the risk for the development of first AF, and reduce or delay the progression from paroxysmal to permanent AF.
Conflict of Interest: none declared.
Funding
Supported by funding from the American Heart Association, the American Society of Echocardiography, and National Institutes of Health.
References
- 1.Mokdad AH, Serdula MK, Dietz WH, Bowman BA, Marks JS, Koplan JP. The continuing epidemic of obesity in the United States. JAMA. 2000;284:1650–1651. doi: 10.1001/jama.284.13.1650. [DOI] [PubMed] [Google Scholar]
- 2.Braunwald E. Shattuck lecture—cardiovascular medicine at the turn of the millennium: triumphs, concerns, and opportunities. N Engl J Med. 1997;337:1360–1369. doi: 10.1056/NEJM199711063371906. [DOI] [PubMed] [Google Scholar]
- 3.Ford ES, Mokdad AH, Giles WH. Trends in waist circumference among U.S. adults. Obes Res. 2003;11:1223–1231. doi: 10.1038/oby.2003.168. [DOI] [PubMed] [Google Scholar]
- 4.Obesity Trends: 1991–2001 Prevalence of Obesity Among U.S Adults, by Characteristics. Behavioral Risk Factor Surveillance System (1991–2001) Atlanta: Centers for Disease Control and Prevention; 2003. [Google Scholar]
- 5.Flegal KM, Carroll MD, Ogden CL, Johnson CL. Prevalence and trends in obesity among US adults, 1999–2000. JAMA. 2002;288:1723–1727. doi: 10.1001/jama.288.14.1723. [DOI] [PubMed] [Google Scholar]
- 6.Mokdad AH, Bowman BA, Ford ES, Vinicor F, Marks JS, Koplan JP. The continuing epidemics of obesity and diabetes in the United States. JAMA. 2001;286:1195–1200. doi: 10.1001/jama.286.10.1195. [DOI] [PubMed] [Google Scholar]
- 7.Wolf PA, Benjamin EJ, Belanger AJ, Kannel WB, Levy D, D’Agostino RB. Secular trends in the prevalence of atrial fibrillation: The Framingham Study. Am Heart J. 1996;131:790–795. doi: 10.1016/s0002-8703(96)90288-4. [DOI] [PubMed] [Google Scholar]
- 8.Tsang TS, Petty GW, Barnes ME, O’Fallon WM, Bailey KR, Wiebers DO, Sicks JD, Christianson TJ, Seward JB, Gersh BJ. The prevalence of atrial fibrillation in incident stroke cases and matched population controls in Rochester, Minnesota: changes over three decades. J Am Coll Cardiol. 2003;42:93–100. doi: 10.1016/s0735-1097(03)00500-x. [DOI] [PubMed] [Google Scholar]
- 9.Mokdad AH, Ford ES, Bowman BA, Dietz WH, Vinicor F, Bales VS, Marks JS. Prevalence of obesity, diabetes, and obesity-related health risk factors, 2001. JAMA. 2003;289:76–79. doi: 10.1001/jama.289.1.76. [DOI] [PubMed] [Google Scholar]
- 10.Krahn AD, Manfreda J, Tate RB, Mathewson FA, Cuddy TE. The natural history of atrial fibrillation: incidence, risk factors, and prognosis in the Manitoba follow-up study. Am J Med. 1995;98:476–484. doi: 10.1016/S0002-9343(99)80348-9. [DOI] [PubMed] [Google Scholar]
- 11.Stewart S, Hart CL, Hole DJ, McMurray JJ. Population prevalence, incidence, and predictors of atrial fibrillation in the Renfrew/Paisley study. Heart. 2001;86:516–521. doi: 10.1136/heart.86.5.516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wilhelmsen L, Rosengren A, Lappas G. Hospitalizations for atrial fibrillation in the general male population: morbidity and risk factors. J Intern Med. 2001;250:382–389. doi: 10.1046/j.1365-2796.2001.00902.x. [DOI] [PubMed] [Google Scholar]
- 13.Benjamin EJ, Levy D, Vaziri SM, D’Agostino RB, Belanger AJ, Wolf PA. Independent risk factors for atrial fibrillation in a population-based cohort. The Framingham Heart Study. JAMA. 1994;271:840–844. [PubMed] [Google Scholar]
- 14.Psaty BM, Manolio TA, Kuller LH, Kronmal RA, Cushman M, Fried LP, White R, Furberg CD, Rautaharju PM. Incidence of and risk factors for atrial fibrillation in older adults. Circulation. 1997;96:2455–2461. doi: 10.1161/01.cir.96.7.2455. [DOI] [PubMed] [Google Scholar]
- 15.Wang TJ, Parise H, Levy D, D’Agostino RB, Sr, Wolf PA, Vasan RS, Benjamin EJ. Obesity and the risk of new-onset atrial fibrillation. JAMA. 2004;292:2471–2477. doi: 10.1001/jama.292.20.2471. [DOI] [PubMed] [Google Scholar]
- 16.Melton LJ., 3rd History of the Rochester Epidemiology Project. Mayo Clin Proc. 1996;71:266–274. doi: 10.4065/71.3.266. [DOI] [PubMed] [Google Scholar]
- 17.World Health Organization Expert Committee. Physical status, the use and interpretation of anthropometry. 1995. WHO Tech Rep Ser. 1995:854. [PubMed] [Google Scholar]
- 18.Fuster V, Ryden LE, Asinger RW, Cannom DS, Crijns HJ, Frye RL, et al. ACC/AHA/ESC guidelines for the management of patients with atrial fibrillation: executive summary. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines and Policy Conferences (Committee to Develop Guidelines for the Management of Patients with Atrial Fibrillation) developed in collaboration with the North American Society of Pacing and Electrophysiology. J Am Coll Cardiol. 2001;38:1231–1265. doi: 10.1016/s0735-1097(01)01587-x. [DOI] [PubMed] [Google Scholar]
- 19.McKee PA, Castelli WP, McNamara PM, Kannel WB. The natural history of congestive heart failure: The Framingham study. N Engl J Med. 1971;285:1441–1446. doi: 10.1056/NEJM197112232852601. [DOI] [PubMed] [Google Scholar]
- 20.Schiller NB, Shah PM, Crawford M, DeMaria A, Devereux RB, Feigenbaum H, Gutgesell H, Reichek N, Sahn D, Schnittger I, Silverman NH, Tajik AJ. Recommendations for quantitation of the left ventricle by two-dimensional echocardiography. American Society of Echocardiography Committee on Standards, Subcommittee on Quantitation of Two-Dimensional Echocardiograms. J Am Soc Echocardiogr. 1989;2:358–367. doi: 10.1016/s0894-7317(89)80014-8. [DOI] [PubMed] [Google Scholar]
- 21.Schabelman S, Schiller NB, Silverman NH, Ports TA. Left atrial volume estimation by two-dimensional echocardiography. Cathet Cardiovasc Diagn. 1981;7:165–178. doi: 10.1002/ccd.1810070206. [DOI] [PubMed] [Google Scholar]
- 22.Warnberg J, Nova E, Moreno LA, Romeo J, Mesana MI, Ruiz JR, Ortega FB, Sjostrom M, Bueno M, Marcos A. Inflammatory proteins are related to total and abdominal adiposity in a healthy adolescent population: the AVENA Study. Am J Clin Nutr. 2006;84:505–512. doi: 10.1093/ajcn/84.3.505. [DOI] [PubMed] [Google Scholar]
- 23.Juge-Aubry CE, Henrichot E, Meier CA. Adipose tissue: a regulator of inflammation. Best Pract Res Clin Endocrinol Metab. 2005;19:547–566. doi: 10.1016/j.beem.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 24.Juge-Aubry CE, Somm E, Giusti V, Pernin A, Chicheportiche R, Verdumo C, Rohner-Jeanrenaud F, Burger D, Dayer JM, Meier CA. Adipose tissue is a major source of interleukin-1 receptor antagonist: upregulation in obesity and inflammation. Diabetes. 2003;52:1104–1110. doi: 10.2337/diabetes.52.5.1104. [DOI] [PubMed] [Google Scholar]
- 25.Kahn SE, Zinman B, Haffner SM, O’Neill MC, Kravitz BG, Yu D, Freed MI, Herman WH, Holman RR, Jones NP, Lachin JM, Viberti GC. Obesity is a major determinant of the association of C-reactive protein levels and the metabolic syndrome in type 2 diabetes. Diabetes. 2006;55:2357–2364. doi: 10.2337/db06-0116. [DOI] [PubMed] [Google Scholar]
- 26.Aviles RJ, Martin DO, Apperson-Hansen C, Houghtaling PL, Rautaharju P, Kronmal RA, Tracy RP, Van Wagoner DR, Psaty BM, Lauer MS, Chung MK. Inflammation as a risk factor for atrial fibrillation. Circulation. 2003;108:3006–3010. doi: 10.1161/01.CIR.0000103131.70301.4F. [DOI] [PubMed] [Google Scholar]
- 27.Dernellis J, Panaretou M. Effect of C-reactive protein reduction on paroxysmal atrial fibrillation. Am Heart J. 2005;150:1064. doi: 10.1016/j.ahj.2005.06.032. [DOI] [PubMed] [Google Scholar]
- 28.Dernellis J, Panaretou M. C-reactive protein and paroxysmal atrial fibrillation: evidence of the implication of an inflammatory process in paroxysmal atrial fibrillation. Acta Cardiol. 2001;56:375–380. doi: 10.2143/AC.56.6.2005701. [DOI] [PubMed] [Google Scholar]
- 29.Chung M, Martin D, Sprecher D, Wazni O, Kanderian A, Carnes C, Bauer J, Tchou P, Niebauer M, Natale A, Van Wagoner D. C-reactive protein elevation in patients with atrial arrhythmias: inflammatory mechanisms and persistence of atrial fibrillation. Circulation. 2001;104:2886–2891. doi: 10.1161/hc4901.101760. [DOI] [PubMed] [Google Scholar]
- 30.Wang TJ, Larson MG, Levy D, Benjamin EJ, Leip EP, Wilson PW, Vasan RS. Impact of obesity on plasma natriuretic peptide levels. Circulation. 2004;109:594–600. doi: 10.1161/01.CIR.0000112582.16683.EA. [DOI] [PubMed] [Google Scholar]
- 31.Dessi-Fulgheri P, Sarzani R, Rappelli A. The natriuretic peptide system in obesity-related hypertension: new pathophysiological aspects. J Nephrol. 1998;11:296–299. [PubMed] [Google Scholar]
- 32.Dessi-Fulgheri P, Sarzani R, Tamburrini P, Moraca A, Espinosa E, Cola G, Giantomassi L, Rappelli A. Plasma atrial natriuretic peptide and natriuretic peptide receptor gene expression in adipose tissue of normotensive and hypertensive obese patients. J Hypertens. 1997;15:1695–1699. doi: 10.1097/00004872-199715120-00074. [DOI] [PubMed] [Google Scholar]
- 33.Baranowska B, Wasilewska-Dziubinska E, Radzikowska M, Plonowski A, Roguski K. Impaired response of atrial natriuretic peptide to acute water load in obesity and in anorexia nervosa. Eur J Endocrinol. 1995;132:147–151. doi: 10.1530/eje.0.1320147. [DOI] [PubMed] [Google Scholar]
- 34.Wildman RP, Farhat GN, Patel AS, Mackey RH, Brockwell S, Thompson T, Sutton-Tyrrell K. Weight change is associated with change in arterial stiffness among healthy young adults. Hypertension. 2005;45:187–192. doi: 10.1161/01.HYP.0000152200.10578.5d. [DOI] [PubMed] [Google Scholar]
- 35.Wykretowicz A, Guzik P, Kasinowski R, Krauze T, Bartkowiak G, Dziarmaga M, Wysocki H. Augmentation index, pulse pressure amplification and superoxide anion production in patients with coronary artery disease. Int J Cardiol. 2005;99:289–294. doi: 10.1016/j.ijcard.2004.01.040. [DOI] [PubMed] [Google Scholar]
- 36.Dudley SC, Jr, Hoch NE, McCann LA, Honeycutt C, Diamandopoulos L, Fukai T, Harrison DG, Dikalov SI, Langberg J. Atrial fibrillation increases production of superoxide by the left atrium and left atrial appendage: role of the NADPH and xanthine oxidases. Circulation. 2005;112:1266–1273. doi: 10.1161/CIRCULATIONAHA.105.538108. [DOI] [PubMed] [Google Scholar]
- 37.Gami AS, Hodge DO, Herges RM, Olson EJ, Nykodym J, Kara T, Somers VK. Obstructive sleep apnea, obesity, and the risk of incident atrial fibrillation. J Am Coll Cardiol. 2007;49:565–571. doi: 10.1016/j.jacc.2006.08.060. [DOI] [PubMed] [Google Scholar]