Abstract
Dimeric galectin-1 (dGal-1) is a homodimeric lectin with multiple proposed functions. Although dGal-1 binds to diverse glycans, it is unclear whether dGal-1 preferentially binds to specific subsets of glycans on cell surfaces to transmit signals. To explore this question, we selectively inhibited major glycan biosynthetic pathways in human HL60, Molt-4, and Jurkat cells. Inhibition of N-glycan processing blocked surface binding of dGal-1 and prevented dGal-1-induced Ca2+ mobilization and phosphatidylserine exposure. By contrast, inhibition of O-glycan or glycosphingolipid biosynthesis did not affect dGal-1 binding or dGal-1-induced Ca2+ mobilization and phosphatidylserine exposure. These results demonstrate that dGal-1 preferentially binds to and signals through glycoproteins containing complex-type N-glycans in at least some leukocyte subsets.
Keywords: galectin, inflammation, leukocytes, N-glycans, signaling
Introduction
Galectins constitute a family of soluble β-galactoside-binding lectins that are expressed by all metazoans (Barondes, Castronovo, et al. 1994; Barondes, Cooper, et al. 1994; Cooper and Barondes 1999; Leffler et al. 2004). Dimeric galectin-1 (dGal-1) is a homodimer consisting of two ∼14.5 kDa subunits that are noncovalently associated (Cho and Cummings 1995, 1996; Giudicelli et al. 1997). dGal-1 functions in cell adhesion (Moiseeva et al. 2003; He and Baum 2004; Martinez et al. 2004), development (Colnot et al. 1996), inflammation (Rubinstein et al. 2004), leukocyte apoptosis (Perillo et al. 1998; Rubinstein et al. 2004), neutrophil turnover (Dias-Baruffi et al. 2003; Stowell et al. 2007; Stowell, Qian, et al. 2008), cancer (Rubinstein et al. 2004; van den Brule et al. 2004), and immunity (Rabinovich et al. 2007; Toscano et al. 2007; Salatino et al. 2008). Like other galectins, dGal-1 weakly recognizes lactose (Galβ1-4Glc) and N-acetyllactosamine disaccharides such as the type 2 sequence Galβ1-4GlcNAcβ-R (LN) and the type 1 sequence Galβ1-3GlcNAcβ-R (Leffler and Barondes, 1986). However, dGal-1 binds with higher affinity to long-chain type 2 sequences (-3Galβ1-4GlcNAcβ1-)n called poly-N-acetyllactosamines (PLs) (Stowell et al. 2004; Stowell, Arthur, et al. 2008; Leppanen et al. 2005).
dGal-1 reportedly binds to both N- and O-glycans on various cell surface glycoproteins as well as to glycosphingolipids and the ganglioside GM1 (Ohannesian et al. 1995; Perillo et al. 1995; Kopitz et al. 1998; Pace et al. 1999; Nguyen et al. 2001; Gauthier et al. 2002; Carlow et al. 2003; Lanteri et al. 2003; Andre et al. 2005; Elola et al. 2005; Ideo et al. 2005; Suzuki et al. 2005b; Walzel et al. 2006). These apparently conflicting results raise questions as to the nature of the glycans required for dGal-1 signaling in cells.
dGal-1 induces Ca2+ mobilization in and phosphatidylserine (PS) exposure on activated human neutrophils and promyelocytic HL60 cells; however, these responses are not accompanied by apoptosis (Dias-Baruffi et al. 2003; Stowell et al. 2007; Stowell, Qian, et al. 2008). The galectin signaling to expose surface PS in the absence of cell death has been termed preaparesis (Stowell, Qian, et al. 2008). These results prompted us to further explore the glycans required for dGal-1-induced preaparesis and Ca2+ mobilization in leukocytes. We used human HL60 cells, leukemic MOLT-4 T cells, and leukemic Jurkat T cells, which have been extensively used as models for dGal-1 signaling. Cell lines are particularly useful because they permit the use of selective inhibitors of glycosylation to remodel the cellular glycome. We examined the effects of defined, commonly used inhibitors of N- and O-glycan and glycosphingolipid biosynthesis and/or elongation on dGal-1 binding and on the ability of dGal-1 to induce Ca2+ mobilization and preaparesis.
Results
dGal-1 binds to complex-type N-glycans on HL60 cells
dGal-1 binds to and signals through glycans containing PL sequences on HL60 cells (Leppanen et al. 2005; Stowell, Qian, et al. 2008). Such sequences might be expressed on N- or O-glycans or on glycosphingolipids. To explore the types of PL-containing glycans required for dGal-1 signaling, we treated HL60 cells with inhibitors of glycosylation and examined the effects of the inhibitors on binding and signaling by dGal-1.
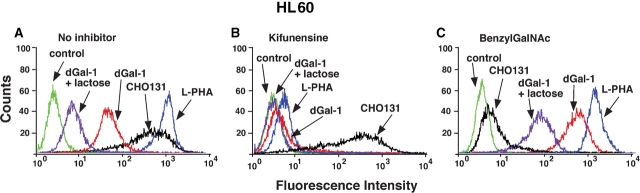
dGal-1 bound well to HL60 cells (Figure 1A). The addition of 20 mM lactose, a weak inhibitor of dGal-1, reduced dGal-1 binding, confirming that binding was to cell surface glycans. We used plant lectins and specific antibodies to explore the effects of biosynthetic inhibitors on glycan structure. Treating cells with kifunensine, an inhibitor of α-mannosidase I, prevents trimming and processing of high mannose-type N-glycans, and thus, blocks the formation of complex-type N-glycans (Elbein et al. 1990). The effects of kifunensine were monitored by reduced binding of the plant lectin, Phaseolus vulgaris leukoagglutinin (L-PHA), which recognizes tri- and tetraantennary complex N-glycans containing outer galactose residues and an α-linked mannose residue substituted at the C-2 and C-6 positions (Cummings and Kornfeld 1982a, 1982b). Treating cells with benzyl-2-acetamido-2-deoxy-α-d-galactopyranoside (benzylGalNAc) affects O-glycan biosynthesis by competing with enzymes that elaborate complex-type O-glycans (Kuan et al. 1989; Huet et al. 1998; Gouyer et al. 2001). The effects of benzylGalNAc were monitored by reduced binding of mAb CHO-131, which recognizes fucosylated and sialylated complex O-glycans on a core 2 backbone (Walcheck et al. 2002).
Fig. 1.
dGal-1 binding to HL60 cells requires expression of complex-type N-glycans. HL60 cells were incubated with dGal-1 in the presence or absence of lactose to measure glycan-specific binding to surface ligands, with L-PHA to measure complex N-glycans, or with mAb CHO-131 to measure complex O-glycans. Binding assays were performed on control cells that were not incubated with a glycosylation inhibitor (A), on cells preincubated with kifunensine for 7 days to inhibit complex-type N-glycan synthesis (B), or with cells preincubated with benzylGalNAc for 3 days to inhibit complex-type O-glycan synthesis (C). The data are representative of three separate experiments.
Both L-PHA and CHO-131 bound to HL60 cells (Figure 1A). Kifunensine treatment of HL60 cells reduced binding of L-PHA but did not affect binding of CHO-131 (Figure 1B). These results demonstrate that kifunensine treatment shifted the distribution of N-glycans from complex, fucosylated, and sialylated structures, known to be expressed in HL60 cells, to high mannose-type structures, but did not detectably alter expression of complex O-glycans identified by mAb CHO-131. Importantly, much less dGal-1 bound to HL60 cells treated with kifunensine, indicating that dGal-1 receptors contain complex-type N-glycans.
BenzylGalNAc treatment of HL60 cells reduced binding of CHO-131 but did not affect binding of L-PHA, indicating that the inhibitor blocked expression of at least some complex O-glycans but did not detectably affect expression of complex N-glycans (Figure 1C). Somewhat more dGal-1 bound to HL60 cells treated with benzylGalNAc than to untreated cells (Figure 1C), which could be due to enhanced access of dGal-1 to cell surface N-glycans in the absence of O-glycans. These results demonstrate that the receptors for dGal-1 on HL60 cells require expression of complex N-glycans.
dGal-1-induced exposure of surface phosphatidylserine on HL60 cells requires expression of complex-type N-glycans
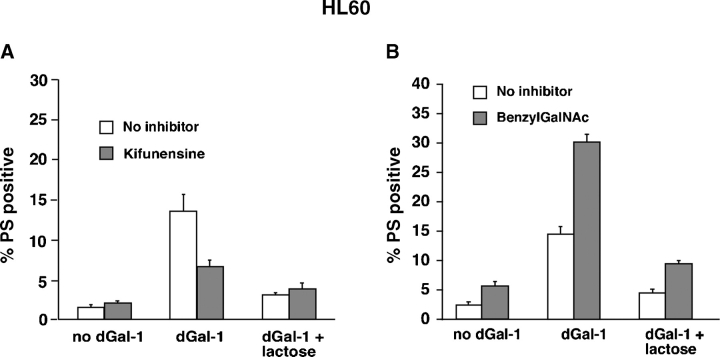
Binding of dGal-1 to HL60 cells and activated human neutrophils signals exposure of PS on their surfaces, without accompanying apoptosis (Dias-Baruffi et al. 2003; Stowell et al. 2007). Compared to nontreated cells, kifunensine-treated HL60 cells exposed much less PS after incubation with dGal-1 for 4 h (Figure 2A), whereas benzylGalNAc-treated HL60 cells exposed more PS after incubation with dGal-1 (Figure 2B). The inclusion of lactose with dGal-1 decreased PS exposure to control levels, indicating the specificity of dGal-1-induced PS mobilization.
Fig. 2.
dGal-1-induced exposure of PS on HL60 cells requires expression of complex-type N-glycans. HL60 cells were treated with 10 μM dGal-1 for 4 h, disengaged with lactose, stained with FITC-annexin V and PI, and then analyzed by flow cytometry. The indicated cells were preincubated with kifunensine for 7 days (A) or with benzylGalNAc for 3 days (B). The results are depicted as the percentage of cells that stained with annexin V above a threshold level, but that remained viable as assessed by staining with PI below a threshold level. The data represents the mean ± SD of three experiments.
dGal-1-induced Ca2+ mobilization in HL60 cells requires expression of complex-type N-glycans
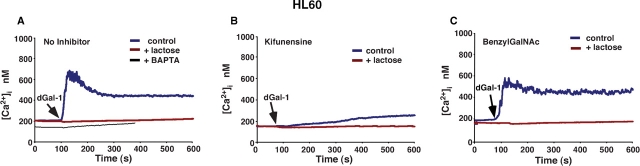
Binding of dGal-1 to neutrophils induces a Ca2+ flux (Karmakar et al. 2005). To determine whether dGal-1 similarly mobilized Ca2+ in HL60 cells, Fluo-4-loaded HL60 cells were incubated with dGal-1. The addition of dGal-1 elicited an immediate rapid rise in cytoplasmic Ca2+, which triggered an influx of extracellular Ca2+ that maintained Ca2+ above basal levels for several minutes (Figure 3A). dGal-1 agglutinated the HL60 cells, as manifested by small oscillations in the fluorimeter tracing. Inclusion of 20 mM lactose reversed dGal-1-induced agglutination and prevented the Ca2+ flux, indicating that signaling required binding of dGal-1 to cell surface glycoconjugates. Preloading HL60 cells with the intracellular Ca2+ chelator BAPTA-AM (1,2-bis(o-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid/acetoxymethyl ester) abrogated the sustained Ca2+ flux (Figure 3A), indicating that dGal-1 first released Ca2+ from intracellular stores, which triggered the extracellular Ca2+ influx.
Fig. 3.
dGal-1-induced elevation of cytosolic Ca2+ in HL60 cells requires expression of complex-type N-glycans. Cytosolic Ca2+ levels in stirred Fluo-4-labeled HL60 cells were continuously measured in a fluorimeter. (A) Control or BAPTA-loaded HL60 cells in a Ca2+-containing buffer were incubated with 10 μM dGal-1 in the presence or absence of 20 mM lactose. (B) Kifunensine-treated HL60 cells were incubated with 10 μM dGal-1 in the presence or absence of 20 mM lactose. (C) BenzylGalNAc-treated HL60 cells were incubated with 10 μM dGal-1 in the presence or absence of 20 mM lactose. The data are representative of three independent experiments.
The addition of dGal-1 to kifunensine-treated HL60 cells elicited much smaller Ca2+ fluxes than those in untreated cells (Figure 3B), indicating that the major Ca2+ signaling receptors for dGal-1 required expression of complex-type N-glycans. Thus, the loss of Ca2+ mobilization correlated strongly with the loss of surface binding of dGal-1 and abrogation of dGal-1-induced PS exposure on kifunensine-treated HL60 cells. In contrast, the addition of dGal-1 to benzylGalNAc-treated HL60 cells triggered Ca2+ fluxes similar to those in untreated cells (Figure 3C).
dGal-1 binding and signaling in Molt-4 and Jurkat cells also require expression of complex-type N-glycans
The above results show that binding and signaling through dGal-1 require expression of complex-type N-glycans on HL60 cells. To explore the generality of cellular responses to dGal-1, we examined two lymphocytic cell lines, Molt-4 and Jurkat, both of which are known to respond to dGal-1 (Walzel et al. 1996, 2006; Dias-Baruffi et al. 2003; Hahn et al. 2004; van der Leij et al. 2007). Notably, Jurkat cells lack even simple core 1 O-glycans (Piller et al. 1990) because of a mutation in the chaperone Cosmc (Ju and Cummings 2002), which is required for correct folding and expression of core 1 β1-3-galactosyltransferase (T-synthase). As observed for HL60 cells, kifunensine treatment of Molt-4 and Jurkat cells markedly decreased binding of L-PHA and dGal-1 (data not shown). CHO-131 did not bind to Molt-4 or Jurkat cells, indicating that these cells lacked complex sialylated and fucosylated O-glycans recognized by this antibody (data not shown). The plant lectin peanut agglutinin (PNA), which recognizes the core 1 O-glycan disaccharide (Galβ1-3GalNAcα-Ser/Thr) (Lotan et al. 1975; Novogrodsky et al. 1975), bound well to both Molt-4 and Jurkat cells and also bound to benzylGalNAc-1-treated cells (data not shown). This illustrates the lack of specificity of PNA for core 1 Galβ1-3GalNAcα-Ser/Thr, which is not present on Jurkat cells. The lack of specificity of PNA for the core 1 O-glycan is further documented by binding of PNA to a variety of galactose-containing glycans on microarrays, as summarized on the website of the Consortium for Functional Glycomics (http://www.functionalglycomics.org/glycomics/).
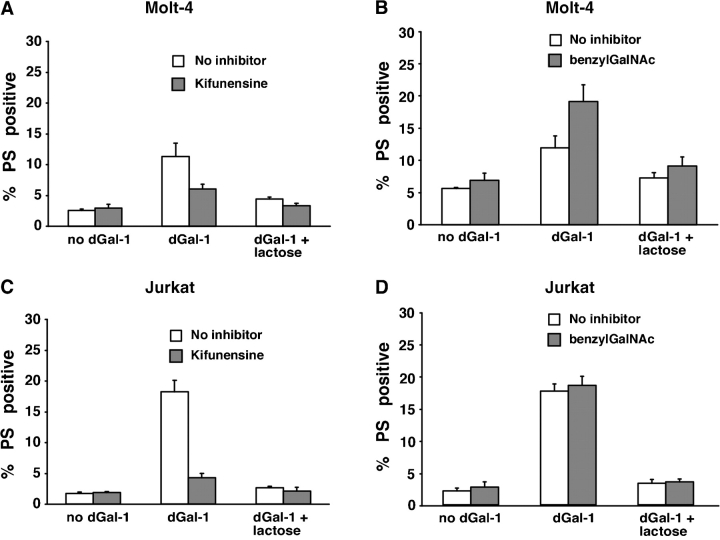
Kifunensine treatment of Molt-4 cells decreased PS exposure after incubation with dGal-1 (Figure 4A), whereas benzylGalNAc did not inhibit PS exposure (Figure 4B). Similarly, kifunensine treatment of Jurkat cells decreased PS exposure after incubation with dGal-1 (Figure 4C), whereas benzylGalNAc did not inhibit PS exposure (Figure 4D).
Fig. 4.
dGal-1-induced exposure of PS on Molt-4 and Jurkat cells requires expression of complex-type N-glycans. Molt-4 cells (A, B) and Jurkat cells (C, D) were treated with 10 μM dGal-1 for 4 h, disengaged with lactose, incubated with a mixture of FITC-conjugated annexin V and PI, and analyzed by flow cytometry. The indicated cells were preincubated with kifunensine for 3 days or with benzylGalNAc for 3 days. The data are depicted as the percentage of cells that stained with annexin V above a threshold level, but that remained viable as assessed by staining with PI below a threshold level. The data represent the mean ± SD of three experiments.
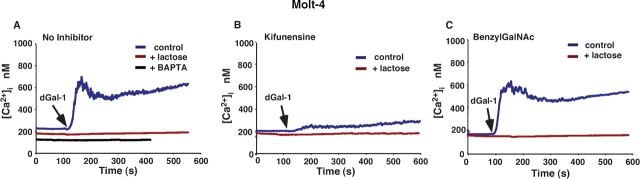
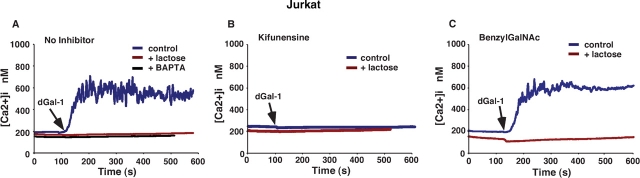
The addition of dGal-1 to Fluo-4-loaded Molt-4 cells (Figure 5A) and to Jurkat cells (Figure 6A) caused an immediate rapid rise in cytoplasmic Ca2+, which triggered an influx of extracellular Ca2+ that maintained Ca2+ above basal levels for several minutes. Inclusion of 20 mM lactose reversed dGal-1-induced agglutination and prevented the Ca2+ flux, indicating that signaling required binding of dGal-1 to cell surface glycoconjugates. Preloading cells with the intracellular Ca2+ chelator BAPTA-AM abrogated the sustained Ca2+ flux (Figure 5A and Figure 5B), indicating that dGal-1 first released Ca2+ from intracellular stores, which triggered the extracellular Ca2+ influx. The addition of dGal-1 to kifunensine-treated cells elicited much smaller weak Ca2+ fluxes than those in untreated cells (Figure 5B and Figure 6B), indicating that the major Ca2+ signaling receptors for dGal-1 required expression of complex-type N-glycans. Thus, the loss of Ca2+ mobilization correlated strongly with the loss of surface binding of dGal-1 and abrogation of dGal-1-induced PS exposure on kifunensine-treated Molt-4 and Jurkat cells. In contrast, the addition of dGal-1 to benzylGalNAc-treated cells elicited Ca2+ fluxes similar to those in untreated cells (Figure 5C and Figure 6C).
Fig. 5.
dGal-1-induced elevation of cytosolic Ca2+ in Molt-4 cells requires expression of complex-type N-glycans. Cytosolic Ca2+ levels in stirred Fluo-4-labeled Molt-4 cells were continuously measured in a fluorimeter. (A) Control or BAPTA-loaded Molt-4 cells in a Ca2+-containing buffer were incubated with 10 μM dGal-1 in the presence or absence of 20 mM lactose. (B) Kifunensine-treated Molt-4 cells were incubated with 10 μM dGal-1 in the presence or absence of 20 mM lactose. (C) BenzylGalNAc-treated Molt-4 cells were incubated with 10 μM dGal-1 in the presence or absence of 20 mM lactose. The data are representative of three independent experiments.
Fig. 6.
dGal-1-induced elevation of cytosolic Ca2+ in Jurkat cells requires expression of complex-type N-glycans. Cytosolic Ca2+ levels in stirred Fluo-4-labeled Jurkat cells were continuously measured in a fluorimeter. (A) Control or BAPTA-loaded Jurkat cells in a Ca2+-containing buffer were incubated with 10 μM dGal-1 in the presence or absence of 20 mM lactose. (B) Kifunensine-treated Jurkat cells were incubated with 10 μM dGal-1 in the presence or absence of 20 mM lactose. (C) BenzylGalNAc-treated Jurkat cells were incubated with 10 μM dGal-1 in the presence or absence of 20 mM lactose. The data are representative of three independent experiments.
Taken together, the data with Molt-4 and Jurkat cells closely parallel those obtained with HL60 cells. For all three cell lines, the kifunensine-mediated loss of complex N-glycans abrogated binding of dGal-1 and prevented dGal-1-mediated Ca2+ mobilization and exposure of PS.
dGal-1 binding to and signaling in HL60 cells do not require expression of glycosphingolipids
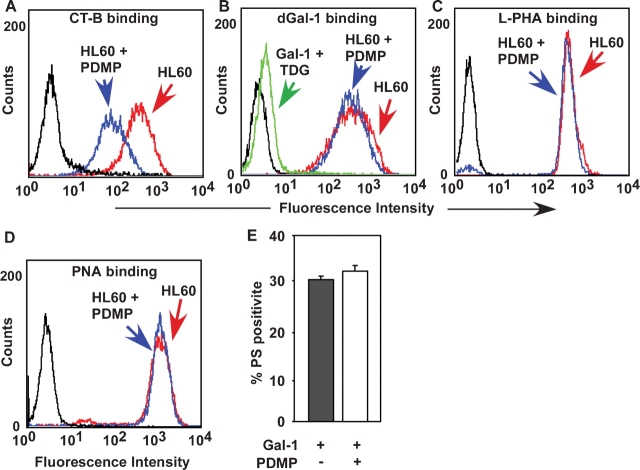
Since it has been reported that dGal-1 binds to the ganglioside GM1 (Kopitz et al. 1998; Siebert et al. 2005), we explored whether glycosphingolipids on HL60 cells contribute to dGal-1 binding and signaling. To reduce expression of glycosphingolipids, we used the inhibitor 1-phenyl-2-decanoylamino-3-morpholino-1-propanol (PDMP), which blocks the addition of glucose to ceramide in the first step in glycosphingolipid synthesis (Rosenwald et al. 1992; Radin et al. 1993). PDMP treatment inhibited glycosphingolipid synthesis in HL60 cells, as documented by reducing the staining of cells with cholera toxin B subunit, which binds specifically to the ganglioside GM1 (Van Heyningen et al. 1976; Spangler 1992) (Figure 7A). However, PDMP treatment did not reduce binding of dGal-1 (Figure 7B), L-PHA (Figure 7C), or PNA (Figure 7D). These results demonstrate that PDMP treatment reduced GM1 on the cell surface but did not affect expression of N- or O-glycans and dGal-1 receptors. Furthermore, PDMP treatment did not affect exposure of PS after incubation with dGal-1 (Figure 7E). These results demonstrate that inhibition of glycosphingolipid expression on HL60 cells does not significantly affect either dGal-1 binding or signaling.
Fig. 7.
dGal-1 binding to HL60 cells and dGal-1-induced exposure of PS on HL60 cells do not require expression of gangliosides. Control HL60 cells or HL60 cells incubated with PDMP for 3 days were analyzed by flow cytometry for binding of cholera toxin subunit B (CT-B) (A), dGal-1 (B), L-PHA (C), or PNA (D). (E) Control or PDMP-incubated HL60 cells were treated with 10 μM dGal-1 for 4 h, disengaged with lactose, incubated with a mixture of FITC-conjugated annexin V and PI, and analyzed by flow cytometry. The data are depicted as the percentage of cells that stained with annexin V above a threshold level, but that remained viable as assessed by staining with PI below a threshold level. The data represent the mean ± SD of three experiments.
Discussion
We have demonstrated that dGal-1 preferentially bound to kifunensine-sensitive complex N-glycans on HL60, Molt-4, and Jurkat cells. Furthermore, the receptors for dGal-1 on these cells that signal Ca2+ mobilization and exposure of PS required complex-type N-glycans. By contrast, dGal-1 did not require benzylGalNAc-sensitive O-glycans or PDMP-sensitive glycosphingolipids to bind to and signal in these cells.
Our results are consistent with studies of glycans recognized by dGal-1 in Jurkat cells (Walzel et al. 2006) although these authors did not note that Jurkat cells lack core 1 O-glycans. Our data also support previous findings that binding of rat dGal-1 and several other mammalian galectins to glycosylation mutants of Chinese hamster ovary (CHO) cells correlates with expression of complex-type N-glycans (Patnaik et al. 2006). Galectin-3 receptors on T cells require proper N-glycosylation and N-glycan branching for effective crosslinking and formation of the T-cell synapse (Demetriou et al. 2001; Dennis et al. 2002; Partridge et al. 2004; Lau et al. 2007). Deficiency of β1,6 N-acetylglucosaminyltransferase V (Mgat5), a key enzyme involved in N-glycan biosynthesis, lowers the threshold for T-cell activation by enhancing the clustering of the T-cell receptor (Demetriou et al. 2001). Mgat5 initiates β1,6 GlcNAc branching on N-glycans, thereby increasing expression of PL ligands that are important for dGal-1 binding and signaling (Leppanen et al. 2005; Suzuki et al. 2005a, 2006; Bianco et al. 2006; Lagana et al. 2006; Chen et al. 2007; Stowell, Arthur, et al. 2008). The modification of complex-type N-glycans on CD45 by ST6Gal I sialyltransferase negatively regulates dGal-1-induced signaling (Amano et al. 2003). Human dGal-1 does not bind to sialylated N-glycans with terminal α2,6-linked sialic acids of the type elaborated by ST6Gal I (Stowell, Arthur, et al. 2008). A recent study tested the ability of dGal-1 to bind to a very large panel of N- and O-glycans on a microarray. Of the many glycans examined, dGal-1 bound to a subset of N-glycans but failed to bind to core 1 O-glycans, including the disaccharide Galβ1-3GalNAcα (Stowell, Arthur, et al. 2008). Although these studies and our current data demonstrate the importance of complex-type N-glycans for interactions with dGal-1, some studies have questioned the roles of N- or O-glycans in dGal-1 binding and signaling (Carlow et al. 2003; Siebert et al. 2003, 2005; Elola et al. 2005). Therefore, it remains possible that some cell types express dGal-1 receptors that do not require complex-type N-glycans.
The specific glycoprotein signaling receptors for dGal-1 on HL60, Molt-4, and Jurkat cells are not yet defined. It is possible that each cell displays different receptors that share common complex-type N-glycans recognized by dGal-1. dGal-1 mobilizes cytosolic Ca2+ and exposes PS on the surfaces of human neutrophils through a pathway that requires action of Src family kinases and phospholipase C-γ (Karmakar et al. 2005).
The major dGal-1 receptors on HL60 cells are PL-containing glycans that are sensitive to endo-β-galactosidase, which degrades linear, unmodified PL (Leppanen et al. 2005; Stowell, Arthur, et al. 2008). The expression of PL-containing glycans is regulated by Mgat5 (Pierce and Arango 1986; Dennis et al. 2002; Guo et al. 2003; Partridge et al. 2004) and by β1,3-N-acetylglucosaminyltransferases and β1-4-galactosyltransferases (Ishida et al. 2005; Togayachi et al. 2007). How these enzymes cooperatively synthesize the structures that galectins recognize is an important topic for future studies.
Materials and methods
Materials
The chemicals used and their sources were as follows: kifunensine (Roche Diagnostics, Mannheim, Germany); benzyl-2-acetamido-2-deoxy-α-d-galactopyranoside (Calbiochem, San Diego, CA); PNA-biotin and PHA-L-biotin (Vector Laboratories, Burlingame, CA); fluorescein isothiocyanate (FITC)-conjugated annexin V and streptavidin, RPMI 1640 medium (Roche Diagnostics); Fluo-4 AM and propidium iodide (PI); biotinylated cholera toxin subunit B, streptavidin ALEXA 488 (Molecular Probes Invitrogen, Carlsbad, CA); DL-PDMP (Biomol, Plymouth Meeting, PA); Hank's balanced salt solution (Mediatech, Manassas, VA); human serum albumin (ZLB Bioplasma, Glendale, CA); and BAPTA-AM (Amersham, Piscataway, NJ).
Preparation of dGal-1
Recombinant human dGal-1 expressed in Escherichia coli was purified on lactosyl-Sepharose and dGal-1 was biotinylated as described (Dias-Baruffi et al. 2003).
Cell lines
HL60, Molt-4, and Jurkat cells from the American Type Culture Collection were maintained at 37°C and 5% CO2 in a complete RPMI 1640 medium containing 10% fetal calf serum, glutamine (2 mM), penicillin (100 milliunits/mL), and streptomycin (100 μg/mL).
Treatment of cells with kifunensine or benzylGalNAc to inhibit complex N-glycan and complex O-glycan biosynthesis
HL60 cells were incubated in a medium containing 10 μg/mL kifunensine for 7 days before use in experiments. Molt-4 and Jurkat cells were incubated in a medium containing kifunensine for 3 days before use. In other experiments, cells were incubated in a medium containing 2 mM benzylGalNAc for 3 days before use. Control, treated cells were incubated with dGal-1 in the presence or absence of 20 mM lactose. HL60, Molt-4, and Jurkat cells were suspended in either the complete RPMI medium or Ca2+–Mg2+-containing Hank's balanced salt solution (HBSS) with 0.5% human serum albumin (HSA). To monitor changes in PS exposure, control- and inhibitor-treated cells were incubated with 10 μM dGal-1 at 37°C in the presence or absence of 20 mM lactose for the times indicated. Prior to flow cytometry analysis, agglutination of dGal-1-treated cells was reversed by the addition of 20 mM lactose.
Treatment of cells with PDMP to inhibit glycosphingolipid synthesis
HL60 cells were treated with 1.2 μM PDMP for 72 h. Following treatment, the cells were washed twice in a HBSS buffer before flow cytometry analysis. To determine the extent of ganglioside inhibition following PDMP treatment, cells were resuspended in HBSS containing 1 μg/mL biotinylated cholera toxin subunit B, a specific marker for GM1 (Reed et al. 1987), and incubated for 1 h at 4°C. After incubation, cells were washed twice with HBSS, incubated with 2 μg/mL streptavidin ALEXA 488 for 1 h at 4°C, washed twice again, and resuspended in HBSS. To analyze changes in PS exposure following PDMP treatment, control and treated HL60 cells were incubated with 10 μM dGal-1 for 4 h in complete RPMI and then analyzed for PS exposure by flow cytometry as detailed below.
Flow cytometry
Cells were incubated with biotinylated dGal-1 (2 μg/mL), PHA-L, PNA, or MAL (Maackia amurensis agglutinin) lectins (10 μg/ mL), or CHO-131 mAb (5 μg/mL) for 45 min on ice in HBSS/HSA, washed once with HBSS, and then incubated with streptavidin-FITC or anti-mouse (Fab)2-FITC for 30 min on ice in HBSS/HSA. After washing twice with HBSS, cells were resuspended in HBSS and analyzed by flow cytometry. To measure PS exposure, a mixture of FITC-conjugated annexin V and PI was incubated with cells for 15 min on ice as described previously (Dias-Baruffi et al. 2003). The cells were diluted into HBSS and analyzed immediately on a FACS Calibur instrument (Becton–Dickinson) using Cell Quest software. The fluorescence intensity for binding of both annexin V and PI was measured for the entire cell population. For most experiments, the data are represented as the percentage of cells that stained with annexin V above a threshold level, but that remained viable as assessed by staining with PI below a threshold level.
Ca2+ flux measurements
Ca2+ flux experiments were carried out as described (Karmakar et al. 2005). Briefly, HL60, Molt-4, and Jurkat cells were loaded with 3 μM Fluo-4 AM at 37°C for 30 min in the presence of 4 mM probenecid to minimize dye leakage. The cells were washed with HBSS, incubated for 30 min at room temperature to allow the Fluo-4 dye to completely de-esterify, washed twice more, and resuspended at 107 cells/mL in HBSS/HSA. In some cases, cells were incubated with BAPTA in HBSS for 30 min along with Fluo-4-AM. Fluo-4-labeled cells (3 × 106/mL) were treated with 10 μM dGal-1 at 37°C in the presence or absence of 20 mM lactose. Fluorescence readings were obtained in a stirring cell fluorimeter (PerkinElmer Life Sciences LS-50) equipped with a water-jacketed cuvette holder. After obtaining the basal signal, fluorescence intensities were acquired at 0.1 s intervals for 10-15 min with continuous stirring of the cell suspension.
Funding
National Institutes of Health (grant HL085607 to R.D.C. and R.P.M).
Acknowledgments
We thank Cindy Carter, Lisa Mayer, and Todd Walker for technical assistance, Dr. Bruce Walcheck for mAb CHO-131, and Dr. Jamie Heimburg-Molinaro for help in preparing the manuscript.
Conflict of interest statement
None declared.
Abbreviations
- AM
acetoxymethyl ester
- BAPTA
1,2-bis(o-aminophenoxy) ethane-N,N,N′,N′-tetraacetic acid)
- benzylGalNAc
benzyl-2-acetamido-2-deoxy-α-d-galactopyranoside
- CHO
Chinese hamster ovary
- dGal-1
dimeric galectin-1
- FITC
fluorescein isothiocyanate
- HBSS
Hank's balanced salt solution
- L-PHA
Phaseolus vulgaris leukoagglutinin
- MAL
Maackia amurensis agglutinin, PI, propidium iodide
- PDMP
DL-threo-1-phenyl-2-decanoylamino-3-morpholino-1-propanolċHCl
- PL
poly-N-acetyllactosamine
- PNA
peanut agglutinin
- PS
phosphatidylserine
References
- Amano M, Galvan M, He J, Baum LG. The ST6Gal I sialyltransferase selectively modifies N-glycans on CD45 to negatively regulate galectin-1-induced CD45 clustering, phosphatase modulation, and T cell death. J Biol Chem. 2003;278:7469–7475. doi: 10.1074/jbc.M209595200. [DOI] [PubMed] [Google Scholar]
- Andre S, Kaltner H, Lensch M, Russwurm R, Siebert HC, Fallsehr C, Tajkhorshid E, Heck AJ, von Knebel Doeberitz M, Gabius HJ, et al. Determination of structural and functional overlap/divergence of five proto-type galectins by analysis of the growth-regulatory interaction with ganglioside GM1 in silico and in vitro on human neuroblastoma cells. Int J Cancer. 2005;114:46–57. doi: 10.1002/ijc.20699. [DOI] [PubMed] [Google Scholar]
- Barondes SH, Castronovo V, Cooper DN, Cummings RD, Drickamer K, Feizi T, Gitt MA, Hirabayashi J, Hughes C, Kasai K, et al. Galectins: A family of animal beta-galactoside-binding lectins. Cell. 1994;76:597–598. doi: 10.1016/0092-8674(94)90498-7. [DOI] [PubMed] [Google Scholar]
- Barondes SH, Cooper DN, Gitt MA, Leffler H. Galectins. Structure and function of a large family of animal lectins. J Biol Chem. 1994;269:20807–20810. [PubMed] [Google Scholar]
- Bianco GA, Toscano MA, Ilarregui JM, Rabinovich GA. Impact of protein–glycan interactions in the regulation of autoimmunity and chronic inflammation. Autoimmun Rev. 2006;5:349–356. doi: 10.1016/j.autrev.2006.02.003. [DOI] [PubMed] [Google Scholar]
- Carlow DA, Williams MJ, Ziltener HJ. Modulation of O-glycans and N-glycans on murine CD8 T cells fails to alter annexin V ligand induction by galectin 1. J Immunol. 2003;171:5100–5106. doi: 10.4049/jimmunol.171.10.5100. [DOI] [PubMed] [Google Scholar]
- Chen IJ, Chen HL, Demetriou M. Lateral compartmentalization of T cell receptor versus CD45 by galectin-N-glycan binding and microfilaments coordinate basal and activation signaling. J Biol Chem. 2007;282:35361–35372. doi: 10.1074/jbc.M706923200. [DOI] [PubMed] [Google Scholar]
- Cho M, Cummings RD. Galectin-1, a beta-galactoside-binding lectin in Chinese hamster ovary cells: I. Physical and chemical characterization. J Biol Chem. 1995;270:5198–5206. doi: 10.1074/jbc.270.10.5198. [DOI] [PubMed] [Google Scholar]
- Cho M, Cummings RD. Characterization of monomeric forms of galectin-1 generated by site-directed mutagenesis. Biochemistry. 1996;35:13081–13088. doi: 10.1021/bi961181d. [DOI] [PubMed] [Google Scholar]
- Colnot C, Ripoche MA, Scaerou F, Foulis D, Poirier F. Galectins in mouse embryogenesis. Biochem Soc Trans. 1996;24:141–146. doi: 10.1042/bst0240141. [DOI] [PubMed] [Google Scholar]
- Cooper DN, Barondes SH. God must love galectins; he made so many of them. Glycobiology. 1999;9:979–984. doi: 10.1093/glycob/9.10.979. [DOI] [PubMed] [Google Scholar]
- Cummings RD, Kornfeld S. Characterization of the structural determinants required for the high affinity interaction of asparagine-linked oligosaccharides with immobilized Phaseolus vulgaris leukoagglutinating and erythroagglutinating lectins. J Biol Chem. 1982;257:11230–11234. [PubMed] [Google Scholar]
- Cummings RD, Kornfeld S. Fractionation of asparagine-linked oligosaccharides by serial lectin-Agarose affinity chromatography. A rapid, sensitive, and specific technique. J Biol Chem. 1982;257:11235–11240. [PubMed] [Google Scholar]
- Demetriou M, Granovsky M, Quaggin S, Dennis JW. Negative regulation of T-cell activation and autoimmunity by Mgat5 N-glycosylation. Nature. 2001;409:733–739. doi: 10.1038/35055582. [DOI] [PubMed] [Google Scholar]
- Dennis JW, Pawling J, Cheung P, Partridge E, Demetriou M. UDP-N-acetylglucosamine:alpha-6-d-mannoside beta1,6 N-acetylglucosaminyltransferase V (Mgat5) deficient mice. Biochim Biophys Acta. 2002;1573:414–422. doi: 10.1016/s0304-4165(02)00411-7. [DOI] [PubMed] [Google Scholar]
- Dias-Baruffi M, Zhu H, Cho M, Karmakar S, McEver RP, Cummings RD. Dimeric galectin-1 induces surface exposure of phosphatidylserine and phagocytic recognition of leukocytes without inducing apoptosis. J Biol Chem. 2003;278:41282–41293. doi: 10.1074/jbc.M306624200. [DOI] [PubMed] [Google Scholar]
- Elbein AD, Tropea JE, Mitchell M, Kaushal GP. Kifunensine, a potent inhibitor of the glycoprotein processing mannosidase I. J Biol Chem. 1990;265:15599–15605. [PubMed] [Google Scholar]
- Elola MT, Chiesa ME, Alberti AF, Mordoh J, Fink NE. Galectin-1 receptors in different cell types. J Biomed Sci. 2005;12:13–29. doi: 10.1007/s11373-004-8169-5. [DOI] [PubMed] [Google Scholar]
- Gauthier L, Rossi B, Roux F, Termine E, Schiff C. Galectin-1 is a stromal cell ligand of the pre-B cell receptor (BCR) implicated in synapse formation between pre-B and stromal cells and in pre-BCR triggering. Proc Natl Acad Sci USA. 2002;99:13014–13019. doi: 10.1073/pnas.202323999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giudicelli V, Lutomski D, Levi-Strauss M, Bladier D, Joubert-Caron R, Caron M. Is human galectin-1 activity modulated by monomer/dimer equilibrium? Glycobiology. 1997;7:viii–x. doi: 10.1093/glycob/7.3.323-a. [DOI] [PubMed] [Google Scholar]
- Gouyer V, Leteurtre E, Delmotte P, Steelant WF, Krzewinski-Recchi MA, Zanetta JP, Lesuffleur T, Trugnan G, Delannoy P, Huet G. Differential effect of GalNAcalpha-O-bn on intracellular trafficking in enterocytic HT-29 and Caco-2 cells: Correlation with the glycosyltransferase expression pattern. J Cell Sci. 2001;114:1455–1471. doi: 10.1242/jcs.114.8.1455. [DOI] [PubMed] [Google Scholar]
- Guo HB, Lee I, Kamar M, Pierce M. N-Acetylglucosaminyltransferase V expression levels regulate cadherin-associated homotypic cell–cell adhesion and intracellular signaling pathways. J Biol Chem. 2003;278:52412–52424. doi: 10.1074/jbc.M308837200. [DOI] [PubMed] [Google Scholar]
- Hahn HP, Pang M, He J, Hernandez JD, Yang RY, Li LY, Wang X, Liu FT, Baum LG. Galectin-1 induces nuclear translocation of endonuclease G in caspase- and cytochrome c-independent T cell death. Cell Death Differ. 2004;11:1277–1286. doi: 10.1038/sj.cdd.4401485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He J, Baum LG. Presentation of galectin-1 by extracellular matrix triggers T cell death. J Biol Chem. 2004;279:4705–4712. doi: 10.1074/jbc.M311183200. [DOI] [PubMed] [Google Scholar]
- Huet G, Hennebicq-Reig S, de Bolos C, Ulloa F, Lesuffleur T, Barbat A, Carriere V, Kim I, Real FX, Delannoy P, et al. GalNAc-alpha-O-benzyl inhibits NeuAcalpha2-3 glycosylation and blocks the intracellular transport of apical glycoproteins and mucus in differentiated HT-29 cells. J Cell Biol. 1998;141:1311–1322. doi: 10.1083/jcb.141.6.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ideo H, Seko A, Yamashita K. Galectin-4 binds to sulfated glycosphingolipids and carcinoembryonic antigen in patches on the cell surface of human colon adenocarcinoma cells. J Biol Chem. 2005;280:4730–4737. doi: 10.1074/jbc.M410362200. [DOI] [PubMed] [Google Scholar]
- Ishida H, Togayachi A, Sakai T, Iwai T, Hiruma T, Sato T, Okubo R, Inaba N, Kudo T, Gotoh M, et al. A novel beta1,3-N-acetylglucosaminyltransferase (beta3Gn-T8), which synthesizes poly-N-acetyllactosamine, is dramatically upregulated in colon cancer. FEBS Lett. 2005;579:71–78. doi: 10.1016/j.febslet.2004.11.037. [DOI] [PubMed] [Google Scholar]
- Ju T, Cummings RD. A unique molecular chaperone Cosmc required for activity of the mammalian core 1 beta 3-galactosyltransferase. Proc Natl Acad Sci USA. 2002;99:16613–16618. doi: 10.1073/pnas.262438199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karmakar S, Cummings RD, McEver RP. Contributions of Ca2+ to galectin-1-induced exposure of phosphatidylserine on activated neutrophils. J Biol Chem. 2005;280:28623–28631. doi: 10.1074/jbc.M414140200. [DOI] [PubMed] [Google Scholar]
- Kopitz J, von Reitzenstein C, Burchert M, Cantz M, Gabius HJ. Galectin-1 is a major receptor for ganglioside GM1, a product of the growth-controlling activity of a cell surface ganglioside sialidase, on human neuroblastoma cells in culture. J Biol Chem. 1998;273:11205–11211. doi: 10.1074/jbc.273.18.11205. [DOI] [PubMed] [Google Scholar]
- Kuan SF, Byrd JC, Basbaum C, Kim YS. Inhibition of mucin glycosylation by aryl-N-acetyl-alpha-galactosaminides in human colon cancer cells. J Biol Chem. 1989;264:19271–19277. [PubMed] [Google Scholar]
- Lagana A, Goetz JG, Cheung P, Raz A, Dennis JW, Nabi IR. Galectin binding to Mgat5-modified N-glycans regulates fibronectin matrix remodeling in tumor cells. Mol Cell Biol. 2006;26:3181–3193. doi: 10.1128/MCB.26.8.3181-3193.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanteri M, Giordanengo V, Hiraoka N, Fuzibet JG, Auberger P, Fukuda M, Baum LG, Lefebvre JC. Altered T cell surface glycosylation in HIV-1 infection results in increased susceptibility to galectin-1-induced cell death. Glycobiology. 2003;13:909–918. doi: 10.1093/glycob/cwg110. [DOI] [PubMed] [Google Scholar]
- Lau KS, Partridge EA, Grigorian A, Silvescu CI, Reinhold VN, Demetriou M, Dennis JW. Complex N-glycan number and degree of branching cooperate to regulate cell proliferation and differentiation. Cell. 2007;129:123–134. doi: 10.1016/j.cell.2007.01.049. [DOI] [PubMed] [Google Scholar]
- Leffler H, Barondes SH. Specificity of binding of three soluble rat lung lectins to substituted and unsubstituted mammalian beta-galactosides. J Biol Chem. 1986;261:10119–10126. [PubMed] [Google Scholar]
- Leffler H, Carlsson S, Hedlund M, Qian Y, Poirier F. Introduction to galectins. Glycoconj J. 2004;19:433–440. doi: 10.1023/B:GLYC.0000014072.34840.04. [DOI] [PubMed] [Google Scholar]
- Leppanen A, Stowell S, Blixt O, Cummings RD. Dimeric galectin-1 binds with high affinity to alpha2,3-sialylated and non-sialylated terminal N-acetyllactosamine units on surface-bound extended glycans. J Biol Chem. 2005;280:5549–5562. doi: 10.1074/jbc.M412019200. [DOI] [PubMed] [Google Scholar]
- Lotan R, Sharon N, Mirelman D. Interaction of wheat-germ agglutinin with bacterial cells and cell-wall polymers. Eur J Biochem. 1975;55:257–262. doi: 10.1111/j.1432-1033.1975.tb02158.x. [DOI] [PubMed] [Google Scholar]
- Martinez VG, Pellizzari EH, Diaz ES, Cigorraga SB, Lustig L, Denduchis B, Wolfenstein-Todel C, Iglesias MM. Galectin-1, a cell adhesion modulator, induces apoptosis of rat Leydig cells in vitro. Glycobiology. 2004;14:127–137. doi: 10.1093/glycob/cwh025. [DOI] [PubMed] [Google Scholar]
- Moiseeva EP, Williams B, Goodall AH, Samani NJ. Galectin-1 interacts with beta-1 subunit of integrin. Biochem Biophys Res Commun. 2003;310:1010–1016. doi: 10.1016/j.bbrc.2003.09.112. [DOI] [PubMed] [Google Scholar]
- Nguyen JT, Evans DP, Galvan M, Pace KE, Leitenberg D, Bui TN, Baum LG. CD45 modulates galectin-1-induced T cell death: Regulation by expression of core 2 O-glycans. J Immunol. 2001;167:5697–5707. doi: 10.4049/jimmunol.167.10.5697. [DOI] [PubMed] [Google Scholar]
- Novogrodsky A, Lotan R, Ravid A, Sharon N. Peanut agglutinin, a new mitogen that binds to galactosyl sites exposed after neuraminidase treatment. J Immunol. 1975;115:1243–1248. [PubMed] [Google Scholar]
- Ohannesian DW, Lotan D, Thomas P, Jessup JM, Fukuda M, Gabius HJ, Lotan R. Carcinoembryonic antigen and other glycoconjugates act as ligands for galectin-3 in human colon carcinoma cells. Cancer Res. 1995;55:2191–2199. [PubMed] [Google Scholar]
- Pace KE, Lee C, Stewart PL, Baum LG. Restricted receptor segregation into membrane microdomains occurs on human T cells during apoptosis induced by galectin-1. J Immunol. 1999;163:3801–3811. [PubMed] [Google Scholar]
- Partridge EA, Le Roy C, Di Guglielmo GM, Pawling J, Cheung P, Granovsky M, Nabi IR, Wrana JL, Dennis JW. Regulation of cytokine receptors by Golgi N-glycan processing and endocytosis. Science. 2004;306:120–124. doi: 10.1126/science.1102109. [DOI] [PubMed] [Google Scholar]
- Patnaik SK, Potvin B, Carlsson S, Sturm D, Leffler H, Stanley P. Complex N-glycans are the major ligands for galectin-1, -3, and -8 on Chinese hamster ovary cells. Glycobiology. 2006;16:305–317. doi: 10.1093/glycob/cwj063. [DOI] [PubMed] [Google Scholar]
- Perillo NL, Marcus ME, Baum LG. Galectins: Versatile modulators of cell adhesion, cell proliferation, and cell death. J Mol Med. 1998;76:402–412. doi: 10.1007/s001090050232. [DOI] [PubMed] [Google Scholar]
- Perillo NL, Pace KE, Seilhamer JJ, Baum LG. Apoptosis of T cells mediated by galectin-1. Nature. 1995;378:736–739. doi: 10.1038/378736a0. [DOI] [PubMed] [Google Scholar]
- Pierce M, Arango J. Rous sarcoma virus-transformed baby hamster kidney cells express higher levels of asparagine-linked tri- and tetraantennary glycopeptides containing [GlcNAc-beta (1,6)Man-alpha (1,6)Man] and poly-N-acetyllactosamine sequences than baby hamster kidney cells. J Biol Chem. 1986;261:10772–10777. [PubMed] [Google Scholar]
- Piller V, Piller F, Fukuda M. Biosynthesis of truncated O-glycans in the T cell line Jurkat. Localization of O-glycan initiation. J Biol Chem. 1990;265:9264–9271. [PubMed] [Google Scholar]
- Rabinovich GA, Toscano MA, Jackson SS, Vasta GR. Functions of cell surface galectin-glycoprotein lattices. Curr Opin Struct Biol. 2007;17:513–520. doi: 10.1016/j.sbi.2007.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radin NS, Shayman JA, Inokuchi J. Metabolic effects of inhibiting glucosylceramide synthesis with PDMP and other substances. Adv Lipid Res. 1993;26:183–213. [PubMed] [Google Scholar]
- Reed RA, Mattai J, Shipley GG. Interaction of cholera toxin with ganglioside GM1 receptors in supported lipid monolayers. Biochemistry. 1987;26:824–832. doi: 10.1021/bi00377a025. [DOI] [PubMed] [Google Scholar]
- Rosenwald AG, Machamer CE, Pagano RE. Effects of a sphingolipid synthesis inhibitor on membrane transport through the secretory pathway. Biochemistry. 1992;31:3581–3590. doi: 10.1021/bi00129a005. [DOI] [PubMed] [Google Scholar]
- Rubinstein N, Ilarregui JM, Toscano MA, Rabinovich GA. The role of galectins in the initiation, amplification and resolution of the inflammatory response. Tissue Antigens. 2004;64:1–12. doi: 10.1111/j.0001-2815.2004.00278.x. [DOI] [PubMed] [Google Scholar]
- Salatino M, Croci DO, Bianco GA, Ilarregui JM, Toscano MA, Rabinovich GA. Galectin-1 as a potential therapeutic target in autoimmune disorders and cancer. Expert Opin Biol Ther. 2008;8:45–57. doi: 10.1517/14712598.8.1.45. [DOI] [PubMed] [Google Scholar]
- Siebert HC, Andre S, Lu SY, Frank M, Kaltner H, van Kuik JA, Korchagina EY, Bovin N, Tajkhorshid E, Kaptein R, et al. Unique conformer selection of human growth-regulatory lectin galectin-1 for ganglioside GM1 versus bacterial toxins. Biochemistry. 2003;42:14762–14773. doi: 10.1021/bi035477c. [DOI] [PubMed] [Google Scholar]
- Siebert HC, Born K, Andre S, Frank M, Kaltner H, von der Lieth CW, Heck AJ, Jimenez-Barbero J, Kopitz J, Gabius HJ. Carbohydrate chain of ganglioside GM1 as a ligand: Identification of the binding strategies of three 15 mer peptides and their divergence from the binding modes of growth-regulatory galectin-1 and cholera toxin. Chemistry. 2005;12:388–402. doi: 10.1002/chem.200500505. [DOI] [PubMed] [Google Scholar]
- Spangler BD. Structure and function of cholera toxin and the related Escherichia coli heat-labile enterotoxin. Microbiol Rev. 1992;56:622–647. doi: 10.1128/mr.56.4.622-647.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stowell SR, Arthur CM, Mehta P, Slanina KA, Blixt O, Leffler H, Smith DF, Cummings RD. Galectins-1, -2 and -3 exhibit differential recognition of sialylated glycans and blood group antigens. J Biol Chem. 2008;180:3091–3102. doi: 10.1074/jbc.M709545200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stowell SR, Dias-Baruffi M, Penttila L, Renkonen O, Nyame AK, Cummings RD. Human galectin-1 recognition of poly-N-acetyllactosamine and chimeric polysaccharides. Glycobiology. 2004;14:157–167. doi: 10.1093/glycob/cwh018. [DOI] [PubMed] [Google Scholar]
- Stowell SR, Karmakar S, Stowell CJ, Dias-Baruffi M, McEver RP, Cummings RD. Human galectin-1, -2, and -4 induce surface exposure of phosphatidylserine in activated human neutrophils but not in activated T cells. Blood. 2007;109:219–227. doi: 10.1182/blood-2006-03-007153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stowell SR, Qian Y, Karmakar S, Koyama NS, Dias-Baruffi M, Leffler H, McEver RP, Cummings RD. Differential roles of galectin-1 and galectin-3 in regulating leukocyte viability and cytokine secretion. J Immunol. 2008;180:3091–3102. doi: 10.4049/jimmunol.180.5.3091. [DOI] [PubMed] [Google Scholar]
- Suzuki O, Nozawa Y, Abe M. Altered N-glycosylation in CD45 and regulatory roles of altered N-glycosylation in galectin-1-induced growth inhibition in human diffuse large B cell lymphoma. Oncol Rep. 2005;13:109–114. [PubMed] [Google Scholar]
- Suzuki O, Nozawa Y, Abe M. Regulatory roles of altered N- and O-glycosylation of CD45 in galectin-1-induced cell death in human diffuse large B cell lymphoma. Int J Oncol. 2005;26:1063–1068. [PubMed] [Google Scholar]
- Suzuki O, Nozawa Y, Abe M. The regulatory roles of cell surface sialylation and N-glycans in human B cell lymphoma cell adhesion to galectin-1. Int J Oncol. 2006;28:155–160. [PubMed] [Google Scholar]
- Togayachi A, Kozono Y, Ishida H, Abe S, Suzuki N, Tsunoda Y, Hagiwara K, Kuno A, Ohkura T, Sato N, et al. Polylactosamine on glycoproteins influences basal levels of lymphocyte and macrophage activation. Proc Natl Acad Sci USA. 2007;104:15829–15834. doi: 10.1073/pnas.0707426104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toscano MA, Bianco GA, Ilarregui JM, Croci DO, Correale J, Hernandez JD, Zwirner NW, Poirier F, Riley EM, Baum LG, et al. Differential glycosylation of TH1, TH2 and TH-17 effector cells selectively regulates susceptibility to cell death. Nat Immunol. 2007;8:825–834. doi: 10.1038/ni1482. [DOI] [PubMed] [Google Scholar]
- Van Den Brule F, Califice S, Castronovo V. Expression of galectins in cancer: A critical review. Glycoconj J. 2004;19:537–542. doi: 10.1023/B:GLYC.0000014083.48508.6a. [DOI] [PubMed] [Google Scholar]
- Van Der Leij J, Van Den Berg A, Harms G, Eschbach H, Vos H, Zwiers P, van Weeghel R, Groen H, Poppema S, Visser L. Strongly enhanced IL-10 production using stable galectin-1 homodimers. Mol Immunol. 2007;44:506–513. doi: 10.1016/j.molimm.2006.02.011. [DOI] [PubMed] [Google Scholar]
- Van Heyningen WE, Van Heyningen S, King CA. The nature and action of cholera toxin. Ciba Found Symp. 1976. pp. 73–88. [DOI] [PubMed]
- Walcheck B, Leppanen A, Cummings RD, Knibbs RN, Stoolman LM, Alexander SR, Mattila PE, McEver RP. The monoclonal antibody CHO-131 binds to a core 2 O-glycan terminated with sialyl-Lewis x, which is a functional glycan ligand for P-selectin. Blood. 2002;99:4063–4069. doi: 10.1182/blood-2001-12-0265. [DOI] [PubMed] [Google Scholar]
- Walzel H, Fahmi AA, Eldesouky MA, Abou-Eladab EF, Waitz G, Brock J, Tiedge M. Effects of N-glycan processing inhibitors on signaling events and induction of apoptosis in galectin-1-stimulated Jurkat T lymphocytes. Glycobiology. 2006;16:1262–1271. doi: 10.1093/glycob/cwl037. [DOI] [PubMed] [Google Scholar]
- Walzel H, Hirabayashi J, Kasai K, Brock J, Neels P. Cell calcium signalling induced by endogenous lectin carbohydrate interaction in the Jurkat T cell line. Glycoconj J. 1996;13:99–105. doi: 10.1007/BF01049685. [DOI] [PubMed] [Google Scholar]