Abstract
Primary open angle glaucoma (POAG) is a major blindness-causing disease, characterized by elevated intraocular pressure due to an insufficient outflow of aqueous humor. The trabecular meshwork (TM) lining the aqueous outflow pathway modulates the aqueous outflow facility. TM cell adhesion, cell–matrix interactions, and factors that influence Rho signaling in TM cells are thought to play a pivotal role in the regulation of aqueous outflow. In a recent study, we demonstrated that galectin-8 (Gal8) modulates the adhesion and cytoskeletal arrangement of TM cells and that it does so through binding to β1 integrins and inducing Rho signaling. The current study is aimed at the characterization of the mechanism by which Gal8 mediates TM cell adhesion and spreading. We demonstrate here that TM cells adhere to and spread on Gal8-coated wells but not on galectin-1 (Gal1)- or galectin-3 (Gal3)-coated wells. The adhesion of TM cells to Gal8-coated wells was abolished by a competing sugar, β-lactose, but not by a noncompeting sugar, sucrose. Also, a trisaccharide, NeuAcα2-3Galβ1-4GlcNAc, which binds specifically to the N-CRD of Gal8, inhibited the spreading of TM cells to Gal8-coated wells. In contrast, NeuAcα2-6Galβ1-4GlcNAc which lacks affinity for Gal8 had no effect. Affinity chromatography of cell extracts on a Gal8-affinity column and binding experiments with plant lectins, Maakia Amurensis and Sambucus Nigra, revealed that α3β1, α5β1, and αvβ1 integrins are major counterreceptors of Gal8 in TM cells and that TM cell β1 integrins carry predominantly α2-3-sialylated glycans, which are high-affinity ligands for Gal8 but not for Gal1 or Gal3. These data lead us to propose that Gal8 modulates TM cell adhesion and spreading, at least in part, by interacting with α2-3-sialylated glycans on β1 integrins.
Keywords: cell adhesion, galectin-8, glaucoma, integrins, trabecular meshwork
Introduction
Primary open angle glaucoma (POAG) is a leading cause for irreversible blindness worldwide. Factors that lead to the development of POAG are not yet fully known. It is clear, however, that elevated intraocular pressure (IOP) is a critical risk factor (Weinreb and Khaw 2004). Elevation in the IOP is due to the dysfunction of the outflow pathway tissues which modulate the clearance of aqueous humor from the anterior chamber of the eye. Prominent among the outflow pathway tissues is the trabecular meshwork (TM). The TM resides in the ocular limbus between the cornea and the sclera and comprises perforated, interlacing collagenous lamellae, called the TM beams. Between the beams there are spaces that serve as flow channels for aqueous humor. The beams are encapsulated by a single layer of endothelial-like cells (Polansky and Alvarado 1994). Glaucomatous eyes exhibit a high level of TM cell loss, above and beyond that of age-matched controls (Alvarado et al. 1984).
In nonocular studies, a carbohydrate-binding protein, galectin-8 (Gal8), has been shown to form high-affinity interactions with integrins, modulate cell–matrix interactions, and promote cell spreading by activating PI3K and the small GTPases, Ras, and Rac (Levy et al. 2001; 2003; Carcamo et al. 2006). In a recent study, we have shown that Gal8 is expressed in the TM and a function-blocking anti-β1 integrin antibody inhibits the adhesion and spreading of TM cells to Gal8-coated wells and that Gal8 modulates Rho signaling in TM cells (Diskin et al., in preparation). In an effort to characterize the mechanism by which Gal8 mediates TM cell adhesion and spreading, here we report studies on (i) identification of TM cell counterreceptors of Gal8, (ii) carbohydrate-binding specificity of Gal8, and (iii) the function of TM cell Gal8 in the context of the glycosylation pattern of β1 integrins.
Material and methods
Preparation of human TM cell cultures
TM cells were cultured using normal human cadaver eyes (donor age 71–89 years, Central Florida Lions Eye Bank, Tampa, FL) collected within 6 h postmortem. TM cell cultures were prepared as previously described (Stamer et al. 1995) and were used at third to fourth passage.
Preparation of recombinant galectin-8
Recombinant human glutathione-S-transferase (GST)-tagged Gal8 was expressed in Escherichia coli as previously described (Carcamo et al. 2006) and was purified by affinity chromatography on a β-lactose-conjugated Sepharose column (EY Labs, San Mateo, CA; 1 mL bed volume) as described by Diskin et al. (in preparation). Purified preparations of GST-Gal8 contained a single 60-kDa anti-Gal8-reactive component (expected m.w.: Gal8, 36 kDa; GST, 26 kDa). Recombinant human 6-histidine-tagged Gal8 (His-Gal8) was expressed in the E. coli strain BL21 (Novagen, Gibbstown, NJ) using the expression vector PET28a LysS (Novagen). Bacteria were induced by 1 mM IPTG for 4 h at 37°C, and protein was purified by affinity chromatography on a β-lactose-conjugated Sepharose column as described above. Purified preparations of His-Gal8 contained a single 36-kDa anti-Gal8-reactive component.
Quantification of cell adhesion and spreading
To assess the adhesion and spreading of TM cells onto different substrates, 96-well microtiter plates were coated with Gal1, Gal3, and Gal8 (30 μg/mL, in PBS, 4°C, 16 h), human fibronectin from placenta (Sigma, St. Louis, MO) (30 μg/mL), poly-l-lysine (Sigma) (100 μg/mL), and GST tag. Gal8 was prepared as described above. Gal1 and Gal3 were prepared as previously described (Cao et al. 2002). Confluent TM cultures were dissociated with freshly prepared 5 mM EDTA (Invitrogen) in Ca2+-free PBS (Invitrogen, Carlsbad, CA) (7 min, 37°C) and plated on microtiter plates coated with Gal8 and other substrates described above (50,000 cells/well, 3 wells/group). Following incubation (4 h, 37°C), cells were washed with PBS and stained with Giemsa stain (Diff Quik stain set, Dade Behring, Newark, DE). Plates were scanned with an Epson Perfection 4490 scanner and approximate cell density was assessed using image analysis software (Gene Tools, Synoptics, Cambridge, England). Micrographs were taken on a Nikon Eclipse TE200 inverted microscope (Nikon, Melville, NY) equipped with a spot camera (Diagnostic Instruments, Sterling Heights, MI). Ten random fields of each experimental condition were photographed and spread cells were counted manually. One thousand cells were counted under each condition and the percent spread cells were calculated. Statistical significance was calculated with an unpaired Student's t-test. To assess the involvement of carbohydrate recognition in cell adhesion and spreading on Gal8, cells were incubated on Gal8-coated microtiter wells in the presence of either β-lactose (10 mM, 100 mM in DMEM) (Sigma), NeuAcα2-3Galβ1-4GlcNAc (3-SLN, 5 mM, 10 mM in DMEM) (V-labs, Covington, LA) or asialofetuin (ASF, 5 mg/mL in DMEM) (Sigma). Noncompeting saccharides, sucrose and NeuAcα2-6Galβ1-4GlcNAc (6-SLN, V-labs) served as negative controls for these experiments.
Characterization of the carbohydrate-binding specificity of galectin-8
To characterize the carbohydrate-binding specificity of Gal8, glycan array v3 was used (Consortium of Functional Glycomics (CFG), Scripps Institute, La Jolla, CA). The array is printed with 320 glycan targets (Blixt et al. 2004). A full list of glycans printed on the v3 array is available at http:// glycomics.scripps.edu/CoreH/CoreHarray033007V3.pdf. Glycan array analyses were performed by the CFG core facility. Briefly, the arrays were sequentially incubated with GST-Gal8 (0.01, 0.05, 0.1, 0.2, 0.5, 1, 2, and 5 μg/mL), goat anti-Gal8 (R&D Systems, Minneapolis, MN), and FITC-labeled anti-goat IgG. The highest measurable signal (saturating) was ∼60,000 RFU. The average background RFU values, defined as the average signal of glycans that structurally cannot bind to Gal8 (Carlsson, Oberg, et al. 2007), were ∼300 and ∼1200 RFU for the lowest and the highest Gal8 concentrations, respectively. Four times the background values were subtracted from the RFU value of each glycan. Then, to compare binding abilities of different glycans to Gal8, significant binding was arbitrarily defined as that giving over 11,000 RFU (20% of maximum signal). All saccharides exhibiting significant binding also produced the appropriate dose response curve at higher Gal8 concentrations. Incubation of the array with goat anti-Gal8 followed by a FITC-labeled anti-goat IgG, without preincubation with Gal8, served as a negative control.
Identification of galectin-8 counterreceptors in TM cells by affinity chromatography
Gal8 counterreceptors of TM cells were identified using a GST-Gal8 affinity matrix (Einarson 2004). First, to prevent nonspecific binding, TM cell extracts from six 10-cm dishes in the GST lysis buffer (20 mM Tris–HCl, pH 8.0, 200 mM EDTA, pH 8.0, 0.5% Nonidet P-40) were incubated with glutathione-conjugated agarose beads in the presence of GST (2 h, 4°C). Unbound proteins were divided into two equal fractions, one was incubated with glutathione beads in the presence of GST-Gal8 and the other with glutathione beads in the presence of GST alone (control) (2 h, 4°C). Counterreceptors were released using 100 mM β-lactose in the GST lysis buffer (15 min, 4°C). To ensure that the proteins eluted from the Gal8 affinity matrix are specifically Gal8 counterreceptors, prior to elution with β-lactose, the matrix was eluted with a noncompeting sugar, sucrose. Eluted proteins were dialyzed against water (24 h, 4°C), lyophilized, and electrophoresed on 10% polyacrylamide gels in the presence of SDS. Gels were stained with Coomassie brilliant blue (Candiano et al. 2004) and photographed. Protein bands were isolated from the gel, subjected to in-gel trypsin digestion, and analyzed by liquid chromatography/mass spectrometry/mass spectrometry (LC/MS/MS) on ion-trap mass spectrometers. The MS/MS spectra were searched against the NCBI nonredundant protein sequence database using the SEQUEST computer algorithm (Yates et al. 1995). A minimum of four unique, nonoverlapping peptides were set as the threshold for a match.
Analysis of integrin sialylation
The sialylation status of β1 integrins in TM cells was assessed by binding studies with plant lectins (Seales et al. 2003). Briefly, TM cell lysates (600 μg protein) were incubated with 4 μg of biotin-labeled Maakia Amurensis (MAA) lectin (Vector labs, Burlingame, CA), which binds selectively to α2-3-linked sialic acids (Wang and Cummings 1988) or with 4 μg of biotin-labeled Sambucus Nigra (SNA) lectin (Vector labs) that binds selectively to α2-6-linked sialic acids (Shibuya et al. 1987), for 2 h with shaking at 4°C. The samples were then incubated with streptavidin–agarose beads (2 h, 4°C) and centrifuged. For controls, the samples were incubated with agarose beads alone. Proteins bound to the beads were released by boiling in a sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS–PAGE) sample buffer and were electrophoresed in 10% polyacrylamide gels in the presence of SDS. Protein blots of the gels were processed for immunostaining using a mouse anti-β1 integrin (JB1A, Chemicon, Temecula, CA, 1:5000 in the blocking buffer, 2 h, 25°C) as the primary antibody and anti-mouse IgG (Vector Labs) as a secondary antibody. Control blots were processed the same way except that the step involving the incubation with the primary antibody was omitted.
Results
TM cells adhere to galectin-8 but not to galectin-1 or -3
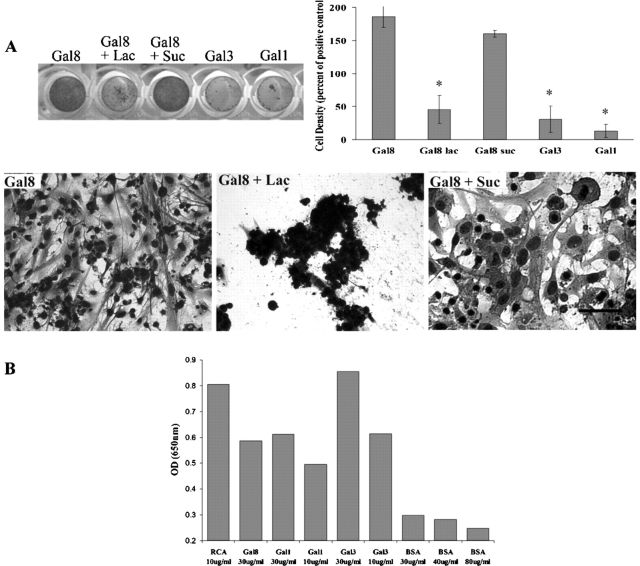
To determine whether TM cells adhere to Gal8 in a carbohydrate-dependent manner, and if so, whether other galectins also serve as permissive substrates for TM cells, we performed cell adhesion assays in the presence and absence of saccharides. TM cells adhered to Gal8 (Figure 1A). Similar results were obtained regardless of whether the wells were coated with GST-Gal8 (Figure 1A) or His-Gal8 (not shown, data indistinguishable from that shown in Figure 1A for wells coated with GST-Gal8). In contrast, the adhesion of TM cells to wells coated with GST tag alone was negligible (<5%) compared to the wells coated with GST-Gal8 or His-Gal8 (not shown). Cell adhesion to GST- as well as His-Gal8 was inhibited by β-lactose, a competing sugar, but not by a noncompeting sugar, sucrose (Figure 1A). In contrast to Gal8, TM cells did not adhere to Gal1 and Gal3, both of which are present in the TM (Fautsch et al. 2003). The lack of cell adhesion to Gal1 and Gal3 was not due to the loss of carbohydrate-binding activity of the galectins following adsorption onto cell culture plates. When plates were developed with HRP-labeled asialofetuin (ASF), which contains numerous terminal N-acetyllactosamine (Gal-GlcNAc) residues and binds to most galectins (Dam et al. 2005), wells coated with Gal1 and Gal3 yielded equal or greater (OD: 0.61 and OD: 0.85, respectively) intensity than Gal8 (OD: 0.587) (Figure 1B). Also, the Gal1 and Gal3 preparations used in this assay were not cytotoxic (NP, unpublished data). TM cells did not adhere to noncoated plastic wells in the absence of serum (not shown). Cells incubated on uncoated wells in the presence of serum served as a positive control (not shown). Since results obtained using GST-Gal8 and His-Gal8 were comparable, remainder of the studies were carried out using GST-Gal8.
Fig. 1.
TM cells adhere to and spread on galectin-8 but not to galectins-1 or -3. (A) Normal human TM cells were plated on microtiter wells (50,000 cells/well) coated with different galectins (30 μg/mL) and the plates were incubated in serum-free DMEM in the presence and absence of β-lactose or sucrose (100 mM in DMEM, 37°C, 7% CO2, 16 h). Following incubation, the cells were stained with Giemsa and photographed. Top left panel shows the photograph of the plate and lower panel shows the light micrographs of representative areas of the wells. Approximate cell density of adhered cells in each well is shown in the top-right panel. Note that cells adhered and spread on Gal8 and that the binding of cells to Gal8 was abolished by the presence of β-lactose (Gal8 + Lac), a competing sugar, but not by the presence of sucrose, a noncompeting sugar (Gal8 + Suc). Cells did not adhere or spread on Gal1 or Gal3. Similar results were obtained with six cultures from different donors. Results obtained with His-Gal8 were indistinguishable from those obtained with GST-Gal8 (not shown). No cell adhesion was observed in a serum-free medium on uncoated wells or when wells were coated with GST alone (not shown). Cells incubated on uncoated wells in the presence of serum served as a positive control (not shown). Cell density in the positive control wells was set as 100% and cell density in other cells was presented as percent of positive control. Mean values ± standard deviation are shown (N = 3); *, P = 0.04 compared to Gal8 and Gal8+Suc groups. (B) Carbohydrate-binding activity of Galectins-1 and -3 is not lost upon adsorption onto plates. To assure that the lack of adhesion of TM cells to Gal1- and Gal3-coated wells is a not an artifact stemming from the loss of carbohydrate-binding activity of the lectins upon adsorption onto the culture wells, plates coated with galectins were probed with horseradish peroxidase-labeled asialofetuin (ASF), developed with 3,3′,5,5′-tetramethylbenzidine (TMB) substrate and absorbance was read at 650 nm on a plate reader. Ricinus communis agglutinin (RCA), a plant lectin with galactose-binding specificity, served as a positive control, and bovine serum albumin (BSA) served as a negative control. Note that the binding activity of Gal1 and Gal3 is the same as or higher than that of Gal8. Thus, the lack of binding of TM cells to Gal1 and Gal3 is not due to the loss of carbohydrate-binding activity of the lectins.
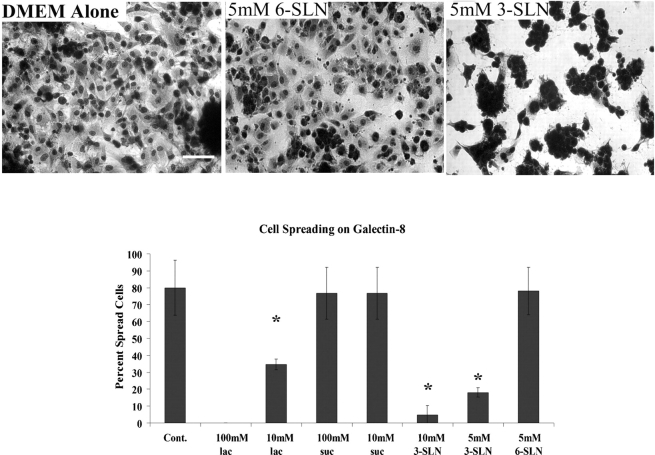
Cells attached to Gal8-coated wells showed a fully spread morphology (Figure 1A). The few cells that adhered to Gal8-coated wells in the presence of β-lactose clumped together and none of them spread (Figure 1A). To further establish the involvement of carbohydrate recognition in TM cell spreading on Gal8, the assays were performed in the presence and absence of ASF, this time providing the competing saccharides in the protein-bound form. Wells coated with human fibronectin (known to promote TM cell adhesion and spreading) and poly-l-lysine (known to promote TM cell adhesion but not spreading) (Zhou et al. 2000) served as positive and negative controls, respectively. Microscopic examination revealed that ASF inhibited the attachment of TM cells and severely inhibited cell spreading on Gal8-coated wells (80% reduction of cell spreading) (Figure 2). In contrast, ASF had no effect on cell spreading on fibronectin-coated wells (Figure 2). Cells did not spread on poly-l-lysine-coated wells regardless of the presence of ASF (not shown).
Fig. 2.
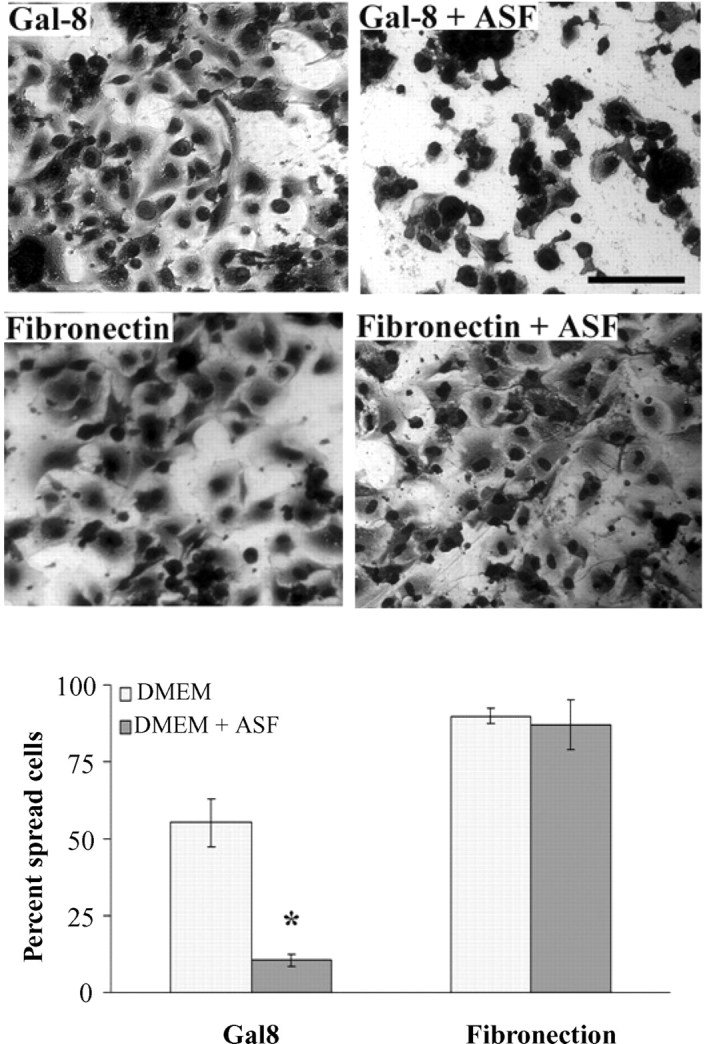
Asialofetuin inhibits the adhesion of TM cells to Gal8 but not to fibronectin. Normal human TM cells were incubated on microtiter wells coated with Gal8 or fibronectin (37°C, 7% CO2, 4 h) in serum-free DMEM, in the presence and absence of asialofetuin (ASF). Following incubation, the plates were stained with Giemsa. In the top panel are micrographs of representative fields from each group showing inhibition of cell spreading by ASF on Gal8-coated wells but not on fibronectin-coated wells. Ten random fields of each experimental condition were photographed, and spread cells were counted manually. Note that ASF inhibited cell spreading on Gal8 by about 80%, whereas it had no effect on the spreading of cells adhered to fibronectin. Mean values ± standard deviation are shown (N = 3); *P = 0.004 compared to the cells incubated on Gal8 in the absence of ASF. This experiment was performed twice with reproducible results. Magnification bars: 50 μm.
Carbohydrate-binding specificity of Gal8
The inhibitory effect of lactose and ASF (Figures 1 and 2) suggests that the carbohydrate-binding activity of the lectin is critical for the adhesion and spreading of TM cells on Gal8. The lack of adhesion and spreading of TM cells on Gal1 and Gal3 (Figure 1A) suggests that unique features of Gal8 are required. Gal8 has two carbohydrate recognition domains (N-CRD and C-CRD), and the specificity of both has been studied in detail (Ideo et al. 2003; Carlsson, Oberg, et al. 2007). The N-CRD has a unique preference for 3-sulfated and 3-sialylated galactosides, not shared by any other animal galectin; such structures are tolerated by Gal1 and Gal3 but not preferred. The C-CRD prefers blood group A- and B-type determinants, and, in this regard, Gal8 is similar to Gal3. Both Gal8 CRDs also bind to a range of saccharides with lower affinity than the best ligands, and these low-/moderate-affinity ligands may become important when the two CRDs act together, e.g., when intact Gal8 interacts with a cell surface, where the best ligands may not always be required (Carlsson, Oberg, et al. 2007). To shed further light on this complex issue, in the current study, we performed the saccharide-binding analysis of intact Gal8 on a glycan array to include galectin concentrations low enough to permit ranking of the saccharides that gave saturating binding in a recently published study (Carlsson, Oberg, et al. 2007). As shown in Table I, the best binding saccharides were those that have been previously shown to exhibit good binding affinity for both CRDs (Carlsson, Oberg, et al. 2007). These included 3′-O sulfated glycans, Galα1-3Gal containing glycans and LnNT (Galβ1-3GalNAcβ1-4Galβ1-4Glcβ). The combined action of both CRDs was probably responsible for Gal8 binding to these glycans even at very low concentration (∼10 nM Gal8, Table I). Other good binders were those with the highest affinity for either N-CRD such as 3′-O-sialylated galactosides or C-CRD such as GalNacα1-3Gal containing glycans. NeuAcα2-3Galβ1-4GlcNAcβ (3-SLN), the saccharide expected to be found in glycoproteins such as integrins, showed binding at 800 nM Gal8 concentration on the array (Table I, glycan no. 237). The entire list of glycans printed on the array with the respective signal intensities of Gal8 binding at each concentration is provided as supplementary Table S1.
Table I.
Glycan array data showing saccharide motifs that exhibited affinity to Gal8
| Glycan number | ||||
|---|---|---|---|---|
| on array | Structure | Binding at (nM)a | Motif in site B-Cb | Motif in site C-Db |
| 3-Sulfated | ||||
| 30 | [3OSO3]Galβ1-4(6OSO3)Glcβ-Sp8 | 8 | 3-Su-Galβ | Galβ1-4Glc |
| 28 | [3OSO3]Galβ1-4Glcβ-Sp8 | 300 | 3-Su-Galβ | Galβ1-4Glc |
| 32 | [3OSO3]Galβ1-3GalNAcα-Sp8 | 300 | 3-Su-Galβ | Galβ1-3GalNAc |
| 33 | [3OSO3]Galβ1-3GlcNAcβ-Sp8 | 300 | 3-Su-Galβ | Galβ1-3GlcNAc |
| 35 | [3OSO3]Galβ1-4[6OSO3]GlcNAcβ-Sp8 | 80 | 3-Su-Galβ | Galβ1-4GlcNAc |
| 36 | [3OSO3]Galβ1-4GlcNAcβ-Sp0 | 800 | 3-Su-Galβ | Galβ1-4GlcNAc |
| 3-Sialylated | ||||
| 240 | Neu5Acα2-3Galβ1-4Glcβ-Sp8 | 300 | Siaα2-3Galβ | Galβ1-4Glc |
| 226 | Neu5Acα2-3Galβ1-3GlcNAcβ-Sp8 | 300 | Siaα2-3Galβ | Galβ1-3GlcNAc |
| 202 | Neu5Acα2-3Galβ1-3GalNAcα-Sp8 | 300 | Siaα2-3Galβ | Galβ1-3GalNAc |
| 227 | Neu5Acα2-3Galβ1-4[6OSO3]GlcNAcβ-Sp8 | 200 | Siaα2-3Galβ | Galβ1-4GlcNAc |
| 237 | Neu5Acα2-3Galβ1-4GlcNAcβ-Sp8 | 800 | Siaα2-3Galβ | Galβ1-4GlcNAc |
| Blood group A and B | ||||
| 98 | Galα1-3(Fucα1-2)Galβ1-4Glcβ-Sp0 | 8 | Gal(NAc)α1-3Galβ | Galβ1-4Glc |
| 83 | GalNAcα1-3(Fucα1-2)Galβ1-4Glcβ-Sp0 | 80 | Gal(NAc)α1-3Galβ | Galβ1-4Glc |
| 95 | Galα1-3(Fucα1-2)Galβ1-3GlcNAcβ-Sp0 | 20 | Gal(NAc)α1-3Galβ | Galβ1-3GlcNAc |
| 79 | GalNAcα1-3(Fucα1-2)Galβ1-3GlcNAcβ-Sp0 | 80 | Gal(NAc)α1-3Galβ | Galβ1-3GlcNAc |
| 97 | Galα1-3(Fucα1-2)Galβ1-4GlcNAc-Sp0 | 20 | Gal(NAc)α1-3Galβ | Galβ1-4GlcNAc |
| 81 | GalNAcα1-3(Fucα1-2)Galβ1-4GlcNAcβ-Sp0 | 80 | Gal(NAc)α1-3Galβ | Galβ1-4GlcNAc |
| Other | ||||
| 149 | Galβ1-4GlcNAcβ1-3Galβ1-4Glcβ-Sp8 | 20 | GlcNAcβ1-3Galβ | Galβ1-4Glc |
| 283 | Galβ1-4GlcNAcβ1-3Galβ1-3GlcNAcβ-Sp0 | 80 | GlcNAcβ1-3Galβ | Galβ1-3GlcNAc |
| 147 | Galβ1-4GlcNAcβ1-3Galβ1-4GlcNAcβ-Sp0 | 200 | GlcNAcβ1-3Galβ | Galβ1-4GlcNAc |
| 235 | Neu5Acα2-3Galβ1-4GlcNAcβ1-3Galβ1-4GlcNAcβ1 | 40 | Siaα2-3Galβ or | Galβ1-4GlcNAc or |
| -3Galβ1-4GlcNAcβ-Sp0 | GlcNAcβ1-3Galβ | Galβ1-4Glc |
aThe lowest Gal8 concentration required to give above 20% of maximum signal. All binding glycans showed an appropriate dose response curve at higher concentrations, reaching maximum signal if possible within the galectin concentration range tested. Average background was <1% and highest <10%.
bThe likely motif bound in subsite B-C or C-D of the galectin CRD as described earlier (Leffler et al. 2004; Carlsson, Oberg, et al. 2007). Subsite B binds to β-lactose and defines a conserved binding site shared by all members of galectin family; the remaining subsites define the fine specificity for neighboring residues and vary among galectin CRDs.
To determine the importance of the Gal8 N-CRD for adhesion and/or spreading of TM cells on Gal8-coated wells, the assays were conducted in the presence and absence of 3-SLN, which binds to N-CRD but not to C-CRD of Gal8 (Carlsson, Oberg, et al. 2007). At 5 mM concentration, the trisaccharide inhibited cell adhesion and cell spreading to Gal8 by 48 and 90%, respectively (Figure 3). In contrast, 6-SLN which does not bind to either CRD had no effect (Figure 3). Compared to 3-SLN, a significantly higher concentration of β-lactose was required for a comparable inhibitory effect (Figure 3) as expected from the relative affinity of these saccharides for the N-CRD in solution (Carlsson, Oberg, et al. 2007).
Fig. 3.
α2-3-Sialylated glycans inhibit the adhesion and spreading of TM cells on Gal8-coated wells. Normal human TM cells were plated on microtiter wells (50,000 cells/well) coated with Gal8 (30 μg/mL), and the plates were incubated in serum-free DMEM in the presence and absence of β-lactose (100 mM, 10 mM), sucrose (100 mM), NeuAcα2-3Galβ1-4GlcNAc (3-SLN; 5 mM, 10 mM) or NeuAcα2-6Galβ1-4GlcNAc (6-SLN; 5 mM) (37°C, 7% CO2, 4 h). Following incubation, the plates were stained with Giemsa.Ten random fields of each experimental condition were photographed, and spread cells were counted manually. The top panel shows representative micrographs of cells. The bottom panel shows quantitation of spread cells under each experimental condition. Note that at 10 mM, β-lactose abolished only 50% of cell spreading whereas 3-SLN at 5 mM abolished 90% of cell spreading; 6-SLN, which does not bind to Gal8, did not interfere with TM cell spreading on Gal8. Sucrose at 100 mM did not inhibit cell spreading. Mean values ± standard deviation are shown (N = 3); *P < 0.005 compared to cells incubated on Gal8 in media alone or in the presence of sucrose or 6-SLN. Magnification bar: 50 μm.
β1 integrins are major counterreceptors for galectin-8 in human trabecular meshwork cells
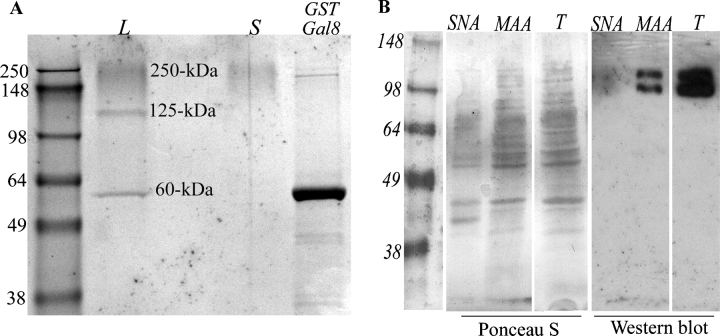
In an effort to understand the mechanism by which Gal8 mediates TM cell adhesion and spreading, information with respect to its counterreceptors on TM cells is prerequisite. To determine what the counterreceptors are for Gal8 that are expressed by TM cells, TM cell lysates were chromatographed on a Gal8-affinity matrix and the bound proteins were electrophoresed and identified by LC/MS/MS. On electrophoresis gels, three components (250, 125, and 60 kDa) appeared in the bound fraction eluted with β-lactose (Figure 4A, lane L). The 250-kDa component was also detected in the fraction eluted with the irrelevant sugar, sucrose (Figure 4A, lane S), and was, therefore, deemed nonspecific and not pursued further. The 125- and 60-kDa components eluted with lactose were excised, subjected to in-gel trypsin digestion, and analyzed by mass spectrometry. The 125-kDa band contained integrins β1, α3, α5, and αv. Protein sequences with identified peptides are available in supplemental material (Figure S1). These findings show that Gal8 has specific, carbohydrate-dependent interactions with α3β1, α5β1, and αvβ1 integrins in TM cells. In nonocular studies, Gal8 has been previously shown to bind integrins β1, α5, α3, αM, and α6 (Hadari et al. 2000; Nishi et al. 2003; Carcamo et al. 2006). The current study is the first report of Gal8 binding to integrin αv. The 60-kDa band was identified as GST-Gal8 that probably leached in a small amount from the column. None of the above components were detected when agarose beads conjugated with GST alone were used as the affinity matrix (not shown).
Fig. 4.
(A) β1 integrins are major counterrecptors for galectin-8 in TM cells. Protein extracts from confluent cultures of normal human TM cells were incubated with a Gal8-affinity matrix. Proteins bound to the matrix were eluted first with sucrose, a noncompeting sugar, and then with β-lactose, a competing sugar. Following elution, beads were boiled in the electrophoresis sample buffer and centrifuged, and supernatants were electrophoresed on a SDS–PAGE. Protein bands were visualized by staining with Coomassie brilliant blue and identified by mass spectrometry. Three main components (250, 125, and 60 kDa) were detected in the β-lactose eluate (L). The 250-kDa component was also detected in the sucrose eluted (S) fraction and was therefore deemed nonspecific. The 125-kDa band which was unique to the β-lactose eluate-contained integrins β1, α3, α5, and αv as determined by mass spectrometry. The 60-kDa band was identified as GST-Gal8, which probably leached from the column in small amounts. However, most of the GST-Gal8 remained on the column and was released after beads were boiled (GST-Gal8). This experiment was performed twice, with reproducible results. (B) TM cell β1 integrins contain α2-3-sialylated glycans. Protein extracts from confluent cultures of normal human TM cells were incubated with biotin-labeled SNA or biotin-labeled MAA (2 h, 4°C) and avidin-conjugated agarose beads. Following incubation, the beads were washed, boiled in the SDS–PAGE sample buffer to release bound proteins, and centrifuged; supernatants were electrophoresed on SDS–PAGE gels. The protein blot of the gel was stained with Ponceau S, and then processed for immunostaining with mouse anti β1 integrin. Note that the MAA (specificity: α2-3-sialylated glycans)-bound fraction as well as the total cell extract (T) contained an anti-β1 integrin-reactive doublet, characteristic of a glycosylated β1 integrin. In contrast, no anti-β1 integrin-reactive components were detected in the SNA (specificity: α2-6-sialylated glycans)-bound fraction. Also, no anti-β1 integrin-reactive components were detected in the supernatants of cell extracts incubated with the agarose beads alone or in the blots of unfractionated, whole cell extract not exposed to the primary antibody (not shown).
TM cell β1 integrins contain 3-sialylated glycans
Having shown that Gal8 binds to β1 integrins in TM cells and that the carbohydrate-binding activity of the N-CRD of Gal8 was required for the functional effects, it was of interest to determine whether β1 integrins in TM cells bear 3-sialylated glycans. Pull down experiments conducted using biotinylated plant lectins (MAA and SNA) in conjunction with Western blot analysis using anti-β1 integrin revealed that TM cell β1 integrins bind largely to MAA which binds selectively to 3-sialylated glycans, but not to SNA which binds selectively to 6-sialylated glycans (Figure 4B). These data suggest that β1 integrins in TM cells predominantly contain 3-sialylated glycans, the preferred ligands for the N-CRD of Gal8.
Discussion
The goal of the present study was to shed light on the mechanism by which Gal8 modulates TM cell adhesion and spreading. Our finding that the adhesion and spreading of TM cells on Gal8 substrate is inhibited specifically by β-lactose, 3-SLN, and ASF suggests that Gal8 promotes TM cell adhesion and spreading in a carbohydrate-dependent manner. The striking finding that Gal1 and Gal3, which can act as permissive substrates and promote adhesion of nonocular cells (Pesheva et al. 1998; Ramkumar and Podder 2000), did not promote the adhesion of TM cells suggests that Gal8-mediated TM cell adhesion events involve the affinity of Gal8 N-CRD for 3-sialylated galactosides (Ideo et al. 2003; Carlsson, Oberg, et al. 2007) that is unique among animal galectins. In support of this conclusion, the TM cell β1 integrins were found to carry predominantly 3-linked sialic acids. Moreover, specific inhibition of the Gal8 N-CRD with 3-SLN reduced adhesion and abolished spreading of TM cells on Gal8-coated wells.
Recent studies have demonstrated that sialylation distinctively modulates the recognition of cell surface glycans and biological signaling by different galectins. For example, Gal2 exhibits little or no binding to either α2-3- or α2-6-sialylated glycans (Stowell et al. 2008b), whereas both Gal1 and Gal3 tolerate the presence of terminal sialic acid in α2-3-linkage. Also, Gal1 does not tolerate the presence of terminal sialic acid in α2-6-linkage, and while Gal3 can tolerate the presence of α2-6-linked sialic acid (Stowell et al. 2008b), it preferentially binds to unsialylated, as compared to α2-6-sialylated glycans (Zhuo et al. 2008). Differential recognition of sialylated cell surface glycans by different galectins correlates well with their biological signaling activity as measured by cellular sensitivity to galectin-induced phosphatidylserine exposure (Stowell et al. 2008a), cell death (Toscano et al. 2007), and macrophage differentiation (Woodard-Grice et al. 2008). Although the more detailed relationship between Gal8 specificity and its effects on TM cells is expected to be complex (Carlsson, Oberg, et al. 2007; also current study), it appears that 3′-linked sialic acids on N-glycans of the TM cells bind Gal8 well enough to promote the TM cell adhesion and spreading effects observed in the current study. While initially, the N-CRD was reported to bind to glycolipids preferentially, it also binds to glycoproteins (Ideo et al. 2003). Despite the fact that 3-SLN binds Gal8 with significantly less affinity than the best Gal8 ligands (Table I), it is, most likely, still the relevant ligand for the Gal8-mediated effects observed in the current study. In fact, Gal8 affinity for 3-SLN (Table I) is similar to or better than the affinity that Gal1 and Gal3 have for their best ligands mediating various biological effects (Pesheva et al. 1998; Ramkumar and Podder 2000). Also, in nonocular studies utilizing human monocytic and T-lymphoblast cell lines (Carlsson, Oberg, et al. 2007) and Chinese hamster ovary cells (Patnaik et al. 2006), the best ligands of Gal8 were not required for Gal8-mediated binding and activation of signaling at cell surfaces. In these cells, Gal8-mediated binding occurs mainly to N-glycans, and the combined interactions of N- and C-CRDs with moderate affinity ligands appears to be enough for Gal8-mediated effects to occur (Patnaik et al. 2006; Carlsson, Carlsson, et al. 2007). In contrast, the best N-CRD ligands were found to modulate intracellular sorting after endocytosis instead (Carlsson, Carlsson, et al. 2007). Moreover, simultaneous interactions between (i) sialylated-N-glycans and N-CRD and (ii) C-CRD and close enough nonsialylated saccharide branches on the cell surface may result in a much higher combined affinity that would not be observed on the glycan array because the spots contain only one glycan each.
However, the above argument does not rule out the possibility that high-affinity Gal8 ligands may, at least to some degree, have also contributed to the interaction of Gal8 with the TM cell surface. For example, in contrast to Gal1 and Gal3, the core disaccharide binding site of the Gal8-CRDs (site C-D in Table I) prefers lactose that is commonly found in glycolipids and Galβ1-3GalNAc that is found in O-linked glycans, and the interaction between such glycans on TM cell surface and Gal8 can conceivably contribute to the TM cell–Gal8 interactions. Moreover, other Gal8 binding enhancing features, such as the 6-O-sulfate (e.g., glycan nos. 30, 35, and 227, Table I), also have the potential to contribute to the Gal8-mediated effects reported here. Even if the details of Gal8–cell surface interactions are likely to be more complex than previously anticipated, it is highly likely that Gal8 selectively mediates adhesion and spreading of TM cells, at least in part, due to the unique affinity of the N-CRD for 3-sialylated galactosides on β1 integrins based on the following: (i) α3β1, α5β1, and αvβ1 were identified as major counterreceptors of Gal8, (ii) TM cell β1 integrins carry predominantly 3-linked sialic acids; and (iii) an anti-β1 integrin antibody inhibits TM cell adhesion to Gal8 (Diskin et al., in preparation). The sialylation pattern of integrins in different cell types may vary, and this could have an impact on the function of integrins (Semel et al. 2002; Seales et al. 2003; 2005). For example, β1 integrin from myeloid cells (Semel et al. 2002), colon adenocarcinoma (Seales et al. 2005), and neutrophils, all contain 6-sialylated glycans which do not have affinity for Gal8. In contrast, as described above, in the current study, TM cell β1 integrins were found to contain largely 3-sialylated glycans which bind specifically to Gal8 N-CRD. In fact, hypersialylation with 6-glycans inhibits the binding of integrin to fibronectin (Seales et al. 2005). In contrast, the current study suggests that in TM cells, the presence of α2-3-sialylated glycans promotes the function of β1 integrins via a Gal8-mediated pathway. We note that in a recent study, Stowell et al. (2008a) found that cell signaling activity of Gal8, as measured by phosphatidylserine exposure in leukocytes, resides in the C-terminal CRD and that dimerization of Gal8 occurs through the N-terminal CRD. We hope to perform in depth studies aimed at the characterization of the role of the individual C-CRD and N-CRD in TM cell adhesion and spreading in the future.
Relatively few studies have thus far investigated the role of galectins in ocular diseases. It is known that Gal1 and Gal3 are expressed on both normal and glaucomatous TM cells (Fautsch et al. 2003). Although the role of these galectins in the pathogenesis of glaucoma remains to be investigated, both galectins are well known for their role in a variety of biological processes including the regulation of cell growth, apoptosis, and signaling, and therefore, have the potential to play a pivotal role in the control of IOP and the pathogenesis of ocular hypertension. The current study suggests that Gal8 modulates TM cell adhesion and spreading by interacting with α2-3-sialylated glycans on β1 integrins. This function may allow Gal8 to participate in the regulation of aqueous outflow.
Supplementary Data
Supplementary data for this article is available online at http://glycob.oxfordjournals.org/.
Funding
National Institutes of Health Grants R03EY015168 (N.P.); vision research P30EY13078; Mass Lions Eye Research fund; Research to Prevent Blindness.
Acknowledgments
We thank Dr. F. T Liu for bacteria expressing Gal1 and purified recombinant Gal3, Drs. Alfonso Gonzalez and Andrea Soza for bacteria expressing GST-Gal8, and Drs. Linda Baum, David Smith, Jon Degnore, Teresa Borrás, and Richard Alvarez, for helpful discussions. Glycan array analysis was performed by the Consortium for Functional Glycomics.
Conflict of interest statement
None declared.
Abbreviations
- 3-SLN
NeuAcα2-3Galβ1-4GlcNAc
- 6-SLN
NeuAcα2-6Galβ1-4GlcNAc
- ASF
asialofetuin
- CRD
carbohydrate recognition domain
- DMEM
Dulbecco's modified eagle medium
- ECM
extracellular matrix
- FBS
fetal bovine serum
- Gal1
galectin-1
- Gal3
galectin-3
- Gal8
galectin-8
- GST
glutathione-S-transferase
- HRP
horseradish peroxidase
- IOP
intraocular pressure
- POAG
primary open angle glaucoma
- RFU
relative fluorescence units
- RIPA
radioimmunoprecipitation
- SDS–PAGE
sodium dodecyl sulfate– polyacrylamide gel electrophoresis
- TBS
Tris buffered saline
- TM
trabecular meshwork
References
- Alvarado J, Murphy C, Juster R. Trabecular meshwork cellularity in primary open-angle glaucoma and nonglaucomatous normals. Ophthalmology. 1984;91:564–579. doi: 10.1016/s0161-6420(84)34248-8. [DOI] [PubMed] [Google Scholar]
- Blixt O, Head S, Mondala T, Scanlan C, Hufle ME Jr, Alvarez R, Bryan MC, Fazio F, Calarese D, Stevens J, et al. Printed covalent glycan array for ligand profiling of diverse glycan binding proteins. Proc Natl Acad Sci USA. 2004;101:17033–17038. doi: 10.1073/pnas.0407902101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Candiano G, Bruschi M, Musante L, Santucci L, Ghiggeri GM, Carnemolla B, Orecchia P, Zardi L, Righetti PG. Blue silver: A very sensitive colloidal Coomassie G-250 staining for proteome analysis. Electrophoresis. 2004;25:1327–1333. doi: 10.1002/elps.200305844. [DOI] [PubMed] [Google Scholar]
- Cao Z, Said N, Amin S, Wu HK, Bruce A, Garate M, Hsu DK, Kuwabara I, Liu FT, Panjwani N. Galectins-3 and -7, but not galectin-1, play a role in re-epithelialization of wounds. J Biol Chem. 2002;277:42299–42305. doi: 10.1074/jbc.M200981200. [DOI] [PubMed] [Google Scholar]
- Carcamo C, Pardo E, Oyanadel C, Bravo-Zehnder M, Bull P, Caceres M, Martinez J, Massardo L, Jacobelli S, Gonzalez A, et al. Galectin-8 binds specific beta1 integrins and induces polarized spreading highlighted by asymmetric lamellipodia in Jurkat T cells. Exp Cell Res. 2006;312:374–386. doi: 10.1016/j.yexcr.2005.10.025. [DOI] [PubMed] [Google Scholar]
- Carlsson S, Carlsson MC, Leffler H. Intracellular sorting of galectin-8 based on carbohydrate fine specificity. Glycobiology. 2007;17:906–912. doi: 10.1093/glycob/cwm059. [DOI] [PubMed] [Google Scholar]
- Carlsson S, Oberg CT, Carlsson MC, Sundin A, Nilsson UJ, Smith D, Cummings RD, Almkvist J, Karlsson A, Leffler H. Affinity of galectin-8 and its carbohydrate recognition domains for ligands in solution and at the cell surface. Glycobiology. 2007;17:663–676. doi: 10.1093/glycob/cwm026. [DOI] [PubMed] [Google Scholar]
- Dam TK, Gabius HJ, Andre S, Kaltner H, Lensch M, Brewer CF. Galectins bind to the multivalent glycoprotein asialofetuin with enhanced affinities and a gradient of decreasing binding constants. Biochemistry. 2005;44:12564–12571. doi: 10.1021/bi051144z. [DOI] [PubMed] [Google Scholar]
- Diskin S, Cao Z, Gong H, Soza A, González A, Panjwani N. Galectin-8 promotes cytoskeletal rearrangement in trabecular meshwork cells through activation of Rho signaling. Invest Ophthalmol Vis Sci. doi: 10.1371/journal.pone.0044400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Einarson MB. Detection of protein–protein interactions using the GST fusion protein pull-down technique. Nat Meth. 2004;1:275–276. [Google Scholar]
- Fautsch MP, Silva AO, Johnson DH. Carbohydrate binding proteins galectin-1 and galectin-3 in human trabecular meshwork. Exp Eye Res. 2003;77:11–16. doi: 10.1016/s0014-4835(03)00107-6. [DOI] [PubMed] [Google Scholar]
- Hadari YR, Arbel-Goren R, Levy Y, Amsterdam A, Alon R, Zakut R, Zick Y. Galectin-8 binding to integrins inhibits cell adhesion and induces apoptosis. J Cell Sci. 2000;113(Pt 13):2385–2397. doi: 10.1242/jcs.113.13.2385. [DOI] [PubMed] [Google Scholar]
- Ideo H, Seko A, Ishizuka I, Yamashita K. The N-terminal carbohydrate recognition domain of galectin-8 recognizes specific glycosphingolipids with high affinity. Glycobiology. 2003;13:713–723. doi: 10.1093/glycob/cwg094. [DOI] [PubMed] [Google Scholar]
- Leffler H, Carlsson S, Hedlund M, Qian Y, Poirier F. Introduction to galectins. Glycoconj J. 2004;19:433–440. doi: 10.1023/B:GLYC.0000014072.34840.04. [DOI] [PubMed] [Google Scholar]
- Levy Y, Arbel-Goren R, Hadari YR, Eshhar S, Ronen D, Elhanany E, Geiger B, Zick Y. Galectin-8 functions as a matricellular modulator of cell adhesion. J Biol Chem. 2001;276:31285–31295. doi: 10.1074/jbc.M100340200. [DOI] [PubMed] [Google Scholar]
- Levy Y, Ronen D, Bershadsky AD, Zick Y. Sustained induction of ERK, protein kinase B, and p70 S6 kinase regulates cell spreading and formation of F-actin microspikes upon ligation of integrins by galectin-8, a mammalian lectin. J Biol Chem. 2003;278:14533–14542. doi: 10.1074/jbc.M207380200. [DOI] [PubMed] [Google Scholar]
- Nishi N, Shoji H, Seki M, Itoh A, Miyanaka H, Yuube K, Hirashima M, Nakamura T. Galectin-8 modulates neutrophil function via interaction with integrin alphaM. Glycobiology. 2003;13:755–763. doi: 10.1093/glycob/cwg102. [DOI] [PubMed] [Google Scholar]
- Patnaik SK, Potvin B, Carlsson S, Sturm D, Leffler H, Stanley P. Complex N-glycans are the major ligands for galectin-1, -3, and -8 on Chinese hamster ovary cells. Glycobiology. 2006;16:305–317. doi: 10.1093/glycob/cwj063. [DOI] [PubMed] [Google Scholar]
- Pesheva P, Kuklinski S, Schmitz B, Probstmeier R. Galectin-3 promotes neural cell adhesion and neurite growth. J Neurosci Res. 1998;54:639–654. doi: 10.1002/(SICI)1097-4547(19981201)54:5<639::AID-JNR9>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Polansky J, Alvarado J. Cellular mechanisms influencing the aqueous humor outflow pathway. In: Albert DM, Jakobiec FA, editors. Principles and Practice of Ophthalmology: Basic Science. Philadelphia: WB Saunders; 1994. pp. 226–251. [Google Scholar]
- Ramkumar R, Podder SK. Elucidation of the mechanism of interaction of sheep spleen galectin-1 with splenocytes and its role in cell–matrix adhesion. J Mol Recognit. 2000;13:299–309. doi: 10.1002/1099-1352(200009/10)13:5<299::AID-JMR504>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Seales EC, Jurado GA, Burnson BA, Wakefield JK, Frost AR, Bellis SL. Ras oncogene directs expression of a differentially sialylated, functionally altered beta1 integrin. Oncogene. 2003;22:7137–7145. doi: 10.1038/sj.onc.1206834. [DOI] [PubMed] [Google Scholar]
- Seales EC, Jurado GA, Singhal A, Bellis SL. Hypersialylation of beta1 integrins, observed in colon adenocarcinoma, may contribute to cancer progression by up-regulating cell motility. Cancer Res. 2005;65:4645–4652. doi: 10.1158/0008-5472.CAN-04-3117. [DOI] [PubMed] [Google Scholar]
- Semel AC, Seales EC, Singhal A, Eklund EA, Colley KJ, Bellis SL. Hyposialylation of integrins stimulates the activity of myeloid fibronectin receptors. J Biol Chem. 2002;277:32830–32836. doi: 10.1074/jbc.M202493200. [DOI] [PubMed] [Google Scholar]
- Shibuya N, Goldstein IJ, Broekaert WF, Nsimba-Lubaki M, Peeters B, Peumans WJ. The elderberry (Sambucus nigra L.) bark lectin recognizes the Neu5Ac(alpha 2-6)Gal/GalNAc sequence. J Biol Chem. 1987;262:1596–1601. [PubMed] [Google Scholar]
- Stamer WD, Seftor RE, Williams SK, Samaha HA, Snyder RW. Isolation and culture of human trabecular meshwork cells by extracellular matrix digestion. Curr Eye Res. 1995;14:611–617. doi: 10.3109/02713689508998409. [DOI] [PubMed] [Google Scholar]
- Stowell SR, Arthur CM, Mehta P, Slanina KA, Blixt O, Leffler H, Smither DF, Cummings RD. Dimeric galectin-8 induces phosphatidylserine exposure in leukocytes through polylactosamine recognition by the C-terminal domain. J Biol Chem. 2008a;283:20547–20559. doi: 10.1074/jbc.M802495200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stowell SR, Arthur CM, Slnina KA, Horton JR, Smith DF, Cummings RD. Galectin-1, -2, and -3 exhibit differential recognition of sialylated glycans and blood group antigens. J Biol Chem. 2008b;283:10109–10123. doi: 10.1074/jbc.M709545200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toscano MA, Bianco GA, Ilarregui JM, Corci DO, Correale J, Hernandez JD, Zwirner NW, Poirier F, Riley EM, Baum LG, et al. Differential glycosylation of TH1, TH2 and TH-17 effector cells selectively regulates susceptibility to cell death. Nat Immunol. 2007;8:825–834. doi: 10.1038/ni1482. [DOI] [PubMed] [Google Scholar]
- Wang WC, Cummings RD. The immobilized leukoagglutinin from the seeds of Maackia Amurensis binds with high affinity to complex-type Asn-linked oligosaccharides containing terminal sialic acid-linked alpha-2,3 to penultimate galactose residues. J Biol Chem. 1988;263:4576–4585. [PubMed] [Google Scholar]
- Weinreb RN, Khaw PT. Primary open-angle glaucoma. Lancet. 2004;363:1711–1720. doi: 10.1016/S0140-6736(04)16257-0. [DOI] [PubMed] [Google Scholar]
- Woodard-Grice AV, McBrayer AC, Wakefield JK, Zhou Y, Bellis SL. Proteolytic shedding of ST6Gal-I by BACE1 regulates the glycosylation and function of alpha 4 beta 1 integrins. J Biol Chem. 2008;283:26364–26373. doi: 10.1074/jbc.M800836200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yates JR, 3rd, Eng JK, McCormack AL, Schieltz D. Method to correlate tandem mass spectra of modified peptides to amino acid sequences in the protein database. Anal Chem. 1995;67:1426–1436. doi: 10.1021/ac00104a020. [DOI] [PubMed] [Google Scholar]
- Zhou L, Cheng EL, Rege P, Yue BY. Signal transduction mediated by adhesion of human trabecular meshwork cells to extracellular matrix. Exp Eye Res. 2000;70:457–465. doi: 10.1006/exer.1999.0806. [DOI] [PubMed] [Google Scholar]
- Zhuo Y, Chammas R, Bellis SL. Sialylation of {beta}1 integrins blocks cell adhesion to galectin-3 and protects cells against galectin-3-induced apoptosis. J Biol Chem. 2008;283:22177–22185. doi: 10.1074/jbc.M8000015200. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.