Abstract
Glycopeptidolipids (GPLs) are a class of glycolipids produced by several nontuberculosis-causing members of the Mycobacterium genus including pathogenic and nonpathogenic species. GPLs are expressed in different forms with production of highly antigenic, typeable serovar-specific GPLs in members of the Mycobacterium avium complex (MAC). M. avium and M. intracellulare, which comprise this complex, are slow-growing mycobacteria noted for producing disseminated infections in AIDS patients and pulmonary infections in non-AIDS patients. Previous studies have defined the gene cluster responsible for GPL biosynthesis and more recent work has characterized the function of the individual genes. Current research has also focused on the GPL's role in colony morphology, sliding motility, biofilm formation, immune modulation and virulence. These topics, along with new information on the enzymes involved in GPL biosynthesis, are the subject of this review.
Keywords: biofilm, GPL, morphology, mycobacteria, review
Introduction
Glycopeptidolipids (sGPLs) are a class of glycolipids produced by several nontuberculosis-causing (NTM) members of the Mycobacterium genus. Rapidly growing mycobacteria associated with human disease, such as M. abscessus and M. chelonae, express GPLs (Howard and Byrd 2000; Ripoll et al. 2007). Moreover, GPLs are produced by rapidly growing saprophytic M. smegmatis as well as by animal pathogens, including M. porcinum and M. senegalense (Lopez Marin et al. 1993; Howard and Byrd 2000; Ripoll et al. 2007). Members of the Mycobacterium avium complex (MAC), which includes M. avium and M. intracellulare, are slow-growing mycobacteria noted for producing disseminated infections in AIDS patients as well as pulmonary infections in non-AIDS patients (Horsburgh 1999; Field et al. 2004; Wagner and Young 2004). These species are characterized by their production of highly antigenic, typeable serovar-specific GPLs (ssGPLs). The identification and characterization of GPLs was initiated by the work of Schaefer, Marks, and Jenkins in the 1960s and 1970s. Schaefer and colleagues observed that opportunistic mycobacteria could be classified by seroagglutination reactions (Schaefer 1965). Later, it was shown that thin-layer chromatography (TLC) could be utilized to characterize the different lipid profiles of NTM and to complement the use of seroagglutination in NTM classification (Jenkins et al. 1971; Marks et al. 1971). This provided a link between serovar specificity and surface lipid composition. Since mycobacterial species which express GPLs include some important pathogens, significant effort has been invested in determining the structure of GPLs as well as investigating their functions and role in pathogenesis. This review will focus on various aspects of GPLs, including their structure and the biosynthetic pathway, as well as their effect on colony morphology, sliding motility, biofilm formation, and immune modulation.
GPL structure
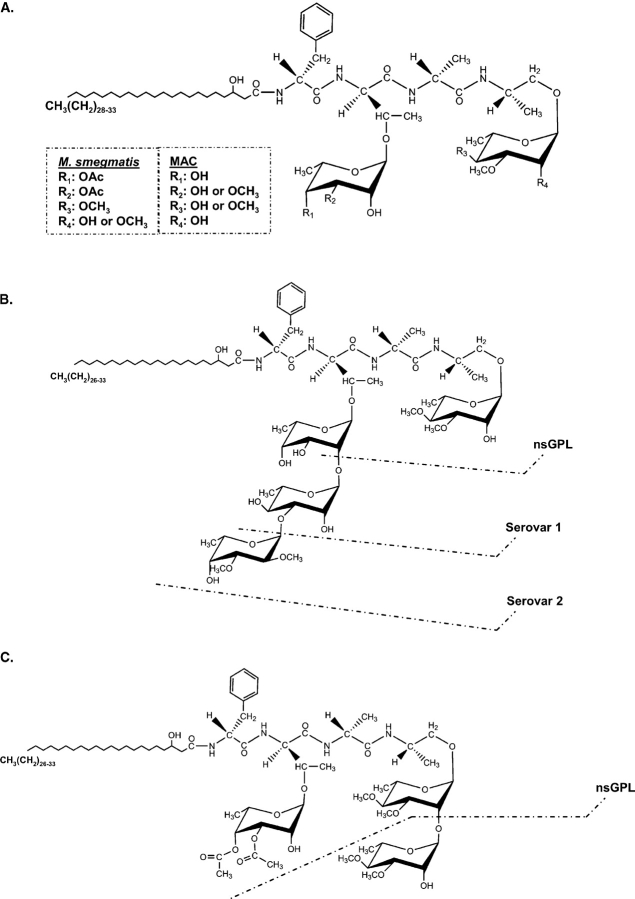
GPLs are composed of a lipopeptide core structure containing a 3-hydroxy or a 3-methoxy C26-C33 fatty acyl chain N-linked to a tripeptide-amino-alcohol core generally made up of d-phenylalanine-d-allo-threonine-d-alanine-l-alaninol. This lipopeptide core is glycosylated with the allo-threonine glycosidically linked to a 6-deoxy-α-l-talose (6-deoxytalose) and the alaninol glycosidically linked to an α-l-rhamnose (rhamnose) (Figure 1A and Table I). These di-glycosylated GPLs make up the apolar or nonseovar-specific (ns) species. In the case of the MAC, the 6-deoxytalose may be nonmethylated or 3-O-methylated, and the rhamnose is either 3-O-methylated or 3,4-di-O-methylated (Figure 1A). The nsGPLs may also be O-acetylated at various locations, depending on the strain. In contrast, M. smegmatis, M. abscessus, and M. chelonae produce nonspecific GPLs (nsGPLs) that contain a 6-deoxytalose, that is 3,4-di-O-acetylated, and a rhamnose, that is 2,3,4-tri-O-methylated or 3,4-di-O-methylated (Patterson et al. 2000; Villeneuve et al. 2003; Ripoll et al. 2007) (Table I). M. senegalense, M. porcinum, and M. peregrinum contain a different GPL core structure with a 3-O-methyl rhamnose attached to the allo-threonine instead of a 6-deoxytalose (Lopez Marin et al. 1993) (Table I).
Fig. 1.
GPL structures. (A) The nonspecific GPLs (nsGPLs) of MAC and M. smegmatis. These GPLs share the same di-glycosylated N-linked fatty acyl tripeptide-amino-alcohol core. However, there are slight differences in the modification of the α-l-rhamnose (rhamnose) and 6-deoxy-α-l-talose (6-deoxytalose). In the case of MAC, the rhamnose is either 3-O-methylated or 3,4-di-O-methylated, and the 6-deoxytalose is 3-O-methylated or nonmethylated. In the case of M. smegmatis, its nsGPLs contain a 3,4-di-O-methyl rhamnose or a 2,3,4-tri-O-methyl rhamnose, and the 6-deoxytalose is usually 3,4-di-O-acetylated. MAC strains may also have acetyl groups at various undefined locations on the GPL depending on the strain of mycobacteria. (B) MAC strains produce polar, serovar-specific GPLs (ssGPLs). ssGPLs contain an α-l-rhamnose that is always 3,4-di-O-methylated, and the nonmethylated 6-deoxy-α-l-talose is extended with additional carbohydrate moieties. Different ssGPLs possess different oligosaccharide appendages. For example, the serovar 1 GPL contains an α-l-rhamnose-(1→2)-6-deoxy- α-l-talose linked to the allo-threonine of the lipopeptide core, and the serovar 2 GPL contains a 2,3-di-O-methyl-α-l-fucose-(1→3) linked to the rhamnose of the serovar 1 GPL (reviewed by Chatterjee and Khoo 2001). (C) M. smegmatis produces a polar GPL. A nsGPL is modified with an additional methylated rhamnose residue; however, unlike the ssGPLs of MAC, glycosylation does not occur at the 6-deoxytalose. A 3,4-di-O-methyl-α-l-rhamnose is (1→2) linked to the 3,4-di-O-methyl-α-l-rhamnose attached to the alaninol of the lipopeptide core, and the 6-deoxy-α-l-talose is 3,4-di-O-acetylated. Ac: acetyl.
Table I.
GPLs are produced by different species of Mycobacterium. These species of mycobacteria produce GPLs with the same lipopeptide core, but which vary in glycosylation, methylation, and acetylation. Generally, a methylated rhamnose (Rha) is glycosidically linked to the lipopeptide core at the alaninol (Aol), and an O-methylated (OMe) or O-acetylated (OAc) 6-deoxytalose (6dTal) is linked to the allo-threonine (T). However, some species contain a 3-O-methyl-rhamnose attached to the allo-threonine. Additional carbohydrates glycosidically linked to the 6-deoxytalose constitute the polar GPLs in the case of M. avium and M. intracellulare.
| Species | Nonspecific GPLs | Polar GPLs | Serovar-specific GPLs | References |
|---|---|---|---|---|
| M. avium M. intracellulare | Aol: Rha (3,4-OMe2 or 3-OMe) T: 6dTal (3-OMe or nonmethylated | See serovar-specific GPLs | Aol: 3,4-OMe2Rha T: 6dTal extended with oligosaccharide (usually beginning w/Rha) | Chatterjee and Khoo 2001 |
| M. abscessus M. chelonae M. smegmatis | Aol: Rha (2,3,4-OMe3 or 3,4-OMe2) T: 3,4-OAc26dTal | Aol: 3,4-OMe2Rha (1→2)3,4OMe2Rha T: 3,4-OAc26dTal | N/A | Villeneuve et al. 2003 Ripoll et al. 2007 |
| M. peregrinum M. porcinum M. senegalense | Aol: 3,4OMe2Rha T: 3-OMeRha | Aol: 3,4-OMe2Rha (1→2)Rha (methylated or nonmethylated) T: 3-OMeRha | N/A | Lopez Marin et al. 1993 |
In addition to the nsGPLs, M. avium and M. intracellulare produce a variety of polar or ssGPLs. The terminal rhamnose linked to the alaninol is always 3,4-di-O-methylated, and the 6-deoxytalose is extended with various oligosaccharide residues, usually beginning with a rhamnose (reviewed by Chatterjee and Khoo 2001) (Figure 1B). A variety of ssGPLs may be produced depending on the carbohydrate modifications, and accordingly, they distinguish the different typeable, serovar-specific MAC strains. For example, MAC serovar 1 produces GPLs that contain a rhamnose attached to the 6-deoxytalose, whereas serovar 2 strains produce GPLs that have a di-O-methylated α-l-fucose attached to the rhamnose of the serovar 1 strain (Figure 1B). Individual strains of MAC produce only one species of ssGPL.
Some rapidly growing mycobacteria, such as M. smegmatis, M. abscessus, and M. chelonae, also create polar GPLs. However, these polar GPLs are different from the ssGPLs of MAC, as they are produced by the addition of a 3,4-di-O-methyl rhamnose attached to the alaninol-linked 3,4-di-O-methyl rhamnose (Table I). They also contain a di-O-acetylated 6-deoxytalose, which is not further glycosylated (Villeneuve et al. 2003; Ripoll et al. 2007) (Figure 1C and Table I). In the case of M. smegmatis, this polar GPL is produced under conditions of carbon starvation (Ojha et al. 2002; Mukherjee et al. 2005), and unlike the ssGPLs of the MAC, the structure does not vary and therefore does not render serovar specificity to M. smegmatis. M. senegalense, M. peregrinum, and M. porcinum also produce triglycosylated GPLs; however, the rhamnosyl disaccharide attached to the alaninol varies in methylation, and a 3-O-methyl rhamnose is attached to the allo-threonine in place of the di-O-acetylated 6-deoxytalose as found in M. smegmatis (Lopez Marin et al. 1993) (Table I).
GPL biosynthesis
A strong understanding of the GPL biosynthetic pathways is fundamental to defining GPLs’ role in mycobacterial virulence. Much work has been completed to elucidate the biosynthesis of both nsGPLs and ssGPLs of the MAC, M. smegmatis, M. abscessus, and M. chelonae. The M. smegmatis mc2155 strain has often been used for biosynthetic studies as this strain is easy to manipulate and it mostly produces simple, nsGPL structures.
Formation of the lipopeptide core
In 1999, Billman-Jacobe and colleagues isolated a mycobacterial peptide synthetase (mps) gene required for the formation of the lipopeptide core of GPLs in M. smegmatis and showed that the MPS enzyme functions by transferring amino acids to a peptide acceptor, where the first three domains also contain racemase activity involved in converting the first three amino acids to the d-isomers (Billman-Jacobe et al. 1999). A more recent study indicates that the M. smegmatis MPS activity is encoded by two genes designated mps1 and mps2 (Sonden et al. 2005) (Figure 2). In the same study, it was also shown that a gene encoding a polyketide synthase (pks) was important for the formation of the lipopeptide core, and another gene called gap was important in transporting GPLs to the surface of M. smegmatis (Sonden et al. 2005). In the case of M. avium, pstA and pstB were shown to be similar to the M. smegmatis mps genes in that they are essential for lipopeptide core formation (Freeman et al. 2006) (Figure 2).
Fig. 2.
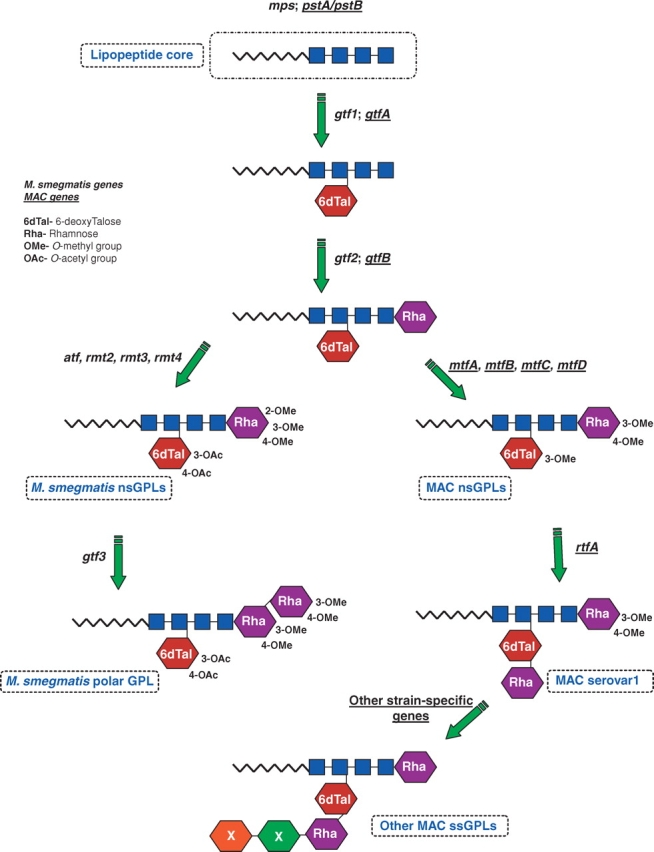
The biosynthetic pathways for formation of GPLs. The biosynthesis of GPLs produced by M. smegmatis and MAC has largely been delineated. Genes involved in producing nsGPLs of M. smegmatis and MAC (genes underlined) share homology and function; however, there are slight differences in the methylation, acetylation, and glycosylation pathways. MAC and M. smegmatis encode genes specific to producing the respective ssGPLs. Boxes denote peptides; 6dTal: 6-deoxytalose; Rha: rhamnose; OMe: O-methyl; OAc: O-acetyl; mps: mycobacterial peptide synthetase (M. smegmatis); pks: polyketide synthase; pst: peptide synthetase (M. avium); gtf: glycosyltransferase; atf: acetyltransferase; mtf: methyltransferase (M. avium); rmt: rhamnose methyltransferase (M. smegmatis); rtf: rhamnosyltransferase.
Glycosylation of GPLs
Original genetic studies resulted in the isolation of a 22–27 kb gene cluster (designated ser2 cluster) which encoded the genes required to produce serovar 2 GPL (Belisle et al. 1991). Later studies by this group lead to isolation of rough M. avium 2151 mutants with deletions of the entire ser2 cluster. These mutants expressed the lipopeptide core devoid of carbohydrates and failed to express the ns- or ssGPL (Belisle, Klaczkiewicz, et al. 1993; Belisle, McNeil, et al. 1993). These studies delineated the region of the M. avium genome responsible for glycosylation of the lipopeptide core. Subsequent studies have addressed the role of individual genes within the cluster (Mills et al. 1994). Eckstein and colleagues (1998) expressed M. avium rtfA in M. smegmatis, whereby it produced ssGPLs, and demonstrated that the rtfA gene functioned as a rhamnosyltransferase by adding a rhamnose to the 6-deoxytalose, the first step necessary for generating ssGPLs (Figure 2). The specificity of rtfA was later confirmed by generating M. avium rtfA mutants (Maslow et al. 2003).
After the findings by Eckstein et al. (1998), other studies were carried out to identify other glycosyltransferases involved in the synthesis of M. smegmatis and M. avium GPLs. The glycosyltransferases responsible for adding the 6-deoxytalose and the rhamnose to the core lipopeptide in MAC were identified, GtfA and GtfB, respectively (Eckstein et al. 2003). Miyamoto et al. (2006) observed that M. smegmatis gtf1 and gtf2 were functionally equivalent to MAC gtfA and gtfB, respectively. In addition, the glycosyltransferase involved in creating the polar GPL of M. smegmatis under carbon-starved conditions, namely Gtf3, was also discovered, and this enzyme functions by adding the 3,4-di-O-methyl-rhamnose to the terminal 3,4-di-O-methyl rhamnose (Ojha et al. 2002; Deshayes et al. 2005; Mukherjee et al. 2005; Miyamoto et al. 2006) (Figure 2). Other glycosyltransferases involved in the formation of ssGPLs have also been identified, such as those involved in the formation of serovar 2 GPL (Eckstein et al. 2003; Miyamoto et al. 2007), serovar 7 GPL (Fujiwara et al. 2007), serovar 8 GPL (Irani et al. 2004), serovar 12 GPL (Nakata et al. 2008), and any fucose-containing ssGPLs, including serovars 2, 3, 4, and 9 (Miyamoto et al. 2007). The GPL biosynthetic clusters of different MAC serovars of diverse virulence have also been compared and show slight modifications; however, a correlation between the gene sequence and virulence was not observed (Krzywinska and Schorey 2003). Interestingly, these studies resulted in the identification of homologous gene transfer between two different M. avium serotypes (Krzywinska et al. 2004). This was the first study to show naturally occurring homologous recombination in a pathogenic species of Mycobacterium.
GPL modifications
In addition to identifying the glycosyltransferases involved in GPL biosynthesis, various studies have identified the genes responsible for the methylation and acetylation of the GPLs. Patterson et al. (2000) created a M. smegmatis transposon mutant with a disrupted mtf1 gene, which completely lacked methylated GPLs. The authors concluded that the disrupted mtf1 gene encoded a rhamnosyl 3-O-methyl transferase, and that methylation at the C3 carbon of the rhamnose was necessary for subsequent methylation at C2 and C4. The M. smegmatis mtf1 gene, later re-named rmt3 by the same group, also showed homology to the M. avium mtfD gene. Jeevarajah and colleagues (2004) defined the remaining methyltransferases, including those involved in methylating the fatty acyl moiety and the terminal rhamnosyl residue at C2 and C4 (fmt, rmt2, and rmt4, respectively). In the same study, the authors performed complementation studies to confirm the methyltransferase activity of homologous M. avium methyltransferases, namely mtfB and mtfC with 4-O-methyltransferase activity and mtfD with 3-O-methyltransferase activity (Jeevarajah et al. 2002, 2004). mtfA also has high similarity to mtfD, suggesting that the MtfA enzyme likely methylates the 6-deoxytalose on the C3 carbon (Eckstein et al. 2003) (Figure 2).
Krzywinska et al. (2005) also produced a 3-O-methyl transferase M. avium mutant (mtfD deficient) via homologous recombination, which not only lacked nsGPLs with methylated rhamnose residues but also lacked the ability to produce ssGPLs. How the loss of methylated rhamnose on the nsGPLs results in a failure to produce ssGPLs is currently unknown; however, it supports the hypothesis that mature nsGPLs are the substrate for forming ssGPLs. Eckstein et al. (2003) have determined that M. avium 724 possesses a putative O-acetyltransferase (atfA), whereas M. avium 2151 lacks this gene. Comparative genomic studies showed that M. smegmatis has one acetyltransferase gene, whereas both M. abscessus and M. chelonae have two. The single M. smegmatis acetyltransferase and the two present in M. abscessus and M. chelonae function to acetylate the 6-deoxytalose at the C3 and C4 carbons (Ripoll et al. 2007). As predicted, a M. smegmatis mutant deficient in this acetyltransferase lacked both acetyl groups on the 6-deoxytalose (Recht and Kolter 2001).
Effects of drugs on GPL composition
The question of how GPL composition might affect antibiotic efficacy, or how antibiotics might alter GPL profiles, has stimulated recent interest. In 1999, Khoo and colleagues determined that ethambutol-susceptible and -resistant M. avium serovar 1 strains had different GPL profiles. The susceptible strain had fewer and only polar GPLs, whereas the resistant strain had mostly nsGPLs (Khoo et al. 1999). This suggests that more hydrophobic GPLs may render the cell wall less permeable to antibiotics. Another study demonstrated that treatment with the antifungal azole inhibitors, econazole and clotrimazole, which function by inhibiting cytochrome p450, were not only effective at eliminating M. smegmatis growth but also at decreasing the production of GPLs by M. smegmatis (Burguiere et al. 2005). It is thought that cytochrome p450 catalyzes the hydroxylation of fatty acids, and this may be necessary for the production of GPLs (Burguiere et al. 2005). Although these observations are intriguing, more studies are needed to connect GPL composition with antibiotic resistance. Whether certain antibiotics are able to alter GPL profiles sufficiently to modulate the immune response has yet to be determined.
MAC GPLs in AIDS and non-AIDS patients
Studies to elucidate the GPLs’ role in virulence have focused primarily on MAC, as these organisms are some of the most common sources of bacterial disseminated infections in AIDS patients, causing significant morbidity and mortality (Horsburgh 1991). Members of MAC also cause pulmonary infections in non-AIDS patients, sometimes referred to as hot-tub lung or Lady Windermere's syndrome (Field et al. 2004; Wagner and Young 2004). Due to the nature of their hydrophobic, waxy cell wall, these mycobacteria are able to withstand harsh environmental conditions, are chlorine resistant, and therefore survive and form biofilms in filtered drinking water systems (Hilborn et al. 2006). Drinking water is a major source of infection, especially in hospitals, whereupon ingestion, the bacteria can colonize and invade the gastrointestinal tract. Colonization in the lungs can also occur following inhalation of aerosolized droplets containing NTM. Dissemination occurs in immunocompromised individuals as the mycobacteria enter the vascular system and colonize various organs (Horsburgh 1999).
Interest in serovar-specific GPLs (ssGPLs) stems in part from studies that have shown certain MAC strains to be isolated more frequently from AIDS patients. For example, MAC serovars 4 and 8 were the most common isolates from AIDS patients (Horsburgh et al. 1986; Tsang et al. 1992). However, studies have also demonstrated AIDS and non-AIDS patients to have the same serovar-specific isolates, suggesting that the prevalence of certain MAC strains may be due to their geographic distribution (Yakrus and Good 1990; Torrelles et al. 2000). A study by Lee et al. (1991) showed that both HIV-negative and HIV-positive homosexual individuals without mycobacterial disease had antibodies reactive against different MAC serovars, indicating that patients were exposed to and mounted an immune response to different strains of MAC. Another study examined the role of ssGPLs in vitro, where human macrophages were infected with different patient-derived serovar-specific M. avium strains, and the authors concluded that there was no correlation between serovar specificity and virulence (Crowle et al. 1986). Additionally, a study by Maekura et al. (2005) focused on non-AIDS patients exhibiting MAC pulmonary disease and observed that patients containing serovar 4 strains were more frequently nonresponsive to multidrug chemotherapy and had a shorter survival time; however, patients with other serovar-specific isolates also exhibited disease. From these studies, it is not possible to link specific ssGPLs to the development or severity of MAC-induced disease in AIDS and non-AIDS patients. Nevertheless, the studies have sparked significant interest in the role that GPLs play in the disease process and how GPL structure may affect Mycobacterium virulence.
GPLs and colony morphology
Members of the MAC are noted for their ability to change colony morphologies. Many different morphologies have been described, and include, but are not limited to, smooth opaque (SmO), smooth transparent (SmT), rough (Rg), rough transparent (RgT), and pinpoint (Kansal et al. 1998; Torrelles et al. 2000). Mycobacteria defective in GPL biosynthesis often possess altered colony morphologies. For example, MAC 2151 serovar 2 produces isogenic SmO, SmT, and Rg strains, where the Rg is devoid of core lipopeptide molecules or glycosylated lipopeptides, suggesting that glycosylation of the lipopeptide core is necessary for rendering colony smoothness (Belisle, Klaczkiewicz, et al. 1993, Belisle, McNeil, et al. 1993). The mps mutant of M. smegmatis also completely lacked GPLs and exhibited a rough morphotype (Billman-Jacobe et al. 1999). A Rg M. avium mutant isolated from a patient with a chronic lung infection was also devoid of GPLs but produced an unusual lipopeptide (Riviere et al. 1996). In contrast, other GPL-defective mutants that displayed a Rg colony morphology, as compared to the smooth parent strains, had only slightly modified GPLs. Some of these include the MAC 104 serovar 1 Rg mutant, which lacks gtfA (Torrelles et al. 2002; Eckstein et al. 2003), the M. smegmatis gtf3 mutant (Deshayes et al. 2005), and the M. smegmatis atf1 mutant (Recht and Kolter 2001), among others. However, other strains with slight GPL modifications, such as the MAC 104 mtfD mutant, still produce smooth morphotypes (Krzywinska et al. 2005). Nevertheless, these observations suggest a correlation between GPL production/structure and colony morphology.
In contrast, the relationship between colony morphology and virulence remains unclear. Most strains isolated from MAC-infected patients have a transparent morphotype (Crowle et al. 1986; Reddy et al. 1996). Animal studies have also compared SmT strains of M. avium to SmO and Rg isogenic strains. In 1970, Schaefer and colleagues measured the pathogenicity of SmT, SmO, and Rg variants of M. avium in mice and chickens. The results demonstrated, as a whole, that the Rg variants were more virulent than both the SmT and SmO, and the SmT were more virulent than the SmO variants (Schaefer et al. 1970). Appelberg and colleagues also tested various M. avium strains and morphotypes and found, in general, that the SmT morphotypes were most virulent in mouse infection models compared to the SmO and Rg variants (Pedrosa et al. 1994). Another study demonstrated that the M. avium 2151 SmT strain was more virulent and less proinflammatory as compared to the SmO and Rg GPL-deficient isogenic strains (Bhatnagar and Schorey 2006). However, in a study by Torrelles et al. (2002), the M. avium 104 Rg isogenic strain, which produced a modified GPL lacking 6-deoxytalose residues (gtfA deficient), was as virulent as the SmT morphotype. These observations indicate that although some Rg or SmO strains are less virulent in a mouse or macrophage infection model compared to the isogenic SmT strain, this correlation may not always hold true. For example, studies involving M. abscessus demonstrated isogenic Rg, GPL-deficient variants to be more virulent in mice than the smooth counterparts (Byrd and Lyons 1999; Howard et al. 2006; Catherinot et al. 2007), although Catherinot et al. (2007) showed that the Rg variant was more proinflammatory in vitro than the smooth strain. Finally, a study by Kansal et al. (1998) compared the SmT and RgT isogenic strains of M. avium 101. They demonstrated that the RgT strain was phagocytosed more readily by macrophages and was more virulent in vivo (Kansal et al. 1998).
The variation between studies may be due not only to differences in the mycobacterial strains used but also in the underlying causes of the morphological changes, particularly for SmO variants, as the cell wall changes responsible for this morphology remain mostly undefined. Some authors suggest that SmT and SmO strains have different GPL compositions on the cell surface, or that the GPLs of the SmT strain are more surface exposed compared to the SmO strain (Reddy et al. 1996; Bhatnagar and Schorey 2006). Belisle and Brennan (1994) demonstrated that three different SmO strains produced greater amounts of nsGPL compared to the respective SmT strains. However, others studies suggest that there is no difference in the surface GPL composition between the isogenic SmT and SmO M. avium 104 strains (Torrelles et al. 2002), and one study showed that both MAC 2151 SmT and SmO colonies were reactive to ssGPL-specific antibody (Belisle, McNeil, et al. 1993).
Other studies propose that different colony morphologies can be determined by the lipooligosaccharide (LOS) composition (Belisle and Brennan 1989), the presence of a 66-kDa protein found only in SmT isolates (Prinzis et al. 1994), or by the composition of the capsule (Rastogi et al. 1981). Unfortunately, no SmO or SmT morphotype-specific antibodies have been generated, which would certainly facilitate the biochemical studies (Belisle, McNeil, et al. 1993; Prinzis et al. 1994). In summary, the varied results obtained with the different morphotypes suggest that comparing M. avium Rg to SmT/O as a mechanism to evaluate the GPLs’ role in virulence is problematic. Clarification calls for a more controlled approach, such as the generation of M. avium mutants, which lack genes involved in GPL biosynthesis, to utilize purified native and/or modified GPLs to define any immunomodulatory functions.
GPLs and sliding motility and biofilm formation
M. smegmatis and M. avium have been observed to exhibit sliding motility, appearing as multilayered halos on agar surfaces (Martinez et al. 1999; Recht et al. 2001). In addition, this sliding motility also seems to be correlated with the ability to produce biofilms on PVC surfaces in drinking water systems and in vitro (Carter et al. 2003). These two properties may be important in facilitating mycobacterial virulence, as their presence in drinking water is a major source of infection (Hilborn et al. 2006), and their motility may contribute to epithelial cell invasion after ingestion or inhalation (Yamazaki, Danelishvili, Wu, Hidaka, et al. 2006).
A number of studies suggest that GPLs may play an important role in these processes. Martinez et al. (1999) found that M. avium 2151 smooth strains spread more than the Rg morphotypes, suggesting a role for GPLs in motility. In addition, other studies have implicated GPLs in M. smegmatis sliding motility and biofilm formation. M. smegmatis transposon mutants defective in mps and GPL membrane transport proteins, such as those encoded by gap, lacked GPL expression and were nonmotile compared to the GPL-producing parent strains (Recht et al. 2000; Etienne et al. 2002; Sonden et al. 2005). Some of these mutants were also defective in biofilm formation on PVC plastic (Recht et al. 2000). Similar results have been observed for the M. avium pstA/pstB mutants, which displayed a Rg morphotype and did not bind to PVC plastic in contrast to the parental SmT strains (Freeman et al. 2006). Moreover, a GPL-producing wild-type strain of M. abscessus displayed sliding motility and biofilm formation whereas the GPL-deficient Rg strain lacked both functions (Howard et al. 2006).
Recht et al. (2000) proposed a model for sliding motility in which GPLs located on the cell surface, with their hydrophobic fatty acyl tails exposed, created a hydrophobic environment that decreased friction between the bacterium and hydrophilic surface. Their model also proposed that GPL-defective mutants had more hydrophilic products exposed, such as polysaccharides, thus decreasing their motility due to an increase in friction. However, this model implies that the GPL carbohydrate moieties would have only limited exposure to the environment, an unlikely prospect as published data supports exposure of the carbohydrate moieties on the bacterial surface (Kolk et al. 1989). The same group, however, produced an atf1-deficient M. smegmatis strain that produced nonacetylated GPLs. This mutant had an “intermediate rough morphotype” and reduced sliding motility and biofilm formation on agar (Recht and Kolter 2001). This observation is interesting as it suggests that sliding motility and biofilm formation can be affected by slight structural modifications of the GPL carbohydrates. Other mutants have been produced that are defective in sliding motility and/or biofilm formation and these may also be defective in GPL biosynthesis; however, additional biochemical analyses are needed to address this possibility (Yamazaki, Danelishvili, Wu, Macnab, et al. 2006; Mukherjee and Chatterji 2008; Gopalaswamy et al. 2008).
A correlation between biofilm formation and virulence has also been observed. Carter et al. (2003) tested a number of M. avium strains originally isolated from AIDS patients for their ability to form biofilms on PVC plastic. They found that all strains could form biofilms, but to varying degrees, and that all expressed GPLs. Interestingly, the M. avium strain A5 was able to bind to and translocate across epithelial cells; however, biofilm-defective mutants were diminished in this capacity relative to the wild-type strain (Yamazaki, Danelishvili, Wu, Hidaka, et al. 2006). These mutants were defective in their GPL biosynthetic pathways (Yamazaki, Danelishvili, Wu, Macnab, et al. 2006) suggesting a role for GPLs in epithelial cell invasion as well as in biofilm formation.
Immunomodulation by GPLs
GPL-defective MAC mutants have been useful in elucidating the role of GPLs in immunomodulation (Irani et al. 2004; Krzywinska et al. 2005; Bhatnagar and Schorey 2006). Moreover, purified GPLs have been used to directly evaluate their effect on immune cells. GPLs can be readily extracted from the mycobacterial cell wall using a chloroform:methanol extraction procedure and subsequently purified using high-performance thin-layer chromatography (HP-TLC) or high-performance liquid chromatography (HPLC).
Several studies have addressed whether GPLs could function in modulating a T helper-1 (Th1) response, with some concluding that GPLs downregulate Th1 type responses, thus benefiting the pathogen (Pourshafie et al. 1993; Horgen et al. 2000). In contrast, other studies indicate that intact GPLs were not inhibitory (Barrow et al. 1993). Moreover, GPLs whose oligosaccharides were removed from the allo-threonine by β-elimination were capable of downregulating a Th1-type response, whereas intact GPL failed to modulate the response (Tassell et al. 1992; Rastogi and Barrow 1994).
Additional studies have scrutinized how GPLs direct or modulate a proinflammatory response. Total lipid and ssGPL fractions have been observed to induce the release of various proinflammatory mediators, such as prostaglandins, leukotrienes, IL-1, IL-6, and TNF-α (Barrow et al. 1993; Pourshafie et al. 1993; Barrow et al. 1995; Horgen et al. 2000; Sweet and Schorey 2006). The ability of ssGPLs to stimulate the release of proinflammatory mediators appears to be structure specific, as certain ssGPLs are proinflammatory while others are not (Barrow et al. 1995; Sweet and Schorey 2006). This indicates that slight structural modifications can alter the way in which the GPL interacts with host-cell receptors. Our laboratory also observed that the proinflammatory response was MyD88 and Toll-like receptor 2 (TLR2) dependent, suggesting that certain GPLs have the necessary structure to signal through this receptor (Sweet and Schorey 2006). These results corroborate other studies where M. avium was shown to stimulate an inflammatory response in macrophages through TLR2, and that TLR2-deficient mice are more susceptible to an M. avium infection (Quesniaux et al. 2004). Interestingly, we observed that in addition to the carbohydrate requirement for TLR2 signaling, the number and position of O-methyl and O-acetyl groups on the rhamnose and 6-deoxytalose, respectively, were also important. For example, we have observed that de-acetylated GPLs were no longer biologically active (unpublished results). These observations are significant as they suggest that slight structural modifications of the carbohydrates may alter the overall conformation of the GPL and consequently lead to the loss of, or a change in, the positioning of functional groups required for signaling through TLR2. This also suggests that slight modifications of the GPLs during an infection may affect the ability of MAC to induce a proinflammatory response; however, this prediction awaits characterization of GPLs isolated at different times postinfection.
In addition to TLRs, M. avium and other pathogenic mycobacteria can engage macrophages via a wide variety of receptors, including the complement receptor-3 and -4 (CR3/CR4) (Schorey et al. 1997) and the mannose receptor (MR) (Schlesinger et al. 1994). GPLs have also been shown to alter cellular functions through their interaction with host receptors including the CRs and MR (Irani and Maslow 2005; Villeneuve et al. 2005; Shimada et al. 2006). For example, de-acetylated or succinylated polar GPLs of M. smegmatis, but not nsGPLs, inhibited the phagocytosis of M. smegmatis or M. avium (Villeneuve et al. 2003). In a subsequent study, the same group showed that the CR3 and MR were involved in the phagocytosis of GPL-coated beads (Villeneuve et al. 2005). Together, the studies implicate that GPLs are able to associate with and potentially signal through different receptors; however, the precise molecular determinants of the GPL-receptor interactions have yet to be fully elucidated.
Following receptor-mediated phagocytosis, pathogenic mycobacteria block the phagosome–lysosome (P–L) fusion which is essential for their survival inside phagocytic cells. Some mechanisms have been proposed for this block in the P–L fusion by M. tuberculosis and include limiting the production of PI-3 phosphate on the mycobacterial phagosome, thus preventing the recruitment of early endosome antigen-1 and other host proteins required for fusion with late endosomes and lysosomes (Malik et al. 2001; Fratti et al. 2003; Kang et al. 2005). Whether M. avium performs this function in a similar fashion has not been defined. Recent studies have implicated a role for GPLs in the P–L delay (Kano et al. 2005) and the involvement of the MR in this process (Shimada et al. 2006). However, the results from these studies are relatively inconclusive since the GPLs were de-acetylated and therefore do not provide information about the function of the native molecules. We have shown that native nsGPLs can also function to delay P–L biogenesis in macrophages. Although the mechanism remains unclear, our results suggest a role for MR (unpublished results), again supporting that GPLs can interact with different receptors. GPLs may also function to aid in the intracellular survival of MAC by serving as a protective barrier against lysosomal enzymes (Tereletsky and Barrow 1983), or by interfering with P–L biogenesis by disrupting the phagosomal membrane (Sut et al. 1990; Vergne et al. 1995).
Together the results using purified GPLs support a role for these glycolipids in modulating the innate, as well as T-cell immune responses. Nevertheless, it is unclear from these studies what function is associated with GPLs during the course of a macrophage or host infection. Most studies to address this question have used Rg isogenic strains which differ in the presence or structure of the GPLs. However, as indicated above, these studies have not resulted in a clear picture of how or whether GPLs function in immune modulation during the course of an infection. One possible complication in using these isogenic strains is the potential for additional genetic changes to occur during culturing of the SmT and Rg isolates. Unfortunately, no studies have been performed using Rg variants genetically reconstituted to produce wild-type GPL which would allow one to confirm that the responses observed were the result of changes in GPL. Nevertheless there have been two studies which have used M. avium mutants which express modified GPLs due to a specific deletion of a gene involved in GPL biosynthesis. In studies by Krzywinska et al. (2005), murine macrophages infected with a mtfD-deficient M. avium 104 strain produced increased levels of TNF-α and RANTES relative to wild-type or reconstituted SmT M. avium 104. The mutant was also attenuated in a mouse infection model. The mtfD mutant expressed an unusual undermethylated form of the nsGPL. Using a SmO variant of the M. avium strain 920A6, Irani and Maslow (2005) observed a decrease in TNF-α production by J774 cells infected with an rtfA-deficient mutant relative to cells infected with the wild-type or reconstituted SmO M. avium 920A6. The rtfA mutant lacked expression of the serotype 8 GPL but had a normal nsGPL expression profile. Together these results confirm that modifying GPL expression patterns in the context of whole mycobacteria can affect an immune response; however, the type of response varied depending on the serotype/morphotype of the parental M. avium strain.
Conclusion
Studies using purified GPLs and GPL mutants have highlighted the importance of this glycolipid in various biological processes. Moreover, studies using mutants defective in GPL glycosylation and modification and studies using different purified GPLs have highlighted the importance of the carbohydrate residues, as well as the carbohydrate modifications, in the GPL-mediated functions. However, clear results have been difficult to obtain for M. avium and M. intracellulare stemming in part from our limited ability to do genetic manipulation on these pathogenic mycobacterial species. Nevertheless, biochemical and genetic studies, primarily using M. smegmatis, have significantly increased our understanding of the GPL biosynthetic pathway. This foundation should facilitate future studies to better define GPLs’ role in biofilm formation, cell invasion, and immune modulation, and how variations in GPL structure may affect the pathogenicity of MAC and other GPL-expressing mycobacteria.
Funding
National Institute of Allergy and Infectious Diseases (AI056979 and AI052439)
Acknowledgments
We thank Kathleen Eggleson for careful review of the manuscript.
Glossary
Abbreviations
- AIDS
acquired immune deficiency syndrome
- CR3
complement receptor 3
- CR4
complement receptor 4
- GPLs
glycopeptidolipids
- IL-1
interleukin-1
- IL-6
interleukin-6
- LOS
lipooligosaccharide
- MAC
Mycobacterium avium complex
- MR
mannose receptor
- MyD88
myeloid differentiation primary response gene 88
- nsGPLs
nonspecific glycopeptidolipids
- NTM
nontuberculosis mycobacteria
- P–L
phagosome—lysosome
- PVC
polyvinylchloride
- Rg
rough
- RgT
rough transparent
- SmO
smooth opaque
- SmT
smooth transparent
- ssGPLs
serovar-specific glycopeptidolipids
- TLR2
toll-like receptor 2
- TNF-α
tumor necrosis factor-α
Conflict of interest statement
None declared.
References
- Barrow WW, Davis TL, Wright EL, Labrousse V, Bachelet M, Rastogi N. Immunomodulatory spectrum of lipids associated with Mycobacterium avium serovar 8. Infect Immun. 1995;63:126–133. doi: 10.1128/iai.63.1.126-133.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrow WW, de Sousa JP, Davis TL, Wright EL, Bachelet M, Rastogi N. Immunomodulation of human peripheral blood mononuclear cell functions by defined lipid fractions of Mycobacterium avium. Infect Immun. 1993;61:5286–5293. doi: 10.1128/iai.61.12.5286-5293.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belisle JT, Brennan PJ. Chemical basis of rough and smooth variation in mycobacteria. J Bacteriol. 1989;171:3465–3470. doi: 10.1128/jb.171.6.3465-3470.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belisle JT, Brennan PJ. Molecular basis of colony morphology in Mycobacterium avium. Res Microbiol. 1994;145:237–242. doi: 10.1016/0923-2508(94)90024-8. [DOI] [PubMed] [Google Scholar]
- Belisle JT, Klaczkiewicz K, Brennan PJ, Jacobs WR, Jr, Inamine JM. Rough morphological variants of Mycobacterium avium. Characterization of genomic deletions resulting in the loss of glycopeptidolipid expression. J Biol Chem. 1993;268:10517–10523. [PubMed] [Google Scholar]
- Belisle JT, McNeil MR, Chatterjee D, Inamine JM, Brennan PJ. Expression of the core lipopeptide of the glycopeptidolipid surface antigens in rough mutants of Mycobacterium avium. J Biol Chem. 1993;268:10510–10516. [PubMed] [Google Scholar]
- Belisle JT, Pascopella L, Inamine JM, Brennan PJ, Jacobs WR., Jr Isolation and expression of a gene cluster responsible for biosynthesis of the glycopeptidolipid antigens of Mycobacterium avium. J Bacteriol. 1991;173:6991–6997. doi: 10.1128/jb.173.21.6991-6997.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatnagar S, Schorey JS. Elevated mitogen-activated protein kinase signalling and increased macrophage activation in cells infected with a glycopeptidolipid-deficient Mycobacterium avium. Cell Microbiol. 2006;8:85–96. doi: 10.1111/j.1462-5822.2005.00602.x. [DOI] [PubMed] [Google Scholar]
- Billman-Jacobe H, McConville MJ, Haites RE, Kovacevic S, Coppel RL. Identification of a peptide synthetase involved in the biosynthesis of glycopeptidolipids of Mycobacterium smegmatis. Mol Microbiol. 1999;33:1244–1253. doi: 10.1046/j.1365-2958.1999.01572.x. [DOI] [PubMed] [Google Scholar]
- Burguiere A, Hitchen PG, Dover LG, Dell A, Besra GS. Altered expression profile of mycobacterial surface glycopeptidolipids following treatment with the antifungal azole inhibitors econazole and clotrimazole. Microbiology. 2005;151:2087–2095. doi: 10.1099/mic.0.27938-0. [DOI] [PubMed] [Google Scholar]
- Byrd TF, Lyons CR. Preliminary characterization of a Mycobacterium abscessus mutant in human and murine models of infection. Infect Immun. 1999;67:4700–4707. doi: 10.1128/iai.67.9.4700-4707.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter G, Wu M, Drummond DC, Bermudez LE. Characterization of biofilm formation by clinical isolates of Mycobacterium avium. J Med Microbiol. 2003;52:747–752. doi: 10.1099/jmm.0.05224-0. [DOI] [PubMed] [Google Scholar]
- Catherinot E, Clarissou J, Etienne G, Ripoll F, Emile JF, Daffe M, Perronne C, Soudais C, Gaillard JL, Rottman M. Hypervirulence of a rough variant of the Mycobacterium abscessus type strain. Infect Immun. 2007;75:1055–1058. doi: 10.1128/IAI.00835-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee D, Khoo KH. The surface glycopeptidolipids of mycobacteria: Structures and biological properties. Cell Mol Life Sci. 2001;58:2018–2042. doi: 10.1007/PL00000834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowle AJ, Tsang AY, Vatter AE, May MH. Comparison of 15 laboratory and patient-derived strains of Mycobacterium avium for ability to infect and multiply in cultured human macrophages. J Clin Microbiol. 1986;24:812–821. doi: 10.1128/jcm.24.5.812-821.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshayes C, Laval F, Montrozier H, Daffe M, Etienne G, Reyrat JM. A glycosyltransferase involved in biosynthesis of triglycosylated glycopeptidolipids in Mycobacterium smegmatis: Impact on surface properties. J Bacteriol. 2005;187:7283–7291. doi: 10.1128/JB.187.21.7283-7291.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckstein TM, Belisle JT, Inamine JM. Proposed pathway for the biosynthesis of serovar-specific glycopeptidolipids in Mycobacterium avium serovar 2. Microbiology. 2003;149:2797–2807. doi: 10.1099/mic.0.26528-0. [DOI] [PubMed] [Google Scholar]
- Eckstein TM, Silbaq FS, Chatterjee D, Kelly NJ, Brennan PJ, Belisle JT. Identification and recombinant expression of a Mycobacterium avium rhamnosyltransferase gene (rtfA) involved in glycopeptidolipid biosynthesis. J Bacteriol. 1998;180:5567–5573. doi: 10.1128/jb.180.21.5567-5573.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etienne G, Villeneuve C, Billman-Jacobe H, Astarie-Dequeker C, Dupont MA, Daffe M. The impact of the absence of glycopeptidolipids on the ultrastructure, cell surface and cell wall properties, and phagocytosis of Mycobacterium smegmatis. Microbiology. 2002;148:3089–3100. doi: 10.1099/00221287-148-10-3089. [DOI] [PubMed] [Google Scholar]
- Field SK, Fisher D, Cowie RL. Mycobacterium avium complex pulmonary disease in patients without HIV infection. Chest. 2004;126:566–581. doi: 10.1378/chest.126.2.566. [DOI] [PubMed] [Google Scholar]
- Fratti RA, Chua J, Vergne I, Deretic V. Mycobacterium tuberculosis glycosylated phosphatidylinositol causes phagosome maturation arrest. PNAS. 2003;100:5437–5442. doi: 10.1073/pnas.0737613100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman R, Geier H, Weigel KM, Do J, Ford TE, Cangelosi GA. Roles for cell wall glycopeptidolipid in surface adherence and planktonic dispersal of Mycobacterium avium. Appl Environ Microbiol. 2006;72:7554–7558. doi: 10.1128/AEM.01633-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiwara N, Nakata N, Maeda S, Naka T, Doe M, Yano I, Kobayashi K. Structural characterization of a specific glycopeptidolipid containing a novel N-acyl-deoxy sugar from Mycobacterium intracellulare serotype 7 and genetic analysis of its glycosylation pathway. J Bacteriol. 2007;189:1099–1108. doi: 10.1128/JB.01471-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gopalaswamy R, Narayanan S, Jacobs WR, Jr, Av-Gay Y. Mycobacterium smegmatis biofilm formation and sliding motility are affected by the serine/threonine protein kinase PknF. FEMS Microbiol Lett. 2008;278:121–127. doi: 10.1111/j.1574-6968.2007.00989.x. [DOI] [PubMed] [Google Scholar]
- Hilborn ED, Covert TC, Yakrus MA, Harris SI, Donnelly SF, Rice EW, Toney S, Bailey SA, Stelma GN., Jr Persistence of nontuberculous mycobacteria in a drinking water system after addition of filtration treatment. Appl Environ Microbiol. 2006;72:5864–5869. doi: 10.1128/AEM.00759-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horgen L, Barrow EL, Barrow WW, Rastogi N. Exposure of human peripheral blood mononuclear cells to total lipids and serovar-specific glycopeptidolipids from Mycobacterium avium serovars 4 and 8 results in inhibition of TH1-type responses. Microb Pathog. 2000;29:9–16. doi: 10.1006/mpat.2000.0358. [DOI] [PubMed] [Google Scholar]
- Horsburgh CR., Jr Mycobacterium avium complex infection in the acquired immunodeficiency syndrome. N Engl J Med. 1991;324:1332–1338. doi: 10.1056/NEJM199105093241906. [DOI] [PubMed] [Google Scholar]
- Horsburgh CR., Jr The pathophysiology of disseminated Mycobacterium avium complex disease in AIDS. J Infect Dis. 1999;179(Suppl 3):S461–S465. doi: 10.1086/314804. [DOI] [PubMed] [Google Scholar]
- Horsburgh CR, Jr, Cohn DL, Roberts RB, Masur H, Miller RA, Tsang AY, Iseman MD. Mycobacterium avium–M. intracellulare isolates from patients with or without acquired immunodeficiency syndrome. Antimicrob Agents Chemother. 1986;30:955–957. doi: 10.1128/aac.30.6.955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard ST, Byrd TF. The rapidly growing mycobacteria: Saprophytes and parasites. Microbes Infect. 2000;2:1845–1853. doi: 10.1016/s1286-4579(00)01338-1. [DOI] [PubMed] [Google Scholar]
- Howard ST, Rhoades E, Recht J, Pang X, Alsup A, Kolter R, Lyons CR, Byrd TF. Spontaneous reversion of Mycobacterium abscessus from a smooth to a rough morphotype is associated with reduced expression of glycopeptidolipid and reacquisition of an invasive phenotype. Microbiology. 2006;152:1581–1590. doi: 10.1099/mic.0.28625-0. [DOI] [PubMed] [Google Scholar]
- Irani VR, Lee SH, Eckstein TM, Inamine JM, Belisle JT, Maslow JN. Utilization of a ts-sacB selection system for the generation of a Mycobacterium avium serovar-8 specific glycopeptidolipid allelic exchange mutant. Ann Clin Microbiol Antimicrob. 2004;3:18. doi: 10.1186/1476-0711-3-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irani VR, Maslow JN. Induction of murine macrophage TNF-alpha synthesis by Mycobacterium avium is modulated through complement-dependent interaction via complement receptors 3 and 4 in relation to M. avium glycopeptidolipid. FEMS Microbiol Lett. 2005;246:221–228. doi: 10.1016/j.femsle.2005.04.008. [DOI] [PubMed] [Google Scholar]
- Jeevarajah D, Patterson JH, McConville MJ, Billman-Jacobe H. Modification of glycopeptidolipids by an O-methyltransferase of Mycobacterium smegmatis. Microbiology. 2002;148:3079–3087. doi: 10.1099/00221287-148-10-3079. [DOI] [PubMed] [Google Scholar]
- Jeevarajah D, Patterson JH, Taig E, Sargeant T, McConville MJ, Billman-Jacobe H. Methylation of GPLs in Mycobacterium smegmatis and Mycobacterium avium. J Bacteriol. 2004;186:6792–6799. doi: 10.1128/JB.186.20.6792-6799.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins PA, Marks J, Schaefer WB. Lipid chromatography and seroagglutination in the classification of rapidly growing mycobacteria. Am Rev Respir Dis. 1971;103:179–187. doi: 10.1164/arrd.1971.103.2.179. [DOI] [PubMed] [Google Scholar]
- Kang PB, Azad AK, Torrelles JB, Kaufman TM, Beharka A, Tibesar E, DesJardin LE, Schlesinger LS. The human macrophage mannose receptor directs Mycobacterium tuberculosis lipoarabinomannan-mediated phagosome biogenesis. J Exp Med. 2005;202:987–999. doi: 10.1084/jem.20051239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kano H, Doi T, Fujita Y, Takimoto H, Yano I, Kumazawa Y. Serotype-specific modulation of human monocyte functions by glycopeptidolipid (GPL) isolated from Mycobacterium avium complex. Biol Pharm Bull. 2005;28:335–339. doi: 10.1248/bpb.28.335. [DOI] [PubMed] [Google Scholar]
- Kansal RG, Gomez-Flores R, Mehta RT. Change in colony morphology influences the virulence as well as the biochemical properties of the Mycobacterium avium complex. Microb Pathog. 1998;25:203–214. doi: 10.1006/mpat.1998.0227. [DOI] [PubMed] [Google Scholar]
- Khoo KH, Jarboe E, Barker A, Torrelles J, Kuo CW, Chatterjee D. Altered expression profile of the surface glycopeptidolipids in drug-resistant clinical isolates of Mycobacterium avium complex. J Biol Chem. 1999;274:9778–9785. doi: 10.1074/jbc.274.14.9778. [DOI] [PubMed] [Google Scholar]
- Kolk AH, Evers R, Groothuis DG, Gilis H, Kuijper S. Production and characterization of monoclonal antibodies against specific serotypes of Mycobacterium avium and the Mycobacterium avium–Mycobacterium intracellulare–Mycobacterium scrofulaceum complex. Infect Immun. 1989;57:2514–2521. doi: 10.1128/iai.57.8.2514-2521.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krzywinska E, Bhatnagar S, Sweet L, Chatterjee D, Schorey JS. Mycobacterium avium 104 deleted of the methyltransferase D gene by allelic replacement lacks serotype-specific glycopeptidolipids and shows attenuated virulence in mice. Mol Microbiol. 2005;56:1262–1273. doi: 10.1111/j.1365-2958.2005.04608.x. [DOI] [PubMed] [Google Scholar]
- Krzywinska E, Krzywinski J, Schorey JS. Naturally occurring horizontal gene transfer and homologous recombination in Mycobacterium. Microbiology. 2004;150:1707–1712. doi: 10.1099/mic.0.27088-0. [DOI] [PubMed] [Google Scholar]
- Krzywinska E, Schorey JS. Characterization of genetic differences between Mycobacterium avium subsp. avium strains of diverse virulence with a focus on the glycopeptidolipid biosynthesis cluster. Vet Microbiol. 2003;91:249–264. doi: 10.1016/s0378-1135(02)00292-4. [DOI] [PubMed] [Google Scholar]
- Lee BY, Chatterjee D, Bozic CM, Brennan PJ, Cohn DL, Bales JD, Harrison SM, Andron LA, Orme IM. Prevalence of serum antibody to the type-specific glycopeptidolipid antigens of Mycobacterium avium in human immunodeficiency virus-positive and -negative individuals. J Clin Microbiol. 1991;29:1026–1029. doi: 10.1128/jcm.29.5.1026-1029.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez Marin LM, Laneelle MA, Prome D, Daffe M. Structures of the glycopeptidolipid antigens of two animal pathogens: Mycobacterium senegalense and Mycobacterium porcinum. Eur J Biochem. 1993;215:859–866. doi: 10.1111/j.1432-1033.1993.tb18103.x. [DOI] [PubMed] [Google Scholar]
- Maekura R, Okuda Y, Hirotani A, Kitada S, Hiraga T, Yoshimura K, Yano I, Kobayashi K, Ito M. Clinical and prognostic importance of serotyping Mycobacterium avium–Mycobacterium intracellulare complex isolates in human immunodeficiency virus-negative patients. J Clin Microbiol. 2005;43:3150–3158. doi: 10.1128/JCM.43.7.3150-3158.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malik ZA, Shankar SI, Kusner DJ. Mycobacterium tuberculosis phagosomes exhibit altered calmodulin-dependent signal transduction: contribution to inhibition of phagosome-lysosome fusion and intracellular survival in human macrophages. J Immunol. 2001;166:3392–3401. doi: 10.4049/jimmunol.166.5.3392. [DOI] [PubMed] [Google Scholar]
- Marks J, Jenkins PA, Schaefer WB. Thin-layer chromatography of mycobacterial lipids as an aid to classification: Technical improvements: Mycobacterium avium, M. intracellulare (Battey bacilli) Tubercle. 1971;52:219–225. doi: 10.1016/0041-3879(71)90044-4. [DOI] [PubMed] [Google Scholar]
- Martinez A, Torello S, Kolter R. Sliding motility in mycobacteria. J Bacteriol. 1999;181:7331–7338. doi: 10.1128/jb.181.23.7331-7338.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maslow JN, Irani VR, Lee SH, Eckstein TM, Inamine JM, Belisle JT. Biosynthetic specificity of the rhamnosyltransferase gene of Mycobacterium avium serovar 2 as determined by allelic exchange mutagenesis. Microbiology. 2003;149:3193–3202. doi: 10.1099/mic.0.26565-0. [DOI] [PubMed] [Google Scholar]
- Mills JA, McNeil MR, Belisle JT, Jacobs WR, Jr, Brennan PJ. Loci of Mycobacterium avium ser2 gene cluster and their functions. J Bacteriol. 1994;176:4803–4808. doi: 10.1128/jb.176.16.4803-4808.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto Y, Mukai T, Maeda Y, Nakata N, Kai M, Naka T, Yano I, Makino M. Characterization of the fucosylation pathway in the biosynthesis of glycopeptidolipids from Mycobacterium avium complex. J Bacteriol. 2007;189:5515–5522. doi: 10.1128/JB.00344-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto Y, Mukai T, Nakata N, Maeda Y, Kai M, Naka T, Yano I, Makino M. Identification and characterization of the genes involved in glycosylation pathways of mycobacterial glycopeptidolipid biosynthesis. J Bacteriol. 2006;188:86–95. doi: 10.1128/JB.188.1.86-95.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee R, Chatterji D. Proteomics and mass spectrometric studies reveal planktonic growth of Mycobacterium smegmatis in biofilm cultures in the absence of rpoZ. J Chromatogr B Anal Technol Biomed Life Sci. 2008;861:196–202. doi: 10.1016/j.jchromb.2007.08.009. [DOI] [PubMed] [Google Scholar]
- Mukherjee R, Gomez M, Jayaraman N, Smith I, Chatterji D. Hyperglycosylation of glycopeptidolipid of Mycobacterium smegmatis under nutrient starvation: Structural studies. Microbiology. 2005;151:2385–2392. doi: 10.1099/mic.0.27908-0. [DOI] [PubMed] [Google Scholar]
- Nakata N, Fujiwara N, Naka T, Yano I, Maeda S. Identification and characterization of two novel methyltransferase genes that determine the serotype 12-specific structure of glycopeptidolipids of Mycobacterium intracellulare. J Bacteriol. 2008;190:1064–1071. doi: 10.1128/JB.01370-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ojha AK, Varma S, Chatterji D. Synthesis of an unusual polar glycopeptidolipid in glucose-limited culture of Mycobacterium smegmatis. Microbiology. 2002;148:3039–3048. doi: 10.1099/00221287-148-10-3039. [DOI] [PubMed] [Google Scholar]
- Patterson JH, McConville MJ, Haites RE, Coppel RL, Billman-Jacobe H. Identification of a methyltransferase from Mycobacterium smegmatis involved in glycopeptidolipid synthesis. J Biol Chem. 2000;275:24900–24906. doi: 10.1074/jbc.M000147200. [DOI] [PubMed] [Google Scholar]
- Pedrosa J, Florido M, Kunze ZM, Castro AG, Portaels F, McFadden J, Silva MT, Appelberg R. Characterization of the virulence of Mycobacterium avium complex (MAC) isolates in mice. Clin Exp Immunol. 1994;98:210–216. doi: 10.1111/j.1365-2249.1994.tb06127.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pourshafie M, Ayub Q, Barrow WW. Comparative effects of Mycobacterium avium glycopeptidolipid and lipopeptide fragment on the function and ultrastructure of mononuclear cells. Clin Exp Immunol. 1993;93:72–79. doi: 10.1111/j.1365-2249.1993.tb06499.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prinzis S, Rivoire B, Brennan PJ. Search for the molecular basis of morphological variation in Mycobacterium avium. Infect Immun. 1994;62:1946–1951. doi: 10.1128/iai.62.5.1946-1951.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quesniaux V, Fremond C, Jacobs M, Parida S, Nicolle D, Yeremeev V, Bihl F, Erard F, Botha T, Drennan M, et al. Toll-like receptor pathways in the immune responses to mycobacteria. Microbes Infect. 2004;6:946–959. doi: 10.1016/j.micinf.2004.04.016. [DOI] [PubMed] [Google Scholar]
- Rastogi N, Barrow WW. Cell envelope constituents and the multifaceted nature of Mycobacterium avium pathogenicity and drug resistance. Res Microbiol. 1994;145:243–252. doi: 10.1016/0923-2508(94)90025-6. discussion 252–261. [DOI] [PubMed] [Google Scholar]
- Rastogi N, Frehel C, Ryter A, Ohayon H, Lesourd M, David HL. Multiple drug resistance in Mycobacterium avium: Is the wall architecture responsible for exclusion of antimicrobial agents? Antimicrob Agents Chemother. 1981;20:666–677. doi: 10.1128/aac.20.5.666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Recht J, Kolter R. Glycopeptidolipid acetylation affects sliding motility and biofilm formation in Mycobacterium smegmatis. J Bacteriol. 2001;183:5718–5724. doi: 10.1128/JB.183.19.5718-5724.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Recht J, Martinez A, Torello S, Kolter R. Genetic analysis of sliding motility in Mycobacterium smegmatis. J Bacteriol. 2000;182:4348–4351. doi: 10.1128/jb.182.15.4348-4351.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Recht J, Martinez A, Torello S, Kolter R. Sliding motility and biofilm formation in mycobacteria. Acta Cient Venez. 2001;52(Suppl 1):45–49. [PubMed] [Google Scholar]
- Reddy VM, Luna-Herrera J, Gangadharam PR. Pathobiological significance of colony morphology in Mycobacterium avium complex. Microb Pathog. 1996;21:97–109. doi: 10.1006/mpat.1996.0046. [DOI] [PubMed] [Google Scholar]
- Ripoll F, Deshayes C, Pasek S, Laval F, Beretti JL, Biet F, Risler JL, Daffe M, Etienne G, Gaillard JL, et al. Genomics of glycopeptidolipid biosynthesis in Mycobacterium abscessus and M. chelonae. BMC Genomics. 2007;8:114. doi: 10.1186/1471-2164-8-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riviere M, Puzo G, Wright EL, Barrow WW. A unique phenylalanine-containing lipopeptide isolated from a rough-colony variant of Mycobacterium avium. Eur J Biochem. 1996;241:682–690. doi: 10.1111/j.1432-1033.1996.00682.x. [DOI] [PubMed] [Google Scholar]
- Schaefer WB. Serologic identification and classification of the atypical mycobacteria by their agglutination. Am Rev Resp Dis. 1965;92:85. doi: 10.1164/arrd.1965.92.6P2.85. [DOI] [PubMed] [Google Scholar]
- Schaefer WB, Davis CL, Cohn ML. Pathogenicity of transparent, opaque, and rough variants of Mycobacterium avium in chickens and mice. Am Rev Resp Dis. 1970;102:499–506. doi: 10.1164/arrd.1970.102.4.499. [DOI] [PubMed] [Google Scholar]
- Schlesinger LS, Hull SR, Kaufman TM. Binding of the terminal mannosyl units of lipoarabinomannan from a virulent strain of Mycobacterium tuberculosis to human macrophages. J Immunol. 1994;152:4070–4079. [PubMed] [Google Scholar]
- Schorey JS, Carroll MC, Brown EJ. A macrophage invasion mechanism of pathogenic mycobacteria. Science. 1997;277:1091–1093. doi: 10.1126/science.277.5329.1091. [DOI] [PubMed] [Google Scholar]
- Shimada K, Takimoto H, Yano I, Kumazawa Y. Involvement of mannose receptor in glycopeptidolipid-mediated inhibition of phagosome–lysosome fusion. Microbiol Immunol. 2006;50:243–251. doi: 10.1111/j.1348-0421.2006.tb03782.x. [DOI] [PubMed] [Google Scholar]
- Sonden B, Kocincova D, Deshayes C, Euphrasie D, Rhayat L, Laval F, Frehel C, Daffe M, Etienne G, Reyrat JM. Gap, a mycobacterial specific integral membrane protein, is required for glycolipid transport to the cell surface. Mol Microbiol. 2005;58:426–440. doi: 10.1111/j.1365-2958.2005.04847.x. [DOI] [PubMed] [Google Scholar]
- Sut A, Sirugue S, Sixou S, Lakhdar-Ghazal F, Tocanne JF, Laneelle G. Mycobacteria glycolipids as potential pathogenicity effectors: Alteration of model and natural membranes. Biochemistry. 1990;29:8498–8502. doi: 10.1021/bi00488a042. [DOI] [PubMed] [Google Scholar]
- Sweet L, Schorey JS. Glycopeptidolipids from Mycobacterium avium promote macrophage activation in a TLR2- and MyD88-dependent manner. J Leukoc Biol. 2006;80:415–423. doi: 10.1189/jlb.1205702. [DOI] [PubMed] [Google Scholar]
- Tassell SK, Pourshafie M, Wright EL, Richmond MG, Barrow WW. Modified lymphocyte response to mitogens induced by the lipopeptide fragment derived from Mycobacterium avium serovar-specific glycopeptidolipids. Infect Immun. 1992;60:706–711. doi: 10.1128/iai.60.2.706-711.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tereletsky MJ, Barrow WW. Postphagocytic detection of glycopeptidolipids associated with the superficial L1 layer of Mycobacterium intracellulare. Infect Immun. 1983;41:1312–1321. doi: 10.1128/iai.41.3.1312-1321.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torrelles JB, Chatterjee D, Lonca JG, Manterola JM, Ausina VR, Brennan PJ. Serovars of Mycobacterium avium complex isolated from AIDS and non-AIDS patients in Spain. J Appl Microbiol. 2000;88:266–279. doi: 10.1046/j.1365-2672.2000.00958.x. [DOI] [PubMed] [Google Scholar]
- Torrelles JB, Ellis D, Osborne T, Hoefer A, Orme IM, Chatterjee D, Brennan PJ, Cooper AM. Characterization of virulence, colony morphotype and the glycopeptidolipid of Mycobacterium avium strain 104. Tuberculosis (Edinb) 2002;82:293–300. doi: 10.1054/tube.2002.0373. [DOI] [PubMed] [Google Scholar]
- Tsang AY, Denner JC, Brennan PJ, McClatchy JK. Clinical and epidemiological importance of typing of Mycobacterium avium complex isolates. J Clin Microbiol. 1992;30:479–484. doi: 10.1128/jcm.30.2.479-484.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vergne I, Prats M, Tocanne JF, Laneelle G. Mycobacterial glycopeptidolipid interactions with membranes: A monolayer study. FEBS Lett. 1995;375:254–258. doi: 10.1016/0014-5793(95)01219-5. [DOI] [PubMed] [Google Scholar]
- Villeneuve C, Etienne G, Abadie V, Montrozier H, Bordier C, Laval F, Daffe M, Maridonneau-Parini I, Astarie-Dequeker C. Surface-exposed glycopeptidolipids of Mycobacterium smegmatis specifically inhibit the phagocytosis of mycobacteria by human macrophages. Identification of a novel family of glycopeptidolipids. J Biol Chem. 2003;278:51291–51300. doi: 10.1074/jbc.M306554200. [DOI] [PubMed] [Google Scholar]
- Villeneuve C, Gilleron M, Maridonneau-Parini I, Daffe M, Astarie-Dequeker C, Etienne G. Mycobacteria use their surface-exposed glycolipids to infect human macrophages through a receptor-dependent process. J Lipid Res. 2005;46:475–483. doi: 10.1194/jlr.M400308-JLR200. [DOI] [PubMed] [Google Scholar]
- Wagner D, Young LS. Nontuberculous mycobacterial infections: A clinical review. Infection. 2004;32:257–270. doi: 10.1007/s15010-004-4001-4. [DOI] [PubMed] [Google Scholar]
- Yakrus MA, Good RC. Geographic distribution, frequency, and specimen source of Mycobacterium avium complex serotypes isolated from patients with acquired immunodeficiency syndrome. J Clin Microbiol. 1990;28:926–929. doi: 10.1128/jcm.28.5.926-929.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki Y, Danelishvili L, Wu M, Hidaka E, Katsuyama T, Stang B, Petrofsky M, Bildfell R, Bermudez LE. The ability to form biofilm influences Mycobacterium avium invasion and translocation of bronchial epithelial cells. Cell Microbiol. 2006;8:806–814. doi: 10.1111/j.1462-5822.2005.00667.x. [DOI] [PubMed] [Google Scholar]
- Yamazaki Y, Danelishvili L, Wu M, Macnab M, Bermudez LE. Mycobacterium avium genes associated with the ability to form a biofilm. Appl Environ Microbiol. 2006;72:819–825. doi: 10.1128/AEM.72.1.819-825.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]