Summary
Drosophila embryonic dorsal-ventral (DV) polarity is controlled by a group of sequentially acting serine proteases located in the fluid-filled perivitelline space between the embryonic membrane and the eggshell, which generate the ligand for the Toll receptor on the ventral side of the embryo [1, 2, 3]. Spatial control of the protease cascade relies on the Pipe sulfotransferase, a fly homologue of vertebrate glycosaminoglycan modifying enzymes [4, 5, 6], which is expressed in ventral cells of the follicular epithelium surrounding the developing oocyte. The identification of the Pipe enzymatic target has remained a major gap in our understanding of the mechanism controlling the perivitelline protease cascade, and hence embryonic DV patterning. Here we show that the protein Vitelline Membrane-Like (VML) [7] undergoes Pipe-dependent sulfation and, consistent with a role in conveying positional information from the egg chamber to the embryo, becomes incorporated into the eggshell at a position corresponding to the location of the follicle cells from which it was secreted. Although VML influences embryonic DV pattern in a sensitized genetic background, VML is not essential for DV axis formation, suggesting that there is redundancy in the composition of the Pipe enzymatic target. Correspondingly, we find that additional structural components of the vitelline membrane undergo Pipe-dependent sulfation. In identifying the elusive targets of Pipe, this ork points to the vitelline membrane as the source of signals that generate the Drosophila DV axis and provides a framework for understanding the mechanism controlling spatially-specific activation of serine protease activity during embryonic pattern formation.
Results and Discussion
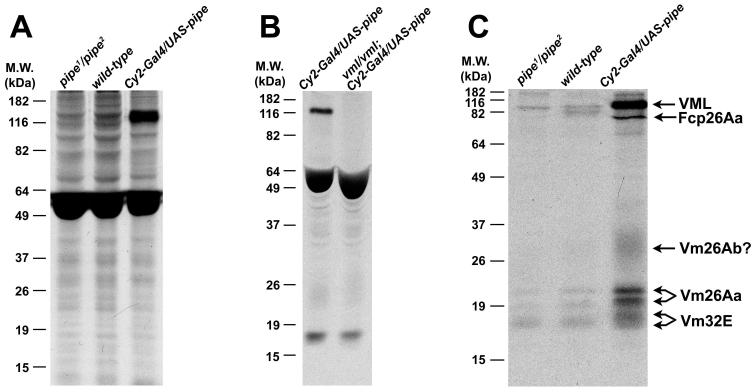
Previous genetic approaches have failed to identify genes encoding putative enzymatic targets of Pipe, the identities of which could provide important insights into the molecular mechanism defining Drosophila embryonic DV polarity. We reasoned that the target of Pipe enzymatic activity should exhibit Pipe-dependent sulfation in ovarian extracts from females fed radioactive 35S-containing Na2SO4[8]. While no differences in the patterns of sulfation were observed in ovarian extracts from wild-type and pipe1/pipe2 females fed on yeast containing [35S]Na2SO4, ovarian extracts from transgenic females expressing high levels of the Pipe-ST2 isoform [4] (henceforth Pipe protein) throughout the follicle cell layer displayed a novel, strongly labeled band of about 120 kDa following SDS-PAGE and autoradiography (Fig. 1A).
Figure 1.
Pipe dependent labeling of vitelline membrane proteins
(A) Autoradiogram of ovarian extracts from [35S]Na2SO4-fed pipe mutant (left lane), wild-type (middle lane) and Pipe overexpressing (right lane) females. Note the presence of the 120 kDa band associated with Pipe overexpression. Maternal genotypes are indicated at top. (B) The 120 kDa band is absent from ovarian extracts of [35S]Na2SO4-fed VmlEPgy2 /Vml EPgy2 mutant females that over-expressed Pipe (right lane). The heavily labeled bands between 49 and 64 kDa in all lanes in panels A and B correspond to yolk proteins, which are known to undergo sulfation [32]. (C) Detection of Pipe dependent labeled species from purified eggshell/extracellular matrix preparations of ovarian extracts [14] from [35S]Na2SO4-fed pipe mutant (left lane), wild-type (middle lane), and Pipe over-expressing females (right lane).
Mass spectrometric analysis of an SDS-PAGE gel slice containing the 120 kDa band identified four peptide sequences located within the predicted open reading frame encoded by the previously identified gene Vml [7](also designated CG34333)(Fig. S1). The predicted protein product of Vml is 578 amino acids in length and carries a hydrophobic N-terminal predicted signal peptide, suggesting that VML is secreted. The C-terminal region of the protein contains a 25 amino acid long stretch that exhibits substantial similarity to the “VM domain” present in several previously characterized components of the vitelline membrane layer of the eggshell [9]. The central region of VML is extremely rich in serine, proline, alanine and tyrosine residues and contains 30 perfect (and additional imperfect) repeats of the amino acid sequence SYSAPAAP. The repeat region contains 127 serine residues and 4 threonine residues that are predicted by the program NetOGly (http://www.cbs.dtu.dk/services/NetOGlyc/) to undergo mucin-type O-linked addition of N-acetylgalactosamine. Extensive glycosylation could provide an explanation for the discrepancy between the predicted MW of 56.1 kDa, based on the primary amino acid sequence of VML, and the observed apparent MW of approximately 120 kDa based on SDS-PAGE. Confirming that the 120 kDa band corresponds to VML, a mutation containing a P-element insertion in the VML open reading frame (P{EPgy2}CG2879EY21650, henceforth referred to as VmlEPgy2) resulted in the loss of the radio-labeled 120 kDa band from ovarian extracts of [35S]Na2SO4-fed females that over-express Pipe (Fig. 1B).
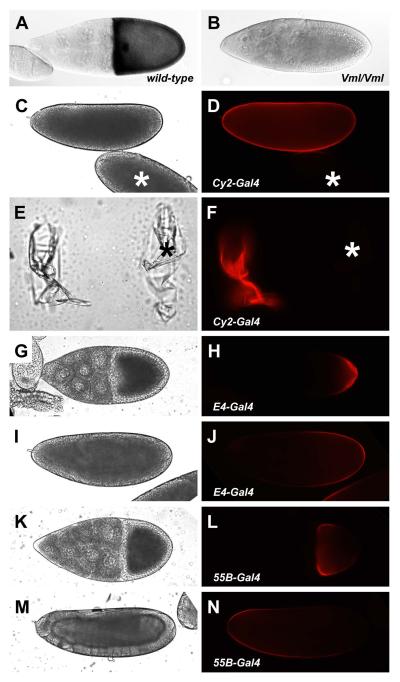
Whole mount mRNA in situ hybridization to fixed ovarian egg chambers demonstrated that Vml mRNA is expressed uniformly throughout the follicle cell layer of stage 10 egg chambers (Fig. 2A), the stage at which pipe is expressed, and that Vml mRNA is absent from females homozygous for the VmlEPgy2 mutation (Fig. 2B). To determine the localization of VML protein following its synthesis, we expressed a transgenic version of VML fused to Red Fluorescent Protein (RFP) [10] in follicle cells under Gal4-mediated transcriptional control. Dechorionated eggs from females expressing VML-RFP under the control of the strong, uniformly expressed follicle cell enhancer trap element, CY2-Gal4 [11], displayed red fluorescence that appeared to be associated with the vitelline membrane (Fig. 2C, D). Confirming this assignment, dechorionated eggs that were mounted on cover slips, from which larvae were allowed to hatch, exhibited bright red fluorescence (Fig. 2E, F). VML-RFP expressed under the control of the E4-Gal4 enhancer trap insertion [11], which drives expression in a posterior subpopulation of follicle cells, was efficiently secreted into the perivitelline space between the posterior follicle cells and the oocyte (Fig. 2G, H) and was stably incorporated into the vitelline membrane in the posterior half of the egg (Fig. 2I, J). Conversely, expression of VML-RFP in anterior follicle cells under the control of the 55B-Gal4 enhancer trap [12] led to its secretion into the perivitelline space at the anterior end of the stage 10 oocyte (Fig. 2K, L) and to its stable incorporation into the vitelline membrane in the anterior half of the egg (Fig. 2M, N). Assuming that endogenous wild-type VML behaves like VML-RFP, these data indicate that VML secreted into the perivitelline space remains near its site of secretion and ultimately becomes stably incorporated into the vitelline membrane in the corresponding position.
Figure 2.
VML is expressed in follicle cells and becomes stably localized in the vitelline membrane layer of the eggshell
Whole mount in situ hybridization to Vml mRNA in stage 10 egg chambers from wild-type (A) and VmlEPgy2/VmlEPgy2 mutant (B) females. Distribution of VML-RFP expressed under the control of CY2-Gal4 in eggs (C, D) and vitelline membranes (E, F). Asterisks indicate the position of an egg and a vitelline membrane from non-expressing females. Distribution of E4-Gal4 driven VML-RFP in a stage 10 egg chamber (G, H) and egg (I, J). Distribution of 55B-Gal4 driven VML-RFP in a stage 10 egg chamber (K, L) and egg (M, N). Bright field images of eggs (C, I, M), empty vitelline membranes (E) and stage 10 egg chambers (G, K) are shown at left, while corresponding fluorescent images are shown in panels D, J, N, F, H, and L, respectively.
Surprisingly, females homozygous for VmlEPgy2 produce hatching embryos. This finding, which suggests that VML acts redundantly with other substrates of Pipe, could explain the failure of previous genetic screens to identify mutations in genes encoding potential enzymatic targets of Pipe. To investigate whether VML influences embryonic DV patterning, we examined the effect of eliminating VML on the phenotype produced by the hypomorphic pipe7 allele. We have previously shown that the severity of the pipe7 phenotype is sensitive to the availability of PAPS, the biological sulfate donor [13]. We reasoned that a reduction in the availability of the Pipe sulfation target might similarly result in an enhancement of the pipe7 mutant phenotype.
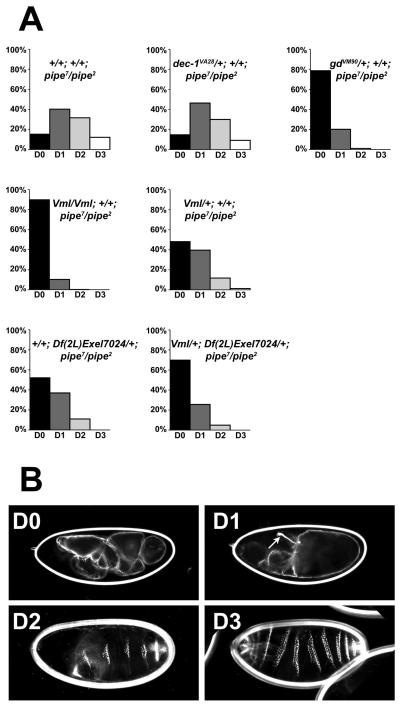
Loss of VML expression led to a dramatic increase in the dorsalization of embryonic progeny of pipe7/pipe2 mutant females (Fig. 3, Table 1). Although the embryos from pipe7/pipe2 females exhibited phenotypes ranging from weakly dorsalized to completely dorsalized (Fig. 3A) (see legend to Fig. 3 for classification criteria and Fig. 3B for representative embryonic cuticles), the great majority of embryos from VmlEPgy2/VmlEPgy2; pipe7/pipe2 females were completely dorsalized, with a small proportion being strongly dorsalized. This enhancement of the pipe7/pipe2 mutant phenotype by the loss of VML protein is consistent with VML acting as a functional substrate of Pipe.
Figure 3.
Reduction in the expression of VML and other vitelline membrane components influences the dorsal-ventral phenotypes of embryos produced by pipe7/pipe2 mutant females (A) Percentage of embryos exhibiting dorsal-ventral cuticular phenotypes of varying severity, produced by females of various genotypes. DV phenotypes of embryonic cuticles were classified according to Roth et al. 1991 [33], as follows. Embryos lacking all lateral or ventral pattern elements were scored as completely dorsalized (D0). Embryos bearing Filzkörper or Filzkörper material but lacking ventral denticles were scored as strongly dorsalized (D1). Embryos with Filzkörper/Filzkörper material and ventral denticle bands of narrower than normal width were scored as moderately dorsalized (D2). Finally, weakly embryos that failed to hatch but otherwise appeared almost wild-type, typically exhibiting twisted or tail-up phenotypes, were scored as weakly dorsalized (D3). Maternal genotypes are indicated above each histogram. Numbers of embryos examined and scored are listed in Table 1. (B) Representative DO, D1, D2 and D3 embryos. Maternal genotypes: (D0, D1) VmlEPgy2/ VmlEPgy2; pipe7/pipe2, (D2, D3) pipe7/pipe2. An arrow indicates the position of Filzkörper in the D1 embryo. Filzkörper present in the D2 and D3 embryos are out of the plane of focus.
Table 1.
The effects of reducing the expression of VML and other eggshell components on the distribution of DV phenotypes of embryos produced by pipe2/pipe7 females
| Maternal mutant background (number of embryos scored) |
Proportion of embryos exhibiting denoted DV phenotypes* percentage ± estimated standard error** |
|||
|---|---|---|---|---|
| D0 | D1 | D2 | D3 | |
|
+/+; +/+; pipe7/pipe2 (n=1681) |
15.7% ± 0.9% |
40.7% ± 1.2% |
31.5% ± 1.1% |
12.1% ± 0.8% |
|
Vml/Vml; +/+; pipe7/pipe2 (n=554) |
89.5% ± 1.3% |
10.3% ± 1.3% |
0.2% ± 0.2% |
0 |
|
Vml/+; +/+; pipe7/pipe2 (n=327) |
48.0% ± 2.8% |
39.1% ± 2.7% |
11.6% ± 1.8% |
1.2% ± 0.6% |
|
+/+; Df(2L)Exel7024/+; pipe7/pipe2 (n=662) |
52.1% ± 1.9% |
36.9% ± 1.9% |
11.0% ± 1.2% |
0 |
|
Vml/+; Df(2L)Exel7024/+; pipe7/pipe2 (n=600) |
69.5% ± 1.9% |
25.7% ± 1.8% |
4.8% ± 0.9% |
0 |
|
dec-1 VA28/+; +/+; pipe7/pipe2 (n=222) |
14.4% ± 2.4% |
46.8% ± 3.3% |
29.7% ± 3.1% |
9.0% ± 1.9% |
|
gd VM90/+; +/+; pipe7/pipe2 (n=208) |
78.8% ± 2.8% |
20.2% ± 2.8% |
1.0% ± 0.7% |
0 |
Embryonic phenotypes are scored according to Roth et al., 1991 [33].
Standard errors are estimated as (percentage x (1-percentage) / n) 1/2
To investigate whether the dispensability of VML results from the ability of one or more other vitelline membrane to substitute for it, we utilized a labeling strategy similar to the one used to identify VML, but in this case we subjected the dissected ovaries to a fractionation procedure [14] that enriches for ovarian extracellular matrix proteins and proteins of the forming eggshell. In addition to VML, a number of other radioactive bands were detected in extracts from females over-expressing Pipe which were either absent, or much less strongly labeled, in the extracts from wild-type and pipe mutant females (Fig. 1C). Mass spectrometric analysis indicated that two of the labeled bands corresponded to Vm26Aa, which is known to undergo processing [15], while a lower molecular weight doublet of bands present in the extract from Pipe overexpressing, which are difficult to resolve from one another in the figure, were both identified as Vm32E. A fifth labeled band corresponded to Fcp26Aa (Palisade) [14, 16]. Finally, a diffuse radioactive band ranging from about 20 kDa to 25 kDa, although not identified by mass spectrometry, is likely to correspond to Vm26Ab, based on its diffuse appearance and size [15]. Additional bands observed in the wild-type derived extract at about 82 kDa, and in the extract from females overexpressing Pipe at about 70kDa, may represent additional targets of Pipe-mediated sulfation. However, these bands were not reproducibly observed following labeling reactions and were not characterized thoroughly. In the case of VML, Vm26Aa, and Vm32E, we note labeling of the bands even in extracts from pipe mutant females. This labeling might result from residual activity of the protein produced by one or both of the mutant alleles carried by these females, or to the production of alternative splicing isoforms that are known to be produced by the pipe locus, but which do not appear to have a role in DV patterning [13; Z. Zhang and D. Stein, unpublished]. However, the interval of time that we have used to feed females with [35S]Na2SO4 is long enough to allow some incorporation of radioactive label into methionine and cysteine residues. As vitelline membrane proteins are known to be expressed at high levels during a short interval of oogenesis, and since the fractionation procedure that we have used enriches for eggshell proteins, we consider it likely that the labeling of these proteins in pipe mutant females results from the incorporation of radioactive amino acids during their synthesis. The absence of a conspicuous increase in the level of sulfation of these proteins in wild-type females, in comparison to pipe mutant females, suggests that under normal circumstances, the proportion of Pipe substrate that is sulfated is very low. Interestingly, in extracts from Pipe overexpressing females, Vm32E is present as a doublet of bands, both of which have been identified as Vm32E, while the lower of the two bands corresponding to Vm26A exhibits a slight increase in molecular weight upon Pipe overexpression. Both observations are consistent with a situation in which Pipe-mediated sulfation results in the formation of protein with a slightly larger molecular weight, that can be distinguished from protein that exhibits labeling due to radioactive amino acid incorporation. Taken together, however, the results of these labeling studies allow us to conclude that in addition to VML, Pipe sulfates at least four additional components of the vitelline membrane.
No conventional loss-of-function alleles specifically affecting Vm26Aa, Vm32E or Fcp26Aa have been reported. Females homozygous for the single available loss of function mutation affecting Vm26Ab generate flaccid, collapsed eggs that do not undergo embryonic development [17], which precludes examination of the effects of complete loss of Vm26Ab activity on DV patterning. However, the chromosomal deficiency Df(2L)Exel7024 deletes the genes encoding Vm26Aa, Vm26Ab and Fcp26Aa, the additional putative vitelline membrane proteins Fcp26Ac(Vm26Ac) and CG13992 [7, 14], and 11 additional annotated genes. We reasoned that if vitelline membrane proteins encoded by the genes uncovered by this deficiency represented additional functioning targets of Pipe, then introduction of Df(2L)Exel7024 into the pipe7 mutant background would enhance the mutant phenotype. As seen in Figure 4A and Table 1, in comparison to the progeny of pipe7/pipe2 mutant females, the introduction of one copy of Df(2L)Exel7024 into the pipe7/pipe2 mutant background led to a significant increase in the proportion of strongly dorsalized embryos and to a complete absence of weakly dorsalized embryos. Strikingly, the combination of Df(2L)Exel7024 with a single mutant copy of Vml led to an increase in the severity of dorsalization that was greater than the effect of the deficiency alone or of a single mutant copy of Vml.
A mutation affecting Dec-1, an eggshell structural protein [18] that is not sulfated by Pipe, did not enhance the severity of the pipe7/pipe2 mutant phenotype (Fig. 3, Table 1). This result suggests that the enhancing effects of the Vml mutation and of Df(2L)Exel7024 result from a specific effect on DV patterning rather than from a general alteration in eggshell integrity. In contrast, introduction of a mutant copy of gastrulation defective (gd) [19] dramatically increased the severity of the pipe7/pipe2 mutant phenotype (Fig. 3, Table 1), consistent with the involvement of GD in the pathway. Together, these data strongly suggest that in addition to VML, the vitelline membrane proteins Vm26Aa, Vm26Ab, VM32E and Fcp26Aa represent enzymatic substrates of Pipe, one or more of which may contribute to the localized cue that controls the DV serine protease cascade. Like VML, these proteins are likely to become incorporated into the eggshell at positions corresponding to the location of follicle cells from which they were secreted, thus stabilizing positional information conferred upon them by Pipe-mediated sulfation.
Several of the additional vitelline membrane proteins sulfated by Pipe contain stretches of amino acids similar or identical to the SYSAPAAP motif that is present in multiple copies in VML, which may represent attachment sites for carbohydrates sulfated by Pipe. Despite the similarity of Pipe protein isoforms to vertebrate GAG-modifying enzymes, the nature of the carbohydrate species that are likely to represent the direct substrate of Pipe remains controversial. While the expression in follicle cells of genes required for GAG synthesis does not appear to be essential for DV patterning, and while VML does not carry any copies of the Ser-Gly-Xaa-Gly consensus sequence for GAG addition [20], the composition of heparan sulfate obtained from extracellular matrix/eggshell preparations of wild-type and pipe mutant ovaries appear to differ [21]. The identification of carbohydrates and other modifications associated with VML and with the other vitelline membrane proteins modified by Pipe should resolve this issue.
In some respects, the effects of the vitelline membrane components modified by Pipe resemble those of Torsolike, a protein involved in Terminal patterning in Drosophila. torsolike is expressed in two populations of follicle cells that are adjacent to the anterior and posterior poles of the developing oocyte [22, 23] and becomes incorporated into the vitelline membrane at the two poles of the egg [24]. However, how Torsolike leads to the formation of pattern at the anterior and posterior ends of the embryo remains a mystery.
In contrast, our identification of modified targets of Pipe enables the formulation of specific and testable models of how they might act to influence embryonic DV pattern. We consider the most likely scenario to be one in which the sulfate modified carbohydrates localized by their carrier proteins to the ventral side of the egg act as cofactors necessary for the activity of one or more of the perivitelline proteases. A number of instances are known in which carbohydrate binding is required for activation of serine proteolytic activity [25, 26]. Alternatively, the modified Pipe target(s) may be necessary for stable localization of one or more of the proteases. In a third model, the ventral cue may inactivate or sequester an inhibitor of one or more of the perivitelline proteases. Indeed, a serine protease inhibitor of DV patterning, Serpin27A (Spn27A), has been identified in the perivitelline space [27, 28]. However, the effects of Spn27A are not affected by Pipe activity [27]. This does not rule out the possibility that another serine protease inhibitor that is inhibited by the ventral cue may be present in the perivitelline fluid. Ultimately, the elucidation of the mechanism that restricts serine proteolytic activity and Toll activation to the ventral side of the egg and embryo will rely on studies of the interaction between the ventral cue and the perivitelline serine proteases, an avenue of investigation that is now feasible with the identification of VML and other targets sulfated by Pipe.
Experimental Procedures
Drosophila stocks and strain maintenance
All stocks were maintained employing standard conditions and procedures. The wild-type D. melanogaster stock used was Oregon R. Mutants and transgenic lines used in this study are described on Flybase and are as follows: dec-1VA28, gdVM90, pipe1, pipe2, pipe7, Df(2L)Exel7024, P{EPgy2}CG2879EY21650, UAS-pipe-ST2 (P{UAS-pip.ST2}. The CY2-Gal4, E4-Gal4 and 55B-Gal4 enhancer trap insertions [11, 12] were obtained from Dr. Trudi Schüpbach.
Metabolic labeling of ovarian proteins
In vivo labeling with 35S was carried out using a modification of the protocol for labeling of glycosaminoglycans described by Pinto et al. (2004) [8]. 50 adult males and 50 adult females were combined and allowed to mate en masse for 24 hours. The females were then transferred to a plastic tube containing 10 ml of fly medium supplemented with a feeding mixture containing 200 μg yeast, 200 μl distilled water and 200 μCi [35S]Na2SO4 placed on the wall of the tube. Females were fed on the labeling mixture for 40 hr at 29°C which included one exchange of the feeding mixture after the first 20 hr of feeding. Following radioactive labeling, the females were anesthetized with carbon dioxide and their ovaries were dissected in cold Ringer’s solution.
Protein purification and mass spectrometric analysis
500 pairs of radio-labeled ovaries from adult females carrying the Gal4 driver CY2-Gal4 together with an insertion of pUAST-pipe-ST2 were homogenized in 1.5 ml of NP-40 buffer (1% NP-40, 0.15M NaCl, 62.5 mM Tris-HCl, pH 7.0), and the pellet was collected after 5 min of centrifugation at 12,000 rpm. The pellet was resuspended and washed 6 times in 1.5 mls NP-40 buffer. The pellet was dissolved in 0.5 ml 3M Urea and subjected ion exchange chromatography with DEAE-sephacyl (GE Healthcare, Piscataway, NJ). The solubilized material was loaded onto a column (8 cm in height. 0.8 cm in diameter) containing DEAE-Sephacryl pre-equilibrated in 3M Urea. The sample was loaded onto the column and eluted in 3 successive volumes of 15 ml of 3M Urea containing 0M, 0.25M and 0.5 M NaCl respectively, collected in 1ml column fractions. An aliquot of each collected fraction was examined by SDS-PAGE followed by autoradiography. Fractions that contained a radio-labeled band of approximately 120 kDa were combined and dialyzed twice against 1 l of 5 mM Tris-HCl (pH 6.8) buffer containing 0.1% SDS. The dialyzed sample was dried in a vacuum, resuspended in sample buffer and subjected to SDS-PAGE (10%).
Following electrophoresis, the gel was stained with Coomassie Brilliant Blue and the stained band corresponding to the 120 kDa radio-labeled band was excised for Mass Spectrometric analysis. Protein identification was performed at the Analytical Instrumentation Facility Core of the University of Texas at Austin using a MALDI-TOF/TOF Mass Spectrometer. MASCOT V2.0 was used to search the non-redundant Swiss-Prot, TrEMBL and NCBI databases for proteins containing peptides predicted by the Mass Spectrometric analysis.
In some experiments, ovarian extracellular matrix and eggshell proteins were isolated, starting with 50 pairs of ovaries from [35S]Na2SO4-fed females, using the protocol of Fakhouri et al. (2006) [14] including treatment with DNase I and RNase A.
In situ hybridization analysis
The two oligonucleotides 5′ - GATCGAGGATCCATGTGTGGACGACGACTGCTGTTC - 3′ and 5′ - GATCGAGAATTCGTAACCATATCCCTGACTGCACGG - 3′ were used for PCR amplification of the intronless VML open reading frame using Oregon R genomic DNA as a template and Phusion high fidelity DNA polymerase (New England Biolabs, Ipswich, MA). The DNA fragment resulting from PCR amplification was purified, digested with BamHI and EcoRI, and ligated to BamHI/EcoRI digested pBluescriptKS+ (Stratagene, La Jolla, CA) yielding pBluescript-VML.
For in situ hybridization studies, the BamHI/EcoRI VML fragment was excised from pBluescript-VML and used as a template to generate a random primed digoxygenin-dUTP-labelled DNA probe using the DIG High Prime labeling mix (Roche Applied Science, Indianapolis, IN). In situ hybridization and detection of fixed, dissected ovaries from OregonR and VmlEPgy2/ VmlEPgy2 mutant females was carried out according to Hong and Hashimoto (1995) [29].
Construction of pUAST-VML-RFP
The two oligonucleotides 5′ - CACCAAAATGTGTGGACGACGACTGCTG - 3′ and 5′ - GTAACCATATCCCTGACTGCACGG - 3′ were used for PCR amplification of the VML open reading frame using pBluescript-VML as a template and Phusion high fidelity DNA polymerase (New England Biolabs, Ipswich, MA). The amplification product was introduced into the Gateway system entry vector pENTR using the pENTR/D-TOPO Directional Cloning Kit (Invitrogen, Carlsbad, CA). The resulting plasmid, pENTR-VML, carries the VML open reading frame flanked by attL1 and attL2 site-specific recombination target sites for Bacteriophage Lambda Integrase.
To construct pUAST-VML-RFP, the Gateway LR Clonase Enzyme Mix (Invitrogen, Carlsbad, CA) was used to accomplish site-site specific recombination between pENTR-VML and the Gateway destination vector, pTWR (Terence Murphy, Carnegie Institute of Washington, Baltimore, MD). Clonase-mediated site-specific recombination between pENTR-VML and pTWR resulted in the formation of pUAST-VML-RFP, which carries an in-frame fusion of mRFP1 [10] to the C-terminus of VML, downstream of Gal4 transcription factor binding sites in plasmid pUAST [30]. Transformant flies carrying pUAST-VML-RFP were generated at GenetiVision, Inc. (Houston, TX).
Examination of Ovary, Egg and Embryonic Phenotypes
Ovaries were dissected from yeast-fed adult females directly into PBS and examined under bright field illumination or under UV illumination using a TRITC filter set on a Zeiss Axioplan II microscope outfitted with an Axiocam digital camera.
For the examination of the localization of VML-FRP, eggs were collected on yeasted apple juice agar plates, dechorionated in a 50% solution of Clorox bleach in water and placed directly into halocarbon 27 oil (Sigma Life Science, St. Louis, MO) for examination and photography under bright field or fluorescence illumination. Alternatively, the dechorionated eggs were glued to cover slips, covered with halocarbon 200 oil (Halocarbon Products Corp., River Edge, NJ) and allowed to continue development until the larvae hatched from the vitelline membranes. The empty eggshells were then photographed under bright field and fluorescence illumination.
For the examination of embryonic phenotypes, larval cuticles were prepared according to Van der Meer (1977) [31].
Supplementary Material
Acknowledgements
We are grateful to Drs. Kathryn Anderson, Robert DeLotto, Carl Hashimoto, Donald Morisato, Trudi Schüpbach and to the Drosophila Stock Center in Bloomington Indiana for providing Drosophila stocks essential to this study. We also thank Dr. Maria Person, Director of the Analytical Instrumentation Facility Core, administered jointly by the Institute for Cellular and Molecular Biology and the College of Pharmacy of the University of Texas at Austin, and by the University of Texas M.D. Anderson Cancer Center Science Park, for her assistance in the mass spectrometric analysis leading to the identification of VML. Plasmid pTWR, the Gateway-compatible expression vector produced by Dr. Terence Murphy (Carnegie Institute of Washington, Baltimore, MD) and containing mRFP1, which was generated in the lab of Dr. Roger Tsien (HHMI/UC San Diego), was obtained from the Drosophila Genomics Resource Center, located at Indiana University in Bloomington, IN. This work was supported by a grant from the National Institutes of Health (GM077337).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Moussian B, Roth S. Dorsoventral axis formation in the Drosophila embryo-shaping and transducing a morphogen gradient. Curr. Biol. 2005;15:R887–R899. doi: 10.1016/j.cub.2005.10.026. [DOI] [PubMed] [Google Scholar]
- 2.Hashimoto C, Hudson KL, Anderson KV. The Toll gene of Drosophila, required for dorsal-ventral embryonic polarity appears to encode a transmembrane protein. Cell. 1988;52:269–279. doi: 10.1016/0092-8674(88)90516-8. [DOI] [PubMed] [Google Scholar]
- 3.Schneider DS, Jin Y, Morisato D, Anderson KV. A processed form of the Spätzle protein defines dorsal-ventral polarity in the Drosophila embryo. Development. 1994;120:1243–1250. doi: 10.1242/dev.120.5.1243. [DOI] [PubMed] [Google Scholar]
- 4.Sen J, Goltz JS, Stevens L, Stein D. Spatially restricted expression of pipe in the Drosophila egg chamber defines embryonic dorsal-ventral polarity. Cell. 1998;95:471–481. doi: 10.1016/s0092-8674(00)81615-3. [DOI] [PubMed] [Google Scholar]
- 5.Kobayashi M, Habuchi H, Yoneda M, Habuchi O, Kimata K. Molecular cloning and expression of Chinese hamster ovary cell heparan-sulfate 2-sulfotransferase. J. Biol. Chem. 1997;272:13980–13985. doi: 10.1074/jbc.272.21.13980. [DOI] [PubMed] [Google Scholar]
- 6.Kobayashi M, Sugumaran G, Liu J, Shworak NW, Silbert JE, Rosenberg RD. Molecular cloning and characterization of a human uronyl 2-sulfotransferase that sulfates iduronyl and glucuronyl residues in dermatan/chondroitin sulfate. J. Biol. Chem. 1999;274:10474–10480. doi: 10.1074/jbc.274.15.10474. [DOI] [PubMed] [Google Scholar]
- 7.Alatortsev VE. New Genes for Vitelline Membrane Proteins in Drosophila. Mol. Biol. 2006;40:330–332. [PubMed] [Google Scholar]
- 8.Pinto DO, Ferreira PL, Andrade LR, Petrs-Silva H, Linden R, Abdelhay E, Araujo HM, Alonso CE, Pavao MS. Biosynthesis and metabolism of sulfated glycosaminoglycans during Drosophila melanogaster development. Glycobiol. 2004;14:529–536. doi: 10.1093/glycob/cwh070. [DOI] [PubMed] [Google Scholar]
- 9.Scherer LJ, Harris DH, Petri WH. Drosophila vitelline membrane genes contain a 114 base pair region of highly conserved coding sequence. Dev. Biol. 1988;130:786–788. doi: 10.1016/0012-1606(88)90367-3. [DOI] [PubMed] [Google Scholar]
- 10.Campbell RE, Tour O, Palmer AE, Steinbach PA, Baird GS, Zacharias DA, Tsien RY. A monomeric red fluorescent protein. Proc. Natl. Acad. Sci. USA. 2002;99:7877–7882. doi: 10.1073/pnas.082243699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Queenan AM, Ghabrial A, Schüpbach T. Ectopic activation of Torpedo/EGFR, a Drosophila receptor tyrosine kinase, dorsalizes both the eggshell and the embryo. Development. 1997;124:3871–3880. doi: 10.1242/dev.124.19.3871. [DOI] [PubMed] [Google Scholar]
- 12.Brand AH, Perrimon N. Raf acts downstream of the EGF receptor to determine dorsoventral polarity during Drosophila oogenesis. Genes Dev. 1994;8:629–639. doi: 10.1101/gad.8.5.629. [DOI] [PubMed] [Google Scholar]
- 13.Zhu X, Sen J, Stevens L, Goltz JS, Stein D. Drosophila Pipe protein activity in the ovary and the embryonic salivary gland does not require heparan sulfate glycosaminoglycans. Development. 2005;132:3813–3822. doi: 10.1242/dev.01962. [DOI] [PubMed] [Google Scholar]
- 14.Fakhouri M, Elalayli M, Sherling D, Hall JD, Miller E, Sun X, Wells L, LeMosy EK. Minor proteins and enzymes of the Drosophila eggshell matrix. Dev. Biol. 2006;293:127–141. doi: 10.1016/j.ydbio.2006.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pascucci T, Perrino J, Mahowald AP, Waring GL. Eggshell assembly in Drosophila: processing and localization of vitelline membrane and chorion proteins. Dev. Biol. 1996;177:590–598. doi: 10.1006/dbio.1996.0188. [DOI] [PubMed] [Google Scholar]
- 16.Elalayli M, Hall JD, Fakhouri M, Neiswender H, Ellison TT, Han Z, Roon P, LeMosy EK. Palisade is required in the Drosophila ovary for assembly and function of the protective vitelline membrane. Dev. Biol. 2008;319:359–369. doi: 10.1016/j.ydbio.2008.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schüpbach T, Wieschaus E. Female sterile mutations on the second chromosome of Drosophila melanogaster. II. Mutations blocking oogenesis or altering egg morphology. Genetics. 1991;129:1119–1136. doi: 10.1093/genetics/129.4.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hawley RJ, Waring GL. Cloning and analysis of the dec-1 female-sterile locus, a gene required for proper assembly of the Drosophila eggshell. Genes Dev. 1988;2:341–349. doi: 10.1101/gad.2.3.341. [DOI] [PubMed] [Google Scholar]
- 19.Konrad KD, Goralski TJ, Mahowald AP, Marsh JL. The gastrulation defective gene of Drosophila melanogaster is a member of the serine protease superfamily. Proc. Natl. Acad. Sci. U S A. 1998;95:6819–6824. doi: 10.1073/pnas.95.12.6819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bourdon MA, Krusius T, Campbell S, Schwartz NB, Ruoslahti E. Identification and synthesis of a recognition signal for the attachment of glycosaminoglycans to proteins. Proc. Natl. Acad. Sci. USA. 1987;84:394–398. doi: 10.1073/pnas.84.10.3194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Park Y, Zhang Z, Linhardt RJ, Lemosy EK. Distinct heparan sulfate compositions in wild-type and pipe-mutant eggshell matrix. Fly. 2008;2:175–179. doi: 10.4161/fly.6706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Savant-Bhonsale S, Montell DJ. torso-like encodes the localized determinant of Drosophila terminal pattern formation. Genes Dev. 1993;7:2548–2555. doi: 10.1101/gad.7.12b.2548. [DOI] [PubMed] [Google Scholar]
- 23.Martin JR, Raibaud A, Ollo R. Terminal pattern elements in Drosophila embryo induced by the Torso-like protein. Nature. 1994;367:741–745. doi: 10.1038/367741a0. [DOI] [PubMed] [Google Scholar]
- 24.Stevens LM, Beuchle D, Jurcsak J, Tong X, Stein D. The Drosophila embryonic patterning determinant Torsolike is a component of the eggshell. Curr. Biol. 2003;13:1058–1063. doi: 10.1016/s0960-9822(03)00379-8. [DOI] [PubMed] [Google Scholar]
- 25.Hallgren J, Karlson U, Poorafshar M, Hellman L, Pejlar G. Mechanism for activation of mouse cell tryptase: dependence on heparin and acidic pH for formation of active tetramers of mouse mast cell protease 6. Biochemistry. 2000;39:13068–13077. doi: 10.1021/bi000973b. [DOI] [PubMed] [Google Scholar]
- 26.Takahashi M, Mori S, Shigeta S, Fujita T. Role of MBL-associated serine protease (MASP) on activation of the lectin complement pathway. In: Lambris JD, editor. Current Topics in Innate Immunity. Springer; New York: 2007. pp. 93–104. [DOI] [PubMed] [Google Scholar]
- 27.Ligoxygakis P, Roth S, Reichart J-M. A serpin regulates dorsal-ventral axis formation in the Drosophila embryo. Curr. Biol. 2003;13:2097–2102. doi: 10.1016/j.cub.2003.10.062. [DOI] [PubMed] [Google Scholar]
- 28.Hashimoto C, Kim DR, Weiss LA, Miller JW, Morisato D. Spatial regulation of developmental signaling by a serpin. Dev. Cell. 2003;5:945–950. doi: 10.1016/s1534-5807(03)00338-1. [DOI] [PubMed] [Google Scholar]
- 29.Hong CC, Hashimoto C. An unusual mosaic protein with a protease domain, encoded by the nudel gene, is involved in defining embryonic dorsoventral polarity in Drosophila. Cell. 1995;82:785–794. doi: 10.1016/0092-8674(95)90475-1. [DOI] [PubMed] [Google Scholar]
- 30.Brand AH, Perrimon N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development. 1993;118:401–415. doi: 10.1242/dev.118.2.401. [DOI] [PubMed] [Google Scholar]
- 31.van der Meer JM. Optical clean and permanent whole mount preparations for phase-contrast microscopy of cuticular structures of insect larvae. Dros. Inf. Serv. 1977;52:160. [Google Scholar]
- 32.Bauerle PA, Huttner WB. Tyrosine sulfation of yolk proteins 1, 2 and 3 in Drosophila melanogaster. J. Biol. Chem. 1985;260:6434–6439. [PubMed] [Google Scholar]
- 33.Roth S, Hiromi Y, Godt D, Nüsslein-Volhard C. cactus, a maternal gene required for proper formation of the dorsoventral morphogen gradient in Drosophila embryos. Development. 1991;112:371–388. doi: 10.1242/dev.112.2.371. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.