Abstract
Neurofibromatosis type I (NF1) is a genetic disorder caused by mutations in the NF1 tumor suppressor gene. Neurofibromin is encoded by NF1 and functions as a negative regulator of Ras activity. Somatic mutations in the residual normal NF1 allele within cancers of NF1 patients is consistent with NF1 functioning as a tumor-suppressor. However, the prevalent non-malignant manifestations of NF1, including learning and bone disorders emphasize the importance of dissecting the cellular and biochemical effects of NF1 haploinsufficiency in multiple cell lineages. One of the least studied complications of NF1 involves cardiovascular disorders, including arterial occlusions that result in cerebral and visceral infarcts. NF1 vasculopathy is characterized by vascular smooth muscle cell (VSMC) accumulation in the intima area of vessels resulting in lumen occlusion. We recently showed that Nf1 haploinsufficiency increases VSMC proliferation and migration via hyperactivation of the Ras-Erk pathway, which is a signaling axis directly linked to neointima formation in diverse animal models of vasculopathy. Given this observation, we tested whether heterozygosity of Nf1 would lead to vaso-occlusive disease in genetically engineered mice in vivo. Strikingly, Nf1+/− mice have increased neointima formation, excessive vessel wall cell proliferation and Erk activation after vascular injury in vivo. Further, this effect is directly dependent on a Gleevec sensitive molecular pathway. Therefore, these studies establish an Nf1 model of vasculopathy, which mirrors features of human NF1 vaso-occlusive disease, identifies a potential therapeutic target and provides a platform to further dissect the effect of Nf1 haploinsufficiency in cardiovascular disease.
INTRODUCTION
Neurofibromatosis type 1 (NF1) is an autosomal disorder that affects 1 in 3500 individuals (1,2). NF1 results from mutations in the NF1 tumor suppressor gene, which encodes the protein neurofibromin (3). Neurofibromin functions as a p21ras (Ras) GTPase activating protein (GAP) to negatively regulate Ras signaling (4–8). The detection of somatic mutations in the residual normal NF1 allele within the cancers of individuals with NF1 is consistent with NF1 functioning as a tumor-suppressor gene (9). However, evidence in selected lineages now indicates that analogous to recent discoveries in p53 and p27, gene dosage effects of NF1 alter cell fates and functions (10,11). The most recent studies using Nf1+/− cells have focused on the role of Nf1 haploinsufficiency in lineages of the tumor micro-environment of plexiform neurofibromas and optic gliomas (12–14). However, a phenotype in long-term learning in Nf1+/− mice, similar to a spatial–visual discoordination observed in NF1 patients, has been established (15). The high frequency of non-malignant manifestations in NF1 patients, including learning deficits and osseous abnormalities, such as osteoporosis, suggest the importance of NF1 haploinsufficiency in multiple cell lineages (15,16). Recognition of the cellular and biochemical underpinnings of these physical findings is important in identifying specific molecular therapies and in disease treatment and prevention.
One of the least studied complications of NF1 involves disorders of the cardiovascular system. Although the exact frequency of vascular lesions is unknown, vasculopathy is an under-recognized complication of the disease and contributes to excess morbidity and mortality particularly among younger patients (17–20). Specifically, NF1 patients develop renal artery stenosis and arterial occlusions that result in cerebral and visceral infarcts (17–21). NF1 vascular lesions are characterized by an accumulation of vascular smooth muscle cells (VSMCs) in the intima area of the vessel (termed neointima formation) resulting in lumen occlusion (17,22,23).
We recently demonstrated that neurofibromin deficient VSMCs have increased proliferation and migration in response to platelet-derived growth factor-BB (PDGF-BB) via hyperactivation of the canonical Ras-Erk pathway (24). This observation is intriguing and provides potential insights into NF1 vasculopathy given the emerging paradigm in vascular biology where tight control of the PDGF-BB-Ras-Erk signaling axis in VSMCs is critical for maintaining VSMC homeostasis in blood vessel walls and preventing premature development of vascular occlusive disease (25–31). Specifically, mice harboring genetic mutations that increase signaling through the PDGF-BB-Ras-Erk signaling axis develop exaggerated neointimal hyperplasia and arterial occlusive disease reminiscent of the cerebrovascular complications, which develop in some NF1 patients (26–29,31–36). Despite these prior observations, it remains unclear whether heterozygous inactivation of Nf1 leads to increased neointima formation after vascular injury in vivo. This is an important question since a major obstacle in understanding the pathogenesis and treatment of NF1 vasculopathies is the lack of an animal model that recapitulates the human disease (17).
Therefore, we performed a well-established murine carotid artery injury model of neointima formation in Nf1+/− and wild-type (WT) mice to compare their vascular responses. Strikingly, Nf1+/− mice have increased neointima formation, excessive vascular wall cellular proliferation and Erk activation in response to vascular injury. Further, we provide evidence that treatment of Nf1+/− mice with imatinib mesylate (Gleevec), a pharmacological inhibitor of the PDGF-BB-Ras-Erk pathway, inhibits neointima formation after arterial injury providing a novel molecular target for NF1 vasculopathies.
RESULTS
Nf1+/− mice have increased neointima formation and vessel lumen occlusion in response to mechanical arterial injury
Heterozygous inactivation of Nf1 increases VSMC proliferation and migration in response to PDGF-BB stimulation in vitro (24), which are cellular functions linked to neointima formation in vivo (26–29,31–33). Therefore, based on these prior studies, we tested whether Nf1+/− mice had increased neointima formation in response to mechanical arterial injury in vivo compared with WT controls utilizing a well established surgical model (37). The C57BL/6 murine strain was utilized for these experiments since previous studies indicate that C57BL/6 mice are more resistant to neointima formation in response to injury compared with other strains (38). Therefore, we hypothesized that use of C57BL/6 mice would allow us to interrogate whether Nf1 haploinsufficiency would enhance neointima formation on a relatively resistant murine genetic strain.
Briefly, the endothelium of the common carotid artery of WT and Nf1+/− mice was denuded using a beaded wire and the mice were allowed to recover for 21 days postoperatively. Whole carotid arteries were then harvested and analyzed to quantify the animal's response to injury. For each animal, the contralateral carotid artery served as an uninjured control compared with the injured vessel. To quantitate differences between the two experimental genotypes, morphometric analysis on arterial cross-sections was completed by measuring lumen, intima and media area. From these measurements, the intima-to-media (I/M) ratio was calculated for the injured and uninjured arteries. The I/M ratio is a widely used measurement to predict the development of cardiovascular morbidity (39). Of note, animals in which the internal elastic lamina (IEL) was damaged by the mechanical injury were excluded from the study.
Histological examination of the uninjured carotid arteries from WT and Nf1+/− mice demonstrated the absence of a neointima and revealed no structural differences in vessel architecture between the two experimental genotypes (Fig. 1A). However, in response to arterial injury, Nf1+/− mice had increased vessel occlusion compared with WT controls (Fig. 1A). A representative low and high power image of a hematoxylin–eosin (H&E) stain of an arterial cross-section of uninjured and injured vessels harvested from WT and Nf1+/− mice is shown in Figure 1A. Detailed morphometric analysis revealed that the injured arteries isolated from Nf1+/− mice had a 5-fold increase in intima area compared with WT controls (Fig. 1B). No significant difference was observed in the media area in response to injury between the two genotypes (data not shown) indicating Nf1+/− mice have increased accumulation of cells in the intima area resulting in partial lumen occlusion. Of note, the C57/BL6 WT mice formed a small neointima after arterial injury, which is similar to previously published reports utilizing this murine strain (38,40). Based on intima and media measurements, the I/M ratios of the injured arteries harvested from Nf1+/− mice were 3.5-fold higher than WT controls (Fig. 1C). Further, Nf1+/− mice had a 6-fold increase in percent lumen stenosis in response to injury compared with WT mice (Fig. 1D). Therefore, this data clearly demonstrates that heterozygous inactivation of Nf1 greatly accelerates neointima formation and vessel lumen occlusion after arterial injury.
Figure 1.
Histological and morphometric analysis of injured carotid arteries from WT and Nf1+/− mice. (A) Representative photomicrographs of carotid arteries from WT (top panels) and Nf1+/− (bottom panels) mice stained with H&E 21 days following no injury (left panels) or injury (right panels). Red arrows indicate boundary of neointima. Scale bars represent 50 µm. Results are representative of five independent experiments. (B) Neointima area of uninjured and injured carotid artery cross-sections from WT and Nf1+/− mice. Data represent mean neointima area of five cross-sections±SEM, n = 5. For Nf1+/− uninjured versus injured, *P < 0.05, and for WT injured versus Nf1+/− injured, **P < 0.05 by one-way ANOVA. (C) I/M ratio of uninjured and injured carotid artery cross-sections from WT and Nf1+/− mice. Data represent mean I/M ratio of five cross-sections±SEM, n = 5. For Nf1+/− uninjured versus injured, *P < 0.01, and for WT injured versus Nf1+/− injured, **P < 0.05 by one-way ANOVA. (D) Percentage of carotid artery stenosis 21 days following injury in WT and Nf1+/− mice. Data represent mean percent stenosis of five cross-sections±SEM, n = 5, *P < 0.04 by Student's unpaired t-test with Welch correction.
Nf1+/− mice have increased numbers of VSMCs, proliferating cells and augmented Erk activation in the evolving neointima
PDGF-BB binding to its receptor activates both the Ras-Erk and PI-3 kinase-Akt signaling pathways, which regulate the proliferation and migration of VSMCs (41–43). Our previous data demonstrates that heterozygous inactivation of Nf1 increases both murine and human VSMC proliferation and migration via hyperactivation of the canonical Ras-Erk pathway and not the Ras –PI-3 kinase-Akt pathway in vitro (24). Therefore, in order to better understand the mechanism of increased neointima formation in Nf1+/− mice, we identified VSMCs, proliferating resident neointima cells and Erk activation after arterial injury utilizing immunohistochemistry.
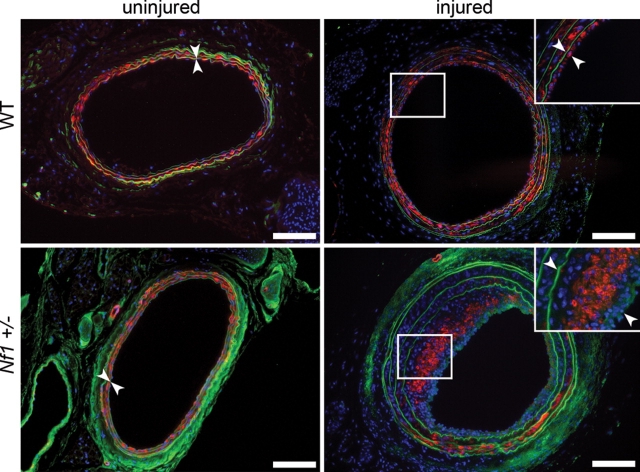
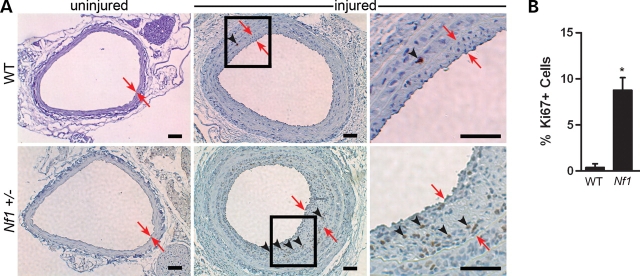
In response to arterial injury, the evolving neointima in both WT and Nf1+/− mice was composed primarily of VSMCs, as detected by immunohistochemical staining of cells with an anti-alpha smooth muscle actin (α-SMA) antibody in arterial cross-sections (Fig. 2). For both WT and Nf1+/− mice, VSMCs accounted for at least 75% of the cells in the neointima, which is consistent with previously published reports (30,40,44). This, however, may be an underestimation of the total number of VSMCs in the neointima because vascular injury produces phenotypic changes in VSMCs that down regulate expression of proteins including α-SMA (45). Further, the injured vessels harvested from Nf1+/− mice had increased cellular proliferation in the neointima compared with WT controls (Fig. 3A). Cellular proliferation in the neointima was determined by immunohistochemical staining of arterial cross-sections for the presence of Ki67, a nuclear protein that is expressed in all cells active in cell cycle (46). Specifically, Nf1+/− injured vessels had an ∼20-fold increase in the percent of proliferating cells in the neointima, which expressed the Ki67 antigen, compared with WT controls 21 days post injury (Fig. 3B). Co-staining of arterial cross-sections with Ki67 and α-SMA indicated that the cells which express Ki67 also co-expressed α-SMA (data not shown). Therefore, the increased neointima area in Nf1+/− mice compared with WT mice can be attributed to increased proliferation of VSMCs in response to mechanical injury.
Figure 2.
α-SMA analysis of injured carotid arteries from WT and Nf1+/− mice. Representative photomicrographs of uninjured (left panels) and injured (right panels) carotid arteries from WT (top panels) and Nf1+/− (bottom panels) mice stained with α-SMA (red). Cell nuclei are counterstained with DAPI (blue) and autofluorescence of murine tissue is visible (green). White boxes indicate area magnified in inset. White arrowheads indicate neointima boundary. Scale bars represent 100 µm. Results are representative of five independent experiments.
Figure 3.
Analysis of cellular proliferation within the neointima of injured carotid arteries from WT and Nf1+/− mice. (A) Representative photomicrographs of uninjured (left panels) and injured (right panels) carotid arteries from WT (top panels) and Nf1+/− (bottom panels) mice stained with anti-Ki67 (brown) antibody and hematoxylin (blue). Black boxes in middle panels indicate areas that are magnified in the far right panels. Red arrows indicate neointima boundary. Black arrowheads represent positive Ki67 staining of proliferating cells. Results are representative of five independent experiments. Scale bars represent 50 µm. (B) Quantification of percent Ki67 positive cells within the neointima of injured carotid arteries of WT and Nf1+/− mice. Data represent mean percentage of Ki67 positive cells in the neointima±SEM, n = 3, *P < 0.005 by Student's unpaired t-test with Welch correction.
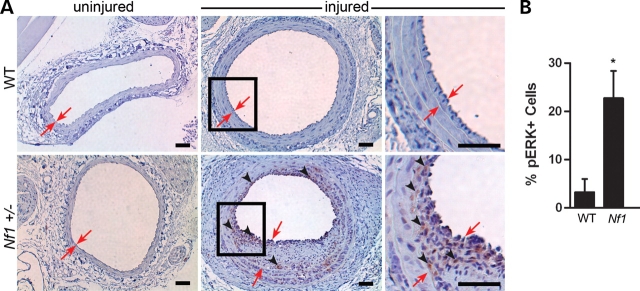
Activation of the Ras-Erk pathway in vivo in response to arterial injury was determined by staining injured arterial cross-sections with an antibody directed against phosphorylated-Erk. Consistent with increased numbers of VSMCs and cellular proliferation in the neointima, Nf1+/− mice had a 7-fold increase in Erk phosphorylation compared with WT controls 21 days after arterial injury (Fig. 4). Therefore, Nf1+/− mice have excessive cell proliferation, VSMC accumulation and Erk activation in the evolving neointima compared with WT controls after mechanical arterial injury.
Figure 4.
Analysis of Erk phosphorylation within the neointima of injured carotid arteries of WT and Nf1+/− mice. (A) Representative photomicrographs of uninjured (left panels) and injured (right panels) carotid arteries from WT (top panels) and Nf1+/− (bottom panels) mice stained with anti-phosphorylated-Erk (brown) antibody and counterstained with hematoxylin (blue). Black boxes in middle panels indicate areas that are magnified in the far right panels. Red arrows indicate neointima boundary. Black arrowheads represent positive phosphorylated-Erk staining. Results are representative of five independent experiments. Scale bars represent 50 µm. (B) Quantification of percent phosphorylated-Erk positive cells within the neointima of injured carotid arteries of WT and Nf1+/− mice. Data represent mean percentage of phosphorylated-Erk positive cells in the neointima±SEM, n = 3, *P < 0.04 by Student's unpaired t-test.
Administration of Gleevec inhibits neointima formation in Nf1+/− mice after mechanical arterial injury
Gleevec is a potent inhibitor of the PDGF-BB signaling axis in VSMCs and prevents neointima formation and aneurysms in vivo in mice genetically predisposed to diverse vasculopathies (32). Given our prior experimental observations implicating hyperactivation of the PDGF-BB-Ras-Erk signaling pathway in the neointima formation in Nf1+/− mice, we tested whether pre-administration of Gleevec would inhibit neointima formation in Nf1+/− mice after arterial mechanical injury. We utilized a Gleevec treatment protocol which had previously been shown to prevent neointima formation in other murine models of vascular disease (47). Specifically, either PBS or 50 mg/kg Gleevec was administered daily to WT and Nf1+/− mice intraperitoneally beginning 3 days prior to arterial injury and continued for 7 days post injury. Mice were then sacrificed 21 days postoperatively for analysis.
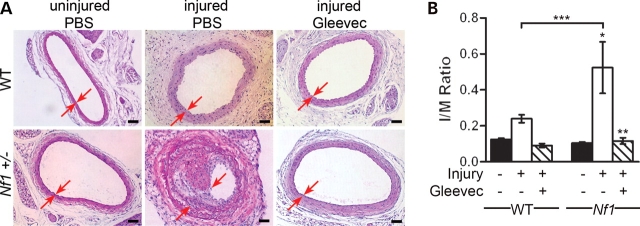
In response to carotid injury, Gleevec treatment greatly reduced neointima formation in Nf1+/− mice compared with PBS treatment (Fig. 5A). Specifically, Gleevec-treated Nf1+/− mice had a 4-fold reduction in I/M ratio compared with PBS-treated Nf1+/− mice (Fig. 5B) in response to carotid injury. No significant difference was seen in response to injury in the I/M ratios of WT mice when comparing PBS with Gleevec treatment. This is consistent with the fact that C57BL/6 WT mice are resistant to neointima formation in response to mechanical injury (38,40). Further, immunohistochemistry indicates that Gleevec treatment reduced cellular proliferation as well as Erk phosphorylation in Nf1+/− mice in response to injury compared with PBS treatment (Fig. 6). Thus, this data indicates that enhanced neointima formation in Nf1+/− mice in response to vascular injury is mediated via a Gleevec sensitive pathway.
Figure 5.
Histological and morphometric analysis of injured carotid arteries from Gleevec-treated WT and Nf1+/− mice. (A) Representative photomicrographs of H&E-stained carotid arteries from WT (top panels) and Nf1+/− (bottom panels) mice 21 days following no injury and PBS treatment (left panels), injury and PBS treatment (middle panels) or injury and Gleevec treatment (right panels). Red arrows indicate boundary of neointima. Scale bars represent 50 µm. Results are representative of five independent experiments. (B) I/M ratio of injured carotid artery cross-sections from PBS and Gleevec-treated WT and Nf1+/− mice. Data represent mean I/M ratio of five cross-sections±SEM, n = 4–6 mice. For Nf1+/− uninjured versus injured with PBS treatment, *P < 0.001; for Nf1+/− injured with PBS versus injured with Gleevec treatment, **P < 0.001; and for WT injured with PBS treatment versus Nf1+/− injured with PBS treatment, ***P < 0.05 by one-way ANOVA.
Figure 6.
Analysis of α-SMA, Ki67 and Erk phosphorylation within the neointima of injured carotid arteries from Gleevec-treated Nf1+/− mice. Representative photomicrographs of α-SMA staining (left panels) of carotid artery cross-sections from injured Nf1+/− PBS-treated (top panels) and Nf1+/− Gleevec-treated (bottom panels) mice. α-SMA staining is seen in red, DAPI nuclear dye is blue and murine tissue autofluorescence is green. White arrows indicate neointima boundary. White boxes indicate area magnified in inset. Representative images of Ki67 (middle panels) and phoshophorylated-Erk (right panels) staining of carotid artery cross-sections counter-stained with hematoxylin (blue). Red arrows indicate neointima boundary. Black arrowheads represent positive Ki67 or phosphorylated-Erk staining (brown). Black boxes indicate areas magnified in insets. Results are representative of five independent experiments. Scale bars represent 50 µm.
DISCUSSION
NF1 is an autosomal dominant genetic disorder with a wide range of both malignant and non-malignant clinical manifestations. In contrast to some NF1 cancers where loss of heterozygosity at the NF1 allele is a prerequisite for tumor development, many of the non-malignant complications of NF1, including learning disorders, skeletal abnormalities and vascular disease occur secondary to aberrant NF1+/− cellular functions within a specific tissue microenvironment (15,16,22,48,49). Thus, study of the effects of NF1 haploinsufficiency in different cell lineages is imperative for understanding the diverse morbidities associated with NF1. Vasculopathy associated with NF1 is an under-recognized complication of the disease and contributes to significant premature morbidity and mortality in patients. An important tool for understanding the pathogenesis of NF1 vasculopathy is the development of in vivo animal models, which accurately recapitulate some, if not all, aspects of the clinical disease. In this study, we show that Nf1+/− mice have increased neointima formation in response to mechanical arterial injury compared with WT mice with vessel morphology resembling NF1 vascular lesions. Consistent with our previous in vitro data, we show that Nf1+/− mice have increased cellular proliferation and Erk phosphorylation in response to vascular injury compared with WT mice. Further, we show that the increased neointima formation is mediated via a Gleevec sensitive molecular signaling pathway.
NF1 vasculopathy was first described in 1945 (22). Since the original report, multiple clinical studies demonstrate that different locations within the entire arterial tree may be affected in NF1 patients (18,21). In 2001, an analysis of 3253 death certificates of persons with NF1 indicated that the median age of death for NF1 patients was 15 years less than that of the general population (19). In this report, a diagnosis suggestive of NF1 vasculopathy was listed 7.2 times more often than expected among NF1 patients <30 years old at the time of death and 2.2 times more often than expected among patients 30–40 years old at the time of death (19). Another study demonstrated that 2.5% of children with NF1, who had undergone brain MRI, were found to have cerebrovascular system abnormalities including narrowed vessels, moya-moya, vascular stenosis and aneurysm (20). Although rare, sudden death is also linked to NF1 vascular disease secondary to vessel occlusion (23). Despite numerous clinical observations of NF1 vasculopathy, the molecular mechanisms which control the development and progression of vascular disease in NF1 patients are incompletely understood.
An emerging paradigm in vascular biology is that neointima formation in response to injury is tightly controlled by the Ras-Mek-Erk signaling axis in resident cells within the vascular wall including endothelial cells (ECs) and VSMCs (26–29,35,43). Specifically, prior animal studies indicate that increased Ras activation augments VSMC proliferation and migration, and the subsequent development of vascular lesions (26,31,32,34). These vascular lesions are characterized by intimal wall hyperplasia and neointima formation, which ultimately leads to occlusive vascular disease. In support of the importance of Ras in occlusive vascular disease, adenoviral-mediated transfer of a dominant negative H-Ras into VSMCs prevents the development of stenotic lesions in rats after mechanical arterial injury by inhibiting the proliferation and migration of VSMCs (26). Similar results were obtained when animals were treated with a chemical Ras farnesyltransferase inhibitor prior to arterial mechanical injury (27).
The importance of Ras activation in neointima formation was further highlighted in studies utilizing Grb2+/− mice (43). Grb2 is a signaling protein that facilitates Ras activation by receptor tyrosine kinases including the PDGF-BB receptor (PDGF-βR) (31). PDGF-BB is released locally by platelets and other cells in arteries after injury (33). Similar to prior observations utilizing Ras inhibitors, Grb2+/− mice were resistant to neointima development following vessel injury (43). Further, VSMCs harvested from Grb2+/− mice demonstrated decreased proliferation and Erk activation in response to PDGF-BB in vitro (43).
Finally, when genetically engineered mice harboring constitutively active PDGF-βR only in VSMCs were intercrossed with low-density lipoprotein receptor knockout mice, which have a predisposition to developing atherosclerosis, the mutant progeny developed aneurysms and a marked susceptibility to atherosclerosis (32). The mutant mice also showed hyperproliferation of VSMCs and increased Erk activation in vivo (32). Consistent with the central pathogenic role of hyperactivation of the PDGF-βR and Erk signaling pathway in controlling VSMC proliferation in vivo, mutant mice treated with Gleevec, an inhibitor of PDGF-βR signaling, did not develop atherosclerosis or aneurysms (32). Collectively, these studies provide a paradigm where hyperactivation of Ras and the PDGF-βR signaling pathway activates a discrete set of biochemical effectors which potentiates VSMC proliferation and migration in vivo.
Recent genetic and biochemical studies from our laboratory demonstrated that neurofibromin functions as a formal Ras GAP in both murine and human VSMCs in vitro (24). Specifically Nf1+/− VSMCs have increased migration and proliferation in response to PDGF-BB stimulation via hyperactivation of the Ras-Erk signaling pathway (24). Consistent with our prior in vitro observations, we now provide data in the present study to demonstrate that haploinsufficiency at Nf1 in vascular wall resident cells leads to hyperactivation of Erk, increased VSMC proliferation, and formation of an enlarged neointima in vivo in response to injury. This observation is consistent with the concept that precise regulation of the Ras-Erk signaling axis is critical for maintenance of vascular wall homeostasis and lumen integrity.
Gleevec is a potent inhibitor of both PDGF-BB-Ras-Erk signaling and c-kit receptor activation in vivo (50) and prevents neointima formation in other mouse models after arterial injury (32,47). Based on these prior studies and the fact that Nf1+/− VSMCs are hypersensitive to PDGF-BB stimulation, we tested whether Gleevec could prevent or delay neointima formation in Nf1+/− mice after vessel injury. In the present study, we show that Gleevec greatly diminishes neointima formation in Nf1+/− mice and reduces proliferation and Erk activation in VSMCs in vivo. While we do detect inhibition of Erk activation and proliferation in VSMCs in the vessel wall with Gleevec treatment in vivo, it is possible that Gleevec also prevents the influx of bone-marrow-derived VSMCs or inflammatory cells to the evolving neointima in Nf1+/− mice to account for the observed treatment effect. In support of this concept, recent murine genetic studies demonstrate that activation of the c-kit receptor in bone-marrow-derived cells mobilizes either VSMCs or cells that express VSMC antigens to enhance the progression of an evolving neointima by either direct cellular integration or paracrine stimulation of resident cells in the vessel wall (47,51). This observation is especially intriguing since previous studies from our group demonstrate that Nf1+/− bone-marrow-derived cells have increased proliferation, migration and survival in response to Kit ligand stimulation both in vitro and in vivo (49,52,53). Utilizing adoptive bone-marrow-transplant techniques, we are currently testing the contribution of Nf1+/− bone-marrow-derived cells to neointima formation in other studies.
While these studies establish that Nf1 haploinsufficiency is sufficient to predispose mice to an enlarged neointima compared with controls in vivo, several questions remain. Blood vessels are composed primarily of a continuous monolayer of ECs surrounded by VSMCs. Dysfunction of either cell type can result in intimal hyperplasia, which can ultimately lead to occlusive vascular disease. Further, animal studies of arterial mechanical injury have now shown that mobilization and transmigration of bone-marrow-derived cells into the intima in response to vascular injury directly contributes to neointima formation (47,51,54–56). Therefore, based on the complex interaction between cells in response to arterial injury and the global control of Ras signaling, it is essential that the predominant cell lineage(s) involved in neointima formation in Nf1+/− mice be dissected in order to determine the optimal treatment and prevention of NF1 vasculopathy.
Towards this goal, the function of neurofibromin has been interrogated in vivo in both ECs and VSMCs by lineage specific ablation of both Nf1 alleles utilizing murine cre/lox technology (30,57). Loss of heterozygosity by cre induced ablation at the Nf1 locus in ECs resulted in embryonic lethality secondary to the development of complex cardiac defects (57). Interestingly, when both Nf1 alleles were ablated in VSMCs utilizing SM22 cre mice, the murine progeny developed normally with no overt vascular phenotype (30). However, genetically engineered mice in which both Nf1 alleles were ablated in VSMCs while all other cell lineages were haploinsufficient at Nf1 had enhanced neointima formation characterized by VSMC accumulation in response to carotid ligation (30). While these studies offer significant insight into the function of neurofibromin in both VSMCs and ECs in vivo, ablation of both Nf1 alleles in either VSMCs or ECs may overestimate the contribution of each individual cell lineage to neointima formation or mask the complex cellular phenotypes, which interact in the vessel wall since differences in signaling strength and duration have previously been shown between Nf1−/− and Nf1+/− cells (10,11). Loss of heterozygosity of NF1 in ECs or VSMCs has not been described in NF1 patients, therefore it remains important to dissect how Nf1+/− resident cells in the vessel wall and other cells potentially recruited to areas of arterial occlusion interact to produce vascular pathology.
In sum, this study provided evidence that heterozygous inactivation of Nf1 results in enhanced Erk phosphorylation, cellular proliferation and subsequent neointima formation in response to arterial injury via a Gleevec sensitive molecular pathway and provides an expandable platform to further understand NF1 vasculopathy. Studies are ongoing to dissect the role of specific cell lineages in the development NF1 vaso-occlusive disease utilizing cre/lox technology to generate mice that are haploinsufficient at Nf1 in ECs, VSMCs or bone-marrow-derived cells.
MATERIAL AND METHODS
Animals
Nf1+/− mice were obtained from Tyler Jacks at the Massachusetts Institute of Technology (Cambridge, MA) in a C57BL/6.129 background and backcrossed for 13 generations into the C57BL/6J strain. The Nf1 allele was genotyped by polymerase chain reaction (PCR) as previously described (53,58). All protocols for this study were approved by the Indiana University Laboratory Animal Research Center.
Carotid artery injury
The carotid arteries of 12–15-week-old C57BL/6 WT and Nf1+/− male mice were mechanically injured by use of a beaded guidewire as previously described with minor modifications (31,37). In brief, animals were anesthetized by inhalation of isoflurane (2%)-oxygen (98%) mixture. Under a dissecting microscope (Leica, Bannockburn, IL), the entire left carotid artery was exposed via an anterior incision of the neck. Microvascular clamps were used to temporarily occlude the common and internal carotid artery and a 6/O silk ligature was used to tie off the distal external carotid artery. An epoxy resin-beaded probe (0.45–0.6 mm diameter beads) was introduced through a transverse arteriotomy in the external carotid artery. The probe was inserted and withdrawn into the common carotid artery three times with rotation to denude the endothelium and stretch the artery ∼2-fold. The external carotid artery was immediately ligated proximal to the arteriotomy site. Microvascular clamps were removed and normal blood flow through the common carotid and internal carotid artery was re-established. The skin was closed with running 6/O suture.
Gleevec administration
When stated, Gleevec (50 mg/kg/day; Novartis International AG, Basel, Switzerland) was administrated once per day by intraperitoneal (I.P.) injection. Gleevec treatment began 3 days prior to carotid injury and continued through day 7 post injury. Mice recovered to 21 days post injury. Phosphate buffered saline (PBS; Invitrogen, Grand Island, NY) was given in an equivalent volume as described for Gleevec as a control.
Histopathology and immunohistochemistry
Twenty-one days after carotid injury, mice were anesthetized with 1.25% Avertin (Sigma-Aldrich, St Louis, MO) and were perfusion fixed in situ with PBS for 5 min followed by 4% paraformaldehyde in PBS (pH 7.3) for 10 min at a constant pressure of 100 mmHg. The injured left and control uninjured right common carotid arteries were excised under a dissecting microscope, fixed in 4% paraformaldehyde for 8–12 h at 4°C and embedded in paraffin or snap-frozen in OCT (Sakura Finetek USA, Inc. Torrance, CA) in liquid nitrogen. Serial 5 µm cross-sections were made at 100 µm intervals across the length of the artery. H&E staining was conducted according to standard methods (Anatech, Ltd, Battle Creek, MI).
For immunostaining, sections were blocked for endogenous peroxidase activity with 3% hydrogen peroxide in methanol following antigen retrieval in Antigen Unmasking Solution (Vector Laboratories, Berlingame, CA) at 95° C. Sections were blocked in 3% bovine serum albumin (BSA, Sigma) for 1 h and were stained for smooth muscle cells (anti-SMA, Sigma, 1:400), cellular proliferation (anti-Ki67, DAKO Corp, Carpinteria, CA, 1:50) or Erk phosphorylation (anti-phosphorylated-Erk, Cell Signaling, Danvers, MA, 1:100). Purified class- and species-matched immunoglobulins (BD Pharmingen, San Jose, CA) were used for isotype controls. Sections were incubated with appropriate biotinylated secondary antibody (Vector Laboratories) followed by incubation with either 3,3′-Diaminobenidine (DAB, Vector Laboratories) and counterstained with hematoxylin, or were mounted in 90% glycerol/10% PBS, pH 8.0, containing, 6-diamidino-2-phenylindole dihydrochloride (DAPI; Sigma) to permit nuclear identification. Sections were examined and images of sections were collected using a Zeiss Axioskop microscope (Carl Zeiss, Chester, VA) with a 20× or 40× CP-ACHROMAT/0.12NA objective. Images were acquired using a SPOT RT color camera (Diagnostic Instruments, Sterling Heights, MI). Percent positive Ki67 and phosphorylated-Erk cells in the neointima were calculated as: (total number positive cells in neoinitma/total cell number in neointima)7×100.
Morphometric analysis
For morphometric analyses, images of H&E stained cross-sections of injured and control arteries were analyzed using Metamorph 6.1 (Universal Imaging System Corp, Westerchester, PA). Lumen area, area inside IEL and area inside external elastic lamina were measured. Intima area and media area were calculated as previously described (38). 5–10 sections along the length of each artery were measured by a person blinded to animal genotypes and the (I/M) ratio was calculated for each artery. Percent lumen stenosis was calculated as: (intima area/IEL area)×100.
Statistical analyses
All values are presented as mean±S.E.M. Intima area and I/M ratio analysis was assessed by One-way ANOVA with a Tukey post test was performed using GraphPad InStat version 3.00 (GraphPad Software, San Diego, CA, USA). Percent lumen stenosis, percent Ki67 positive cells, and percent phosphorylated Erk positive cells analyses were assessed by Students unpaired t test. P-values <0.05 were considered significant.
FUNDING
NIH P50 NS052606 (D.A.I.), Department of Defense W81XWH-08-1-0129 (D.A.I.), Riley Children's Foundation (D.A.I., D.W.C., S.J.C.), P30 CA82709 (D.A.I.), AHA -8156237 (E.A.L., D.A.I.).
ACKNOWLEDGEMENTS
We thank Janice Walls for her expert administrative assistance in the preparation of the manuscript.
Conflict of Interest statement. None declared.
REFERENCES
- 1.Riccardi V.M. Neurofibromatosis: past, present, and future. N. Engl. J. Med. 1991;324:1283–1285. doi: 10.1056/NEJM199105023241812. [DOI] [PubMed] [Google Scholar]
- 2.Friedman J.M., Gutmann D.H., MacCollin M., Riccardi V.M. Neurofibromatosis: Phenotype, Natural History, and Pathogenesis. 3rd edn. Baltimore, MD: The Johns Hopkins University Press; 1999. [Google Scholar]
- 3.Brodeur G.M. The NF1 gene in myelopoiesis and childhood myelodysplastic syndromes. N. Engl. J. Med. 1994;330:637–638. doi: 10.1056/NEJM199403033300912. [DOI] [PubMed] [Google Scholar]
- 4.Boguski M.S., McCormick F. Proteins regulating Ras and its relatives. Nature. 1993;366:643–654. doi: 10.1038/366643a0. [DOI] [PubMed] [Google Scholar]
- 5.Clark C.J., Drugan J.K., Terrell R.S., Bradham C., Der C.J., Bell R.M., Campbell S. Peptides containing a consensus RAS binding sequence from Raf-1 and GTPase activating protein NF1 Ras function. Proc. Natl Acad. Sci. USA. 1996;93:1577–1581. doi: 10.1073/pnas.93.4.1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.DeClue J.E., Papageorge A.G., Fletcher J.A., Diehl S.R., Ratner N., Vaas W.C., Lowry D.R. Abnormal regulation of mammalian p21 ras contributes to malignant tumor growth in von Recklinghausen (Type 1) neurofibromatosis. Cell. 1992;69:265–273. doi: 10.1016/0092-8674(92)90407-4. [DOI] [PubMed] [Google Scholar]
- 7.Hall A. Signal transduction through small GTPases—a tale of two GAPs. Cell. 1992;69:389–391. doi: 10.1016/0092-8674(92)90441-e. [DOI] [PubMed] [Google Scholar]
- 8.Xu G., O'Connell P., Viskochil D., Cawthon R., Robertson M., Culver M., Dunn D., Stevens J., Gesteland R., White R., et al. The Neurofibromatosis type 1 gene encodes a protein related to GAP. Cell. 1990;62:599–608. doi: 10.1016/0092-8674(90)90024-9. [DOI] [PubMed] [Google Scholar]
- 9.Le D.T., Kong N., Zhu Y., Lauchle J.O., Aiyigari A., Braun B.S., Wang E., Kogan S.C., Le Beau M.M., Parada L., et al. Somatic inactivation of Nf1 in hematopoietic cells results in a progressive myeloproliferative disorder. Blood. 2004;103:4243–4250. doi: 10.1182/blood-2003-08-2650. [DOI] [PubMed] [Google Scholar]
- 10.Cichowski K., Santiago S., Jardim M., Johnson B.W., Jacks T. Dynamic regulation of the Ras pathway via proteolysis of the NF1 tumor suppressor. Genes Dev. 2003;17:449–454. doi: 10.1101/gad.1054703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hiatt K., Ingram D.A., Zhang Y., Bollag G., Clapp D.W. Neurofibromin GTPase-activating protein-related domains restore normal growth in Nf1 −/− cells. J. Biol. Chem. 2001;276:7240–7245. doi: 10.1074/jbc.M009202200. [DOI] [PubMed] [Google Scholar]
- 12.Le L.Q., Parada L.F. Tumor microenvironment and neurofibromatosis type I: connecting the GAPs. Oncogene. 2007;26:4609–4616. doi: 10.1038/sj.onc.1210261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gutmann D.H., Loehr A., Zhang Y., Kim J., Henkemeyer M., Cashen A. Haploinsufficiency for the neurofibromatosis 1 (NF1) tumor suppressor results in increased astrocyte proliferation. Oncogene. 1999;18:4450–4459. doi: 10.1038/sj.onc.1202829. [DOI] [PubMed] [Google Scholar]
- 14.Zhu Y., Ghosh P., Charnay P., Burns D.K., Parada L.F. Neurofibromas in NF1: Schwann cell origin and role of tumor environment. Science. 2002;296:920–922. doi: 10.1126/science.1068452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Silva A.J., Frankland P.W., Marowitz Z., Friedman E., Lazlo G., Cioffi D., Jacks T., Bourtchuladze R. A mouse model for the learning and memory deficits associated with neurofibromatosis type I. Nat. Genet. 1997;15:281–284. doi: 10.1038/ng0397-281. [DOI] [PubMed] [Google Scholar]
- 16.Yang F.C., Chen S., Robling A.G., Yu X., Nebesio T.D., Yan J., Morgan T., Li X., Yuan J., Hock J., et al. Hyperactivation of p21ras and PI3K cooperate to alter murine and human neurofibromatosis type 1-haploinsufficient osteoclast functions. J. Clin. Invest. 2006;116:2880–2891. doi: 10.1172/JCI29092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Friedman J.M., Arbiser J., Epstein J.A., Gutmann D.H., Huot S.J., Lin A.E., McManus B., Korf B.R. Cardiovascular disease in neurofibromatosis 1: report of the NF1 Cardiovascular Task Force. Genet. Med. 2002;4:105–111. doi: 10.1097/00125817-200205000-00002. [DOI] [PubMed] [Google Scholar]
- 18.Lehrnbecher T., Gassel A.M., Rauh V., Kirchner T., Huppertz H.I. Neurofibromatosis presenting as a severe systemic vasculopathy. Eur. J. Pediatr. 1994;153:107–109. doi: 10.1007/BF01959219. [DOI] [PubMed] [Google Scholar]
- 19.Rasmussen S.A., Yang Q., Friedman J.M. Mortality in neurofibromatosis 1: an analysis using U.S. death certificates. Am. J. Hum. Genet. 2001;68:1110–1118. doi: 10.1086/320121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rosser T.L., Vezina G., Packer R.J. Cerebrovascular abnormalities in a population of children with neurofibromatosis type 1. Neurology. 2005;64:553–555. doi: 10.1212/01.WNL.0000150544.00016.69. [DOI] [PubMed] [Google Scholar]
- 21.Greene J.F., Jr, Fitzwater J.E., Burgess J. Arterial lesions associated with neurofibromatosis. Am. J. Clin. Pathol. 1974;62:481–487. doi: 10.1093/ajcp/62.4.481. [DOI] [PubMed] [Google Scholar]
- 22.Hamilton S.J., Friedman J.M. Insights into the pathogenesis of neurofibromatosis 1 vasculopathy. Clin. Genet. 2000;58:341–344. doi: 10.1034/j.1399-0004.2000.580501.x. [DOI] [PubMed] [Google Scholar]
- 23.Kanter R.J., Graham M., Fairbrother D., Smith S.V. Sudden cardiac death in young children with neurofibromatosis type 1. J. Pediatr. 2006;149:718–720. doi: 10.1016/j.jpeds.2006.07.046. [DOI] [PubMed] [Google Scholar]
- 24.Li F., Munchhof A.M., White H.A., Mead L.E., Krier T.R., Fenoglio A., Chen S., Wu X., Cai S., Yang F.C., et al. Neurofibromin is a novel regulator of RAS-induced signals in primary vascular smooth muscle cells. Hum. Mol. Genet. 2006;15:1921–1930. doi: 10.1093/hmg/ddl114. [DOI] [PubMed] [Google Scholar]
- 25.Raines E.W. PDGF and cardiovascular disease. Cytokine Growth Factor Rev. 2004;15:237–254. doi: 10.1016/j.cytogfr.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 26.Jin G., Chieh-Hsi Wu J., Li Y.S., Hu Y.L., Shyy J.Y., Chien S. Effects of active and negative mutants of Ras on rat arterial neointima formation. J. Surg. Res. 2000;94:124–132. doi: 10.1006/jsre.2000.6014. [DOI] [PubMed] [Google Scholar]
- 27.Kouchi H., Nakamura K., Fushimi K., Sakaguchi M., Miyazaki M., Ohe T., Namba M. Manumycin A, inhibitor of ras farnesyltransferase, inhibits proliferation and migration of rat vascular smooth muscle cells. Biochem. Biophys. Res. Commun. 1999;264:915–920. doi: 10.1006/bbrc.1999.1546. [DOI] [PubMed] [Google Scholar]
- 28.Ueno H., Yamamoto H., Ito S., Li J.J., Takeshita A. Adenovirus-mediated transfer of a dominant-negative H-ras suppresses neointimal formation in balloon-injured arteries in vivo. Arterioscler. Thromb. Vasc. Biol. 1997;17:898–904. doi: 10.1161/01.atv.17.5.898. [DOI] [PubMed] [Google Scholar]
- 29.Wu C.H., Lin C.S., Hung J.S., Wu C.J., Lo P.H., Jin G., Shyy Y.J., Mao S.J., Chien S. Inhibition of neointimal formation in porcine coronary artery by a Ras mutant. J. Surg. Res. 2001;99:100–106. doi: 10.1006/jsre.2001.6159. [DOI] [PubMed] [Google Scholar]
- 30.Xu J., Ismat F.A., Wang T., Yang J., Epstein J.A. NF1 regulates a Ras-dependent vascular smooth muscle proliferative injury response. Circulation. 2007;116:2148–2156. doi: 10.1161/CIRCULATIONAHA.107.707752. [DOI] [PubMed] [Google Scholar]
- 31.Zhang S., Ren J., Khan M.F., Cheng A.M., Abendschein D., Muslin A.J. Grb2 is required for the development of neointima in response to vascular injury. Arterioscler. Thromb. Vasc. Biol. 2003;23:1788–1793. doi: 10.1161/01.ATV.0000085015.49110.85. [DOI] [PubMed] [Google Scholar]
- 32.Boucher P., Gotthardt M., Li W.P., Anderson R.G., Herz J. LRP: role in vascular wall integrity and protection from atherosclerosis. Science. 2003;300:329–332. doi: 10.1126/science.1082095. [DOI] [PubMed] [Google Scholar]
- 33.Bowen-Pope D.F., Ross R., Seifert R.A. Locally acting growth factors for vascular smooth muscle cells: endogenous synthesis and release from platelets. Circulation. 1985;72:735–740. doi: 10.1161/01.cir.72.4.735. [DOI] [PubMed] [Google Scholar]
- 34.Chen K.H., Guo X., Ma D., Guo Y., Li Q., Yang D., Li P., Qiu X., Wen S., Xiao R.P., et al. Dysregulation of HSG triggers vascular proliferative disorders. Nat. Cell Biol. 2004;6:872–883. doi: 10.1038/ncb1161. [DOI] [PubMed] [Google Scholar]
- 35.Chien K.R., Hoshijima M. Unravelling Ras signals in cardiovascular disease. Nat. Cell Biol. 2004;6:807–808. doi: 10.1038/ncb0904-807. [DOI] [PubMed] [Google Scholar]
- 36.Indolfi C., Chiariello M., Avvedimento E.V. Selective gene therapy for proliferative disorders: sense and antisense. Nat. Med. 1996;2:634–635. doi: 10.1038/nm0696-634. [DOI] [PubMed] [Google Scholar]
- 37.Werner N., Junk S., Laufs U., Link A., Walenta K., Bohm M., Nickenig G. Intravenous transfusion of endothelial progenitor cells reduces neointima formation after vascular injury. Circ. Res. 2003;93:e17–e24. doi: 10.1161/01.RES.0000083812.30141.74. [DOI] [PubMed] [Google Scholar]
- 38.Kuhel D.G., Zhu B., Witte D.P., Hui D.Y. Distinction in genetic determinants for injury-induced neointimal hyperplasia and diet-induced atherosclerosis in inbred mice. Arterioscler. Thromb. Vasc. Biol. 2002;22:955–960. doi: 10.1161/01.atv.0000017994.77066.75. [DOI] [PubMed] [Google Scholar]
- 39.Burke G.L., Evans G.W., Riley W.A., Sharrett A.R., Howard G., Barnes R.W., Rosamond W., Crow R.S., Rautaharju P.M., Heiss G. Arterial wall thickness is associated with prevalent cardiovascular disease in middle-aged adults. The Atherosclerosis Risk in Communities (ARIC) Study. Stroke. 1995;26:386–391. doi: 10.1161/01.str.26.3.386. [DOI] [PubMed] [Google Scholar]
- 40.Zhu B., Reardon C.A., Getz G.S., Hui D.Y. Both apolipoprotein E and immune deficiency exacerbate neointimal hyperplasia after vascular injury in mice. Arterioscler. Thromb. Vasc. Biol. 2002;22:450–455. doi: 10.1161/hq0302.105377. [DOI] [PubMed] [Google Scholar]
- 41.Huang J., Kontos C.D. Inhibition of vascular smooth muscle cell proliferation, migration, and survival by the tumor suppressor protein PTEN. Arterioscler. Thromb. Vasc. Biol. 2002;22:745–751. doi: 10.1161/01.atv.0000016358.05294.8d. [DOI] [PubMed] [Google Scholar]
- 42.Mourani P.M., Garl P.J., Wenzlau J.M., Carpenter T.C., Stenmark K.R., Weiser-Evans M.C. Unique, highly proliferative growth phenotype expressed by embryonic and neointimal smooth muscle cells is driven by constitutive Akt, mTOR, and p70S6K signaling and is actively repressed by PTEN. Circulation. 2004;109:1299–1306. doi: 10.1161/01.CIR.0000118462.22970.BE. [DOI] [PubMed] [Google Scholar]
- 43.Zhan Y., Kim S., Izumi Y., Izumiya Y., Nakao T., Miyazaki H., Iwao H. Role of JNK, p38, and ERK in platelet-derived growth factor-induced vascular proliferation, migration, and gene expression. Arterioscler. Thromb. Vasc. Biol. 2003;23:795–801. doi: 10.1161/01.ATV.0000066132.32063.F2. [DOI] [PubMed] [Google Scholar]
- 44.Zhu W.H., MacIntyre A., Nicosia R.F. Regulation of angiogenesis by vascular endothelial growth factor and angiopoietin-1 in the rat aorta model: distinct temporal patterns of intracellular signaling correlate with induction of angiogenic sprouting. Am. J. Pathol. 2002;161:823–830. doi: 10.1016/S0002-9440(10)64242-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Regan C.P., Adam P.J., Madsen C.S., Owens G.K. Molecular mechanisms of decreased smooth muscle differentiation marker expression after vascular injury. J. Clin. Invest. 2000;106:1139–1147. doi: 10.1172/JCI10522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Scholzen T., Gerdes J. The Ki-67 protein: from the known and the unknown. J. Cell. Physiol. 2000;182:311–322. doi: 10.1002/(SICI)1097-4652(200003)182:3<311::AID-JCP1>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 47.Wang C.H., Anderson N., Li S.H., Szmitko P.E., Cherng W.J., Fedak P.W., Fazel S., Li R.K., Yau T.M., Weisel R.D., et al. Stem cell factor deficiency is vasculoprotective: unraveling a new therapeutic potential of imatinib mesylate. Circ. Res. 2006;99:617–625. doi: 10.1161/01.RES.0000243210.79654.fd. [DOI] [PubMed] [Google Scholar]
- 48.Cichowski K., Jacks T. NF1 tumor suppressor gene function: narrowing the GAP. Cell. 2001;104:593–604. doi: 10.1016/s0092-8674(01)00245-8. [DOI] [PubMed] [Google Scholar]
- 49.Ingram D.A., Yang F.C., Travers J.B., Wenning M.J., Hiatt K., New S., Hood A., Shannon K., Williams D.A., Clapp D.W. Genetic and biochemical evidence that haploinsufficiency of the Nf1 tumor suppressor gene modulates melanocyte and mast cell fates in vivo. J. Exp. Med. 2000;191:181–188. doi: 10.1084/jem.191.1.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Buchdunger E., Cioffi C.L., Law N., Stover D., Ohno-Jones S., Druker B.J., Lydon N.B. Abl protein-tyrosine kinase inhibitor STI571 inhibits in vitro signal transduction mediated by c-kit and platelet-derived growth factor receptors. J. Pharmacol. Exp. Ther. 2000;295:139–145. [PubMed] [Google Scholar]
- 51.Zernecke A., Schober A., Bot I., von Hundelshausen P., Liehn E.A., Mopps B., Mericskay M., Gierschik P., Biessen E.A., Weber C. SDF-1alpha/CXCR4 axis is instrumental in neointimal hyperplasia and recruitment of smooth muscle progenitor cells. Circ. Res. 2005;96:784–791. doi: 10.1161/01.RES.0000162100.52009.38. [DOI] [PubMed] [Google Scholar]
- 52.Ingram D.A., Hiatt K., King A.J., Fisher L., Shivakumar R., Derstine C., Wenning M.J., Diaz B., Travers J.B., Hood A., et al. Hyperactivation of p21ras and the hematopoietic-specific Rho GTPase, Rac2, cooperate to alter the proliferation of neurofibromin deficient mast cells in vivo and in vitro. J. Exp. Med. 2001;194:57–70. doi: 10.1084/jem.194.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang Y.Y., Vik T.A., Ryder J.W., Srour E.F., Jacks T., Shannon K., Clapp D.W. Nf1 regulates hematopoietic progenitor cell growth and ras signaling in response to multiple cytokines. J. Exp. Med. 1998;187:1893–1902. doi: 10.1084/jem.187.11.1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Roque M., Fallon J.T., Badimon J.J., Zhang W.X., Taubman M.B., Reis E.D. Mouse model of femoral artery denudation injury associated with the rapid accumulation of adhesion molecules on the luminal surface and recruitment of neutrophils. Arterioscler. Thromb. Vasc. Biol. 2000;20:335–342. doi: 10.1161/01.atv.20.2.335. [DOI] [PubMed] [Google Scholar]
- 55.Simon D.I., Dhen Z., Seifert P., Edelman E.R., Ballantyne C.M., Rogers C. Decreased neointimal formation in Mac-1(−/−) mice reveals a role for inflammation in vascular repair after angioplasty. J. Clin. Invest. 2000;105:293–300. doi: 10.1172/JCI7811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tanaka H., Sukhova G.K., Swanson S.J., Clinton S.K., Ganz P., Cybulsky M.I., Libby P. Sustained activation of vascular cells and leukocytes in the rabbit aorta after balloon injury. Circulation. 1993;88:1788–1803. doi: 10.1161/01.cir.88.4.1788. [DOI] [PubMed] [Google Scholar]
- 57.Gitler A.D., Zhu Y., Ismat F.A., Lu M.M., Yamauchi Y., Parada L.F., Epstein J.A. Nf1 has an essential role in endothelial cells. Nat. Genet. 2003;33:75–79. doi: 10.1038/ng1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bollag G., Clapp D.W., Shih S., Adler F., Zhang Y.Y., Thompson P., Lange B.J., Freedman M.H., McCormick F., Jacks T., et al. Loss of NF1 results in activation of the Ras signaling pathway and leads to aberrant growth in haematopoietic cells. Nat. Genet. 1996;12:144–148. doi: 10.1038/ng0296-144. [DOI] [PubMed] [Google Scholar]