Abstract
BACKGROUND
Previously, we found that genistein at low concentrations stimulates the growth of human uterine leiomyoma (LM) cells, but not uterine smooth muscle (myometrial) cells (SMC). The aim of this study was to understand the molecular mechanism whereby genistein causes hyperproliferation of LM cells.
METHODS
The effects of genistein at 1 µg/ml on LM cells and SMC were evaluated using estrogen response element gene reporter, real-time RT–PCR, western blot, immunoprecipitation and cell proliferation assays.
RESULTS
Elevated estrogen receptor (ER) transactivation, increased mRNA expression of early estrogen-responsive genes, progesterone receptor and insulin-like growth factor-I (IGF-I), and decreased protein levels of ER-alpha (ERα) were found in genistein-treated LM cells, but not SMC. Additionally, extracellular regulated kinase (ERK), Src homology/collagen (Shc) and ERα were transiently activated, and interactions between ERα and IGF-I receptor (IGF-IR) were rapidly induced by genistein in LM cells. Using ER antagonist ICI 182,780 and MAPK/ERK kinase (MEK) inhibitor PD98059, we found that these early events were inhibited and the proliferative effect of genistein on LM cells was abrogated.
CONCLUSIONS
ERα is involved in the transient activation of ERK/mitogen activated protein kinase (MAPK) by genistein via its early association with IGF-IR, leading to hyper-responsiveness of LM cells and confirming that ER signaling is enhanced by activation of ERK/MAPK in LM cells.
Keywords: Genistein, LM cells, SMC, ERα, IGF-IR
Introduction
Uterine leiomyomas (LMs) (fibroids or myomas) are the most common tumors originating in the smooth muscle wall of the uterus and the major cause of hysterectomy in women of reproductive age (Newbold et al., 2000; Maruo et al., 2004; Walker and Stewart, 2005; Bukulmez and Doody, 2006; Marsh and Bulun, 2006; Payson et al., 2006). Although it has been established that uterine LMs are steroid-dependent and that sensitivity/responsiveness is enhanced in both rodent and human tumors and tumor-derived cell lines in response to estrogen or growth factors (van der Ven et al., 1994; Strawn et al., 1995; Walker, 2002; Maruo et al., 2004; Marsh and Bulun, 2006), the potential role of environmental estrogens in the etiology of LMs has only recently been explored in experimental animal models (Newbold, 1995; Hunter et al., 1999; Hodges et al., 2000; Newbold et al., 2002).
Studies have shown that rodents treated neonatally with environmental estrogens, such as natural plant compounds (phytoestrogens) and industrial byproducts (industrial estrogens), have an increased incidence of uterine LMs and uterine adenocarcinoma later in life (Hodges et al., 2001; Newbold, 2004; Newbold et al., 2001a,b, 2002). Direct evidence for a role of environmental estrogens in the pathogenesis of LMs is limited. However, enhanced sensitivity of uterine LMs to environmental estrogens is suggested to be modulated via the estrogen receptor (ER) in both in vivo and in vitro rodent studies (Hunter et al., 2000; Newbold et al., 2002).
The phytoestrogen genistein, an estrogenic soy-derived compound belonging to the isoflavone class, is commonly consumed in the diet. Since it was reported that plasma concentrations of genistein can reach 4 µM (≈1 µg/ml) in Japanese consuming a soy-rich diet (Adlercreutz et al., 1993), there is much concern as to whether this compound has beneficial and/or adverse physiologic effects. In vivo studies have shown that developmental exposure to genistein at environmentally relevant concentrations (1 or 10 µg/pup/day) alters murine reproductive differentiation, resulting in abnormal ovarian development and uterine neoplasia later in life (Newbold et al., 2001a,b; Jefferson et al., 2006). At cellular and molecular levels, genistein at concentrations ∼1 µM (∼0.27 µg/ml) stimulates the growth of human breast cancer cells (Wang et al., 1996; Hsieh et al., 1998; Chen and Wong, 2004). Similar effects of genistein (≤1 µg/ml) have also been observed in human uterine LM cells in our laboratory; whereas this proliferative effect was not observed in normal human uterine smooth muscle cells (SMC) (Moore et al., 2007). It has been reported that LM primary cultures have elevated transcriptional activity in response to 17β-estradiol (E2) compared with autologous myometrial cultures (Andersen et al., 1995). Although cytokines and growth factors are thought to foster LM growth by autocrine–paracrine mechanisms (Giudice et al., 1993; Strawn et al., 1995; Dixon et al., 2000; Sozen and Arici, 2006), the molecular mechanisms of the hyper-responsiveness of LM cells to estrogens are not fully understood.
Generally, it is accepted that estrogen exerts its effects in estrogen-responsive tissues by binding to ER and modulating the transcription of target genes including growth factors (Curtis et al., 1996). Nevertheless, genomic effects of estrogen which are attributable to transcriptional activation following ligand receptor binding can be supplemented or augmented by cytoplasmic signaling pathways (Driggers and Segars, 2002), such as the mitogen activated protein kinase (MAPK) pathway transducted from growth factor receptors or plasma membrane localized ERs (Kato et al., 1995; Segars and Driggers, 2002; Levin, 2005). Therefore, estrogen signaling might be coupled to growth factor signaling through feedback mechanisms in which the regulation of growth factors by estrogen could be enhanced by the activation of growth factor receptors. Insulin-like growth factor-I (IGF-I) receptor is one of the growth factors receptors, which is highly expressed in human uterine LM tissues compared to their normal counterparts (van der Ven et al., 1994; Dixon et al., 2000). IGF-I has been reported to play an important role in the growth of human uterine LM (van der Ven et al., 1994; Strawn et al., 1995; Englund et al., 2000; Gao et al., 2001). Cross-talk between ERα and IGF-I has been demonstrated in murine uterus in response to genistein (Klotz et al., 2000; Hewitt et al., 2005). However, no study has investigated the direct associations between ER and IGF-IR signaling pathways in human uterine LM.
In the present study, we investigate the role(s) of ERα, IGF-IR and extracellular regulated kinase (ERK) and their early activation in genistein-induced LM cell proliferation, and explore the possibility that early interactions between ERα and IGF-IR, and activation of ERK might explain the increased proliferation observed in LM cells in response to low-dose genistein treatment, when compared with SMC.
Materials and Methods
Culture of human uterine LM and myometrial cells
Human LM cells (GM10964; Coriell Institute for Medical Research, Camden, NJ, USA) and SMC (Clonetics Corporation, San Diego, CA, USA) were used for the experiments at passage 14 and were kept in a standard tissue culture incubator at 37°C and 5% CO2. Both cell lines were cultured similarly as previously reported (Swartz et al., 2005). The SMC were cultured using a Smooth Muscle Cell Growth Media System (SmGM-2 BulletKit®; Clonetics). Twenty-four hours prior to the treatment of both cell lines with either the vehicle control, containing 0.3% dimethylsulfoxide (DMSO) (Sigma Chemical Company, St Louis, MO, USA) or 1 µg/ml genistein (4′, 5, 7-trihydroxyisoflavone; Sigma), the media were changed to Dulbecco’s modified Eagle’s medium/nutrient mixture F-12 Ham (DMEM/F-12) (Hyclone Laboratories, Logan, UT, USA) phenol red-free with 10% charcoal dextran treated fetal bovine serum (FBS) (Sigma).
Transient transfection assays
LM cells and SMC were plated on 12-well tissue-culture plates (Corning Incorporated, Corning, NY, USA) at 70% confluency. The medium was replaced with DMEM/F-12 without phenol red, containing 10% charcoal-stripped FBS (Sigma) for at least 24 h before transfection. In a standard transfection, 0.5 µg of the reporter plasmid, 3×-Vit-ERE-TATA-Luc, which contains three copies of the vitellogenin estrogen response element (ERE) in the pGL2-TATA-Inr plasmid (a gift from Dr. McDonnell, Duke University Medical Center, Durham, NC, USA) and 10 ng of the pRL-CMV Renilla luciferase normalization vector (Promega Corporation, Madison, WI, USA) were used for each well, with 3 µl FuGENE 6 Transfection Reagent (Roche Diagnostics, Indianapolis, IN, USA). The medium was changed 8 h after transfection, and both cell lines were treated with phenol red-free DMEM/F-12 containing 10% charcoal-stripped fetal calf serum in the presence or absence of 1 µg/ml genistein, or 10 nM E2 (Sigma) for 48 h. The anti-estrogen ICI 182,780 (AstraZeneca, Wilmington, DE, USA) and the MAPK/ERK kinase (MEK) inhibitor, PD 98059 (Calbiochem, San Diego, CA, USA) were used at a concentration of 1 and 10 µM, respectively. Luciferase assays were performed using the dual-luciferase reporter assay system (Promega) according to the manufacturer’s protocols. A vehicle control of 0.3% DMSO and a control of PD 98059 at 10 µM were included for each set. Each value was normalized to the Renilla luciferase control, and each data point generated represented the average of duplicate determinations. All experiments were repeated a minimum of three times, and reproducible results were obtained in independent experiments.
RNA isolation
After the treatment of LM cells and SMC with either the vehicle control or 1 µg/ml genistein for 24 h, total RNA was extracted LMSMC using a Rneasy MidiKit (QIAGEN, Valencia, CA, USA), as suggested by the manufacturer. The RNA concentrations were determined spectrophotometrically, and the quality of the RNA was checked on a formaldehyde agarose gel.
Real-time RT–PCR
Total RNA extracted from LM cells and SMC were reverse transcribed into cDNA in a final volume of 20 µl containing 1×PCR buffer II, 5.5 mM MgCl2, 2 mM deoxy-NTP, 8 U of ribonuclease inhibitor, 2.5 µM random hexamers, 25 U of murine leukemia virus reverse transcriptase, and 1 µg total RNA, according to the instructions of Applied Biosystems (Applied Biosystems, Foster City, CA, USA). PCR primers were designed with the Primer Express software package that accompanies the Applied Biosystems Model 7900 sequence detector (Perkin–Elmer Life Sciences, Foster City, CA, USA) and are listed in Supplementary Table 1. All reactions were carried out using an ABI Prism 7900 Sequence Detection System (Applied Biosystems) in 50 µl reaction mixture containing 100 ng cDNA template, 2.9 mM MgCl2, 1×SYBR Green PCR Master Mix (Applied Biosystems), 0.2 µM of each forward and reverse primer. The PCR conditions were set up as follows: after an incubation at 50°C for 2 min and denaturing at 95°C for 10 min, 40 cycles (95°C for 30 s, 60°C for 1 min). All PCR were performed twice in triplicate experiments, and the results were averaged. The averaged threshold cycle (CT) values of the real-time PCR were used in subsequent calculations. To quantify transcripts of the genes precisely, we monitored glyceraldehyde-3-phosphate dehydrogenase (GAPDH) transcript as the internal quantitative control. Each sample was normalized by quantitation against its GAPDH content. The relative difference between control and genistein treatment was determined using the ΔΔCT method as outlined in the Applied Biosystems protocol, and the results are presented as fold change above control.
Cell proliferation assay
Cell proliferation was determined using a colorimetric assay (CellTiter 96R AQueous One Solution Cell Proliferation assay, Promega). LM cells were seeded into 96-well plates at 4 × 103 cells per well, and cultured in phenol red-free DMEM/F-12 containing 10% charcoal-stripped FBS for 24 h. Cells were treated either with vehicle, genistein or the inhibitors, 1 µM ICI 182,780 or 10 µM PD 98059 alone, or with genistein in combination with 1 µM ICI 182,780 or 10 µM PD 98059. Cell proliferation was determined according to the manufacturer’s protocol at 24, 72 and 168 h. Spectrophotometric analysis was measured by using a microplate reader (Molecular Devices Corporation, Sunnyvale, CA, USA). The absorbance is directly proportional to the number of living cells present. The experiments were repeated three times, each with at least six replicates.
Western blotting analysis and immunoprecipitation
LM cells and SMC were treated with genistein alone or genistein in combination with PD 98059 or ICI 182,780 for 5, 10 and 15 min. The control groups were treated with 0.3% DMSO. Protein expression levels were determined by western blot analysis as previously described (Swartz et al., 2005). Briefly, protein lysates were obtained with a radioimmunoprecipitation assay (RIPA) buffer, and protein concentrations were determined by bicinchorinic acid assay (BCA) protein assay (Pierce Biochnology, Rockford, IL, USA). For western blotting, the membranes were blocked and probed overnight with a specific primary antibody: 1:1000 of phospho-MAPK P44/42 (Cell Signaling Technology, Beverly, MA, USA) or total MAPK P44/42 (Cell Signaling); 1:2000 of ERα (H-184, Santa Cruz); 1:1000 of IGF-IRβ (Sc-713, Santa Cruz); 1:1000 of ERβ (P168, CalBiochem); 1:1000 of phospho-ERα (Serine-118, Cell Signaling); 1:200 of Src homology/collagen (Shc) (Sc-967; Santa Cruz); and 1:1000 of phospho-Shc (Cell Signaling). Blots were stripped using Western Reprobe (G-Biosciences, St Louis, MO, USA) and reprobed with a rabbit anti-hypoxanthine phosphoribosyl-transferase (anti-HPRT) antibody (1:300, Santa Cruz). HPRT served as a loading control. Densitometric analyses were performed using a densitometer (Fluor ChemTM 8900, Alpha Innotech, San Leandro, CA, USA). For immunoprecipitation studies, 0.5 mg of cell lysates from each treatment were incubated with 5 µg polyclonal anti-IGF-IRβ antibody (C-20; Santa Cruz) overnight at 4°C, followed by capturing the immunocomplex by adding 50 µl of 50% slurry protein-A Sepharose beads (Life Technologies Inc., Gaithersburg, MD, USA) for 1 h at 4°C. The proteins were eluted from the beads and analyzed on 10% SDS–polyacrylamide gels, then blotted with ERα and IGF-IRβ antibodies.
Statistical analysis
The experiments for transient transfection, real-time RT–PCR, cell proliferation, western blot analysis and immunoprecipitation assays were repeated on at least three independent occasions. The data obtained from transient transfection, real-time RT–PCR and cell proliferation assays were expressed as mean ± SEM, and the data obtained from western blot analysis and immunoprecipitation assays were expressed as mean ± SD. Statistical analysis was performed using one-way analysis of variance (ANOVA) with Dunnett’s multiple comparisons test and Newman–Keuls test, respectively. A probability level of P < 0.05 was considered as statistically significant.
Results
ER transactivation by genistein in LM cells and SMC
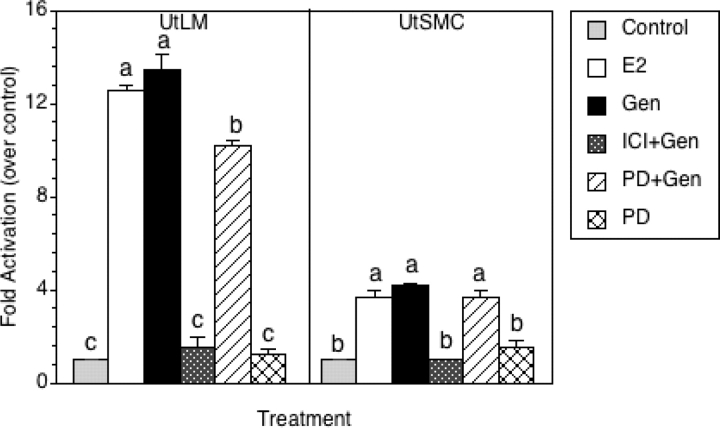
To determine whether genistein is able to directly activate gene transcription of a classical estrogen-responsive promoter element (the vitellogenin ERE) in LM cells and SMC, both cell types were transiently transfected with a firefly luciferase reporter gene driven by three vitellogenin EREs. Transient transfection was performed and activation of luciferase transcription was measured by using the dual-luciferase reporter assay after LM cells and SMC were treated with vehicle, E2, genistein or 10 µM PD 98059 alone, or with genistein in the presence of 1 µM ICI 182,780 or 10 µM PD 98059 for 48 h. As shown in Fig. 1, transcriptional activation of endogenous ER was induced by E2 and genistein in LM cells and SMC. The transcriptional activation of ER was higher in LM cells than that in SMC when compared with controls. The transactivational potential of genistein at approximately 4 µM (1 µg/ml) was found to be equivalent to E2 (10 nM) in both LM cells and SMC. However, in the presence of ER antagonist, ICI 182,780, the transactivational potential of genistein was completely blocked in both cell lines (Fig. 1), demonstrating that the transactivational potential of genistein is dependent on ER. When LM cells and SMC were treated with a MEK inhibitor, PD98059 alone, the transactivational activity was not increased or decreased in either cell type, suggesting that PD98059 has no effect on ER transactivational activity. Interestingly, in the presence of PD98059, the transactivational potential of genistein was significantly inhibited in LM cells, but not in SMC (Fig. 1), suggesting that MAPK/ERK signaling might be activated in the presence of genistein, leading to elevated ER transactivational activity in LM cells.
Figure 1:
ER transactivation in human uterine LM cells and human uterine SMC.
LM cells and SMC were transfected with pRL-CMV normalization plasmid and 3×-Vit-ERE-TATA-Luc reporter plasmid. Following transfection, cells were treated with 0.3% DMSO (Control), 10 nM E2, 1 µg/ml of genistein (Gen) and 10 µM PD 98059 (PD), or Gen in combination with 1 µM ICI 182,780 (ICI+Gen) or 10 µM PD 98059 (PD+Gen) in phenol red-free DMEM/F-12 containing 10% charcoal-stripped fetal calf serum for 48 h. Luciferase assays were performed by using the dual-luciferase reporter assay. Each value was normalized to the internal luciferase control, and fold activation was calculated by dividing values by the vehicle control (set at 1). All experiments were repeated at least three independent times with duplicate samples, and the values represent the mean ± SEM and the average coefficient of variance for each value is <10%. a, b or c, groups which are statistically different (P < 0.05).
Up-regulation of estrogen-responsive genes by genistein in LM cells
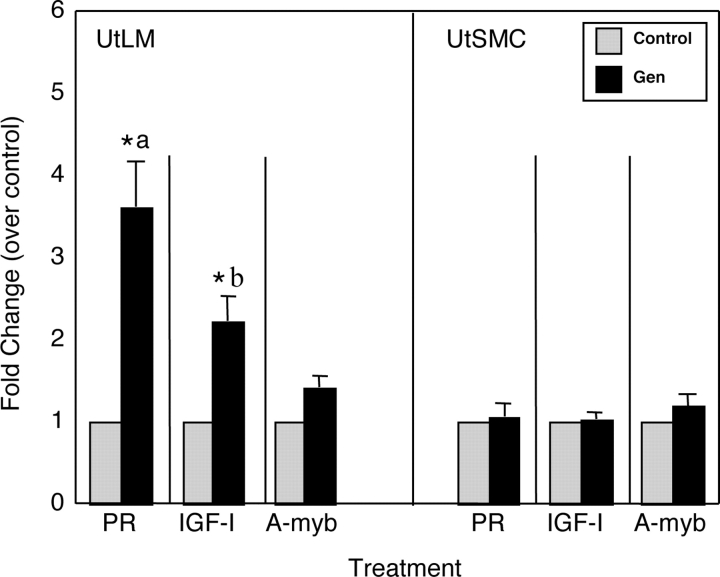
Relative mRNA expression of estrogen-responsive genes, PR, IGF-I and A-myb genes was determined by real-time RT–PCR in LM cells and SMC treated with genistein for 24 h (Fig. 2). The mRNA expression of IGF-I and PR was significantly increased (3.5- and 2.5-fold changes, respectively) in genistein-treated compared with vehicle-treated LM cells, whereas the mRNA expression of IGF-I and PR was not increased in genistein-treated SMC (Fig. 2). However, no statistically significant changes of mRNA expression of A-myb were found in either cell line following genistein treatment.
Figure 2:
Real-time PCR measurement of selected estrogen-responsive genes expressed in human uterine LM cells and human uterine SMC treated with 0.3% DMSO (Control) or 1 µg/ml of genistein (Gen) in phenol red-free DMEM/F-12 containing 10% charcoal-stripped fetal calf serum for 24 h.
The relative mRNA expression levels of PR, IGF-I and A-myb in LM cells and SMC were determined by using ΔΔCT method as described in Materials and Methods. All experiments were repeated at least three independent times with duplicate samples. Fold changes were calculated by dividing values on the y-axis with the vehicle control (set at 1). Results are presented as mean ± SEM (n = 3). a and b, statistical differences between LM cells and SMC (P < 0.05). *P < 0.05 versus control.
Effects of genistein on expression of ERs in LM cells and SMC
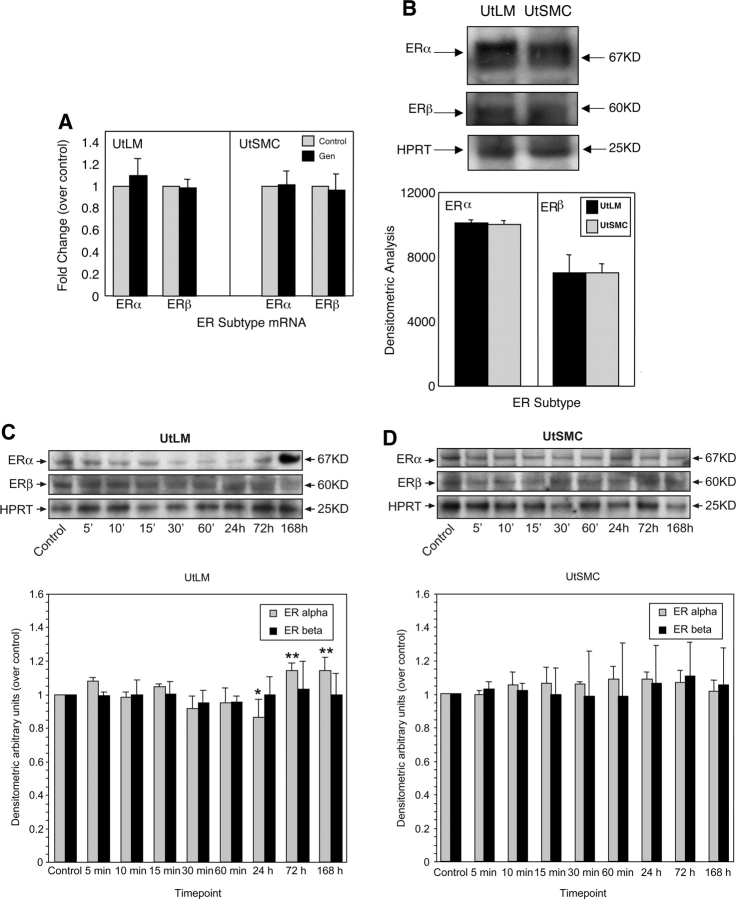
It has been reported that two ER subtypes, ERα and ERβ are differentially expressed between LMs and myometrium (Kovacs et al., 2001; Jakimiuk et al., 2004). Therefore, we investigated whether ERα and ERβ were differently affected by genistein in LM cells and SMC. Using real-time RT–PCR, we found that the mRNA expression of ERα and ERβ was not significantly changed in both LM cells and SMC following vehicle- or genistein-treatment for 24 h (Fig. 3A). At the protein level, we found that when LM cells and SMC were cultured in phenol red-free DMEM/F-12 containing 10% charcoal-stripped FBS for 24 h, the protein expression of ERα and ERβ showed similar basal expression levels for each receptor type for the two cell lines (Fig. 3B). However, when LM cells and SMC were treated with genistein, ERα and ERβ protein levels were differentially expressed in these cells. We found that in the LM cells, ERα protein expression levels tended to decrease, and reached statistical significance by 24 h compared with control (P < 0.05) (Fig. 3C). Moreover, it was found that the protein expression of ERα was increased by 72 and 168 h in the LM cells following genistein treatment, compared to ERα protein expression at 24 h (P < 0.05). However, ERβ protein levels remained unchanged in genistein-treated LM cells (Fig. 3C). In SMC, we found that the protein expression levels of ERα and ERβ were not changed in the presence of genistein (Fig. 3D), suggesting that the protein expression of ERα and ERβ were less effected by genistein in SMC.
Figure 3:
Effects of genistein on ERα and ERβ expression levels in human uterine LM cells and human uterine SMC.
(A) Real-time RT–PCR analysis of the relative expression of ERα and ERβ mRNA in LM cells and SMC in 0.3% DMSO (Control) or treated with 1 µg/ml of genistein (Gen) in phenol red-free DMEM/F-12 containing 10% charcoal-stripped fetal calf serum for 24 h. HPRT served as a loading control. Experiments were repeated at least three independent times with duplicate samples. Fold changes were calculated by dividing values on the y-axis with the vehicle control (set at 1). Results are presented as mean ± SEM (n = 3). (B) A representative western blot of the protein expression of ERα and ERβ in LM cells and SMC cultured in phenol red-free DMEM/F-12 containing 10% charcoal-stripped FBS for 24 h. Molecular weight markers are indicated on the right of the blot. (C) Time course of the protein expression of ERα and ERβ in LM cells in 0.3% DMSO (Control) at 0 min or treated with 1 µg/ml of genistein in phenol red-free DMEM/F-12 containing 10% charcoal-stripped fetal calf serum for 5, 10, 15, 30, 60 min, 24, 72 and 168 h. HPRT was served as a loading control. (D) Time course of the protein expression of ERα and ERβ in SMC treated with 0.3% DMSO (Control) at 0 min or 1 µg/ml of genistein in phenol red-free DMEM/F-12 containing 10% charcoal-stripped fetal calf serum for 5, 10, 15, 30, 60 min, 24, 72 and 168 h. The blots presented are representative examples of experiments that were performed at least three times with repetitive results. Molecular weight markers are indicated on the right of the blot. The histograms shown below are the quantitative representation after densitometry of data (mean ± SD) of three independent experiments. Values of ERα and ERβ band densities in LM cells and SMC were divided by the vehicle control (set at 1).*P < 0.05 versus vehicle control containing 0.3% DMSO. **P < 0.05 compared to 24 h.
Activation of ERK/MAPK, ERα and Shc by genistein in LM cells
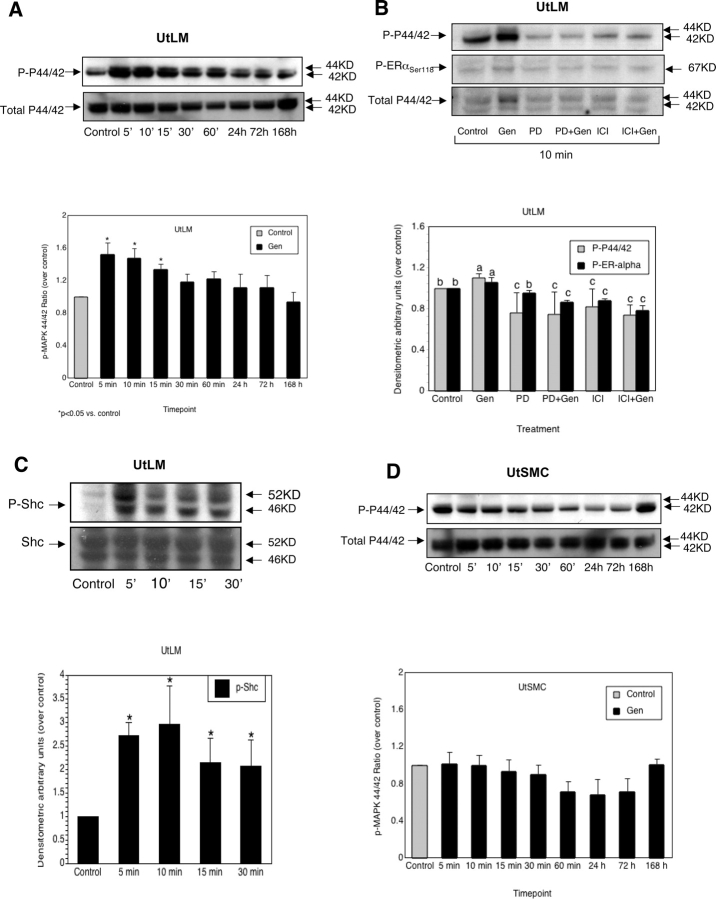
The influence of the MAPK/ERK signaling pathway on ER transactivation in LM cells but not SMC was tested by activation of P44/42 MAPK (ERK1/2) in both cell lines using western blotting. After genistein treatment, phosphorylation of P44/42 MAPK was increased in LM cells, and the increase was observed as early as 5 min, with robust activity around 10–15 min, returning to approximate basal levels by 30 min and remaining at 24, 72 and 168 h, when compared with vehicle-treated control (Fig. 4A). We chose the 10 min time point to evaluate whether the phosphorylation of P44/42 MAPK is associated with the phosphorylation of ERα in LM cells. LM cells were treated with the estrogen antagonist ICI 182,780 or with a MEK inhibitor PD98059, either alone or in the presence of genistein for 10 min. The phosphorylation of P44/42 MAPK was significantly increased only in the presence of genistein alone at 10 min, and did not occur in the presence of PD98059 and ICI 182,780 (Fig. 4B). Phosphorylation of ERα was also increased by genistein at 10 min in LM cells, and this effect was partially inhibited by ICI 182,780 and PD98059. Also, PD, but not ICI, alone had no effect on ERα phosphorylation, although they both inhibited phosphorylated P44/42 MAPK activity. Since the Shc proteins contain specific tyrosine phosphorylation sites necessary for their association with Grb2 and the subsequent activation of the downstream GTP-binding protein RAS (Pelicci et al., 1992; Sasaoka et al., 1994), which results in the dual phosphorylation of the ERK1 and ERK2 (P44/42) MAPK (Lamphere and Lienhard, 1992), we determined the phosphorylation status of Shc in the LM cells. We found that genistein activated Shc at early time points from 5 to 30 min in LM cells (Fig. 4C), suggesting that the upstream targets of genistein might transduct signaling to Shc to activate P44/42 MAPK. To determine if the changes in phosphorylation status of P44/42 MAPK under genistein treatment occurs in SMC, SMC were exposed to genistein in a time course manner similar to the genistein treatment of LM cell (Fig. 4D).
Figure 4:
Effects of genistein on phosphorylation of P44/42 MAPK, ERα and Shc in human uterine LM cells and phosphorylation of P44/42 MAPK in human uterine SMC.
(A) Time course of the protein expression of phosphorylation of P44/42 MAPK (P-P44/42) and total P44/42 MAPK (total P44/42) in LM cells treated with 0.3% DMSO (Control) at 0 min or 1 µg/ml of genistein in phenol red-free DMEM/F-12 containing 10% charcoal-stripped fetal calf serum for 5, 10, 15, 30, 60 min, 24, 72 and 168 h. (B) Protein expression of phosphorylation of P44/42 MAPK (P-P44/42), phospho-ERα Serine-118 (P-ERα) and total P44/42 MAPK (Total P44/42) in LM cells treated with 0.3% DMSO (Control), 1 µg/ml of genistein (Gen), 1 µM ICI 182,780 (ICI) and 10 µM PD 98059 (PD) alone, or Gen in combination with 1 µM ICI 182,780 (ICI+Gen) or Gen in combination with 10 µM PD 98059 (PD+Gen) for 10 min. (C) Protein expression of phosphorylated Shc (P-Shc) and total Shc (Shc) in LM cells treated with 0.3% DMSO (Control) at 0 min, or 1 µg/ml of Gen at 5, 10, 15 and 30 min. (D) Time course of the protein expression of P-P44/42 and total P44/42 in SMC treated with 0.3% DMSO (Control) at 0 min or 1 µg/ml of Gen phenol red-free DMEM/F-12 medium containing 10% charcoal-stripped fetal calf serum for 5, 10, 15, 30, 60 min, 24, 72 and 168 h. The blots presented are representative examples of experiments that were performed at least three times with repetitive results. Molecular weight markers are indicated on the right of the blot. The bar graphs indicated below are the quantitative representation after densitometry of data (mean ± SD) (n = 3) of three independent experiments. Values of P-P44/42, P-ERα and P-Shc band densities in LM cells or SMC were divided by the vehicle control (set at 1), respectively. *P < 0.05 versus vehicle control containing 0.3% DMSO. a, b, c and d indicate groups which are statistically different (P < 0.05).
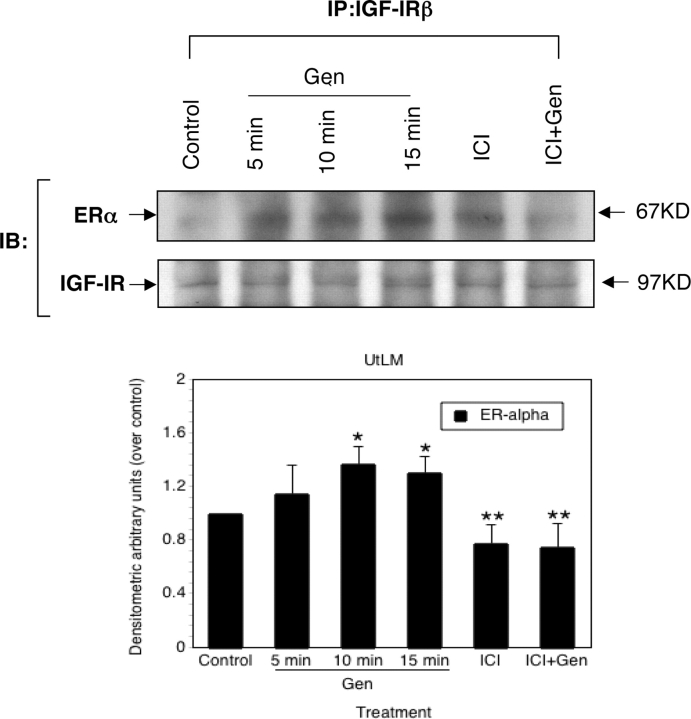
Interactions between ERα and IGF-IR induced by genistein in LM cells
To define the early events involved in genistein-induced phosphorylation of Shc and P44/42 MAPK in LM cells, protein–protein interactions between ERα and IGF-IR were examined at early time points after genistein treatment by using immunoprecipitation. As shown in Fig. 5, interactions between ERα and IGF-IR were demonstrated when LM cells were treated with genistein at early time points, and the interactions of ERα and IGF-IR were significantly increased at 10 and 15 min as evidenced by densitometric analysis of data. However, in the presence of estrogen antagonist, ICI 182,780, the association between ERα and IGF-IR was inhibited (Fig. 5). Moreover, at these time points, the protein expression of IGF-IR was not changed in the presence of either genistein or ICI 182,780.
Figure 5:
Genistein (Gen)-induced interactions between ERα and IGF-IR in human uterine LM cells.
Interactions between ERα and IGF-IR were determined by using immunoprecipitation (IP) as described in Materials and Methods. LM cells were treated with 0.3% DMSO (Control) at 0 min, or 1 µg/ml of Gen at 5, 10 and 15 min, or 1 µM ICI 182,780 (ICI) for 10 min, or Gen in combination with 1 µM ICI 182,780 (ICI+Gen) for 10 min. The blots presented are representative examples of experiments that were performed at least three times with repetitive results. Molecular weight markers are indicated on the right of the blot. The histograms indicated below are the quantitative representation after densitometry of data (mean ± SD) (n= 3) of three independent experiments. Values of ERα band densities in LM cells were divided by the vehicle control (set at 1), *P< 0.05 versus vehicle control containing 0.3% DMSO. **P< 0.05 versus Gen-treated at 10 min.
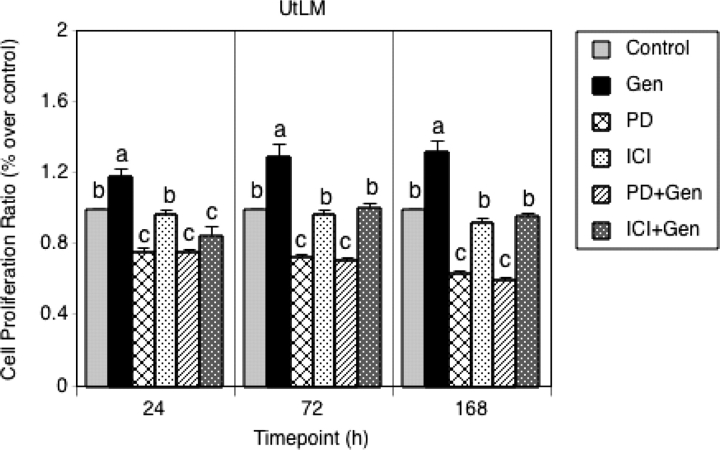
Effects of genistein on ER and ERK1/2 MAPK signaling pathways in LM cell proliferation
To demonstrate that both ER and ERK1/2 MAPK signaling pathways are involved in genistein-induced LM cells proliferation, selective inhibitors, ICI 182,780 and PD98059 were used in cell proliferation assays. As shown in Fig. 6, in the presence of PD98059 and ICI 182,780, the stimulatory effect of a low concentration of genistein on the growth of LM cells was significantly inhibited at 24, 72 and 168 h (P < 0.05). Moreover, when LM cells were treated with PD98059 or ICI 182,780 alone, LM proliferation was inhibited compared with control, and the inhibitory effect of PD98059 was more potent than that of ICI 182,780 at 24, 72 and 168 h (P < 0.05). Importantly, PD98059 significantly (P < 0.05) inhibited genistein-stimulated proliferation of LM cells at 72 and 168 h compared with ICI 182,780, suggesting that even though the proliferation of LM cells is believed to be estrogen-dependent, activation of ERK1/2 MAPK signaling pathway may be important for proliferation LM and may enhance transactivational activity of ERα by phosphorylation of ER serine sites.
Figure 6:
Effects of genistein (Gen) on human uterine LM cell proliferation. Cell proliferation assays were performed as described in Materials and Methods.
LM cells were treated with vehicle (control), 1 µM ICI 182,780 (ICI), 10 µM PD 98 059 (PD) and 1 µg/ml of Gen alone, or Gen in combination with 1 µM ICI 182,780 (ICI+Gen) or 10 µM PD 98 059 (PD+Gen) for 24, 72 and 168 h, and then cell proliferation was assessed. All experiments were repeated at least four independent times with six samples. Cell proliferation ratios were calculated by dividing absorbance values on the y-axis with the vehicle control (set at 1). Results are presented as mean ± SEM (n = 4). a, b and c indicate groups which are statistically different (P < 0.05).
Discussion
Genistein is known to exhibit estrogenic properties in both in vivo and in vitro studies. Growth-stimulatory effects of genistein at low concentrations (<1 µM) have been reported in several cell types, including human breast cancer cells (Wang et al., 1996; Hsieh et al., 1998; Chen and Wong, 2004) and Eker rat uterine LM (ELT3) cells (Hunter et al., 1999). Interestingly, data from earlier studies in our laboratory show that low concentrations (≤1 µg/ml) of genistein have a stimulatory effect on human LM cells, but not SMC (Moore et al., 2007). The growth advantage and elevated transcriptional activity in response to estrogenic compounds in uterine LM cells have been reported in former studies (van der Ven et al., 1994; Andersen et al., 1995); however, no study has yet proposed a mechanism for the differences in estrogenic responses between LM cells and SMC in a comprehensive manner. In the present study, we report a mechanism that might contribute to the hyper-responsiveness of LM cells to estrogens versus SMC in the presence of 1 µg/ml of genistein.
Transactivation assays have contributed much to the characterization of compounds with estrogen-like activity. Most of the information currently available on the transcriptional activities of genistein through ERs has been derived from transactivational studies on synthetic estrogen-responsive reporters (Kuiper et al., 1998; Hall and Korach, 2002; Jefferson et al., 2002; Mueller et al., 2004). In the present study, using a specific reporter with three copies of the vitellogenin A consensus ERE (3×ERE), which enables the detection of very weak xenoestrogens that may lack measurable activity on natural and single EREs (Norris et al., 1997; Hall and McDonnell, 1999; Hall et al., 2000), we analysed transcriptional activities of genistein in both cell lines. Consistent with the weak estrogenic effects of genistein that have been characterized in several mammalian cell types by ER binding affinity and transactivation assays (Kuiper et al., 1998; Hall and Korach, 2002; Jefferson et al., 2002; Mueller et al., 2004), it is notable that genistein showed similar transactivational potencies to E2 in LM cells and SMC, respectively, at a concentration (1 µg/ml ≈ 3.7 µM) nearly 400 times greater than that of E2 (10 nM). It was reported that ER initiates transcriptional activation upon binding of a ligand (Klein-Hitpass et al., 1986), and the transcriptional activity of liganded ER can also be supplemented or augmented by the cytoplasmic signaling pathway, MAPK pathway (Kato et al., 1995; Driggers and Segars, 2002; Segars and Driggers, 2002; Levin, 2005). The inhibitory effect of PD98059 on genistein-induced transactivation in LM cells suggests that the cytoplasmic signaling pathway, MAPK, might be involved in enhancing ER transactivation activity in LM cells. In addition, genistein also induced ER transactivation in SMC, suggesting that ER in SMC also initiates transcriptional activation after genistein treatment. However, when we analysed the antagonistic or synergistic activity of genistein by co-incubating cells with genistein and E2, no inhibitory or additive effect was found on ER transactivation in either cell line (data not shown).
Up-regulation by genistein of endogenous estrogen-responsive genes, PR or IGF-I, have been reported in Eker rat uterine LM cells (Hunter et al., 1999), and in human LM cells by E2 (Swartz et al., 2005). We confirmed the elevated ‘estrogenic' effects of genistein in LM cells compared to SMC. Since IGF-I is important in the growth of LMs (van der Ven et al., 1994; Strawn et al., 1995; Dixon et al., 2000; Englund et al., 2000; Gao et al., 2001), the increased expression of mRNA IGF-I in genistein-treated LM cells might act as a feedback mechanism directly impacting on the function of growth factor receptors, leading to cell proliferation. Although Swartz et al. (2005) found a significant increase in the mRNA and protein expression of A-myb in E2-treated LM cells, in the present study the increase in mRNA expression of A-myb was not significant in genistein-treated LM cells. This might be due to the regulation of A-myb expression in LM cells being more sensitive to potent estrogens, such as E2.
ERα and ERβ, two isoforms of ER, are not functionally equivalent and play different roles in ER action in vitro and in vivo (Couse et al., 1999; Hall and McDonnell, 1999; Mueller et al., 2004). The mRNA and protein expression of these ERs are still controversial in LMs and myometrium (Kovacs et al., 2001; Gargett et al., 2002; Jakimiuk et al., 2004). Herein, we detected the mRNA and protein expression levels of ERα and ERβ in both LM cells and SMC under our culture conditions in the absence or presence of genistein to assess whether ERα and ERβ play different roles in these cell lines. The basal expression of ERα and ERβ at both mRNA and protein levels is interesting, in that the mRNA expression patterns of ERα and ERβ in LM cells and SMC are similar to results reported by Jakimiuk et al. (2004), in which the mRNA expression levels of ERα and ERβ were found not to differ in tissue samples from premenopausal women. Because of a lack of good quality commercially available ERβ antibody previously (Gargett et al., 2002), for the first time, we have reported the protein expression of ERβ in both LM and UtSMC cell types in vitro. At the protein level, our in vitro results indicate that the basal expression levels of ERα are consistent with the results from tissues examined during menopause, in which ERα expression was not significantly enhanced in human LM compared to adjacent myometrium (Kovacs et al., 2001). However, in contrast to results that state protein expression levels of ERβ are elevated in LMs in comparison to adjacent myometrium during menopause (Kovacs et al., 2001), we found that the protein expression of ERβ was similar between LM cells and SMC under our culture conditions. We thought the reason that we saw no differences in the basal protein expression levels of ERα and ERβ might be due to the absence of exogenous estrogens in our culture system and this might represent a ‘menopausal state'. Genistein was reported to preferentially bind to ERβ and activation of ERβ was reported to inhibit cellular proliferation (Paech et al., 1997; Kuiper et al., 1998). The mRNA and protein expression levels of ERβ did not change in either cell line after genistein treatment. This may reflect very low expression levels of ERβ mRNA and protein in LM cells and SMC. Slight changes in ERβ might be undetectable, resulting in no significant differences in either cell line. Although genistein can induce ER transactivation in SMC, the minor changes of the mRNA and protein expression of ERα after genistein treatment suggest that the ERα transcript is less regulated by genistein treatment in SMC, or that ERα expression might be affected by ERK1/2 MAPK signaling. Since ERα protein degradation represents a hallmark of ERα activation by an agonist (Santagati et al., 1997), the decreased ERα protein expression in LM cells after genistein treatment suggests that more ERα was activated in LM cells compared to SMC, followed by degradation of the ERα protein that was subsequently reversed. This might represent a feedback mechanism of the regulation of ERα expression in LM cells. Although we showed that the mRNA expression of ERα was not changed significantly after genistein treatment at 24 h, additional studies showed that the mRNA expression of ERα was increased after 24 h up to 168 h in genistein-treated LM cells (data not shown).
Activation of the ERK1/2 (also referred to as P44/42) MAPK signaling pathway can phosphorylate ERα and leads to augmentation of the genomic effects of estrogen (Kato et al., 1995; Driggers and Segars, 2002). E2 and xenoestrogens were reported to activate a variety of kinases leading to the activation of ERK1/2 in vitro (Dos Santos et al., 2002; Bulayeva and Watson, 2004; Chambliss et al., 2005; Wozniak et al., 2005), though the rapid and transient activation of the MAPK pathway was determined by E2-stimulation in quiescent LM cells (Barbarisi et al., 2001). However, in the present study, we demonstrated that a low concentration of genistein-induced P44/42 MAPK activation in LM cells, but not in UtSMC, providing evidence for the elevated transactivational activity of genistein in LM cells. In several cell types, ERα was reported to be involved in the early activation of P44/42 MAPK by estrogens (Song et al., 2002; Bulayeva and Watson, 2004; Levin, 2005). Activation of Shc, an early signaling intermediate of IGF-IR, is associated with ERα-mediated rapid P44/42 MAPK activation (Song et al., 2004). Herein, the phosphorylation of Shc and ERα in LM cells at early time points suggests that the IGF-IR/MAPK/ERK signaling pathways might be activated early by genistein treatment. In addition, inhibition of the phosphorylation of P44/42 MAPK and ERα by both a MEK inhibitor and an ER antagonist highlights the importance of ERα in the rapid phosphorylation of P44/42 MAPK.
An increasing body of evidence suggests that IGF-IR is involved in the facilitation of ERα-mediated rapid E2 action (Song et al., 2004; Levin, 2005). Studies have shown that IGF-IR is over-expressed in LMs compared with matched myometrium (Dixon et al., 2000). In this study, we demonstrated the existence of associations between IGF-IR and ERα, and suggest that this interaction might be important in the early activation of IGF-IR signaling after genistein treatment for a short period of time. Inhibition of the interaction between ERα and IGF-IR by ICI 182,780 suggests that the early interactions between ERα and IGF-IR might occur when ERα is bound with a ligand in LM cells. Although it was reported that ICI 182,780 decreases the mRNA expression level of IGF-IR (Huynh et al., 1996), the protein expression of IGF-IR was not changed by genistein or ICI 182,780 in the present study (Fig. 5). The difference may be due to the time of ICI 182,780 treatment of 10 min is shorter in our study. It was reported that IGF-I signaling pathway was involved in genistein-induced MCF-7 cell growth (Chen and Wong, 2004). However, in our study, we reported the different effects of genistein, including induction of early interactions between ERα and IGF-IR in LM cells, leading to increased ER transcriptional activity.
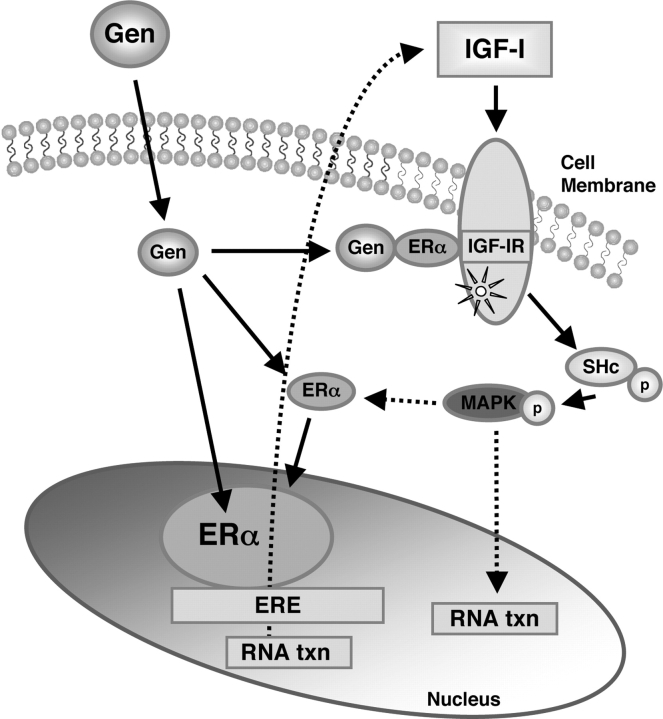
In summary, our studies show the differential responses between LM cells and SMC after treatment with a low concentration of genistein. As is proposed in Fig. 7, this mechanism would provide a novel explanation for the hyper-responsiveness of LM cells to a low dose of genistein, compared to SMC that we have previously reported (Moore et al., 2007). Consistent with the previous findings in our laboratory, we provide further evidence that IGF-IR signaling pathway might play an important role in the growth of LM cells in response to estrogens and that interactions between the IGF-I receptor and ERα and their signaling pathways may also be involved. Further investigations are needed to fully elucidate the mechanism(s) involved and the biological significance of the interactions between ERα and IGF-IR in LM cells following exposure to genistein.
Figure 7:
Proposed model: genistein (Gen) initiates cytosolic signaling from IGF-IR and ERα interaction to enhance ER transcription in uterine LM cells.
Gen binding with ERα in cytoplasm induces interactions between ERα and IGF-IR, leading to early phosphorylation of Shc (P-Shc) and mitogen activated protein kinase MAPK, which may then in turn lead to activation of ER at Ser sites resulting in enhanced ER transcriptional activity and up-regulation of IGF-I expression, thus resulting in a positive feedback regulatory mechanism and/or autocrine mechanism that stimulates LM cell proliferation.
Funding
This research was supported by the Intramural Research Program of the NIH, National Institute of Environmental Health Sciences.
Supplementary material
Supplementary material is available at http://molehr.oxfordjournals.org/
Acknowledgements
The authors would like to thank Ms. Retha Newbold and Dr. Wendy Jefferson for their critical review of this manuscript.
References
- Adlercreutz H, Markkanen H, Watanabe S. Plasma concentrations of phyto-oestrogens in Japanese men. Lancet. 1993;342:1209–1210. doi: 10.1016/0140-6736(93)92188-y. [DOI] [PubMed] [Google Scholar]
- Andersen J, DyReyes VM, Barbieri RL, Coachman DM, Miksicek RJ. Leiomyoma primary cultures have elevated transcriptional response to estrogen compared with autologous myometrial cultures. J Soc Gynecol Invest. 1995;2:542–551. doi: 10.1016/1071-5576(94)00053-4. [DOI] [PubMed] [Google Scholar]
- Barbarisi A, Petillo O, Di Lieto A, Melone MA, Margarucci S, Cannas M, Peluso G. 17-beta estradiol elicits an autocrine leiomyoma cell proliferation: evidence for a stimulation of protein kinase-dependent pathway. J Cell Physiol. 2001;186:414–424. doi: 10.1002/1097-4652(2000)9999:999<000::AID-JCP1040>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Bukulmez O, Doody KJ. Clinical features of myomas. Obstet Gynecol Clin North Am. 2006;33:69–84. doi: 10.1016/j.ogc.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Bulayeva NN, Watson CS. Xenoestrogen-induced ERK-1 and ERK-2 activation via multiple membrane-initiated signaling pathways. Environ Health Perspect. 2004;112:1481–1487. doi: 10.1289/ehp.7175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambliss KL, Simon L, Yuhanna IS, Mineo C, Shaul PW. Dissecting the basis of nongenomic activation of endothelial nitric oxide synthase by estradiol: role of ERalpha domains with known nuclear functions. Mol Endocrinol. 2005;19:277–289. doi: 10.1210/me.2004-0008. [DOI] [PubMed] [Google Scholar]
- Chen WF, Wong MS. Genistein enhances insulin-like growth factor signaling pathway in human breast cancer (MCF-7) cells. J Clin Endocrinol Metab. 2004;89:2351–2359. doi: 10.1210/jc.2003-032065. [DOI] [PubMed] [Google Scholar]
- Couse JF, Hewitt SC, Bunch DO, Sar M, Walker VR, Davis BJ, Korach KS. Postnatal sex reversal of the ovaries in mice lacking estrogen receptors alpha and beta. Science. 1999;286:2328–2331. doi: 10.1126/science.286.5448.2328. [DOI] [PubMed] [Google Scholar]
- Curtis SW, Washburn T, Sewall C, DiAugustine R, Lindzey J, Couse JF, Korach KS. Physiological coupling of growth factor and steroid receptor signaling pathways: estrogen receptor knockout mice lack estrogen-like response to epidermal growth factor. Proc Natl Acad Sci USA. 1996;93:12626–12630. doi: 10.1073/pnas.93.22.12626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon D, He H, Haseman JK. Immunohistochemical localization of growth factors and their receptors in uterine leiomyomas and matched myometrium. Environ Health Perspect. 2000;108(Suppl 5):795–802. doi: 10.1289/ehp.00108s5795. [DOI] [PubMed] [Google Scholar]
- Dos Santos EG, Dieudonne MN, Pecquery R, Le Moal V, Giudicelli Y, Lacasa D. Rapid nongenomic E2 effects on p42/p44 MAPK, activator protein-1, and cAMP response element binding protein in rat white adipocytes. Endocrinology. 2002;143:930–940. doi: 10.1210/endo.143.3.8678. [DOI] [PubMed] [Google Scholar]
- Driggers PH, Segars JH. Estrogen action and cytoplasmic signaling pathways. Part II: the role of growth factors and phosphorylation in estrogen signaling. Trends Endocrinol Metab. 2002;13:422–427. doi: 10.1016/s1043-2760(02)00634-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Englund K, Lindblom B, Carlstrom K, Gustavsson I, Sjoblom P, Blanck A. Gene expression and tissue concentrations of IGF-I in human myometrium and fibroids under different hormonal conditions. Mol Hum Reprod. 2000;6:915–920. doi: 10.1093/molehr/6.10.915. [DOI] [PubMed] [Google Scholar]
- Gao Z, Matsuo H, Wang Y, Nakago S, Maruo T. Up-regulation by IGF-I of proliferating cell nuclear antigen and Bcl-2 protein expression in human uterine leiomyoma cells. J Clin Endocrinol Metab. 2001;86:5593–5599. doi: 10.1210/jcem.86.11.8008. [DOI] [PubMed] [Google Scholar]
- Gargett CE, Bucak K, Zaitseva M, Chu S, Taylor N, Fuller PJ, Rogers PA. Estrogen receptor-alpha and -beta expression in microvascular endothelial cells and smooth muscle cells of myometrium and leiomyoma. Mol Hum Reprod. 2002;8:770–775. doi: 10.1093/molehr/8.8.770. [DOI] [PubMed] [Google Scholar]
- Giudice LC, Irwin JC, Dsupin BA, Pannier EM, Jin IH, Vu TH, Hoffman AR. Insulin-like growth factor (IGF), IGF binding protein (IGFBP), and IGF receptor gene expression and IGFBP synthesis in human uterine leiomyomata. Hum Reprod. 1993;8:1796–1806. doi: 10.1093/oxfordjournals.humrep.a137937. [DOI] [PubMed] [Google Scholar]
- Hall JM, Korach KS. Analysis of the molecular mechanisms of human estrogen receptors alpha and beta reveals differential specificity in target promoter regulation by xenoestrogens. J Biol Chem. 2002;277:44455–44461. doi: 10.1074/jbc.M200849200. [DOI] [PubMed] [Google Scholar]
- Hall JM, McDonnell DP. The estrogen receptor beta-isoform (ERbeta) of the human estrogen receptor modulates ERalpha transcriptional activity and is a key regulator of the cellular response to estrogens and antiestrogens. Endocrinology. 1999;140:5566–5578. doi: 10.1210/endo.140.12.7179. [DOI] [PubMed] [Google Scholar]
- Hall JM, Chang CY, McDonnell DP. Development of peptide antagonists that target estrogen receptor beta-coactivator interactions. Mol Endocrinol. 2000;14:2010–2023. doi: 10.1210/mend.14.12.0561. [DOI] [PubMed] [Google Scholar]
- Hewitt SC, Collins J, Grissom S, Deroo B, Korach KS. Global uterine genomics in vivo: microarray evaluation of the estrogen receptor alpha-growth factor cross-talk mechanism. Mol Endocrinol. 2005;19:657–668. doi: 10.1210/me.2004-0142. [DOI] [PubMed] [Google Scholar]
- Hodges LC, Bergerson JS, Hunter DS, Walker CL. Estrogenic effects of organochlorine pesticides on uterine leiomyoma cells in vitro. Toxicol Sci. 2000;54:355–364. doi: 10.1093/toxsci/54.2.355. [DOI] [PubMed] [Google Scholar]
- Hodges LC, Hunter DS, Bergerson JS, Fuchs-Young R, Walker CL. An in vivo/in vitro model to assess endocrine disrupting activity of xenoestrogens in uterine leiomyoma. Ann N Y Acad Sci. 2001;948:100–111. doi: 10.1111/j.1749-6632.2001.tb03991.x. [DOI] [PubMed] [Google Scholar]
- Hsieh CY, Santell RC, Haslam SZ, Helferich WG. Estrogenic effects of genistein on the growth of estrogen receptor-positive human breast cancer (MCF-7) cells in vitro and in vivo. Cancer Res. 1998;58:3833–3838. [PubMed] [Google Scholar]
- Hunter DS, Hodges LC, Vonier PM, Fuchs-Young R, Gottardis MM, Walker CL. Estrogen receptor activation via activation function 2 predicts agonism of xenoestrogens in normal and neoplastic cells of the uterine myometrium. Cancer Res. 1999;59:3090–3099. [PubMed] [Google Scholar]
- Hunter DS, Hodges LC, Eagon PK, Vonier PM, Fuchs-Young R, Bergerson JS, Walker CL. Influence of exogenous estrogen receptor ligands on uterine leiomyoma: evidence from an in vitro/in vivo animal model for uterine fibroids. Environ Health Perspect. 2000;108(Suppl 5):829–834. doi: 10.1289/ehp.00108s5829. [DOI] [PubMed] [Google Scholar]
- Huynh H, Nickerson T, Pollak M, Yang X. Regulation of insulin-like growth factor I receptor expression by the pure antiestrogen ICI 182780. Clin Cancer Res. 1996;2:2037–2042. [PubMed] [Google Scholar]
- Jakimiuk AJ, Bogusiewicz M, Tarkowski R, Dziduch P, Adamiak A, Wrobel A, Haczynski J, Magoffin DA, Jakowicki JA. Estrogen receptor alpha and beta expression in uterine leiomyomas from premenopausal women. Fertil Steril. 2004;82(Suppl 3):1244–1249. doi: 10.1016/j.fertnstert.2004.02.130. [DOI] [PubMed] [Google Scholar]
- Jefferson WN, Padilla-Banks E, Clark G, Newbold RR. Assessing estrogenic activity of phytochemicals using transcriptional activation and immature mouse uterotrophic responses. J Chromatogr B Analyt Technol Biomed Life Sci. 2002;777:179–189. doi: 10.1016/s1570-0232(02)00493-2. [DOI] [PubMed] [Google Scholar]
- Jefferson WN, Padilla-Banks E, Newbold RR. Studies of the effects of neonatal exposure to genistein on the developing female reproductive system. J AOAC Int. 2006;89:1189–1196. [PubMed] [Google Scholar]
- Kato S, Endoh H, Masuhiro Y, Kitamoto T, Uchiyama S, Sasaki H, Masushige S, Gotoh Y, Nishida E, Kawashima H, et al. Activation of the estrogen receptor through phosphorylation by mitogen-activated protein kinase. Science. 1995;270:1491–1494. doi: 10.1126/science.270.5241.1491. [DOI] [PubMed] [Google Scholar]
- Klein-Hitpass L, Schorpp M, Wagner U, Ryffel GU. An estrogen-responsive element derived from the 5’ flanking region of the Xenopus vitellogenin A2 gene functions in transfected human cells. Cell. 1986;46:1053–1061. doi: 10.1016/0092-8674(86)90705-1. [DOI] [PubMed] [Google Scholar]
- Klotz DM, Hewitt SC, Korach KS, Diaugustine RP. Activation of a uterine insulin-like growth factor I signaling pathway by clinical and environmental estrogens: requirement of estrogen receptor-alpha. Endocrinology. 2000;141:3430–3439. doi: 10.1210/endo.141.9.7649. [DOI] [PubMed] [Google Scholar]
- Kovacs KA, Oszter A, Gocze PM, Kornyei JL, Szabo I. Comparative analysis of cyclin D1 and oestrogen receptor (alpha and beta) levels in human leiomyoma and adjacent myometrium. Mol Hum Reprod. 2001;7:1085–1091. doi: 10.1093/molehr/7.11.1085. [DOI] [PubMed] [Google Scholar]
- Kuiper GG, Lemmen JG, Carlsson B, Corton JC, Safe SH, van der Saag PT, van der Burg B, Gustafsson JA. Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor beta. Endocrinology. 1998;139:4252–4263. doi: 10.1210/endo.139.10.6216. [DOI] [PubMed] [Google Scholar]
- Lamphere L, Lienhard GE. Components of signaling pathways for insulin and insulin-like growth factor-I in muscle myoblasts and myotubes. Endocrinology. 1992;131:2196–2202. doi: 10.1210/endo.131.5.1385098. [DOI] [PubMed] [Google Scholar]
- Levin ER. Integration of the extranuclear and nuclear actions of estrogen. Mol Endocrinol. 2005;19:1951–1959. doi: 10.1210/me.2004-0390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh EE, Bulun SE. Steroid hormones and leiomyomas. Obstet Gynecol Clin North Am. 2006;33:59–67. doi: 10.1016/j.ogc.2005.12.001. [DOI] [PubMed] [Google Scholar]
- Maruo T, Ohara N, Wang J, Matsuo H. Sex steroidal regulation of uterine leiomyoma growth and apoptosis. Hum Reprod Update. 2004;10:207–220. doi: 10.1093/humupd/dmh019. [DOI] [PubMed] [Google Scholar]
- Moore AB, Castro L, Yu L, Zheng X, Di X, Sifre MI, Kissling GE, Newbold RR, Bortner CD, Dixon D. Stimulatory and inhibitory effects of genistein on human uterine leiomyoma cell proliferation are influenced by the concentration. Hum Reprod. 2007;22:2623–2631. doi: 10.1093/humrep/dem185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller SO, Simon S, Chae K, Metzler M, Korach KS. Phytoestrogens and their human metabolites show distinct agonistic and antagonistic properties on estrogen receptor alpha (ERalpha) and ERbeta in human cells. Toxicol Sci. 2004;80:14–25. doi: 10.1093/toxsci/kfh147. [DOI] [PubMed] [Google Scholar]
- Newbold R. Cellular and molecular effects of developmental exposure to diethylstilbestrol: implications for other environmental estrogens. Environ Health Perspect. 1995;103(Suppl 7):83–87. doi: 10.1289/ehp.95103s783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newbold RR. Lessons learned from perinatal exposure to diethylstilbestrol. Toxicol Appl Pharmacol. 2004;199:142–150. doi: 10.1016/j.taap.2003.11.033. [DOI] [PubMed] [Google Scholar]
- Newbold RR, DiAugustine RP, Risinger JI, Everitt JI, Walmer DK, Parrott EC, Dixon D. Advances in uterine leiomyoma research: conference overview, summary, and future research recommendations. Environ Health Perspect. 2000;108(Suppl 5):769–773. doi: 10.1289/ehp.00108s5769. [DOI] [PubMed] [Google Scholar]
- Newbold RR, Banks EP, Bullock B, Jefferson WN. Uterine adenocarcinoma in mice treated neonatally with genistein. Cancer Res. 2001;a 61:4325–4328. [PubMed] [Google Scholar]
- Newbold RR, Jefferson WN, Padilla-Banks E. The mouse uterotrophic assay: other end points. Environ Health Perspect. 2001;b 109:A569–A570. doi: 10.1289/ehp.109-a569a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newbold RR, Moore AB, Dixon D. Characterization of uterine leiomyomas in CD-1 mice following developmental exposure to diethylstilbestrol (DES) Toxicol Pathol. 2002;30:611–616. doi: 10.1080/01926230290105839. [DOI] [PubMed] [Google Scholar]
- Norris JD, Fan D, Kerner SA, McDonnell DP. Identification of a third autonomous activation domain within the human estrogen receptor. Mol Endocrinol. 1997;11:747–754. doi: 10.1210/mend.11.6.0008. [DOI] [PubMed] [Google Scholar]
- Paech K, Webb P, Kuiper GG, Nilsson S, Gustafsson J, Kushner PJ, Scanlan TS. Differential ligand activation of estrogen receptors ERalpha and ERbeta at AP1 sites. Science. 1997;277:1508–1510. doi: 10.1126/science.277.5331.1508. [DOI] [PubMed] [Google Scholar]
- Payson M, Leppert P, Segars J. Epidemiology of myomas. Obstet Gynecol Clin North Am. 2006;33:1–11. doi: 10.1016/j.ogc.2005.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelicci G, Lanfrancone L, Grignani F, McGlade J, Cavallo F, Forni G, Nicoletti I, Pawson T, Pelicci PG. A novel transforming protein (SHC) with an SH2 domain is implicated in mitogenic signal transduction. Cell. 1992;70:93–104. doi: 10.1016/0092-8674(92)90536-l. [DOI] [PubMed] [Google Scholar]
- Santagati S, Gianazza E, Agrati P, Vegeto E, Patrone C, Pollio G, Maggi A. Oligonucleotide squelching reveals the mechanism of estrogen receptor autologous down-regulation. Mol Endocrinol. 1997;11:938–949. doi: 10.1210/mend.11.7.9936. [DOI] [PubMed] [Google Scholar]
- Sasaoka T, Rose DW, Jhun BH, Saltiel AR, Draznin B, Olefsky JM. Evidence for a functional role of Shc proteins in mitogenic signaling induced by insulin, insulin-like growth factor-1, and epidermal growth factor. J Biol Chem. 1994;269:13689–13694. [PubMed] [Google Scholar]
- Segars JH, Driggers PH. Estrogen action and cytoplasmic signaling cascades. Part I: membrane-associated signaling complexes. Trends Endocrinol Metab. 2002;13:349–354. doi: 10.1016/s1043-2760(02)00633-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song RX, McPherson RA, Adam L, Bao Y, Shupnik M, Kumar R, Santen RJ. Linkage of rapid estrogen action to MAPK activation by ERalpha-Shc association and Shc pathway activation. Mol Endocrinol. 2002;16:116–127. doi: 10.1210/mend.16.1.0748. [DOI] [PubMed] [Google Scholar]
- Song RX, Barnes CJ, Zhang Z, Bao Y, Kumar R, Santen RJ. The role of Shc and insulin-like growth factor 1 receptor in mediating the translocation of estrogen receptor alpha to the plasma membrane. Proc Natl Acad Sci USA. 2004;101:2076–2081. doi: 10.1073/pnas.0308334100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sozen I, Arici A. Cellular biology of myomas: interaction of sex steroids with cytokines and growth factors. Obstet Gynecol Clin North Am. 2006;33:41–58. doi: 10.1016/j.ogc.2005.12.005. [DOI] [PubMed] [Google Scholar]
- Strawn EY, Jr, Novy MJ, Burry KA, Bethea CL. Insulin-like growth factor I promotes leiomyoma cell growth in vitro. Am J Obstet Gynecol. 1995;172:1837–1843. doi: 10.1016/0002-9378(95)91420-x. Discussion 1843–1834. [DOI] [PubMed] [Google Scholar]
- Swartz CD, Afshari CA, Yu L, Hall KE, Dixon D. Estrogen-induced changes in IGF-I, Myb family and MAP kinase pathway genes in human uterine leiomyoma and normal uterine smooth muscle cell lines. Mol Hum Reprod. 2005;11:441–450. doi: 10.1093/molehr/gah174. [DOI] [PubMed] [Google Scholar]
- van der Ven LT, Gloudemans T, Roholl PJ, van Buul-Offers SC, Bladergroen BA, Welters MJ, Sussenbach JS, den Otter W. Growth advantage of human leiomyoma cells compared to normal smooth-muscle cells due to enhanced sensitivity toward insulin-like growth factor I. Int J Cancer. 1994;59:427–434. doi: 10.1002/ijc.2910590323. [DOI] [PubMed] [Google Scholar]
- Walker CL. Role of hormonal and reproductive factors in the etiology and treatment of uterine leiomyoma. Recent Prog Horm Res. 2002;57:277–294. doi: 10.1210/rp.57.1.277. [DOI] [PubMed] [Google Scholar]
- Walker CL, Stewart EA. Uterine fibroids: the elephant in the room. Science. 2005;308:1589–1592. doi: 10.1126/science.1112063. [DOI] [PubMed] [Google Scholar]
- Wang TT, Sathyamoorthy N, Phang JM. Molecular effects of genistein on estrogen receptor mediated pathways. Carcinogenesis. 1996;17:271–275. doi: 10.1093/carcin/17.2.271. [DOI] [PubMed] [Google Scholar]
- Wozniak AL, Bulayeva NN, Watson CS. Xenoestrogens at picomolar to nanomolar concentrations trigger membrane estrogen receptor-alpha- mediated Ca2+ fluxes and prolactin release in GH3/B6 pituitary tumor cells. Environ Health Perspect. 2005;113:431–439. doi: 10.1289/ehp.7505. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.