Abstract
BACKGROUND
Inactivating LH receptor (LHR) mutations have been described so far in men as well as in women. Phenotypes in men have been variable with in nearly all cases impairment of sex differentiation or azoospermia. We report a milder reproductive phenotype both in a male patient and his sister.
METHODS AND RESULTS
We describe a family that carries a homozygous mutation G→A at position −1 at the intron 10–exon 11 boundary of the LHR gene. The male patient presented with delayed puberty, micropenis and oligospermia. Two of his sisters were homozygous for the same mutation and were infertile. Surprisingly, one of them was found to have had regular ovarian cycles for years and showed normal LH values (6.5 and 10.6 mIU/ml for LH and FSH, respectively). In vitro analysis showed that this altered splicing resulted in an LHR from which eight amino acids are deleted from the extracellular domain (ΔTyr317-Ser324). In vitro expression has shown that the receptor was expressed and capable of LH-induced signaling, albeit with reduced potency (P < 0.001).
CONCLUSIONS
LHR mutations may represent an underestimated cause of infertility in women, in addition to being responsible for male hypogonadism with reduced spermatogenesis.
Keywords: LH receptor, splice mutation, male hypogonadism, menstrual cycle
Introduction
The glycoprotein hormones LH and hCG play an essential role in male and female gonadal function, pregnancy and male fetal sex differentiation (Themmen and Huhtaniemi, 2000). LH acts through the LH receptor (LHR), a member of the G-protein-coupled receptor (GPCR) family. In men, LHR inactivating mutations are associated with severe phenotypes such as 46, XY disorders of sex development (Kremer et al., 1995; Latronico et al., 1996; Themmen and Huhtaniemi, 2000). In most cases, the XY individuals have female external genitalia as a result of a lack of testosterone during the critical fetal period of sexual differentiation. Testicular histology shows Leydig cell hypoplasia/agenesis/dysplasia with a normal number of Sertoli cells. In milder cases, the phenotype ranges from micropenis (Martens et al., 1998) to severe hypospadias (Laue et al., 1996). In women, inactivating mutations of LHR result in a phenotype with normal development of primary and secondary sexual characteristics, with primary or, more often, secondary amenorrhea and infertility (Toledo et al., 1996; Latronico and Arnhold, 2006).
Here we describe a family that carries a single base change located in intron 10, at the intron 10–exon 11 boundary. In the male propositus, this mutation is responsible for hypogonadism with oligospermia. One of the sisters, although homozygous for the mutation, presented with regular menstruations for several years. We further demonstrate that the mutation results in altered splicing, and thereby production of an LHR with impaired hormone responsiveness.
Materials and Methods
Informed consent
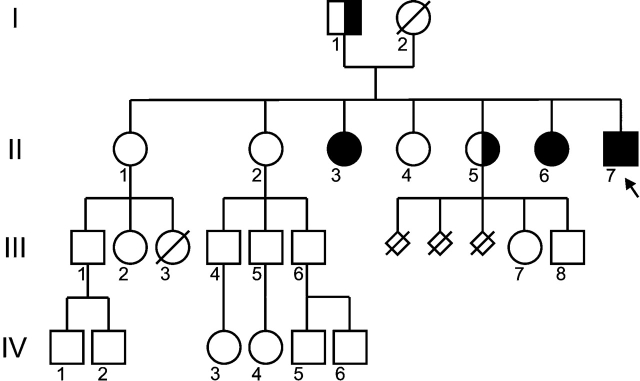
The propositus, three of his sisters and his father (Fig. 1) gave informed consent for the study.
Figure 1:
Family pedigree of the propositus, indicating the mutation in intron 10 (−1) G→A.
Propositus II-7 is indicated by an arrow.
Molecular analysis
DNA was extracted from peripheral blood leukocytes with the QiaAmp DNA blood mini kit (Qiagen, Courtaboeuf, France).The 11 exons and flanking intronic regions of the LHR gene were amplified by PCR and sequenced using ABI Prism BigDye terminator sequencing kit and the ABI 310 genetic analyzer (Applied Biosystems, Courtaboeuf, France).
Materials
Unless stated otherwise, cell culture reagents were purchased from Gibco, Invitrogen Corporation, Paisley, UK; enzymes, restriction enzymes and polymerases from Roche, Almere, The Netherlands; fine chemicals from Sigma Aldrich, Zwijndrecht, The Netherlands; and oligonucleotides from Biololegio, Nijmegen, The Netherlands. hCG and LH are a kind gift of NV Organon, Oss, The Netherlands.
Construction of minigenes and expression plasmids
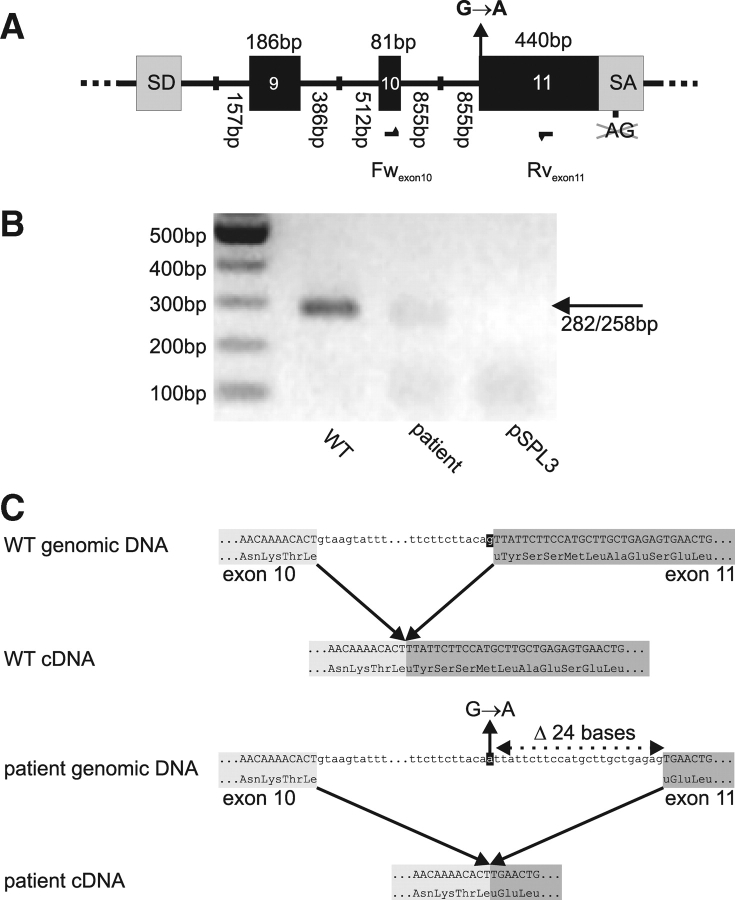
Patient and wild type (WT) minigenes were created based on the pSPL3 vector (Gibco, Invitrogen Corporation). Fig. 2A depicts an overview of the final constructs. In short, the cryptic exon was deleted from pSPL3 (NdeI, NheI), then exon 9, exon 10 and exon 11 fragments, with flanking intronic sequences, were inserted. Fragments for exon 9, exon 10 and exon 11 were created with primer pairs (forward Fw and reverse Rv): FwEcoRI-intron8 5′GAATTCAGATCTTAGTGAGAG/Rvintron9-EcoRI 5′GAATTCGGTAAATTTTGCCCTCCTGAGG; FwXhoI-intron9 5′CTCGAGGAGTAGGGAGCACCCACGTATG/Rvintron10-XhoI 5′CTCGAGGCGATACTCTGTTACAGGAAGG; FwBamHI-intron10 5′GGATCCGTGGCAAAGGAAGGACTGTTC/Rvexon11-BglII 5′AGATCTGGTGCCATGCAGGTGAAATCGG, respectively (restriction sites in bold face). Finally, to avoid splicing to the original splice acceptor (SAv) in pSPL3, XbaI (exon 11)–BglII (behind SAv) was deleted.
Figure 2:
In vitro splicing of WT and patient minigenes.
(A) Schematic overview of the wild type (WT) and patient minigenes derived from pSPL3. For clarity, only sequence from pSPL3 splice donor (SD) to splice acceptor (SA) is represented. To allow splicing to exon 11, part of pSPL3 SA was deleted. Location of oligonucleotides forward Fwexon10 and reverse Rvexon11 is depicted as arrows. In the patient minigene, mutation G(−1)→A at intron 10–exon 11 boundary is present. (B) RT–PCR on complementary DNA (cDNA) derived from in vitro spliced minigenes using oligonucleotides Fwexon10–Rvexon11. (C) Reconstruction of in vitro splicing using WT and patient minigenes, based on sequencing of in vitro splice products.
Construction of the expression plasmid pSG5-LHRWT is described elsewhere (Kraaij et al., 1995). An N-terminal HA-tag was included by inserting the double-stranded oligonucleotides (5′ GTACCCATACGATGTTCCAGATTACGC 3′ and complement) into the Eco47III site, directly behind the signal peptide of the LHR. The patient LHR was constructed by deletion of the first 24 bp of exon 11, through fusion PCR of fragments generated with the following primer pairs: Fwupstream 5′GAATTCCGGCCATGAAGC/Rvfusion1 GCCACTCAGTTCAAGTGTTTTGTTATTCACTTTCC and Fwfusion2 CAAAACACTTGAACTGAGTGGCTGGGAC/Rvdownstream 5′GGTGCCATGCAGGTGAAA (non-complimentary parts in bold face). All constructs were verified by dideoxynucleotide sequencing.
Cell culture and transfection
HEK293 cells were maintained as described earlier (Martens et al., 1998). Two days prior to transfection, cells are plated at a density of 20% in 25 cm2 tissue culture flasks (Nalge Nunc International, Rochester, NY, USA). For transfection, medium was replaced by serum free medium and supplemented with 700 µl complementary DNA (cDNA)–PEI mixture (150 mM NaCl supplemented with 10 ng PEI (polyetyleneimine, linear MW ∼25.000; Polysciences Inc., Warrington, PA, USA), and 7 µg pSG5-LHR/1.4 µg pCRE6lux/1.4 µg pRL-SV40 (reporter gene assay/enzyme-linked immunosorbent assay (ELISA)) or 9 µg minigene (in vitro splicing)). After 4 h, medium was supplemented with fetal bovine serum to a final concentration of 10%.
In vitro splicing
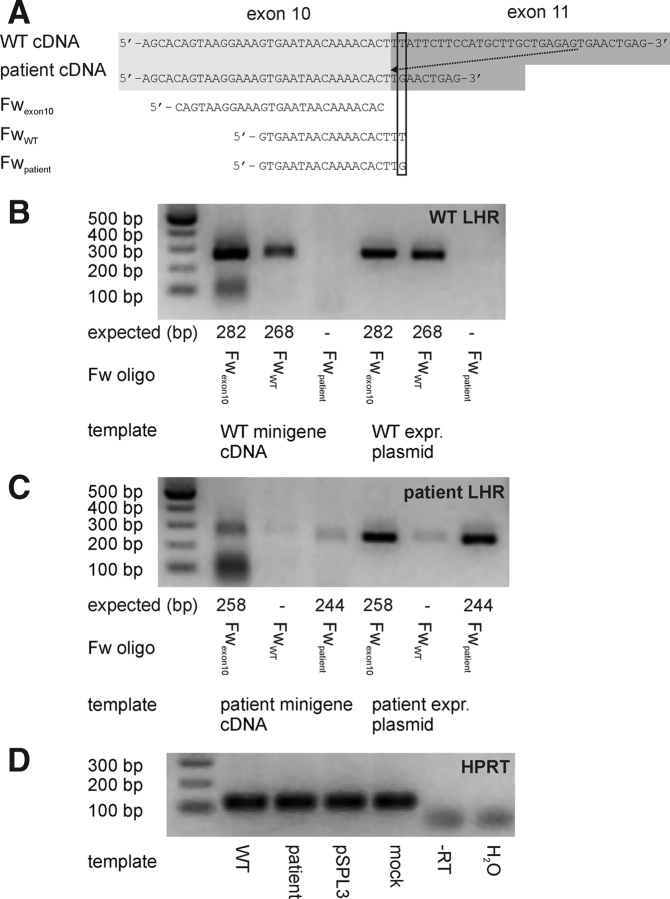
WT and patient minigenes were transfected to HEK293 cells (see above) and after 72 h mRNA was isolated using Trizol according to the manufacturers instruction (Gibco, Invitrogen Corporation) and products were studied by RT–PCR with pSPL3-based oligonucleotides, FwSD6 and RvSA2 (Exon Trappping System, Gibco, Invitrogen Corporation), and the internal oligonucleotides Fwex10, FwWT and Fwpatient (Fig. 3A) and Rvexon11 (5′ GATTGCACATGAGAAAACGAGG 3′). Products were characterized by agarose gel electrophoresis and, after cloning to pGEM-T-Easy (Promega Corp., Madison, WI, USA), analysed by dideoxynucleotide sequencing.
Figure 3:
Allele-specific amplification of alternative splice products.
Design of general (Fwexon10) and WT (FwWT) or patient (Fwpatient) specific oligonucleotides that, in combination with Rvexon11, should result in an allele-discriminating PCR (A). The most 3′ base of the specific oligonucleotides and corresponding sequence in WT and patient cDNA are boxed. General and allele-specific amplification of WT (B) or patient (C) cDNA (obtained after in vitro splicing). Parallel PCR on expression plasmids (1 ng) is used for validation of PCR selectivity. The size of the expected PCR products is depicted below the gel pictures. As a control for cDNA synthesis, the housekeeping enzyme hypoxanthine–guanine phosphoribosyl transferase (HPRT) is amplified (D), this control is also shown for non-transfected (mock) cells, pSPL3-transfected cells, as well as samples without reverse transcriptase (-RT).
Reporter gene analysis
Twenty-four hours post-transfection, cells were detached using trypsin–EDTA and transferred to white μClear TC-treated plates (Greiner Bio-one Alphen a/d Rijn, The Netherlands). Stimulation and readout are described earlier (Piersma et al., 2006). Firefly relative light units (RLUs) were normalized using renilla RLU and analysed using Prism3 (Graphpad Software Inc., San Diego, CA, USA). Statistical analysis of EC50 values was carried out by Student's t-test. P-values <0.05 were considered to indicate a significant difference.
ELISA
Twenty-four hours post-transfection, cells were detached using trypsin–EDTA and transferred to 96-well assay plates (poly-lysine coated clear plates (Corning Inc., Corning, NY, USA) for ELISA. Forty-eight hours post-transfection, cells were washed with PBS, fixed with 4% paraformaldehyde in phosphate-buffered saline (PBS) (30 min, room temperature) and blocked with 3% bovine serum albumin (BSA) in PBS (1 h, 37°C). Wells were washed 4× with PBS and incubated (1 h, 37°C) with 1:1000 dilution of Anti-HA-Peroxidase (3F10, Roche, Mannheim, Germany) in PBS, 3% BSA. Wells were washed 6× with PBS and peroxidase activity was assayed using 1-Step Ultra tetramethylbenzidine-ELISA (Pierce, Rockford, IL, USA).
Results
The family tree is shown in Fig. 1. No consanguinity is known in this family, originating from Portugal.
Patient II-7
The proband (Patient II-7), a 34-year-old man was referred to the Reproductive Endocrine Unit for hypogonadism. He mentioned that his puberty started at the age of 13 years. Upon examination, he was obese with a weight of 88 kg and a height of 165 cm (BMI 32.3). He had rare pubic and axillary hair, matching a Tanner stage P2. The frequency of his shaving had always been once a month. He had a small penis, reaching 8 cm during penile erection. His descended testes had normal volume (25 ml each; normal range (N): 14–30 ml). This was confirmed by ultrasound showing a testicular size of 49 × 28 and 50 × 30 mm for the right and the left testis, respectively. His hormonal evaluation is reported in Table I. His ejaculate displayed on two occasions a reduced volume with oligospermia, 0.35 and 0.5 ml with 3 and 3.2 million spermatozoa per ml, respectively (N: 3–5 ml for volume and >30 million/ml for sperm count). Sperm vitality was low, respectively, 14 and 20% of normally motile sperm (N> 50%) on two different sperm evaluations. His karyotype was 46, XY. For ethical reasons, a testicular biopsy could not be performed in this patient, as sperm was present in his semen.
Table I.
Hormonal evaluation of the propositus (II-7) and one of his sister (II-6).
| Patient | Male II-7 | Female II-6 |
|---|---|---|
| Testosterone (nmol/l) | ||
| Base line | 0.9 (N: 11–40) | 1.02 (N: 0.35–1.85) |
| 96 h post hCG | 2.8 | |
| 8 weeks post 5000 IU hCG, 3 times per week | 2 | |
| Estradiol (pg/ml) | 138 (50–150) | |
| LH (mIU/ml) | 30 (N: 0.5–11) | 6.5 (N: 4–10) |
| FSH (mIU/mL) | 10 (N: 0.8–13) | 10.6 (N: 3–10) |
| Inhibin B (ng/ml) | 153 (135–350) |
N, normal range of values.
Patient II-6
One of the proband's sisters (Patient II-6) was 42 years old when she was evaluated in our unit. Upon examination, her weight was 53 kg with a height of 150 cm (BMI 23.6). She had normal external genitalia, Tanner grade 5. Her karyotype was 46, XX. She mentioned a normal, but late puberty as her menarche occurred at the age of 16 years. Her menses had been regular, every 28 days, until the age of 20. Thereafter, she developed oligomenorrhea with intervals from 2 to 6 months between menses. Several progestin tests had been performed and had always been positive, followed by bleeding. Basal body temperature charts had always failed to identify a rise in body temperature. Although she was not using any contraception, no pregnancy occurred. A laparoscopy had been performed when she was 30-year-old, identifying two enlarged ovaries measuring 40 × 25 and 40 × 20 mm, without any scar of ovulation. No cyst was described. Ultrasonography of the pelvis following clomiphene citrate therapy revealed some follicular development, as follicles reached 10, 12 and 15 mm. However, endometrial thickness had always remained <8 mm. No ovulation had occurred under this treatment as progesterone levels always remained <1 ng/ml. Thereafter, she underwent two IVF attempts. Multifollicular development was obtained with an estradiol (E2) serum level reaching 270 pg/ml. No oocyte could be retrieved following hCG administration. Therefore, ovulation induction has always been unsuccessful. Subsequently, she exhibited regular menses following sequential progestin therapy (Duphaston®), 20 mg daily, 10 days per month. Hormone levels, reported in Table I, were measured in our clinic on Day 2 of the cycle, after progesterone withdrawal. They were identical to her previous serum results, obtained when she was 20 and 30 years old.
Patient II-3
Another proband's sister (Patient II-3) was 53 years old when the propositus was evaluated. Her weight was 55 kg and her height 153 cm. Her menarche occurred at 16–17 years, she displayed oligomenorrhea (3–4 months interval) all her reproductive life and was infertile. Her breast development was normal; she had scarce pubic and axillary hair (P2A1). She had several episodes of functional ovarian cysts. She underwent several unsuccessful ovarian stimulation cycles.
Patient II-5
This proband's sister was aged 44 years when she was evaluated. She had had menarche at the age of 13 years and had always presented regular menses. She presented three spontaneous abortions occurring on Month 3, 4 and 6 of gestation. No cause could be identified for those abortions.
Sequencing of the LHR gene
This clinical case of male hypogonadism (Patient II-7) with a low testosterone level in combination with high LH and normal FSH prompted us to sequence all exons and proximal parts of the introns of the LHR gene of Patient II-7. No mutations were found within the exons of the LHR gene. However, a G to A substitution was present in intron 10 at position −1, 5′ to the splice acceptor site of exon 11, thereby changing the most conserved part of the consensus splice acceptor sites C−3 (78%), A−2 (100%), G−1 (100%) and G+1 (55%) (Krawczak et al., 1992). Also his sisters II-3 and II-6 were homozygous for this mutation, whereas father and sister II-5 were heterozygous. DNA from the mother was not available. The mutation we describe has never been identified in our control population, including several hundreds of European male and females. Furthermore, in the hapmap database, this variation has not been reported.
In vitro splicing
The base change at position −1 of the intron 10–exon 11 boundary suggested that the splice acceptor site of exon 11 is not functional. Therefore, we decided to study whether alternative splice products exist. Since patient tissue expressing the LHR was unavailable, we constructed minigenes (Fig. 2A), which allowed the study of alternative splicing in vitro. After transfecting the WT and patient-derived minigenes to HEK293 cells, mRNA was isolated and, transcripts were analysed by RT–PCR using a primer-pair targeting exons 10 and 11 (Fwexon10, Rvexon11). Transfection of the WT minigene resulted in a 282 bp product, corresponding to the correct removal of intron 10 (Fig. 2B). In contrast, the product from the patient minigene appeared slightly smaller and less intense (Fig. 2B). Cloning and sequencing of the Fwexon10–Rvexon11 products validated that the expected 282 bp band was formed from the WT minigene and that the G→A mutation caused not only intron 10, but also the first 24 bases of exon 11 to be spliced out (Fig. 2C). The deletion of 24 bases does not result in a frame shift or in a premature stop codon, but in an 8-amino-acid deletion (ΔTyr317-Ser324). This deletion is located in the C-terminal cysteine rich region of the LHR, linking the leucine-rich repeat ectodomain to the transmembrane domain. Additional attempts were made to study the splicing of exons 9–10–11 using pSPL3-based oligonucleotides. However, we did not find products corresponding to the expected removal of the intronic sequences, but observed, in both WT and patient-derived transfections, bands that were shorter (∼350, 550 and 650 bp) than the expected 775 bp (data not shown).
Allele-specific PCR
To study whether altered splicing is exclusively occurring in the patient minigene and not in the WT (and vice versa), two forward oligonucleotides were designed, overlapping either the WT or patient exon 10–exon 11 boundary, thus creating allele-specific oligonucleotides (Fig. 3A). The patient-specific oligonucleotide was able to amplify patient expression plasmid DNA (Fig. 3C), but did not yield a product with WT expression plasmid DNA (Fig. 3B), demonstrating the specificity of Fwpatient. Using WT cDNA from pSPL3-WT transfected HEK293 cells, no product was found with Fwpatient (Fig. 3B), indicating that altered splicing is restricted to the patient.
Using FwWT, we could amplify the expected product in WT cDNA, however, also a fainter product was observed in cDNA from pSPL3-patient transfected HEK293 cells. Since the WT-specific oligonucleotide also amplified patient expression plasmid DNA (Fig. 3C), albeit much less than with WT plasmid DNA as a template (Fig. 3B), FwWT did not appear to be fully specific. In the PCRs using cDNA derived from pSPL3-patient transfected cells, always less intense bands were obtained than for WT cDNA, either with a specific or a general forward oligonucleotide, while for WT or patient plasmid DNA, this difference is not observed. The RT–PCRs of the housekeeping enzyme hypoxanthine–guanine phosphoribosyl transferase (HPRT) gene yielded equal amounts of products (Fig. 3D). Therefore, it may be speculated that splicing from the patient minigene occurs less efficiently than from the WT minigene.
Pharmacological characterization
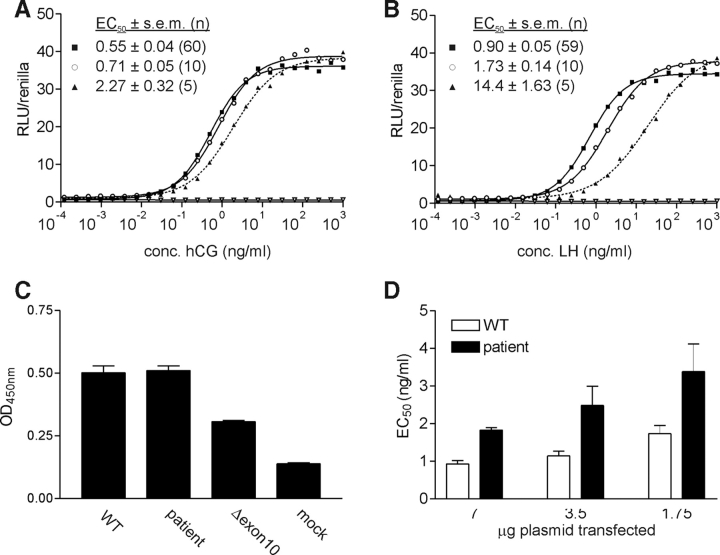
Altered splicing in the patient results in a shorter form of the LHR protein, of which 8 amino acids are deleted at the N-terminal end of exon 11 (ΔTyr317-Ser324). To study potential changes in hormone responsiveness of this aberrant form of the LHR, protein site-directed mutagenesis was employed to recreate the mutant receptor in an expression plasmid. Transient transfection of WT and ΔTyr317-Ser324 LHR expression plasmids in HEK293 cells resulted in similar expression levels as determined by ELISA (Fig. 4C). Upon hCG (Fig. 4A) or LH (Fig. 4B) stimulation, both receptors could activate the cAMP-dependent reporter plasmid to the same extent (identical Emax). Despite the absence of large effects on the potencies of hCG and LH, the potency of LH, but not of hCG, for the ΔTyr317-Ser324 LHR was slightly (1.9-fold) but significantly (P < 0.001) reduced (Fig. 4B). The effects of the removal of the first eight amino acids (Tyr317-Ser324) on the potencies of LH and hCG were much milder than those for the LHR of another, previously described, patient (Gromoll et al., 2000; Müller et al., 2003) in which exon 10 is deleted (ΔGln290-Leu316; 3.9- and 15-fold, respectively), which was tested as an additional control (Fig. 4).
Figure 4:
Pharmacological characterization of WT and patient LHRs.
Dose–response curves of hCG (A) or LH (B) for WT (▪), patient (○) or Δexon 10 (▴)* LHRs or mock transfected controls (▿), as measured by a CAMP response element (CRE) luc-driven reporter gene assay. A representative experiment is shown. A parallel enzyme-linked immunosorbent assay, targeting an N-terminal HA-tag is used to evaluate receptor expression (C). Potencies (EC50) of LH for WT and patient LHRs after transfecting 7, 3.5 or 1.75 µg expression plasmids as measured by a CRE luc-driven reporter gene assay (D). Total amount of transfected plasmid is kept constant by supplementing empty pSG5. (*Gromoll et al., 2000; Müller et al., 2003).
The possibility that changes in expression level may further affect hormone sensitivity was explored by transfecting decreasing amounts (2- and 4-fold) of plasmid DNA. For both WT and patient LHRs, a stepwise decrease in expression resulted in a stepwise rightward shift of LH dose–response curve and thus decreased potency (Fig. 4D). For hCG, identical decreases were observed. For both the patient and WT LHR, transfection of 4-fold less plasmid DNA resulted in 2-fold decrease in potency of LH.
Discussion
In this study, we report three cases of homozygous LHR mutations in one brother and two of his sisters. Because the male hypogonadism was associated with high LH levels, near normal FSH levels and weak response to hCG stimulation, an LHR gene defect was suspected and confirmed.
The phenotype of one of the sisters, homozygous for the LHR mutation, was unexpected. The phenotype of Patient II-3 is similar to the previous phenotypes described in other women with LH resistance, but Patient II-6 has a much milder phenotype than previously described. Thus far, all women described carrying inactivating LHR mutations were sisters of patients with 46, XY disorders of sex development (Arnhold et al., 1999; Themmen and Huhtaniemi, 2000; Themmen, 2005; Latronico and Arnhold, 2006), and all presented with normal breast development and primary or secondary amenorrhea with E2 levels within the normal range for the follicular phase. In some cases, normal follicular development has been reported. Lack of ovulation was described in all cases. The female phenotypes in our family are very unusual for two main reasons: first, one of the sisters presented with regular menses for at least 4 years and second, her LH levels always remained within the normal range. Therefore, the diagnosis of an LHR mutation could not have been suspected. This is in contrast to all cases described before where women with LHR mutations had elevated LH levels (>10 mIU/ml). Interestingly, the female phenotype we report is close to the female phenotype of the patient with an LHβ mutation, recently described by Lofrano-Porto et al. (2007); however, this patient had sporadic menses in contrast to our patient who had regular menses for several years.
In Patient II-6, the lack of ovulation was identified during spontaneous cycles or following clomiphene citrate treatment, thus confirming the mandatory role of LH for ovulation in women. Furthermore, during ovarian hyperstimulation, serum E2 reached the modest level of 270 pg/ml. These data support a mild inactivation of the LHR, as a complete inactivating LHR mutation would abolish ovarian androgen production, and therefore E2 production obtained from androgen aromatization. The absence of oocyte retrieval in IVF suggests an abnormal follicular development.
The male patient we report has a much milder phenotype than males previously reported with inactivating LHR mutations, as he did not present 46, XY disorders of sex development. He even had spontaneous spermatogenesis. These symptoms were even milder than the male patient described by Gromoll and coworkers, with a homozygous deletion of exon 10, resulting in hypogonadism and azoospermia (Gromoll et al., 2000). Our proband's phenotype suggests a partial inactivating mutation of LHR. Furthermore, spontaneous spermatogenesis indicates that the minimum testosterone levels in the testis allowing spermatogenesis may be lower than discussed before. In contrast to the LHR knockout mice (Ahtiainen et al., 2007), high doses of androgens given to the propositus did not improve the sperm parameters.
Both the propositus and his sisters, Patients II-3 and II-6, are homozygous for a mutation in the LHR on the intron 10–exon 11 boundary (−1 from exon 11; G→A). In the consensus splice acceptor site, the nucleotides A−2G−1 are fully conserved (Krawczak et al., 1992). Single base-pair substitutions in the splice sites constitute some 9.5% of all mutations causing inherited disease (Krawczak et al., 1992; Stenson et al., 2003; Krawczak et al., 2007). These mutations generally result in skipping of the adjacent exon, which appears to be the most frequent, or utilization of a cryptic splice site (Krawczak et al., 2007). Although splice site mutations are not frequently observed in GPCR genes, both cryptic splice site utilization (Thomas et al., 1995) and exon skipping (Silveira et al., 2002) have been reported. Thus, the mutation in the LHR in Patients II-3, II-6 and II-7 is likely to disrupt the intron 10 splice acceptor site and may thereby result in altered splicing.
Since proband's testicular tissue, and thus LHR mRNA is unavailable, we could not evaluate altered splicing directly. As an alternative, we applied an in vitro splicing assay and compared the splicing products derived from WT and patient minigenes. Using primers that focus on the removal of the mutation-carrying intron 10, altered splicing was observed, resulting in a transcript shorter by 24 bp (Fig. 2). Consequently, the patient LHR would display an 8-amino-acid deletion within the extracellular domain, near the start of transmembrane domain.
We constructed a mutant receptor ΔTyr317-Ser324 LHR expression plasmid, representing the protein product after cryptic splice site usage, and tested it for functionality in our CAMP response element (CRE)-driven reporter gene assay. The potencies of LH and hCG were decreased, albeit marginally (1.8- and 1.2-fold, respectively). This decrease is smaller than was observed in an earlier patient from which exon 10 of the LHR was deleted, which correspond to the milder clinical phenotype. Whereas exon 10 deletion resulted in delayed puberty with azoospermia (Gromoll et al., 2000), in our Patient II-7 the symptoms were mainly restricted to severe hypogonadism; his sister (II-6) had even displayed regular menstrual cycles.
Our in vitro splicing experiments suggest that LHR splicing in the patient not only results in a different product, but also occurs less efficiently. We observed less spliced LHR cDNA derived from the patient minigene than for the WT minigene. This does not appear to result from changes in PCR amplification efficiency since identical quantities of the various plasmid DNAs resulted in identical PCR yields. Also experimental variation in cDNA synthesis can be ruled out as the housekeeping enzyme HPRT yielded bands with equal intensity in all conditions. We further demonstrate that a decrease in receptor expression results in a reduction of LH potency, for both WT and Δ(Tyr317-Ser325) LHRs. We therefore conclude that also in vivo, the small decrease in LH potency may be further aggravated by reduced LHR expression. This view is supported by previous findings indicating that overall receptor signal capacity is a combination of both cell surface expression and coupling efficiency (Martens et al., 1998).
In conclusion, we describe a new inactivating LHR splice mutation. The phenotype is milder than previously described cases, especially in a woman who had regular menses and normal LH levels. This suggests the heterogeneity of the phenotype of this rare genetic disease. In most cases, LHR mutation is associated with 46, XY disorders of sex development or hypogonadism in men and with amenorrhea with abnormal follicular development in women. The new female phenotype of inactivating LHR includes infertility, with regular cycles and normal LH levels.
Funding
The work was supported by NIH grant 5R01DK069711-02 (M.B. and A.P.N.T.).
Acknowledgements
Jan Willem Kooper is acknowledged for helpful discussions.
References
- Ahtiainen P, Rulli S, Pakarainen T, Zhang FP, Poutanen M, Huhtaniemi I. Phenotypic characterisation of mice with exaggerated and missing LH/hCG action. Mol Cell Endocrinol. 2007;260–262:255–263. doi: 10.1016/j.mce.2005.11.047. [DOI] [PubMed] [Google Scholar]
- Arnhold IJ, Latronico AC, Batista MC, Mendonca BB. Menstrual disorders and infertility caused by inactivating mutations of the luteinizing hormone receptor gene. Fertil Steril. 1999;71:597–601. doi: 10.1016/s0015-0282(98)00517-2. [DOI] [PubMed] [Google Scholar]
- Gromoll J, Eiholzer U, Nieschlag E, Simoni M. Male hypogonadism caused by homozygous deletion of exon 10 of the luteinizing hormone (LH) receptor: differential action of human chorionic gonadotropin and LH. J Clin Endocrinol Metab. 2000;85:2281–2286. doi: 10.1210/jcem.85.6.6636. [DOI] [PubMed] [Google Scholar]
- Kraaij R, Post M, Kremer H, Milgrom E, Epping W, Brunner HG, Grootegoed JA, Themmen APN. A missense mutation in the second transmembrane segment of the luteinizing hormone receptor causes familial male-limited precocious puberty. J Clin Endocrinol Metab. 1995;80:3168–3172. doi: 10.1210/jcem.80.11.7593421. [DOI] [PubMed] [Google Scholar]
- Krawczak M, Reiss J, Cooper DN. The mutational spectrum of single base-pair substitutions in mRNA splice junctions of human genes: causes and consequences. Hum Genet. 1992;90:41–54. doi: 10.1007/BF00210743. [DOI] [PubMed] [Google Scholar]
- Krawczak M, Thomas NS, Hundrieser B, Mort M, Wittig M, Hampe J, Cooper DN. Single base-pair substitutions in exon-intron junctions of human genes: nature, distribution, and consequences for mRNA splicing. Hum Mutat. 2007;28:150–157. doi: 10.1002/humu.20400. [DOI] [PubMed] [Google Scholar]
- Kremer H, Kraaij R, Toledo SP, Post M, Fridman JB, Hayashida CY, van Reen M, Milgrom E, Ropers HH, Mariman E, et al. Male pseudohermaprhoditism due to a homozygous missense mutation of the luteinizing hormone receptor gene. Nat Genet. 1995;9:160–164. doi: 10.1038/ng0295-160. [DOI] [PubMed] [Google Scholar]
- Latronico AC, Arnhold IJ. Inactivating mutations of LH and FSH receptors—from genotype to phenotype. Pediatr Endocrinol Rev. 2006;4:28–31. [PubMed] [Google Scholar]
- Latronico AC, Anasti J, Arnhold IJ, Rapaport R, Mendoca BB, Bloise W, Castro M, Tsigos C, Chrousos G. Brief report: testicular and ovarian resistance to luteinizing hormone caused by inactivating mutations of the luteinizing hormone receptor. N Engl J Med. 1996;334:507–512. doi: 10.1056/NEJM199602223340805. [DOI] [PubMed] [Google Scholar]
- Laue LL, Wu SM, Kudo M, Bourdony CJ, Cutler GB, Hsueh AJ, Chan WY. Coumpound heterozygous mutations of the luteinizing hormone receptor gene in Leydig cell hypoplasia. Mol Endocrinol. 1996;10:987–997. doi: 10.1210/mend.10.8.8843415. [DOI] [PubMed] [Google Scholar]
- Lofrano-Porto A, Barra GB, Giacomini LA, Nascimento PP, Latronico AC, Casulari LA, da Rocha Neves Fde A. Luteinizing hormone beta mutation and hypogonadism in men and women. N Engl J Med. 2007;357:897–904. doi: 10.1056/NEJMoa071999. [DOI] [PubMed] [Google Scholar]
- Martens JWM, Verhoef-Post M, Abelin N, Ezabella M, Toledo SPA, Brunner HG, Themmen APN. A homozygous mutation in the luteinizing hormone receptor causes partial Leydig cell hypoplasia: Correlation between receptor activity and phenotype. Molecular Endocrinology. 1998;12:775–784. doi: 10.1210/mend.12.6.0124. [DOI] [PubMed] [Google Scholar]
- Müller T, Gromoll J, Simoni M. Absence of exon 10 of the human luteinizing hormone (LH) receptor impairs LH, but not human chorionic gonadotropin action. J Clin Endocrinol Metab. 2003;88:2242–2249. doi: 10.1210/jc.2002-021946. [DOI] [PubMed] [Google Scholar]
- Piersma D, Berns EM, Verhoef-Post M, Uitterlinden AG, Braakman I, Pols HA, Themmen APN. A common polymorphism renders the luteinizing hormone receptor protein more active by improving signal peptide function and predicts adverse outcome in breast cancer patients. J Clin Endocrinol Metab. 2006;91:1470–1476. doi: 10.1210/jc.2005-2156. [DOI] [PubMed] [Google Scholar]
- Silveira LF, Stewart PM, Thomas M, Clark DA, Bouloux PM, MacColl GS. Novel homozygous splice acceptor site GnRH receptor (GnRHR) mutation: human GnRHR “knockout”. J Clin Endocrinol Metab. 2002;87:2973–2977. doi: 10.1210/jcem.87.6.8535. [DOI] [PubMed] [Google Scholar]
- Stenson PD, Ball EV, Mort M, Phillips AD, Shiel JA, Thomas NS, Abeysinghe S, Krawczak M, Cooper DN. Human Gene Mutation Database (HGMD): 2003 update. Hum Mutat. 2003;21:577–581. doi: 10.1002/humu.10212. [DOI] [PubMed] [Google Scholar]
- Themmen APN. An update of the pathophysiology of human gonadotrophin subunit and receptor gene mutations and polymorphisms. Reproduction. 2005;130:263–274. doi: 10.1530/rep.1.00663. [DOI] [PubMed] [Google Scholar]
- Themmen APN, Huhtaniemi IT. Mutations of gonadotropins and gonadotropin receptors: elucidating the physiology and pathophysiology of pituitary-gonadal function. Endocr Rev. 2000;21:551–583. doi: 10.1210/edrv.21.5.0409. [DOI] [PubMed] [Google Scholar]
- Thomas PM, Cote GJ, Wohllk N, Haddad B, Mathew PM, Rabl W, Aguilar-Bryan L, Gagel RF, Bryan J. Mutations in the sulfonylurea receptor gene in familial persistent hyperinsulinemic hypoglycemia of infancy. Science. 1995;268:426–429. doi: 10.1126/science.7716548. [DOI] [PubMed] [Google Scholar]
- Toledo SP, Brunner HG, Kraaij R, Post M, Dahia PL, Hayashida CY, Kremer HTAP. An inactivating mutation of the luteinizing hormone receptor causes amenorrhea in a 46,XX female. J Clin Endocrinol Metab. 1996;81:3850–3854. doi: 10.1210/jcem.81.11.8923827. [DOI] [PubMed] [Google Scholar]