Abstract
BACKGROUND
In this study, levels and rates of change in total testosterone (T), sex hormone-binding globulin (SHBG) and free androgen index (FAI) were related to chronological age and to the final menstrual period (FMP) as an indicator of ovarian aging.
METHODS
Data were annually acquired over a 15-year period in 629 women of the Michigan Bone Health and Metabolism Study cohort. Data were censored for hormone therapy use. Endogenous androgen patterns over time were described with stochastic processes and bootstrapping.
RESULTS
With ovarian aging, T levels rose from a mean of 18 ng/dl commencing 10 years prior to the FMP to 27 ng/dl at the FMP. Over the 20-year period encompassing the FMP, modeled mean SHBG levels changed from 58 to 34 nM and the FAI ratio increased from 1.6 to 2.9 in a non-linear manner. With chronological aging, total T levels increased (P < 0.0001) from 43 to 50 years, but not thereafter. SHBG declined steadily with age with a modestly greater rate of change between 49 and 54 years. The FAI increased from 1.3 to 2.5 from 34 to 58 years.
CONCLUSIONS
T increased from approximately age 40 until the FMP whereas SHBG had rate of change patterns reflecting both chronological and ovarian aging components. These data provide new insight into the endogenous androgen patterns at mid-life.
Keywords: reproductive senescence, hormone trajectories, total testosterone, androgens, ovarian aging
Introduction
The relative importance of testosterone (T) to women’s health, especially during the late reproductive years, the menopause transition, and the postmenopause has been a matter of speculation. Studies have variously suggested both positive and negative roles for androgens in women with respect to sexual functioning (Bancroft and Cawood, 1996; Davis and Tran, 2001), bone health (Steinberg et al., 1989; Slemenda et al., 1996), vasomotor symptoms (Sherwin and Gelfand, 1984), breast cancer (Secreto et al., 1991; Berrino et al., 1996) and cardiovascular health (Worboys et al., 2001), as reviewed by Morley and Perry (2003) and Labrie et al. (2003).
In women, there is controversy about the direction of circulating T levels across the life-span. Although some cross-sectional studies indicated a decline with age (Zumoff et al., 1995), endogenous T did not decline when linked to the menopause transition as a measure of ovarian aging and evaluated longitudinally in women aged 45–55 years (Burger et al., 2000). Considering only total T levels is inadequate for assessing the androgen environment because about 65% of T is carried in peripheral blood bound to sex hormone-binding globulin (SHBG), with approximately 1–2% of T free and the remaining T loosely bound to albumin. Because SHBG declines during the menopause transition, there is likely a positive change in the free androgen index (FAI) (Burger et al., 2000), reflecting an increase in biologically available androgen among women.
In this paper, we have described the levels and rates of change of total T, SHBG and FAI in relation to chronological age and to time around the FMP. The latter provides a framework for reproductive aging. We hypothesized: (1) There would be a decrease in T levels at the FMP as the loss of gonadotrophin-responsive follicles resulted in a decrease of the androgen-producing theca cells. (2) There would be a discernible difference in the rate of SHBG change at 2 years prior to the FMP as estradiol levels diminish (Sowers et al., 2008a) and do not stimulate SHBG synthesis in the liver at a rate comparable to that found in premenopausal women.
Materials and Methods
Study population and sample size
The Michigan Bone Health and Metabolism Study (MBHMS) is a population-based longitudinal natural history study of folliculogenesis and the sex steroid hormones in relation to the initiation of musculoskeletal and metabolic diseases and development of functional limitations (Sowers et al., 1992, 1999). MBHMS includes 664 age-eligible (24–44 years in 1992/1993) women identified from two sampling frames: (1) the family records of Tecumseh (Michigan) Community Health Study which evolved from a community census (1959–1985); and, (2) a 1992 Tecumseh community listing. This report includes data annually collected during the 15-year period from 1992/1993 through 2007/2008, excluding the 18- and 14-month lapses in funding in 1997 and 2003, respectively. During those months, neither data nor specimens were collected.
There were 629 women with 5932 androgen data points available for analyses to describe chronological aging. There were 181 women with a definitive FMP date (i.e. no ambiguity generated by exogenous hormone use or reproductive surgery) whose 1672 data points were analyzed for ovarian aging. The 27 data points 10 years after the FMP were truncated to ensure availability of data.
Blood was not drawn (thereby precluding hormone analyses) when participants were pregnant or breastfeeding. Over time, information from 2 to 7% of women was limited to interview data in any given year because participants were too ill for an in-person visit, were more than 2.5 h from the research clinic or refused phlebotomy (<0.5%). Data were censored at the time of death for 14 (2%) participants. Of the participants, 59% had 11 or 12 of the 12 possible visits with phlebotomy; 11% had 9–10 visits, 24% had 3–8 visits; and 6% of participants had 1–2 visits.
This study was approved by the University of Michigan Institutional Review Board and informed consent was obtained from all participants.
Measures of menopausal transition status
A woman was classified as premenopausal if she had no increase in menstrual irregularity in the previous year and at least nine menstrual cycles in a 12-month period. Perimenopause was defined as having menstrual irregularity and having less than nine menstrual cycles in a 12-month time period. Postmenopause was characterized as having at least 12 consecutive months of amenorrhea associated with no other medical cause. Final menstrual period (FMP) was defined retrospectively as that before 12 months of amenorrhea with no alternative physiologically normal explanation such pregnancy or lactation, consistent with the World Health Organization recommendation (WHO Scientific Group, 1994).
Menopause associated with chemotherapy and surgery was identified as such. Hysterectomy and oophorectomy procedures were verified by medical record abstraction. Hormone therapy and oral contraceptive (HT/OC) use was assessed at each visit and included preparation components and duration of use.
T and SHBG assays
Blood and urine specimens were collected from participants in a fasting state in days 2–7 of the follicular phase of the menstrual cycle. If women were post-menopausal or could not be linked to menses, specimens were collected on the anniversary of study enrollment ±15 days. Specimens were aliquoted and stored at −80°C without thawing until the assay.
Serum total T concentrations were determined by competitive binding of a 2-dimethylacridinuimester (DMAE)-labeled T-derivative to a rabbit polyclonal anti-T antibody premixed with monoclonal anti-rabbit IgG antibody immobilized on solid phase paramagnetic particles. Inter- and intra-assay coefficients of variation were 10.5 and 8.5%, respectively, with a lower limit of detection of 2 ng/dl (Sowers et al., 2001).
The de novo two-site chemiluminescent assay for serum SHBG concentrations involved competitive binding of DMAE-labeled SHBG to a commercially available rabbit anti-SHBG antibody and a solid phase of goat anti-rabbit IgG conjugated to paramagnetic particles. Inter- and intra-assay coefficients of variation for SHBG were 9.9 and 6.1%, respectively, and the lower limit of detection was 2 nM. Total T was indexed to SHBG to calculate the FAI (FAI = 100 total T/28.84 * SHBG).
Other measures
At each study visit, height (cm) and weight (kg) were measured with a stadiometer and balance-beam scale, respectively. Body mass index (BMI) was calculated by dividing the weight (kg) by height (m) squared. Women were interviewed about selected aspects of their personal behavior and reproductive history, including smoking practice, number of live births over 28 weeks of age and age at menarche.
Data analysis
Variable distributions were examined for normality, the presence of non-plausible outliers and changing variability over time. Univariate analyses were used to determine if transformations of outcome measures were necessary for satisfying model assumptions. Hormone values, including T, sex SHBG and FAI, were log transformed (natural) for statistical modeling but back-transformed when presenting results to facilitate ease of communication.
Data to assess the hormone associations with ovarian aging came from 181 women with a ‘clean’ FMP unobscured by HT use or gynecological surgery, although data to assess chronological aging included the entire cohort. By setting the FMP to be the time origin (t = 0), the maximum time span for the 181 individual women with FMP was 15 years but their data permits a 20-year time span coverage around the FMP. The data modeling processes allow making estimations. Data were censored at the time of initiation of HT (if any).
A multiple-step process, more fully described in Appendix 1, was used to organize hormone mean profile and rates of change into epochs related to ovarian aging characterized by the FMP and to chronological aging characterized by natural age. In the first step, non-parametric stochastic mixed modeling was used to estimate a cubic spline function as well as 95% confidence intervals (95% CIs) around the mean hormone values in relation to years around the FMP and chronological age (Zhang et al., 1998).
In the second step, to estimate the instantaneous rate of change which are the first- and second-order derivatives of log(Hormone), respectively, we solved differential equations associated with the cubic spline function. The curvature of the mean profile of log(Hormone) over time, representing the degree of bend in the line, was approximated by integrating both rate of change and acceleration/deceleration. The 95% confidence bands of these characteristics were obtained using a bootstrapping approach with 100 bootstrap samples (Efron and Tibshirani, 1986; Claeskens and Van Keilegom, 2003).
In the third step, data were organized into epochs by setting nodes for piecewise linear mixed models using inflection points defined by differentiating the cubic spline smoothing functions (Neter et al., 1985). This estimated the mean rate of change at each segment in relation to time to FMP and allowed statistical comparisons of slopes from two consecutive intervals around an inflection point to ascertain whether two adjacent slopes differed. The population mean and standard errors for log Hormone at turning time points were presented in back-transformed scale for clinical interpretation using the Delta method Taylor series approach.
All analyses included adjustment for BMI and its change over time. We also tested for effect modification by obesity status for the associations of T with ovarian and chronological aging. To do this, analyses were stratified to allow comparison of profiles of women according to BMI status and mean hormone (standard errors) values. We used generalized linear mixed models, including pairwise comparisons, to assess statistical differences in the group means. There was no statistical evidence of effect modification and so analyses findings are not reported. We also conducted post hoc analyses to determine if censoring the data upon diagnosis of diabetes would alter the findings. We identified that the findings were not altered.
Analyses were implemented in SAS version 9.1 and SAS macro language (SAS Institute, Cary, NC, USA), or Matlab7.0 (The MathWorks, Inc.).
Results
Table I depicts data from the initial and most recent examinations for the 629 woman of the total sample. At baseline, when they were aged 24–44 years, 75% of women were pre- or perimenopausa1 and menstrual status was not classified in 25% because of contraceptive use. Not surprisingly, the 181 women with a subsequent clean FMP were older [median age (interquartile range) is 41 (4)] at study initiation than women in the total sample [median age (interquartile range) is 38 (7); P-value for difference is <0.0001].
Table I.
MBHMS cohort characteristics (median, IQRa) or N (%) at the initial (1992/1993)b and most recent follow-up (2007/2008)c for the total sample and for women with an FMP
| Total sample |
Women with FMP |
|||
|---|---|---|---|---|
| Initial examb (n = 600) | Most recentc (n = 506) | Initial examb (n = 181) | Most recentc (n = 181) | |
| Age (years) | 38 (7) | 51 (9) | 41 (4) | 55 (5) |
| BMI (kg/m2) | 25.5 (7.5) | 28.5 (8.9) | 25.6 (7.3) | 29.4 (9.2) |
| Skeletal muscle mass (kg) | 20.1 (3.4) | 20.5 (3.8) | 20.0 (3.0) | 20.2 (3.4) |
| Testosterone (ng/ml) | 19.9 (16.4) | 32.7 (18.6) | 19.6 (15.1) | 37.5 (17.9) |
| SHBG | 65 (53) | 46 (33) | 58.7 (52) | 40.9 (26) |
| FAI (T/SHBG) | 1.84 (2.37) | 2.64 (2.48) | 1.68 (1.81) | 3.12 (2.27) |
| Obesity (%) | ||||
| <30 kg/cm2 | 76% | 59% | 74% | 53% |
| >30 kg/cm2 | 24% | 41% | 26% | 47% |
| Parity (%) | ||||
| Nulliparous | 18% | 13% | 13% | 12% |
| Parity = 1–2 | 51% | 53% | 49% | 50% |
| Parity >2 | 31% | 34% | 38% | 38% |
| Smoking status (%) | ||||
| Never | 57% | 54% | 54% | 50% |
| Former | 20% | 31% | 23% | 37% |
| Current | 23% | 15% | 23% | 13% |
| Menopausal status (%) | ||||
| Premenopause | 68% | 29% | 81% | – |
| Perimenopause | 7% | 11% | 5% | – |
| Postmenopause | 1% | 29% | 2% | 100% |
| Surgical menopause | 0% | 15% | – | – |
| Exogenous hormone used | 24% | 16% | 12% | – |
FAI: free androgen index; FMP: final menstrual period; HT: hormone therapy; IQR: inter-quartile range; MBHMS: Michigan Bone Health and Metabolism Study; OC: oral contraceptive; SHBG: sex hormone binding globulin; T: testosterone.
aIQR is interquartile range.
bInitial examination for more than 95% of cohort (n = 600/629) is 1992/1993 (excluding potential information from women who were pregnant or lactating).
cCurrent examination is 2007/2008 for the 80.4% of the cohort enrollees (n = 506/629) who are still alive, living within 2.5 h of the clinical site, and participating in the study.
dOC or HT use.
Ovarian aging in T, SHBG and FAI
Based on statistical models, total T levels rose from a mean value of 17.7 ng/dl (standard error [SE] = 0.88) to 31.5 ng/dl (SE = 2.27) in the 20-year study interval around the FMP, including an increase from a mean of 17.7 ng/dl commencing 10 years prior to the FMP to 27 ng/dl at the FMP. The total rise in the 20-year time span is 13.8 ng/dl (P < 0.0001) or an increase of 44% (Fig. 1A).
Figure 1.
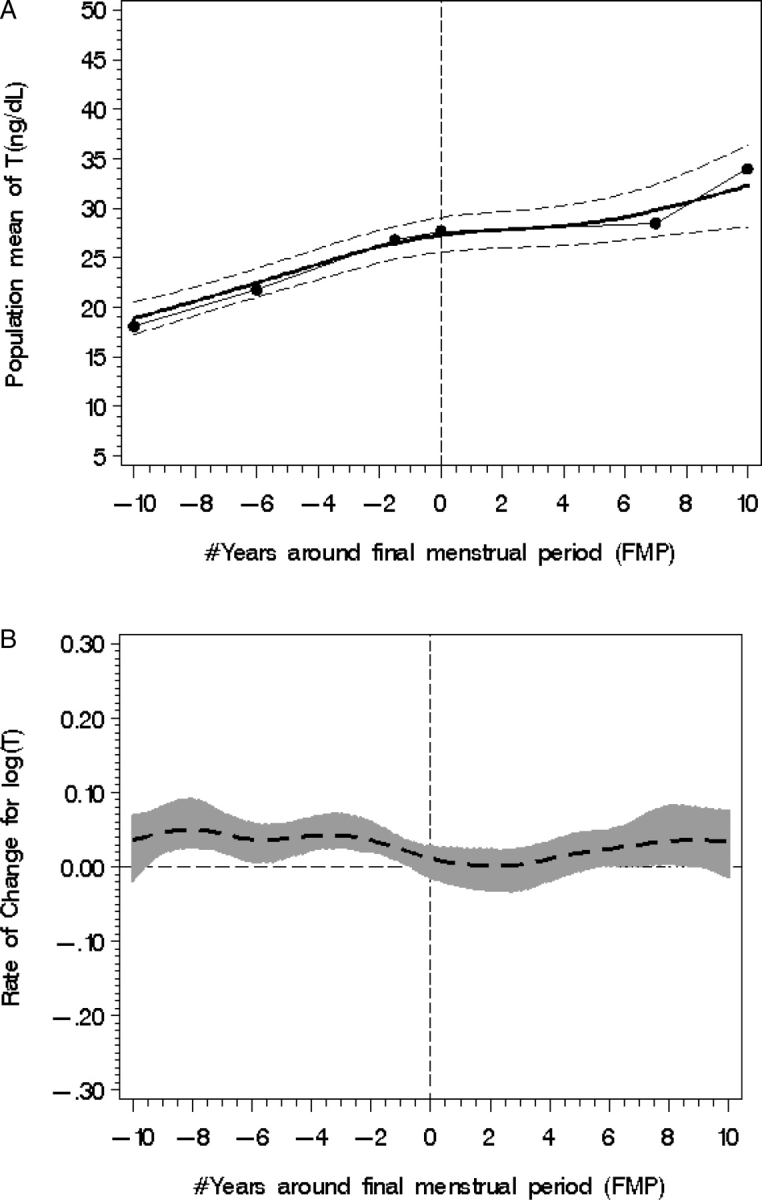
Population mean T (A) with fitted model (smooth line), piecewise line (dotted line) and 95% CI from 10 years before to 10 years after the FMP, designated with a zero year. (B) T rate of change (95% CI shown with shading); statistical significance occurs when the 95% CI does not overlap the line of no change (at −10 to −2 years before the FMP).
Statistical modeling revealed two time spans (Fig. 1B) when the log T rate of change increased significantly. The first occurred in the span from 10 to 1.5 years prior to the FMP [mean T rate of change (0.053, SE = 0.013, P < 0.0001)]. Around 7 years after the FMP, there was another statistically significant increase in log T with a mean rate of change of 0.063, SE = 0.03, P = 0.04.
In statistical models of ovarian aging, the mean SHBG level declined from 57.9 nM (SE = 4.35) to 34.2 nM (SE = 2.83), the later value being 23.7 nM (P < 0.0001) lower or 41% less than the initial value (Fig. 2A). In the years between 10 years prior to the FMP, SHBG decreased 14.3%, based on piecewise regression analyses. SHBG was about 31% lower 10 years after the FMP compared with the SHBG value at the FMP (P < 0.0001).
Figure 2.
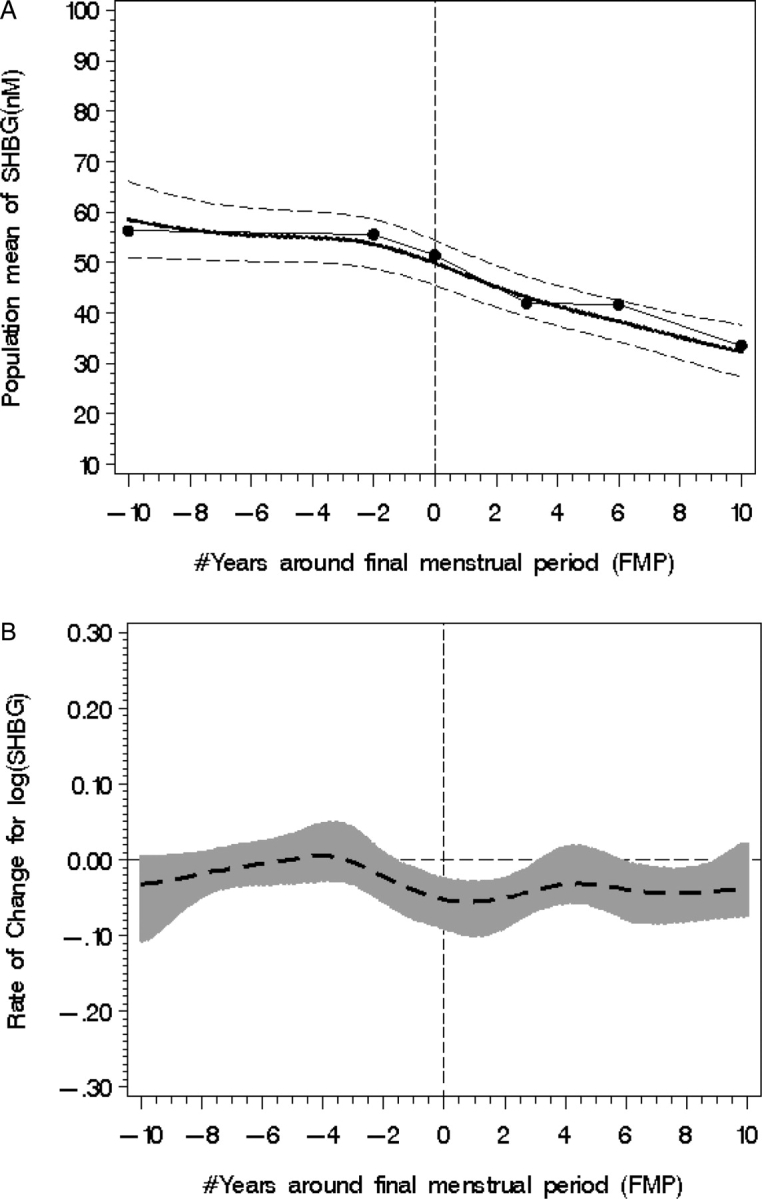
Population mean SHBG (A) with fitted model (smooth line), piecewise line (dotted line) and 95% CI from 10 years before to 10 years after the FMP, designated with zero year. (B) SHBG rate of change (95% CI shown with shading); statistical significance occurs when the 95% CI does not overlap with the line of no change (occurring between −2 years < FMP < +3 and >6 years FMP).
Modeling revealed two time spans (Fig. 2B) when the SHBG rate of change increased significantly. There was no statistically significant acceleration in the SHBG rate of change until 2 years prior to the FMP; then the SHBG rate of change increased [log SHBG mean rate of change 0.072 (SE = 0.025), P = 0.005]. There was a second acceleration in SHBG loss between 6.5 and 10 years after the FMP [log SHBG mean rate of change: −0.058 (SE = 0.027), P = 0.03]. Thus, SHBG levels progressively fell but with greater rapidity in the time periods around the FMP (−2 < FMP+3) and between 6.5 and 10 years after the FMP.
FAI increased from 1.58 (SE = 0.13) to 2.94 (SE = 0.28) over the 20-year period around the FMP in a non-linear manner (see Fig. 3A) with total rise of FAI 1.36 (P < 0.0001) or an 86% increase. log FAI did not change in the interval from 10 to 3 years prior to the FMP mean [log FAI rate of change: 0.0013 (SE = 0.012), P = 0.91]; however, log FAI significantly increased from 3 years prior to the FMP to the FMP [log FAI rate of change: 0.053 (SE = 0.023), P = 0.02] as shown in Fig. 3B and somewhat during the interval from the FMP to 2 years after the FMP [log FAI rate of change: 0.064 (SE = 0.038, P = 0.09)]. At 5.5 years after the FMP, log FAI increased again with a significant mean rate of change [0.083 (SE = 0.0275), P = 0.003]. Thus, the rates of FAI change increased in the time period around the FMP (−3 < FMP+2), equilibrated, and then began to increase again at approximately 6 years after the FMP. FAI had increased from 1.58 (SE = 0.13) to 1.88 (SE = 0.11), about 0.3 (P < 0.0001) higher or an increase of 16% in the interval from 10 years before the FMP to the FMP, and from 1.88 (SE = 0.11) to 2.94 (SE = 0.28), about1.06 higher (P < 0.0001) or an increase of 56% in the interval from the FMP to 10 years after the FMP.
Figure 3.
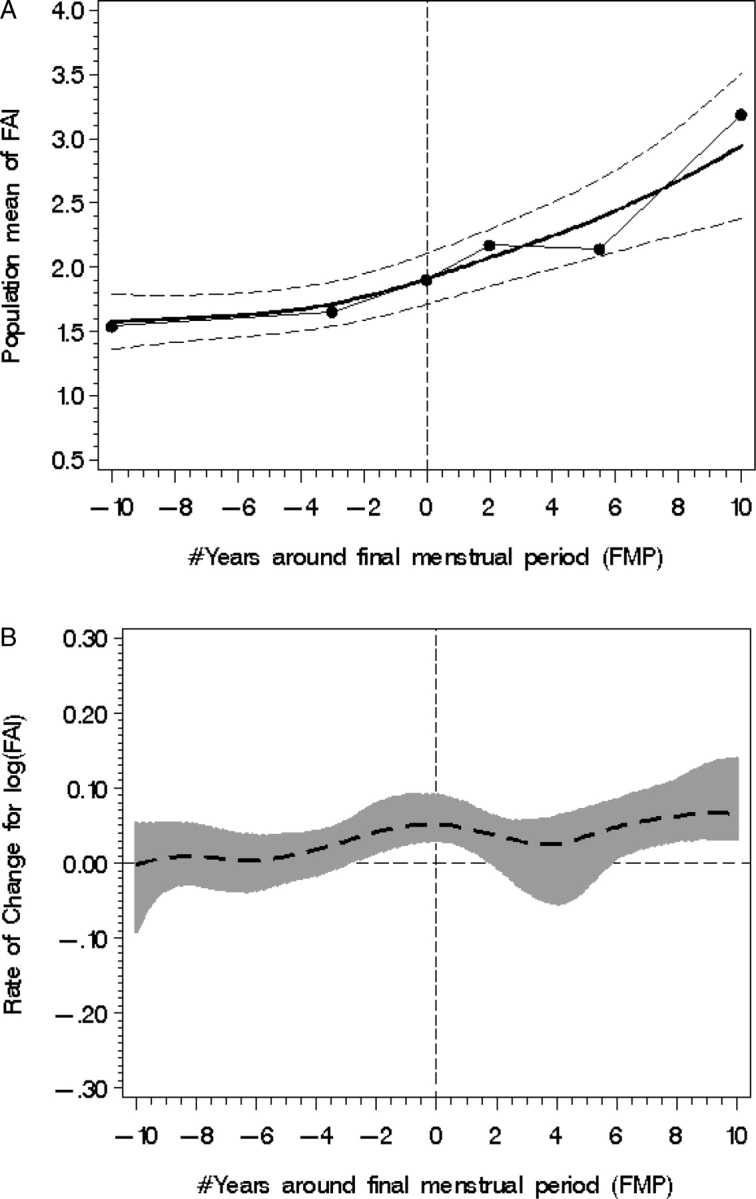
Population mean FAI (A) with fitted model (smooth line), piecewise line (dotted line) and 95% CI from 10 years before to 10 years after the FMP, designated with zero year. (B) FAI rate of change (95% CI shown with shading); statistical significance occurs when the 95% CI does not overlap with the line of no change (occurring between −3 years < FMP < +2 and >6 years FMP).
Chronological aging and the androgens
As seen in Fig. 4A, there was a modest increase in total T with age [an increase of 0.029 (SE = 0.003) per year, P < 0.0001]. A significant increase in the total log T rate of change occurred between the ages of 43 and 50 years (Fig. 4B).
Figure 4.
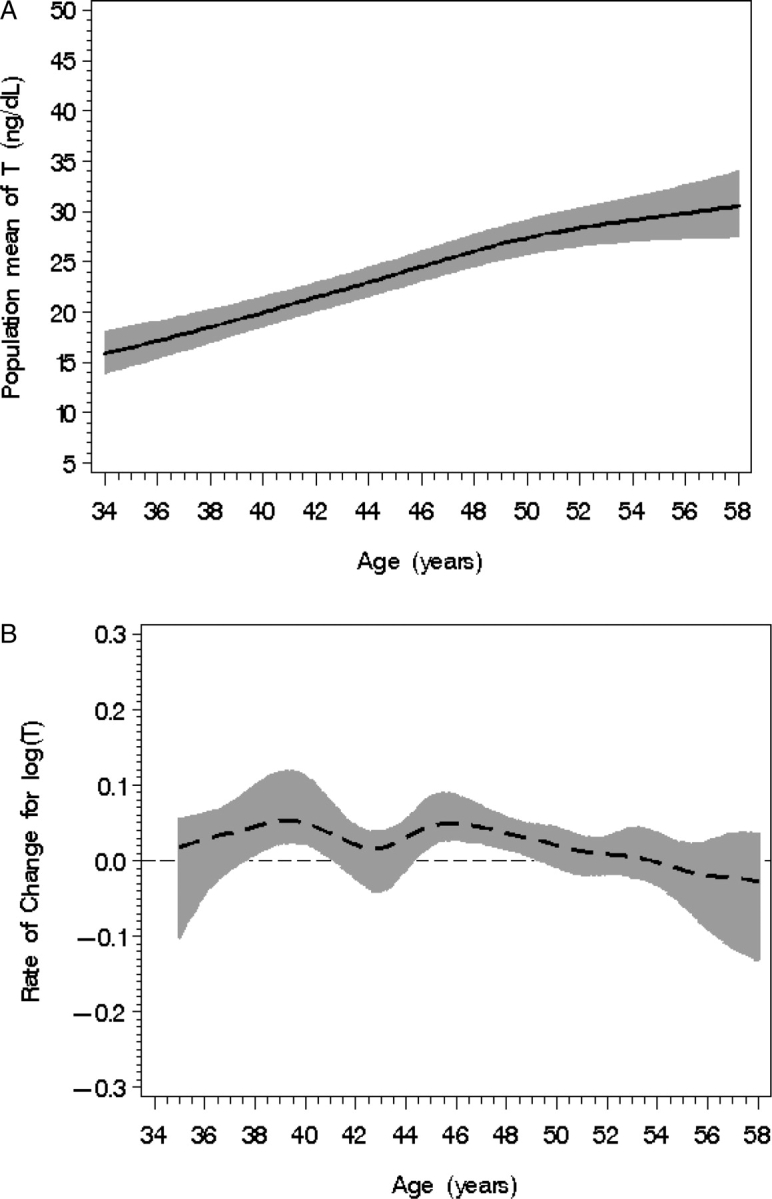
Population mean T level (A) according to chronological age with 95% confidence bands (shaded). (B) T rate of change with 95% CIs (shaded) according to age; statistical significance occurs when the 95% CI does not overlap with the line of no change (occurring between 43 and 50 years of age).
There was a steady, almost constant decline of SHBG with age [−0.031 (SE = 0.004)] as shown in Fig. 5A. There was a very modest increase in the log SHBG rate of change between the ages of 49 and 54 years (Fig. 5B).
Figure 5.
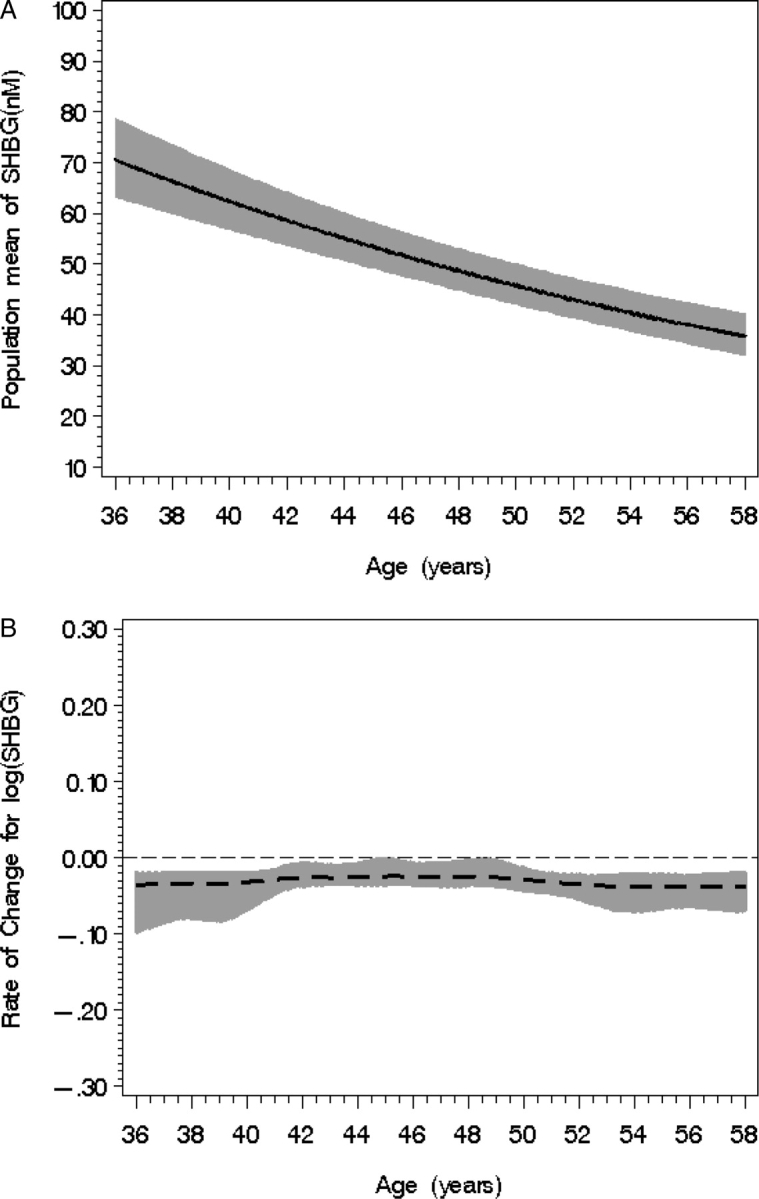
Population mean SHBG level (A) according to chronological age with 95% confidence bands (shaded). (B) SHBG rate of change with 95% CIs (shaded) according to age; statistical significance occurs when the 95% CI does not overlap with the line of no change (notably occurring between 49 and 54 years of age).
FAI increased from 1.3 to 2.5 over across the age span from 34 to 58 years (Fig. 6A). The highly irregular rates of change observed in the age ranges of 45–47, 49–50 and 53–54 (Fig. 6B) likely includes multiple ovarian aging events in addition to the chronological aging contribution.
Figure 6.
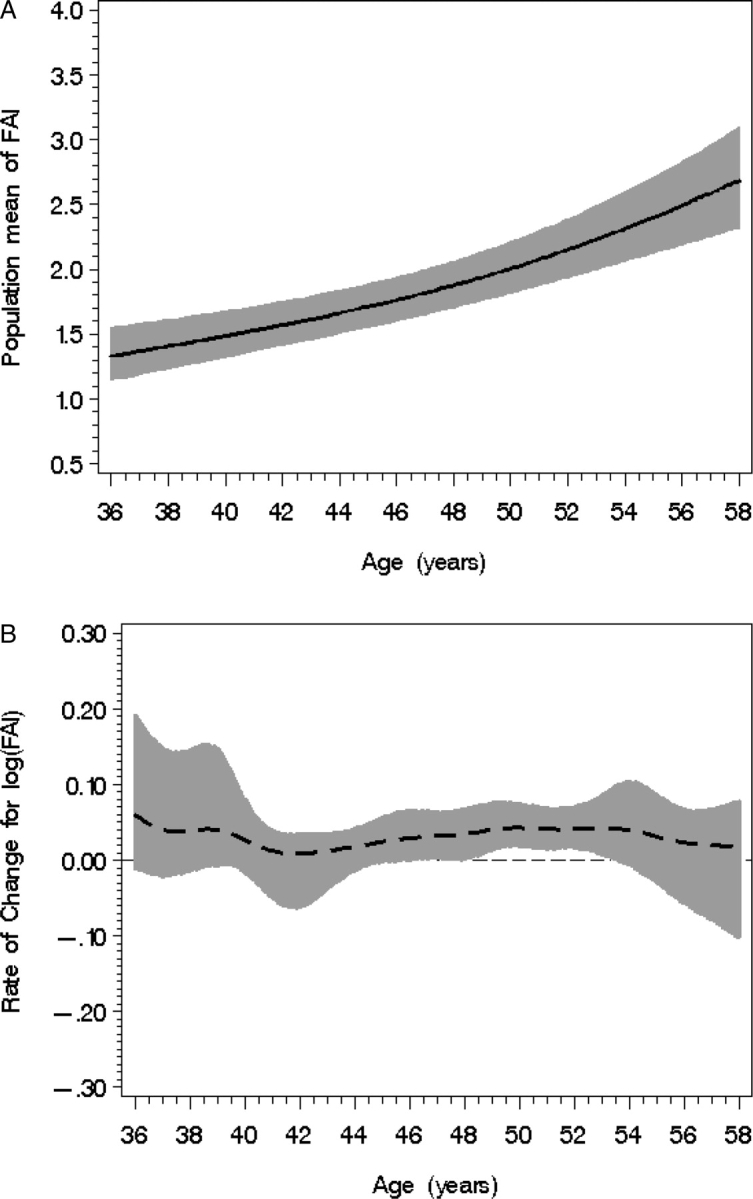
Population mean FAI level (A) according to chronological age with 95% confidence bands (shaded). (B) FAI rate of change with 95% CIs (shaded) according to age; statistical significance occurs when the 95% CI does not overlap with the line of no change.
The age-related patterns for T, SHBG and FAI were similar when comparing patterns of the 181 women with a clean FMP and the overall sample of 629 women.
Discussion
It is well-known that the endocrine environment in women after 55 years of age is relatively more androgenic. We identified that, in contrast to conventional wisdom, total T values in women from ages 38–50 years were increasing, not decreasing; nonetheless, values were still within expected ranges. Of further interest, the expected SHBG decline with age also appears to include a ‘menopause’ transition component. An increasingly lower SHBG level occurred in the 4-year period around the FMP; this may be accounted for by the plummeting estradiol levels and notably, this same pattern also appeared at the time when there is a secondary, recently identified, drop in estradiol (Sowers et al., 2008a). We also observed a rise in the FAI in relation to the FMP and a secondary increase in rate of change about 6–8 years after the FMP.
The literature includes mixed findings about the change in circulating androgens using the FMP as an index of ovarian aging. Three studies have undertaken the longitudinal approach of following women with multiple measures before and after the FMP (Longcope et al., 1986; Rannevik et al., 1986; Burger et al., 1995) with one of those studies appropriately fitting statistical models for longitudinal data (Burger et al., 2000). In one study, mean T levels in 172 women remained unchanged through the menopausal transition to the early postmenopause (Burger et al., 2000). If our sample were constrained to their age range and evaluated over the same time period, we would have reached a similar conclusion. However, our women had a broader age range and could be evaluated for time trends up to 10 years on either side of the FMP. Longcope et al. (1986) did not observe any change in T during 80 months following the FMP but noted that the mean concentrations of T in all their subjects, including those still having menses, were significantly less than values in normal young women sampled on days 5–7 of the cycle. Thus, the 30% rise in mean serum T levels over the 10 years prior to the FMP that we report has not been described previously, potentially because there is no comparable study with longitudinal observations over a long time-period in a large enough population with observed FMPs.
It is notable that most studies reporting a decline in T across age are based on a cross-sectional comparison of values from mid-aged women referenced to values identified in young adult women. For example, in a cross-sectional study, Zumoff et al. (1995) identified a steep decline in total serum T with age, such that levels in a woman aged 40 were approximately 50% of those in a woman aged 21 years. Closer examination of the published data indicates that potentially there was a decline among women in the age range of 20–30 years but not in participants in the age range from 30 to 50 years. Vermeulen (1976) showed that post-menopausal women aged 51–65 years had lower mean levels of T than women aged 18–25, not inconsistent with the observation of Zumoff et al. (1995).
Integrating our findings with those in the literature (Jiroutek et al., 1998; Laughin et al., 2000; Davison et al., 2005) suggests that T values over the reproductive life of women are potentially compatible with a U-shaped distribution. Though our study includes a more extended age range and longer observation period than most studies, we do not have sufficient data in the earliest reproductive years to describe the breadth of this U-shaped distribution. Likewise, we cannot extrapolate our findings into mature women more than 10 years following their FMP. Thus, additional work is needed to describe the natural history of T in women across adulthood.
The explanation as to why T levels actually begin rising during the perimenopause is speculative. However, a rise in circulating T during the perimenopausal is consistent with an evolving model of ovarian aging suggested in our observations of an accelerating rise in FSH (Sowers et al., 2008b) following a decline to undetectability of AMH and inhibin B levels (Sowers et al., 2008c) and sustained serum E2 levels until the final 2 years prior to the FMP (Sowers et al., 2008a). A declining number of gonadotrophin-responsive follicles could require a higher concentration of androgens from theca cells to help drive aromatization in the reduced number of granulosa cells, thereby increasing the androgen gradient across the basal lamina (Ryan and Petro, 1966). This rise in theca-cell-derived androgens would be driven by the rise in LH that accompanies the observed menopause transition rise in FSH and concordant our understanding that LH stimulates androgen secretion. This potential mechanism of serum LH and rising T levels in the late reproductive and perimenopausal periods remains to be studied as we did not measure LH in the current study.
Our study also suggests the importance of ovarian aging with androgens. We identified the expected age-related decline in SHBG; however, there were two time periods when there was a departure from the linear age-related decline in SHBG. One period occurred around the FMP and the second period occurred approximately 6–8 years after the FMP. We found that these periods of greater SHBG rates of change, though modest, were timed to concomitant declines in serum estradiol levels reported in this cohort (Sowers et al., 2008a). Although FAI patterns in the population also appeared to reflect some influence of hormone changes with the menopause transition, this was associated with the SHBG patterns and not the T patterns. Burger et al. (2000) also concluded that SHBG and FAI levels change at the time of menopause, at least partially due to the decline in E2. Their data was not of sufficient duration to include the 6–8 year estradiol decline we have reported (Sowers et al., 2008a).
SHBG, synthesized in the liver, not only provides transport for steroids in the blood, but also regulates hormone access to target tissues through varied degrees of binding affinity (Siiteri et al., 1982). Mean SHBG was more than 50% higher among the HT users compared with premenopausal or post-menopausal women (Sowers et al., 2008d), consistent with literature that has identified increases in SHBG levels following the administration of exogenous estrogens and during pregnancy (Anderson 1974; Mathur et al., 1985; Casson et al., 1997). Earlier research indicated that T increases with increasing body size (Sowers et al., 2001). Recognizing this, we evaluated BMI and its change as both a confounder and as an effect modifier. First, statistical models were adjusted for BMI to control for confounding. Then, we assessed whether BMI was an effect modifier in the associations of T to chronological or ovarian aging in obese women versus non-obese women. We found no evidence of effect modification.
There are a number of issues about the interpretation of T values in women being debated (Wierman et al., 2006). T is at its lowest concentration in the early follicular phase of the cycle, rising at the mid-cycle peak (Abraham, 1974). Sensitive assays indicate there is circadian variation with peak T levels in the early morning hours. Recognizing this, our specimens were collected in days 2–7 of the menstrual cycle when women were still menstruating. Further, specimens were collected fasted and in the morning before 11 a.m. by protocol. Although the adherence to this rigorous protocol enhances the likelihood of having optimal T levels for interpretation, the negative aspect is that the day 2–7 specimen poses an additional requirement on the assay to provide meaningful values in the lowest range. In correlation studies using gas chromatographic-mass spectrometry (GC-MS) (McConnell et al., 2007), there was good correlation between our assay and low values by GC-MS and excellent correlation in the 10–90 ng/dl range reported here. There is also a growing appreciation that the secretion and target tissue action for androgens may occur in the same tissue, with minimal involvement of the circulating peripheral distribution (Labrie et al., 2003). We have measured and are reporting values in the peripheral distribution where some degree of metabolism has already occurred and where selected local activity cannot be assessed. Finally, we do not have supporting measures of DHEA, DHEAS, or free T to relate to time and/or ovarian aging.
In summary, our data support a more complex androgen environment in women through the menopause transition such that after age 55 the environment may become more androgenic than has been previously described. We found increased, not decreased, total T values before the FMP compared with 10 years prior to the FMP. The FAI biomarker of bioavailable T had about an 80% increase from 10 years before the FMP to 10 years after the FMP. Although there was the expected age-related decline in SHBG, this decline appeared to include a ‘menopause’ transition component identifiable as a greater decline in the 4-year period around the FMP and a secondary decline about 6 years after the FMP, and both time frames were coincident with drops in the circulating estradiol levels. Understanding these patterns may help in interpreting the androgen environment observed in studies of women’s health.
Funding
Grants supporting the writing of this manuscript include AR051384, AR040888, AR20557 (Sowers, PI).
Appendix 1
In the analysis, hormone values, including T, SHBG and FAI, were log transformed (natural) to relate their change patterns to ovarian aging (characterized by FMP) and chronological aging (characterized by natural age). Analyses were implemented in Matlab7.0 (The MathWorks, Inc.), SAS version 9.1, SAS macro language and SAS/IML (SAS Institute, Cary, NC, USA).
Semi/non-parametric stochastic mixed model
Relationships between hormone simultaneous changes over time and FMP and chronological age could not be appropriately modeled by using quadratic or cubic terms; therefore, a semi-parametric stochastic mixed modeling was used. In general, the semi-parametric stochastic mixed model can be formulated by:
| A.1 |
where: tij is the time variable (ovarian age or chronological age) for subject i at jth measurement; β is a p × 1 vector of regression coefficients associated with covariates Xij; f(t) is a twice-differentiable smooth function of time; bi are independent q × 1 vectors of random effects associated with covariates Zij; Ui (tij) are independent random processes used to model serial correlation; εij are independent measurement errors.
The fundamental assumptions for this model are: bi, Ui (tij), and εij are mutually independent. bi ∼ normal (0, D(φ)), D is a positive definite matrix depending on a parameter vector φ; Ui (tij) is a mean zero Gaussian process with covariance function or a non-homogeneous Ornstein-Uhlenbeck (NOU) process, cov(Ui (t), Ui (s)) = γ(ζ, α; t, s) depending on a parameter vector ζ and a scalar α, which is used to characterize the variance and correlation of the process Ui (t); εij ∼ iid N (0, σ2).
More specifically, to capture the characteristics of hormone mean profile and variance varying over time, the modeling of hormone values (or rate of change) was formulated as:
| A.2 |
where Ui (t) is a non-homogeneous NOU process satisfying:
| A.3 |
| A.4 |
.
This assumed each woman’s serial correlation was the same. The smoothing function f(t) represents the mean profile of log(Hormone) for the population of women over time.
In this study, the potential covariates considered included BMI, smoking behavior and exogenous hormone users.
Change characteristics of mean profile log Hormone trajectory
Hormone trajectory changes over time following a non-linear pattern. The instantaneous changes of these trajectories (i.e. mean log Hormone profile) can be characterized by rate of change, acceleration/deceleration and curvature, where are first-, second-order derivatives of the mean curve, and the hinge/bend of the mean curve integrating the rate of change and acceleration, respectively. The cubic spline approach was used to estimate the rate of change as well as acceleration or deceleration.
Assume the time t was equally spaced with step h= tk+1 − tk (k = 1, 2, … , n−1), where n was the total number of distinguishable time points. Let f(t) be the log(Hormone) mean profile (trajectory) and S3 (t) be its cubic spline approximation. The rate of change can be approximated by solving ‘m’ equations:
| A.5 |
where mk is the cubic spline approximation to f′(tk) with errors O(h4), k = 2, 3, … ,n−1. The m1 and mn can be given or computed by suitable forward and backward finite difference formulas, respectively, e.g. using first 5-points and last 5-points
| A.6 |
| A.7 |
.
The acceleration/deceleration can be approximated by solving ‘M ’ equations:
| A.8 |
where Mk is the second derivative approximations f′′(tk) with O(h2) errors, k = 2, 3, … , n−1. The M1 and Mn satisfied the boundary conditions 2M1+ M2 = (6/h) ((f(t2) − f(t1)/h) − m1) and Mn−1+ 2Mn = (6/h)(mn − (f(tn) − f(tn−1)/h)).
The curvature of mean profile of log Hormone over time represents the degree of bend and is approximated by integrating both rate of change and acceleration/deceleration 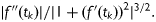
The 95% confidence bands of these characteristics were obtained using bootstrapping approach with 100 bootstrap samples by replicating the above processes (2,3).
Piecewise linear mixed model related time (ovarian aging and chronological aging)
The nodes (or turning points) of the population log Hormone profile were identified based on the changing characteristics obtained by the above processes and they were further used to segment the hormone trajectory into stages. Piecewise linear mixed model was developed to capture these segmented characteristics (i.e. rate of change at each segment). Statistical comparisons of these slopes from two consecutive intervals around a turning point were tested to ascertain if one slope was different than the adjacent slope. The piece wise linear mixed model was formulated as follows.
Assume the independent time variable of interest t ∈ T ⊂ R1 (e.g. the time to FMP, or chronological age in this study). Let 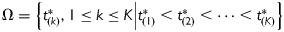 be one known division of T, where K is the total number of turning points used to split T into (K+1) non-overlapped intervals. The mean structure of piecewise linear mixed effect model was given by:
be one known division of T, where K is the total number of turning points used to split T into (K+1) non-overlapped intervals. The mean structure of piecewise linear mixed effect model was given by:
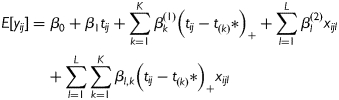 |
A.9 |
where 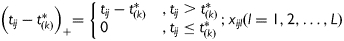 are the covariates of interest. If there are no other covariates (e.g. pure ‘time’ effect is of interest) then βl(2) and βl,k can be dropped from the model. The random effects will be appropriately specified, e.g. random intercept and random slopes. The variance–covariance structure and model assumptions follow general linear mixed models.
are the covariates of interest. If there are no other covariates (e.g. pure ‘time’ effect is of interest) then βl(2) and βl,k can be dropped from the model. The random effects will be appropriately specified, e.g. random intercept and random slopes. The variance–covariance structure and model assumptions follow general linear mixed models.
References
- Abraham GE. Ovarian and adrenal contribution to peripheral androgens during the menstrual cycle. J Clin Endocrinol Metab. 1974;39:340–346. doi: 10.1210/jcem-39-2-340. [DOI] [PubMed] [Google Scholar]
- Anderson DC. Sex hormone binding globulin. Clin Endocrinol. 1974;3:69–96. doi: 10.1111/j.1365-2265.1974.tb03298.x. [DOI] [PubMed] [Google Scholar]
- Bancroft J, Cawood EH. Androgens and the menopause; a study of 40–60-year-old women. Clin Endocrinol. 1996;45:577–587. doi: 10.1046/j.1365-2265.1996.00846.x. [DOI] [PubMed] [Google Scholar]
- Berrino F, Muti P, Micheli A, Bolelli G, Krogh V, Sciajno R, Pisani P, Panico S, Secreto G. Serum sex hormone levels after menopause and subsequent breast cancer. J Natl Cancer Inst. 1996;88:291–296. doi: 10.1093/jnci/88.5.291. [DOI] [PubMed] [Google Scholar]
- Burger HG, Dudley EC, Hopper JL, Shelley JM, Green A, Smith A, Dennerstein L, Morse C. The endocrinology of the menopausal transition: a cross-sectional study of a population-based sample. J Clin Endocrinol Metab. 1995;80:3537–3545. doi: 10.1210/jcem.80.12.8530596. [DOI] [PubMed] [Google Scholar]
- Burger HG, Dudley EC, Cui J, Dennerstein L, Hopper JL. A prospective longitudinal study of serum testosterone, dehydroepiandrosterone sulfate, and sex hormone-binding globulin levels through the menopause transition. J Clin Endocrinol Metab. 2000;85:2832–2838. doi: 10.1210/jcem.85.8.6740. [DOI] [PubMed] [Google Scholar]
- Casson PR, Elkind-Hirsch KE, Buster JE, Hornsby PJ, Carson SA, Snakes MC. Effect of postmenopausal estrogen replacement on circulating androgens. Obstet Gynecol. 1997;90:995–998. doi: 10.1016/s0029-7844(97)00538-3. [DOI] [PubMed] [Google Scholar]
- Claeskens G, Van Keilegom I. Bootstrap confidence bands for regression curves and their derivatives. Ann Stat. 2003;31:1852–1884. [Google Scholar]
- Davis SR, Tran J. Testosterone influences libido and well being in women. Trends Endocrinol Metab. 2001;12:33–37. doi: 10.1016/s1043-2760(00)00333-7. [DOI] [PubMed] [Google Scholar]
- Davison SL, Bell R, Donath S, Montalto JG, Davis SR. Androgen levels in adult females: changes with age, menopause, and oophorectomy. J Clin Endocrinol Metab. 2005;90:3847–3853. doi: 10.1210/jc.2005-0212. [DOI] [PubMed] [Google Scholar]
- Efron B, Tibshirani R. Bootstrap methods for standard errors, confidence intervals, and other measures of statistical accuracy. Stat Sci. 1986;1:54–77. [Google Scholar]
- Jiroutek MR, Chen MH, Johnston CC, Longcope C. Changes in reproductive hormones and sex hormone-binding globulin in a group of postmenopausal women measured over 10 years. Menopause. 1998;5:90–94. [PubMed] [Google Scholar]
- Labrie F, Luu-the V, Labrie C, Belanger A, Simard J, Lin SX, Pelletier G. Endocrine and intracrine sources of androgens in women: inhibition of breast cancer and other roles of androgens and their precursor dehydroepiandrosterone. Endocr Rev. 2003;24:152–182. doi: 10.1210/er.2001-0031. [DOI] [PubMed] [Google Scholar]
- Laughin GA, Barrett-Connor E, Kritz-Silverstein D, von Muhlen D. Hysterectomy, oophorectomy, and endogenous sex hormone levels in older women: the Rancho Bernardo Study. J Clin Endocrinol Metab. 2000;85:645–651. doi: 10.1210/jcem.85.2.6405. [DOI] [PubMed] [Google Scholar]
- Longcope C, Franz C, Morello C, Baker R, Johnston CC., Jr Steroid and gonadotropin levels in women during the peri-menopausal years. Maturitas. 1986;8:189–196. doi: 10.1016/0378-5122(86)90025-3. [DOI] [PubMed] [Google Scholar]
- Mathur RS, Landgrebe SC, Moody LO, Semmens JP, Williamson HO. The effect of estrogen treatment on plasma concentrations of steroid hormones, gondadotropins, prolactin and sex hormone-binding globulin in post-menopausal women. Maturitas. 1985;7:129–133. doi: 10.1016/0378-5122(85)90018-0. [DOI] [PubMed] [Google Scholar]
- McConnell D, Sowers M, Chen J, Lasley B. Assessment of circulating androgens in mid-aged women: direct immunoassay for testosterone correlates with GCMS and RIA. 89th Annual Meeting of the Endocrine Society; 2007; Toronto, Canada. Abstract 852253. [Google Scholar]
- Morley JE, Perry HM., III Androgens and women at the menopause and beyond. J Gerontol A Biol Sci Med Sci. 2003;58A:409–416. doi: 10.1093/gerona/58.5.m409. [DOI] [PubMed] [Google Scholar]
- Neter J, Wasserman W, Kutner M. Applied Linear Statistical Models. 2nd edn. Homewood, IL: Irwin; 1985. [Google Scholar]
- Rannevik G, Carlstrom K, Jeppsson S, Bjerre B, Svanberg L. A prospective long-term study in women from pre-menopause to post-menopause: changing profiles of gonadotrophins, oestrogens and androgens. Maturitas. 1986;8:297–307. doi: 10.1016/0378-5122(86)90038-1. [DOI] [PubMed] [Google Scholar]
- Ryan KJ, Petro Z. Steroid biosynthesis by human ovarian granulosa and theca cells. J Clin Endocrinol Metab. 1966;26:46–52. doi: 10.1210/jcem-26-1-46. [DOI] [PubMed] [Google Scholar]
- Secreto G, Toniolo P, Berrino F, Recchione C, Cavalleri A, Pisani P, Toris A, Fariselli G, Di Pietro S. Serum and urinary androgens and risk of breast cancer in postmenopausal women. Cancer Res. 1991;51:2572–2576. [PubMed] [Google Scholar]
- Sherwin BB, Gelfand MM. Effects of parenteral administration of estrogen and androgen on plasma hormone levels and hot flashes in the surgical menopause. Am J Obstet Gynecol. 1984;148:552–557. doi: 10.1016/0002-9378(84)90746-4. [DOI] [PubMed] [Google Scholar]
- Siiteri PK, Murai JT, Hammond GL, Nisker JA, Raymoure WJ, Kuhn RW. The serum transport of steroid hormones. Recent Prog Horm Res. 1982;38:457–510. doi: 10.1016/b978-0-12-571138-8.50016-0. [DOI] [PubMed] [Google Scholar]
- Slemenda C, Longcope C, Peacock M, Hui S, Johnston CC. Sex steroids, bone mass, and bone loss. A prospective study of pre-, peri-, and postmenopausal women. J Clin Invest. 1996;97:14–21. doi: 10.1172/JCI118382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowers MF, Kshirsagar A, Crutchfield MM, Updike S. Joint influence of fat and lean body composition compartments on femoral bone mineral density in premenopausal women. Am J Epidemiol. 1992;136:257–265. doi: 10.1093/oxfordjournals.aje.a116491. [DOI] [PubMed] [Google Scholar]
- Sowers M, Willing M, Burns T, Deschenes S, Hollis B, Crutchfield M, Jannausch M. Genetic markers, bone mineral density, and serum osteocalcin levels. J Bone Miner Res. 1999;14:1411–1419. doi: 10.1359/jbmr.1999.14.8.1411. [DOI] [PubMed] [Google Scholar]
- Sowers MF, Beebe JL, McConnell D, Randolph J, Jannausch M. Testosterone concentrations in women, aged 25–50 years: associations with lifestyle, body composition and ovarian status. Am J Epidemiol. 2001;153:256–264. doi: 10.1093/aje/153.3.256. [DOI] [PubMed] [Google Scholar]
- Sowers MR, Zheng H, McConnell D, Nan B, Harlow SD, Randolph JF., Jr Estradiol rates of change in relation to the final menstrual period in a population-based cohort of women. J Clin Endocrinol Metab. 2008a;93:3847–3852. doi: 10.1210/jc.2008-1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowers MR, Zheng H, McConnell D, Nan B, Harlow SD, Randolph JF., Jr Follicle stimulating hormone and its rate of change in defining menopause transition stages. J Clin Endocrinol Metab. 2008b;93:3958–3964. doi: 10.1210/jc.2008-0482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowers MR, Eyvazzadeh AD, McConnell D, Yosef M, Jannausch ML, Zhang D, Harlow S, Randolph JF., Jr Anti-mullerian hormone and inhibin B in the definition of ovarian aging and the menopause transition. J Clin Endocrinol Metab. 2008c;93:3478–3483. doi: 10.1210/jc.2008-0567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowers MR, Randolph J, Jr, Jannausch M, Lasley B, Jackson E, McConnell D. Levels of sex steroid and cardiovascular disease measures in premenopausal and hormone-treated women at mid-life: implications for the “timing hypothesis”. Arch Intern Med. 2008d;168:2146–2153. doi: 10.1001/archinte.168.19.2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg KK, Freni-Titulaer LW, DePuey EG, Miller DT, Sgoutas DS, Coralli CH, Phillips DL, Rogers TN, Clark RV. Sex steroids and bone density in premenopausal and perimenopausal women. J Clin Endocrinol Metab. 1989;69:533–539. doi: 10.1210/jcem-69-3-533. [DOI] [PubMed] [Google Scholar]
- Vermeulen A. The hormonal activity of the postmenopausal ovary. J Clin Endocrinol Metab. 1976;42:247–253. doi: 10.1210/jcem-42-2-247. [DOI] [PubMed] [Google Scholar]
- WHO Scientific Group on Research on the Menopause in the 1990's. Geneva, Switzerland: 1994. [Google Scholar]
- Wierman ME, Basson R, Davis SR, Khosla S, Miller KK, Rosner W, Santoro N. Androgen therapy in women: an Endocrine Society Clinical Practice guideline. J Clin Endocrinol Metab. 2006;91:3697–3710. doi: 10.1210/jc.2006-1121. [DOI] [PubMed] [Google Scholar]
- Worboys S, Kotsopoulos D, Teede H, McGrath B, Davis SR. Evidence that parenteral testosterone therapy may improve endothelium-dependent and –independent vasodilation in postmenopausal women already receiving estrogen. J Clin Endocrinol Metab. 2001;86:158–161. doi: 10.1210/jcem.86.1.7103. [DOI] [PubMed] [Google Scholar]
- Zhang D, Lin X, Sowers M. Semiparametric stochastic mixed models for longitudinal data. J Am Stat Assoc. 1998;93:710–719. [Google Scholar]
- Zumoff B, Strain GW, Miller LK, Rosner W. Twenty-four hour mean plasma testosterone concentration declines with age in normal premenopausal women. J Clin Endocrinol Metab. 1995;80:1429–1430. doi: 10.1210/jcem.80.4.7714119. [DOI] [PubMed] [Google Scholar]


