Abstract
An acute brain insult such as traumatic head/brain injury, stroke, or an episode of status epilepticus can trigger epileptogenesis, which, after a latent, seizure-free period, leads to epilepsy. The discovery of effective pharmacological interventions that can prevent the development of epilepsy requires knowledge of the alterations that occur during epileptogenesis in brain regions that play a central role in the induction and expression of epilepsy. In the present study, we investigated pathological alterations in GABAergic interneurons in the rat basolateral amygdala (BLA), and the functional impact of these alterations on inhibitory synaptic transmission, on days 7 to 10 after SE induced by kainic acid. Using design-based stereology combined with GAD67 immunohistochemistry, we found a more extensive loss of GABAergic interneurons compared to the loss of principal cells. Fluoro-Jade C staining showed that neuronal degeneration was still ongoing. These alterations were accompanied by an increase in the levels of glutamate decarboxylase and the α1 subunit of the GABAA receptor, and a reduction in the GluK1 (previously known as GluR5) subunit, as determined by Western blots. Whole-cell recordings from BLA pyramidal neurons showed a significant reduction in the frequency and amplitude of action potential-dependent spontaneous IPSCs, a reduced frequency but not amplitude of miniature IPSCs, and impairment in the modulation of IPSCs via GluK1-containing kainate receptors (GluK1Rs). Thus, in the BLA, GABAergic interneurons are more vulnerable to seizure-induced damage than principal cells. Surviving interneurons increase their expression of glutamate decarboxylase and the α1 GABAA receptor subunit, but this does not compensate for the interneuronal loss; the result is a dramatic reduction of tonic inhibition in the BLA circuitry. As activation of GluK1Rs by ambient levels of glutamate facilitates GABA release, the reduced level and function of these receptors may contribute to the reduction of tonic inhibitory activity. These alterations at a relatively early stage of epileptogenesis may facilitate the progress towards the development of epilepsy.
Keywords: basolateral amygdala, interneurons, epileptogenesis, status epilepticus, kainate receptors, inhibitory synaptic transmission, glutamate decarboxylase, GluR5 subunit, GluK1 subunit, GABAA receptor subunits
The epilepsies are episodic neurological disorders characterized by the occurrence of recurrent seizures. In a significant proportion of patients, epilepsy follows an acute brain insult such as traumatic brain injury, stroke, or a period of prolonged and intense seizures (status epilepticus; SE). When an acute brain insult is the etiological factor, the symptoms of epilepsy often appear after a seizure-free latent period following the acute injury (Annegers et al., 1980; Salazar et al., 1985; Angeleri et al., 1999; Statler et al., 2006). During this seizure-free period, which can vary from months to years (French et al., 1993; Mathern et al., 1995; Treib et al., 1996), neuronal networks in certain brain regions undergo structural and functional changes that lead to hyperexcitability and eventually to the expression of spontaneous seizures; this process is referred to as epileptogenesis.
The latent period of epileptogenesis offers the opportunity for therapeutic intervention that may prevent the development of epilepsy, or reduce the severity of the developing disease. However, the development of effective pharmacological treatments that will inhibit epileptogenesis requires knowledge of the alterations that occur in neuronal networks during epileptogenesis, which will eventually be responsible for the expression of epilepsy. Two brain structures that undergo such alterations and are well known to play a central role in the development and expression of epilepsy are the hippocampus and the amygdala. The hippocampus and/or the amygdala are the brain regions that harbor the epileptic focus and drive the expression of the most common type of epilepsy, the temporal lobe epilepsy (TLE) (Quesney, 1986; Goldring et al., 1992; Gotman and Levtova, 1996; Bragin et al., 2005; Bragin et al., 2007). TLE commonly develops after an initial precipitating brain injury, such as febrile seizures or head trauma (French et al., 1993). In an effort to understand the epileptogenic process that leads to TLE, a number of studies have focused on alterations occurring in the hippocampus after an acute brain insult in animal models. One of the hallmarks of such alterations is loss of GABAergic interneurons (Sloviter, 1987; Lowenstein et al., 1992; Best et al., 1993; Obenhaus et al., 1993; Houser and Esclapez, 1996; Morin et al., 1998; Sun et al., 2007). Most often this is accompanied by reduced inhibitory activity (Rice et al., 1996; Hirsch et al., 1999; Cossart et al., 2001; Kobayashi and Buckmaster, 2003; Shao and Dudek, 2005; Sun et al., 2007), but unaltered or enhanced inhibition has also been observed in some areas of the epileptic hippocampus (Gibbs et al., 1997; Nusser et al., 1998; Cossart et al., 2001; Shao and Dudek, 2005), as mechanisms come into play that attempt to compensate for the loss of interneurons.
Although the amygdala also plays a central role in epilepsy, and it actually appears to have a higher propensity for generation of seizures compared to the hippocampus (Goddard, 1967; Goddard et al., 1969; Kairiss et al., 1984; Racine et al., 1988) there is limited knowledge of the alterations occurring in the amygdala during the course of epileptogenesis (for a review see Aroniadou-Anderjaska et al., 2008). Neuronal damage and, particularly, loss of GABAergic neurons have been demonstrated in the amygdala after prolonged SE (Tunnanen et al., 1996). Whether or not there are other alterations compensating for the loss of interneurons, and what the net impact is on the excitability of the amygdala during the process of epileptogenesis is not clear. In addition, it is important to note that the changes occurring in certain brain circuits after an initial insult that triggers epileptogenesis do not seem to follow a linear progression towards reduced inhibition and enhanced excitation (see Straessle et al., 2003; Sloviter et al., 2006; El-Hassar et al., 2007; Rocha et al., 2007). For example, in the CA1 hippocampal area, inhibitory activity has been found to be enhanced or reduced at different stages of epileptogenesis (El-Hassar et al., 2007). Therefore, the alterations that are found at a particular time point/period of epileptogenesis are not necessarily the same –changing only quantitatively– throughout epileptogenesis.
In the present study, we investigated pathological and pathophysiological alterations of the GABAergic system in the amygdala of young adult rats, at 7 to 10 days after SE induced by kainic acid (KA). We focused on the basolateral nucleus of the amygdala (BLA), which, from all the amygdala nuclei, plays the most important role in the initiation and spread of seizures (White and Price, 1993a, 1993b; Mohapel et al., 1996). We determined the extent of neuronal and interneuronal loss, as well as alterations in the levels of glutamate decarboxylase (GAD), the enzyme that catalyzes GABA synthesis, and the α1 subunit of the GABAA receptor (GABAA α1), which is important for the sedative and anticonvulsant actions of benzodiazepines (Rudolph et al., 1999; Crestani et al., 2000, Da Settimo et al., 2007). In addition, we examined alterations in the level and function of the kainate receptors that contain the GluK1 subunit (GluK1Rs; the GluK1 subunit was until recently referred to as GluR5 kainate receptor subunit), and the overall, net impact of these alterations on tonic inhibitory activity in the BLA circuitry. We were interested in alterations of GluK1R expression and function because these receptors are highly expressed in the amygdala (Bettler et al., 1990; Li et al., 2001; Braga et al., 2003), and are present on both principal cells (Gryder and Rogawski, 2003) and GABAergic interneurons in the BLA, modulating inhibitory synaptic transmission and neuronal excitability (Braga et al., 2003, 2004; Aroniadou-Anderjaska et al., 2007). In addition, there is evidence that GluK1Rs are significantly involved in epilepsy (Smolders et al., 2002; Rogawski et al., 2003; Kaminski et al., 2004), and there are alterations in both the function of GluK1Rs (Palma et al., 2002) and the expression of the GluK1 subunit in TLE patients (Mathern et al., 1998; Kortenbruck et al., 2001) and epileptic rats (Ullal et al., 2005). We found that (1) there is a significant loss of BLA interneurons, which are more vulnerable than principal cells to SE-induced damage, (2) GluK1R receptor level and function are reduced, and (3) the result of these alterations is a dramatic reduction in tonic inhibition, despite that GAD and GABAA α1 levels are increased.
Experimental Procedures
Animals
Experiments were performed on male Sprague-Dawley rats (Taconic Farms, Rockville, MD), 5–6 weeks old, weighing 170–200 g at the start of the experiments. Animals were individually housed in an animal facility approved by the Association for Assessment and Accreditation of Laboratory Animal Care, in an environmentally controlled room (20–23 °C, 12-h light/12-h dark cycle, lights on 07:00 a.m.), with food and water available ad libitum. All animal use procedures were in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals, and were approved by the Animal Care and Use Committees of the National Institute of Neurological Disorders and Stroke and Uniformed Services University of the Health Sciences.
Surgery
Following 5 days of acclimatization, the rats were stereotaxically implanted with 5 cortical stainless steel screw electrodes under general anesthesia [ketamine 60 mg/kg intraperitonally (i.p.) and medetomidine 0.5 mg/kg i.p.], using the following coordinates, in mm, after Paxinos and Watson (1998): Two frontal electrodes AP = +1.5, ML = ±2.5 from bregma; two parietal electrodes AP = −5.0, ML = ±4 from bregma; and a single cerebellar reference electrode midline, AP = −1.0 from lambda. Each screw electrode with socket (E363/20; Plastics ONE Inc., Roanoke, VA) was placed in a plastic pedestal (MS363; Plastics ONE Inc., Roanoke, VA) and attached to the skull with dental acrylic cement. Anesthesia was reversed by i.p. injection of 1 mg/kg atipamezol.
Induction of status epilepticus
After 1 week of recovery, 25 rats were injected (i.p.) with KA to induce SE (“KA-SE rats”). Fifteen implanted rats were injected with corresponding amounts of saline at corresponding times as the KA-treated rats; this group of rats served as controls (“sham control rats”). SE was induced following a modified titration protocol after Hellier et al. (1998). In order to maximize consistency of the SE expression between rats, EEG seizure activity rather than the behavioral seizure correlate was used to determine the necessity of additional KA injections. Rats were initially treated with 7.5 mg/kg KA dissolved in 0.1 M phosphate buffered normal saline (PBS; 5mg/ml), followed by additional 5 mg/kg doses every 60 min, until SE began, defined by 5 min of continuous generalized electrographic seizure activity (all four cortical electrodes involved; no breaks longer than 10 sec). Following this protocol, rats received between 12.5 and 32.5 mg/kg KA. SE was allowed to continue without intervention for 180 min, and then 25–30 mg/kg of diazepam was injected i.p. for termination of SE. Fluid was substituted with 6 ml lactated Ringer’s solution injected subcutaneously (s.c.) after resolution of continuous convulsive seizures, usually less than 10 min after the diazepam injection. The sham control rats also received diazepam injections. For three days after SE, rats were offered fruits and soft chow. Additional lactated Ringer’s solution was administered by s.c. injection if dehydration was apparent. The wooden bedding was changed to iso-PADs™ (Harlan Teklad, Madison, WI) to avoid aspiration in case of frequent seizures.
EEG recordings and analysis
Recordings were performed in freely moving rats using a commercially available Stellate® EEG monitoring system modified for the use in rodents (Respitech Medical Inc., Lancaster, PA; sampling rate, 200 Hz). EEG recordings were visually analyzed offline with filter settings set to 0.3 Hz low frequency filter, 60 Hz notch filter, and 70 Hz high frequency filter, using the Harmonie Viewer 6.1c from Stellate® (Montreal, Quebec, Canada). An electrographic seizure was defined as a period of EEG changes marked by an abrupt starting and ending, that included a minimum of 10 sec of consistent, repetitive discharges at least double the amplitude of the background activity and not less than 1 Hz frequency. A single graphoelement was classified as sharp wave when it was clearly distinguishable from movement artifacts, had twice the amplitude of the background EEG, and was less than 200 ms in duration. Animals were recorded starting in the early morning, throughout SE, and continuing for at least two hours after the diazepam injection (7 to 9 hours total recording time per animal). The EEG seizure activity had stopped at the end of the recording period in 20 of the 25 KA-treated rats, with four mortalities. Post-SE EEG monitoring for detection of spontaneous sharp waves and electrographic seizure activity was performed either for 24 hours, 6 to 9 days after status epileptics (n = 16 rats) or on days 6 and 7 for 8 hours per day (n = 5 rats). The non-occurrence or occurrence of spontaneous sharp waves, spikes and seizures was noted during each recording period.
Fixation and tissue processing
Five rats from the KA-SE group and five sham control rats were used for histological studies of the BLA. Six to 9 days after SE, rats were EEG monitored for 24 hours. On the day after monitoring, the animals were deeply anesthetized using ketamine (60 mg/kg i.p.) and medetomidine (0.5 mg/kg i.p.) and transcardially perfused with PBS (100 mL) followed by 4% paraformaldehyde (250 mL). The brains were removed and post-fixed overnight at 4° C, then transferred to a solution of 30% sucrose in PBS for 72 hours, and frozen with dry ice before storage at −80° C until sectioning. A 1-in-6 series of sections containing the rostro-caudal extent of the BLA was cut at 40 μm on a sliding microtome. One series of sections was mounted on slides (Superfrost Plus; Daigger, Vernon Hills, IL) in PBS for Nissl staining with cresyl violet. An adjacent series of sections was also mounted on slides for Fluoro-Jade C staining. The remaining series of sections were placed in a cryoprotectant solution (30% ethylene glycol and 30% glycerol in 0.05 M sodium phosphate buffer) and stored at −20° C until processing for immunohistochemistry.
Glutamic acid decarboxylase-67 (GAD67) immunohistochemistry
To label GAD67-immunoreactive neurons, a l-in-6 series of free-floating sections was collected from the cryoprotectant solution, washed three times for 5 min each in 0.1 M phosphate buffered saline (PBS), then incubated in a blocking solution containing 10% normal goat serum (NGS; Chemicon), and 0.5% Triton X-100 in PBS for one hour at room temperature. The sections were then incubated with mouse anti-GAD67 serum (1:1000, MAB5406; Chemicon), 5% NGS, 0.3% Triton X-100, and 1% bovine serum albumin, overnight at 4° C. After rinsing three times in 0.1% Triton X-100 in PBS, the sections were incubated with Cy3-conjugated goat anti-mouse antibody (1:1000; Jackson ImmoResearch) and 0.0001% DAPI (Sigma, St. Louis, MO) in PBS for one hour at room temperature. After a final rinse in PBS, sections were mounted on slides, air dried for 30 min, then coverslipped with ProLong Gold antifade reagent (Invitrogen).
Fluoro-Jade C(FJ-C) staining
FJ-C (Histo-Chem, Jefferson, AK) was used to identify irreversibly dying neurons in the BLA, on days 7–10 after SE. Mounted sections were air-dried overnight, then immersed in a solution of 1% sodium hydroxide in 80% ethanol for 5 min. The slides were then rinsed for 2 min in 70% ethanol, 2 min in dH20, and incubated in 0.06% potassium permanganate solution for 10 min. After a 2 min rinse in dH20, the slides were transferred a 0.0001% solution of FJ-C dissolved in 0.1% acetic acid for 10 min. Following three 1-min rinses in dH20, the slides were dried on a slide warmer, cleared in xylene for at least 1 min and coverslipped with DPX (Sigma).
Stereological quantification
Design-based stereology was used to quantify the total number of neurons on Nissl-stained sections and total number of inhibitory neurons on GAD67-stained sections in the BLA of KA-SE and sham control rats. Sections were viewed with a Zeiss Axioplan 2ie (Oberkochen, Germany) fluorescent microscope with a motorized stage, interfaced with a computer running StereoInvestigator 7.5 (MicroBrightField, Williston, VT). The BLA was identified on slide-mounted sections and delineated under a 2.5× objective, based on the atlas of Paxinos and Watson (1998). Estimated totals were determined using the optical fractionator probe, and all sampling was done under a 63× oil immersion objective.
For Nissl-stained neurons in the BLA of both KA-SE and sham control rats, a 1-in-6 series of sections was analyzed (on average, 6 sections). The counting frame was 35 × 35 μm, the counting grid was 190 × 190 μm, and the disector height was 12 μm. Nuclei were counted when the top of the nucleus came into focus within the disector which was placed 2 μm below the section surface. Section thickness was measured at every counting site, and the average mounted section thickness was 19 μm. An average of 288 Nissl-stained neurons per rat was counted.
For GAD67-stained neurons in BLA, of both KA-SE and sham control rats, a 1-in-6 series of sections was analyzed (on average, 6 sections), the counting frame was 60 × 60 μm. The counting grid was 100 × 100 μm for KA-SE rats and 110 × 110 μm for sham control rats. The disector height was 12 μm (n = 1), 16 μm (n = 5), or 20 μm (n = 4), depending on extent of antibody penetration in the tissue. Cells were counted if they came into focus within the disector, which was placed 2 μm below the section surface. Section thickness was measured at every fifth counting site, and the average mounted section thickness was 40 μm. An average of 207 GAD67-stained neurons per rat was counted. The coefficient of error (CE) for the estimated total of Nissl-stained and GAD67-stained neurons in the BLA was calculated using both the Gunderson (m = 1; Gundersen et al., 1999) and Schmitz-Hof (2nd Estimation; Schmitz and Hof, 2000) equations.
Quantification of FJ-C stained neurons
Tracings of the BLA from an adjacent series of Nissl-stained sections were superimposed on the sections stained with FJ-C. FJ-C positive cells were counted at 20×, and recorded as number of cells per section from, on average, 6 sections containing the BLA of sham control rats and KA-SE rats.
Western Blotting
Six rats from the KA-SE group and three sham controls were used for measuring the levels of the GluK1 subunit, the GABA synthesizing enzyme GAD65/67, and the α1 subunit of the GABAA receptor, in the BLA. The CA3 subfield of the hippocampus and the ventral posteromedial and ventral posterolateral (VPM/VPL) thalamic nuclei were also included in the Western blot analysis, for comparison. Six to nine days after SE, rats were video-EEG monitored for 24 hours. On the day after monitoring, rats were anesthetized with CO2 and then decapitated. Discrete brain regions were micro-dissected from individual 800 μm-thick brain sections cut with a Vibratome (Technical Products International). The tissues were sonicated in lysis buffer (1% NP-40, 20 mM Tris, pH 8.0, 137 nM NaCl, 10% Glycerol, Tyr &Ser/Thr Phosphatase Inhibitor Cocktails; Upstate, Temecula, CA). After removal of cellular debris by centrifugation, protein levels in the lysates were measured by the Bradford Coomassie Blue colorimetric assay (Bio-Rad Laboratories, Hercules, CA) and equalized accordingly. Aliquots (60 μg) were boiled for 5 minutes in the presence of loading buffer (NuPAGE LDS Sample Buffer (4×), Invitrogen, Carlsbad, CA), then placed on ice for 1 minute. Each brain region was loaded in triplicate and proteins were separated on a 7.5% SDS-PAGE under reducing conditions using the Bio-Rad Mini-Protean3 cell system. Proteins were transferred to nitrocellulose membranes (0.45μm; Invitrogen). After blocking with 5% nonfat dry milk in Tris-buffered saline containing 0.1% Tween 20 (TBS-T) at room temperature for 1 hour, blots were incubated overnight at 4°C with specific primary antibodies: anti-GluK1, 1:500 (Tocris Bioscience, Ellisville, Missouri), anti-GAD65/67 (1:10,000; Chemicon), and anti-GABAA α1 (1:200, Chemicon), prepared in 5% bovine serum albumin (BSA) in TBS-T. After washing in TBS-T, membranes were incubated with peroxidase-conjugated goat anti-rabbit (1:10,000; Jackson Immuno Research, West Grove, PA) prepared in 5% BSA in TBS-T for 2 hours, at room temperature. Anti-β-actin (1:500; Cell Signaling Technology, Danvers, MA) was used as a loading control in all experiments. After washing in TBS-T, blots were developed using enhanced chemiluminescence detection according to the manufacture’s recommendation (Pierce, Rockford, IL) and exposed to BioMax MR Film (Kodak Biomax, Rochester, NY) under non-saturating conditions. The blots were stripped with Restore Western blot Stripping Buffer (Pierce) and then incubated with subsequent antibodies (see above). Absorbance values of bands for GluK1, GAD65/67, and GABAA α1 were analyzed by densitometry using Image J analysis systems (NIH, Bethesda, MD), and normalized relative to the β-actin absorbance value.
Amygdala slice electrophysiology
Coronal slices containing the amygdala were prepared from rats, on days 7–10 after SE. The rats were anesthetized with CO2 and then decapitated. The brain was rapidly removed and placed in ice-cold artificial cerebrospinal fluid (ACSF) composed of (in mM): 125 NaCl, 2.5 KCl, 2.0 CaCl2, 2.0 MgCl2, 25 NaHCO3, 1.25 NaH2PO4, and 22 glucose, bubbled with 95% O2 and 5% CO2 to maintain a pH of 7.4. A block containing the amygdala region was prepared, and 400 μm slices were cut with a Vibratome (Series 1000; Technical Products International, St. Louis, MO). Slices were kept in a holding chamber containing oxygenated ACSF at room temperature, and recordings were initiated ≥1 hr after slice preparation. Slices were transferred to a submersion-type recording chamber, where they were continuously perfused with oxygenated ACSF, at a rate of 3–4 ml/min. Neurons were visualized with an upright microscope (Nikon Eclipse E600fn; Nikon, Tokyo, Japan) using Nomarski-type differential interference optics through a 60× water immersion objective. All experiments were performed at room temperature (28°C). Tight-seal (>1 GΩ) whole-cell recordings were obtained from the cell body of pyramidal-shaped neurons in the BLA region. Patch electrodes were fabricated from borosilicate glass and had a resistance of 1.5–5.0 MΩ when filled with a solution containing (in mM): 135 Cs-gluconate, 10 MgCl2, 0.1 CaCl2, 1 EGTA, 10 HEPES, 2 Na-ATP, 0.2 Na3GTP, pH 7.3 (285–290 mOsm). Neurons were voltage-clamped using an Axopatch 200B amplifier (Axon Instruments, Foster City, CA). IPSCs were pharmacologically isolated and recorded at a -70 mV holding potential. Access resistance (5–24 MΩ) was regularly monitored during recordings, and cells were rejected if it changed by >15% during the experiment. The signals were filtered at 2 kHz, digitized (Digidata 1322A; Axon Instruments), and stored on a computer using pClamp9 software (Axon Instruments). The peak amplitude, 10–90% rise time, and decay time constant of IPSCs were analyzed off-line using pClamp9 software and the Mini Analysis Program (Synaptosoft, Inc., Leonia, NJ). Miniature IPSCs (mIPSCs) were analyzed offline using the Mini Analysis Program and detected by manually setting the mIPSC threshold (~1.5 times the baseline noise amplitude) after visual inspection.
Drugs
The following drugs were used: kainic acid (Tocris Cookson, Ballwin, MO), diazepam (Hospira Inc., IL), D-APV (Tocris; an NMDA receptor antagonist), (+)-(2S)-5,5-dimethyl-2-morpholineacetic acid (SCH50911; Tocris; a GABAB receptor antagonist), ATPA (Sigma; a GluK1R agonist; see Clarke et al., 1997), GYKI 52466 (Tocris; an AMPA receptor antagonist), and tetrodotoxin (TTX; Sigma; a sodium channel blocker).
Statistical analysis
All statistical values are presented as mean ± S.E.M. Results from sham control and KA-SE groups were compared using the unpaired Student’s t test; p < 0.05 was considered statistically significant. Sample sizes (n) refer to the number of rats, except for the electrophysiology results where “n” refers to the number of slices or recorded cells.
Results
Status epilepticus
SE by EEG and behavioral criteria occurred in 24 out of 25 rats treated with KA. One rat experienced only focal seizures with unilateral forelimb clonus and was not studied. None of the saline-treated sham control rats displayed EEG or behavioral seizures during the observation period (n=21). Diazepam (25–30 mg/kg) was administered 180 min after the beginning of SE in the KA-treated rats to terminate the behavioral and EEG seizure activity. The mean cumulative duration of EEG seizure activity in the KA treated rats was 257.1 ± 13.9 min (n=23). One rat died during SE, and three rats died after diazepam treatment.
EEG indicators of epileptogenesis
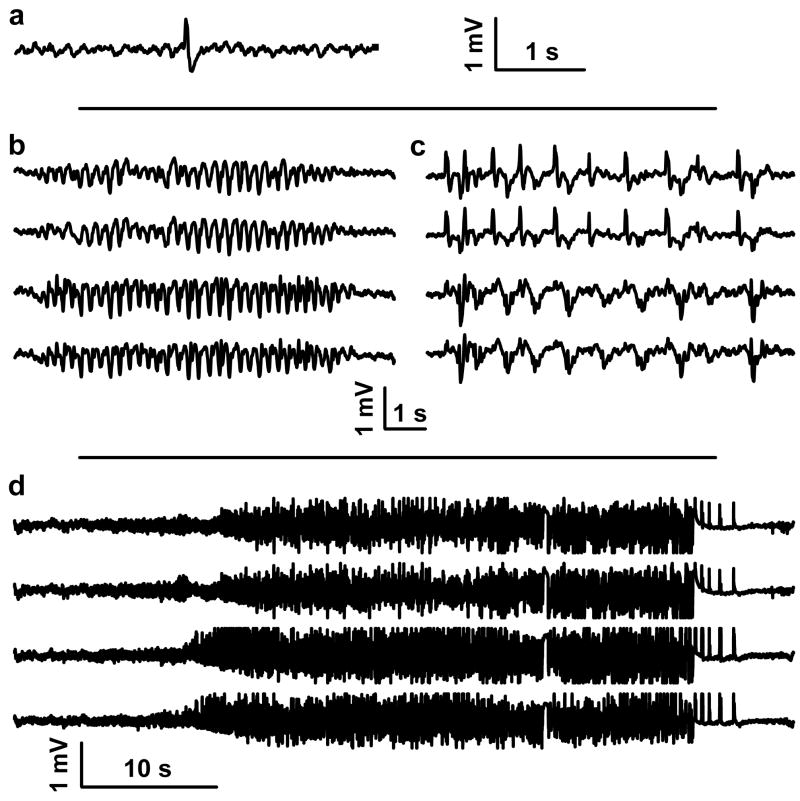
In the post-SE model as conducted according to the protocol we used, a high proportion of animals eventually develop spontaneous motor seizures (Hellier et al., 1998). To confirm that epileptogenesis was in progress in the KA-SE rats we recorded EEG activity during the latent period before the development of overt behavioral seizures. Six to 9 days after SE, EEG monitoring of the KA-SE rats indicated the presence of spontaneous sharp waves and/or spike wave complexes (n = 20 rats; Fig. 1a, b), whereas spontaneous EEG seizures and/or episodes of periodic generalized epileptic discharges also occurred in 55 % of the rats (n = 11; Fig. 1c, d)
Figure 1. EEG traces recorded from the cortex of rats, 6 days after KA-SE.
(a) Single sharp wave, (b) spike-wave complexes, (c) periodic generalized epileptic discharges, and (d) seizures. Trace in (a) was recorded from a left frontal cortical screw electrode. Traces in (b), (c), and (d) were recorded (top to bottom) from a left frontal, right frontal, left parietal, and right parietal cortical screw electrode.
Neuronal loss and degeneration
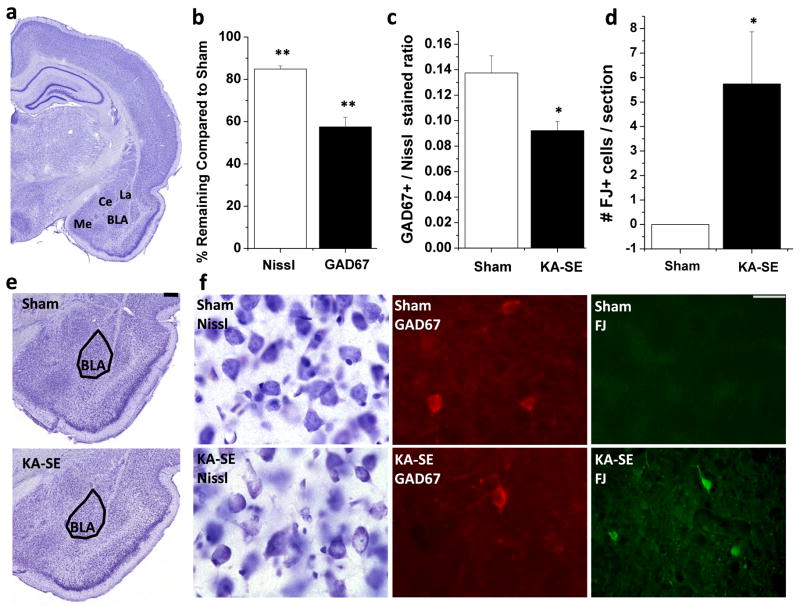
Previous studies have shown that 2 weeks after prolonged SE (> 3 hours) there is extensive loss of GABAergic interneurons in the amygdala (Tunnanen et al., 1996). To determine the extent of neuronal loss in our study, 7 to 10 days after SE which was terminated at 3 hours, we counted the total number of neurons and total number of GABAergic neurons in the BLA of the sham control and the KA-SE groups. The total number of neurons in the BLA of sham control rats (n = 5), estimated by stereologically counting Nissl stained neurons, was 85,877 ± 2,913 (Table 1; Fig. 2). In the KA-SE rats (n = 5), the total number of neurons was 72,904 ± 1,261, a 15% reduction compared to sham control rats (p < 0.004). The total number of GABAergic neurons in the BLA was estimated by counting GAD67-immunoreactive cells. GAD67 is the higher molecular weight (67 kD) isoform of glutamate decarboxylase (GAD), the enzyme that synthesizes GABA (Erlander and Tobin, 1991), and is predominantly localized in cell bodies of GABAergic neurons (Esclapez et al., 1994; Soghomonian and Martin, 1998). The total number of GABAergic neurons in the BLA of sham control rats was 11,685 ± 864 (Table 1, Fig. 2). In KA-SE rats, the total number of GABAergic neurons was 6,718 ± 537, a 43% reduction compared to controls (p < 0.002). Thus, in the BLA of sham control rats, GABAergic interneurons represent 13.7 ± 1.4% of the total population of neurons, but after KA-SE this is significantly reduced to 9.2 ± 0.7% (Fig. 6; p < 0.02), indicating that GABAergic neurons in the BLA are more vulnerable to KA-SE induced injury than principal cells.
Table 1.
Stereological estimation of total Nissl-stained and GAD67-positive neurons in the BLA of sham control rats and KA-SE rats
| Sham Control (n = 5) | KA-SE (n = 5) | p value (t test) | % Neurons Remaining | |
|---|---|---|---|---|
| Nissl-stained | ||||
| Mean | 85,877 | 72,904 | ||
| S.E.M. | 2,913 | 1,261 | ||
| Mean Gundersen C.E. (m=1) | .060 | .062 | <.004 | 84.9 |
| Mean Schmitz-Hof C.E. (2nd est) | .056 | .061 | ||
|
| ||||
| GAD67+ | ||||
| Mean | 11,685 | 6,718 | ||
| S.E.M. | 864 | 537 | ||
| Mean Gundersen C.E. (m = 1) | .066 | .076 | <.002 | 57.4 |
| Mean Schmitz-Hof C.E. (2nd est) | .065 | .076 | ||
Means are values from the BLA of both hemispheres; n indicates number or rats per group. S.E.M., standard error of the mean; C.E., coefficient of error as calculated by Gundersen et al. (1999) and Schmitz and Hof (2000).
Figure 2. GABAergic interneurons are preferentially lost in the BLA after KA-SE, and neurodegeneration continues on days 7 to 10 after KA-SE.
a Location of the amygdala nuclei in a coronal brain section (Me=medial amygdala; La=Lateral amygdala; BLA=Basolateral amygdala; Ce=Central amygdala). (b) Number of Nissl-stained neurons and GAD67-immunoreactive neurons in the BLA of the KA-SE rats (n = 5), expressed as a percentage of the number of neurons in the sham-control group. (c) Ratio of GAD67-immunoreactive neurons to Nissl-stained neurons in the BLA, demonstrating that a lower number of GABAergic neurons make up the total population of neurons in the BLA of the KA-SE rats compared to sham rats. Mean ± SEM values come from 5 sham rats and 5 KA-SE rats. (d) Number of FJ-C positive cells in the BLA of KA-SE rats (n = 5) versus the sham rats (n = 5). There was no evidence for neurodegeneration in sham rats, while neurodegeneration was ongoing in the KA-SE rats. (e) A panoramic view of the Nissl-stained amygdaloid complex from a sham rat and a KA-SE rat (scale bar is 250 μm). (f) Photomicrographs of Nissl-stained, GAD67-immunoreactive, and FluoroJade-C (FJ-C)-stained sections from the BLA of sham control rats and KA-SE rats, 7–10 days after KA-SE (scale bar = 25 μm). * p<0.05, **p<0.005.
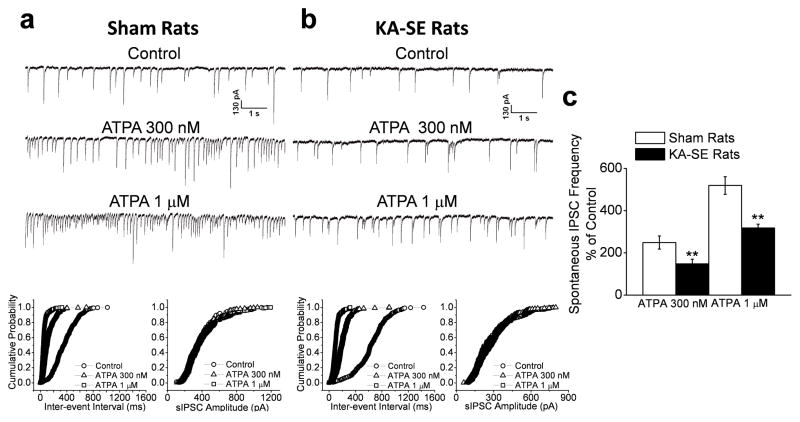
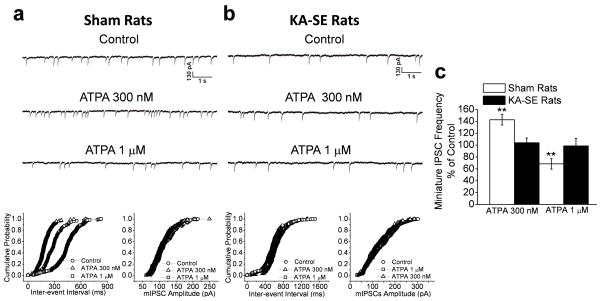
Figure 6. The GluK1R-mediated enhancement in the frequency of sIPSCs in the BLA is reduced after KA-SE.
The GluK1R agonist ATPA, at 300 nM or 1 μM, increased the frequency of sIPSCs recorded from BLA pyramidal neurons in the sham controls (a1), but its effect was less pronounced in the KA-SE rats (b2). Cumulative probability plots of interevent intervals and amplitudes of sIPSCs corresponding to the traces in a1 and b1 are shown in a2 and b2, respectively. (c) Pooled sIPSC frequency-data of 10 slices from KA-SE rats and 11 slices from sham rats, expressed as a percentage of the sIPSC frequency during control conditions (before application of ATPA). The increase in sIPSC frequency by either concentration of ATPA was significantly lower in the KA-SE rats compared to the sham rats (**p< 0.01).
FJ-C was used to determine the extent to which neurons are still degenerating in the BLA, 7–10 days after KA-SE. Whereas no FJ-C positive staining was found in the BLA of sham control rats (n = 5), all KA-SE rats (n = 5) demonstrated positive staining in the BLA (5.7 ± 2.1 FJ-C positive cells/section; Fig. 2; p < 0.03). In addition, positive staining was found, to a greater extent, in other amygdala nuclei, including the medial, lateral, posterior cortical, and central amygdala nuclei, as well as in other brain regions, such as the hilus, CA1, and CA3 subfields of the hippocampus, the piriform cortex, and the endopiriform cortex, in all KA-SE rats, but not in the control sham rats. These results demonstrate that neurodegeneration was still occurring in the BLA and other brain areas on days 7 to 10 after KA-induced SE.
Alterations in the levels of glutamate decarboxylase (GAD) and the GABAA α1 subunit
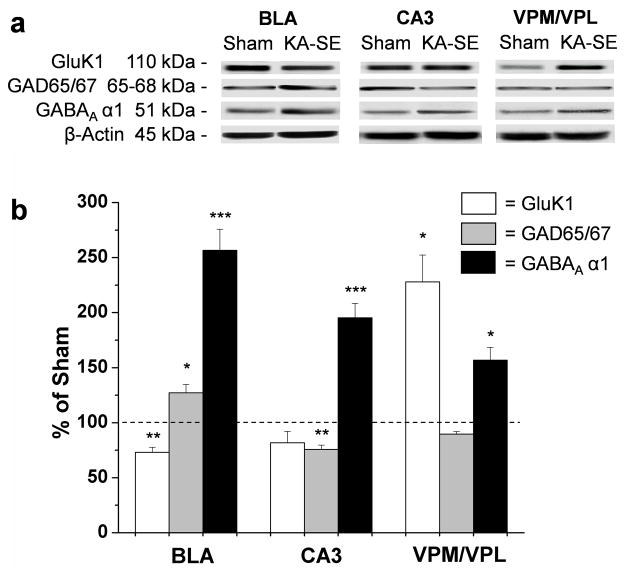
When a brain insult damages GABAergic interneurons, a number of mechanisms often come into play which, presumably, attempt to compensate for the inhibitory loss. Such mechanisms may include an increase in GAD (Baran et al., 2004), and/or an increase in the expression of subunits that form the GABAA receptor complex (Gilby et al., 2005). In the present study, we compared the levels of GAD and the α1 subunit of the GABAA receptor in the KA-SE rats with those in the sham control rats. To probe the Western blot for the quantification of GAD we used an antibody that recognizes both the 65 kD and the 67 kD isoforms, which are predominantly localized in the nerve terminals and somata of GABAergic neurons, respectively (Esclapez et al., 1994; Soghomian and Martin,1998). GAD65/67 protein levels were elevated in the BLA of the KA-SE rats (n = 6) to 127.1 ± 7.8% of the sham rats (Fig. 3; p < 0.04). In contrast, in the CA3 subfield of the hippocampus, there was a reduction to 75.8 ± 3.4% of the sham group (Fig. 3; p < 0.01), while no significant changes in GAD65/67 protein levels were observed in the ventral posteromedial and ventral posterolateral (VPM/VPL) thalamic nuclei. Thus, GAD65/67 is up-regulated in the surviving GABAergic interneurons in the BLA, 7 to 10 days after KA-SE.
Figure 3. GAD65/67 and GABAA α1 subunit levels are increased in the BLA, while the GluK1 kainate receptor subunit is reduced, on days 7 to 10 after SE.
a Representative Western blots showing GluK1, GAD65/67, GABAA α1, and β-actin antibody binding, in the BLA. For comparison, the levels of these proteins were also measured in the CA3 subfield of the hippocampus, and the ventral posteromedial and ventral posterolateral thalamic nuclei (VPM/VPL). (b) Quantification of GluK1, GAD65/67, and GABAA α1 protein densities relative to β-actin density in KA-SE rats, normalized relative to sham controls. Values are mean ± SEM (n = 6) * p<0.05, **p<0.01 *** p<0.005.
Quantification of the GABAA α1 protein levels by Western blot analysis showed a significant increase in the BLA of the KA-SE rats to 257 ± 19% of the level in the sham control group (p < 0.003; Fig. 3). GABAA α1 levels were also increased in the CA3 subfield of the hippocampus (195 ±13% of shams; p < 0.003; Fig. 3), as well as in the VPM/VPL thalamic nuclei (157 ± 12% of shams; p < 0.04; Fig. 3) of the KA-SE rats. Thus, the GABAA α1 subunit is up-regulated in the surviving GABAergic interneurons in the BLA, 7 to 10 days after KA-SE.
Alterations in the level of the GluK1 subunit
GluK1Rs are significantly involved in epilepsy (Smolders et al., 2002; Rogawski et al., 2003; Kaminski et al., 2004), and their expression or function are altered in TLE patients and epileptic rats (Mathern et al., 1998; Kortenbruck et al., 2001; Palma et al., 2002; Ullal et al., 2005). In the BLA, these receptors are present on both postsynaptic and presynaptic sites of GABAergic interneurons (Braga et al., 2003), and they appear to play an important role in the regulation of neuronal excitability (Gryder and Rogawski, 2003; Braga et al., 2003, 2004; Aroniadou-Anderjaska et al., 2007). In the present study, to determine if along with the loss of GABAergic interneurons the level of the GluK1 subunit was also reduced, or if compensatory mechanisms increased the expression of this subunit, we used Western blot analysis to compare the level of the GluK1 protein in the BLA of KA-SE and sham control rats. We found that the GluK1 subunit in the BLA of KA-SE rats was reduced to 73.0 ± 4.6% of the level in the sham rats (p < 0.006; Fig. 3). For comparison, we also examined the GluK1 levels in two other brain regions. In the CA3 hippocampal area, GluK1 levels were reduced in KA-SE rats but the change was not statistically significant, whereas in the VPM/VPL thalamic nuclei there was a significant elevation in the GluK1 protein (228.0 ± 24.4% of the sham group; p < 0.02; Fig. 3). Thus, in the BLA, the level of the GluK1 subunit is significantly reduced, on days 7 to 10 after SE.
Alterations in GABAA receptor-mediated IPSCs
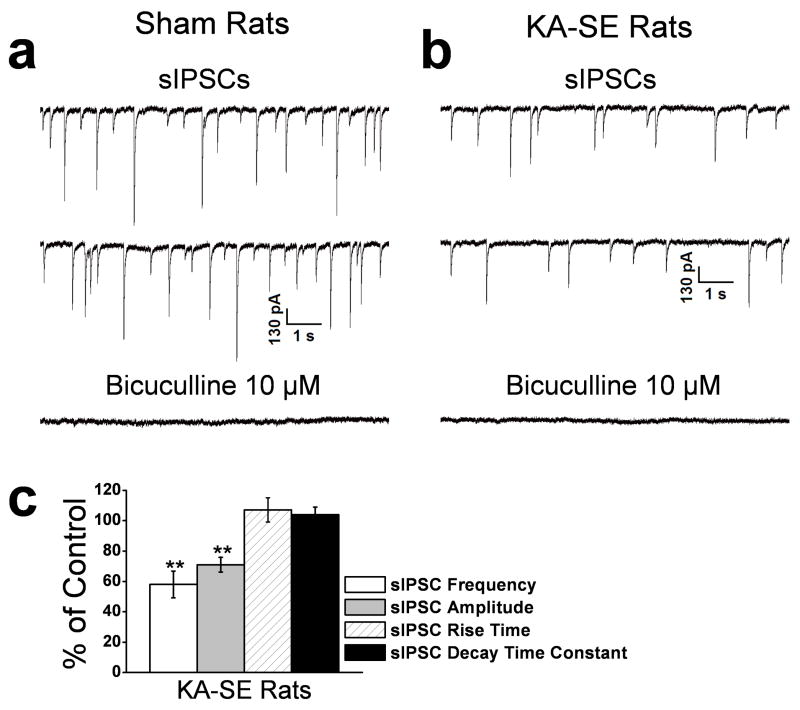
To determine the impact of the pathological changes described above on the excitability of the BLA circuitry we recorded action potential-dependent, spontaneous IPSCs (sIPSCs) and miniature IPSCs (mIPSCs) from the somata of BLA pyramidal-shaped neurons of KA-SE rats and sham control rats. Spontaneous IPSCs were recorded at a holding potential of −70 mV, and in the presence of D-APV (50 μM), GYKI 52466 (50 μM), and SCH50911 (20 μM) to block NMDA, AMPA, and GABAB receptors, respectively. The mean frequency of sIPSCs was 1.9 ± 0.7 Hz (n = 23) in sham control rats, and 1.1 ± 0.4 Hz in KA-SE rats, 42.1 ± 5.1 % lower than in the sham group (n = 21; p < 0.01; Fig. 4). The mean amplitude of sIPSCs in KA-SE rats was also reduced to 71.0 ± 4.8% of the control value (n = 21; p < 0.01; Fig. 4). There were no significant differences between KA-SE rats and sham control rats in the rise time and the decay time constant of the sIPSCs. These results suggest a reduced inhibitory tone of BLA pyramidal cells, on days 7 to 10 after KA-SE.
Figure 4. The frequency and amplitude of spontaneous IPSCs (sIPSCs) in the BLA are reduced on days 7 to 10 after KA-SE.
sIPSCs were recorded from pyramidal-shaped neurons in the presence of D-APV (50 μM), SCH50911 (20 μM), and GYKI 52466 (50 μM), at a holding potential of −70 mV. Representative examples are shown in (a) and (b), and group data in (c). The frequency and the amplitude of the sIPSCs were reduced in the KA-SE rats (b) compared to the sham controls (a). The recorded currents were blocked by the GABAA receptor antagonist bicuculline. (c) Group data (n = 21) from KA-SE rats, normalized relative to the sham controls. The frequency and amplitude, but not the rise time and the decay time constant of the sIPSCs were significantly lower in the KA-SE group compared to the sham controls (**p < 0.01).
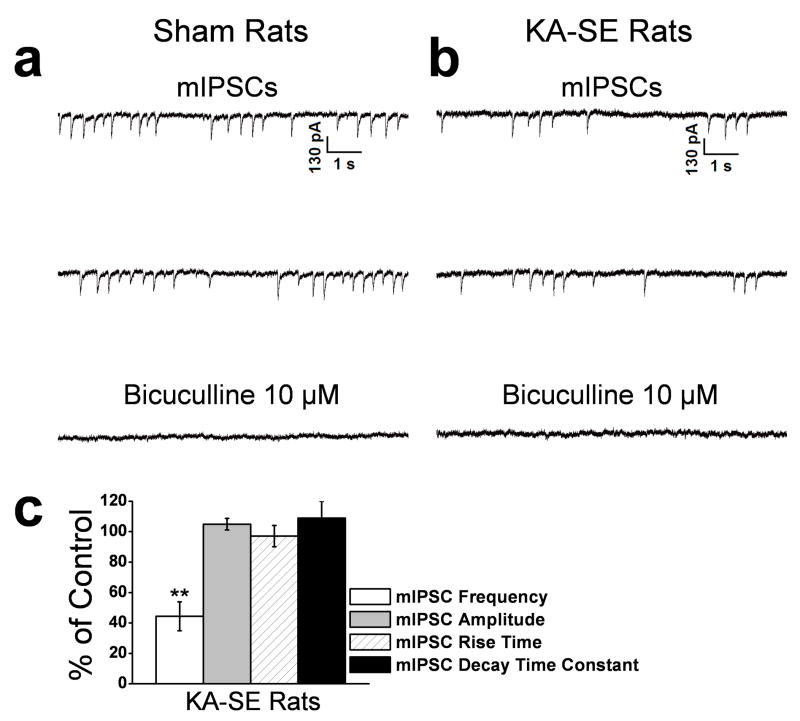
To determine whether the reduction of sIPSCs was associated with a decreased responsiveness of postsynaptic GABAA receptors we recorded action potential-independent, mIPSCs from the soma of BLA pyramidal-shaped neurons of KA-SE rats and sham control rats. Miniature IPSCs were recorded at a holding potential of −70 mV, and in the presence of D-APV (50 μM), SCH50911 (20 μM), GYKI 52466 (50 μM), and TTX (1 μM). The mean frequency of mIPSCs was 1.2 ± 0.4 Hz (n = 14) in sham control rats and 0.5 ± 0.2 Hz in KA-SE rats (56.7 ± 3.9 % lower than the sham control group; n = 15, p < 0.05; Fig. 5). There was no significant difference in mIPSC amplitude, between sham control and KA-SE rats (Fig. 5). Thus, the reduced frequency and amplitude of sIPSCs (Fig. 4) did not involve a decreased responsiveness of postsynaptic GABAA receptors.
Figure 5. The frequency but not the amplitude of miniature IPSCs (mIPSCs) in the BLA is reduced on days 7 to 10 after KA-SE.
mIPSCs were recorded in the presence of D-APV (50 μM), SCH50911 (20 μM), GYKI 52466 (50 μM), and TTX (1 μM), at a holding potential of −70 mV. Representative examples are shown in (a) and (b), and group data in (c). The frequency of the mIPSCs was reduced in the KA-SE rats (b) compared to the sham controls (a). The GABAA receptor antagonist bicuculline blocked the mIPSCs. (c) Group data (n = 15) from KA-SE rats, normalized relative to the sham controls. The frequency, but not the amplitude, rise time, or decay time constant of the mIPSCs were significantly lower in the KA-SE group compared to the sham controls (**p < 0.01).
Alterations in the GluK1R-mediated modulation of GABAergic transmission
The effect of GluK1R activation on inhibitory transmission in the BLA depends on the intensity of activation of these receptors (the concentration of the endogenous agonist glutamate). Activation of postsynaptic (somatodendritic) GluK1Rs enhances GABA release via depolarization of interneurons (Braga et al., 2003). However, presynaptic GluK1Rs on GABAergic terminals increase the probability of GABA release when activated weakly or by ambient concentrations of extracellular glutamate, but have the opposite effect (inhibiting GABA release) when the agonist concentration increases (Braga et al., 2003). It seems, therefore, that the activity of these receptors reduces neuronal excitability in the BLA at the “resting state”, while promotes/exacerbates hyperexcitability when the concentrations of glutamate rise (Braga et al., 2003; Aroniadou-Anderjaska et al., 2007). To determine the functional impact of the reduced level of the GluK1 protein in the KA-SE rats, we compared the effects of the GluK1R agonist ATPA on sIPSCs and mIPSCs in the KA-SE group and the sham control group.
Bath application of ATPA, at 300 nM, produced a 149 ± 31 % increase in the frequency of sIPSCs recorded from BLA pyramidal cells (n = 11) of sham control rats, while 1 μM ATPA further increased the sIPSC frequency to 419 ± 42 % from baseline (n = 11; Fig. 6). In the KA-SE rats, 300 nM ATPA produced only a 48 ± 22 % increase in sIPSC frequency from baseline (n = 10), and 1 μM ATPA increased sIPSC frequency to 218 ± 19 % from baseline (n = 10; Fig. 6). Thus, the enhancement in the frequency of sIPSCs in the BLA, produced by activation of GluK1Rs was significantly lower in KA-SE rats compared to sham control rats, at agonist concentrations of either 300 nM (p < 0.01) or 1 μM (p < 0.01). As these effects of ATPA were probably produced by a depolarizing action on postsynaptic GluK1Rs of GABAergic interneurons, these results suggest that either the function of these receptors is impaired in the KA-SE rats, or that the surviving GABAergic interneurons in the KA-SE group have a lower number of these receptors, which could be due to reduced expression of the GluK1 subunit, or other mechanisms of downregulation.
When mIPSCs were recorded from BLA pyramidal cells, in the presence of TTX, 300 nM ATPA produced a 42.8 ± 7.2% facilitation in the mIPSC frequency (n = 9) in sham control rats, with no change in the amplitude or decay time constant of the mIPSCs. In contrast, in the KA-SE rats, 300 nM ATPA failed to produce a significant effect on mIPSCs (n = 9; Fig. 7). Application of 1 μM ATPA produced a 31.6 ± 5.9 % reduction in the mIPSC frequency (n = 9) in sham control rats, with no change in the amplitude or decay time constant of the mIPSCs, but had no significant effect on mIPSCs recorded from the KA-SE rats (n = 9; Fig. 7). These results suggest that presynaptic GluK1Rs on GABAergic terminals in the KA-SE group are either functionally impaired, or that the surviving GABAergic interneurons in the KA-SE group have a lower number of these receptors, which could be due to reduced expression of the GluK1 subunit, or other mechanisms of downregulation.
Figure 7. The GluK1R-mediated effects on presynaptic GABA release in the BLA are nearly absent after KA-SE.
In the sham control group, the GluK1R agonist ATPA, at 300 nM, increased the frequency of mIPSCs recorded from BLA pyramidal neurons (a1); this effect was almost absent in the KA-SE rats (b1). At 1 μM, ATPA reduced the frequency of mIPSCs in the sham group (a1), but, again, had virtually no effect in the KA-SE group (b1). Cumulative probability plots of interevent intervals and amplitudes of mIPSCs corresponding to the traces in a1 and b1 are shown in a2 and b2, respectively. (c) Pooled mIPSC frequency-data of 9 slices from KA-SE rats and 9 slices from sham rats expressed as a percentage of the mIPSC frequency during control conditions (before application of ATPA); **p< 0.01.
Discussion
The present study shows that in the BLA, the amygdala nucleus which plays a central role in the generation and spread of seizures, there is already a dramatic decrease in tonic inhibitory activity, 7 to 10 days after the initiation of epileptogenesis triggered by SE. The reduction of tonic inhibition is primarily due to the loss of GABAergic interneurons. A reduction and/or impaired function of GluK1Rs is also likely to contribute to the reduction of tonic GABA release. The increase in the level of GAD and the GABAA α1 subunit suggests an increased expression of these proteins in the surviving interneurons, but these alterations are not sufficient to compensate for the interneuronal loss. These findings are summarized in Table 2.
Table 2.
Summary of statistically significant alterations in the rat BLA at 7–10 after KA-SE
| Total number of neurons | 15% ↓ |
| Number of interneurons | 43% ↓ |
| GAD level | 27% ↑ |
| GABAA subunit level | 157% ↑ |
| GluK1 subunit level | 27% ↓ |
| sIPSC frequency | 42% ↓ |
| sIPSC amplitude | 29% ↓ |
| mIPSC frequency | 56% ↓ |
| Enhancement of sIPSC frequency by the GluK1R agonist ATPA (1μM) | 52% ↓ |
| Change in mIPSC frequency by the GluK1R agonist ATPA | No effect in KA-SE rats |
Loss of GABAergic interneurons
In agreement with prior studies (Tuunanen et al., 1996, 1999), we observed a substantial reduction in the number of neurons in the BLA after KA-induced SE. However, the magnitude of the reduction was less than in these previous studies most likely because the duration of SE in our experiments was shorter. It is well recognized that the severity of neuronal damage in SE models is dependent on the duration of seizure activity (Lemos and Cavalheiro, 1995; Gorter et al., 2003). In the present study, we used diazepam to terminate seizures within 3 hours after onset, whereas Tuunanen et al. (1996) recorded seizure activity lasting on average 13 hours. In addition, in Tuunanen et al., 1996, neuronal loss was examined 2 weeks after SE. Since neurons continue degenerating on days 7 to 10 after SE (Fig. 2a, d), the time-point after SE that neuronal loss is examined must also play a role in the severity of the observed loss. It is noteworthy that loss of neurons in the BLA, as observed in animal models (Tuunanen et al., 1996, 1999; Pitkänen et al., 1998; Covolan and Mello, 2000; present study), is also a common pathological feature in human TLE (Cendes et al., 1993; Hudson et al., 1993; Wolf et al., 1997; Pitkänen et al., 1998; Guerreiro et al., 1999).
The damage of GABAergic neurons in the present study was significantly greater than that of principal neurons, suggesting a higher vulnerability of GABAergic neurons to SE-induced injury. It has been speculated that certain interneuron populations are vulnerable to seizure-induced damage because of a low capacity to buffer calcium (Scharfman and Schwartzkroin, 1989; Sloviter, 1989). Calcium binding proteins (CBPs) play an important role in calcium buffering (Blaustein, 1988), however no clear relationship has been established between CBPs and vulnerability to damage by prolonged seizures (Freund et al., 1992). A high susceptibility of somatostatin-containing interneurons to SE-induced damage has been reported in the hippocampus (Sloviter, 1987; Buckmaster and Dudek, 1997; Sun 2007), and has been associated with the high level of a tyrosine phosphatase present in these neurons, which blocks a latent neuroprotective response initiated by the ERK/MAPK signaling pathway (Choi et al., 2007). A similar mechanism may also be involved in the high vulnerability of somatostatin-containing interneurons in the BLA (Tuunanen et al., 1996, 1997), the majority of which are GABAergic inhibitory neurons (McDonald and Pearson, 1989; Muller et al., 2007). The proportion of somatostatin-containing neuron loss was not determined in the present study. However, since only 11–18% of BLA GABAergic neurons contain somatostatin (McDonald and Pearson, 1989), it is likely that other types of GABA neurons were also lost.
Compensatory mechanisms
In the present study, despite the 43% decrease in the number of GABAergic neurons in the BLA of the KA-SE rats, the level of GAD was significantly increased, suggesting an increased expression of the enzyme in the remaining interneurons. Others, however, have found a decrease in the activity of GAD, 9 days after KA-induced SE (Sperk et al., 1983). This difference may imply that when GAD expression is increased in the BLA, the same enzyme is downregulated in the other nuclei of the amygdala, resulting in an overall decreased activity when the whole amygdala is examined. Alternatively, differences between the two studies in the extent of the interneuronal loss could account for differences in the level and/or the activity of GAD.
The level of the α1 subunit of the GABAA receptor was also increased in the BLA of the KA-SE rats, despite the loss of both principal neurons and interneurons, suggesting an increased expression of this subunit by the surviving cells. Previous studies have reported SE-induced alterations in the expression of different GABAA receptor subunits or GABAA receptor binding in different brain regions (Schwarzer et al., 1997; Tsunashima et al., 1997; Gilby et al., 2005; Rocha et al., 2007; Brooks-Kayal et al., 1998; Fritschy et al., 1999; Houser and Esclapez, 2003; Raol et al., 2006), including increases in the GABAA α1 subunit in the amygdala (Gilby et al., 2005). This subunit is important for the sedative and anticonvulsant actions of benzodiazepines (Rudolph et al., 1999; Crestani et al., 2000, Da Settimo et al., 2007), and, therefore, alterations in this subunit could affect responsivity to certain pharmacological treatments, in epileptic patients. However, in the present study, the increase in the level of the GABAA α1 subunit in the BLA, did not appear to produce an increase in GABAA receptor number, or to alter the stoichiometry of the GABAA receptors in such a way as to increase the efficacy of inhibitory transmission, at least in the principal BLA neurons. This conclusion can be derived from the lack of a difference between sham and KA-SE rats in the amplitude of mIPSCs recorded from pyramidal-shaped neurons in the BLA (Fig. 5). Thus, 7 to 10 days after the initiation of epileptogenesis by SE, the increase in the expression of the GABAA α1 subunit does not appear to affect inhibitory transmission. It is possible that the GABAA α1 subunit fails to assemble into the receptor complex, or undergo phosphorylation (Pumain and Laschet, 2006).
Reduced inhibitory tone
In the hippocampus, reduced inhibitory activity is a hallmark of epileptogenesis and epilepsy (Rice et al., 1996; Hirsch et al., 1999; Cossart et al., 2001; Kobayashi and Buckmaster, 2003; Shao and Dudek, 2005; Sun et al., 2007), but unaltered or enhanced inhibitory transmission have also been observed in certain hippocampal regions (Gibbs et al., 1997; Nusser et al., 1998; Cossart et al., 2001; Shao and Dudek, 2005). Importantly, the changes that take place in different brain regions during epileptogenesis do not seem to follow a linear progression towards reduced inhibition and enhanced excitation, but, rather, they can be qualitatively different at different time points during the course of epileptogenesis (Covolan and Mello, 2000; Straessle et al., 2003; Sloviter et al., 2006; El-Hassar et al., 2007; Rocha et al., 2007). For example, in the CA1 hippocampal area, inhibitory activity has been found to be enhanced or reduced at different stages of epileptogenesis (El-Hassar et al., 2007). In the amygdala, GABAergic inhibitory transmission is impaired when epilepsy has developed (Gean et al., 1989; Smith and Dudek, 1997; Mangan et al., 2000; Benini and Avoli, 2006; for a review see Aroniadou-Anderjaska et al., 2008), but it is unclear how inhibitory activity and the excitability of the amygdala change during the course of epileptogenesis. The present study shows that the inhibitory tone in the BLA network is already dramatically reduced 1 week to 10 days after triggering epileptogenesis by KA-SE.
The reduced frequency and amplitude of action potential-dependent sIPSCs in the BLA of the KA-SE rats was consistent with the loss of GABAergic interneurons. Alterations in postsynaptic GABAA receptors, which are often associated with epilepsy (McDonald et al., 1991; Henry et al., 1993; Rocha et al., 2007), could produce a reduction in both the frequency and the amplitude of sIPSCs; however, the unaltered amplitude of mIPSCs in the KA-SE rats suggests that changes in the number or function of postsynaptic GABAA receptors were not a contributing factor. Other mechanisms related to the excitation of interneurons or presynaptic GABA release may also contribute to the reduction in tonic inhibition, and as we found in the present study one of these mechanisms involves the modulation of GABAergic transmission by GluK1Rs (see next section).
Reduced level the GluK1 subunit and impaired GluK1R-mediated modulation of GABA release
Unlike other ionotropic glutamate receptor subunits, the GluK1 subunit of kainate receptors has a relatively restricted distribution in the central nervous system, but is highly expressed in the BLA (Bettler et al., 1990; Li et al., 2001; Braga et al., 2003), where it plays a prominent role in the modulation of GABA release (Braga et al., 2003, 2004; Aroniadou-Anderjaska et al., 2007). Activation of postsynaptic GluK1Rs on somatodendritic sites of BLA interneurons enhances GABA release by depolarizing interneurons; presynaptic GluK1Rs on GABAergic terminals also facilitate GABA release when activated by low concentrations of an agonist, but inhibit GABA release when activated by higher agonist concentrations (≥ 1 μM ATPA; Braga et al., 2003). GluK1Rs are also present on principal neurons where they have a depolarizing action (Gryder and Rogawski, 2003). Thus, the net effect of GluK1R activation on the BLA network can be expected to be a reduction in excitability when agonist concentrations are low, while epileptiform activity is promoted with high agonist concentrations due to direct excitation of principal neurons and reduced GABA release from interneurons. Accordingly, GluK1R antagonists inhibit epileptiform and epileptic activity in the amygdala (Rogawski et al., 2003; Apland et al., 2007).
Due to the loss of principal neurons and primarily interneurons in the KA-SE group, the level of the GluK1 subunit in the BLA of the KA-SE rats was reduced. This finding alone, however, does not say whether or not there was a lower or an increased expression of the GluK1 subunit in the surviving interneurons. Expression of the GluK1 subunit could increase, but not sufficiently to overcome the overall loss in the protein due to neuronal loss. However, the electrophysiological experiments can exclude this possibility. When, in the KA-SE rats, 1) the frequency of sIPSCs recorded due to the spontaneous activity of the surviving interneurons is minimally enhanced by ATPA compared to the enhancement in the sham control group (Fig. 6), and 2) ATPA in the KA-SE group has no significant effect on the frequency of mIPSCs, when in the sham group ATPA increases or decreases the frequency of mIPSCs depending on the concentration (Fig. 7), the conclusion is that the GluK1Rs in the surviving interneurons are either functionally impaired or downregulated (reduced expression, internalization, or desensitization). Considering that GluK1Rs are activated by ambient concentrations of extracellular glutamate and facilitate GABA release (Braga et al., 2003), the reduced level and expression or function of GluK1Rs in the KA-SE rats can certainly contribute to the diminished frequency and amplitude of sIPSCs (Fig. 4) and frequency of mIPSCs (Fig. 5).
Functional Implications
The present study has demonstrated that due to a high vulnerability of the BLA interneurons to seizure-induced damage, the inhibitory tone in the BLA is already significantly compromised on days 7 to 10 after SE, before epilepsy is fully expressed. Expression of GAD and the GABAA α1 subunit are increased, but these alterations do not compensate for the interneuronal loss and the reduced inhibitory activity. The reduced inhibitory tone in the BLA at this relatively early stage of epileptogenesis may facilitate episodes of hyperexcitability and the associated excitotoxicity, thus contributing to delayed neuropathology (Nairismagi et al., 2004), and the ongoing neuronal degeneration that was observed in the present study. It is also reasonable to speculate that chronic loss/reduction of the inhibitory tone could alter the physiology of the surviving neurons, such as intrinsic membrane properties affecting excitability. Since GluK1Rs are activated by basal concentrations of extracellular glutamate facilitating GABA release (Braga et al., 2003), the reduced level and function of GluK1Rs during epileptogenesis may imply impairment in one of the “safety mechanisms” that prevent hyperactivity in the network. These changes can enhance the propensity of the BLA to generate seizures, which can spread to other limbic structures via the extensive projections from the BLA (Amaral et al., 1992; Pitkänen, 2000), facilitating the progression of epileptogenesis.
Acknowledgments
This work was supported by the National Institutes of Health CounterACT Program (NINDS award U01 NS058162-01 to MFMB), the Defense Threat Reduction Agency-Joint Science and Technology Office, Medical S&T Division (grant 1.E0021_07_US_C to MFMB), and the NINDS Intramural Research Program (MAR).
Abbreviations
- SE
status epilepticus
- BLA
basolateral amygdala
- ACSF
artificial cerebrospinal fluid
- FJ-C
FluoroJade-C
- GAD
glutamic acid decarboxylase
- GluK1Rs
GluK1-containing kainate receptors
- KA
kainic acid
- TLE
temporal lobe epilepsy
- TTX
tetrodotoxin
- sIPSCs
spontaneous inhibitory postsynaptic currents
- mIPSCs
miniature inhibitory postsynaptic currents
- C.E
coefficient of error
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amaral DG, Price JL, Pitkänen A, Carmichael TS. Anatomical organization of the primate amygdaloid complex. In: Aggleton J, editor. The Amygdala: Neurobiological Aspects of Emotion, Memory, and Mental Dysfunction. New York: Wiley-Liss; 1992. pp. 1–66. [Google Scholar]
- Angeleri F, Majkowski J, Cacchio G, Sobieszek A, D’Acunto S, Gesuita R, Bachleda A, Polonara G, Krolicki L, Signorino M, Salvolini U. Posttraumatic epilepsy risk factors: one-year prospective study after head injury. Epilepsia. 1999;40:1222–30. doi: 10.1111/j.1528-1157.1999.tb00850.x. [DOI] [PubMed] [Google Scholar]
- Annegers J, Grabow J, Groover R, Laws E, Elveback L, Kurland L. Seizures after head trauma: A population study. Neurology. 1980;30:683–89. doi: 10.1212/wnl.30.7.683. [DOI] [PubMed] [Google Scholar]
- Apland JP, Aroniadou-Anderjaska V, Braga MF. Soman induces ictogenesis in the amygdala and interictal activity in the hippocampus that are blocked by a GluR5 kainate receptor antagonist in vitro. Neuroscience. 2009;159:380–9. doi: 10.1016/j.neuroscience.2008.11.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aroniadou-Anderjaska V, Qashu F, Braga MF. Mechanisms regulating GABAergic inhibitory transmission in the basolateral amygdala: implications for epilepsy and anxiety disorders. Amino Acids. 2007;32:305–15. doi: 10.1007/s00726-006-0415-x. [DOI] [PubMed] [Google Scholar]
- Aroniadou-Anderjaska V, Fritsch B, Qashu F, Braga MF. Pathology and pathophysiology of the amygdala in epileptogenesis and epilepsy. Epilepsy Res. 2008;78:102–16. doi: 10.1016/j.eplepsyres.2007.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baran H, Kepplinger B, Draxler M, Skofitsch G. Choline acetyltransferase, glutamic acid decarboxylase and somatostatin in the kainic acid model for chronic temporal lobe epilepsy. Neurosignals. 2004;13:290–97. doi: 10.1159/000081964. [DOI] [PubMed] [Google Scholar]
- Benini R, Avoli M. Altered inhibition in lateral amygdala networks in a rat model of Temporal Lobe Epilepsy. J Neurophysiol. 2006;95:2143–54. doi: 10.1152/jn.01217.2005. [DOI] [PubMed] [Google Scholar]
- Best N, Mitchell J, Baimbridge KG, Wheal HV. Changes in parvalbumin-immunoreactive neurons in the rat hippocampus following a kainic acid lesion. Neurosci Lett. 1993;155:1–6. doi: 10.1016/0304-3940(93)90660-d. [DOI] [PubMed] [Google Scholar]
- Bettler B, Boulter J, Hermans-Borgmeyer I, O’Shea-Greenfield A, Deneris ES, Moll C, Borgmeyer U, Hollmann M, Heinemann S. Cloning of a novel glutamate receptor subunit, GluR5: expression in the nervous system during development. Neuron. 1990;5:583–95. doi: 10.1016/0896-6273(90)90213-y. [DOI] [PubMed] [Google Scholar]
- Blaustein MP. Calcium transport and buffering in neurons. Trends Neurosci. 1988;11:438–43. doi: 10.1016/0166-2236(88)90195-6. [DOI] [PubMed] [Google Scholar]
- Braga MF, Aroniadou-Anderjaska V, Xie J, Li H. Bidirectional modulation of GABA release by presynaptic glutamate receptor 5 kainate receptors in the basolateral amygdala. J Neurosci. 2003;23:442–52. doi: 10.1523/JNEUROSCI.23-02-00442.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braga MF, Aroniadou-Anderjaska V, Li H. The physiological role of kainate receptors in the amygdala. Mol Neurobiol. 2004;30:127–41. doi: 10.1385/MN:30:2:127. [DOI] [PubMed] [Google Scholar]
- Bragin A, Wilson CL, Fields T, Fried I, Engel J., Jr Analysis of seizure onset on the basis of wideband EEG recordings. Epilepsia. 2005;46(S5):59–63. doi: 10.1111/j.1528-1167.2005.01010.x. [DOI] [PubMed] [Google Scholar]
- Bragin A, Claeys P, Vonck K, Van Roost D, Wilson C, Boon P, Engel J., Jr Analysis of initial slow waves (ISWs) at the seizure onset in patients with drug resistant temporal lobe epilepsy. Epilepsia. 2007;48:1883–94. doi: 10.1111/j.1528-1167.2007.01149.x. [DOI] [PubMed] [Google Scholar]
- Brooks-Kayal AR, Shumate MD, Jin H, Rikhter TY, Coulter DA. Selective changes in single cell GABA(A) receptor subunit expression and function in temporal lobe epilepsy. Nat Med. 1998;4:1166–72. doi: 10.1038/2661. [DOI] [PubMed] [Google Scholar]
- Buckmaster PS, Dudek FE. Neuron loss, granule cell axon reorganization, and functional changes in the dentate gyrus of epileptic kainate-treated rats. J Comp Neurol. 1997;385:385–404. [PubMed] [Google Scholar]
- Cendes F, Andermann F, Gloor P, Evans A, Jones-Gotman M, Watson C, Melanson D, Olivier A, Peters T, Lopes-Cendes I. MRI volumetric measurement of amygdala and hippocampus in temporal lobe epilepsy. Neurology. 1993;43:719–25. doi: 10.1212/wnl.43.4.719. [DOI] [PubMed] [Google Scholar]
- Choi YS, Lin SL, Lee B, Kurup P, Cho HY, Naegele JR, Lombroso PJ, Obrietan K. Status epilepticus-induced somatostatinergic hilar interneuron degeneration is regulated by striatal enriched protein tyrosine phosphatase. J Neurosci. 2007;27:2999–3009. doi: 10.1523/JNEUROSCI.4913-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke VR, Ballyk BA, Hoo KH, Mandelzys A, Pellizzari A, Bath CP, Thomas J, Sharpe EF, Davies CH, Ornstein PL, Schoepp DD, Kamboj RK, Collingridge GL, Lodge D, Bleakman D. A hippocampal GluR5 kainate receptor regulating inhibitory synaptic transmission. Nature. 1997;389:599–603. doi: 10.1038/39315. [DOI] [PubMed] [Google Scholar]
- Cossart R, Dinocourt C, Hirsch JC, Merchan-Perez A, De Felipe J, Ben-Ari Y, Esclapez M, Bernard C. Dendritic but not somatic GABAergic inhibition is decreased in experimental epilepsy. Nat Neurosci. 2001;4:52–62. doi: 10.1038/82900. [DOI] [PubMed] [Google Scholar]
- Coulter DA. Chronic epileptogenic cellular alterations in the limbic system after status epilepticus. Epilepsia. 1999;40(S1):23–33. doi: 10.1111/j.1528-1157.1999.tb00875.x. [DOI] [PubMed] [Google Scholar]
- Covolan L, Mello LE. Temporal profile of neuronal injury following pilocarpine or kainic acid-induced status epilepticus. Epilepsy Res. 2000;39:133–52. doi: 10.1016/s0920-1211(99)00119-9. [DOI] [PubMed] [Google Scholar]
- Crestani F, Martin JR, Möhler H, Rudolph U. Mechanism of action of the hypnotic zolpidem in vivo. Br J Pharmacol. 2000;131:1251–56. doi: 10.1038/sj.bjp.0703717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Da Settimo F, Taliani S, Trincavelli ML, Montali M, Martini C. GABA A/Bz receptor subtypes as targets for selective drugs. Curr Med Chem. 2007;14:2680–701. doi: 10.2174/092986707782023190. [DOI] [PubMed] [Google Scholar]
- El-Hassar L, Milh M, Wendling F, Ferrand N, Esclapez M, Bernard C. Cell domain-dependent changes in the glutamatergic and GABAergic drives during epileptogenesis in the rat CA1 region. J Physiol. 2007;578:193–211. doi: 10.1113/jphysiol.2006.119297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erlander MG, Tobin AJ. The structural and functional heterogeneity of glutamic acid decarboxylase: a review. Neurochem Res. 1991;16:215–26. doi: 10.1007/BF00966084. [DOI] [PubMed] [Google Scholar]
- Esclapez M, Tillakaratne NJ, Kaufman DL, Tobin AJ, Houser CR. Comparative localization of two forms of glutamic acid decarboxylase and their mRNAs in rat brain supports the concept of functional differences between the forms. J Neurosci. 1994;14:1834–55. doi: 10.1523/JNEUROSCI.14-03-01834.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- French JA, Williamson PD, Thadani MD, Darcey TM, Mattson RH, Spenser SS, Spencer DD. Characteristics of medial temporal lobe epilepsy: I. Results of history and physical examination. Ann Neurol. 1993;34:774–80. doi: 10.1002/ana.410340604. [DOI] [PubMed] [Google Scholar]
- Freund TF, Ylinen A, Miettinen R, Pitkänen A, Lahtinen H, Baimbridge KG, Riekkinen PJ. Pattern of neuronal death in the rat hippocampus after status epilepticus. Relationship to calcium binding protein content and ischemic vulnerability. Brain Res Bull. 1992;28:27–38. doi: 10.1016/0361-9230(92)90227-o. [DOI] [PubMed] [Google Scholar]
- Fritschy JM, Keiner T, Bouilleret V, Loup F. Gabaergic neurons and GABA(A)-receptors in temporal lobe epilepsy. Neurochem Int. 1999;34:435–45. doi: 10.1016/s0197-0186(99)00040-6. [DOI] [PubMed] [Google Scholar]
- Gean PW, Shinnick-Gallagher P, Anderson AC. Spontaneous epileptiform activity and alteration of GABA- and NMDA-mediated neurotransmission in amygdala neurons kindled in vivo. Brain Research. 1989;494:177–81. doi: 10.1016/0006-8993(89)90160-1. [DOI] [PubMed] [Google Scholar]
- Gibbs JW, III, Shumate MD, Coulter DA. Differential epilepsy-associated alterations in postsynaptic GABA(A) receptor function in dentate granule and CA1 neurons. J Neurophysiol. 1997;77:1924–38. doi: 10.1152/jn.1997.77.4.1924. [DOI] [PubMed] [Google Scholar]
- Gilby KL, Da Silva AG, McIntyre DC. Differential GABA(A) subunit expression following status epilepticus in seizure-prone and seizure-resistant rats: a putative mechanism for refractory drug response. Epilepsia. 2005;46(S5):3–9. doi: 10.1111/j.1528-1167.2005.01001.x. [DOI] [PubMed] [Google Scholar]
- Goddard GV. Development of epileptic seizures through brain stimulation at low intensity. Nature. 1967;214:1020–21. doi: 10.1038/2141020a0. [DOI] [PubMed] [Google Scholar]
- Goddard GV, McIntyre DC, Leech CK. A permanent change in brain function resulting from daily electrical stimulation. Exp Neurol. 1969;25:295–330. doi: 10.1016/0014-4886(69)90128-9. [DOI] [PubMed] [Google Scholar]
- Goldring S, Edwards I, Harding GW, Bernardo KL. Results of anterior temporal lobectomy that spares the amygdala in patients with complex partial seizures. J Neurosurg. 1992;77:185–93. doi: 10.3171/jns.1992.77.2.0185. [DOI] [PubMed] [Google Scholar]
- Gorter JA, Goncalves Pereira PM, van Vliet EA, Aronica E, Lopes da Silva FH, Lucassen PJ. Neuronal cell death in a rat model for mesial temporal lobe epilepsy is induced by the initial status epilepticus and not by later repeated spontaneous seizures. Epilepsia. 2003;44:647–58. doi: 10.1046/j.1528-1157.2003.53902.x. [DOI] [PubMed] [Google Scholar]
- Gotman J, Levtova V. Amygdala-hippocampus relationships in temporal lobe seizures: a phase-coherence study. Epilepsy Res. 1996;25:51–57. doi: 10.1016/0920-1211(96)00021-6. [DOI] [PubMed] [Google Scholar]
- Gryder DS, Rogawski MA. Selective antagonism of GluR5 kainate-receptor-mediated synaptic currents by topiramate in rat basolateral amygdala neurons. J Neurosci. 2003;23:7069–74. doi: 10.1523/JNEUROSCI.23-18-07069.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerreiro C, Cendes F, Li LM, Jones-Gotman M, Andermann F, Dubeau F, Piazzini A, Feindel W. Clinical patterns of patients with temporal lobe epilepsy and pure amygdalar atrophy. Epilepsia. 1999;40:453–61. doi: 10.1111/j.1528-1157.1999.tb00740.x. [DOI] [PubMed] [Google Scholar]
- Gundersen HJ, Jensen EB, Kieu K, Nielsen J. The efficiency of systematic sampling -reconsidered. J Microsc. 1999;193:199–211. doi: 10.1046/j.1365-2818.1999.00457.x. [DOI] [PubMed] [Google Scholar]
- Hellier JL, Patrylo PR, Buckmaster PS, Dudek FE. Recurrent spontaneous motor seizures after repeated low-dose systemic treatment with kainate: assessment of a rat model of temporal lobe epilepsy. Epilepsy Res. 1998;31:73–84. doi: 10.1016/s0920-1211(98)00017-5. [DOI] [PubMed] [Google Scholar]
- Henry TR, Frey KA, Sackellares JC, Gilman S, Koeppe RA, Brunberg JA, Ross DA, Berent S, Young AB, Kuhl DE. In vivo cerebral metabolism and central benzodiazepine receptor binding in temporal lobe epilepsy. Neurology. 1993;43:1998–2006. doi: 10.1212/wnl.43.10.1998. [DOI] [PubMed] [Google Scholar]
- Hirsch JC, Agassandian C, Merchán-Pérez A, Ben-Ari Y, DeFelipe J, Esclapez M, Bernard C. Deficit of quantal release of GABA in experimental models of temporal lobe epilepsy. Nat Neurosci. 1999;2:499–500. doi: 10.1038/9142. [DOI] [PubMed] [Google Scholar]
- Houser CR, Esclapez M. Vulnerability and plasticity of the GABA system in the pilocarpine model of spontaneous recurrent seizures. Epilepsy Res. 1996;26:207–18. doi: 10.1016/s0920-1211(96)00054-x. [DOI] [PubMed] [Google Scholar]
- Houser CR, Esclapez M. Downregulation of the alpha5 subunit of the GABA(A) receptor in the pilocarpine model of temporal lobe epilepsy. Hippocampus. 2003;13:633–45. doi: 10.1002/hipo.10108. [DOI] [PubMed] [Google Scholar]
- Hudson LP, Munoz DG, Miller L, McLachlan RS, Girvin JP, Blume WT. Amygdaloid sclerosis in temporal lobe epilepsy. Ann Neurol. 1993;33:622–31. doi: 10.1002/ana.410330611. [DOI] [PubMed] [Google Scholar]
- Kairiss EW, Racine RJ, Smith GK. The development of the interictal spike during kindling in the rat. Brain Res. 1984;322:101–10. doi: 10.1016/0006-8993(84)91185-5. [DOI] [PubMed] [Google Scholar]
- Kaminski RM, Banerjee M, Rogawski MA. Topiramate selectively protects against seizures induced by ATPA, a GluR5 kainate receptor agonist. Neuropharmacology. 2004;46:1097–1104. doi: 10.1016/j.neuropharm.2004.02.010. [DOI] [PubMed] [Google Scholar]
- Kobayashi M, Buckmaster PS. Reduced inhibition of dentate granule cells in a model of temporal lobe epilepsy. J Neurosci. 2003;23:2440–52. doi: 10.1523/JNEUROSCI.23-06-02440.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kortenbruck G, Berger E, Speckmann EJ, Musshoff U. RNA editing at the Q/R site for the glutamate receptor subunits GLUR2, GLUR5, and GLUR6 in hippocampus and temporal cortex from epileptic patients. Neurobiol Dis. 2001;8:459–68. doi: 10.1006/nbdi.2001.0394. [DOI] [PubMed] [Google Scholar]
- Lemos T, Cavalheiro EA. Suppression of pilocarpine-induced status epilepticus and the late development of epilepsy in rats. Exp Brain Res. 1995;102:423–28. doi: 10.1007/BF00230647. [DOI] [PubMed] [Google Scholar]
- Li H, Chen A, Xing G, Wei ML, Rogawski MA. Kainate receptor-mediated heterosynaptic facilitation in the amygdala. Nat Neurosci. 2001;4:612–20. doi: 10.1038/88432. [DOI] [PubMed] [Google Scholar]
- Lowenstein DH, Thomas MJ, Smith DH, McIntosh TK. Selective vulnerability of dentate hilar neurons following traumatic brain injury: a potential mechanistic link between head trauma and disorders of the hippocampus. J Neurosci. 1992;12:4846–53. doi: 10.1523/JNEUROSCI.12-12-04846.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangan PS, Scott CA, Williamson JM, Bertram EH. Aberrant neuronal physiology in the basal nucleus of the amygdala in a model of chronic limbic epilepsy. Neuroscience. 2000;101:377–91. doi: 10.1016/s0306-4522(00)00358-4. [DOI] [PubMed] [Google Scholar]
- Mathern GW, Babb TL, Vickery BG, Melendez M, Pretorious JK. The clinical-pathogenic mechanisms of hippocampal neuron loss and surgical outcomes in temporal lobe epilepsy. Brain. 1995;118:105–18. doi: 10.1093/brain/118.1.105. [DOI] [PubMed] [Google Scholar]
- Mathern GW, Pretorius JK, Kornblum HI, Mendoza D, Lozada A, Leite JP, Chimelli L, Born DE, Fried I, Sakamoto AC, Assirati JA, Peacock WJ, Ojemann GA, Adelson PD. Altered hippocampal kainate-receptor mRNA levels in temporal lobe epilepsy patients. Neurobiol Dis. 1998;5:151–76. doi: 10.1006/nbdi.1998.0200. [DOI] [PubMed] [Google Scholar]
- McDonald AJ, Pearson JC. Coexistence of GABA and peptide immunoreactivity in non-pyramidal neurons of the basolateral amygdala. Neurosci Lett. 1989;100:53–58. doi: 10.1016/0304-3940(89)90659-9. [DOI] [PubMed] [Google Scholar]
- McDonald JW, Garofalo EA, Hood T, Sackellares JC, Gilman S, McKeever PE, Troncoso JC, Johnston MV. Altered excitatory and inhibitory amino acid receptor binding in hippocampus of patients with temporal lobe epilepsy. Ann Neurol. 1991;29:529–41. doi: 10.1002/ana.410290513. [DOI] [PubMed] [Google Scholar]
- Mohapel P, Dufresne C, Kelly ME, McIntyre DC. Differential sensitivity of various temporal lobe structures in the rat to kindling and status epilepticus induction. Epilepsy Res. 1996;23:179–87. doi: 10.1016/0920-1211(95)00084-4. [DOI] [PubMed] [Google Scholar]
- Morin F, Beaulieu C, Lacaille JC. Selective loss of GABA neurons in area CA1 of the rat hippocampus after intraventricular kainate. Epilepsy Res. 1998;32:363–69. doi: 10.1016/s0920-1211(98)00033-3. [DOI] [PubMed] [Google Scholar]
- Muller JF, Mascagni F, Mcdonald AJ. Postsynaptic targets of somatostatin-containing interneurons in the rat basolateral amygdala. J Comp Neurol. 2007;500:513–29. doi: 10.1002/cne.21185. [DOI] [PubMed] [Google Scholar]
- Nairismägi J, Gröhn OH, Kettunen MI, Nissinen J, Kauppinen RA, Pitkänen A. Progression of brain damage after status epilepticus and its association with epileptogenesis: a quantitative MRI study in a rat model of temporal lobe epilepsy. Epilepsia. 2004;45:1024–34. doi: 10.1111/j.0013-9580.2004.08904.x. [DOI] [PubMed] [Google Scholar]
- Nusser Z, Hajos N, Somogyi P, Mody I. Increased number of synaptic GABA(A) receptors underlies potentiation at hippocampal inhibitory synapses. Nature. 1998;395:172–77. doi: 10.1038/25999. [DOI] [PubMed] [Google Scholar]
- Obenhaus A, Esclapez M, Houser CR. Loss of glutamate decarboxylase mRNA-containing neurons in the rat dentate gyrus following pilocarpine-induced seizures. J Neurosci. 1993;13:4470–85. doi: 10.1523/JNEUROSCI.13-10-04470.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palma E, Esposito V, Mileo AM, Di Gennaro G, Quarato P, Giangaspero F, Scoppetta C, Onorati P, Trettel F, Miledi R, Eusebi F. Expression of human epileptic temporal lobe neurotransmitter receptors in Xenopus oocytes: An innovative approach to study epilepsy. Proc Natl Acad Sci USA. 2002;99:15078–83. doi: 10.1073/pnas.232574499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 4. New York NY: Elsevier; 1998. [DOI] [PubMed] [Google Scholar]
- Pitkänen A, Tuunanen J, Kälviäinen R, Partanen K, Salmenperä T. Amygdala damage in experimental and human temporal lobe epilepsy. Epilepsy Res. 1998;32:233–53. doi: 10.1016/s0920-1211(98)00055-2. [DOI] [PubMed] [Google Scholar]
- Pitkänen A. Connectivity of the rat amygdaloid complex. In: Aggleton JP, editor. The Amygdala: A functional analysis. New York: Oxford UP; 2000. pp. 31–115. [Google Scholar]
- Pumain R, Laschet J. A key glycolytic enzyme plays a dual role in GABAergic neurotransmission and in human epilepsy. Crit Rev Neurobiol. 2006;18:197–203. doi: 10.1615/critrevneurobiol.v18.i1-2.200. [DOI] [PubMed] [Google Scholar]
- Quesney LF. Clinical and EEG features of complex partial seizures of temporal lobe origin. Epilepsia. 1986;27(S2):27–45. doi: 10.1111/j.1528-1157.1986.tb05738.x. [DOI] [PubMed] [Google Scholar]
- Racine RJ, Paxinos G, Mosher JM, Kairiss EW. The effects of various lesions and knife-cuts on septal and amygdala kindling in the rat. Brain Res. 1988;454:264–74. doi: 10.1016/0006-8993(88)90826-8. [DOI] [PubMed] [Google Scholar]
- Raol YH, Zhang G, Lund IV, Porter BE, Maronski MA, Brooks-Kayal AR. Increased GABA(A)-receptor alpha1-subunit expression in hippocampal dentate gyrus after early-life status epilepticus. Epilepsia. 2006;47:1665–73. doi: 10.1111/j.1528-1167.2006.00640.x. [DOI] [PubMed] [Google Scholar]
- Rice A, Rafiq A, Shapiro SM, Jakoi ER, Coulter DA, DeLorenzo RJ. Long-lasting reduction of inhibitory function and gamma-aminobutyric acid type A receptor subunit mRNA expression in a model of temporal lobe epilepsy. Proc Natl Acad Sci USA. 1996;93:9665–69. doi: 10.1073/pnas.93.18.9665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocha L, Suchomelová L, Mares P, Kubová H. Effects of LiCl/pilocarpine-induced status epilepticus on brain mu and benzodiazepine receptor binding: regional and ontogenetic studies. Brain Res. 2007;1181:104–17. doi: 10.1016/j.brainres.2007.08.062. [DOI] [PubMed] [Google Scholar]
- Rogawski MA, Gryder D, Castaneda D, Yonekawa W, Banks MK, Li H. GluR5 kainate receptors, seizures, and the amygdala. Ann N Y Acad Sci. 2003;985:150–62. doi: 10.1111/j.1749-6632.2003.tb07079.x. [DOI] [PubMed] [Google Scholar]
- Rudolph U, Crestani F, Benke D, Brunig I, Benson JA, Fritschy JM, Martin JR, Bluethmann H, Möhler H. Benzodiazepine actions mediated by specific gamma-aminobutyric acid(A) receptor subtypes. Nature. 1999;401:796–800. doi: 10.1038/44579. [DOI] [PubMed] [Google Scholar]
- Salazar AM, Jabbari B, Vance SC, Grafman J, Amin D, Dillon JD. Epilepsy after penetrating head injury. I. Clinical correlates: a report of the Vietnam Head Injury Study. Neurology. 1985;35:1406–14. doi: 10.1212/wnl.35.10.1406. [DOI] [PubMed] [Google Scholar]
- Scharfman HE, Schwartzkroin PA. Protection of dentate hilar cells from prolonged stimulation by intracellular calcium chelation. Science. 1989;246:257–60. doi: 10.1126/science.2508225. [DOI] [PubMed] [Google Scholar]
- Schwarzer C, Tsunashima K, Wanzenböck C, Fuchs K, Sieghart W, Sperk G. GABA(A) receptor subunits in the rat hippocampus II: altered distribution in kainic acid-induced temporal lobe epilepsy. Neuroscience. 1997;80:1001–17. doi: 10.1016/s0306-4522(97)00145-0. [DOI] [PubMed] [Google Scholar]
- Shao LR, Dudek FE. Changes in mIPSCs and sIPSCs after kainate treatment: evidence for loss of inhibitory input to dentate granule cells and possible compensatory responses. J Neurophysiol. 2005;94:952–960. doi: 10.1152/jn.01342.2004. [DOI] [PubMed] [Google Scholar]
- Schmitz C, Hof PR. Recommendations for straightforward and rigorous methods of counting neurons based on a computer simulation approach. J Chem Neuroanat. 2000;20:93–114. doi: 10.1016/s0891-0618(00)00066-1. [DOI] [PubMed] [Google Scholar]
- Sloviter RS. Decreased hippocampal inhibition and a selective loss of interneurons in experimental epilepsy. Science. 1987;235:73–76. doi: 10.1126/science.2879352. [DOI] [PubMed] [Google Scholar]
- Sloviter RS. Calcium-binding protein (calbindin-D28k) and parvalbumin immunocytochemistry: localization in the rat hippocampus with specific reference to the selective vulnerability of hippocampal neurons to seizure activity. J Comp Neurol. 1989;280:183–96. doi: 10.1002/cne.902800203. [DOI] [PubMed] [Google Scholar]
- Sloviter RS, Zappone CA, Harvey BD, Frotscher M. Kainic acid-induced recurrent mossy fiber innervation of dentate gyrus inhibitory interneurons: possible anatomical substrate of granule cell hyper-inhibition in chronically epileptic rats. J Comp Neurol. 2006;494:944–60. doi: 10.1002/cne.20850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith BN, Dudek FE. Enhanced population responses in the basolateral amygdala of kainate-treated, epileptic rats in vitro. Neurosci Lett. 1997;222:1–4. doi: 10.1016/s0304-3940(97)13326-2. [DOI] [PubMed] [Google Scholar]
- Smolders I, Bortolotto ZA, Clarke VR, Warre R, Khan GM, O’Neill MJ, Ornstein PL, Bleakman D, Ogden A, Weiss B, Stables JP, Ho KH, Ebinger G, Collingridge GL, Lodge D, Michotte Y. Antagonists of GLU(K5)-containing kainate receptors prevent pilocarpine-induced limbic seizures. Nat Neurosci. 2002;5:796–804. doi: 10.1038/nn880. [DOI] [PubMed] [Google Scholar]
- Soghomonian JJ, Martin DL. Two isoforms of glutamate decarboxylase: why? Trends Pharmacol Sci. 1998;19:500–05. doi: 10.1016/s0165-6147(98)01270-x. [DOI] [PubMed] [Google Scholar]
- Sperk G, Lassmann H, Baran H, Kish SJ, Seitelberger F, Hornykiewicz O. Kainic acid induced seizures: neurochemical and histopathological changes. Neuroscience. 1983;10:1301–1315. doi: 10.1016/0306-4522(83)90113-6. [DOI] [PubMed] [Google Scholar]
- Statler KD. Pediatric posttraumatic seizures: epidemiology, putative mechanisms of epileptogenesis and promising investigational progress. Dev Neurosci. 2006;28:354–63. doi: 10.1159/000094162. [DOI] [PubMed] [Google Scholar]
- Straessle A, Loup F, Arabadzisz D, Ohning GV, Fritschy JM. Rapid and long-term alterations of hippocampal GABAB receptors in a mouse model of temporal lobe epilepsy. Eur J Neurosci. 2003;18:2213–26. doi: 10.1046/j.1460-9568.2003.02964.x. [DOI] [PubMed] [Google Scholar]
- Sun C, Mtchedlishvili Z, Bertram EH, Erisir A, Kapur J. Selective loss of dentate hilar interneurons contributes to reduced synaptic inhibition of granule cells in an electrical stimulation-based animal model of temporal lobe epilepsy. J Comp Neurol. 2007;500:876–93. doi: 10.1002/cne.21207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treib J, Haass A, Grauer MT. High-velocity bullet causing indirect trauma to the brain and symptomatic epilepsy. Mil Med. 1996;161:61–64. [PubMed] [Google Scholar]
- Tsunashima K, Schwarzer C, Kirchmair E, Sieghart W, Sperk G. GABA(A) receptor subunits in the rat hippocampus III: altered messenger RNA expression in kainic acid-induced epilepsy. Neuroscience. 1997;80:1019–32. doi: 10.1016/s0306-4522(97)00144-9. [DOI] [PubMed] [Google Scholar]
- Tuunanen J, Halonen T, Pitkänen A. Status epilepticus causes selective regional damage and loss of GABAergic neurons in the rat amygdaloid complex. Eur J Neurosci. 1996;8:2711–25. doi: 10.1111/j.1460-9568.1996.tb01566.x. [DOI] [PubMed] [Google Scholar]
- Tuunanen J, Halonen T, Pitkänen A. Decrease in somatostatin-immunoreactive neurons in the rat amygdaloid complex in a kindling model of temporal lobe epilepsy. Epilepsy Res. 1997;26:315–27. doi: 10.1016/s0920-1211(96)00900-x. [DOI] [PubMed] [Google Scholar]
- Tuunanen J, Lukasiuk K, Halonen T, Pitkänen A. Status epilepticus-induced neuronal damage in the rat amygdaloid complex: distribution, time-course, and mechanisms. Neuroscience. 1999;94:473–95. doi: 10.1016/s0306-4522(99)00251-1. [DOI] [PubMed] [Google Scholar]
- Ullal G, Fahnestock M, Racine R. Time-dependent effect of kainate-induced seizures on glutamate receptor GluR5, GluR6, and GluR7 mRNA and protein expression in rat hippocampus. Epilepsia. 2005;46:616–23. doi: 10.1111/j.1528-1167.2005.49604.x. [DOI] [PubMed] [Google Scholar]
- White LE, Price JL. The functional anatomy of limbic status epilepticus in the rat. I. Patterns of 14C-2-deoxyglucose uptake and Fos immunocytochemistry. J Neurosci. 1993a;13:4787–4809. doi: 10.1523/JNEUROSCI.13-11-04787.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White LE, Price JL. The functional anatomy of limbic status epilepticus in the rat. II. The effects of focal deactivation. J Neurosci. 1993b;13:4810–30. doi: 10.1523/JNEUROSCI.13-11-04810.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf HK, Aliashkevich AF, Blümcke I, Wiestler OD, Zentner J. Neuronal loss and gliosis of the amygdaloid nucleus in temporal lobe epilepsy: a quantitative analysis of 70 surgical specimens. Acta Neuropathol. 1997;93:606–10. doi: 10.1007/s004010050658. [DOI] [PubMed] [Google Scholar]