Abstract
It is possible that motor adaptation in the timescales of minutes is supported by two distinct processes: one process that learns slowly from error but has strong retention, and another that learns rapidly from error but has poor retention. This two-state model makes the prediction that if a period of adaptation is followed by a period of reverse-adaptation, then in the subsequent period in which errors are clamped to zero (error-clamp trials) there will be a spontaneous recovery, i.e., a rebound of behavior toward the initial level of adaptation. Here we tested and confirmed this prediction during double-step, on-axis, saccade adaptation. When people adapted their saccadic gain to a magnitude other than one (adaptation) and then the gain was rapidly reversed back to one (reverse-adaptation), in the subsequent error-clamp trials (visual target placed on the fovea after the saccade) the gain reverted toward the initially adapted value and then gradually reverted toward normal. We estimated that the fast system was about 20 times more sensitive to error than the slow system, but had a time constant of 28 seconds while the slow system had a time constant of nearly 8 minutes. Therefore, short-term adaptive mechanisms that maintain accuracy of saccades rely on a memory system that has characteristics of a multi-state process with a logarithmic distribution of timescales.
Keywords: Saccade adaptation, motor memory, computational neuroscience, extinction
Introduction
The phenomenon of spontaneous recovery has been extensively observed in memory research, particularly in the classical conditioning paradigm (Myers and Davis, 2002). In this paradigm, the animal is presented with a paired conditioned and unconditioned stimuli (CS and US) until the association is learned. Subsequently, the CS is presented without the US until it no longer produces a response (termed extinction). Interestingly, in the post-extinction period the animal increasingly shows some of its previously learned response to the CS. In the literature on motor control, a similar phenomenon has been observed in control of eye movements: when monkeys adapt control of their saccades to an artificial manipulation of the target, and then are rapidly de-adapted back to the baseline state, subsequent passage of time in complete darkness (in which no errors are available) makes the oculomotor system revert back toward its initially adapted state (Kojima et al., 2004).
Recently, we proposed that a simple model of memory can explain this general phenomenon (Smith et al., 2006). In this model, learning is affected by two factors: prediction error, which causes the brain to adapt and change its behavior in order to minimize errors in subsequent trials, and passage of time, which causes forgetting. The model explained that in a typical short-term training paradigm (many minutes), behavior was supported by two processes: a fast adaptive process that was highly sensitive to error but had poor retention, and a slow adaptive process that had poor sensitivity to error but had robust retention. Behavior was the sum of these two processes. The model explained that spontaneous recovery occurred because a typical adaptation/reverse-adaptation protocol triggered a specific chain of events: during the initial long period of adaptation, most of the behavior became dependent on the slow adaptive process. Reverse-adaptation training forced behavior to return to baseline not through washing out of the learning, but by introducing a fast process that competed with the slow process. With passage of time after reverse-adaptation, the fast process faded, allowing an apparent recovery of the initial response.
To test the model, we needed a protocol to continuously assay the state of the learner’s memory during the spontaneous recovery period. This is difficult, however, because the state of the learner can change because of errors that might occur on various trials and because of the passage of time from trial to trial. Theoretically, spontaneous recovery is best observed if passage of time is the only factor that influences behavior. Therefore, we needed a protocol in which behavior was recorded continuously while errors were eliminated in every trial. These ‘error-clamp’ trials were recently introduced in the context of reaching (Scheidt et al., 2000; Smith et al., 2006). Here, we employed a similar approach in a saccade adaptation experiment to test whether behavior exhibited spontaneous recovery after adaptation/reverse-adaptation training.
Materials and Methods
Subjects were recruited from our medical school community. The experiments were composed of two complementary double-step adaptation paradigms, together lasting a total of 45 minutes. Group 1 subjects trained in a gain-down (decrease) paradigm and then in a gain-up (increase) paradigm until the gain returned to baseline (n=9, including authors DZ and RS). Group 2 subjects (n=8, including authors RS) trained in a gain-up paradigm and then in a gain-down paradigm. Four subjects participated in both groups. There was at least a break of one day between the two experiments. Subjects gave written consent and the protocol was approved by the Johns Hopkins Institutional Review Board.
Experimental Setup
The position of either the right or the left eye was measured with a magnetic-field search-coil system (Robinson, 1963) using directional scleral annuli (Skalar Medical BV, Delft, Netherlands). Raw coil signals were filtered in hardware (90-Hz low-pass Butterworth), digitized (1,000 Hz), and saved on computer for later analysis. Targets were rear-projected using a mirror-controlled laser beam of 2mm-diameter projected onto a translucent screen located 1m in front of the subject with a 15deg step-response of faster than 10ms. The room was otherwise dark. The head of the subjects was stabilized with a bite bar of dental impression material.
Experimental Paradigms
Saccade adaptation was induced using the standard double-step paradigm (McLaughlin, 1967). The experiment began with a sequence of error-clamp trials, then adaptation trials, and concluded with another set of error-clamp trials, as illustrated in Fig. 1.
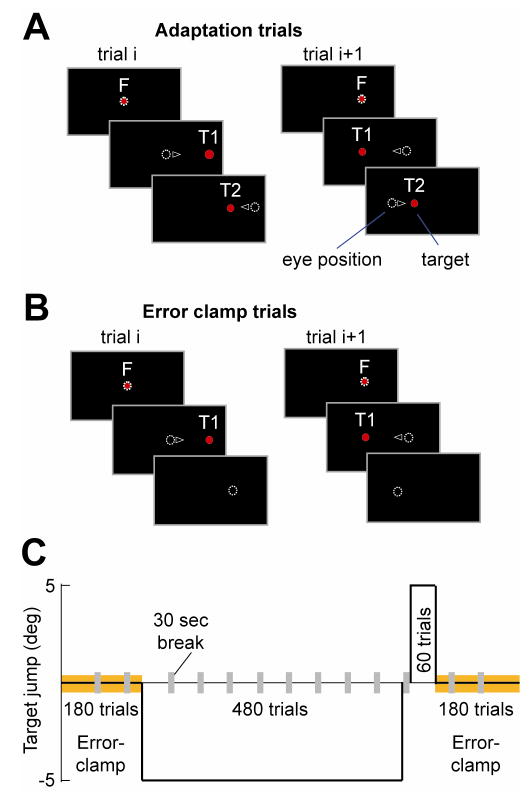
Figure 1.
Experimental paradigm. A. Adaptation trials (gain-down shown here). F: fixation. T1: target of the saccade. T2: remapped target during the saccade. Small triangle: direction of eye movement. The trial ended with the eyes at T2, which then became the fixation for the next trial. B. Error-clamp trials. T1 disappeared upon saccade initiation. After a brief period in the dark (500 or 800ms), it reappeared at the exact position of the fovea. That point became the fixation point for the next trial. C. The training sets began with 180 error-clamp trials, followed by 480 adaptation trials, 60 extinction trials, and finally 180 error-clamp trials. Adaptation for Group 1 was gain-down (as shown here) and for Group 2 was gain-up (not shown).
Adaptation trials
Trials (Fig. 1A) began with a fixation target. At a random time in the range of 500–1000ms, the fixation target was turned off and target T1 appeared at 15° horizontal. Once the eye began moving toward T1, T1 was displaced to T2. The displacement was either 5° away from (gain-up) or 5° closer to (gain-down) the initial fixation point. The jump was triggered near the onset of the eye movement, defined as the moment where the eye crossed a virtual 2° window placed around the fixation point (only visible to the experimenter). Target T2 was maintained for 500ms, at which time the trial ended and T2 became the fixation point for the next trial in the opposite direction. Over time, subjects learned to make a saccade in response to T1 that was smaller (gain-down paradigm) or larger (gain-up paradigm). Each adaptation set consisted of 60 trials. The mean inter-trial time was 1250ms. Between sets, subjects received 30 seconds of rest, in which they were asked to close their eyes.
Error-clamp trials
Trials (Fig. 1B) began with a fixation target. At a random time in the range of 500–1000ms, the fixation target was turned off and target T1 was displayed at 15° horizontal. Once the saccade began, T1 disappeared and at 500 or 800ms later (Groups 1 and 2, respectively) it reappeared at the position where the eye was located at 10ms prior (i.e., 490 or 790ms). In this way, first no and then a zero visual error was present after the saccade. Due to slight amplitude asymmetry between leftward and rightward saccade, occasionally a drift away from the center developed over time. We restrained eye position inside a +/−20-degree range by resetting the fixation point to +/−5 degrees whenever the eye landed out-of-bounds. The mean inter-trial time was 1250ms and 1550ms (for Groups 1 and 2, respectively).
Control trials
At the start of each experiment, subjects performed 20 trials in which the visual target at 15° horizontal remained present throughout the saccade and post-saccadic periods.
Group 1: Gain-down then gain-up
The sequence of trials is illustrated in Fig. 1C. Subjects performed 180 error-clamp trials consisting of 90, 60 and then 30 trials. They then had 480 gain-down trials followed by 60 gain-up trials. The gain-up trials were followed by a sequence of 30, 60 and 90 error-clamp trials. A brief rest period (30 seconds) was inserted between sets as shown in Fig. 1C.
Group 2: Gain-up then gain-down
For this group, the pattern of training was the same as in Group 1 except that gain-down training followed gain-up training.
Data analysis
The duration of saccades was determined by a 20-deg/sec speed threshold. Discriminating criteria were used to dismiss abnormal saccades. Saccades were rejected (i) if they didn’t reach a peak velocity higher than 90 deg/sec; (ii) if they had a latency less than 100ms; (iii) if they displayed multiple peaks in their speed profile; (iv) and finally if they were shorter than 50% of the target displacement. Most subjects had less than 5% of their saccade falling under one or more of these aforementioned criteria, with none of them exceeding 10% of all saccades.
The system identification problem
We assumed that the learner’s behavior (saccade amplitude) on any given trial depended on the values of two hidden states (effectively, the states of the memory). We represent these states with vector x (a 2×1). The states were affected by three factors: visual error ỹ at end of a saccade, passage of time between trials, and Gaussian noise εx. If we assume that the inter-trial interval is constant, then we can write the change in states from trial to trial as:
| (1) |
In this equation, the matrix A (a diagonal 2×2) specifies how the states will change from trial n to n+1 because of passage of time, and the vector b specifies how the states will change because of the error observed on trial n. We cannot directly observe the states, but can measure saccade amplitude on trial n as y(n), which we assume is affected by target displacement p(n), some inherent bias that the subject may have yb, a weighted sum of all memory states (the weights are unknown), plus execution noise εy. This is written as:
| (2) |
In Eq. (2), the vector c specifies the relative weight of each state in influencing the saccade amplitude. Our next step is to transform these equations so that they can be easily fitted to our data (the sequence of saccade amplitudes y(n)).
The error on trial n is due to the intra-saccadic displacement that we imposed on the target. If we write that displacement as u(n), then the error on that trial is:
| (3) |
Inserting Eq. (3) into Eq. (1) produces our state space model of the task:
| (4) |
In our experiment, for each subject we gave a sequence of targets p(n), displaced that target during the saccade by amount u(n), and measured the saccade amplitude y(n). In adaptation trials, u(n) was the intra-saccadic target displacement. In error-clamp trials u(n)= y(n)− p(n)+ yb
In summary, the mathematical problem consists in finding the parameters of the adaptive system of Eq. (4), given a sequence of inputs u(n) (target displacements) and measurements y(n) (saccade amplitudes)..
A recent breakthrough called sub-space identification (van Overschee and De Moor, 1996) provides elegant, closed form solutions for identification of stochastic linear systems. [A tutorial on the subject is available in the lecture notes of the last author’s web page: webhost5.nts.jhu.edu/reza/Courses/learningtheory_files/subspace.ppt] What we did in the above derivation is to transform our adaptive system equations into a form that can easily be solved by this approach. Once the parameters of the system are identified, we can estimate the state vector (i.e., the memory state) at each time step using a Kalman filter.
It is important to note that there is an infinite space of solutions to our problem, i.e., the same set of input and output data can be generated by an infinite number of systems that, from the point of view of Eq. (4), are indistinguishable. A particularly useful solution among these is one in which the matrix A is diagonal, assigning a unique time constant to each state. After estimation of the parameters, we transformed the system to one where A was diagonal. This produced a time constant of forgetting for each state. To interpret the time scales, we translated the state update equation of the discrete system of Eq. (4) to continuous time:
where Ac =Δ−1(A−bcT− I), bc =Δ−1b, and Δ is the inter-trial interval, set to 1250ms. If we represent vector x as [xf, xs]T, i.e., the fast and slow states, then λs and λf refer to the time constant of the solution to this differential equation.
Results
We employed two kinds of trials in our experiments. In an adaptation trial, the visual target was displaced during the saccade (e.g., Fig. 1A, gain-down training). In an error-clamp trial (Fig. 1B), visual cues were removed at saccade onset and withheld until 500ms (Group 1) or 800ms (Group 2) after the saccade. At that time, the target was shown at precisely the current eye position. The idea was to assay the state of the saccadic system without introducing endpoint errors. The protocol consisted of the following blocks of trials: error-clamp, adaptation training, reverse-adaptation training, and error-clamp (Fig. 1C).
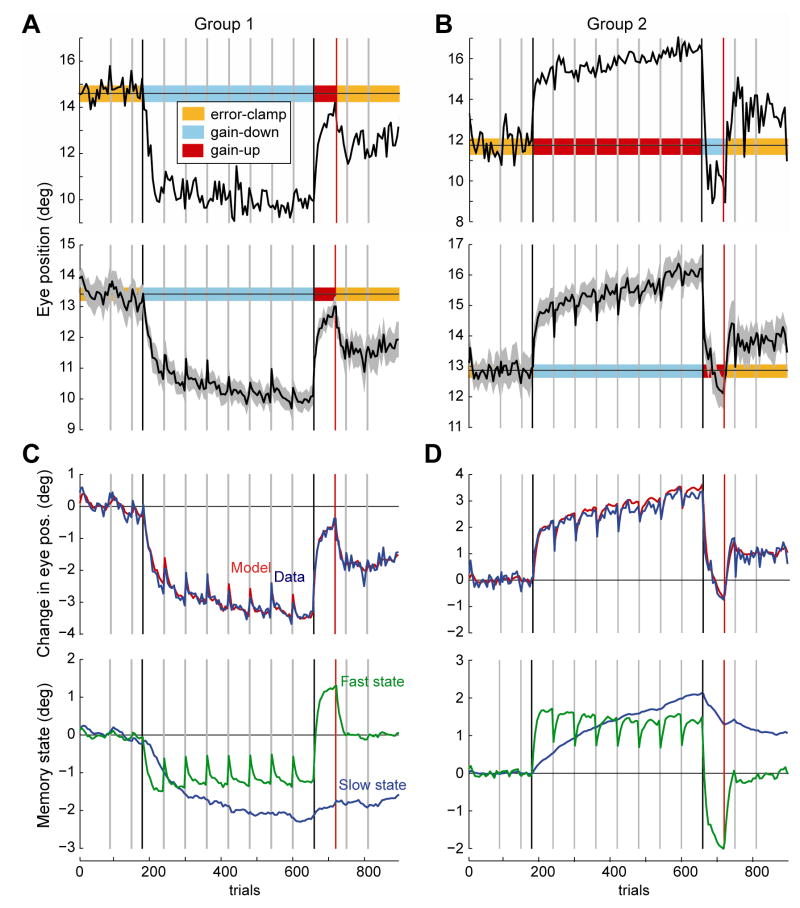
Figs. 2A and 2B show representative and average saccade amplitude data for the two groups. In Group 1, saccades were hypometric (13.2° ± 0.47, mean and SEM) in the initial set of error-clamp trials. The ensuing gain-down adaptation induced a rapid decrease in amplitude in the first set, followed by a slower decrease in the seven remaining sets. By the last set of gain-down training, saccade amplitude had dropped to 10.0° ± 0.32. It took only 60 trials in the following gain-up training to bring the saccade amplitudes to near baseline (13.0° ± 0.30 for the last six trials). However, in the subsequent error-clamp trials saccade amplitudes did not remain stationary. Rather, they sharply declined (p=0.002, one-sided t-test, within subject comparison of the last six trials of gain-up vs. last six-trials of the immediately following the set of error-clamp trials). In the last two sets of the error-clamp trials amplitudes were 11.4° ± 0.52 (p<0.0001, one-sided t-test, within subject comparison of first and last two sets of error-clamp trials). Therefore, when gain-down training was followed by a rapid period of gain-up training, in the following error-clamp trials the amplitudes reverted back toward the values achieved in the initial gain-down period.
Figure 2.
Adaptation, extinction, and spontaneous recovery of saccade gains. In all plots, the vertical gray lines indicate 30 second set breaks. The two vertical black lines mark the beginning and end of the adaptation trials. The vertical red line marks the end of the extinction trials. A and B. Representative saccade amplitudes and group data (with SEM). Data was averaged using variable bin widths to show the rapid changes that occur at set breaks: In each set, the bin size was two trials for the first bin, then four trials, then six trials for all subsequent bins for that set. C and D. The top sub-plot shows the fit of Eq. (4) to the mean data. The bottom plot shows the contribution of each hidden state to the motor output. The bin sizes are the same as parts A and B.
We observed a similar pattern of spontaneous recovery when gain-up training was followed by gain-down training (Group 2, Fig. 2B). In the initial error-clamp trials saccades were hypometric (12.9° ± 0.51). Amplitudes increased rapidly in the initial gain-up set and then gradually increased to 16.0° ± 0.41 by the final set. The 60 trials of reverse-adaptation training were sufficient to rapidly reduce saccade amplitudes to slightly below baseline levels (12.1° ± 0.51 by the last six trials of the gain-down training). In the subsequent error-clamp trials, however, saccade amplitudes sharply increased (p=0.004 one-sided t-test, within subject comparison of the last six trials of gain-down vs. last six-trials of the immediately following error-clamp trials). Amplitudes remained significantly larger than baseline in the final two error-clamp trials (13.8° ± 0.56, p=0.003, one-sided t-test, within subject comparison of first and last two sets of error-clamp trials). In summary, when adaptation was followed by a rapid period of reverse-adaptation training, in the following period of error-clamp trials saccade amplitude continued to change, reverting toward the behavior exhibited during initial adaptation.
Effect of error-clamp trials
Saccades in the initial error-clamp trials were hypometric, as is typical for saccades made in the dark to single sources of light (Collewijn et al., 1988). However, no visual feedback was available in error-clamp trials for 500–800ms. Did this delay in feedback influence saccade amplitudes? A recent report suggests that for large saccades (22°–34°), lack of visual feedback at saccade termination may result in an increase in saccade amplitudes (Bonnetblanc and Baraduc, 2007). To check for this, we compared the amplitudes of saccades in the initial sets of error-clamp trials to a set of control saccades immediately preceding these trials: Before the main experiment began, subjects in both groups performed 20 trials in which the visual target at 15° remained lit throughout the saccade and post-saccade inter-trial periods. For Group 1, amplitudes in the control period (13.3° ± 0.13) were indistinguishable from the amplitudes in the following error-clamp trials (13.2° ± 0.47, t-test p>0.5). To examine this question further, we increased the delay period in Group 2 to 800ms from the value of 500ms in Group 1. Similar to Group 1, amplitudes in the control period in Group 2 (13.3° ± 0.24) were not reliably distinguishable from the amplitudes in the following error-clamp trials (12.9° ± 0.51, p>0.1). Therefore, error-clamp trials did not appear to significantly bias the amplitudes of saccades.
Effect of the 30sec break between sets
The group data shown in Figs. 2A and 2B suggests that the brief set breaks might have had a highly repeatable effect on saccade amplitudes. That is, in Group 1 the set breaks appeared to coincide with an increase in the amplitudes during the gain-down adaptation blocks, whereas in Group 2 the set breaks coincided with a decrease in the amplitudes during the gain-up adaptation blocks. To quantify this effect, for each subject we compared the last four saccades in each set with the first four saccades in the following set during the gain-down trials in Group 1 and gain-up trials in Group 2. On average, subjects in Group 1 showed an increase of 0.6° ± 0.17 (F(7,1)=14.9, p<0.01) and subjects in Group 2 showed a decrease of 0.6° ± 0.21 (F(8,1)=7.84, p<0.05) during the set breaks. Therefore, the 30sec breaks between sets produced a significant forgetting. The amount of forgetting was indistinguishable between the two groups.
The multiple states of motor memory
Spontaneous recovery is a signature of an adaptive system that is supported by multiple states, each learning at a different timescale (Smith et al., 2006; Kording et al., 2007). To estimate these states and their timescales, we fitted the group data to a linear stochastic state-space model of learning (Eq. 4). In this system, the motor output (saccade amplitude) was supported by two hidden states. The changes in these states were a function of time between trials and endpoint errors observed on each trial.
Figs. 2C and 2D show the fit of the model and plot the estimated contribution of each state to motor output, i.e., cTx= cs xs + cf xf, in which the subscripts denote the slow and fast state. In the initial error-clamp trials, both states were near zero and were driven only by noise. In the subsequent adaptation stage, the fast state learned rapidly while the slow state lagged behind. During the subsequent 30sec rest period between sets, the fast state showed a large decay while the slow state showed little or no decay. This model exhibited two fundamental properties of memory: a fast system that learned quickly but showed poor retention with passage of time, and a slow system that learned slowly but had significantly better retention.
As the adaptation trials continued, the slow state over-took the fast state so that the by the end of gain-down training in Group 1 or gain-up training in Group 2, the slow state’s contribution was twice the fast state. Therefore, by the end of 480 adaptation trials (just before start of reverse-adaptation block) performance was dominated by the slow state.
In the subsequent set break, the fast state once again decayed toward baseline. However, in the subsequent training block (reverse-adaptation training) the errors were in the opposite direction of the previous training, forcing the fast state to rapidly learn and acquire values that now competed with the values of the slow state. By the end of the reverse-adaptation block, the sum of the two states was near baseline (top sub-plot of Fig. 2C, line labeled ‘Model’), but of course the two states had not returned to baseline.
In the following error-clamp trials (note that there was no set break here), the fast state rapidly declined to baseline whereas the slow state gradually declined. The sum effect was spontaneous recovery. That is, in Group 1 motor output reverted back toward the gain-down pattern and in Group 2 it reverted back toward the gain-down pattern.
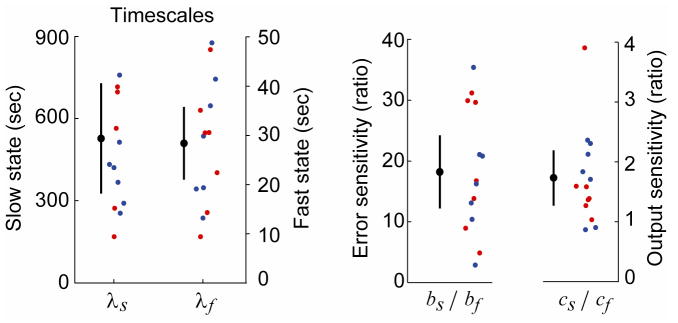
The fit to the group data produced a decay time constant of λf = 28 sec for the fast state and λs = 7 min for the slow state. Error sensitivity of the fast state was 18 times larger for the fast state, i.e., bf/bs =18. Finally, saccade amplitudes relied almost twice as much on the slow than the fast state, i.e., cs/cf =1.7.
We next examined the distribution of these estimates by fitting the model to each subject and condition. The fit was satisfactory in 14 of 17 subjects and their parameter values are plotted in Fig. 3. The data for the three subjects that we could not fit exhibited large variability in saccade amplitudes during the initial error-clamp trials. For the remaining 14 subjects, we found that λf = 28 ± 7 sec (mean ± 95%CI), λs = 7.8 ± 3 min, bf/bs =18 ± 6, and cf/cs =1.7 ± 0.5. Therefore, the time scales of the fast and the slow system, as well as their sensitivity to error, were an order of magnitude apart.
Figure 3.
Distribution of model parameters when Eq. (4) was fitted to each subject data. λs and λf are the time constants of the slow and fast states, which describe the sensitivity of these states to passage of time. bs and bf are the sensitivity of the two states to error. cs and cf are the weighted contribution of these states to the measured behavior.
Discussion
We tested the idea that the memory that contributes to the accuracy of saccades is composed of two functional states: a fast state that learns from error but has poor retention, and a slow state that learns less from the same error but has better retention. The theory predicted that when adaptation is followed by reverse-adaptation, the resulting behavior will show spontaneous recovery of the initially acquired adapted state. To test for this, we began with an adaptation block that used intra-saccadic manipulation of the visual target to introduce endpoint errors. This was followed by a reverse-adaptation block in which the direction of the intra-saccadic target motion was reversed until the saccade amplitudes reached baseline. We then introduced error-clamp trials in which the post-saccadic visual information was withheld for 500 or 800ms, and then shown at the current eye position, signaling zero errors. Saccade amplitudes during this period of error-clamp trials exhibited spontaneous recovery, reverting back toward their initially adapted state.
Is there evidence that in short-term paradigms (up to 1 hour) the memory that supports generation of saccades is affected by both the passage of time and errors? Seeberger et al. (2002) reported that when a block of gain-down adaptation trials (in which the target was moved intra-saccadically and remained visible in the post-saccadic period) was followed by trials in which the post-saccadic target was turned off, the reduced saccade amplitudes caused by gain-down adaptation gradually returned back to baseline. Therefore, passage of time produced a decay in the memory acquired during adaptation. However, the return to baseline was faster if the target remained stationary and the endpoint errors encouraged extinction. This is consistent with a model in which both error and passage of time affect the state of the learner.
If the saccades of the learner are supported by a memory that can be represented as a single state, then adaptation followed by extinction will not produce spontaneous recovery (Smith et al., 2006). Rather, in the post-extinction period saccade amplitudes simply return to baseline. However, if memory is effectively supported by two or more states, then the differential sensitivity of the purported states to error and passage of time can produce spontaneous recovery. That is, in trials in which errors are eliminated, the fast state should rapidly return to baseline, leaving behavior that reflects the slow state. Indeed, in the 30 error-clamp trials post extinction we observed a near 50% recovery of the saccade amplitudes. We estimated that during these trials the learning from the fast state had completely dissipated.
We found that during the 30sec break that separated each adaptation set, saccade amplitudes changed by about 0.6° with a direction that was opposite to the direction of adaptation. The model explained that this forgetting was due to the same fast process that produced spontaneous recovery in the post-extinction error-clamp trials. Therefore, it was crucial not to introduce a break between the extinction block and the error-clamp block. Such a break would prevent us from observing the rapid post-extinction change in saccade amplitudes.
How accurate is our assumption that error-clamp trials eliminated the error signal? The errors that drive saccade adaptation are derived mainly from visual information during the post-saccade period (Wallman and Fuchs, 1998;Noto and Robinson, 2001). When this information is delayed, the resulting adaptation is reduced or eliminated. For example, a 750ms delay reduces the error-dependent adaptive response by 90% (Shafer et al., 2000). In our paradigm, we not only included a delay in presenting the post-saccadic visual information, but we also presented the visual target at the current eye position. Therefore, the combined techniques should reduce any error-driven adaptive response in a given trial even further.
Because saccades in the dark are hypometric, it is conceivable that when a post-saccadic target appears at the current eye position the brain interprets this as an error and attempts to reduce amplitude of the subsequent saccade. This would predict that error-clamp trials should increase hypometria. We checked for this by comparing saccade amplitudes in response to targets that did not disappear (control trials before start of the error-clamp trials) vs. targets that disappeared and then were shown after a delay at the current eye position (error-clamp trials) and did not find a significant difference. If error-clamp trials introduced inadvertent errors, the effect must be quite small because the same model that assumed zero-error in error-clamp trials and accounted for spontaneous recovery also explained the forgetting during the 30sec rest periods during which subjects were in complete darkness.
Perhaps the most significant contribution of the model is that it suggests the possibility that even in brief adaptation experiments, changes in behavior are not only a function of error, but also passage of time. The initially rapid and then gradual changes in the post-extinction trials are largely due to the effect of time passage on the states that supported the memory during error-dependent adaptation. The model predicts that functionally, these states have both fast and slow timescales, with values that are an order of magnitude apart. An order of magnitude also differentiates the sensitivities that these memory states express with respect to error. If one imagines that there are not two but many more states that support a memory, and their sensitivity to passage of time and error is distributed along a logarithmic scale, then such models may be able to account for saccade adaptation data on timescales of weeks and months (Kording et al., 2007). Therefore, such motor memory becomes ‘cemented’ only if it is repeated, causing adaptation in the slowest of the slow states.
The adaptive behavior that we examined here is grossly impaired when there is damage to the oculomotor vermis in the cerebellar cortex (Takagi et al., 1998; Barash et al., 1999) or fastigial oculomotor region in the cerebellar deep nuclei (Robinson et al., 2002). In other cerebellar-dependent paradigms it has been hypothesized that the initially rapid changes in behavior during adaptation may be due to changes in the cerebellar cortex, while the retention of the memory over the long-term (days) may depend on the cerebellar nuclei (Bracha et al., 2000; Medina et al., 2001; Shutoh et al., 2006). Of course, saccade adaptation may also engage structures other than the cerebellum. Therefore, one possibility is that the two timescales reflect changes in distinct anatomical substrates that all contribute to the motor commands that move the eyes.
This line of thinking would be bolstered if there was evidence for the two states in the trajectory of saccades. In a recent examination of cross-axis adaptation, we indeed found that the commands that initiated the saccade adapted more slowly than commands that arrived later in the same saccade (Chen-Harris et al., 2008). For example, the 30sec break between sets produced dramatic forgetting in the late-acting motor commands, yet it had essentially no effect on the motor commands that initiated the saccade. If the late acting motor commands can be attributed to a ‘steering’ mechanism of the eyes via the cerebellum (Kojima et al., 2008), then it is possible that the fast states are a reflection of a changing contribution from this structure.
However, the fact that we have observed two functional states of memory in no way implies involvement of two distinct neural structures. In principle, the two states may reflect mechanisms of plasticity in a single neuron. From the point of view of the two-state model, spontaneous recovery is closely related to ‘savings’: when adaptation is followed by extinction, subjects exhibit faster relearning in a subsequent re-adaptation block. The two-state model explains both phenomena using the concept of fast and slow systems (Smith et al., 2006). Recently, Jirenhed et al. (2007) demonstrated that behavioral changes during adaptation, extinction, and savings in a classical conditioning task that lasted about 16 hours were correlated with discharge of single Purkinje cells in the cerebellar cortex. Therefore, the multiple states that produced savings in that paradigm did not appear to require two or more distinct neural substrates, but were perhaps part of the molecular machinery that supported synaptic plasticity in single Purkinje cells.
Reference List
- Barash S, Melikyan A, Sivakov A, Zhang M, Glickstein M, Thier P. Saccadic dysmetria and adaptation after lesions of the cerebellar cortex. J Neurosci. 1999;19:10931–10939. doi: 10.1523/JNEUROSCI.19-24-10931.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnetblanc F, Baraduc P. Saccadic adaptation without retinal postsaccadic error. Neuroreport. 2007;18:1399–1402. doi: 10.1097/WNR.0b013e3282c48cc1. [DOI] [PubMed] [Google Scholar]
- Bracha V, Zhao L, Irwin KB, Bloedel JR. The human cerebellum and associative learning: dissociation between the acquisition, retention and extinction of conditioned eyeblinks. Brain Res. 2000;860:87–94. doi: 10.1016/s0006-8993(00)01995-8. [DOI] [PubMed] [Google Scholar]
- Chen-Harris H, Joiner WM, Ethier V, Zee DS, Shadmehr R. Adaptive control of saccades via internal feedback. J Neurosci. 2008 doi: 10.1523/JNEUROSCI.5300-07.2008. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collewijn H, Erkelens CJ, Steinman RM. Binocular co-ordination of human horizontal saccadic eye movements. J Physiol. 1988;404:157–182. doi: 10.1113/jphysiol.1988.sp017284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jirenhed DA, Bengtsson F, Hesslow G. Acquisition, extinction, and reacquisition of a cerebellar cortical memory trace. J Neurosci. 2007;27:2493–2502. doi: 10.1523/JNEUROSCI.4202-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima Y, Iwamoto Y, Robinson FR, Noto CT, Yoshida K. Premotor inhibitory neurons carry signals related to saccade adaptation in the monkey. J Neurophysiol. 2008;99:220–230. doi: 10.1152/jn.00554.2007. [DOI] [PubMed] [Google Scholar]
- Kojima Y, Iwamoto Y, Yoshida K. Memory of learning facilitates saccadic adaptation in the monkey. J Neurosci. 2004;24:7531–7539. doi: 10.1523/JNEUROSCI.1741-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kording KP, Tenenbaum JB, Shadmehr R. The dynamics of memory as a consequence of optimal adaptation to a changing body. Nat Neurosci. 2007;10:779–786. doi: 10.1038/nn1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin S. Parametric adjustment in saccadic eye movements. Percept Psychophys. 1967;2:359–362. [Google Scholar]
- Medina JF, Garcia KS, Mauk MD. A mechanism for savings in the cerebellum. J Neurosci. 2001:21. doi: 10.1523/JNEUROSCI.21-11-04081.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers KM, Davis M. Behavioral and neural analysis of extinction. Neuron. 2002;36:567–584. doi: 10.1016/s0896-6273(02)01064-4. [DOI] [PubMed] [Google Scholar]
- Noto CT, Robinson FR. Visual error is the stimulus for saccade gain adaptation. Brain Res Cogn Brain Res. 2001;12:301–305. doi: 10.1016/s0926-6410(01)00062-3. [DOI] [PubMed] [Google Scholar]
- Robinson DA. A method of measuring eye movement using a scleral search coil in a magnetic field. IEEE Trans Biomed Eng. 1963;10:137–145. doi: 10.1109/tbmel.1963.4322822. [DOI] [PubMed] [Google Scholar]
- Robinson FR, Fuchs AF, Noto CT. Cerebellar influences on saccade plasticity. Ann N Y Acad Sci. 2002;956:155–163. doi: 10.1111/j.1749-6632.2002.tb02816.x. [DOI] [PubMed] [Google Scholar]
- Scheidt RA, Reinkensmeyer DJ, Conditt MA, Rymer WZ, Mussa-Ivaldi FA. Persistence of motor adaptation during constrained, multi-joint, arm movements. J Neurophysiol. 2000;84:853–862. doi: 10.1152/jn.2000.84.2.853. [DOI] [PubMed] [Google Scholar]
- Seeberger T, Noto C, Robinson F. Non-visual information does not drive saccade gain adaptation in monkeys. Brain Res. 2002;956:374–379. doi: 10.1016/s0006-8993(02)03577-1. [DOI] [PubMed] [Google Scholar]
- Shafer JL, Noto CT, Fuchs AF. Temporal characteristics of error signals driving saccadic gain adaptation in the macaque monkey. J Neurophysiol. 2000;84:88–95. doi: 10.1152/jn.2000.84.1.88. [DOI] [PubMed] [Google Scholar]
- Shutoh F, Ohki M, Kitazawa H, Itohara S, Nagao S. Memory trace of motor learning shifts transsynaptically from cerebellar cortex to nuclei for consolidation. Neuroscience. 2006;139:767–777. doi: 10.1016/j.neuroscience.2005.12.035. [DOI] [PubMed] [Google Scholar]
- Smith MA, Ghazizadeh A, Shadmehr R. Interacting adaptive processes with different timescales underlie short-term motor learning. PLoS Biol. 2006;4:e179. doi: 10.1371/journal.pbio.0040179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takagi M, Zee DS, Tamargo RJ. Effects of lesions of the oculomotor vermis on eye movements in primate: saccades. J Neurophysiol. 1998;80:1911–1931. doi: 10.1152/jn.1998.80.4.1911. [DOI] [PubMed] [Google Scholar]
- van Overschee P, De Moor B. Subspace identification for linear systems. Boston: Kluwer Academic; 1996. http://homes.esat.kuleuven.be/~smc/sysid/software/ [Google Scholar]
- Wallman J, Fuchs AF. Saccadic gain modification: visual error drives motor adaptation. J Neurophysiol. 1998;80:2405–2416. doi: 10.1152/jn.1998.80.5.2405. [DOI] [PubMed] [Google Scholar]