Abstract
Background/aims
The oral cavity harbors a diverse and complex microbial community. Bacteria accumulate on both the hard and soft oral tissues in sessile biofilms and engage the host in an intricate cellular dialog, which normally constrains the bacteria to a state of commensal harmony. Dendritic cells (DCs) are likely to balance tolerance and active immunity to commensal microorganisms as part of chronic inflammatory responses. While the role played by DCs in maintaining intestinal homeostasis has been investigated extensively, relatively little is known about DC responses to oral bacteria.
Methods
In this study, we pulsed human monocyte-derived immature DCs (iDCs) with cell wall extracts from pathogenic and commensal gram-positive or gram-negative oral bacteria.
Results
Although all bacterial extracts tested induced iDCs to mature and produce cytokines/chemokines including interleukin-12p40, tumor necrosis factor-α, and monocyte chemoattractant protein-1 (MCP-1), the most important factor for programming DCs by oral bacteria was whether they were gram-positive or gram-negative, not whether they were commensal or pathogenic. In general, gram-negative oral bacteria, except for periodontopathic Porphyromonas gingivalis, stimulated DC maturation and cytokine production at lower concentrations than gram-positive oral bacteria. The threshold of bacteria needed to stimulate chemokine production was 100-fold to 1000-fold lower than that needed to induce cytokines. In addition, very low doses of oral commensal bacteria triggered monocytes to migrate toward DC-derived MCP-1.
Conclusion
Oral commensal and pathogenic bacteria do not differ qualitatively in how they program DCs. DC-derived MCP-1 induced in response to oral commensal bacteria may play a role, at least in part, in the maintenance of oral tissue integrity by attracting monocytes.
Keywords: commensal bacteria, dendritic cells, MCP-1
Tissue homeostasis mediated by the interaction between commensal bacteria and hosts has been extensively studied using intestinal epithelial models in mice (19, 25). These studies have revealed that commensal bacterial effects on epithelial cells are important factors for maintaining tissue homeostasis. Gut commensal bacteria trigger anti-inflammatory programs in intestinal epithelial cells by blocking the activation of nuclear factor-κB (NF-κB) by either inhibiting ubiquitination of the inhibitor of NF-κB, I-κB-α (25) or by promoting the nuclear export of transcriptionally active RelA (19).
A small proportion of incoming bacteria evading epithelial barriers are captured by antigen-presenting cells, which are mostly dendritic cells (DCs) (37). Epithelial cells also actively direct DCs (33) to maintain local tissue integrity. DCs can also directly take up luminal bacteria (32). Through this mechanism, DCs open tight junctions between epithelial cells without damaging epithelial tissue integrity and sense bacteria directly. In addition, DCs have been shown to contribute to intestinal homeostasis by inducing regulatory T cells (Tregs) (10). CD4+ CD25+ CTLA-4+ Tregs mediate tolerance to organ specific self-antigens and can prevent auto-immunity and intestinal inflammation in mice (31, 39). DCs stimulated with a commensal strain of Escherichia coli secrete interleukin-10 (IL-10) in a dose-dependent manner (10), and DCs secreting IL-10 induce CD4 T cells differentiate into Tregs (1). These findings support the model that DCs programmed by commensal bacteria contribute to intestinal tissue homeostasis by inducing Tregs. In addition, the frequency of CD4+ CD25+ Foxp3+ Tregs differs between germ-free and specific-pathogen-free mice (28). Germ-free mice have fewer Tregs than specific-pathogen-free mice in mesenteric lymph nodes and in liver-draining celiac lymph nodes, suggesting that the presence of certain microbial flora directs the development of functional Tregs. Tregs are known to migrate towards chemokines, such as thymus and activation-regulated chemokine (TARC)/CCL17 in humans (15) and monocyte chemoattractant protein-1 (MCP-1)/CCL2 in mice (11, 12).
Relatively little is known about how DCs distinguish commensal flora from pathogenic bacteria and mount protective immunity only against the pathogens. The oral cavity harbors a diverse and complex microbial community. The oral microbial flora contains approximately 500 species, and is composed of both commensal and pathogenic species (30). It has been shown that gingival epithelial cells secrete IL-8/CXCL-8, a neutrophil chemoattractant, in response to oral bacteria (14, 20, 44) and this is believed to be beneficial for periodontal tissue. Although DCs also secrete IL-8 in response to bacterial stimuli (40), it is not known how oral commensal bacteria affect IL-8 and other chemokines produced by DCs, and how this may contribute to oral tissue homeostasis. Langerhans cells, a subpopulation of epithelial tissue-specific DCs, can migrate into sites of early gingivitis (26), and migrate out as the gingivitis reaches a chronic status (24). In addition, recent studies have revealed that DCs can infiltrate the lamina propria of pocket epithelium in chronic periodontitis (8, 17). DCs may well be involved in the progression of periodontitis.
Immature DCs sense microbes using pattern-recognition receptors, such as Toll-like receptors and C-type lectin receptors (3). An unresolved question is how commensal and pathogenic oral bacteria differently program DCs. In addition, how oral mucosal homeostasis is maintained by the interaction between oral commensal bacteria and human DCs has not yet been investigated.
We tested if oral commensal and pathogenic bacteria have different effects on programming DCs by stimulating immature human monocyte-derived DCs (iDCs) with cell wall extracts from a series of gram-positive or gram-negative oral bacteria. We evaluated the programming of iDCs by oral bacteria by monitoring DC maturation and by determining the threshold of bacteria needed to induce cytokines or chemokines. We defined the ‘threshold’ of cell wall extract as the dose needed to stimulate DCs to produce 30% or more of the maximum cytokines or chemokines secreted. Our data revealed that the gram-negative oral bacteria, whether they are commensal or pathogenic, could program iDCs more efficiently than gram-positive oral bacteria.
Materials and methods
Bacterial cell wall preparation
Porphyromonas gingivalis (ATCC33277), Actinomyces naeslundii (ATCC19039), Streptococcus gordonii (ATCC51556), Streptococcus mutans (ATCC700610), Haemophilus aphrophilus (ATCC33389), and Aggregatibacter actinomycetemcomitans HK1651 (ATCC700685; serotype b) were purchased from the American Type Culture Collection (Manassas, VA). A. actinomycetemcomitans D7S (serotype a) and Eikenella corrodens (D27p-1) were recovered from a patient with aggressive periodontitis (6, 23, 41). Bacteria were grown and their crude cell wall extracts were prepared using a French pressure cell at 1,054,100 kg/m2. Following centrifugation at 145,000 g, the pellet was resuspended with phosphate-buffered saline and frozen at −80°C until use.
Flow cytometry
The following monoclonal antibodies were used for surface staining: phycoerythrin-conjugated anti-CD1a (Beckman Coulter, Miami, FL, USA), and anti-CD83 (BD Pharmingen, San Diego, CA). The cells were also stained with corresponding phycoerythrin-conjugated isotype-matched control monoclonal antibodies. The cells were analyzed using a FACScalibur flow cytometer and CELLQuest software (BD Biosciences, San Jose, CA, USA).
Cell isolation and culture
Human iDCs were generated from peripheral blood mononuclear cells obtained from buffy coat preparations (Red Cross Blood Bank, Portland, OR, USA) or from leukapheresis products of healthy donors. The iDCs obtained from buffy coat products were isolated as previously described (7). Monocytes (>97–99% CD14+) purified from leukapheresis products by CD14+ immunomagnetic-positive selection were purchased from the Cellular Therapy Laboratory at the Fred Hutchinson Cancer Research Center (Seattle, WA, USA). To obtain iDCs, CD14+ cells were seeded at a density of 3 × 106 cells/ml in six-well plates in 5 ml RPMI-1640 medium supplemented with 10% fetal bovine serum (BioWhittaker, Walkersville, MD, USA), human granulocyte–macrophage colony-stimulating factor (100 ng/ml, Leukine; Amgen, Seattle, WA, USA), and human IL-4 (30 ng/ml; RDI, Flanders, NJ, USA) and cultured for 6 days. Cells were fed on days 2 and 4 by replacing half the medium and adding fresh cytokines. On day 6 the cells exhibited an immature DC phenotype, that is, CD1ahigh CD14−. For maturation of the iDCs, cells were stimulated for 24 h with E. coli lipopolysaccharide (LPS; 1 μg/ml; Sigma-Aldrich Inc., St Louis, MO, USA), or with graded doses of oral bacterial cell wall extracts (0.0001 to ~ 10 μg/ml) or with live A. naeslundii and S. gordonii. The A. naeslundii was grown in Actinomyces Broth for 24 h at 37°C. After washing, bacterial numbers were adjusted to a density of 1.5 × 108. S. gordonii were grown in tryptic soy broth with yeast extract (TSB-YE) medium for 6 h at 37°C following overnight inoculation. Then, bacterial numbers were adjusted to a density of 1.5 × 108.
Cytokine and chemokine enzyme-linked immunosorbent assay
After iDCs were cultured with bacterial cell wall extracts or with live bacteria as described above, supernatants were harvested and levels of cytokines and chemokines were determined by enzyme-linked immunosorbent assay (ELISA). The amounts of IL-1β, IL-2, IL-8, IL-10, IL-12p40, IL-12p70, tumor necrosis factor-α (TNF-α), TARC, MCP-1 (R&D Systems Inc., Minneapolis, MN), and IL-23 (eBioscience Inc., San Diego, CA, USA) were quantified using ELISA kits.
Migration assays
Migration of CD14+ monocytes was measured in duplicate using a transwell system (24-well plates; 5.0-μm pore size; Costar, Corning, NY, USA). A total of 7 × 105 iDCs in 700 μl RPMI-1640 medium containing 1% fetal calf serum was plated in the lower chamber. DCs were stimulated with graded doses of cell wall extracts from either A. naeslundii or S. gordonii; 24 h after DC stimulation, 4 × 105 monocytes in 100 μl medium were added to the upper chamber and incubated at 37°C for 3 h. Cells that migrated into the lower chamber were harvested, concentrated to a volume of 200 μl, and CD14+ monocytes were counted by flow cytometry acquiring events for a fixed time of 90 s. The counts fell within a linear range of the control titration curves obtained by testing increasing concentrations of cells.
Results
Gram-negative oral bacteria are more potent inducers of DC maturation compared to gram-positive oral bacteria
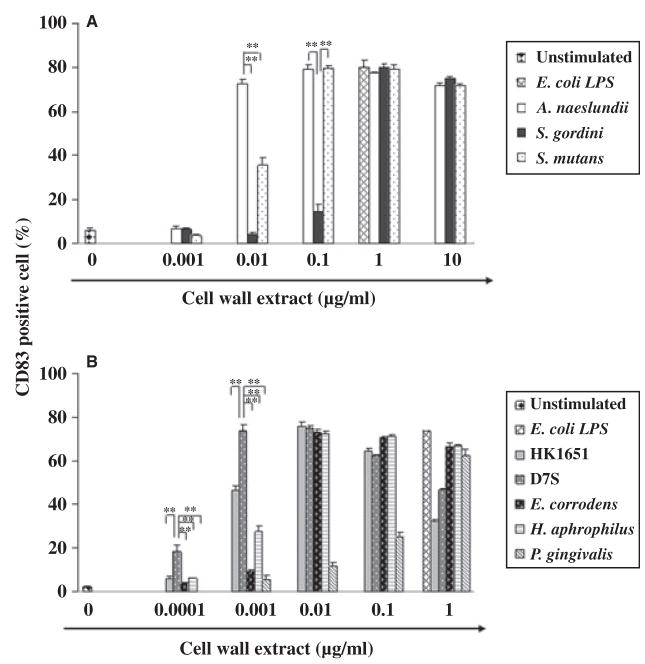
Once iDCs come into contact with pathogenic bacteria they are activated to become mature DCs. Therefore, we first examined the effect of oral bacteria on DC maturation as measured by the expression of the DC maturation marker, CD83. Cell wall extracts obtained from representative gram-positive and gram-negative oral bacteria were employed to examine those cell components that have the potential to interact with DCs. All bacterial extracts tested induced maturation of iDCs and CD83 expression (Fig. 1A,B). When we compared a group of oral gram-positive bacteria, to our surprise the commensal A. naeslundii was the most effective at inducing DC maturation: while A. naeslundii induced DC maturation with as little as 0.01 μg/ml cell wall extract, another commensal S. gordonii and the pathogen S. mutans resembled P. gingivalis and required 10-fold to 100-fold higher concentrations than A. naeslundii to mature DCs (Fig. 1A). The gram-negative oral bacteria, with the exception of P. gingivalis, induced DC maturation at much lower concentrations than the gram-positive oral bacteria tested (Fig. 1B). Among the gram-negative oral bacteria tested, A. actinomycetemcomitans D7S was the most potent at inducing DC maturation. In addition, as little as 0.001 μg/ml A. actinomycetemcomitans D7S induced a similar level of DC maturation as 10 times more of the most effective gram-positive bacterium, A. naeslundii. Gram-negative A. actinomycetemcomitans HK1651, E. corrodens, and H. aphrophilus extracts were somewhat less potent than A. actinomycetemcomitans D7S, but were still much more effective at inducing DC maturation than gram-positive S. mutans and S. gordonii. When iDCs were stimulated with a high dose of 1 μg/ml A. actionomycetemcomitans, the percentage of CD83 cells decreased; this decrease was associated with, and most likely the result of, cell death (data not shown).
Fig. 1.
Dendritic cell (DC) maturation in response to gram-positive (A) and gram-negative (B) oral bacteria. Twenty-four hours after immature DCs (iDCs) were stimulated with graded doses of oral bacterial cell wall extract, the expression of CD83 was examined by flow cytometry. Escherichia coli lipopolysaccharide (LPS) and media were used as positive and negative controls, respectively. Representative results of three independent experiments with three different donors are shown. Asterisks indicate statistically significant difference (**P < 0.01) determined by Student’s t-test.
Gram-negative oral bacteria have lower thresholds for inducing proinflammatory cytokines than gram-positive oral bacteria
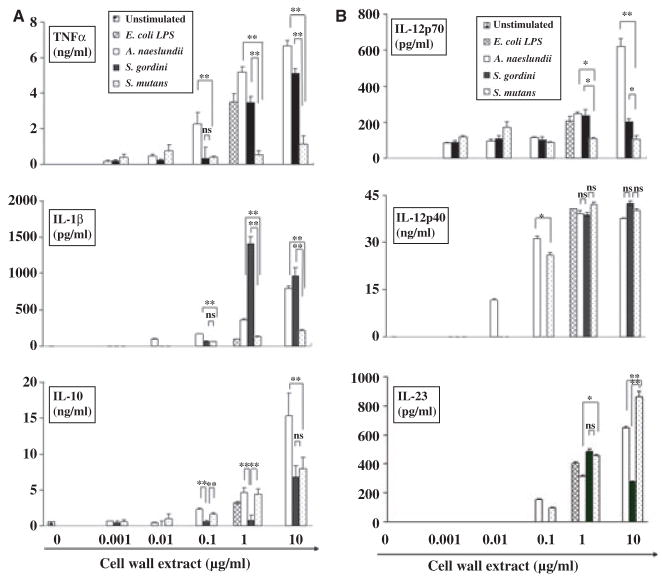
Activated DCs secrete cytokines, many of which are important in the elicitation of primary immune responses. Therefore, we next examined the cytokine profiles of DCs after stimulation with oral bacteria. The gram-positive bacteria A. naeslundii, S. gordonii, and S. mutans induced cytokine secretion by DCs in a dose-dependent manner (Fig. 2). The cell wall extracts of the commensals A. naeslundii and S. gordonii were significantly most effective at stimulating DCs to secrete TNF-α and IL-1β, respectively, while S. mutans induced IL-23 from DCs most effectively. Among all the gram-positive organisms tested, A. naeslundii had the lowest threshold for inducing secretion of all cytokines tested, which was dramatically lower than even a highly periodontopathic bacterium, P. gingivalis (Table 1).
Fig. 2.
Dose-dependent cytokine secretion by dendritic cells (DCs) in response to gram-positive oral bacteria, Actinomyces naeslundii, Streptococcus gordonii, Streptococcus mutans. Escherichia coli lipopolysaccharide (LPS) and media were used as positive and negative controls respectively. Immature DCs stimulated with graded doses of bacterial cell wall extract and culture supernatants were collected after 24 h. The concentration of tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β), IL-10, IL-12p70, IL-12p40, and IL-23 were measured by enzyme-linked immunosorbent assay. All assays were performed in triplicate to obtain mean ± SD. Representative results from three independent experiments with three different donors are shown. Asterisks indicate statistically significant difference (*P < 0.05, **P < 0.01, NS: not significant) determined by Student’s t-test.
Table 1.
Thresholds for proinflammatory cytokines and chemokines of dendritic cells in response to oral bacteria
| Dose of cell wall extract (μg/ml) | ||||||
|---|---|---|---|---|---|---|
| 0.0001 | 0.001 | 0.01 | 0.1 | 1 | 10 | |
| TNF-α | HK1651 D7S E. corrodens |
A. naeslundii2 | S. gordonii2 |
P. gingivalis S. mutans2 |
||
| MCP-1 | D7S |
A. naeslundii2 HK1651 E. corrodens H. aphrophilus |
S. mutans2 |
S. gordoni2 P. gingivalis |
||
| IL-8 | D7S |
HK1651 E. corrodens H. aphrophilus |
A. naeslundii2 S. mutans2 |
S. gordoni2 P. gingivalis |
||
The threshold was defined as the dose of cell wall extract inducing 30% of cytokines/chemokines secreted.
G(+) oral bacteria.
TNF-α, tumor necrosis factor-α; MCP-1, monocyte chemoattractant protein-1; IL-8, interleukin-8; D7S, Aggregatibacter actinomycetemcomitans serotype a; HK 1651, Aggregatibacter actinomycetemcomitans serotype b.
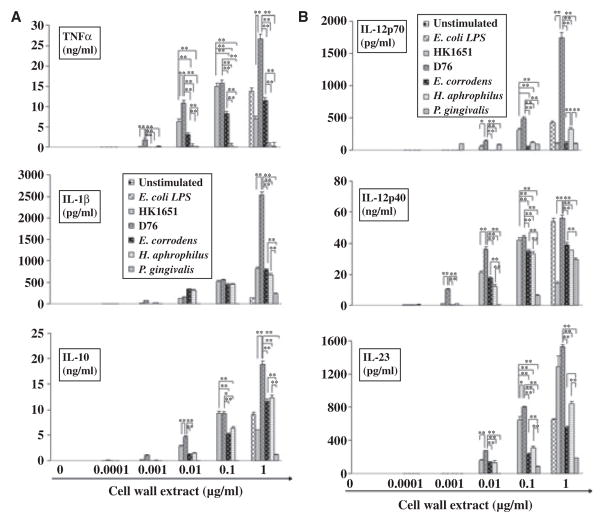
The gram-negative oral bacteria also induced proinflammatory cytokines in a dose-dependent manner and with different thresholds (Fig. 3 and Table 1). Among them, A. actinomycetemcomitans D7S was significantly more effective than the other gram-negative bacteria tested at inducing proinflammatory cytokines, and this was particularly evident at lower doses. Surprisingly, the two commensals tested, E. corrodens and H. aphrophilus, induced significantly more inflammatory cytokines than P. gingivalis did at the same dose, with one exception: P. gingivalis induced more IL-12p70 secretion at lower extract doses than the commensals. Overall, the gram-negative oral bacteria had lower thresholds for inducing proinflammatory cytokines than gram-positive oral bacteria (Table 1). H. aphrophilus did not induce TNF-α secretion by iDCs at any of the dosages tested. Except for IL-1β and IL-23, cytokine levels decreased when iDCs were stimulated with 1 μg/ml of cell wall extract from A. actinomycetemcomitans HK1651, which is more virulent than the A. actinomycetemcomitans D7S strain. This decreased cytokine production was associated with cell death induced by A. actinomycetemcomitans HK1651 (data not shown) as has been previously reported (18).
Fig. 3.
Dose-dependent induction of proinflammatory cytokines by dendritic cells (DCs) in response to gram-negative oral bacteria. Escherichia coli lipopolysaccharide (LPS) and media were used as positive and negative controls, respectively. Immature DCs were stimulated with graded doses of bacterial cell wall extract and culture supernatants were collected after 24 h. The concentration of tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β), IL-10, IL-12p70, IL-12p40, and IL-23 were measured by enzyme-linked immunsorbent assay. All assays were performed in triplicate to obtain mean ± SD. Representative results from three independent experiments with three different donors are shown. Asterisks indicate statistically significant difference (*P < 0.05, **P < 0.01) determined by Student’s t-test.
Fewer oral bacteria are needed to induce iDCs to produce chemokines than to induce production of proinflammatory cytokines
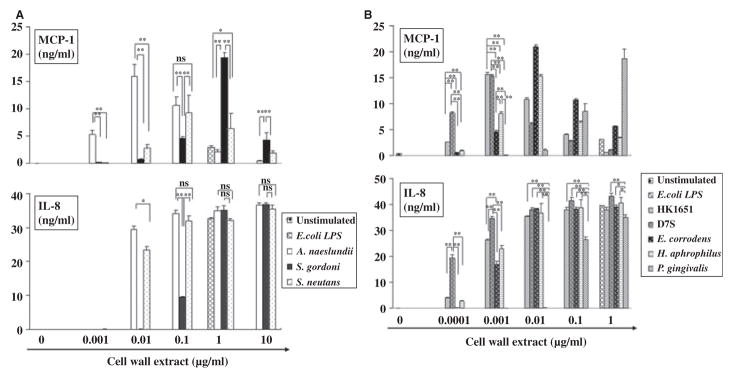
Since activated DCs produce a wide array of chemokines that attract immune and inflammatory cells, we next compared DC production of the chemokines MCP-1, IL-8, and TARC in response to oral bacteria. The dose–response curve for the induction of MCP-1 differed from that of other chemokines and for cytokines in that production decreased at higher doses of bacterial cell wall extracts. This decrease was not the result of cell death, with the exception of A. actinomycetemcomitans HK1651, which through its leukotoxin induced cell death at higher doses (data not shown). For all the bacteria tested the threshold for MCP-1 induction was 100-fold to 1000-fold lower than that for inducing proinflammatory cytokines (Fig. 4A,B, and Table 1).
Fig. 4.
Biphasic secretion pattern of monocyte chemoattractant protein 1 (MCP-1) and dose-dependent manner of interleukin-8 (IL-8) secretion by dendritic cells (DCs) in response to gram-positive (A) and gram-negative (B) oral bacteria. Immature DCs were stimulated with graded doses of oral bacterial cell wall extract and culture supernatants were collected after 24 h. Escherichia coli lipopolysaccharide (LPS) and media were used as positive and negative control, respectively. The concentrations of MCP-1 and IL-8 were measured by enzyme-linked immunosorbent assay. All assays were performed in triplicate to obtain mean ± SD. Representative results from three independent experiments with three different donors are shown. Asterisks indicate statistically significant differences (*P < 0.05, **P < 0.01, NS: not significant) determined by Student’s t-test.
Among the gram-positive bacteria tested, A. naeslundii was significantly more effective at inducing MCP-1 at lower bacterial extract doses; at higher doses S. gordonii induced significantly more MCP-1 than other gram-positive bacteria (Fig. 4A). For gram-negative bacteria, A. actinomycetemcomitans D7S was significantly most potent at inducing MCP-1 and at very low doses. At 0.0001–0.001 μg/ml, two different strains of A. actinomycetemcomitans were significantly more effective at inducing MCP-1 than the other gram-negative bacteria (Fig. 4B). The amount of P. gingivalis required to induce MCP-1 production by DCs was 1000-fold higher than other gram-negative bacteria (Fig. 4B).
All oral bacteria tested induced IL-8 production by DCs in a dose-dependent manner but with different thresholds (Fig. 4A, B). At lower doses, A. naeslundii and S. mutans induced significantly more IL-8 than did S. gordonii. However, at higher doses, these three gram-positive bacteria induced similar amounts of IL-8 (Fig. 4A). A. actinomycetemcomitans D7S was most effective at inducing IL-8 among the gram-negative bacteria tested. Again P. gingivalis was the least effective bacterium in stimulating IL-8 at any doses tested (Fig. 4B). In general, gram-positive oral bacteria and P. gingivalis needed 10-to 100-fold higher concentrations of cell wall extract to induce IL-8 compared to other gram-negative oral bacteria, which induced IL-8 production at approximately 0.0001–0.001 μg/ml (Fig. 4B). The A. actinomycetemcomitans D7S strain had the lowest threshold for MCP-1 and IL-8 induction among the gram-negative oral bacteria tested, while the commensal A. naeslundii had the lowest threshold among the gram-positive oral microorganisms tested. TARC production by DCs in response to oral bacterial cell wall extracts did not differ significantly from levels of TARC produced by unstimulated DCs (data not shown).
Induction of MCP-1 and TNF-α production by live oral bacteria
Next we examined the cytokine/chemokine profile of DCs in response to live oral bacteria. This approach allowed us to compare bacteria in their native form to the bacterial cell wall extracts. Since gram-positive A. naeslundii is the most abundant oral microorganism in the oral cavity (43), and there was approximately a 100-fold difference in the amount of A. naeslundii and gram-positive S. gordonii extracts needed to initiate MCP-1 production by iDCs, we decided to examine MCP-1 and TNF-α secretion by DCs in response to live A. naeslundii and S. gordonii. As with bacterial extracts, live bacteria induced MCP-1 secretion at lower doses and less MCP-1 at higher bacteria numbers (Fig. 5). In addition, live oral bacteria, like bacterial extracts, induced secretion of TNF-α in a bacteria number-dependent manner. Also, as with the bacterial extracts, the threshold of bacteria needed to induce DCs to make MCP-1 was 100-fold lower than that for TNF-α. In several respects, therefore, the stimulation of DCs by live bacteria compared with bacterial extracts was similar. However, the difference in the amount of A. naeslundii and S. gordonii needed to stimulate DCs detected when cell wall extracts were used was not evident with live bacteria.
Fig. 5.
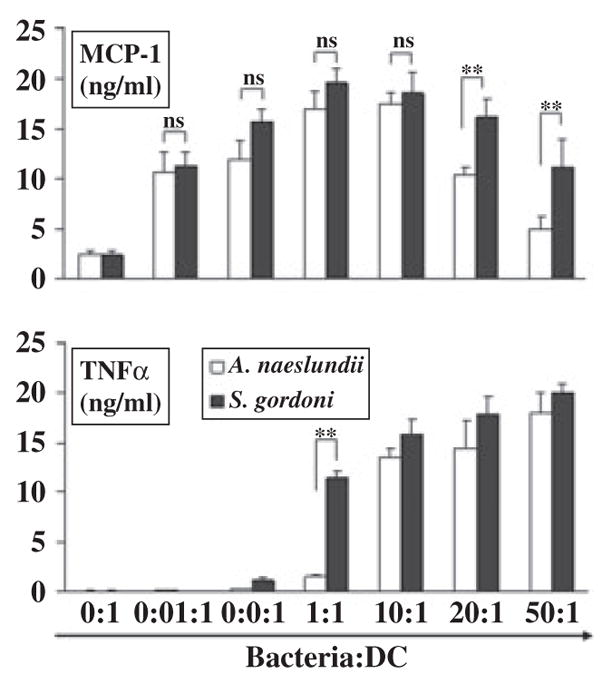
The dose–responses of monocyte chemoattractant protein 1 (MCP-1) and tumor necrosis factor-α (TNF-α) secreted by dendritic cells (DCs) in response to live oral bacteria. Immature DCs were cultured with live Actinomyces naeslundii or Streptococcus gordonii and the supernatants were collected after 18 h. The concentrations of MCP-1 and TNF-α were measured by enzyme-linked immunosorbent assay. Mean ± SD from three independent experiments with three different donors are shown (**P < 0.01, NS: not significant) determined by Student’s t-test.
Monocytes are attracted to MCP-1 secreted by DCs in response to oral commensal bacteria
Since monocytes play a role in tissue homeostasis and migrate in response to MCP-1, we examined next whether the numbers of monocytes induced to migrate corresponded to the levels of MCP-1 secreted by iDCs in response to bacterial cell wall extracts. We tested cell wall extracts from A. naeslundii and S. gordonii. As with MCP-1 secretion, A. naeslundii attracted significantly more monocytes to the iDCs at lower doses than S. gordonii, whereas S. gordonii attracted significantly more monocytes at higher doses (Fig. 6). Therefore, these oral commensals can effectively induce monocyte migration.
Fig. 6.
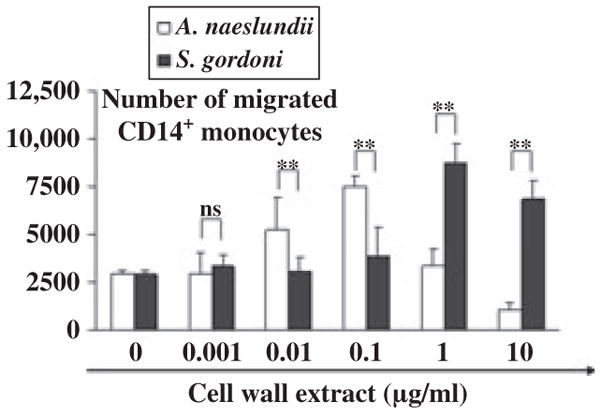
The number of monocytes attracted to monocyte chemoattractant protein 1 (MCP-1) secreted by dendritic cells (DCs). Migration of CD14+ monocytes was measured using tran-swell system. DCs plated to the lower chamber were stimulated with graded doses of oral gram-positive bacteria. After 24 h, monocytes were added to the upper chamber and incubated for an additional 3 h. CD14+ monocytes migrated to the lower chamber were counted by flow cytometry. Mean ± SD from three independent experiments with three different donors are shown. Asterisks indicate statistically significant differences (*P < 0.05, **P < 0.01) determined by Student’s t-test.
Discussion
Although the recognition of microbes by DCs is one of the critical biological responses to induce protective immunity, little is known about how commensal and pathogenic bacteria differ in their programming of DCs. Here we have found that both commensal and pathogenic oral bacteria efficiently stimulate iDCs to mature and produce cytokines and chemokines. Importantly, the biggest differences between stimulation of DCs by oral bacteria were not based on whether they were commensals or pathogens, but whether they were gram-positive or gram-negative.
There are several possible explanations for why oral gram-negative bacteria are more potent at stimulating iDCs than oral gram-positive bacteria. In particular, the presence of LPS in the gram-negative bacteria most likely contributes to the efficiency of DC-programming. LPS is a major component of the outer membrane of gram-negative bacteria (2). LPS in the cell wall extracts from gram-negative bacteria, and not present in the gram-positive bacteria extracts, might help to activate DCs more effectively. P. gingivalis is a gram-negative bacterium; however, it was not as effective as other gram-negative bacteria tested. The low biological activity of P. gingivalis cell wall extract and purified LPS preparations on programming DCs was consistent with the low biological activity of highly purified P. gingivalis LPS on the induction of adhesion molecule expression in endothelial cells (9). Alternatively, other cell type(s) responding to P. gingivalis might generate a cascade of inflammatory responses. Bodet et al. (5) found that P. gingivalis more efficiently triggered mixed macrophage/epithelial cell cultures to produce IL-1β than other periodontopathic bacteria tested. Therefore, the relatively weak effects of P. gingivalis on DCs may not necessarily be the case for all other cell types.
A. actinomycetemcomitans D7S was the most potent at inducing DC maturation among the gram-negative oral bacteria tested. Barbour et al. (4) reported that monocytes isolated from individuals with localized aggressive periodontitis caused by A. actinomycetemcomitans readily differentiate into DCs. Taken together, it may suggest that DCs play a crucial role in the pathogenesis and progression of localized aggressive periodontitis. Stimulation of iDCs with 1 μg/ml cell wall extract from A. actinomycetemcomitans HK1651 resulted in decreased TNF-α, IL-12p70, IL-12p40, and IL-10 production, which was associated with cell death (data not shown). However, even though this periodontopathic bacterium efficiently induced cell death and a drop in most cytokine levels, it was still able to induce IL-1β and IL-23 secretion by DCs. Since the induction of cell death by A. actinomycetemcomitans is caspase-1 dependent (18) and IL-1β production requires caspase-1, it is not surprising that IL-1β levels did not drop in DC cultures with A. actinomycetemcomitans. The fact that IL-23 is efficiently induced by A. actinomycetemcomitans HK1651 is noteworthy. A recent study by Sutton et al. (38) using IL-1 receptor type-I-deficient mice showed that IL-1 signaling is necessary for the induction of IL-17 production by T helper type 17 (Th17) cells. They also showed that IL-1 synergistically functioned with IL-23 to promote the secretion of IL-17 from Th17 cells. IL-1β is also known to induce IL-23p19 transcripts in human colonic subepithelial myofibroblasts (45). Since IL-17 has been implicated in the pathogenesis of periodontal disease (27), DC-derived IL-1β and IL-23 induced by A. actinomycetemcomitans HK1651 and other periodontopathic bacteria may play a role in the induction of IL-17 secretion by Th17 cells and thereby contribute to disease development.
To our surprise, even oral commensal bacteria were very efficient at stimulating DCs to produce proinflammatory cytokines. It is unclear why (or how) oral commensal bacteria activate proinflammatory cytokine secretion. One clue comes from the fact that lamina propria DCs can be activated either by directly sensing gut-associated commensal bacteria (32) or by epithelial cells exposed to commensal bacteria in the gut. While a similar sensing mechanism may be occurring in the oral cavity, unlike gut-associated DCs, the DCs in the oral cavity are faced with keratinized barriers, which in most instances should prevent them from directly contacting bacteria. However, the epithelium that lines the gingival or periodontal pocket is generally thin and lacks keratinized layers, so that DCs in this region may be able to be stimulated by oral commensal bacterial products. In addition, histological examination of periodontal tissues has demonstrated the lack of tight junctions between the oral epithelial cells of the junctional epithelium, providing a tissue permeable to bacterial products (34) so that DCs might directly sense the microbial components. Furthermore, oral commensals may contribute to the programming of DCs while in association with oral pathogens, with which they associate within biofilms (22). Further studies are needed to clarify how oral-associated DCs interact with commensal bacteria and their products.
When DCs were stimulated with live commensal A. naeslundii and S. gordonii differences in cytokine/chemokine responses to the bacteria were not detected that were evident with A. naeslundii and S. gordonii cell wall extracts (Fig. 5). This might be because not all components of the cell wall extract are active or accessible in the live bacteria. Further studies comparing the effects on DCs of purified bacterial components from A. naeslundii and S. gordonii should help to clarify the basis for this difference.
Oral DCs may be affected indirectly by oral bacteria. When gut-associated epithelial cells are exposed to bacteria, they program iDCs to become more inflammatory as measured by cytokine release than iDCs directly exposed to bacteria, which initiate a non-inflammatory program (33). These non-inflammatory DCs secrete TARC (15), but not IL-12. We did not see enhanced TARC production in response to oral bacteria so interactions between iDCs and soluble factor(s) released from epithelial cells may contribute to tissue homeostasis.
Finally, the pathogenicity of bacteria might depend on the niche where the bacteria reside. It is of interest that all the bacteria we tested are known to be associated with extraoral pathology, including cardiovascular disease (13, 21, 29, 36, 42). Consequently, if oral commensal bacteria get into the bloodstream, they may become pathogenic, or at least opportunistic, in other tissues because they possess the ability to potently stimulate inflammatory cytokines and chemokines.
DCs programmed by commensal bacteria contribute to intestinal tissue homeostasis (1, 10) so we examined the chemokine profiles of DCs in response to oral bacteria. Previous studies showed that oral bacteria can stimulate peripheral blood mononuclear cells to induce chemokines, such as MCP-1 and IL-8 (16), and we have extended this finding to DCs and also discovered that irrespective of the bacterium used, less bacterial extract or fewer bacteria are needed to stimulate chemokine production by DCs than are needed to stimulate cytokine production. As with MCP-1 secretion, monocytes were attracted to DCs stimulated with lower doses of A. naeslundii. Our findings suggest that at lower doses of bacteria (that is, under clinically healthy conditions), oral mucosal DCs may produce chemokines, such as MCP-1, in response to oral commensal bacteria, such as A. naeslundii, to attract monocytes into the area of bacterial accumulation. In this way monocytes could phagocytose bacteria without overt inflammatory responses. This could be a novel mechanism for preserving tissue integrity mediated by the interaction between oral bacteria and DCs. MCP-1 also attracts Tregs (11, 12), suggesting that Tregs might be recruited to the subepithelium in the oral mucosal tissue under healthy conditions. In addition, the lower threshold for MCP-1 induction suggests that different receptors/signaling pathways may be used to induce MCP-1 compared with inflammatory cytokines; consistent with this model MCP-1, unlike TNF-α or IL-12p40, is secreted in the absence of MyD88, a key adapter protein for TLR signaling (35).
In conclusion, both oral commensal and pathogenic bacteria can program DCs. Oral gram-negative bacteria are more powerful at inducing DC maturation and proinflammatory cytokine/chemokine secretion from DCs than oral gram-positive bacteria. The interaction between iDCs and oral bacteria seems to play a role in oral mucosal homeostasis. We propose that one innate defense mechanism within oral mucosal tissue is mediated by the interaction between DCs and oral commensal bacteria. Even at lower doses of bacteria, monocytes may be recruited to the sites of injury in response to MCP-1 secreted by iDCs and phagocytose bacteria without causing severe tissue damage. On the other hand, DC-derived IL-1β and IL-23 may contribute to the pathogenesis of periodontal disease by promoting IL-17 secretion by T cells. Further studies are needed to elucidate why oral commensal bacteria induce pro-inflammatory cytokines and how this may differ when DCs are in contact with other cells of the oral cavity.
Acknowledgments
The authors thank Ms Pamela Braham, University of Washington, for preparing bacterial cell wall extract and live bacteria. This study was supported by National Institutes of Health grants DE16381 and AI44257. The authors have no conflicting financial interests.
References
- 1.Akbari O, DeKruyff RH, Umetsu DT. Pulmonary dendritic cells producing IL-10 mediate tolerance induced by respiratory exposure to antigen. Nat Immunol. 2001;2:725–731. doi: 10.1038/90667. [DOI] [PubMed] [Google Scholar]
- 2.Alexander C, Rietschel ET. Bacterial lipopolysaccharides and innate immunity. J Endotoxin Res. 2001;7:167–202. [PubMed] [Google Scholar]
- 3.Appelmelk BJ, van Die I, van Vliet SJ, Vandenbroucke-Grauls CM, Geijtenbeek TB, van Kooyk Y. Cutting edge: carbohydrate profiling identifies new pathogens that interact with dendritic cell-specific ICAM-3-grabbing nonintegrin on dendritic cells. J Immunol. 2003;170:1635–1639. doi: 10.4049/jimmunol.170.4.1635. [DOI] [PubMed] [Google Scholar]
- 4.Barbour SE, Ishihara Y, Fakher M, et al. Monocyte differentiation in localized juvenile periodontitis is skewed toward the dendritic cell phenotype. Infect Immun. 2002;70:2780–2786. doi: 10.1128/IAI.70.6.2780-2786.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bodet C, Chandad F, Grenier D. Inflammatory responses of a macrophage/epithelial cell co-culture model to mono and mixed infections with Porphyromonas gingivalis, Treponema denticola, and Tannerella forsythia. Microbes Infect. 2006;8:27–35. doi: 10.1016/j.micinf.2005.05.015. [DOI] [PubMed] [Google Scholar]
- 6.Chen C, Ashimoto A. Clonal diversity of oral Eikenella corrodens within individual subjects by arbitrarily primed PCR. J Clin Microbiol. 1996;34:1837–1839. doi: 10.1128/jcm.34.7.1837-1839.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen CH, Floyd H, Olson NE, et al. Dendritic-cell-associated C-type lectin 2 (DCAL-2) alters dendritic-cell maturation and cytokine production. Blood. 2006;107:1459–1467. doi: 10.1182/blood-2005-08-3264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cirrincione C, Pimpinelli N, Orlando L, Romagnoli P. Lamina propria dendritic cells express activation markers and contact lymphocytes in chronic periodontitis. J Periodontol. 2002;73:45–52. doi: 10.1902/jop.2002.73.1.45. [DOI] [PubMed] [Google Scholar]
- 9.Darveau RP, Cunningham MD, Bailey T, et al. Ability of bacteria associated with chronic inflammatory disease to stimulate E-selectin expression and promote neutrophil adhesion. Infect Immun. 1995;63:1311–1317. doi: 10.1128/iai.63.4.1311-1317.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Frick JS, Zahir N, Muller M, et al. Colitogenic and non-colitogenic commensal bacteria differentially trigger DC maturation and Th cell polarization: an important role for IL-6. Eur J Immunol. 2006;36:1537–1547. doi: 10.1002/eji.200635840. [DOI] [PubMed] [Google Scholar]
- 11.Fu S, Yopp AC, Mao X, et al. CD4+ CD25+ CD62+ T-regulatory cell subset has optimal suppressive and proliferative potential. Am J Transplant. 2004;4:65–78. doi: 10.1046/j.1600-6143.2003.00293.x. [DOI] [PubMed] [Google Scholar]
- 12.Goulvestre C, Batteux F, Charreire J. Chemokines modulate experimental autoimmune thyroiditis through attraction of autoreactive or regulatory T cells. Eur J Immunol. 2002;32:3435–3442. doi: 10.1002/1521-4141(200212)32:12<3435::AID-IMMU3435>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 13.Haraszthy VI, Zambon JJ, Trevisan M, Zeid M, Genco RJ. Identification of periodontal pathogens in atheromatous plaques. J Periodontol. 2000;71:1554–1560. doi: 10.1902/jop.2000.71.10.1554. [DOI] [PubMed] [Google Scholar]
- 14.Huang GT, Kim D, Lee JK, Kuramitsu HK, Haake SK. Interleukin-8 and intercellular adhesion molecule 1 regulation in oral epithelial cells by selected periodontal bacteria: multiple effects of Porphyromonas gingivalis via antagonistic mechanisms. Infect Immun. 2001;69:1364–1372. doi: 10.1128/IAI.69.3.1364-1372.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Iellem A, Mariani M, Lang R, et al. Unique chemotactic response profile and specific expression of chemokine receptors CCR4 and CCR8 by CD4(+)CD25(+) regulatory T cells. J Exp Med. 2001;194:847–853. doi: 10.1084/jem.194.6.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jiang Y, Russell TR, Graves DT, Cheng H, Nong SH, Levitz SM. Monocyte chemo-attractant protein 1 and interleukin-8 production in mononuclear cells stimulated by oral microorganisms. Infect Immun. 1996;64:4450–4455. doi: 10.1128/iai.64.11.4450-4455.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jotwani R, Palucka AK, Al-Quotub M, et al. Mature dendritic cells infiltrate the T cell-rich region of oral mucosa in chronic periodontitis: in situ, in vivo, and in vitro studies. J Immunol. 2001;167:4693–4700. doi: 10.4049/jimmunol.167.8.4693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kelk P, Johansson A, Claesson R, Hanstrom L, Kalfas S. Caspase 1 involvement in human monocyte lysis induced by Actinobacillus actinomycetemcomitans leukotoxin. Infect Immun. 2003;71:4448–4455. doi: 10.1128/IAI.71.8.4448-4455.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kelly D, Campbell JI, King TP, et al. Commensal anaerobic gut bacteria attenuate inflammation by regulating nuclear-cytoplasmic shuttling of PPAR-gamma and RelA. Nat Immunol. 2004;5:104–112. doi: 10.1038/ni1018. [DOI] [PubMed] [Google Scholar]
- 20.Kusumoto Y, Hirano H, Saitoh K, et al. Human gingival epithelial cells produce chemotactic factors interleukin-8 and monocyte chemoattractant protein-1 after stimulation with Porphyromonas gingivalis via toll-like receptor 2. J Periodontol. 2004;75:370–379. doi: 10.1902/jop.2004.75.3.370. [DOI] [PubMed] [Google Scholar]
- 21.Marques da Silva R, Lingaas PS, Geiran O, Tronstad L, Olsen I. Multiple bacteria in aortic aneurysms. J Vasc Surg. 2003;38:1384–1389. doi: 10.1016/s0741-5214(03)00926-1. [DOI] [PubMed] [Google Scholar]
- 22.Marsh PD. Dental plaque as a microbial biofilm. Caries Res. 2004;38:204–211. doi: 10.1159/000077756. [DOI] [PubMed] [Google Scholar]
- 23.Mena J, Chen C. Identification of strain-specific DNA of Actinobacillus actinomy-cetemcomitans by representational difference analysis. Oral Microbiol Immunol. 2007;22:429–432. doi: 10.1111/j.1399-302X.2007.00371.x. [DOI] [PubMed] [Google Scholar]
- 24.Moughal NA, Adonogianaki E, Kinane DF. Langerhans cell dynamics in human gingiva during experimentally induced inflammation. J Biol Buccale. 1992;20:163–167. [PubMed] [Google Scholar]
- 25.Neish AS, Gewirtz AT, Zeng H, et al. Prokaryotic regulation of epithelial responses by inhibition of IkappaB-alpha ubiquitination. Science. 2000;289:1560–1563. doi: 10.1126/science.289.5484.1560. [DOI] [PubMed] [Google Scholar]
- 26.Newcomb GM, Seymour GJ, Powell RN. Association between plaque accumulation and Langerhans cell numbers in the oral epithelium of attached gingiva. J Clin Periodontol. 1982;9:297–304. doi: 10.1111/j.1600-051x.1982.tb02096.x. [DOI] [PubMed] [Google Scholar]
- 27.Oda T, Yoshie H, Yamazaki K. Porphyromonas gingivalis antigen preferentially stimulates T cells to express IL-17 but not receptor activator of NF-kappaB ligand in vitro. Oral Microbiol Immunol. 2003;18:30–36. doi: 10.1034/j.1399-302x.2003.180105.x. [DOI] [PubMed] [Google Scholar]
- 28.Ostman S, Rask C, Wold AE, Hultkrantz S, Telemo E. Impaired regulatory T cell function in germ-free mice. Eur J Immunol. 2006;36:2336–2346. doi: 10.1002/eji.200535244. [DOI] [PubMed] [Google Scholar]
- 29.Parker MT, Ball LC. Streptococci and aerococci associated with systemic infection in man. J Med Microbiol. 1976;9:275–302. doi: 10.1099/00222615-9-3-275. [DOI] [PubMed] [Google Scholar]
- 30.Paster BJ, Boches SK, Galvin JL, et al. Bacterial diversity in human subgingival plaque. J Bacteriol. 2001;183:3770–3783. doi: 10.1128/JB.183.12.3770-3783.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Read S, Malmstrom V, Powrie F. Cytotoxic T lymphocyte-associated antigen 4 plays an essential role in the function of CD25(+)CD4(+) regulatory cells that control intestinal inflammation. J Exp Med. 2000;192:295–302. doi: 10.1084/jem.192.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rescigno M, Urbano M, Valzasina B, et al. Dendritic cells express tight junction proteins and penetrate gut epithelial monolayers to sample bacteria. Nat Immunol. 2001;2:361–367. doi: 10.1038/86373. [DOI] [PubMed] [Google Scholar]
- 33.Rimoldi M, Chieppa M, Larghi P, Vulcano M, Allavena P, Rescigno M. Monocyte-derived dendritic cells activated by bacteria or by bacteria-stimulated epithelial cells are functionally different. Blood. 2005;106:2818–2826. doi: 10.1182/blood-2004-11-4321. [DOI] [PubMed] [Google Scholar]
- 34.Schwartz J, Stinson FL, Parker RB. The passage of tritiated bacterial endotoxin across intact gingival crevicular epithelium. J Periodontol. 1972;43:270–276. doi: 10.1902/jop.1972.43.5.270. [DOI] [PubMed] [Google Scholar]
- 35.Serbina NV, Kuziel W, Flavell R, Akira S, Rollins B, Pamer EG. Sequential MyD88-independent and -dependent activation of innate immune responses to intracellular bacterial infection. Immunity. 2003;19:891–901. doi: 10.1016/s1074-7613(03)00330-3. [DOI] [PubMed] [Google Scholar]
- 36.Sommer P, Gleyzal C, Guerret S, Etienne J, Grimaud JA. Induction of a putative laminin-binding protein of Streptococcus gordonii in human infective endocarditis. Infect Immun. 1992;60:360–365. doi: 10.1128/iai.60.2.360-365.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Steinman RM. The dendritic cell system and its role in immunogenicity. Annu Rev Immunol. 1991;9:271–296. doi: 10.1146/annurev.iy.09.040191.001415. [DOI] [PubMed] [Google Scholar]
- 38.Sutton C, Brereton C, Keogh B, Mills KH, Lavelle EC. A crucial role for interleukin (IL)-1 in the induction of IL-17-producing T cells that mediate autoimmune encepha-lomyelitis. J Exp Med. 2006;203:1685–1691. doi: 10.1084/jem.20060285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Takahashi T, Tagami T, Yamazaki S, et al. Immunologic self-tolerance maintained by CD25(+)CD4(+) regulatory T cells constitutively expressing cytotoxic T lymphocyte-associated antigen 4. J Exp Med. 2000;192:303–310. doi: 10.1084/jem.192.2.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Verhasselt V, Buelens C, Willems F, De Groote D, Haeffner-Cavaillon N, Goldman M. Bacterial lipopolysaccharide stimulates the production of cytokines and the expression of costimulatory molecules by human peripheral blood dendritic cells: evidence for a soluble CD14-dependent pathway. J Immunol. 1997;158:2919–2925. [PubMed] [Google Scholar]
- 41.Wang Y, Goodman SD, Redfield RJ, Chen C. Natural transformation and DNA uptake signal sequences in Actinobacillus actinomycetemcomitans. J Bacteriol. 2002;184:3442–3449. doi: 10.1128/JB.184.13.3442-3449.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wilson WR, Karchmer AW, Dajani AS, et al. Antibiotic treatment of adults with infective endocarditis due to streptococci, enterococci, staphylococci, and HACEK microorganisms. American Heart Association J Am Med Assoc. 1995;274:1706–1713. [PubMed] [Google Scholar]
- 43.Ximenez-Fyvie LA, Haffajee AD, Socransky SS. Comparison of the microbiota of supra- and subgingival plaque in health and periodontitis. J Clin Periodontol. 2000;27:648–657. doi: 10.1034/j.1600-051x.2000.027009648.x. [DOI] [PubMed] [Google Scholar]
- 44.Yumoto H, Nakae H, Fujinaka K, Ebisu S, Matsuo T. Interleukin-6 (IL-6) and IL-8 are induced in human oral epithelial cells in response to exposure to periodontopathic Eikenella corrodens. Infect Immun. 1999;67:384–394. doi: 10.1128/iai.67.1.384-394.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang Z, Andoh A, Yasui H, et al. Interleukin-1beta and tumor necrosis factor-alpha upregulate interleukin-23 subunit p19 gene expression in human colonic subepithelial myofibroblasts. Int J Mol Med. 2005;15:79–83. [PubMed] [Google Scholar]