Abstract
Background
Improved control efforts are reducing the burden of malaria in Africa, but may result in decreased antimalarial immunity.
Methods
A cohort of 129 children aged 1–10 years in Kampala, Uganda were treated with amodiaquine+sulfadoxine-pyrimethamine for 396 episodes of uncomplicated malaria over a 29 month period as part of a longitudinal clinical trial.
Results
The risk of treatment failure increased over the course of the study from 5% to 21% (HR=2.4/yr, 95%CI=1.3–4.3). Parasite genetic polymorphisms were associated with an increased risk of failure, but their prevalence did not change over time. Three markers of antimalarial immunity were associated with a decreased risk of treatment failure: increased age (HR=0.5/5yrs, 95%CI=0.2–1.2), living in an area of higher malaria incidence (HR=0.26, 95%CI=0.11–0.64), and recent asymptomatic parasitemia (HR=0.06, 95%CI=0.01–0.36). In multivariate analysis, adjustment for recent asymptomatic parasitemia, but not parasite polymorphisms, removed the association between calendar time and the risk of treatment failure (HR=1.5/yr, 95%CI=0.7–3.4), suggesting that worsening treatment efficacy was best explained by decreasing host immunity.
Conclusion
Declining immunity in our study population appeared to be the primary factor underlying decreased efficacy of amodiaquine+sulfadoxine-pyrimethamine. With improved malaria control efforts, decreasing immunity may unmask resistance to partially efficacious drugs.
Keywords: malaria, falciparum, antimalarial, drug resistance, immunity, antifolate, aminoquinoline, malaria control, malaria prevention, combination therapy, amodiaquine, sulfadoxine-pyrimethamine
Background
Increased funding, implementation of effective antimalarial combination therapy, and improved disease prevention efforts appear to be decreasing malarial morbidity and mortality in many areas of Africa [1, 2]. These changes are encouraging, but with partial control of malaria will come new challenges. In particular, malaria is characterized by the development of partial immunity after repeated exposure to parasites [3–5], and improved malaria control efforts are likely to delay and diminish the acquisition of immunity [6]. Limited data exist on the consequences of loss of acquired immunity in African children.
While disease prevention has become an increasingly important component of malaria control efforts in Africa, prompt treatment with effective drugs will remain the cornerstone of control for the foreseeable future [7]. Response to antimalarial therapy depends upon both drug-parasite and host-parasite interactions. Drug-parasite interactions can be altered by parasite mutations which enable the parasites to persist following drug treatment [8–10]. Host-parasite interactions are primarily determined by the acquisition of antimalarial immunity. With increasing immunity, the likelihood of successful response to partially efficacious antimalarials increases, with the immune system helping to clear parasites not killed by antimalarials. Indeed, prior studies have shown associations between increasing age or transmission intensity, both surrogates of acquired immunity [3], and a lower risk of antimalarial treatment failure [11–14].
Treatment for uncomplicated malaria has changed dramatically in Africa in recent years in response to increasing drug resistance, moving from chloroquine or sulfadoxine-pyrimethamine (SP) monotherapy to, recently, broad advocacy for combination therapy [15]. Artemisinin-based combination therapies (ACTs) have shown the greatest efficacy [16], but an older combination regimen, amodiaquine plus SP (AQ+SP), has been highly efficacious in some areas [17–19], and is recommended by the World Health Organization to treat uncomplicated malaria when ACTs are unavailable [15]. We recently observed a rapid decrease in the efficacy of AQ+SP for uncomplicated malaria in children in Kampala, Uganda. To determine whether the decreased efficacy of AQ+SP was due to increasing drug resistance, decreasing host immunity, or a combination of these factors, we analyzed the contributions of both parasite and host factors to treatment outcomes.
Materials and Methods
Recruitment and follow-up of study participants
Between November 2004 and April 2005, children aged 1 to 10 years from households randomly selected from a neighborhood of Kampala, Uganda [20] were enrolled in a randomized trial of combination antimalarial therapies; an interim analysis of comparative results through June 2006 has been published [21]. Briefly, parents or guardians of study participants were asked to bring their children to a designated study clinic for all medical care. Malaria was diagnosed if a child had fever and parasitemia. Upon diagnosis of their first episode of uncomplicated malaria, study participants were randomly assigned to receive 1 of 3 antimalarial regimens (AQ+SP, artesunate plus AQ, or artemether-lumefantrine) for all episodes of uncomplicated malaria over the duration of the study. After treatment for malaria, study participants received active follow up for 28 days and then passive follow up for malaria after 28 days. Children underwent routine assessment and blood smear monthly (or every 3 months after June, 2006) to assess asymptomatic parasitemia. All participants were given long-lasting insecticide-treated bednets between May and June of 2006.
Treatment Outcomes and Laboratory Techniques
Early treatment failures within 3 days of treatment were classified according to 2005 WHO guidelines[22]. Recurrent episodes of malaria occurring more than 63 days after a prior episode were considered new infections. For recurrent episodes of malaria occurring 4–63 days after a prior episode, parasites were genotyped with 6 markers to distinguish new infection from recrudescence [23]. Plasmodium species was evaluated using a species-specific polymerase chain reaction [24]. Parasite polymorphisms were assessed using polymerase chainreaction followed by sequence-specific restriction enzyme digestion [25, 26].
Statistical Analysis
Statistical analysis was performed using Stata SE version 10 (StataCorp., College Station, Texas) and R version 2.5.1 (R Foundation for Statistical Computing, Vienna, Austria). Only data from the AQ+SP study arm were analyzed, as only this arm had adequate numbers of treatment failures for meaningful associations. Treatments with AQ+SP were given from November, 2004 until March, 2007 when, after a planned interim analysis and review by our data and safety monitoring board, this treatment arm was stopped due to an unacceptably high risk of treatment failure.
Our outcome measure was the 63-day risk of treatment failure, defined as recurrent malaria due to recrudescent parasites. We only considered treatments for new P. falciparum infections and excluded treatments for which no genotyping result was obtained. Treatments resulting in early treatment failures were also excluded, as these are commonly due to factors other than drug resistance [26, 27]. Risk of failure was estimated using the Kaplan-Meier product limit formula. Data were censored for subjects who did not complete 63 days of follow-up and for new infections. Predictor variables of interest included calendar time, parasite polymorphisms, age of subjects at enrollment, distance of residence from a swamp, recent asymptomatic parasitemia, and parasite density at the time of treatment. Calendar time was evaluated as a continuous variable. Parasite polymorphisms with mixed alleles were categorized as containing the resistance-mediating polymorphism, as suggested by the similar risk of treatment failure in samples containing mixed alleles or only the polymorphism of interest. Age at enrollment was analyzed instead of age at the time of treatment to enable independent evaluation of the effects of time and age, and was evaluated as a continuous variable. Distance from a swamp, a surrogate marker of parasite exposure [28], was dichotomized at 50 meters to best reflect the relationship with the risk of recrudescence. Asymptomatic parasitemia, a surrogate marker of host immunity[5], was defined as the presence of a positive blood smear in the absence of fever at least 28 days after and 5 days before treatment for malaria. Recent asymptomatic parasitemia was defined as the presence of at least one episode of asymptomatic parasitemia in the prior 180 days. Parasite density was dichotomized at 100,000 parasites/μl to best reflect the relationship with the risk of recrudescence. Associations between predictor variables of interest and the risk of treatment failure were estimated using Cox proportional hazards. Left-censoring of recent asymptomatic parasitemia was accounted for using inverse probability of censoring weighting, with weights determined using logistic regression for covariates which significantly predicted censoring. Robust inference accounting for repeated measurements in the same subject was performed using the grouped jackknife method. Possible confounding or interaction between duration of the interval of assessment for asymptomatic parasitemia and the presence of recent asymptomatic parasitemia was ruled out. Associations between time and the presence of parasite polymorphisms were estimated using logistic regression with time evaluated as a continuous variable. P < 0.05 was considered statistically significant for all tests.
Results
Characteristics of Malaria Episodes
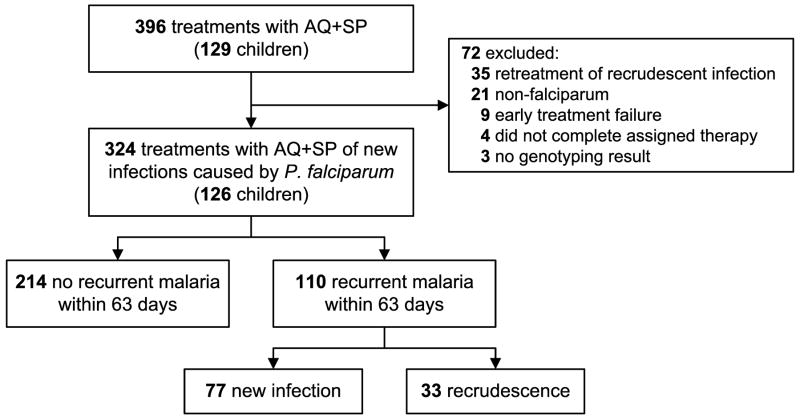
A total of 396 treatments for malaria with AQ+SP were given to 129 children during the course of the study (Figure 1). Of these treatments, 324 given to 126 children were included in the analysis. Considering treatments included in the analysis, 47 children were treated once, 35 were treated twice, and the remaining 44 were treated up to 13 times over the course of the study. The median age at the time of treatment was 7.2 years (IQR 4.9–8.9) and the geometric mean parasite density was 14,515 parasites/μl (range 16–464,000 parasites/μl).
Figure 1.
Malaria Treatments and Outcomes Included in the Analysis.
Increasing Risk of Treatment Failure Over Time
The 63-day risk of recurrent malaria after treatment with AQ+SP was 34%, and the 63-day risk of recrudescence (treatment failure) as determined by genotyping was 11% (Figure 1). The risk of recrudescence after treatment with AQ+SP increased significantly over the 29 months of the study, from 5% during the first quarter of the study to 21% during the last quarter (HR=2.4/yr, 95%CI=1.3–4.3, p=0.002) (Figure 2). In contrast, the risk of new infection after treatment was stable for the first three quarters, and then decreased in the last quarter when compared with the first three quarters (HR=0.49, 95%CI=0.25–0.95, p=0.04). Thus, our results indicate decreasing antimalarial efficacy of AQ+SP, a drug that until recently showed excellent efficacy in Uganda [17, 18].
Figure 2.
Risk of Recurrent Malaria Over Time After Treatment with AQ+SP.
(A) Risk of recrudescence (treatment failure). Vertical bars, 95% confidence intervals.
(B) Risk of new infection. Vertical bars, 95% confidence intervals.
Parasite Polymorphisms are Associated with Treatment Failure but Do Not Explain Changes Over Time
To investigate whether the observed increased risk of failure after treatment with AQ+SP was the result of increased parasite resistance to these drugs, we evaluated parasites collected at the time of each treatment for polymorphisms associated with resistance to SP or AQ. These included single nucleotide polymorphisms (SNPs) in dhfr and dhps that are associated with SP treatment failure [10, 29], and SNPs in pfcrt and pfmdr1 that appear to be associated with AQ treatment failure [30–33].
Considering markers of SP resistance, the SNPs dhfr 51I and dhfr 108N were present in nearly all of a random subset of 90 samples (99% and 100% respectively). We therefore assumed in data analysis that they were present in all samples. Of greater interest were the SNPs dhfr 59R, dhps 437G, and dhps 540E, which have demonstrated varied prevalence across Africa, and which most clearly associate with SP treatment failure [27, 34]. These SNPs were assessed in all 324 samples. Prevalence of all three of these SNPs was high (Table 1). Compared to infections without these polymorphisms, parasites with dhfr 59R, dhps 437G, and dhps 540E were all associated with a higher risk of failure. The presence of all three of these SNPs along with dhfr 51I and dhfr 108N (dhfr/dhps quintuple polymorphism) resulted in almost four times the hazard of treatment failure compared to infections which contained less than 5 of these polymorphisms. However, in the context of high baseline prevalence of the polymorphisms, none of these associations reached statistical significance. We found only 2 samples (0.6%) which contained the dhfr 164L allele, which has been associated with very poor response to SP [35].
Table 1.
Prevalence of Parasite Polymorphisms and Association with Failure After Treatment with AQ+SP.
| Gene | SNP | Prevalence of SNP | Risk of treatment failure with/without SNP | Association with treatment failure |
|
|---|---|---|---|---|---|
| HR (95% CI) | p-value | ||||
| dhfr | 59R | 279/322 (87%) | 12.4%/2.3% | 5.1 (0.7–38) | 0.12 |
| Dhps | 437G | 310/323 (96%) | 11.5%/0.0% | ∞ (0.4–∞) | 0.38 |
| 540E | 304/323 (94%) | 11.2%/7.7% | 2.0 (0.4–11) | 0.42 | |
| pfmdr1 | 86N | 96/322 (30%) | 14.9%/9.4% | 1.6 (0.8–3.2) | 0.15 |
| 184F | 78/322 (24%) | 13.8%/10.2% | 1.4 (0.7–3.0) | 0.39 | |
| 1246Y | 264/321 (82%) | 12.0%/7.3% | 1.7 (0.6–4.8) | 0.35 | |
| dhfr/dhps quintuple | 261/321 (81%) | 12.7%/4.0% | 3.7 (0.9–15) | 0.07 | |
| pfmdr1 double | 40/322 (12%) | 21.2%/9.6% | 2.4 (1.1–5.0) | 0.03 | |
|
Combination of SNPs present |
Association with treatment failure |
||||
| dhfr/dhps quintuple | pfmdr1 double | Prevalence of combination | Risk of treatment failure | HR (95% CI) | p-value |
|
| |||||
| − | +/− | 60/321 (19%) | 4.0% | 1.0 | |
| + | − | 232/321 (72%) | 10.6% | 3.0 (0.7–12) | 0.12 |
| + | + | 29/321 (9%) | 29.0% | 9.1 (2.0–41) | 0.004 |
NOTE. SNP, single nucleotide polymorphism; HR, hazard ratio; dhfr/dhps quintuple, dhfr 51I+59R+108N & dhps 437G+540E; pfmdr1 double, pfmdr1 86N+184F. Associations performed using Cox proportional hazards with inference accounting for repeated measures. Association for dhps 437G is reported as an odds ratio using Fisher’s exact test, as no reliable method of estimating inference for this hazard ratio is available.
Considering potential markers of AQ resistance, all 90 randomly selected samples contained pfcrt 76T, so this SNP was assumed to be present in all samples. Conversely, none of the 90 randomly selected samples contained pfmdr1 1034C or 1042D, so these SNPs were assumed to be absent from all samples. Other relevant pfmdr1 SNPs were evaluated in all 324 samples (Table 1). We found a higher risk of treatment failure with AQ+SP in subjects whose parasites contained pfmdr1 86N, 184F, or 1246Y. None of these individual associations approached statistical significance, however the combined presence of pfmdr1 86N and 184F (pfmdr1 double polymorphism) significantly predicted treatment failure. When considering the combined effects of SNPs associated with SP and AQ resistance, subjects whose parasites did not contain the dhfr/dhps quintuple polymorphism had a low risk of failure regardless of the presence of the pfmdr1 double polymorphism (Table 1). The presence of the dhfr/dhps quintuple polymorphism increased the hazard of failure 3-fold, and the addition of the pfmdr1 double polymorphism increased this hazard another 3-fold.
Since the clinical efficacy of AQ+SP decreased over the course of our study, it was of interest to determine whether the prevalence of key resistance-mediating polymorphisms increased over this time frame. In fact, neither the prevalence of the dhfr/dhps quintuple polymorphism, the pfmdr1 double polymorphism, nor both together changed significantly during the course of the study (p=0.4, 0.3, and 0.7, respectively) (Figure 3). In addition, adjusting for these polymorphism combinations did not change the association between time and the risk of treatment failure (HR=2.4/yr, p=0.004 excluding polymorphisms; HR=2.4/yr, p=0.004 including polymorphisms). These findings indicate that, although there were significant associations between certain parasite polymorphisms and the risk of treatment failure, the increased risk of failure that we observed over the course of our study was not due to an increase in parasite drug resistance mediated by the 12 SNPs that we evaluated.
Figure 3.
Prevalence of Parasite Polymorphisms Over Time. Vertical bars, 95% confidence intervals.
Surrogate Markers of Host Immunity are Associated with Treatment Failure and Explain Changes Over Time
Since the decreasing antimalarial efficacy of AQ+SP could not be explained by known markers of resistance to these agents, we considered the possibility that decreasing treatment response was due to waning host immunity. Although repeated infection is accompanied by the acquisition of clinically relevant antimalarial immunity, there is currently no straightforward marker for this immunity [36]. Therefore, to test the hypothesis that immunity influenced the risk of treatment failure in our study, we measured associations between 3 surrogate markers of immunity and failure: increasing age, exposure to parasites, and history of asymptomatic parasitemia.
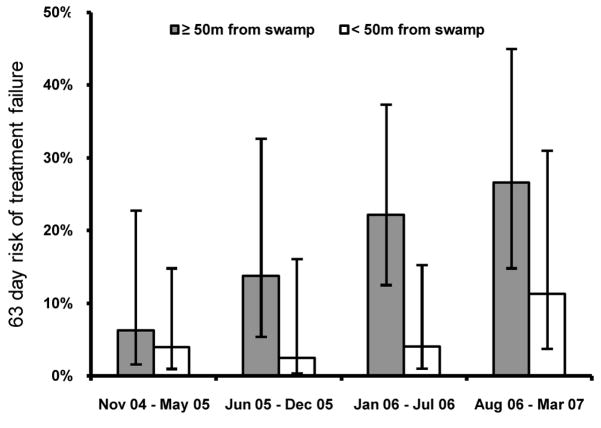
In our cohort, we found an association between increased age at enrollment and the risk of treatment failure, but this association did not reach statistical significance (HR=0.5/5yrs, 95%CI=0.2–1.2, p=0.1). To further consider exposure to malaria parasites in our study population, we used spatial data as a surrogate for exposure. We recently showed that those living close to a swamp bordering the study site had a higher incidence of malaria then those living farther away [28]. Considering AQ+SP treatment outcomes, those living within 50 meters of the swamp (48% of subjects) had a significantly lower risk of treatment failure after therapy then those living at least 50 meters away (5% vs. 18%, HR=0.26, 95%CI=0.11–0.64, p=0.003), consistent with increased immunity in the group with highest exposure to parasites. Those living at least 50 meters from the swamp showed a steady increase in risk of treatment failure through the course of our study, consistent with a gradual decrease in host immunity in this relatively non-immune group (HR=2.0/yr, 95%CI=1.1–3.7, p=0.02; Figure 4). In contrast, those living within 50 meters of the swamp did not show increased risk of treatment failure until late in the course of our study (HR=3.5, 4th quarter vs. 1st three quarters, 95%CI=0.8–14.7, p=0.09). The prevalence of parasite polymorphisms was similar in samples taken from those living within 50 meters and at least 50 meters from the swamp (data not shown). When both surrogates of immunity were considered together, those expected to have the greatest immunity based on age (> 5 years at enrollment) and exposure (living < 50 meters from the swamp) had a much lower risk of treatment failure than those expected to have the least immunity (age < 5 years at enrolment and living > 50 meters from the swamp) (3% vs. 28%, HR=0.12, 95%CI=0.03–0.5, p=0.005). These findings suggest that antimalarial immunity strongly influenced the risk of treatment failure in our study.
Figure 4.
Risk of Treatment Failure in Subjects Over Time Stratified by Distance from the Swamp. Vertical bars, 95% confidence intervals.
Age at enrollment and distance from the swamp appear to be good surrogates for immunity, but they are not markers that could change over the course of the study. To determine whether declining immunity was responsible for the increase in treatment failure over time, a surrogate marker of immunity which might vary over time was required. Another potential surrogate for malarial immunity is asymptomatic parasitemia, since immunity is required to control parasitemia and prevent the development of symptomatic malaria [3–5]. Recent asymptomatic parasitemia (within the prior 180 days, present for 34% of treatments) was strongly associated with a lower risk of failure after therapy with AQ+SP (1% vs. 18%, HR=0.06, 95%CI=0.01–0.36, p=0.002). To test the hypothesis that a decrease in host immunity was responsible for the increasing risk of failure over the course of the study, we performed a multivariate analysis first excluding and then including recent asymptomatic parasitemia as an explanatory variable. After adjusting for parasite resistance-mediating SNPs, parasite density, distance from the swamp, and age at enrollment, the association between time and risk of treatment failure remained strong (HR=2.9/yr, p=0.001) (Table 2). However, the inclusion of recent asymptomatic parasitemia reduced the association between time and the risk of failure (HR=1.5, p=0.3). This finding suggests that a decrease in antimalarial immunity over the course of our study largely drove the observed decrease in efficacy of AQ+SP.
Table 2.
Parasite and Host Factors Associated with Treatment Failure.
| Risk factor | Model not including AP | Model including AP | ||
|---|---|---|---|---|
| HR (95% CI) | p-value | HR (95% CI) | p-value | |
| Calendar time (per year) | 2.9 (1.5–5.4) | 0.001 | 1.5 (0.7–3.4) | 0.3 |
| Parasite SNPs | ||||
| − dhfr/dhps, +/− pfmdr1 | 1.0 | - | 1.0 | - |
| + dhfr/dhps, − pfmdr1 | 2.8 (0.7–11.6) | 0.16 | 3.1 (0.7–12.5) | 0.12 |
| + dhfr/dhps, + pfmdr1 | 7.9 (1.8–35.3) | 0.007 | 7.7 (1.8–33.5) | 0.006 |
| Parasite density > 100,000/μl | 2.2 (0.9–5.5) | 0.08 | 1.9 (0.7–4.8) | 0.18 |
| Living ≤ 50m from swamp | 0.3 (0.1–0.8) | 0.01 | 0.3 (0.1–0.8) | 0.01 |
| Age at enrollment (per 5 years) | 0.6 (0.3–1.2) | 0.15 | 0.7 (0.4–1.4) | 0.3 |
| AP observed in the last 180 days | - | - | 0.06 (0.01–0.5) | 0.006 |
NOTE. AP, asymptomatic parasitemia; HR, hazard ratio; + dhfr/dhps, dhfr 51I+59R+108N & dhps 437G+540E; + pfmdr1, pfmdr1 86N+184F.
Associations performed using Cox proportional hazards with inference accounting for repeated measures.
Discussion
Earlier analysis of data from our ongoing clinical trial showed that AQ+SP was inferior to two ACT regimens [21]. An additional 9 months of follow-up showed further decrease in efficacy (Figure 2), and the AQ+SP arm was subsequently discontinued from our study. Why did the efficacy of AQ+SP decrease so rapidly? An obvious explanation might be increasing resistance of malaria parasites in Kampala to the components of AQ+SP. However, during our study we did not find changes in prevalence of key polymorphisms that mediate diminished responses to AQ or SP. An alternative explanation for the loss of drug efficacy is diminished host immunity, as antimalarial treatment responses to partially efficacious drugs are dependent on host immunity [11–14, 37], and study subjects benefitted from a number of study-specific and national malaria control measures that likely decreased their exposure to parasites. A straightforward measure of antimalarial immunity is not available [36], but a number of reasonable surrogates of immunity have been established. Two such surrogates, increasing age at enrollment and residence near a local area of high transmission, both showed an association with greater treatment efficacy, suggesting that baseline immunity played a major role in efficacy. A surrogate marker of immunity that was able to change over time recent asymptomatic parasitemia appeared to explain the majority of the decrease in AQ+SP efficacy over the course of our study. Thus, our results suggest that, in the setting of pre-existing diminished parasite susceptibility to AQ and SP, worsening drug efficacy was mediated not by increasing parasite resistance, but by diminishing host immunity.
Antimalarial immunity usually increases with increasing age in individuals living in endemic areas, but appeared to wane in our cohort. Factors which may have contributed to declining immunity in our study population included improved access to antimalarial combination therapy, community-wide changes in antimalarial treatment, and distribution of insecticide treated bednets (ITNs). At the time our study began, chloroquine monotherapy was by far the most common antimalarial used in the community [38]. We know from prior data in Kampala that although symptoms often improve after treatment with chloroquine, almost 90% of patients fail to clear their parasites, exposing them to continued parasitemia [39]. Access to prompt combination therapy improved the overall health of children in all three treatment arms of our trial, and dramatically decreased the prevalence of asymptomatic parasitemia [21]. Asymptomatic parasitemia has been associated with protection from subsequent symptomatic malaria [40–44] and, in this study, we now show a strong association with protection from subsequent treatment failure with partially effective therapy. On a community level, the highly effective antimalarial artemether-lumefantrine, which began to be widely dispensed in Kampala in early 2006, may have decreased transmission intensity of parasites in the area as this therapy is highly effective in clearing parasites[21] and artemisinins may provide additional transmission-blocking effects [45]. Finally, we distributed ITNs to all study participants in May through June of 2006, cutting the incidence of malaria in half [28]. Although AQ+SP efficacy began to decline before ITN distribution, the decrease in parasite exposure afforded by this control measure may have further contributed to declining immunity.
It should be emphasized that the effect of immunity on the efficacy of AQ+SP in our study was likely only relevant because local parasites were partially, but not completely, resistant to this therapy. With therapy to which there is a high level of parasite resistance, such as chloroquine or SP, drug efficacy may improve with increased immunity, although not to acceptable levels [46]. With therapy to which there is little or no resistance, efficacy will not vary significantly with immune status since the drug will be able to clear parasites in almost all subjects. Despite our finding of a very high prevalence of parasite polymorphisms that are known to confer moderate resistance to SP, the presence of a polymorphism known to confer high level resistance to SP (dhfr 164L) remains rare in Kampala. We also found polymorphisms in pfcrt and pfmdr1 associated with resistance to amodiaquine; specific associations in prior studies have varied, but none have yet been associated with high level resistance [30–33]. Surprisingly, we found an association between pfmdr1 86N and treatment failure. This relationship was even stronger in infections caused by parasites that also contained the SP resistance-mediating quintuple mutation discussed above. A number of prior studies have noted the 86N polymorphism to associate with a lower risk of failure after treatment with AQ-containing regimens [30–33]. The reason for this discrepancy is unknown. While we cannot exclude the possibility that additional, unmeasured parasite polymorphisms conferring high level resistance to SP or AQ were present, it is unlikely that such polymorphisms would have increased enough in prevalence during the course of our study to explain the rapid decrease in efficacy we observed. Rather, it is most likely that declining immunity in our cohort unmasked pre-existing moderate resistance to AQ+SP in Kampala.
Given the treatment failure rate of 21% at the end of our study, AQ+SP is no longer an appropriate antimalarial therapy in Kampala. Fortunately, data from our ongoing trial have not revealed decreasing efficacy of the ACTs AQ plus artesunate or artemether-lumefantrine (data not shown), and recommendations to use these drugs as first-line therapy across Africa seem to be appropriate. However, with limited availability of ACTs, non-ACT regimens are still widely used to treat uncomplicated malaria in Africa. In addition, the continued efficacy of antifolates in preventing malaria, such as the important role of SP in intermittent preventive therapy of pregnant women, may require underlying antimalarial immunity [47]. Decreasing immunity may additionally increase selective pressure for drug resistant parasites, facilitating further decline in efficacy [48].
With increased malaria control efforts now starting to make a significant impact on malaria transmission in Africa [2, 7] and regional elimination under discussion [49, 50], declines in antimalarial immunity for many living in Africa are likely to be at least as dramatic as that in children in our cohort. Thus, as malaria prevention efforts successfully decrease the burden of malaria in Africa, these efforts must be coupled with access to highly effective antimalarial therapy. In addition, careful monitoring of drug efficacy will be critical to identify emerging drug resistance which may be unmasked due to decreasing antimalarial immunity.
Acknowledgments
We are grateful to all the parents and guardians for kindly giving their consent and the study participants for their cooperation. We thank all the members of the study team in Uganda. GD is a recipient of the Doris Duke Charitable Foundation Clinical Scientist Development Award. PJR is a Doris Duke Charitable Foundation Distinguished Clinical Scientist.
Footnotes
All authors declare no conflict of interest
Funding support: This study received financial support from the National Institutes of Allergy and Infectious Disease (AI052142) and the Doris Duke Charitable Foundation (2004047)
No part of this information has been presented at a meeting
References
- 1.Sharp BL, Kleinschmidt I, Streat E, et al. Seven years of regional malaria control collaboration--Mozambique, South Africa, and Swaziland. Am J Trop Med Hyg. 2007;76:42–7. [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization. Impact of long-lasting insecticidal-treated nets (LLINs) and artemisinin-based combination therapies (ACTs) measured using surveillance data, in four African countries, 2008. World Health Organization; Geneva, Switzerland: http://www.who.int/malaria/docs/ReportGFImpactMalaria.pdf. [Google Scholar]
- 3.Bodker R, Msangeni HA, Kisinza W, Lindsay SW. Relationship between the intensity of exposure to malaria parasites and infection in the Usambara Mountains, Tanzania. Am J Trop Med Hyg. 2006;74:716–23. [PubMed] [Google Scholar]
- 4.Marsh K, Snow RW. Host-parasite interaction and morbidity in malaria endemic areas. Philos Trans R Soc Lond B Biol Sci. 1997;352:1385–94. doi: 10.1098/rstb.1997.0124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schofield L, Mueller I. Clinical immunity to malaria. Curr Mol Med. 2006;6:205–21. doi: 10.2174/156652406776055221. [DOI] [PubMed] [Google Scholar]
- 6.Aponte JJ, Menendez C, Schellenberg D, et al. Age interactions in the development of naturally acquired immunity to Plasmodium falciparum and its clinical presentation. PLoS Med. 2007;4:e242. doi: 10.1371/journal.pmed.0040242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Campbell CC. Halting the toll of malaria in Africa. Am J Trop Med Hyg. 2008;78:851–3. [PubMed] [Google Scholar]
- 8.Duraisingh MT, Roper C, Walliker D, Warhurst DC. Increased sensitivity to the antimalarials mefloquine and artemisinin is conferred by mutations in the pfmdr1 gene of Plasmodium falciparum. Mol Microbiol. 2000;36:955–61. doi: 10.1046/j.1365-2958.2000.01914.x. [DOI] [PubMed] [Google Scholar]
- 9.Pickard AL, Wongsrichanalai C, Purfield A, et al. Resistance to antimalarials in Southeast Asia and genetic polymorphisms in pfmdr1. Antimicrob Agents Chemother. 2003;47:2418–23. doi: 10.1128/AAC.47.8.2418-2423.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sibley CH, Hyde JE, Sims PF, et al. Pyrimethamine-sulfadoxine resistance in Plasmodium falciparum: what next? Trends Parasitol. 2001;17:582–8. doi: 10.1016/s1471-4922(01)02085-2. [DOI] [PubMed] [Google Scholar]
- 11.Djimde AA, Doumbo OK, Traore O, et al. Clearance of drug-resistant parasites as a model for protective immunity in Plasmodium falciparum malaria. Am J Trop Med Hyg. 2003;69:558–63. [PubMed] [Google Scholar]
- 12.Dorsey G, Gasasira AF, Machekano R, Kamya MR, Staedke SG, Hubbard A. The impact of age, temperature, and parasite density on treatment outcomes from antimalarial clinical trials in Kampala, Uganda. Am J Trop Med Hyg. 2004;71:531–6. [PubMed] [Google Scholar]
- 13.Francis D, Nsobya SL, Talisuna A, et al. Geographic differences in antimalarial drug efficacy in Uganda are explained by differences in endemicity and not by known molecular markers of drug resistance. J Infect Dis. 2006;193:978–86. doi: 10.1086/500951. [DOI] [PubMed] [Google Scholar]
- 14.Omar SA, Adagu IS, Warhurst DC. Can pretreatment screening for dhps and dhfr point mutations in Plasmodium falciparum infections be used to predict sulfadoxine-pyrimethamine treatment failure? Trans R Soc Trop Med Hyg. 2001;95:315–9. doi: 10.1016/s0035-9203(01)90250-0. [DOI] [PubMed] [Google Scholar]
- 15.World Health Organization. Guidelines for the Treatment of Malaria Report WHO/HTM/MAL/2006.1108. World Health Organization; Geneva, Switzerland: [Google Scholar]
- 16.Nosten F, White NJ. Artemisinin-based combination treatment of falciparum malaria. Am J Trop Med Hyg. 2007;77:181–92. [PubMed] [Google Scholar]
- 17.Staedke SG, Mpimbaza A, Kamya MR, Nzarubara BK, Dorsey G, Rosenthal PJ. Combination treatments for uncomplicated falciparum malaria in Kampala, Uganda: randomised clinical trial. Lancet. 2004;364:1950–7. doi: 10.1016/S0140-6736(04)17478-3. [DOI] [PubMed] [Google Scholar]
- 18.Yeka A, Banek K, Bakyaita N, et al. Artemisinin versus nonartemisinin combination therapy for uncomplicated malaria: randomized clinical trials from four sites in Uganda. PLoS Med. 2005;2:e190. doi: 10.1371/journal.pmed.0020190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zongo I, Dorsey G, Rouamba N, et al. Randomized comparison of amodiaquine plus sulfadoxine-pyrimethamine, artemether-lumefantrine, and dihydroartemisinin-piperaquine for the treatment of uncomplicated Plasmodium falciparum malaria in Burkina Faso. Clin Infect Dis. 2007;45:1453–61. doi: 10.1086/522985. [DOI] [PubMed] [Google Scholar]
- 20.Davis JC, Clark TD, Kemble SK, et al. Longitudinal study of urban malaria in a cohort of Ugandan children: description of study site, census and recruitment. Malar J. 2006;5:18. doi: 10.1186/1475-2875-5-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dorsey G, Staedke S, Clark TD, et al. Combination therapy for uncomplicated falciparum malaria in Ugandan children: a randomized trial. Jama. 2007;297:2210–9. doi: 10.1001/jama.297.20.2210. [DOI] [PubMed] [Google Scholar]
- 22.Susceptibility of Plasmodium falciparum to antimalarial drugs. World Health Organization; 2005. [Google Scholar]
- 23.Greenhouse B, Dokomajilar C, Hubbard A, Rosenthal PJ, Dorsey G. Impact of transmission intensity on the accuracy of genotyping to distinguish recrudescence from new infection in antimalarial clinical trials. Antimicrob Agents Chemother. 2007;51:3096–103. doi: 10.1128/AAC.00159-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Snounou G, Viriyakosol S, Zhu XP, et al. High sensitivity of detection of human malaria parasites by the use of nested polymerase chain reaction. Mol Biochem Parasitol. 1993;61:315–20. doi: 10.1016/0166-6851(93)90077-b. [DOI] [PubMed] [Google Scholar]
- 25.Duraisingh MT, Curtis J, Warhurst DC. Plasmodium falciparum: detection of polymorphisms in the dihydrofolate reductase and dihydropteroate synthetase genes by PCR and restriction digestion. Exp Parasitol. 1998;89:1–8. doi: 10.1006/expr.1998.4274. [DOI] [PubMed] [Google Scholar]
- 26.Kyabayinze D, Cattamanchi A, Kamya MR, Rosenthal PJ, Dorsey G. Validation of a simplified method for using molecular markers to predict sulfadoxine-pyrimethamine treatment failure in African children with falciparum malaria. Am J Trop Med Hyg. 2003;69:247–52. [PubMed] [Google Scholar]
- 27.Kublin JG, Dzinjalamala FK, Kamwendo DD, et al. Molecular markers for failure of sulfadoxine-pyrimethamine and chlorproguanil-dapsone treatment of Plasmodium falciparum malaria. J Infect Dis. 2002;185:380–8. doi: 10.1086/338566. [DOI] [PubMed] [Google Scholar]
- 28.Clark TD, Greenhouse B, Njama-Meya D, et al. Factors Determining the Heterogeneity of Malaria Incidence in Children in Kampala, Uganda. J Infect Dis. 2008;198:393–400. doi: 10.1086/589778. [DOI] [PubMed] [Google Scholar]
- 29.Gregson A, Plowe CV. Mechanisms of resistance of malaria parasites to antifolates. Pharmacol Rev. 2005;57:117–45. doi: 10.1124/pr.57.1.4. [DOI] [PubMed] [Google Scholar]
- 30.Dokomajilar C, Lankoande ZM, Dorsey G, Zongo I, Ouedraogo JB, Rosenthal PJ. Roles of specific Plasmodium falciparum mutations in resistance to amodiaquine and sulfadoxine-pyrimethamine in Burkina Faso. Am J Trop Med Hyg. 2006;75:162–5. [PubMed] [Google Scholar]
- 31.Happi CT, Gbotosho GO, Folarin OA, et al. Association between mutations in Plasmodium falciparum chloroquine resistance transporter and P. falciparum multidrug resistance 1 genes and in vivo amodiaquine resistance in P. falciparum malaria-infected children in Nigeria. Am J Trop Med Hyg. 2006;75:155–61. [PubMed] [Google Scholar]
- 32.Marfurt J, Muller I, Sie A, et al. The usefulness of twenty-four molecular markers in predicting treatment outcome with combination therapy of amodiaquine plus sulphadoxine-pyrimethamine against falciparum malaria in Papua New Guinea. Malar J. 2008;7:61. doi: 10.1186/1475-2875-7-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tinto H, Guekoun L, Zongo I, Guiguemde RT, D’Alessandro U, Ouedraogo JB. Chloroquine-resistance molecular markers (Pfcrt T76 and Pfmdr-1 Y86) and amodiaquine resistance in Burkina Faso. Trop Med Int Health. 2008;13:238–40. doi: 10.1111/j.1365-3156.2007.01995.x. [DOI] [PubMed] [Google Scholar]
- 34.Dorsey G, Dokomajilar C, Kiggundu M, Staedke SG, Kamya MR, Rosenthal PJ. Principal role of dihydropteroate synthase mutations in mediating resistance to sulfadoxine-pyrimethamine in single-drug and combination therapy of uncomplicated malaria in Uganda. Am J Trop Med Hyg. 2004;71:758–63. [PubMed] [Google Scholar]
- 35.Lynch C, Pearce R, Pota H, et al. Emergence of a dhfr Mutation Conferring High-Level Drug Resistance in Plasmodium falciparum Populations from Southwest Uganda. J Infect Dis. 2008;197:1598–1604. doi: 10.1086/587845. [DOI] [PubMed] [Google Scholar]
- 36.Langhorne J, Ndungu FM, Sponaas AM, Marsh K. Immunity to malaria: more questions than answers. Nat Immunol. 2008;9:725–32. doi: 10.1038/ni.f.205. [DOI] [PubMed] [Google Scholar]
- 37.Staedke SG, Sendagire H, Lamola S, Kamya MR, Dorsey G, Rosenthal PJ. Relationship between age, molecular markers, and response to sulphadoxine-pyrimethamine treatment in Kampala, Uganda. Trop Med Int Health. 2004;9:624–9. doi: 10.1111/j.1365-3156.2004.01239.x. [DOI] [PubMed] [Google Scholar]
- 38.Kemble SK, Davis JC, Nalugwa T, et al. Prevention and treatment strategies used for the community management of childhood fever in Kampala, Uganda. Am J Trop Med Hyg. 2006;74:999–1007. [PubMed] [Google Scholar]
- 39.Kamya MR, Dorsey G, Gasasira A, et al. The comparative efficacy of chloroquine and sulfadoxine-pyrimethamine for the treatment of uncomplicated falciparum malaria in Kampala, Uganda. Trans R Soc Trop Med Hyg. 2001;95:50–5. doi: 10.1016/s0035-9203(01)90331-1. [DOI] [PubMed] [Google Scholar]
- 40.al-Yaman F, Genton B, Reeder JC, Anders RF, Smith T, Alpers MP. Reduced risk of clinical malaria in children infected with multiple clones of Plasmodium falciparum in a highly endemic area: a prospective community study. Trans R Soc Trop Med Hyg. 1997;91:602–5. doi: 10.1016/s0035-9203(97)90046-8. [DOI] [PubMed] [Google Scholar]
- 41.Beck HP, Felger I, Huber W, et al. Analysis of multiple Plasmodium falciparum infections in Tanzanian children during the phase III trial of the malaria vaccine SPf66. J Infect Dis. 1997;175:921–6. doi: 10.1086/513991. [DOI] [PubMed] [Google Scholar]
- 42.Bereczky S, Liljander A, Rooth I, et al. Multiclonal asymptomatic Plasmodium falciparum infections predict a reduced risk of malaria disease in a Tanzanian population. Microbes Infect. 2006 doi: 10.1016/j.micinf.2006.10.014. [DOI] [PubMed] [Google Scholar]
- 43.Farnert A, Rooth I, Svensson, Snounou G, Bjorkman A. Complexity of Plasmodium falciparum infections is consistent over time and protects against clinical disease in Tanzanian children. J Infect Dis. 1999;179:989–95. doi: 10.1086/314652. [DOI] [PubMed] [Google Scholar]
- 44.Males S, Gaye O, Garcia A. Long-term asymptomatic carriage of Plasmodium falciparum protects from malaria attacks: a prospective study among Senegalese children. Clin Infect Dis. 2008;46:516–22. doi: 10.1086/526529. [DOI] [PubMed] [Google Scholar]
- 45.Okell LC, Drakeley CJ, Ghani AC, Bousema T, Sutherland CJ. Reduction of transmission from malaria patients by artemisinin combination therapies: a pooled analysis of six randomized trials. Malar J. 2008;7:125. doi: 10.1186/1475-2875-7-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dorsey G, Kamya MR, Ndeezi G, et al. Predictors of chloroquine treatment failure in children and adults with falciparum malaria in Kampala, Uganda. Am J Trop Med Hyg. 2000;62:686–92. doi: 10.4269/ajtmh.2000.62.686. [DOI] [PubMed] [Google Scholar]
- 47.ter Kuile FO, van Eijk AM, Filler SJ. Effect of sulfadoxine-pyrimethamine resistance on the efficacy of intermittent preventive therapy for malaria control during pregnancy: a systematic review. Jama. 2007;297:2603–16. doi: 10.1001/jama.297.23.2603. [DOI] [PubMed] [Google Scholar]
- 48.Klein EY, Smith DL, Boni MF, Laxminarayan R. Clinically immune hosts as a refuge for drug-sensitive malaria parasites. Malar J. 2008;7:67. doi: 10.1186/1475-2875-7-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Feachem R, Sabot O. A new global malaria eradication strategy. Lancet. 2008;371:1633–5. doi: 10.1016/S0140-6736(08)60424-9. [DOI] [PubMed] [Google Scholar]
- 50.Guerra CA, Gikandi PW, Tatem AJ, et al. The limits and intensity of Plasmodium falciparum transmission: implications for malaria control and elimination worldwide. PLoS Med. 2008;5:e38. doi: 10.1371/journal.pmed.0050038. [DOI] [PMC free article] [PubMed] [Google Scholar]