Abstract
Smoking is an established risk factor for pancreatic cancer; however, detailed examination of the association of smoking intensity, smoking duration, and cumulative smoking dose with pancreatic cancer is limited. The authors analyzed pooled data from the international Pancreatic Cancer Cohort Consortium nested case-control study (1,481 cases, 1,539 controls). Odds ratios and 95% confidence intervals were calculated by using unconditional logistic regression. Smoking intensity effects were examined with an excess odds ratio model that was linear in pack-years and exponential in cigarettes smoked per day and its square. When compared with never smokers, current smokers had a significantly elevated risk (odds ratio (OR) = 1.77, 95% confidence interval (CI): 1.38, 2.26). Risk increased significantly with greater intensity (≥30 cigarettes/day: OR = 1.75, 95% CI: 1.27, 2.42), duration (≥50 years: OR = 2.13, 95% CI: 1.25, 3.62), and cumulative smoking dose (≥40 pack-years: OR = 1.78, 95% CI: 1.35, 2.34). Risk more than 15 years after smoking cessation was similar to that for never smokers. Estimates of excess odds ratio per pack-year declined with increasing intensity, suggesting greater risk for total exposure delivered at lower intensity for longer duration than for higher intensity for shorter duration. This finding and the decline in risk after smoking cessation suggest that smoking has a late-stage effect on pancreatic carcinogenesis.
Keywords: pancreas, pancreatic neoplasms, smoking, tobacco use cessation
Pancreatic adenocarcinoma is the fourth leading cause of cancer mortality in the United States (1) and the fifth worldwide (2). There is no effective screening test for pancreatic cancer; therefore, it is often diagnosed at an advanced stage, contributing to a 5-year survival rate of less than 5% (3). The incidence of pancreatic cancer is higher in men compared with women and, in the United States, in African Americans compared with Caucasians (3).
Cigarette smoking is a consistent risk factor for pancreatic cancer (4–7). History of diabetes, obesity, and family history of pancreatic cancer are also risk factors (8–11). Cigarette smoking may be responsible for approximately 20% of pancreatic cancer cases (12). A recent meta-analysis indicated that current cigarette smokers, compared with never smokers, have about a 2-fold risk of pancreatic cancer and that the risk increases incrementally with the number of cigarettes smoked and the number of years of smoking (12). The magnitude of the effects of the different dimensions of smoking on pancreatic cancer risk varies because of small study sizes and differences in design characteristics (12). In particular, the level of risk associated with intensity, duration, and cumulative dose, and the change in risk with cigarette smoking cessation, need further elucidation to better understand their influence on pancreatic cancer.
We pooled nested case-control samples from the Pancreatic Cancer Cohort Consortium. Our aims were to examine the risk of pancreatic cancer in relation to cigarette smoking, including evaluating the magnitude of effect of smoking intensity, duration, pack-years, and cessation. With over 1,400 cases, this prospective analysis of pancreatic cancer and smoking is one of the largest to date.
MATERIALS AND METHODS
Study population
The Pancreatic Cancer Cohort Consortium is an international initiative that includes investigators from 12 prospective epidemiologic cohorts and one case-control study to identify genetic markers of susceptibility through a genome-wide association study and to investigate environmental and lifestyle risk factors for pancreatic cancer. For this analysis, we included only prospective nested case-control studies that did not match controls to cases by smoking status.
Studies in the pooled analysis included the Alpha-Tocopherol, Beta-Carotene Cancer Prevention (ATBC) Study; CLUE II; Cancer Prevention Study II; European Prospective Investigation into Cancer and Nutrition (EPIC); New York University Women's Health Study; Prostate, Lung, Colorectal, Ovarian Cancer Screening Trial; Shanghai Men's and Women's Health Study; and Women's Health Initiative (Table 1). The Nurses’ Health Study, Health Professionals Follow-up Study, Women's Health Study, and Physicians’ Health Study were excluded because cases and controls were matched on smoking status (never, former, and current cigarette smokers), and the Mayo Clinic study was excluded because it was not prospective. Our final analytic set included 1,481 cases and 1,539 controls from 8 cohorts.
Table 1.
Characteristics of the 8 Cohort Studies Included in the Pooled Analysis of Cigarette Smoking and Pancreatic Cancer
| Cohort | Center | Center Location | Year(s) of Data Collectiona | Cases (n = 1,481) |
Controls (n = 1,539) |
|||||
| No. of Males/No. of Females | Race, % | Age Range, Years | Total No. | Matching Factors | Data Source | No. | ||||
| Alpha-Tocopherol, Beta-Carotene Cancer Prevention Study | National Cancer Institute and the National Public Health Institute | Finland | 1985–1988 | 210/0 | 100% Caucasian | 57–85 | 210 | Age at randomization (1–5 years), baseline blood draw (≥30 days) | Baseline | 211 |
| CLUE II | John Hopkins Bloomberg School of Public Health | United States | 1989 | 38/5 | 100% Caucasian | 42–94 | 83 | Race, gender, age | Baseline | 83 |
| Cancer Prevention Study II | American Cancer Society | United States | 1997 | 89/76 | 97.6% Caucasian, 0.8% African American, 0.8% Asian, 0.8% other | 64–90 | 165 | Race, self-reported ethnicity, gender, date of birth (±6 months), DNA source (blood or buccal), DNA sample provided during the same season and year | Most recent | 165 |
| European Prospective Investigation into Cancer and Nutrition | International Agency for Research on Cancer and Imperial College London | Europe | 1992–2000 (varied by center) | 199/202 | >98% Caucasian | 37–84 | 401 | Gender, center, age at recruitment (±1 month), date of blood donation (±1 month), time of blood draw (±1 hour), hours between blood draw and last food or drink intake (<3, 3–6, >6) | Baseline | 438 |
| New York University Women's Health Study | New York University | United States | 1991–1994 | 0/8 | 89.5% Caucasian, 10.5% other | 48–82 | 8 | Age at enrollment (±6 months), date of enrollment (±3 months), menopausal status at enrollment, race/ethnicity | Most recent | 11 |
| Prostate, Lung, Colorectal, Ovarian Cancer Screening Trial | National Cancer Institute | United States | 1993–2001 | 154/99 | 91.1% Caucasian, 4.7% Asian, 3.2% African American, 1.0% other | 56–84 | 253 | Race, gender, ethnicity, center, frequency samples by calendar year of birth (5-year block), gender, broad categories of race, source of DNA (blood or buccal cell), study arm, study center; for intervention arm, additionally stratified sampled by age | Baseline | 271 |
| Shanghai Men's and Women's Health Study | Vanderbilt University | China | 1996 (F), 2001 (M) | 17/61 | 100% Asian | 43–77 | 78 | Race, ethnicity, gender, year of birth (<2 years), menopausal status at baseline, date of sample collection (<30 days), time of sample collection (AM/PM), time interval after the last meal (<2 hours) | Baseline | 79 |
| Women's Health Initiative | Fred Hutchinson Cancer Research Center | United States | 1992–1998 | 0/283 | 85.6% Caucasian, 7.5% African American, 4.1% Asian, 1.8% other, 1.0% missing | 53–88 | 283 | Gender, center, race, ethnicity, age at screening, enrollment date, study component, hysterectomy status, menopausal status | Baseline | 281 |
Abbreviations: F, female; M, male.
Except for the Cancer Prevention Study II and the New York University Women's Health Study, year(s) smoking data used in this analysis were collected on the baseline questionnaire. For these 2 studies, earliest year smoking data used in this analysis were collected; updated smoking data were also used.
Case ascertainment and data collection
Cases of pancreatic cancer included all incident primary pancreatic adenocarcinomas (International Classification of Diseases for Oncology (ICD-O-3) codes C250–C259 or C25.0–C25.3, C25.7–C25.9). We excluded endocrine pancreatic tumors (code C25.4, histology type, 8150, 8151, 8153, 8155, 8240, 8246) because the etiology of these cancers is thought to be different. Case ascertainment varied between studies but included linking participants to cancer registries, self- and next-of-kin report, and national death indices. Most cases of pancreatic cancer were histologically confirmed (ATBC, CLUE II, Cancer Prevention Study II, EPIC, New York University Women's Health Study, Shanghai Men's and Women's Health Study, Women's Health Initiative) or confirmed through cancer registries (ATBC, EPIC, Shanghai Men's and Women's Health Study), death certificates (Cancer Prevention Study II, EPIC), or review of medical records by medical personnel (ATBC; EPIC; Prostate, Lung, Colorectal Ovarian Cancer Screening Trial; Shanghai Men's and Women's Health Study).
Controls were incidence density sampled with a 1-to-1 control-to-case ratio and were alive and free from pancreatic cancer on the date the matched case was diagnosed. Controls were frequency selected to cases on calendar year of birth (±5 years), gender, race, and ethnicity. Each cohort may have been matched additionally on other relevant factors such as age at baseline or age at blood draw (±5 years), date/time of blood draw, fasting blood draw, and length of follow-up (Table 1).
In each of the 8 studies, data on cigarette smoking history, demographics, and possible confounders were collected through written questionnaires or in-person interviews. Detailed descriptions of data collection methods have been published previously by the individual studies (13–22). From each study, we obtained information on history of cigarette smoking, sex, age, race, body mass index, family history of pancreatic cancer, alcohol consumption, self-reported pancreatitis, and diabetes history. Individual data sets were checked for consistency with previously published results (21).
The Special Studies Institutional Review Board of the National Cancer Institute approved the pooled Pancreatic Cancer Cohort Consortium study. Informed consent was obtained in each of the individual studies. Each study also was approved by its local institutional review board.
Exposure definitions
We classified study participants as ever smokers if they had ever smoked cigarettes (ATBC, CLUE II, EPIC, New York University Women's Health Study), if they had ever smoked more than 100 cigarettes in a lifetime (Cancer Prevention Study II, Women's Health Initiative), or if they had ever smoked cigarettes for 6 months or longer (Prostate, Lung, Colorectal, Ovarian Cancer Screening Trial; Shanghai Men's and Women's Health Study). Former smokers were defined as those who had reported stopping smoking on the most recent questionnaire prior to their diagnosis (cases and matched controls), individually corrected for the time elapsed between questionnaire administration and diagnosis of pancreatic cancer.
For ever smokers, information was also collected on the age at which they began smoking, current smoking habits, and intensity and duration of smoking. Cumulative lifetime exposure to cigarette smoking was computed by using smoking intensity and duration (pack-years = number of packs smoked per day × number of years of smoking). We categorized smoking-related variables into quartiles or quintiles based on ever smokers only (intensity: <10, 10–<20, 20–<25, 25–<30, ≥30; duration: ≤10, >10–20, >20–40, >40–50, >50; pack-years: ≤10, >10–20, >20–30, >30–40, >40; age (years) at start: <15, 15–<20, ≥20; cessation: 1–<10, 10–<15, 15–<20, 20–<30, ≥30); never smokers were used as the reference group.
Statistical analysis
We calculated odds ratios and 95% confidence intervals for pancreatic cancer risk using unconditional logistic regression. We adjusted all models for sex, age (continuous), race (Caucasian, African American, Asian, other, unknown), body mass index (weight (kg)/height (m)2), and self-reported diabetes (yes, no, missing) because they are putative risk factors for pancreatic cancer. We did not adjust for family history of pancreatic cancer because not all cohorts obtained this information. We tested for trend among ever smokers and treated the exposure variable as continuous in the model by entering the median value for each level of the categorical variable among the controls. Tests for trend were 2-sided and were based on the integer scores for smoking cessation (0 for current smokers, from 1 for shorter to as high as 5 for longer cessation time) and age at which smoking started (23).
Heterogeneity in the risk estimates for our study was assessed by using the Q and I2 statistics (24). We considered statistically significant heterogeneity at the P = 0.05 level of association. I2 describes the percentage of variability in point estimates due to heterogeneity rather than sampling error. An I2 of 50% or more was considered to be notably heterogeneous. To investigate whether one single study unduly influenced the pooled estimates, sensitivity analyses also were conducted to compare pooled risk estimates after systematically excluding each study in turn. Interaction was tested by using a multiplicative risk model. We also calculated the attributable proportion of the disease explained by smoking using a multivariate approach (25) (attributable risk = 1 − (((1/never smoker odds ratio (OR)) × number (n) of never smoker pancreatic cancer cases) + ((1/former smoker OR) × n former smoker cases) + ((1/current smoker OR) × n current smoker cases)/total n pancreatic cancer cases)). We evaluated the effects of the delivery rate of cigarette smoking; that is, for subjects with equal total pack-years, particularly whether increasing number of cigarettes per day and decreasing duration of smoking results in greater, the same, or smaller risk of pancreatic cancer. This step is accomplished by applying a recently described 3-parameter model for the excess odds ratio (26) of the form
| (1) |
where d denotes total pack-years of smoking and n denotes cigarettes smoked per day. The parameter β represents the slope of a linear relationship between disease and total pack-years, that is, the excess odds ratio per pack-year at g(n) = 1, whereas g(.) represents the modifying effect of cigarettes per day on β. For each n, β g(n) thus defines a distinct linear relationship for the odds ratios of pancreatic cancer by total pack-years. After a preliminary assessment, we set g(n) = exp{N1 n + N2 n2}, where N1 and N2 are unknown parameters. This function differed from prior analyses (26, 27).
The ATBC study included only smokers. To fit model 1, we therefore adjusted the ATBC study-specific intercept parameter to reflect never smokers using the following procedure. In a mixed population, I = IS=1 P[S = 1] + IS=0 P[S = 0], where I is the disease incidence rate and S = 1 or 0 denotes ever or never smoker, respectively. If RRsmk = IS=1/IS=0 is the relative risk for ever smoked, then I = IS=1 (P[S = 1] + (1/RRsmk) P[S = 0]). We assumed RRsmk = 2 for pancreas cancer and P[S = 1] = 0.70 (27) and included the logarithm of an offset value equal to P[S = 1] + (1/RRsmk) P[S = 0]) = 0.85.
Odds ratio analyses were calculated by using the SAS software program, version 9.1 (SAS Institute, Inc., Cary, North Carolina). Model 1 was fit by using the Epicure program (28).
RESULTS
Cases and controls were similar regarding all matched variables (data not shown). Most study participants were Caucasian, and 89% of the study population was older than age 60 years. Ten percent of our participants were diabetic, and cases and controls tended to be overweight, with a mean body mass index of about 26 kg/m2. The average age at pancreatic cancer onset was 69 years for cases who smoked and 68 years for cases who did not smoke. Thirty-nine percent of the pancreatic cancer cases and 44% of the controls never smoked (data not shown).
There was no interaction by sex; therefore, we present sex-combined results in this paper. Figure 1 (never vs. former smokers) and Figure 2 (never vs. current smokers) show forest plots for cigarette smoking and pancreatic cancer. Compared with never smokers, current smokers had a statistically significant increased risk of pancreatic cancer (OR = 1.77, 95% confidence interval (CI): 1.38, 2.26), whereas former smokers had a nonsignificant risk (OR = 1.09, 95% CI: 0.91, 1.30). The pooled estimates remained stable following the systematic exclusion of one study at a time (data not shown). We found no significant heterogeneity in our study population (pooled estimate for former smokers: Q = 6.7, P = 0.35, I2 = 10%; pooled estimate for current smokers: Q = 7.2, P = 0.30, I2 = 16%). The population attributable risk for smoking (former and current) was 15% (attributable risk = 1 − (((1/1) × 582) + (1/1.09146) × 466) + ((1/1.76450) × 433)/1,481)).
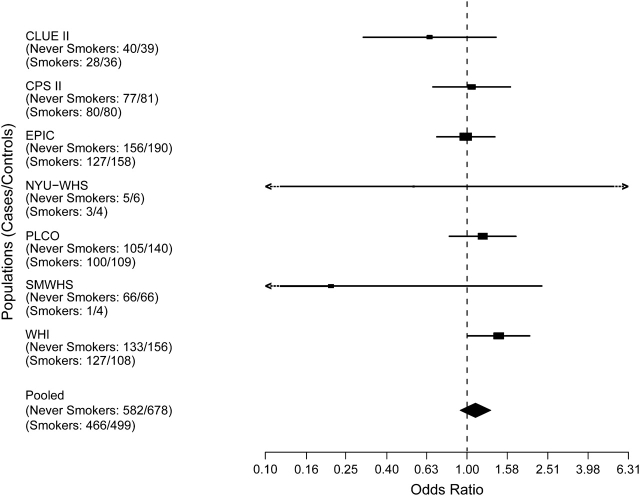
Figure 1.
Risk estimates for pancreatic cancer associated with cigarette smoking for former smokers, by study. Solid shapes represent odds ratios; horizontal lines represent confidence intervals. The magnitude of effect size and width of the confidence intervals affect the size of the solid shapes represented. CPS II, Cancer Prevention Study II; EPIC, European Prospective Investigation into Cancer and Nutrition; NYU–WHS, New York University Women's Health Study; PLCO, Prostate, Lung, Colorectal, Ovarian Cancer Screening Trial; SMWHS, Shanghai Men's and Women's Health Study; WHI, Women's Health Initiative. Confidence intervals including the notation <- -, –> extend beyond the scale provided. For NYU–WHS, odds ratio = 0.46, 95% confidence interval: 0.03, 7.37; for SMWHS, odds ratio = 0.21, 95% confidence interval: 0.02, 2.36.
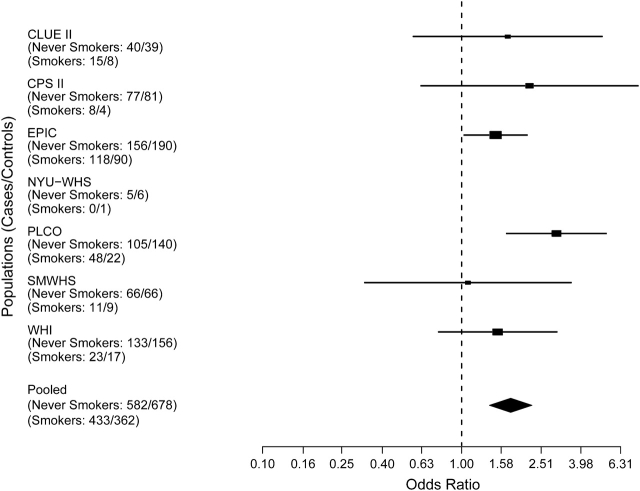
Figure 2.
Risk estimates for pancreatic cancer associated with cigarette smoking for current smokers, by study. Solid shapes represent odds ratios; horizontal lines represent confidence intervals. The magnitude of effect size and width of the confidence intervals affect the size of the solid shapes represented. CPS II, Cancer Prevention Study II; EPIC, European Prospective Investigation into Cancer and Nutrition; NYU–WHS, New York University Women's Health Study; PLCO, Prostate, Lung, Colorectal, Ovarian Cancer Screening Trial; SMWHS, Shanghai Men's and Women's Health Study; WHI, Women's Health Initiative.
For cigarette smokers, compared with never smokers, pancreatic cancer risk increased significantly with increasing intensity, duration, and pack-years (Table 2). Smoking intensity showed a significant risk in the highest exposure category and a significant dose-response trend (≥30 cigarettes/day: OR = 1.75, 95% CI: 1.27, 2.42; P-trend = 0.03). Compared with those for never smokers, the risk estimates for smokers were elevated for the longest duration (>50 years: OR = 2.13, 95% CI: 1.25, 3.62; P-trend <0.001) and cumulative smoking dose (>40 pack-years: OR = 1.78, 95% CI: 1.35, 2.34; P-trend <0.001). Risk estimates for former smokers who had quit smoking for less than 10 years were significantly increased (OR = 2.19, 95% CI: 1.25, 3.83), whereas former smokers who had quit for less than 15 years were at nonsignificant elevated risk compared with never smokers (OR = 1.24, 95% CI: 0.78, 1.98). The risk was nonsignificant for those participants who had quit smoking more than 15 years ago. When current smokers were considered the referent group, risk estimates for former smokers who quit less than 10 years ago were nonsignificantly elevated (OR = 1.24, 95% CI: 0.69, 2.22); odds ratios were significantly reduced after more than 15 years of quitting. When analyses were adjusted by smoking cessation, results were similar for smoking intensity (≥30 cigarettes/day: OR = 2.22, 95% CI: 1.50, 3.26), duration (>50 years: OR = 2.13, 95% CI: 1.25, 3.62), and pack-years (>40 pack-years: OR = 1.96, 95% CI: 1.04, 2.75).
Table 2.
Risk Estimates for Pancreatic Cancer Associated With Cigarette Smoking in the Pooled Study Population, With Never Smokers as the Reference Group
| Smoking Exposure | No. of Cases | No. of Controls | ORa | 95% CI |
| Never smoker | 582 | 678 | 1.00 | Referent |
| Ever smoker | 899 | 861 | 1.24 | 1.06, 1.46 |
| Current | 433 | 362 | 1.77 | 1.38, 2.26 |
| Former | 466 | 499 | 1.09 | 0.91, 1.30 |
| Intensity, cigarettes/day | ||||
| <10 | 142 | 149 | 1.18 | 0.91, 1.55 |
| 10–<20 | 252 | 239 | 1.33 | 1.05, 1.67 |
| 20–<25 | 150 | 145 | 1.30 | 0.98, 1.72 |
| 25–<30 | 70 | 68 | 1.28 | 0.88, 1.88 |
| ≥30 | 121 | 84 | 1.75 | 1.27, 2.42 |
| Ptrend | <0.001 | |||
| Duration, years | ||||
| ≤10 | 86 | 93 | 1.08 | 0.79, 1.49 |
| >10–20 | 113 | 139 | 0.96 | 0.72, 1.27 |
| >20–40 | 406 | 378 | 1.30 | 1.06, 1.60 |
| >40–50 | 161 | 134 | 1.59 | 1.20, 2.11 |
| >50 | 39 | 24 | 2.13 | 1.25, 3.62 |
| Ptrend | <0.001 | |||
| Pack-years | ||||
| ≤10 | 149 | 165 | 1.11 | 0.85, 1.43 |
| >10–20 | 121 | 115 | 1.32 | 0.98, 1.77 |
| >20–30 | 127 | 125 | 1.30 | 0.97, 1.75 |
| >30–40 | 119 | 107 | 1.49 | 1.08, 2.03 |
| >40 | 194 | 146 | 1.78 | 1.35, 2.34 |
| Ptrend | <0.001 | |||
| Years since quitting | ||||
| 1–<10 | 40 | 20 | 2.19 | 1.25, 3.83 |
| 10–<15 | 40 | 37 | 1.24 | 0.78, 1.98 |
| 15–<20 | 36 | 47 | 0.91 | 0.58, 1.44 |
| 20–<30 | 81 | 104 | 0.91 | 0.66, 1.26 |
| ≥30 | 154 | 198 | 0.93 | 0.73, 1.20 |
| Ptrend | <0.001 | |||
| 1–<10b | 40 | 20 | 1.24 | 0.69, 2.22 |
| 10–<15 | 40 | 37 | 0.70 | 0.43, 1.16 |
| 15–<20 | 36 | 47 | 0.52 | 0.32, 0.85 |
| 20–<30 | 81 | 104 | 0.52 | 0.36, 0.75 |
| ≥30 | 154 | 198 | 0.53 | 0.39, 0.73 |
| Ptrend | <0.001 | |||
| Age at start, yearsc | ||||
| <15 | 68 | 64 | 1.24 | 0.85, 1.79 |
| 15–<20 | 361 | 346 | 1.27 | 1.03, 1.56 |
| ≥20 | 410 | 381 | 1.28 | 1.06, 1.56 |
| Ptrend | 0.97 | |||
Abbreviations: CI, confidence interval; OR, odds ratio.
Models were adjusted for age, sex, study center, diabetes, body mass index, and race.
Years since quitting using current smokers as the reference group.
Data were missing for the CLUE II study.
Age at start of smoking was not associated with pancreatic cancer. Patterns were the same and the risk estimate increased when never smokers were compared with current smokers only (>40 pack-years: OR = 2.17, 95% CI: 1.49, 3.16). Using a multiplicative model for risk of pancreatic cancer, we found no significant interaction between smoking and age, sex, body mass index, race, or diabetes (data not shown).
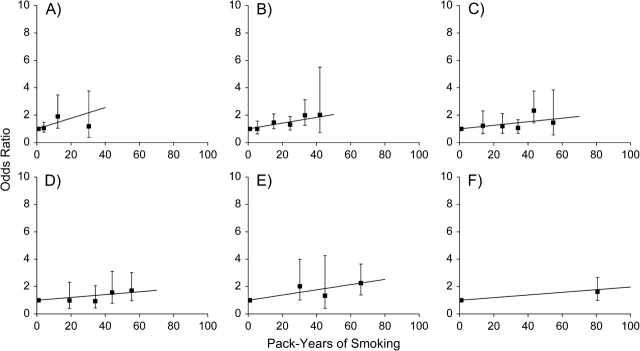
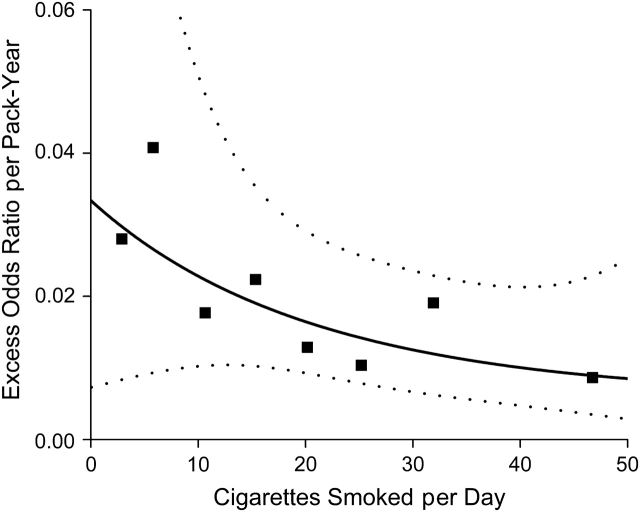
For any given number of cigarettes per day, n, model 1 defines a distinct linear relation for the odds ratios of pancreatic cancer by total pack-years with a slope given by β g(n). We evaluated that assumption in Figure 3, which displays odds ratios for pack-years of smoking within categories of smoking intensity by considering never smokers as the reference. Odds ratios increased with increasing pack-years within each category of intensity, and the associations between odds ratios and pack-years were approximately linear. Within each category of number of cigarettes per day, the slope of the linear estimates show the excess odds ratio per pack-year. The estimated excess odds ratio/pack-year with 95% confidence interval generally decreases with increasing number of cigarettes smoked per day (Figure 4, square symbols). The risk patterns shown in Figure 4 were similar when data were restricted to never smokers and to current and recent former smokers.
Figure 3.
Odds ratios for pancreatic cancer according to pack-years of cigarette smoking (black squares) and fitted linear excess odds ratio (solid line) within categories of number of cigarettes smoked per day in the Pancreatic Cancer Cohort Consortium: A) <10, B) 10–19, C) 20–24, D) 25–29, E) 30–39, F) ≥40. All odds ratios were calculated relative to never smokers; horizontal bars represent 95% confidence intervals.
Figure 4.
Estimated excess odds ratio for pancreatic cancer per pack-year of cigarette smoking (model denoted by the solid line) by cigarettes smoked per day (black squares), with pointwise 95% confidence intervals (dotted lines).
When continuous pack-years and cigarettes per day are used, the fitted model 1 (solid line) with pointwise 95% confidence interval (dotted line) (Figure 4) closely fits the estimates. Although the smoking effects were statistically significant (P < 0.001 for the test of β = 0, N1 = 0, and N2 = 0), variation of the pack-years effect by cigarettes per day was only suggestive (P = 0.16 for the test of N1 = 0 with N2 omitted, and P = 0.35 for the test of N1 = 0 and N2 = 0). The 95% confidence interval for the fitted curve was wide, particularly at low smoking intensities, highlighting the uncertainty of the effects in that range. To further investigate smoking cessation, we used our model to evaluate the excess odds ratio/pack-year for current versus former smokers (data not shown). The risks were lower for former smokers and the excess odds ratio/pack-year varied by smoking status (P = 0.06), but there were too few former smokers with detailed information (324 cases, 331 controls) to estimate the excess odds ratio/pack-year curves with precision.
DISCUSSION
Our findings support published literature showing that current smokers, compared with never smokers, have about an 80% increased risk of pancreatic cancer (12, 29). A recent meta-analysis on tobacco and pancreatic cancer risk analyzed 82 published case-control and cohort studies between 1950 and 2007 (12). The authors of the meta-analysis showed current smokers, compared with never smokers, to have pooled risks of 1.70 (95% CI: 1.53, 1.90) and 1.77 (95% CI: 1.59, 1.97) (12) in cohort and case-control studies, respectively, similar to our study's risk estimates for current smokers. Our risk estimate for former smokers, compared with never smokers (OR = 1.20, 95% CI: 1.11, 1.29), as well as the magnitude of associations with increasing smoking intensity, duration, and pack-years, is consistent with published findings (12).
Most studies suggest that smoking cessation reduces the risk of pancreatic cancer, with the reduction in risk observed 10–15 years after cessation, and our findings are consistent with the literature (12). The recent meta-analysis, which utilized both case-control and cohort data and analyzed smoking cessation in only 3 categories—<10 years, ≥10 years, and ≥20 years—compared with never smokers, showed a nonsignificantly increased pancreatic cancer risk for former smokers for a minimum of 10 years (RR = 1.15, 95% CI: 0.95, 1.40) and a nonsignificant decreased pancreatic cancer risk for those who had quit for 20 or more years (RR = 0.96, 95% CI: 0.85, 1.09) (12). Regardless of the reference group, in our prospective analysis, participants who had quit smoking for more than 15 years were at similar risk as never smokers.
We used an excess odds ratio model for pancreatic cancer that is linear in pack-years and exponential in cigarettes smoked per day and its square. The model isolated the intensity effects for fixed total pack-years, thus enabling the comparison of odds ratios for total exposure delivered at low intensity for long duration and at high intensity for short duration (26, 27, 30, 31). We found that estimates for the excess odds ratio per pack-year generally declined with increasing intensity, suggesting greater risk for a total exposure delivered at lower intensity than for an equivalent exposure delivered at higher intensity. Patterns at lower intensities are subject to increased variability because of limited ranges for pack-years among light smokers. Although the exposure rate variation was not statistically significant, our findings are consistent with previous pancreatic cancer findings from a case-control study that included a large proportion of African-American cases and a cohort study that utilized the ATBC cohort; both studies found similar inverse intensity-exposure-rate patterns (27, 32).
Biologic mechanisms and biases in exposure assessment due to the behavioral influences of nicotine dependency and subsequent nicotine satiation could explain our inverse intensity patterns (26). For example, the intensity pattern noted in our study may be due to underlying biologic processes, such as the activation and detoxification of carcinogenic compounds in cigarette smoke or DNA repair capacity (26). However, patterns of the excess odds ratio/pack-year by intensity of smoking may also reflect modulation of inhalation practices. For example, higher-intensity smokers may inhale less, thereby ingesting relatively fewer carcinogens per cigarette smoked compared with less intense smokers. This difference would result in reduced risks at higher intensities (26). However, studies of other smoking-related cancers have found no evidence of a relation between frequency or depth of inhalation and intensity after controlling for total pack-years (26). Thus, although misclassification of smoking intensity may impact the exposure rate pattern, it is unlikely to fully explain the inverse exposure rate effect (30).
After adjustment for differences in risk with total pack-years of smoking, we observed patterns of decreasing risk with smoking intensity above 15–20 cigarettes per day. A similar pattern has been observed for lung, bladder, oral cavity, kidney, and esophagus as well as pancreatic cancer (26, 27, 30, 31). Although smoking inhalation characteristics may partially explain the smoking intensity patterns (30), the consistency of the results across diverse cancer sites may suggest a common molecular mechanism in the development of smoking-related diseases. Therefore, whereas molecular mechanisms for how smoking could cause pancreatic cancer are not well understood, studies looking at possible smoking-related pathways in other tobacco-related cancers may provide some insights. The main source of exposure to tobacco-related carcinogens for pancreatic cancer is indirect via the bloodstream or bile, so understanding tobacco-related diseases with similar indirect exposure, such as kidney and cervical cancer, may be more informative (12).
This pooled analysis is one of the largest prospective analyses examining the association between smoking and pancreatic cancer, particularly noting the excess risk associated with total exposure delivered at lower intensity and longer duration versus an equivalent exposure delivered at higher intensity and shorter duration. Several additional strengths and limitations should be mentioned when interpreting our findings. Our study includes participants primarily of European decent; therefore, our study results may be generalizable to other Caucasian populations. Furthermore, our findings are consistent with a recent meta-analysis of mostly Caucasian populations, including 65 studies from Australia, Canada, or the United States. Selection bias is unlikely since controls were individually matched to cases in each prospective study. There was variation in questionnaire design and the assessment of smoking exposure in the different studies, which could result in exposure misclassification. Some, but not all cohorts in our analysis had repeated smoking measurements, and individuals may have changed their smoking behavior during follow-up. However, given that our risk estimates for never, former, and current smokers and for smoking duration, intensity, and pack-years were consistent with the literature on pancreatic cancer and smoking (12), misclassification in our study appeared to be minimal. Furthermore, sensitivity analyses revealed that our pooled estimates remained stable following systematic exclusion of each study.
Not all cases of pancreatic cancer were histologically confirmed, and central review of all cases by a study pathologist was not feasible, so it is possible that some disease misclassification could have occurred. However, a review study on pancreatic cancer and smoking has shown that risk estimates in studies in which fewer than 80% of the cases were histologically confirmed, compared with those in which more than 80% of the cases were histologically confirmed, differed by approximately 13% and were still statistically significantly associated with pancreatic cancer (12). Therefore, disease misclassification is unlikely to have changed our results significantly.
Using Pancreatic Cancer Cohort Consortium data, we confirmed previous positive associations between smoking and pancreatic cancer. Moreover, we complemented previous findings that describe an inverse exposure rate pattern for pancreatic cancer and other smoking-related cancers, suggesting commonality in the development of smoking-related diseases. Because it is still unclear exactly when quitting smoking decreases pancreatic cancer risk, this issue should continue to be studied because it may provide insights into the prevention, behavior, and molecular mechanisms associated with pancreatic cancer risk. In the past, there have been arguments about when smoking exhibits its biologic effects, that is, earlier or later in the carcinogenic process (4, 32). Our smoking cessation and intensity pattern and duration data, coupled with existing studies, support a late-stage mechanistic effect in pancreatic cancer development, although the minimum time required for the carcinogenic process to occur is still unknown, and more research is needed to better explain how smoking can lead to pancreatic cancer.
Fifteen percent of pancreatic cancer is explained by cigarette smoking in the Pancreatic Cancer Cohort Consortium study, which is slightly less than previously reported (12). Because smoking is an established pancreatic cancer risk factor, smoking cessation continues to be an effective strategy for decreasing the burden of this disease.
Acknowledgments
Author affiliations: Epidemiology and Genetics Research Program, Division of Cancer Control and Population Sciences, National Cancer Institute, National Institutes of Health, Department of Health and Human Services, Rockville, Maryland (Shannon M. Lynch); National Institute for Public Health and the Environment (RIVM), Bilthoven, the Netherlands (Alina Vrieling, H. Bas Bueno-de-Mesquita); Division of Cancer Epidemiology and Genetics, National Cancer Institute, National Institutes of Health, Department of Health and Human Services, Rockville, Maryland (Jay H. Lubin, Julie Mendelsohn, Patricia Hartge, Rachael Z. Stolzenberg-Solomon, Stephen J. Chanock, Laufey Amundadottir, Demetrius Albanes, Robert N. Hoover, Gilles Thomas, Kevin Jacobs, Geoffrey S. Tobias, Kai Yu); Department of Epidemiology, Harvard School of Public Health, Boston, Massachusetts (Peter Kraft, Dimitrios Trichopoulos); German Cancer Research Center (DKFZ), Heidelberg, Germany (Federico Canzian); Information Management Service, Rockville, Maryland (Emily Steplowski); Department of Environmental Medicine and Cancer Institute, New York University School of Medicine, New York, New York (Alan A. Arlsan, Anne Zeleniuch-Jacquette); Department of Laboratory Medicine/Pathology, School of Medicine, University of Minnesota Prevention Research Center, Minneapolis, Minnesota (Myron Gross); Prevention and Research Center, Mercy Medical Center, Baltimore, Maryland (Kathy Helzlsouer); Department of Epidemiology and Surveillance Research, American Cancer Society, Atlanta, Georgia (Eric J. Jacobs, Alpa V. Patel); Division of Public Health Sciences, Fred Hutchinson Cancer Research Center, Seattle, Washington (Andrea LaCroix, Charles Kooperberg); Department of Health Sciences Research, Mayo Clinic, Rochester, Minnesota (Gloria Petersen); Department of Medicine, Vanderbilt-Ingram Cancer Center, Vanderbilt University, Nashville, Tennessee (Wei Zheng, Xiao-Ou Shu); MRC Centre for Nutrition and Cancer, University of Cambridge, Cambridge, United Kingdom (Sheila A. Bingham); International Agency for Research on Cancer, Lyon, France (Paolo Boffetta, Weimin Ye); Inserm (Institut National de la Santé et de la Recherche Médicale) and Institut Gustave Roussy, Villejuif, France (Marie-Christine Boutron-Ruault); George W. Comstock Center for Public Health Research and Prevention, Hagerstown, Maryland (Sandra Clipp); Bioinformed Consulting Services, Gaithersburg, Maryland (Kevin Jacobs); Core Genotyping Facility, Advanced Technology Program, SAIC-Frederick Inc., National Cancer Institute-Frederick, Frederick, Maryland (Kevin Jacobs); Department of Preventive Medicine, University of Tennessee Health Science Center, Memphis, Tennessee (Karen C. Johnson); Department of Medical Epidemiology and Biostatistics, Karolinska Institute, Stockholm, Sweden (Juhua Luo); Department of Preventive Medicine, Stony Brook University, Stony Brook, New York (Catherine Messina); Molecular and Nutritional Epidemiology Unit, Cancer Research and Prevention Institute ISPO, Florence, Italy (Domenico Palli); Division of Epidemiology, Public Health and Primary Care, Imperial College London, London, United Kingdom (Elio Riboli); Public Health Directorate, Health and Health Care Services Council, Asturias, Spain (Laudina Rodriguez Suarez); Institute of Cancer Epidemiology, Danish Cancer Society, Copenhagen, Denmark (Anne Tjønneland); Division of General Internal Medicine, University of California, Davis, Davis, California (Elissa Tong); Department of Hygiene and Epidemiology, University of Athens Medical School, Athens, Greece (Dimitrios Trichopoulos); and Department of Health Promotion and Chronic Disease Prevention, National Public Health Institute, Helsinki, Finland (Jarmo Virtamo).
This research was supported by the Extramural Research Program of the National Institutes of Health, Division of Cancer Control and Population Sciences, National Cancer Institute and the Intramural Research Program of the National Institutes of Health, Division of Cancer Epidemiology and Genetics, National Cancer Institute. The New York University Women's Health Study is supported by National Cancer Institute research grants (R01CA034588, R01CA098661, P30CA016087) and the National Institute of Environmental Health Sciences Center grant (ES000260). The Women's Health Initiative is funded by the National Heart, Lung, and Blood Institute through contracts (N01WH22110, 24152, 32100-2, 32105-6, 32108-9, 32111-13, 32115, 32118-32119, 32122, 42107-26, 42129-32, and 44221). The Shanghai Men's Health Study was supported by the National Cancer Institute extramural research grant (R01 CA82729). The Shanghai Women's Health Study was supported by the National Cancer Institute extramural research grant (R01 CA70867) and, partially for biologic sample collection, by the Intramural Research Program of the National Cancer Institute (Division of Cancer Epidemiology and Genetics). The Prostate, Lung, Colorectal, Ovarian Cancer Screening Trial was supported by contracts from the National Cancer Institute (University of Colorado Denver, NO1-CN-25514, Georgetown University NO1-CN-25522, Pacific Health Research Institute NO1-CN-25515, Henry Ford Health System NO1-CN-25512, University of Minnesota, NO1-CN-25513, Washington University NO1-CN-25516, University of Pittsburgh NO1-CN-25511, University of Utah NO1-CN-25524, Marshfield Clinic Research Foundation NO1-CN-25518, University of Alabama at Birmingham NO1-CN-75022, Westat, Inc. NO1-CN-25476, University of California, Los Angeles NO1-CN-25404). The Alpha-Tocopherol, Beta-Carotene Cancer Prevention Study was supported by funding provided by the Intramural Research Program of the National Cancer Institute and US Public Health Service contracts (N01-CN-45165, N01-CN-45165, N01-RC-45035, N01-RC-37004). The European Prospective Investigation into Cancer and Nutrition was supported by the European Commission: Public Health and Consumer Protection Directorate 1993–2004; Research Directorate-General 2005; Ligue contre le Cancer, Societé 3M, Mutuelle Générale de l'Education Nationale, Institut National de la Santé et de la Recherche Médicale (INSERM) (France); German Cancer Aid, German Cancer Research Center, Federal Ministry of Education and Research (Germany); Danish Cancer Society (Denmark); Health Research Fund (FIS) of the Spanish Ministry of Health, the participating regional governments and institutions (Spain); Cancer Research UK, Medical Research Council, Stroke Association, British Heart Foundation, Department of Health, Food Standards Agency, the Wellcome Trust (United Kingdom); Greek Ministry of Health and Social Solidarity, Hellenic Health Foundation and Stavros Niarchos Foundation (Greece); Italian Association for Research on Cancer (AIRC) (Italy); Dutch Ministry of Public Health, Welfare and Sports, Dutch Prevention Funds, LK Research Funds, Dutch ZON (Zorg Onderzoek Nederland), World Cancer Research Fund (WCRF) (the Netherlands); Swedish Cancer Society, Swedish Scientific Council, Regional Government of Skane and Västerbotten (Sweden). CLUE II was supported by the National Institute on Aging (5U01AG018033) and National Cancer Institute (CA105069, CA73790). The Cancer Prevention Study II Nutrition Cohort is supported by the American Cancer Society.
Writing committee members: Shannon M. Lynch, Alina Vrieling, Jay H. Lubin, Peter Kraft, Julie B. Mendelsohn, Patricia Hartge, Federico Canzian, Emily Steplowski, and H. Bas Bueno-de-Mesquita; senior author: Rachael Z. Stolzenberg-Solomon.
Poster presentation at the American Congress of Epidemiology meeting, September 14–16, 2008, Tucson, Arizona.
Conflict of interest: none declared.
Glossary
Abbreviations
- ATBC
Alpha-Tocopherol, Beta-Carotene Cancer Prevention
- CI
confidence interval
- EPIC
European Prospective Investigation into Cancer and Nutrition
- OR
odds ratio
- RR
relative risk
References
- 1.Anderson KE, Potter JD, Mack TM. Pancreatic cancer. In: Schottenfeld D, Fraumeni JF Jr, editors. Cancer Epidemiology and Prevention. New York, NY: Oxford University Press; 1996. pp. 725–771. [Google Scholar]
- 2.Johns Hopkins University. The Sol Goldman Pancreatic Cancer Research Center. Johns Hopkins and You. Participating in Research Specific to African Americans. Baltimore, MD: Johns Hopkins University Press; 2003. ( http://pathology.jhu.edu/pancreas/PartAfAm.php). (Accessed December 20, 2007) [Google Scholar]
- 3.Edwards BK, Brown ML, Wingo PA, et al. Annual report to the nation on the status of cancer, 1975–2002, featuring population based trends in cancer treatment. J Natl Cancer Inst. 2005;97(19):1407–1427. doi: 10.1093/jnci/dji289. [DOI] [PubMed] [Google Scholar]
- 4.Silverman DT, Dunn JA, Hoover RN, et al. Cigarette smoking and pancreas cancer: a case-control study based on direct interviews. J Natl Cancer Inst. 1994;86(20):1510–1516. doi: 10.1093/jnci/86.20.1510. [DOI] [PubMed] [Google Scholar]
- 5.Fuchs CS, Colditz GA, Stampfer MJ, et al. A prospective study of cigarette smoking and the risk of pancreatic cancer. Arch Intern Med. 1996;156(19):2255–2260. [PubMed] [Google Scholar]
- 6.Muscat JE, Stellman SD, Hoffmann D, et al. Smoking and pancreatic cancer in men and women. Cancer Epidemiol Biomarkers Prev. 1997;6(1):15–19. [PubMed] [Google Scholar]
- 7.US Department of Health and Human Services. The Health Consequences of Smoking: A Report of the Surgeon General. Atlanta, GA: US Department of Health and Human Services, CDC, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2004. [Google Scholar]
- 8.Huxley R, Ansary-Moghaddam A, Berrington de González A, et al. Type-II diabetes and pancreatic cancer: a meta-analysis of 36 studies. Br J Cancer. 2005;92(11):2076–2083. doi: 10.1038/sj.bjc.6602619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Silverman DT, Schiffman M, Everhart J, et al. Diabetes mellitus, other medical conditions and familial history of cancer as risk factors for pancreatic cancer. Br J Cancer. 1999;80(11):1830–1837. doi: 10.1038/sj.bjc.6690607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Larsson SC, Orsini N, Wolk A. Body mass index and pancreatic cancer risk: a meta-analyis of prospective studies. Int J Cancer. 2007;120(9):1993–1998. doi: 10.1002/ijc.22535. [DOI] [PubMed] [Google Scholar]
- 11.Hart AR, Kennedy H, Harvey I. Pancreatic cancer: a review of the evidence on causation. Clin Gastroenterol Hepatol. 2008;6(3):275–282. doi: 10.1016/j.cgh.2007.12.041. [DOI] [PubMed] [Google Scholar]
- 12.Iodice S, Gandini S, Maisonneuve P, et al. Tobacco and the risk of pancreatic cancer: a review and meta-analysis. Langenbecks Arch Surg. 2008;393(4):535–545. doi: 10.1007/s00423-007-0266-2. [DOI] [PubMed] [Google Scholar]
- 13.The alpha-tocopherol, beta-carotene lung cancer prevention study: design, methods, participant characteristics, and compliance. The ATBC Cancer Prevention Study Group. Ann Epidemiol. 1994;4(1):1–10. doi: 10.1016/1047-2797(94)90036-1. [DOI] [PubMed] [Google Scholar]
- 14.Huang HY, Alberg AJ, Norkus EP, et al. Prospective study of antioxidant micronutrients in the blood and the risk of developing prostate cancer. Am J Epidemiol. 2003;157(4):335–344. doi: 10.1093/aje/kwf210. [DOI] [PubMed] [Google Scholar]
- 15.Riboli E, Hunt KJ, Slimani N, et al. European Prospective Investigation into Cancer and Nutrition (EPIC): study populations and data collection. Public Health Nutr. 2002;5(6B):1113–1124. doi: 10.1079/PHN2002394. [DOI] [PubMed] [Google Scholar]
- 16.Gohagan JK, Prorok PC, Hayes RB, et al. The Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Trial of the National Cancer Institute: history, organization, and status. Control Clin Trials. 2000;21(suppl):251S–272S. doi: 10.1016/s0197-2456(00)00097-0. [DOI] [PubMed] [Google Scholar]
- 17.Thomas DB, Gao DL, Ray RM, et al. Randomized trial of breast self-examination in Shanghai: final results. J Natl Cancer Inst. 2002;94(19):1445–1457. doi: 10.1093/jnci/94.19.1445. [DOI] [PubMed] [Google Scholar]
- 18.Cai H, Zheng W, Xiang YB, et al. Dietary patterns and their correlates among middle-aged and elderly Chinese men: a report from the Shanghai Men's Health Study. Br J Nutr. 2007;98(5):1006–1013. doi: 10.1017/S0007114507750900. [DOI] [PubMed] [Google Scholar]
- 19.Design of the Women's Health Initiative clinical trial and observational study. The Women's Health Initiative Study Group. Control Clin Trials. 1998;19(1):61–109. doi: 10.1016/s0197-2456(97)00078-0. [DOI] [PubMed] [Google Scholar]
- 20.Buring JE, Hennekens CH. The Women's Health Study: rationale and background. J Myocardial Ischemia. 1992;10(4):30–40. [Google Scholar]
- 21.Stolzenberg-Solomon RZ, Albanes D, Nieto FJ, et al. Pancreatic cancer risk and nutrition-related methyl-group availability indicators in male smokers. J Natl Cancer Inst. 1999;91(6):535–541. doi: 10.1093/jnci/91.6.535. [DOI] [PubMed] [Google Scholar]
- 22.Calle EE, Rodriguez C, Jacobs EJ, et al. The American Cancer Society Cancer Prevention Study II Nutrition Cohort—rationale, study design and baseline characteristics. Cancer. 2002;94(9):2490–2501. doi: 10.1002/cncr.101970. [DOI] [PubMed] [Google Scholar]
- 23.Zhou W, Heist RS, Liu G, et al. Smoking cessation before diagnosis and survival in early stage non-small cell lung cancer patients. Lung Cancer. 2006;53(3):375–380. doi: 10.1016/j.lungcan.2006.05.017. [DOI] [PubMed] [Google Scholar]
- 24.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 25.Bruzzi P, Green SB, Byar DP, et al. Estimating the population attributable risk for multiple risk factors using case-control data. Am J Epidemiol. 1985;122(5):904–914. doi: 10.1093/oxfordjournals.aje.a114174. [DOI] [PubMed] [Google Scholar]
- 26.Lubin JH, Caporaso NE. Cigarette smoking and lung cancer: modeling total exposure and intensity. Cancer Epidemiol Biomarkers Prev. 2006;15(3):517–523. doi: 10.1158/1055-9965.EPI-05-0863. [DOI] [PubMed] [Google Scholar]
- 27.Lubin JH, Virtamo J, Weinstein SJ, et al. Cigarette smoking and cancer: intensity patterns in the Alpha-Tocopherol Beta-Carotene Cancer Prevention Study in Finnish men. Am J Epidemiol. 2008;167(8):970–975. doi: 10.1093/aje/kwm392. [DOI] [PubMed] [Google Scholar]
- 28.Preston DL, Lubin JH, Pierce DA, et al. Epicure User's Guide. Seattle, WA: HiroSoft International Corporation; 2006. [Google Scholar]
- 29.Tobacco Smoke and Involuntary Smoking. Lyon, France: International Agency for Research on Cancer; 2004. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans: Summary of Data Reported and Evaluation; vol 83. [Google Scholar]
- 30.Lubin JH, Caporaso N, Wichmann EH, et al. Cigarette smoking and lung cancer: modeling effect modification of total exposure and intensity. Epidemiology. 2007;18(5):639–648. doi: 10.1097/EDE.0b013e31812717fe. [DOI] [PubMed] [Google Scholar]
- 31.Lubin JH, Alavanja MC, Caporaso N. Cigarette smoking and cancer risk: modeling total exposure and intensity. Am J Epidemiol. 2007;166(4):479–489. doi: 10.1093/aje/kwm089. [DOI] [PubMed] [Google Scholar]
- 32.Schottenfeld D, Fraumeni JF. Cancer Epidemiology and Prevention. 3rd ed. New York, NY: Oxford University Press; 2006. [Google Scholar]