Abstract
Although research has demonstrated a relationship between proinflammatory cytokine activity and depressive symptoms, the neurocognitive processes underlying this relationship have remained largely unexplored. Here, we examined the effect of proinflammatory cytokine activation on the neural correlates of socially painful experience and associated depressed mood. Participants received either low-dose endotoxin or placebo through intravenous injection. Levels of the proinflammatory cytokine, IL-6, were repeatedly assessed through hourly blood draws; self-reported depressed mood was assessed hourly as well. Two hours post-injection, participants completed a neuroimaging session in which they were socially excluded during an online ball-tossing game. Replicating previous research, individuals exposed to endotoxin, compared to placebo, showed increases in IL-6 levels and depressed mood. Although there were no meaningful differences between the endotoxin and control groups in neural responses to social exclusion, there were sex differences in the relationships between IL-6 increases and neural responses to exclusion among subjects exposed to endotoxin. Among females, but not males, exposed to endotoxin, increases in IL-6 were associated with increases in social pain-related neural activity (dorsal anterior cingulate cortex, anterior insula) that mediated the relationship between IL-6 increases and depressed mood increases. Implications of these sex differences in the neural correlates of cytokine-associated depressed mood and social pain are discussed.
Introduction
Mounting evidence has demonstrated a relationship between immune system activity and depression (Capuron and Miller, 2004; Raison et al., 2006; Schiepers et al., 2005). Initial support for such a relationship came from observations that cancer patients often developed depression when undergoing treatments that activated proinflammatory cytokines (e.g., interferon-alpha; IFN-α; Bonaccorso et al., 2000; Raison et al., 2005). Subsequent correlational studies have shown that, in some cases, depressed individuals have elevated proinflammatory cytokine levels and that individuals with inflammatory conditions (cardiovascular disease, arthritis) have a high prevalence of depressive disorders (Frommberger et al., 1997; Kronfol, 2002; Levine et al., 1999; Musselman et al., 2001). Moreover, experimental studies have confirmed that cytokines play a causal role in producing depressive symptoms as acute administration of endotoxin (lipopolysaccharide) or typhoid vaccination, which increases proinflammatory cytokines, also leads to concurrent increases in depressed (Reichenberg et al., 2001) or negative mood (Wright et al., 2005) in otherwise healthy male subjects.
Proinflammatory cytokines are hypothesized to contribute to depressed mood, in part, by signaling the central nervous system to initiate “sickness behavior,” a coordinated motivational response that is thought to facilitate recuperation and recovery from illness and disease. Sickness behavior includes symptoms such as fatigue, anhedonia, reduced social activity, and increased pain sensitivity (Dantzer, 2001; Hart, 1988; Kent et al., 1992). Notably, many sickness symptoms share overlapping features with depressive symptoms. Although proinflammatory cytokines are too large to cross the blood-brain barrier and signal the brain directly, cytokines are thought to communicate with the brain through leaky regions in the blood-brain barrier or through the transmission of cytokine signals through the vagus nerve (Dantzer et al., 2008; Maier and Watkins, 1998).
Few studies in humans, however, have investigated the type of neural activity that is altered by proinflammatory cytokines or whether increases in proinflammatory cytokines influence the social or affective neural processes that may make depressed mood more likely. Rather, the few experimental studies of cytokine-induced changes in neural activity have focused primarily on cognitive, as opposed to social or affective changes (Brydon et al., 2008; Capuron et al., 2005). However, socioemotional processes may play a significant role in cytokine-associated depressed mood as well. Psychologists have long noted that individuals with depressive disorders often feel “abandoned, unwanted, and unlovable” (Bowlby, 1980). Moreover, interpersonal hypersensitivity is a strong predictor of depression in both healthy populations as well as those with inflammatory disorders (Boyce et al., 1991; Zautra et al., 1999). Thus, in the current study, we examined whether inflammation alters the neural substrates underlying a specific type of socioemotional sensitivity that may contribute to depressive symptoms. Specifically, based on research showing an overlap in the neural structures underlying physical pain and ‘social pain,’ the pain associated with social loss or rejection (Eisenberger & Lieberman, 2004), we hypothesized that the inflammatory response, which heightens physical pain sensitivity (presumably to prevent further damage to the body in the case of injury or disease) may inadvertently recruit neural systems involved in social pain, increasing social pain sensitivity and potentially increasing vulnerability to depressed mood.
Previous research has shown that the dACC, a neural region associated with the distress of physical pain (Peyron et al., 2000; Ploghaus et al., 1999; Rainville et al., 1997) and the insula, a neural region associated with processing visceral pain and negative affect (Aziz et al., 2000; Cechetto and Saper, 1987; Lane et al., 1997; Phan et al., 2004; Phillips et al., 1997) were also activated in response to being socially excluded (Eisenberger et al., 2003). Moreover, the magnitude of dACC activity has been shown to correlate with self-reported social distress in response to social exclusion (Eisenberger et al., 2003; Eisenberger et al., 2007). Thus, social pain sensitivity and the underlying neural reactivity in the dACC and insula may be an additional consequence of heightened inflammatory responses, which together may contribute to depressed mood.
To examine the effect of proinflammatory cytokine activation on depressed mood as well as the related neural changes, healthy participants were randomly assigned to receive either endotoxin (Escherichia coli), known to increase proinflammatory cytokine levels in a safe manner (Suffredini et al., 1999), or placebo. We then examined the effect of endotoxin vs. placebo on circulating levels of one type of proinflammatory cytokine, interleukin-6 (IL-6)1, and depressed mood throughout the course of a day, hypothesizing that we would replicate previous work (Reichenberg et al., 2001) showing that endotoxin leads to increases in IL-6 levels and depressed mood. However, to further explore the neural mechanisms that link cytokine activation with depressed mood, we also examined the effect of endotoxin vs. placebo on the neural correlates underlying social pain sensitivity and depressed mood.
To do this, we examined neural responses to an episode of social exclusion (at the time of maximal cytokine response) to explore the effect of proinflammatory cytokine activation on the neural systems that underlie social pain sensitivity. We also examined whether this cytokine-associated neural activity was related to the corresponding increases in depressed mood. We hypothesized that proinflammatory cytokine activity would relate to greater activity in social pain-related neural circuitry, such as the dACC and insula, and that activity in these regions would relate to cytokine-associated depressed mood.
In addition, because of the well-known sex differences in the prevalence of depression and inflammatory disorders, with females being twice as likely as males to develop depression (Nolen-Hoeksema, 2001) and, depending on the immune condition, between 2-9 times as likely as males to develop autoimmune disorders (Whitacre et al., 1999), we also examined the relationship between proinflammatory cytokine activity and neural responses to social exclusion in each sex separately. Although cytokine-associated depressed mood has been observed in both male and female patients undergoing medical treatments that increase proinflammatory cytokine activity, studies are split between those that find sex to be a risk factor for developing depression (with females being at greater risk) and those that do not (Raison et al., 2005). In addition, careful investigation of sex differences in experimentally-induced changes in inflammation and depressed mood is lacking; all of the experimental studies of cytokine-associated changes in depressed and/or negative mood have focused exclusively on males (Krabbe et al., 2005; Reichenberg et al., 2001; Wright et al., 2005). Because our sample included equal numbers of males and females, the potential role of sex differences in cytokine-induced depressed mood and the neural correlates that underlie these effects was examined.
Methods
Participants
Thirty-nine participants completed the study (20 female; mean age, 21.8 ± 3.4 years; range, 18-36 years; mean body weight, 72.6 ± 13.0 kg; range, 47.0-98.2 kg), and 36 of those participants completed the neuroimaging session (20 received endotoxin, 16 received placebo; there were equal numbers of males and females in each condition). The three participants who were not scanned were pilot participants who received endotoxin but did not complete the neuroimaging session in order to ensure that participants were well enough to leave the UCLA General Clinical Research Center (GCRC) to complete the neuroimaging session with no nurse supervision.
Participants were recruited from flyers that were posted at the UCLA Medical Center and from advertisements posted in the campus newspaper. Prospective participants with the following conditions were excluded from participation through a structured telephone interview: claustrophobia or metal in their body (relevant for the neuroimaging component of the study), chronic mental or physical illness, serious physical or mental health problems, history of allergies, autoimmune, liver, or other severe chronic diseases, current use of prescription medications, nightshift work or time zone shifts (> 3hrs) within the previous 6 weeks. Participants also had to be right-handed.
After the telephone interview, if still eligible, participants completed an additional face-to-face screening interview to ensure eligibility for the study. Participants received $20 for completing this screening session. During this session, a trained interviewer reviewed the study procedures with the participant, including information on the consequences of endotoxin and the kinds of symptoms that could be expected. After this, participants were asked a series of questions about their physical health and use of medications or drugs. Participants then completed the Structured Clinical Interview for DSM Disorders (SCID; First et al., 1996) to ensure that they were free of mental health problems. Finally, height and weight were measured, vital signs were assessed (e.g., temperature, heart rate, blood pressure), a urine sample was collected to examine drug use (marijuana, opiates, cocaine, amphetamines, methamphetamines), and blood was drawn to screen for pregnancy, if female. A screening laboratory examination was performed including a complete blood cell count, chemistry panel, and liver function tests. Furthermore, any participant who: 1) had a BMI greater than 30, 2) reported physical health problems or medication use, 3) evidenced an Axis I psychiatric disorder based on the SCID assessment, 4) showed evidence of drug use from a positive urine test, 5) had a positive pregnancy test, if female, or 6) showed any abnormalities on their screening laboratory tests were ineligible for the study. The final sample was 39% European-American, 18% Asian, 18% Hispanic, 7% African-American, and 18% “other”. Experimental procedures were approved by the UCLA Human Subjects Protection Committee.
Procedure
The study was conducted at the UCLA GCRC using a randomized, double-blind, placebo-controlled design. Participants arrived at the UCLA GCRC at either 7:30 (n = 31) or 8:30 AM (n = 8) and were assigned to a bed in a single or double occupancy room. Upon arrival, a urine sample was collected and tested for drug use and pregnancy in order to ensure continued eligibility for study participation. No participants tested positive for either. Once this was completed, participants began the study procedure.
A GCRC nurse, who was blind to condition, obtained baseline vital signs (blood pressure, pulse, temperature) as well as height and weight. The nurse then inserted a catheter with a heparin lock into the dominant forearm (right) for hourly blood draws and one into the non-dominant forearm (left) for a continuous saline flush and for drug administration. Once the catheter was in place, nurses began a saline drip to keep participants well hydrated (1000cc of normal saline at 150cc/hr); saline infusion continued throughout the study except for when participants left to complete the neuroimaging session. Participants began the study by completing some demographic questionnaires and eating a light breakfast prior to drug or placebo administration.
Ninety minutes after arrival at the GCRC, each participant was randomly assigned to receive either endotoxin (0.8 ng/kg of body weight) or placebo (same volume of 0.9% saline), which was administered by the nurse as an intravenous bolus. The endotoxin used in this study was derived from Escherichia coli (E. coli group O:113) and provided by the National Institutes of Health Clinical Center as a reference endotoxin for studies of experimental inflammation in humans (Suffredini et al., 1999). Previous research has demonstrated the safe use of this reference endotoxin across many different samples (Andreasen et al., 2008; Suffredini and O'Grady, 1999). No significant differences in age, years of education, or body weight were found between the two groups.
Throughout the study, vital signs (blood pressure, pulse, and temperature) were assessed every half hour (except during the neuroimaging session) and blood draws were collected at baseline (two baseline assessments: 8:30 and 8:45 AM or 9:30 and 9:45 AM, depending on the start time) and then approximately every hour after that for the next six hours. These blood draws were later assayed for levels of IL-6 and cortisol, which have been previously shown to increase as a function of endotoxin (Reichenberg et al., 2001). In addition, the baseline blood draw was later assayed for levels of estradiol and progesterone to control for the phase of the menstrual cycle in females. Participants also completed self-report measures of physical symptoms (e.g., muscle pain, fatigue) and depressed mood with every blood draw.
Approximately two hours post-injection, when proinflammatory cytokines have been shown to peak in previous studies (Krabbe et al., 2005; Reichenberg et al., 2001; Suffredini et al., 1999; Wright et al., 2005), participants completed a neuriomaging session. Participants were escorted to the UCLA Brain Mapping Center where they completed the Cyberball social exclusion task in the scanner (see fMRI paradigm for details). Upon completion of the neuroimaging session, participants returned to the GCRC, had lunch, and completed the rest of the study procedures. For safety reasons, the study physician (M.I.) was aware of each participant's group assignment, but did not take part in the testing procedures. The study physician was on call during each of the experimental sessions. Participants were discharged from the GCRC following the last blood draw upon approval from the study's physician; approval was granted if self-reported physical and psychological symptoms returned to baseline levels. Thus, all participants left the study feeling as good as they did when they started. At the end of the study, participants were thanked, debriefed, and paid for their participation ($200).
Behavioral Assessments
Sickness symptoms
Physical sickness symptoms (muscle pain, shivering, nausea, breathing difficulties, fatigue) were assessed at baseline and then hourly following the endotoxin or placebo adminstration for six hours. Participants rated the extent to which they felt the symptoms listed on a scale from 0 (no symptoms) to 4 (very severe symptoms).
Depressed mood
Depressed mood was assessed at the same time as the sickness symptoms, using an abbreviated version of the Profile of Mood States (McNair et al., 1971). To assess depressed mood, participants rated the extent to which they felt: “unhappy,” “blue,” “lonely,” “gloomy,” and “worthless” on a scale from 0 (not at all) to 4 (extremely). Depressed mood was calculated by averaging scores from each of these items at each timepoint.
fMRI Paradigm
To assess neurocognitive reactivity to social exclusion, participants were scanned while completing the Cyberball social exclusion task, in a manner similar to previous work (Eisenberger et al., 2003, 2007; Williams et al., 2000). While at the GCRC (following endotoxin/placebo administration), participants were told that they would later be escorted to the UCLA Brain Mapping center where they would complete a neuroimaging session. It was explained that during this session, they would be playing a virtual ball-tossing game with two other individuals who were also in fMRI scanners. In reality, however, there were no other players; participants played with a preset computer program. To bolster the cover story that participants were playing with two others, participants (while still at the GCRC) were asked to complete a short questionnaire in which they answered questions about themselves (e.g., “What do you want to do professionally in the future?” “What are your hobbies or other interests?”) and were told that the two other players would see their answers and that they would see the answers of the two other players prior to the scanning session. Upon arriving at the UCLA Brain Mapping Center, each subject read the two other supposed subjects' answers to these questions; in reality, these questionnaires were scripted ahead of time and each subject read the same ones.
After reading the questionnaires from the other supposed subjects, participants were positioned in the scanner and played the Cyberball game. Each game began with a still picture of the two virtual players in the upper corners of the screen and a hand, representing the participant, in the lower-center portion of the screen. After 9s, the cartoon player in the upper left-hand corner started the game by throwing the ball to either the other cartoon player or the participant. The participant could return the ball to one of the players by pressing one of two keys on a button box that was held in his/her right hand. The Cyberball program was set for 60 throws per game, with the computer players waiting 0.5-3.0 seconds (determined randomly) before making a throw to heighten the sense that the participant was actually playing with other individuals.
During the task, participants completed two scans. In the first scan (inclusion), participants played with the two other players for the entire scanning period, with each virtual player throwing the ball to the participant on approximately 50% of the throws. In the second scan (exclusion), participants only received the ball for a total of ten throws and were then excluded for the rest of the scan when the two players stopped throwing the ball to the participant (50-70 seconds). Although it would have been ideal to counterbalance the order of the inclusion and exclusion scans across participants, this would have likely changed the meaning of the psychological experience of rejection for the subjects. Thus to ensure a meaningful experience of exclusion, all subjects completed the inclusion scan first.
fMRI Data Acquisition and Data Analysis
Data were acquired on a Siemens Allegra 3T head-only scanner. Head movements were restrained with foam padding and surgical tape placed across each participant's forehead. For each participant, a high-resolution structural T2-weighted echo-planar imaging volume (spin-echo; TR=5000ms; TE=33ms; matrix size 128×128; 36 axial slices; FOV=20-cm; 3-mm thick, skip 1-mm) was acquired coplanar with the functional scans. Two functional scans were acquired (echo planar T2*-weighted gradient-echo, TR=2000ms, TE=25ms, flip angle=90°, matrix size 64×64, 36 axial slices, FOV=20-cm; 3-mm thick, skip 1-mm), each lasting 2 minutes and 48 seconds.
The imaging data were analyzed using SPM'5 (Wellcome Department of Cognitive Neurology, Institute of Neurology, London, UK). Images for each subject were realigned to correct for head motion, normalized into a standard stereotactic space, and smoothed with an 8mm Gaussian kernel, full width at half maximum, to increase signal-to-noise ratio. The design was modeled using a boxcar function convolved with a canonical hemodynamic response function. For each participant, periods of inclusion and exclusion were modeled as epochs based on the length of that participant's inclusion and exclusion episodes (these varied slightly between participants due to the random delay assigned to the virtual players when throwing the ball as well as the subject's own reaction time to return the throw). After the task was modeled for each participant, planned comparisons were computed as linear contrasts to investigate neural activity during the exclusion compared to the inclusion episode. Random effects analyses of the group were computed using the contrast images generated for each participant.
Plasma Levels of IL-6, Cortisol, Estradiol, and Progesterone
Plasma blood samples were collected in pre-chilled tubes containing sodium ethylenediaminetetraacetic acid and aprotinin and were immediately centrifuged, aliquoted, and placed in a -70°F freezer. Plasma levels of IL-6 were quantified by means of a high sensitivity enzyme-linked immunosorbent assay method (R & D Systems, Minneapolis, MN). All samples were run in duplicate and assayed at the same time, in a single run with a single lot number of reagents and consumables employed by a single operator, with intra-assay coefficients of variation for all variables less than 5%. Plasma levels of cortisol were measured by the DPC IMMULITE 1000 Immunoassay Chemistry Analyzer (Diagnostic Products Corporation CA, USA). IMMULITE 1000 Cortisol assay is a solid-phase competitive chemiluminescent enzyme immunoassay, which was run according to the manufacturer's instructions. The analytical sensitivity of the assay is 0.2 μg/dL (5.5 nmol/L). Hormonal evaluations of estradiol and progesterone were performed on plasma samples taken at baseline. Coated-Tube Radioimmunoassay kits were utilized. The specific kits used were DSL-4300 ACTIVE® Estradiol Coated-Tube Radioimmunoassay Kit for estradiol and DSL-5000 ACTIVE® 17 alphaOH Progesterone Coated-Tube Radioimmunoassay Kit for 17 alphaOH progesterone.
Statistical Analyses
Behavioral outcomes
To assess between-group differences in the effect of endotoxin vs. placebo on IL-6 levels, cortisol levels, physical sickness symptoms (e.g., fatigue), vital signs (e.g., changes in heart rate, blood pressure, temperature), and self-reported depressed mood, we used a standard statistical software program (SPSS) to conduct repeated-measure analyses of variance. Because IL-6 levels were not normally distributed at any of the time points, each value was log-transformed.
To assess the correlation between increases in IL-6 and increases in the relevant dependent variables (from baseline to two hours post-injection, which was immediately prior to the neuroimaging session) for those in the endotoxin condition, Pearson correlation coefficients were calculated. Again, because IL-6 levels were not normally distributed, we calculated a difference score by subtracting the log-transformed baseline values from the log-transformed values at two hours post-injection.
Neuroimaging outcomes
To assess between-group differences in the effect of endotoxin vs. placebo on neural reactivity to the Cyberball game, a one-way ANOVA was computed in SPM'5 contrasting neural activity during exclusion relative to inclusion for those in the endotoxin group compared to those in the control group (p<.001, 10-voxel extent threshold; Forman et al 1995). To explore the relationship between IL-6 increases and neural responses in the endotoxin group, we conducted whole-brain regression analyses to see which neural regions correlated with IL-6 increases. Measures of IL-6 increases (from baseline to two-hours post-injection) were entered as regressors into a random-effects, whole-brain group analysis (for those in the endotoxin condition only), comparing activations during exclusion relative to inclusion (p<.001, 10-voxel extent threshold). Because of our interest in understanding the neural circuitry that plays a role in cytokine-associated depressed mood, neural regions that correlated significantly with IL-6 increases were further examined to see if they also correlated with changes in depressed mood. In addition, because of well-known sex differences in the prevalence of depression and inflammatory disorders (Nolen-Hoeksema, 2001; Whitacre et al., 1999), these analyses were also conducted separately for males and females. All coordinates are reported in Montreal Neurological Institute (MNI) format.
Results
Physiological and Behavioral Responses to Endotoxin
Endotoxin, compared to placebo, led to significant increases in IL-6 levels (peaking at 2-3 hours post-injection), across all time points except for the final one, as evidenced by significant time X condition interactions at each of these time points compared to baseline (T1 (one hour post-injection) through T5 (five hours post-injection): F(1,37) = 23.19, 144.14, 177.11, 63.67, 11.36, all p's <.005; Figure 1a). Endotoxin, compared to placebo, also led to a significant increase in cortisol levels at all of the measured time points except for the first one following baseline (peaking at 3 hours post-injection; time X condition interactions at T2 - T6: F(1,37) = 21.83, 52.78, 20.59, 10.29, 7.96, all p's < .01). In addition, endotoxin, compared to placebo, led to a significant increase in self-reported physical sickness symptoms at all of the measured time points (peaking at 2 hours post-injection; time X condition interactions at T1 –T6: F(1,37) = 9.08, 32.37, 13.60, 8.54, 11.65, 8.01, all p's < .01) as well as significant increases in body temperature (time X condition interactions: T3 - T6: F(1,37) = 14.18, 17.14, 17.93, 5.70, all p's < .05) and pulse (time X condition interactions: T3 - T6: F(1,37) = 48.43, 24.01, 23.74, 14.59, all p's < .001), starting at 3 hours post-injection. Finally, we found a significant increase in self-reported depressed mood in the endotoxin group, compared to the placebo group, at two hours post-injection (time x condition interaction: F(1,35) = 8.13, p < .01; Figure 1b). There were no sex differences in any of these effects. In addition, these effects did not seem to be due to the phase of the menstrual cycle as none of these effects changed after controlling for baseline levels of estradiol and progesterone, which are markers of the phase of the menstrual cycle (either in the full sample or when examining females alone).
Figure 1.
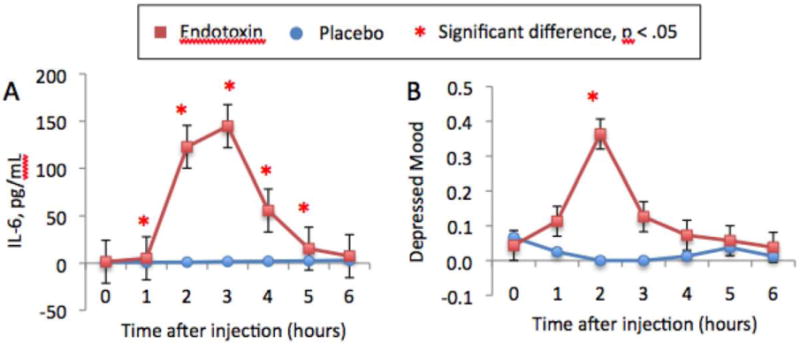
Changes over time in: A) plasma levels of IL-6 (these are raw values that have not been log-transformed) and B) self-reported depressed mood. Time points with asterisks indicate significant time X condition interactions. These figures are published separately and have been adapted from Eisenberger et al., (2009).
Correlations between changes in IL-6 and changes in depressed mood
To examine whether increases in IL-6 correlated with increases in self-reported depressed mood, we examined the correlation between IL-6 increases from baseline to two hours post-injection (right before the neuroimaging session) and self-reported changes in depressed mood across the same time period (both measures were computed as difference scores). For subjects exposed to endotoxin, there was no significant correlation between IL-6 increases and increases in depressed mood. However, when the sample was stratified by sex, increases in IL-6 levels were significantly correlated with increases in self-reported depressed mood in females (r = .70, p < .05) but not in males (r = -.20; p = .59); these correlations were significantly different from each other (Z = 2.12, p < .05). Furthermore, for females, when increases in self-reported physical symptoms (muscle pain, shivering, nausea, breathing difficulties, fatigue) were controlled, the relationship between increases in IL-6 and increases in depressed mood did not change (r = .59, p = .05). Similarly, controlling for increases in temperature or pulse did not significantly change this relationship either (respectively, r = .71, p < .05; r = .73, p < .05). The correlation between IL-6 increases and depressed mood also remained after controlling for baseline levels of estradiol and progesterone (r = .74, p < .001).
Neural Responses to Social Exclusion
Between-group differences in neural activity to social exclusion
To examine the effect of proinflammatory cytokine activation on neural responses to social exclusion, we first examined neural regions that differed in response to social exclusion (compared to inclusion) between those in the endotoxin as compared to those in the placebo condition. Overall, there were no significant differences in social pain-related neural circuitry during exclusion relative to inclusion between the endotoxin and placebo groups. The only between-groups difference in neural activity to exclusion vs. inclusion was found in a region of the occipital cortex (placebo > endotoxin: 52,-78,6, t = 3.70, k = 117; p < .001). Similar effects were observed when examining each sex separately; neither males nor females showed any neural activity that was greater in the endotoxin, compared to the placebo, condition. Females in the placebo group, compared to the endotoxin group showed greater activity in a few neural regions not typically involved in social pain processes (left ventrolateral prefrontal cortex: -34, 32, -8, t = 5.24, k = 14, p < .001; occipital cortex: 20, -72, 30, t = 4.81, k = 16, p < .001 and 48,-78,0, t = 4.31, k = 21, p < .001).
This lack of between-group differences was not surprising given the large amount of variability evidenced in IL-6 responses to endotoxin (see Figure 1a; range: 17.52 – 356.03 pg/ml), a finding that has been demonstrated previously (Stephens et al., 2005). In order to take advantage of this variability, we next examined how individual differences in IL-6 responses to endotoxin (among subjects in the endotoxin group) related to neural responses to social exclusion compared to social inclusion. Furthermore, given the well-known sex differences in the prevalence of depression and immune conditions as well as the observed sex differences in the relationships between IL-6 and depressed mood increases in the current study, correlations between IL-6 increases and neural activity were also examined in each sex separately.
Correlations between IL-6 increases and neural activity in the endotoxin group
We first examined correlations between IL-6 increases and neural activity for all subjects in the endotoxin group during social exclusion compared to social inclusion. With regard to pain-related neural circuitry, we found that greater increases in IL-6 (from baseline to immediately prior to the scanning session) were associated with greater bilateral anterior insula activity (Figure 2a) as well as greater bilateral posterior insula activity (see Table 1). Moreover, activation in the left posterior insula correlated significantly with self-reported increases in depressed mood (r = .51, p < .05), and activation in the right anterior insula correlated marginally significantly with self-reported increases in depressed mood (r = .40, p < .10).2
Figure 2.
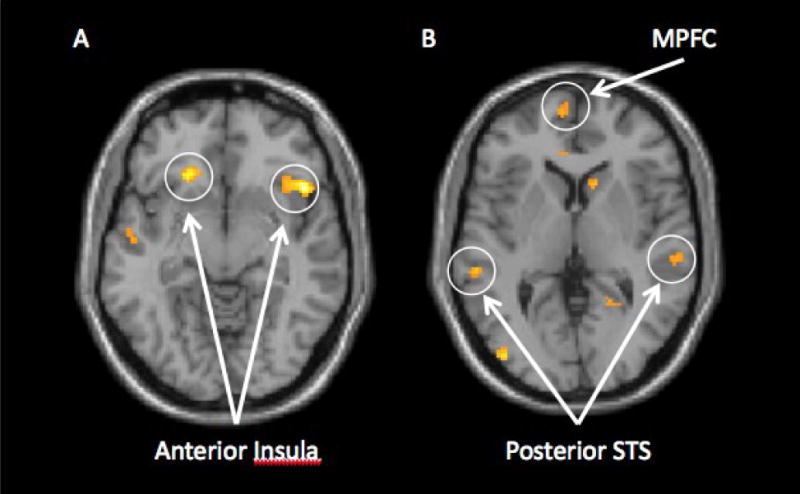
Neural activity that correlated positively with increases in IL-6 levels (from baseline to immediately prior to the neuroimaging session) during exclusion vs. inclusion for the endotoxin subjects: A) Activity in the bilateral insula and B) Activity in the medial prefrontal cortex (MPFC) and posterior superior temporal sulcus (pSTS).
Table 1.
Neural activations during social exclusion vs. inclusion that correlated positively with increases in IL-6 levels from baseline to two hours post-injection for all participants in the endotoxin group, (p < .001, 10-voxel extent threshold). There were no regions that correlated negatively with IL-6 increases. R(ΔDep) refers to the correlation between the parameter estimates from the listed neural activations and self-reported increases in depressed mood. * p< .05; S p < .10
| Region | BA | MNI Coordinate | T | k | R (ΔDep) |
|---|---|---|---|---|---|
| Paralimbic | |||||
| Left Anterior Insula | -20 28 -4 | 7.83 | 158 | .09 | |
| Right Anterior Insula | 48 20 -8 | 7.69 | 306 | .40A | |
| Left Posterior Insula | -46 -18 12 | 4.55 | 81 | .51* | |
| Right Posterior Insula | 46 -14 12 | 4.36 | 31 | .26 | |
| Perigenual Anterior Cingulate | 32 | -8 40 8 | 4.41 | 21 | .33 |
| Posterior Cingulate Cortex | 31 | -16 -22 36 | 4.89 | 14 | .20 |
| Posterior Cingulate Cortex | 31 | 14 -40 14 | 4.14 | 12 | .08 |
| Caudate | 12 16 2 | 4.77 | 58 | .41A | |
| Frontal Lobe | |||||
| Dorsomedial Prefrontal Cortex (PFC) | 9 | -8 60 40 | 6.00 | 52 | .10 |
| Dorsomedial PFC | 9 | -10 46 54 | 5.42 | 23 | .36 |
| Dorsomedial PFC | 9 | 18 56 42 | 5.33 | 33 | .09 |
| Dorsomedial PFC | 8 | 18 24 62 | 5.22 | 23 | .07 |
| Dorsomedial PFC | 8 | -2 28 54 | 4.11 | 17 | .41A |
| Left Dorsolateral PFC | 9 | -34 46 38 | 5.01 | 16 | .17 |
| Right Rostrolateral PFC | 10 | 30 68 12 | 4.15 | 24 | .43A |
| Medial PFC | 10 | -6 62 4 | 4.02 | 18 | .42A |
| Right Dorsal Premotor Cortex | 6 | 60 6 40 | 5.08 | 30 | .25 |
| Supplementary Motor Area | 4/6 | 0 -26 68 | 5.58 | 73 | .07 |
| Supplementary Motor Area | 6 | -6 8 74 | 4.62 | 12 | .49* |
| Supplementary Motor Area | 6 | 12 -20 72 | 4.39 | 31 | .20 |
| Left Premotor Cortex | 4 | -20 -22 54 | 4.29 | 13 | .10 |
| Parietal Lobe | |||||
| Left Somatosensory Cortex | 1/2/3 | -24 -28 64 | 4.32 | 17 | .26 |
| Precuneus | 7 | 16 -42 66 | 4.55 | 50 | .14 |
| Temporal Lobe | |||||
| Right Inferior Temporal Gyrus | 20 | 46 -12 -30 | 4.95 | 47 | .51* |
| Temporal Pole | 38 | -46 10 -40 | 4.65 | 22 | .41A |
| Left Middle Temporal Gyrus | 21 | -54 -8 -12 | 4.40 | 58 | .49* |
| Right Middle Temporal Gyrus | 21 | 62 -10 -12 | 4.19 | 15 | .34 |
| Right Middle Temporal Gyrus | 21 | 58 -2 -16 | 4.06 | 20 | .53* |
| Posterior Superior Temporal Sulcus | 22 | 62 -26 0 | 4.58 | 72 | .18 |
| Posterior Superior Temporal Sulcus | 22 | -58 -32 4 | 4.48 | 21 | .17 |
| Posterior Superior Temporal Sulcus | 22 | -52 -38 10 | 4.16 | 13 | .57* |
| Right Fusiform Gyrus | 37 | 46 -42 -12 | 4.34 | 19 | .35 |
| Occipital Lobe | |||||
| Occipital Cortex | 18 | 0 -74 14 | 4.95 | 48 | .39A |
| Occipital Cortex | 17 | -10 -66 14 | 4.80 | 18 | .38 |
| Occipital Cortex | 19 | 28 -54 2 | 3.88 | 15 | .40A |
| Cerebellum | |||||
| Cerebellum | 20 -60 -38 | 6.03 | 69 | .23 | |
| Cerebellum | -40 -68 -34 | 5.22 | 35 | .15 | |
| Cerebellum | -18 -46 -12 | 5.10 | 57 | .42A | |
| Cerebellum | -24 -56 -42 | 4.88 | 17 | .35 | |
| Cerebellum | -10 -60 -12 | 4.71 | 19 | .48* | |
| Cerebellum | 8 -40 -30 | 4.67 | 42 | .32 | |
| Cerebellum | -4 -84 -38 | 3.91 | 10 | .16 |
Interestingly, increases in IL-6 levels were also correlated with greater activity in several neural regions that have been implicated in ‘mentalizing’—the process of thinking about the contents of other people's minds (Frith and Frith, 1999, 2003, 2006). Individuals who showed greater increases in IL-6 levels from baseline to immediately prior to scanning showed greater activity (in response to social exclusion vs. inclusion) in the medial and dorsomedial prefrontal cortex (MPFC; DMPFC), regions that have been associated with understanding the mental states of others (Frith and Frith, 1999, 2003, 2006; Mitchell et al., 2005), as well as regions of the posterior superior temporal sulcus (pSTS), the temporal pole, posterior cingulate cortex, and precuneus—all of which have also been shown to be involved in understanding the minds or behaviors of others (Figure 2b; Frith and Frith, 1999, 2003, 2006). In addition, several of these regions also correlated positively with increases in self-reported depressed mood (MPFC: r = .42, p < .10; DMPFC: r = .41, p < .10; temporal pole: r = .41, p < .10; pSTS: r = .57, p < .05; see Table 1).
Correlations between IL-6 increases and neural activity for females and for males in the endotoxin group
To further investigate the relationship between increases in IL-6 levels and neural responses to social exclusion among males and females separately, we conducted separate whole-brain regression analyses for males and females. Thus, we examined how increases in IL-6 levels correlated with neural activity to social exclusion vs. inclusion in the female endotoxin subjects and separately in the male endotoxin subjects.
When examining correlations between IL-6 increases and neural activity for females in the endotoxin group, we found only four significant activations in the entire brain, three of which were in social pain-related neural circuitry (Table 2a). Specifically, IL-6 increases were associated with greater activity in the dACC (Figure 3a) as well as the left and right anterior insula (Figure 3b) in response to social exclusion compared to inclusion. Moreover, dACC and right anterior insula clusters identified in this analysis also correlated significantly with increases in self-reported depressed mood from baseline to two hours post-injection (dACC: r = .72, p < .05; Figure 3c; right anterior insula: (r = .69, p < .05; Figure 3d). In addition, left anterior insula activity correlated marginally significantly with increases in self-reported depressed mood (r = .58, p = .08). There was only one other neural region, the somatosensory cortex, that correlated negatively with IL-6 increases (see Table 2a). None of these results changed after controlling for baseline levels of estradiol or progesterone.
Table 2.
Neural activations during social exclusion vs. inclusion that correlated with increases in IL-6 levels from baseline to two hours post-injection for: A) all females in the endotoxin group and B) all males in the endotoxin group (p < .001, 10-voxel extent threshold). R(ΔDep) refers to the correlation between the parameter estimates from the listed neural activations and self-reported increases in depressed mood. * p < .05; S p < .10.
| Region | BA | MNI Coordinate | T | k | R (ΔDep) | |
|---|---|---|---|---|---|---|
| A) Female endotoxin participants | ||||||
| Positive correlations with IL-6 increases | ||||||
| Paralimbic | ||||||
| Left Anterior Insula | -22 32 4 | 6.06 | 17 | .58A | ||
| Right Anterior Insula | 42 22 -2 | 5.70 | 19 | .69* | ||
| Dorsal Anterior Cingulate Cortex (ACC) | 32 | -8 22 46 | 5.82 | 17 | .72* | |
| Temporal Lobe | ||||||
| Right fusiform gyrus | 37 | 44 -40 -12 | 8.57 | 30 | .55 | |
| Negative correlation with IL-6 increases | ||||||
| Right Somatosensory cortex | 1/2/3 | 64 -16 44 | 6.07 | 11 | -.41 | |
| B) Male endotoxin participants | ||||||
| Positive correlations with IL-6 increases | ||||||
| Paralimbic | ||||||
| Right Anterior Insula | 36 18 -12 | 7.75 | 60 | .03 | ||
| Left Anterior Insula | -20 28 -6 | 7.64 | 21 | -.21 | ||
| Right Posterior Insula | 46 -16 12 | 12.02 | 62 | -.25 | ||
| Left Posterior Insula | -36 -12 12 | 6.39 | 34 | -.12 | ||
| Dorsal/Rostral ACC | 32 | 12 34 24 | 6.79 | 23 | -.09 | |
| Frontal Lobe | ||||||
| Left Ventrolateral Prefrontal Cortex | 44 | -60 4 34 | 9.87 | 12 | -.09 | |
| Right Ventrolateral Prefrontal Cortex | 47 | 46 20 -12 | 7.20 | 14 | .00 | |
| Dorsomedial Prefrontal Cortex | 8 | 18 56 40 | 6.26 | 19 | -.24 | |
| Right Dorsal Premotor Cortex | 6 | 54 4 42 | 7.09 | 19 | -.09 | |
| Supplementary Motor Area | 6 | 10 -18 72 | 5.14 | 10 | -.07 | |
| Parietal Lobe | ||||||
| Left Somatosensory Cortex | 1/2/3 | -40 -16 32 | 8.39 | 27 | -.02 | |
| Temporal Lobe | ||||||
| Posterior Superior Temporal Sulcus | 22 | -58 -42 18 | 11.85 | 46 | -.31 | |
| Left Middle Temporal Gyrus | 21 | -56 -4 -22 | 7.84 | 38 | -.08 | |
| Right Inferior Temporal Gyrus | 20 | 56 -20 -24 | 5.97 | 19 | -.15 | |
| Occipital Lobe | ||||||
| Occipital Cortex | 18 | -40 -80 0 | 8.51 | 50 | -.35 | |
| Cerebellum | ||||||
| Cerebellum | -10 -48 -20 | 10.91 | 100 | -.23 | ||
| Cerebellum | 14 -42 -22 | 8.16 | 128 | -.03 | ||
| Cerebellum | 22 -72 -44 | 6.15 | 11 | .18 | ||
| Cerebellum | -8 -54 -38 | 6.07 | 20 | .02 | ||
| Cerebellum | 22 -58 -40 | 5.94 | 61 | -.22 | ||
Figure 3.
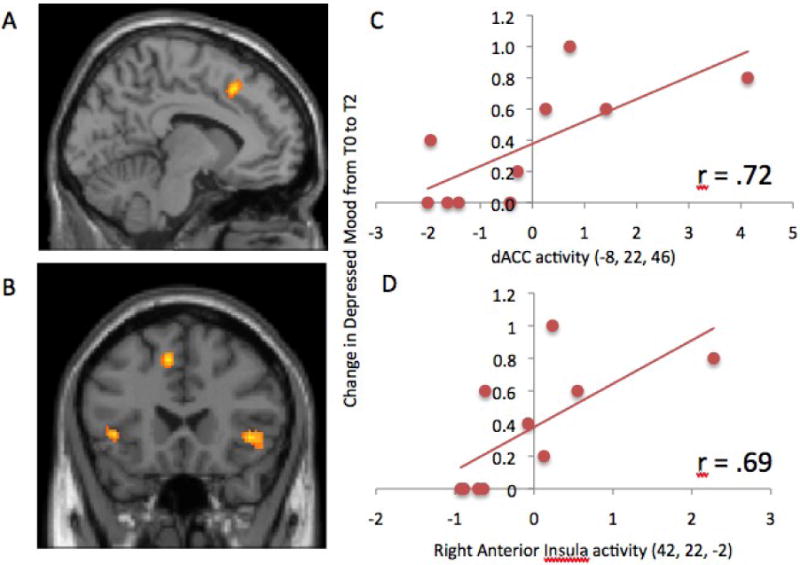
Activity in the A) Dorsal anterior cingulate cortex (dACC; -8, 22, 46) and B) bilateral anterior insula (left: -22, 32, 4; right: 42, 22, -2) that correlated with increases in IL-6 levels from baseline to two-hours post-injection among females in the endotoxin group. Scatterplots showing the relationship between C) dACC activity and D) right anterior insula activity with increases in depressed mood from baseline to two-hours post-injection among females in the endotoxin group.
Because there were significant correlations between IL-6 increases and self-reported depressed mood increases as well as between activity in the dACC and right anterior insula with both of these variables, we conducted statistical mediation analyses to see if activity in these regions mediated the relationship between increases in IL-6 and increases in self-reported depressed mood in females. Both the dACC and right anterior insula were found to significantly or marginally significantly mediate the relationship between IL-6 increases and depressed mood (dACC: Sobel test = 1.71, p < .05, one-tailed; right anterior insula: Sobel test = 1.34, p = .09, one-tailed; MacKinnon et al., 2002); suggesting that, for females, IL-6 may relate to depressed mood, in part, through social pain-related neural activity.
When examining correlations between IL-6 increases and neural activity for males in the endotoxin group, we again found that IL-6 increases correlated with several different social pain processing regions, including anterior and posterior insula as well as a region of the ACC (although this activation was more rostral than the one observed in females; see Table 2b). However, none of these activations correlated significantly with increases in depressed mood (all p's > .16). In addition, for males, but not for females, increases in IL-6 levels were associated with greater activation in several prefrontal regulatory regions (right and left ventrolateral prefrontal cortex (rVLPFC, lVLPFC), as well as several regions involved in mentalizing (DMPFC, pSTS). However, none of these regions correlated significantly with self-reported increases in depressed mood. Interestingly, activity in the rVLPFC, known to be involved in emotion and pain regulation (Eisenberger et al., 2003; Lieberman et al., 2004; Ochsner & Gross, 2005; Wager et al., 2004) correlated negatively with activation in the right anterior insula (34, 24, 14, r = -.89, p < .01), in a region similar to the right anterior insula activation that marginally mediated the relationship between IL-6 increases and depressed mood increases in females (42, 22, -2).
Discussion
The current study aimed to further understand the emerging relationship between proinflammatory cytokine activity and depressed mood by examining some of the possible neurocognitive substrates of these effects. Specifically, we were interested in whether proinflammatory cytokine activity contributed to heightened social pain sensitivity, which may increase vulnerability to depressive symptoms. To do this, we experimentally manipulated proinflammatory cytokine activity through exposure to endotoxin or placebo and then measured self-reported depressive symptoms as well as neural responses to an episode of social exclusion. Although we replicated previous research (Reichenberg et al., 2001) showing that endotoxin, compared to placebo, led to increases in depressed mood, we did not find meaningful differences between the two groups in neural responses to exclusion vs. inclusion. Instead, sex differences emerged, such that, for females, social pain-related neural activity mediated the relationship between IL-6 increases and depressed mood increases; whereas for males, there was no significant relationship between IL-6 increases and depressed mood.
Based on the lack of between-group differences in neural responses to social exclusion, one might conclude that experimentally-induced inflammation does not alter social pain sensitivity. However, our findings suggest that biological variability in the inflammatory response, as measured here by circulating concentrations of IL-6, may be more important in relating to differences in neural responses than the experimental challenge itself. Indeed, variability in the IL-6 response to endotoxin was associated with neural responses to social exclusion such that greater IL-6 increases were associated with greater activity in the anterior and posterior insula, regions involved in both physical and social pain processing (Apkarian et al., 2005; Aziz et al., 2000; Cechetto and Saper, 1987; Eisenberger et al., 2003). Moreover, activity in both of these regions correlated with increases in depressed mood. However, when taking the parameter estimates from the neural activations in this analysis and examining their correlations with depressed mood in each sex separately, the correlations between insula activity and depressed mood in the full endotoxin sample seemed to be driven largely by the females (left posterior insula: females r = .70, p < .05; males r = .33, ns; right anterior insula: females r = .77, p < .05; males r = .03, ns). Thus, although greater IL-6 responses to endotoxin were associated with greater social pain-related neural activity for subjects exposed to endotoxin, the relationship between these neural activations and depressed mood seemed specific to the females exposed to endotoxin. This is consistent with the neural analyses that were run for each sex separately, discussed below.
Unexpectedly, increases in IL-6 levels among those exposed to endotoxin were also associated with greater activity in several neural regions known to play a role in ‘mentalizing’ or understanding the mental states and behaviors of others (Frith and Frith, 1999, 2003, 2006; Mitchell et al., 2005). Thus, it is possible that heightened levels of inflammation are associated with greater efforts at processing the goals and intentions of others in socially threatening situations, possibly to better predict the behaviors of others, thereby protecting oneself in times of compromised physiological functioning. This is consistent with animal models of sickness behavior in which motivational resources are redirected to conserve energy, promote recuperation, and ensure the survival of the sick animal (Hart, 1988). When sick, it may be adaptive to become more sensitive to the goals and intentions of others in order to better predict their behavior and ensure one's own safety with minimal energy expenditure. The effect of proinflammatory cytokine activity on mentalizing ability has not been examined previously and is worthy of further investigation. Future studies would benefit from incorporating specific mentalizing paradigms to more carefully investigate the effects of inflammatory states on the capacity and tendency for mentalizing.
Sex Differences in Cytokine-Associated Depressed Mood and Neural Responses to Exclusion
Interestingly, sex differences emerged in the relationships between proinflammatory cytokine activity, depressed mood, and neural responses to social exclusion. First, for females exposed to endotoxin, increases in IL-6 levels were significantly associated with increases in depressed mood, whereas for males exposed to endotoxin, there was no significant relationship. Moreover, for females exposed to endotoxin, IL-6 increases were associated almost exclusively with greater activity in social pain-related neural activity, and this activity mediated the relationship between IL-6 increases and depressed mood increases. For males, IL-6 increases correlated with increases in some social pain-related neural regions as well as several regions involved in mentalizing and emotion regulation; however, none of these regions correlated significantly with increases in self-reported depressed mood. In other words, while greater increases in levels of IL-6 related to greater social pain-related neural activity in both males and females, the relationship between these neural activations and depressed mood was specific to females.
It is not yet clear what accounts for these sex differences; there are several different possibilities. At a cultural or societal level, it is possible that males in this study were less likely to report feeling depressed as this runs counter to gender stereotypes, which stipulate that it is less socially acceptable for males to express feelings of sadness or depression (Fabes and Martin, 1991). Although there were no sex differences in the effect of endotoxin vs. placebo on self-reported depressed mood, the tendency to downplay subtle changes in depressed mood may have prevented us from finding significant relationships between depressed mood and either IL-6 changes or neural responses to exclusion in males. Future studies should collect behavioral indices of depressed mood in addition to self-reports to reduce biases in how males and females report specific negative mood states.
At the neural level, it is possible that sex differences in cytokine-associated depressed mood are due, in part, to different neural processes typically engaged by males vs. females. In the current study, females primarily showed correlations between IL-6 increases and social pain-related neural activity, whereas males showed correlations between IL-6 increases and several different types of neural activity—including social pain-related neural activity as well as mentalizing and emotion regulatory neural activity. In fact, for males, activity in the rVLPFC, a region involved in emotion regulation, correlated negatively with activity in the anterior insula, in a region similar to the insula activation that mediated the relationship between IL-6 increases and depressed mood increases in females. Thus, it is possible that males engaged more regulatory neural activity during social exclusion, which was associated with reduced affective neural reactivity; we speculate that such enhanced neural regulatory activity may buffer males, in part, from the strong links between IL-6 responses, social pain-related neural activity, and depressed mood that was observed in females. Why males with greater inflammatory responses would recruit more neural regulatory activity than females, however, is not yet clear.
At the neurochemical level, it is possible that sex-specific hormonal substrates contributed to the observed sex differences in cytokine-associated depressed mood. Based on the higher prevalence of both depressive disorders and autoimmune conditions in females (Nolen-Hoeksema, 2001; Whitacre et al., 1999), sex hormones seem a likely contributor to sex differences in cytokine-associated depressed mood. Whether or not this is true is not yet known. However, based on research on sex differences in depression (Nolen-Hoeksema and Girgus, 1994), it is plausible that sex hormones in conjunction with other biological systems, may contribute to sex differences in cytokine-associated depression, although our analyses did not reveal that varying levels of estradiol or progesterone impacted our findings.
Finally, sex differences in inflammatory signaling processes (in addition to peripheral inflammation) may also relate to the neural differences observed. Inflammatory responses in the periphery are transduced to the brain to alter neural activity and depressed mood. Thus, in addition to “signal generated” (cytokines released at the periphery), neural and behavioral responses may reflect “signal heard”, which will be determined in part by the impact of inflammatory cytokines (e.g., IL-6) on inflammatory signaling in the brain. Nuclear factor κ-B (NF-κ-B), a key transcription factor that coordinates the inflammatory signaling cascade, is activated by inflammatory and other challenges. We speculate that sex differences in the neural responses to inflammatory signaling may be due in part to differences in activation of NF-κ-B. Indeed, we have previously found that females, but not males, show a marked activation of NF-κ-B in response to an acute stressor (sleep loss; Irwin et al., 2008), which is known to increase proinflammatory cytokines (Mullington et al., 2009). Future work is needed to determine whether females, as opposed to males, show differential increases in NF-κ-B activation or other nuclear signaling pathways in response to endotoxin.
Limitations and Conclusion
The findings reported here should be interpreted with caution for several reasons. First, the observed sex differences are based on very small sample sizes and thus future studies that include larger numbers of males and females will be needed to determine whether these sex differences replicate. Second, although the goal of this study was to examine the effect of proinflammatory cytokines on neural sensitivity to social exclusion, all of the neural findings are based on correlational analyses conducted among subjects in the endotoxin condition only. Because of this, it is not clear whether proinflammatory cytokines played a causal role in the neural responses observed here or whether some third variable contributed to the correlations between cytokine (IL-6) increases and neural responses to exclusion. It is unlikely, however, that the neural activity observed led to the IL-6 increases as our measure of IL-6 increases occurred prior to the neuroimaging session. Furthermore, it should be emphasized that endotoxin has been shown to increase the production and secretion of several different proinflammatory cytokines, including TNF-α and IL-1 receptor antagonist (Reichenberg et al., 2001). Thus, although we only examined correlations between IL-6 increases and neural activity in the current study, other proinflammatory cytokines may show similar or different patterns of relationships. It will be important for future investigations to examine relationships with other proinflammatory cytokines as well.
In addition, related to the neural activity observed, although we have characterized regions such as the insula or dACC as being involved in social pain processing, these regions have been shown to play a role in many other processes as well (e.g., disgust, performance monitoring; Phillips et al., 2003) and thus the activity observed here may not be specific to social pain. However, given that these neural responses were assessed in response to being socially excluded, it is more likely that these types of activations relate to social pain experience than to other types of processes. Finally, a limitation inherent to this and other studies of experimentally-induced proinflammatory cytokine activity is that we can only examine the effect of cytokines on depressed mood and not full-blown depression. Still, understanding the causal relationships between cytokine activation and depressed mood may provide us with valuable information about the relationships between inflammatory processes and depression.
In sum, experimentally-induced cytokine activation did not increase social pain sensitivity across the board; however, for females, proinflammatory cytokine increases (IL-6) following endotoxin administration were associated with both increases in social pain-related neural activity to social exclusion as well as increases in depressed mood. For males, IL-6 increases following endotoxin administration were associated with increases in neural activity related to social pain sensitivity, mentalizing, and emotion regulation; however none of these activations correlated with changes in depressed mood. Although future work will be needed to determine whether these findings replicate, this study advances our understanding of the social and emotional changes that relate to cytokine activation—changes that may be critical for understanding the contribution of proinflammatory cytokine activity to depressed mood.
Acknowledgments
The authors have no financial gain related to the outcome of this research, and there are no potential conflicts of interest. We would like to thank the staff and support of the UCLA General Clinical Research Center and the UCLA Brain Mapping Center. We would also like to thank Anthony Suffredini, M.D., and George Grimes, R.P., at the National Institutes of Health, Warren Grant Magnuson Clinical Center, for providing the standard reference endotoxin, Thanh Luu for performing the cytokine assays, Richard Olmstead, Ph.D. for statistical advice, and Matthew Lieberman, Ph.D., for feedback on the manuscript. This research was funded by a NARSAD Young Investigator Award (N.I.E.), a Dana Foundation grant (N.I.E), and a postdoctoral research fellowship (N.I.E., T32-MH19925). In addition, the authors acknowledge the additional support provided by grants HL-079955, AG-026364, CA-10014152, CA-116778, P30-AG028748, M01-RR00865, the UCLA Cousins Center at the Semel Institute for Neurosciences, the UCLA Claude D. Pepper Older Americans Independence Center Inflammatory Biology Core, and the General Clinical Research Centers Program (M01-RR00865).
Footnotes
It should be noted that inflammatory challenge (e.g., endotoxin) has been shown to lead to a coordinated pattern of increases across several different proinflammatory cytokines (e.g., IL-6, TNF-α, IL-1 receptor antagonist) and thus we are not hypothesizing that the effects of endotoxin are primarily or uniquely related to IL-6 levels. In the current study, however, we examined IL-6 levels, as this marker of inflammation has previously been shown to correlate with measures of depression (Howren, Lamkin & Suls, 2008) and to map along with other markers of inflammation (e.g., TNF-α) in response to inflammatory challenge (Reichenberg et al. 2001).
It should be noted that although anxiety was not the focus of this investigation, it was measured (using an abbreviated version of the Profile of Mood States (McNair et al., 1971)). Self-reported increases in anxiety did not correlate significantly with the neural activations reported here. In addition, controlling for self-reported increases in anxiety did not significantly change the relationships between these neural activations and self-reported increases in depressed mood.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andreasen AS, Krabbe KS, Krogh-Madsen R, Taudorf S, Pedersen BK, Møller K. Human endotoxemia as a model of systemic inflammation. Current Medicinal Chemistry. 2008;15:1697–1705. doi: 10.2174/092986708784872393. [DOI] [PubMed] [Google Scholar]
- Apkarian AV, Bushnell MC, Treede RD, Zubieta JK. Human brain mechanisms of pain perception and regulation in health and disease. Eur J Pain. 2005;9:463–484. doi: 10.1016/j.ejpain.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Aziz Q, Schnitzler A, Enck P. Functional neuroimaging of visceral sensation. J Clin Neurophysiol. 2000;17:604–612. doi: 10.1097/00004691-200011000-00006. [DOI] [PubMed] [Google Scholar]
- Bonaccorso S, Metzer H, Maes M. Psychological and behavioral effects of interferons. Current Opinion in Psychiatry. 2000;13:673–677. [Google Scholar]
- Bowlby J. Attachment and loss: Vol 3. Loss, sadness, and depression. New York: basic Books; 1980. [Google Scholar]
- Boyce P, Parker G, Barnett B, Cooney M, Smith F. Personality as a vulnerability factor for depression. British J Psychiatry. 1991;159:106–114. doi: 10.1192/bjp.159.1.106. [DOI] [PubMed] [Google Scholar]
- Brydon L, Harrison NA, Walker C, Steptoe A, Critchley HD. Peripheral inflammation is associated with altered substantia nigra activity and psychomotor slowing in humans. Biol Psychiatry. 2008;63:1022–1029. doi: 10.1016/j.biopsych.2007.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capuron L, Miller AH. Cytokines and psychopathology: Lessons from Interferon-α. Biol Psychiatry. 2004;56:819–824. doi: 10.1016/j.biopsych.2004.02.009. [DOI] [PubMed] [Google Scholar]
- Capuron L, Pagnoni G, Demetrashvili M, Woolwine BJ, Nemeroff CB, Berns GS, et al. Anterior cingulate activation and error processing during interferon-alpha treatment. Biol Psychiatry. 2005;58:190–196. doi: 10.1016/j.biopsych.2005.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cechetto DF, Saper CB. Evidence for a viscerotopic sensory representation in the cortex and thalamus in the rat. J Comp Neurol. 1987;262:27–45. doi: 10.1002/cne.902620104. [DOI] [PubMed] [Google Scholar]
- Dantzer R. Cytokine-induced sickness behavior: Mechanisms and implications. Ann N Y Acad Sci. 2001;933:222–234. doi: 10.1111/j.1749-6632.2001.tb05827.x. [DOI] [PubMed] [Google Scholar]
- Dantzer R, O'Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: When the immune system subjugates the brain. Nature Rev Neurosci. 2008;9:46–57. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson SS, Kemeny ME. Acute stressors and cortisol responses: A theoretical integration and synthesis of laboratory research. Psychol Bull. 2004;130:355–391. doi: 10.1037/0033-2909.130.3.355. [DOI] [PubMed] [Google Scholar]
- Eisenberger NI, Lieberman MD. Why rejection hurts: The neurocognitive overlap between physical and social pain. Trends Cogn Sci. 2004;8:294–300. doi: 10.1016/j.tics.2004.05.010. [DOI] [PubMed] [Google Scholar]
- Eisenberger NI, Lieberman MD, Williams KD. Does rejection hurt: An fMRI study of social exclusion. Science. 2003;302:290–292. doi: 10.1126/science.1089134. [DOI] [PubMed] [Google Scholar]
- Eisenberger NI, Taylor SE, Gable SL, Hilmert CJ, Lieberman MD. Neural pathways link social support to attenuated neuroendocrine stress responses. Neuroimage. 2007;35:1601–1612. doi: 10.1016/j.neuroimage.2007.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabes RA, Martin CL. Gender and age stereotypes of emotionality. Pers Soc Psych Bull. 1991;17:532–540. [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JB. Structured clinical interview for DSM-IV Axis I disorders, Patient Edition, Version 2.0. New York State Psychiatric Institute; 1996. [Google Scholar]
- Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): Use of a cluster-size threshold. Magn Reson Med. 1995;33:636–647. doi: 10.1002/mrm.1910330508. [DOI] [PubMed] [Google Scholar]
- Frith CD, Frith U. Interacting minds: A biological basis. Science. 1999;286:1692–1695. doi: 10.1126/science.286.5445.1692. [DOI] [PubMed] [Google Scholar]
- Frith U, Frith CD. Development and neurophysiology of mentalizing. Phiolosophical Transactions of the Royal Society of London. 2003;358:459–473. doi: 10.1098/rstb.2002.1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frith CD, Frith U. How we predict what other people are going to do. Brain Research. 2006;1079:36–46. doi: 10.1016/j.brainres.2005.12.126. [DOI] [PubMed] [Google Scholar]
- Frommberger UH, Bauer J, Haselbauer P, Fräulin A, Riemann D, Berger M. Interleukin-6-(IL-6) plasma levels in depression and schizophrenia: Comparison between the acute state and after remission. Eur Arch Psychiatry Clin Neurosci. 1997;247:228–233. doi: 10.1007/BF02900219. [DOI] [PubMed] [Google Scholar]
- Gillespie CF, Nemeroff CB. Hyoercortisolemia and depression. Psychosom Med. 2005;67:S26–S28. doi: 10.1097/01.psy.0000163456.22154.d2. [DOI] [PubMed] [Google Scholar]
- Gold PW, Chrousos GP. Organization of the stress system and its dysregulation in melancholic and atypical depression: High vs low CRH/NE states. Mol Psychiatry. 2002;7:254–275. doi: 10.1038/sj.mp.4001032. [DOI] [PubMed] [Google Scholar]
- Hart BL. Biological basis of the behavior of sick animals. Neurosci & Biobehavioral Rev. 1988;12:123–137. doi: 10.1016/s0149-7634(88)80004-6. [DOI] [PubMed] [Google Scholar]
- Irwin MR, Wang M, Ribeiro D, Cho HJ, Olmstead R, Breen EC, Martinez-Maza O, Cole S. Sleep loss activates cellular inflammatory signaling. Bio Psychiatry. 2008;64:538–540. doi: 10.1016/j.biopsych.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kent S, Bluthé RM, Kelley KW, Dantzer R. Sickness behavior as a new target of drug development. Trends in Pharmacological Sci. 1992;13:24–28. doi: 10.1016/0165-6147(92)90012-u. [DOI] [PubMed] [Google Scholar]
- Krabbe KS, Reichenberg A, Yirmiya R, Smed A, Pedersen BK, Bruunsgaard H. Low-does endotoxemia and human neuropsychological functions. Brain, Behavior, & Immunity. 2005;19:453–460. doi: 10.1016/j.bbi.2005.04.010. [DOI] [PubMed] [Google Scholar]
- Kronfol Z. Immune dysregulation in major depression: A critical review of existing evidence. Int J Neuropyyshopharmacology. 2002;5:333–343. doi: 10.1017/S1461145702003024. [DOI] [PubMed] [Google Scholar]
- Lane RD, Reiman EM, Ahern GL, Schwartz GE, Davidson RJ. Neuroanatomical correlates of happiness, sadness, and disgust. Am J Psychiatry. 1997;154:926–933. doi: 10.1176/ajp.154.7.926. [DOI] [PubMed] [Google Scholar]
- Levine J, Barak Y, Chengappa KRN, Rapoport A, Antelman SM, Barak V. Low CSF soluble interleukin 2 receptor levels in acute depression. J Neural Transm. 1999;106:1011–1015. doi: 10.1007/s007020050219. [DOI] [PubMed] [Google Scholar]
- Lieberman MD, Jarcho JM, Berman S, Naliboff BD, Suyenobu BY, Mandelkern M, Mayer EA. The neural correlates of placebo effects: A disruption account. Neuroimage. 2004;22:447–455. doi: 10.1016/j.neuroimage.2004.01.037. [DOI] [PubMed] [Google Scholar]
- MacKinnon DP, Lockwood CM, Hoffman JM, West SG, Sheets V. A comparison of methods to test mediation and other intervening variable effects. Psychol Methods. 2002;7:83–104. doi: 10.1037/1082-989x.7.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier SF, Watkins LR. Cytokines for psychologists: Implications of bidirectional immune-to-brain communication for understanding behavior, mood, and cognition. Psychological Rev. 1998;105:83–107. doi: 10.1037/0033-295x.105.1.83. [DOI] [PubMed] [Google Scholar]
- McNair DM, Lorr M, Droppleman LF. Education and Industrial Testing Service. San Diego, CA: 1971. Manual for the Profile of Mood States. [Google Scholar]
- Mitchell JP, Banaji MR, Macrae CN. The link between social cognition and self-referential thought in the medial prefrontal cortex. J Cogn Neuroscience. 2005;18:1306–1315. doi: 10.1162/0898929055002418. [DOI] [PubMed] [Google Scholar]
- Mullington JM, Haack M, Toth M, Serrador JM, Meier-Ewert HK. Cardiovascular, inflammatory, and metabolic consequences of sleep deprivation. Prog Cardiovasc Dis. 2009;51:294–302. doi: 10.1016/j.pcad.2008.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musselman DL, Miller AH, Porter MR, Manatunga A, Gao F, Penna S, et al. Higher than normal plasma interleukin-6 concentrations in cancer patients with depression: Preliminary findings. Am J Psychiatry. 2001;158:1252–1257. doi: 10.1176/appi.ajp.158.8.1252. [DOI] [PubMed] [Google Scholar]
- Nolen-Hoeksema S. Gender differences in depression. Current Directions in Psychological Sci. 2001;10:173–176. [Google Scholar]
- Nolen-Hoeksema S, Girgus JS. The emergence of gender differences in depression during adolescence. Psychol Bull. 1994;115:424–443. doi: 10.1037/0033-2909.115.3.424. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Gross JJ. The cognitive control of emotion. Trends Cogn Sci. 2005;9:242–249. doi: 10.1016/j.tics.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Peyron R, Laurent B, Garcia-Larrea L. Functional imaging of brain responses to pain. A review and meta-analysis. Neurophysiological Clinics. 2000;30:263–288. doi: 10.1016/s0987-7053(00)00227-6. [DOI] [PubMed] [Google Scholar]
- Phan KL, Wager TD, Taylor SF, Liberzon I. Functional neuroimaging studies of human emotions. CNS Spectr. 2004;9:258–266. doi: 10.1017/s1092852900009196. [DOI] [PubMed] [Google Scholar]
- Phillips ML, Drevets WC, Rauch SL, Lane R. Neurobiology of emotion perception I: The neural basis of normal emotion perception. Bio Psychiatry. 2003;54:504–514. doi: 10.1016/s0006-3223(03)00168-9. [DOI] [PubMed] [Google Scholar]
- Phillips ML, Young AW, Senior C, Brammer M, Andrew C, Calder AJ, et al. A specific neural substrate for perceiving facial expressions of disgust. Nature. 1997;389:495–498. doi: 10.1038/39051. [DOI] [PubMed] [Google Scholar]
- Ploghaus A, Tracey I, Gati JS, Clare S, Menon RS, Matthews PM, Rawlins JNP. Dissociating pain from its anticipation in the human brain. Science. 1999;284:1979–1981. doi: 10.1126/science.284.5422.1979. [DOI] [PubMed] [Google Scholar]
- Rainville P, Duncan GH, Price DD, Carrier B, Bushnell MD. Pain affect encoded in human anterior cingulate but not somatosensory cortex. Science. 1997;277:968–971. doi: 10.1126/science.277.5328.968. [DOI] [PubMed] [Google Scholar]
- Raison CL, Capuron L, Miller AH. Cytokines sing the blues: Inflammation and the pathogenesis of depression. Tr Immunology. 2006;27:24–31. doi: 10.1016/j.it.2005.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raison CL, Demetrashvili M, Capuron L, Miller AH. Neuropsychiatric adverse effects of Interferon-alpha: Recognition and Management. Therapy in practice. CNS Drugs. 2005;19:105–123. doi: 10.2165/00023210-200519020-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichenberg A, Yirmiya R, Schuld A, Kraus T, Haack M, Morag A, Pollmächer T. Cytokine-associated emotional and cognitive disturbances in humans. Arch Gen Psychiatry. 2001;58:445–452. doi: 10.1001/archpsyc.58.5.445. [DOI] [PubMed] [Google Scholar]
- Rivier C. Stimulatory effect of interleukin-1 beta on the hypothalamic-pituitary-adrenal axis of the rat: Influence of age, gender and circulating sex steroids. J Endocrinol. 1994;140:365–372. doi: 10.1677/joe.0.1400365. [DOI] [PubMed] [Google Scholar]
- Schiepers OJG, Wichers MC, Maes M. Cytokines and major depression. Progress in Neuro-Psychopharmacology & Biol Psychiatry. 2005;29:201–217. doi: 10.1016/j.pnpbp.2004.11.003. [DOI] [PubMed] [Google Scholar]
- Stephens RCM, O'Malley CMN, Frumento RF, Mythen MG, Bennett-Guerrero E. Low-dose endotoxin elicits variability in the inflammatory response in healthy volunteers. J Endotoxcin Research. 2005;11:204–209. doi: 10.1179/096805105X58661. [DOI] [PubMed] [Google Scholar]
- Suffredini AF, Hochstein HD, McMahon FG. Dose-related inflammatory effects of intravenous endotoxin in humans: Evaluation of a new clinical lot of Escherichia coli O:113 endotoxin. J Infectious Diseases. 1999;179:1278–1282. doi: 10.1086/314717. [DOI] [PubMed] [Google Scholar]
- Suffredini AF, O'Grady NP. Pathophysiologic responses to endotoxin in humans. In: Braude H, Opal SM, Vogel SN, Morrison DC, editors. Endotoxin in Health and Disease. 1st. New York: Marcel Dekker; 1999. pp. 817–830. [Google Scholar]
- Tonelli LH, Holmes A, Postolache TT. Intranasal immune challenge induces sex-dependent depressive-like behavior and cytokine expression in the brain. Neuropsychopharmacology. 2008;33:1039–1048. doi: 10.1038/sj.npp.1301488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager TD, Rilling JK, Smith EE, Sokolik A, Casey KL, Davidson RJ, Kosslyn SM, Rose RM, Cohen JD. Placebo-induced changes in fMRI in the anticipation and experience of pain. Science. 2004;303:1162–1167. doi: 10.1126/science.1093065. [DOI] [PubMed] [Google Scholar]
- Whitacre CC, Reingold SC, O'Looney PA. A gender gap in autoimmunity. Science. 1999;283:1277–1278. doi: 10.1126/science.283.5406.1277. [DOI] [PubMed] [Google Scholar]
- Williams KD, Cheung CKT, Choi W. Cyberostracism: Effects of being ignored over the Internet. J Pers Soc Psychol. 2000;79:748–762. doi: 10.1037//0022-3514.79.5.748. [DOI] [PubMed] [Google Scholar]
- Wright CE, Strike PC, Brydon L, Steptoe A. Acute inflammation and negative mood: Mediation by cytokine activation. Brain, Behavior, & Immunity. 2005;19:345–350. doi: 10.1016/j.bbi.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Zautra AJ, Hamilton NA, Potter P, Smith B. Field research of the relationship between stress and disease activity in rheumatoid arthritis. Ann N Y Acad Sci. 1999;876:397–412. doi: 10.1111/j.1749-6632.1999.tb07664.x. [DOI] [PubMed] [Google Scholar]


